Abstract
Background
Age-specific data on anal, and corresponding cervical, human papillomavirus (HPV) infection are needed to inform female anal cancer prevention.
Methods
We centrally reanalyzed individual-level data from 26 studies reporting HPV prevalence in paired anal and cervical samples by human immunodeficiency virus (HIV) status and age. For women with HIV (WWH) with anal high-grade squamous intraepithelial lesions or worse (HSIL+), we also investigated concurrent cervical cytopathology.
Results
In HIV-negative women, HPV16 prevalence decreased significantly with age, both at anus (4.3% at 15–24 years to 1.0% at ≥55 years; ptrend = 0.0026) and cervix (7.4% to 1.7%; ptrend < 0.0001). In WWH, HPV16 prevalence decreased with age at cervix (18.3% to 7.2%; ptrend = 0.0035) but not anus (11.5% to 13.9%; ptrend = 0.5412). Given anal HPV16 positivity, concurrent cervical HPV16 positivity also decreased with age, both in HIV-negative women (ptrend = 0.0005) and WWH (ptrend = 0.0166). Among 48 WWH with HPV16-positive anal HSIL+, 27 (56%) were cervical high-risk HPV-positive, including 8 with cervical HPV16, and 5 were cervical HSIL+.
Conclusions
Age-specific shifts in HPV16 prevalence from cervix to anus suggest that HPV infections in the anus persist longer, or occur later in life, than in the cervix, particularly in WWH. This is an important consideration when assessing the utility of cervical screening results to stratify anal cancer risk.
Keywords: anus, cervix, HIV, HPV, women
Age-specific shifts in HPV16 prevalence from cervix to anus suggest HPV infections may persist longer, or occur later in life, in the anus than cervix. This is an important consideration for using cervical screening results to direct anal cancer prevention.
Persistent anal high-risk human papillomavirus (HR-HPV) infection, especially HPV16, can lead to anal high-grade squamous intraepithelial lesions (HSIL) that may progress to anal squamous cell carcinoma (ASCC) [1–3]. Anal squamous cell carcinoma incidence has been increasing in many countries, in both genders, a phenomenon attributed to increased population-level sexual transmission of HPV [4]. Twenty-nine thousand ASCC cases, 100% HPV-attributable, were estimated to occur worldwide in 2018, approximately two thirds (19 000) of which occurred in women [5].
Despite the ASCC burden falling disproportionally on women, studies of anal HPV infection, anal lesions, and ASCC have focused largely on men, notably men who have sex with men (MSM), due to their high absolute risk of ASCC [6]. Much of these data were recently collated in a pooled analysis of 64 studies [7], clarifying age-specific prevalence of anal HPV and HSIL in men, by the main known determinants of male ASCC risk, namely, human immunodeficiency virus (HIV) status and sexuality. However, no comparable pooled estimates of age-specific anal HPV prevalence exist for informing ASCC prevention measures in women, among whom the most relevant population-based determinants of ASCC risk are HIV status and history of HPV-related genital (pre)cancer [6].
In a previous pooled analysis, we confirmed cervical HPV (most notably HPV16) and cervical cytopathology (HSIL and cancer) as significant correlates of anal HPV(16) and anal HSIL prevalence, suggesting that cervical screening programs might help stratify women at high ASCC risk [8]. However, overrepresentation of women with cervical HSIL and cancer prevented analysis of age-specific prevalence of anal HPV and HSIL in a population-based manner.
Therefore, we updated our pooled analysis of women with paired anal and cervical samples, with restriction to studies without under-/overrepresentation of cervical abnormalities. Our main objective was to provide population-based age-specific prevalence of anal, and corresponding cervical, HPV infection in women, stratified by HIV status, to inform ASCC prevention programs in women, and also to compare this with men [7]. Our focus was on HPV16, due to its uniquely high anal carcinogenicity [2, 3].
METHODS
Data Collection
We did a systematic literature review for studies reporting paired anal and cervical HPV infection in women, by updating the strategy used in previous pooled analysis [8]. We searched MEDLINE, Embase, and the Cochrane Library for studies published between January 1, 1986 and September 10, 2021, using the terms (“papillomaviridae” OR “papillomavirus” OR “HPV”) AND (“anal canal” OR “anus” OR “anal”).
Eligible studies were required (1) to report type-specific HPV deoxyribonucleic acid (DNA) detected by a polymerase chain reaction-based assay (at least for HPV16) in paired anal and cervical swabs taken at same study visit and (2) to report data from a female population without selections based on cervical cytopathological results or HPV DNA status. Authors of eligible studies were requested to contribute deidentified individual data on age, anal HPV genotypes, cervical HPV genotypes, and HIV status. Although these were not strict inclusion requirements, data on anal and cervical cytopathology were contributed, when available.
Statistical Analysis
Pooled anal and cervical HPV prevalence estimates were calculated for 13 individual HR-HPV types judged carcinogenic or probably carcinogenic (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) [3], as well as for 4 groups of HPV types (any HR-HPV; HPV16 and 18 [2v-HPV]; HPV6, 11, 16, and 18 [4v-HPV]; and HPV6, 11, 16, 18, 31, 33, 45, 52, and 58 [9v-HPV]). Only studies testing for all 13 HR-HPV types contributed to analyses of prevalence for any or multiple HR-HPV types.
Prevalence of HPV infection was stratified by HIV status and age group and compared between strata by prevalence ratios (PRs) with corresponding 95% confidence intervals (CIs), by use of generalized estimating equation, assuming the clustering of individual data within studies. For each type, HPV-positive women were categorized into 3 groups based on HPV-positive site: anus only, cervix only, or both sites. Among anal HPV-positive women, concurrency of HPV infection was estimated as the proportion that were positive for the same HPV type in the cervix. P values for age-specific trends in HPV prevalence were calculated using age group as a continuous variable.
Among a subset of studies with anal and cervical cytopathological results, we evaluated cervical HPV and cytopathology restricted to (1) women with anal HSIL or worse (HSIL+), defined as either cytological diagnosis of atypical squamous cells but cannot exclude HSIL, HSIL, or anal cancer; or histological diagnosis of grade 2 or 3 anal intraepithelial neoplasia, or anal cancer, and (2) women with HPV16-positive anal HSIL+, defined as having a diagnosis of anal HSIL+ and simultaneous detection of HPV16 in anal swabs. Cervical cytopathological diagnoses were classified as normal, low grade, and HSIL+, as previously described [8]. Stata (version 14) was used for all analyses.
RESULTS
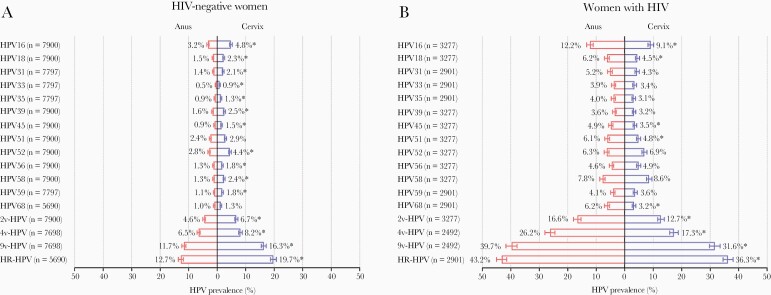
A total of 26 studies were included: 13 studies of 7900 HIV-negative women and 20 studies of 3277 WWH (Supplementary Table S1 and Figure S1). For most individual and groups of HPV types, prevalence was lower at the anus than cervix in HIV-negative women (Figure 1A), but higher at the anus than cervix in WWH (Figure 1B). For example, HPV16 prevalence was 3.2% (255 of 7900) in anus versus 4.8% (379 of 7900) in cervix among HIV-negative women (P < .0001), but 12.2% (399 of 3277) in anus versus 9.1% (299 of 3277) in cervix among WWH (P < .0001). All HR-HPV types were more prevalent in WWH than HIV-negative women, with HPV16 being the most common type, regardless of HIV status and anatomic site.
Figure 1.
Overall prevalence of anal and cervical human papillomavirus (HPV) infection in (A) human immunodeficiency virus (HIV)-negative women and (B) women with HIV. Error bar = 95% confidence interval. *Indicated HPV prevalence was statistically significantly different in the anus and the cervix using χ2 test. 2v-HPV, HPV16 and 18; 4v-HPV, HPV6, 11, 16, and 18; 9v-HPV, HPV6, 11, 16, 18, 31, 33, 45, 52, and 58; HR-HPV, high-risk HPV; HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68.
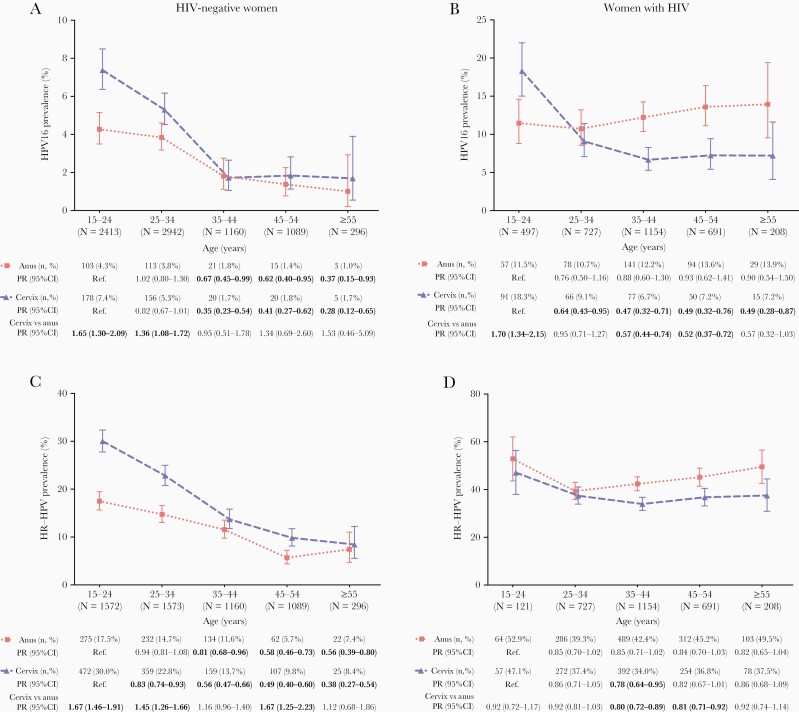
In HIV-negative women, HPV16 prevalence decreased significantly with age, both at anus (from 4.3% at 15–24 years to 1.0% at ≥55 years; ptrend = 0.0026) and cervix (7.4% to 1.7%; ptrend < 0.0001) (Figure 2A). The ratio of cervical HPV16 prevalence over that of anal HPV16 prevalence was higher in young age group (eg, PR = 1.65, 95% CI = 1.30–2.09, at 15–24 years). In WWH, HPV16 prevalence decreased with age at cervix (from 18.3% to 7.2%; ptrend = 0.0035), but it remained constant across all age groups at anus (11.5% to 13.9%; ptrend = 0.5412) (Figure 2B). HPV16 prevalence was higher in cervix than anus at 15–24 years (PR = 1.70, 95% CI = 1.34–2.15), but lower in cervix than anus at older ages (PR = 0.52, 95% CI = 0.37–0.72, at 45–54 years). Age-specific patterns of HR-HPV prevalence in the anus and cervix among HIV-negative women (Figure 2C) and WWH (Figure 2D) were similar to those for HPV16, although the decline in cervical HR-HPV prevalence was not significant in WWH (ptrend = 0.2499). Respective patterns for individual non-16 HR-HPV types and groups of HPV types are shown in Supplementary Figures S2 and S3. For WWH with information on current CD4 count, this did not vary much by age (Supplementary Figure S4), and age-specific trends in HPV16 prevalence were essentially unchanged after adjustment for current CD4 (ptrend = 0.9113 at anus and ptrend = 0.0067 at cervix).
Figure 2.
Age-specific prevalence of anal and cervical human papillomavirus (HPV)16 and high-risk (HR)-HPV infection in human immunodeficiency virus (HIV)-negative women and women with HIV: (A) HPV16 prevalence in HIV-negative women; (B) HPV16 prevalence in women with HIV; (C) HR-HPV prevalence in HIV-negative women; (D) HR-HPV prevalence in women with HIV. Error bar = 95% confidence interval. Significant prevalence ratios (PRs) relative to the reference group are shown in bold. HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68.
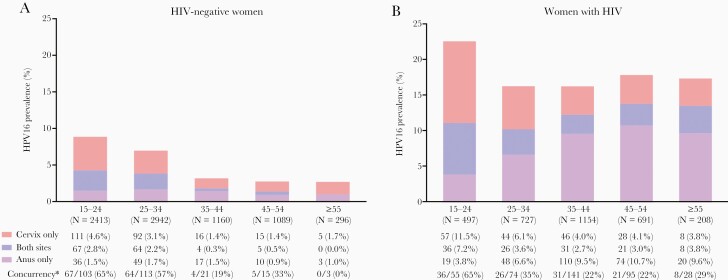
Age-specific patterns of combinations of cervical and anal HPV16 infection are shown in Figure 3, illustrating relative shifts in HPV16 infection from cervix to anus and declines in concurrent infections with increasing age. Among anal HPV16-positive women, concurrent cervical HPV16 infection decreased from 65% (67 of 103) at 15–24 years to 28% (5 of 18) at ≥45 years in HIV-negative women (ptrend = 0.0005) (Figure 3A) and from 65% (36 of 55) to 24% (29 of 123) in WWH (ptrend = 0.0166) (Figure 3B). Respective age-specific patterns of concurrent anal and cervical infection for non-HPV16 HR-HPV types are shown in Supplementary Figure S5.
Figure 3.
Combination of age-specific cervical and anal human papillomavirus (HPV)16 prevalence in (A) human immunodeficiency virus (HIV)-negative women and (B) women with HIV. *Concurrent cervical HPV16 infection in all women with anal HPV16 infection by age group.
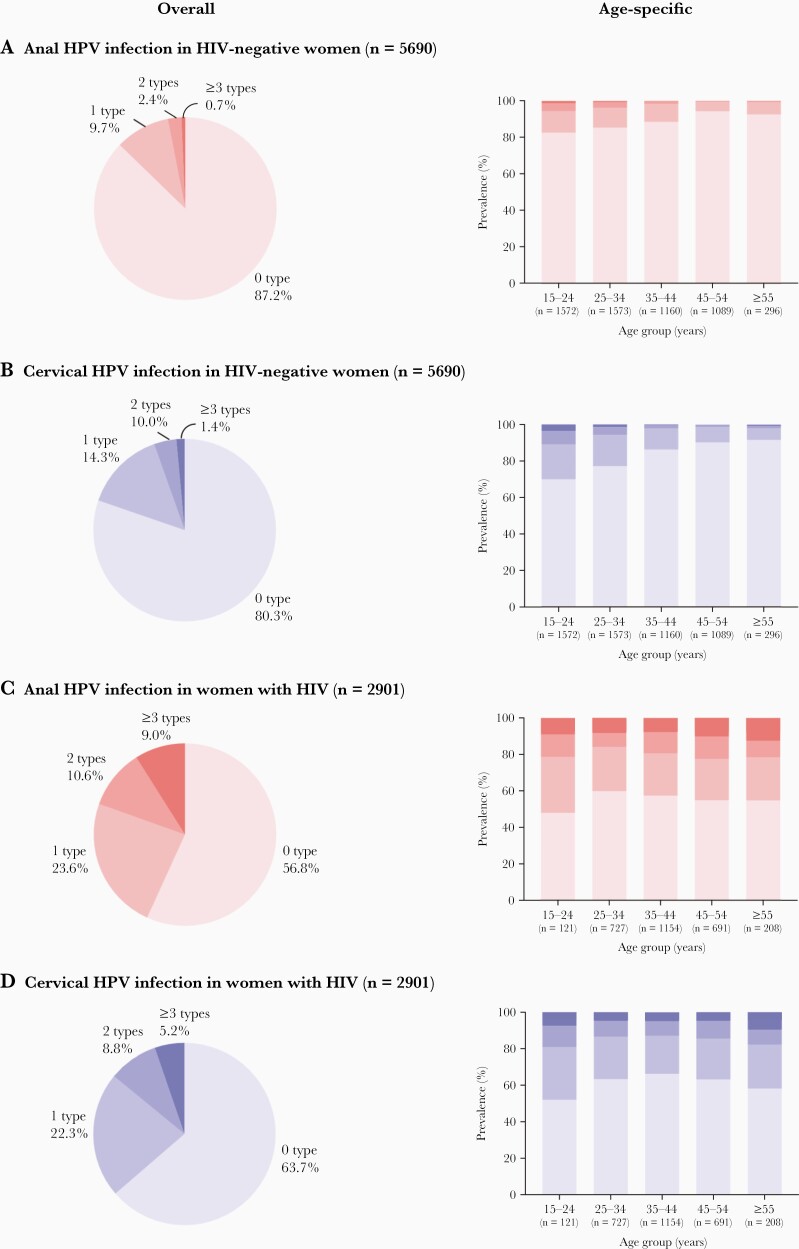
In HIV-negative women, prevalence of multiple HR-HPV infections decreased with age, at both anus and cervix, but no differences by age were observed in WWH (Figure 4).
Figure 4.
Overall and age-specific multiplicity of high-risk human papillomavirus (HPV) infection in women by human immunodeficiency virus (HIV) status and anatomic site: (A) anal HPV infection in HIV-negative women; (B) cervical HPV infection in HIV-negative women; (C) anal HPV infection in women with HIV; (D) cervical HPV infection in women with HIV.
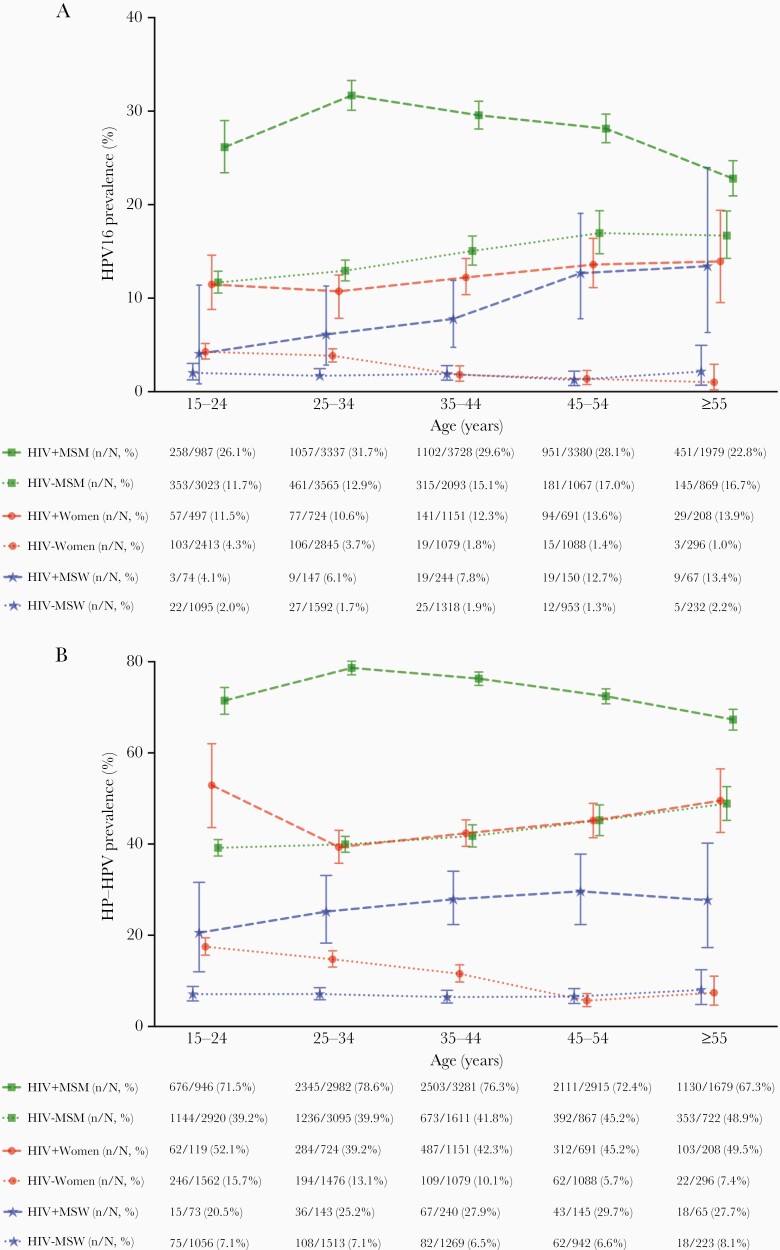
Age-specific prevalence of anal HPV16 and HR-HPV in WWH was lower than that of MSM with HIV (MSMWH) and tended to be similar to that of HIV-negative MSM [7], whereas that of HIV-negative women was lower than that in MSM and in men who have sex with women (MSW) with HIV (MSWWH), but tended to be higher than that in HIV-negative MSW, especially for those younger than 35 years (Figure 5).
Figure 5.
Comparison of age-specific prevalence of anal (A) human papillomavirus (HPV)16 and (B) high-risk (HR) HPV infection among women (in this study) and men [7], according to human immunodeficiency virus (HIV) status and male sexuality. HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68; MSM, men who have sex with men; MSW, men who have sex with women.
Nine of 26 studies contributed data on both anal and cervical cytopathology in WWH (Supplementary Table S1). Prevalence of anal HSIL+ and HPV16-positive anal HSIL+ in WWH was 6% (115 of 1852) and 3% (48 of 1852), respectively, with no differences observed by age group (Table 1). When restricting to 115 WWH diagnosed with anal HSIL+, 64 (56%) were negative for cervical HR-HPV infection, 40 (35%) positive for cervical non-16 HR-HPV only, and 11 (9%) positive for cervical HPV16; 65 (57%) had normal cervical cytology, 42 (36%) cervical LSIL, and 8 (7%) cervical HSIL+. Upon restriction to 48 WWH with HPV16-positive anal HSIL+, 8 (17%) cases were positive for cervical HPV16 and 5 (10%) were cervical HSIL+. No age-specific differences were observed in these proportions.
Table 1.
Overall and Age-Specific Distribution of Cervical High-Risk HPV Infection and Cervical Cytopathology Restricted to WWH With Anal HSIL+ and Anal HPV16-Positive HSIL+
| Overall n (%) |
15–34 Years n (%) |
35–44 Years n (%) |
≥45 Years n (%) |
|
|---|---|---|---|---|
| Anal HSIL+a | N = 115 | N = 28 | N = 47 | N = 40 |
| Cervical HR-HPV Infection | ||||
| Negative | 64 (56%) | 16 (57%) | 24 (51%) | 24 (60%) |
| Positive | 51 (44%) | 12 (43%) | 23 (49%) | 16 (40%) |
| Non-16 HR-HPV only | 40 (35%) | 10 (36%) | 19 (40%) | 11 (28%) |
| HPV16 | 11 (9%) | 2 (7%) | 4 (9%) | 5 (12%) |
| Cervical Cytopathology | ||||
| Normal | 65 (57%) | 14 (50%) | 24 (51%) | 27 (68%) |
| LSIL | 42 (36%) | 13 (46%) | 18 (38%) | 11 (27%) |
| HSIL+ | 8 (7%) | 1 (4%) | 5 (11%) | 2 (5%) |
| HPV16-positive anal HSIL+b | N = 48 | N = 12 | N = 17 | N = 19 |
| Cervical HR-HPV Infection | ||||
| Negative | 21 (44%) | 5 (42%) | 5 (29%) | 11 (58%) |
| Positive | 27 (56%) | 7 (58%) | 12 (71%) | 8 (42%) |
| Non-16 HR-HPV only | 19 (39%) | 6 (50%) | 9 (53%) | 4 (21%) |
| HPV16 | 8 (17%) | 1 (8%) | 3 (18%) | 4 (21%) |
| Cervical Cytopathology | ||||
| Normal | 33 (69%) | 9 (75%) | 10 (59%) | 14 (74%) |
| LSIL | 10 (21%) | 2 (17%) | 5 (29%) | 3 (16%) |
| HSIL+ | 5 (10%) | 1 (8%) | 2 (12%) | 2 (10%) |
Abbreviations: HIV, human immunodeficiency virus; HR-HPV, high-risk human papillomavirus; HSIL+, high-grade squamous intraepithelial lesions or worse; WWH, women with HIV.
The overall prevalence of anal HSIL+ in women with HIV was 6% (115 of 1852). Prevalence was 6% (28 of 466) at age 15–34 years, 7% (47 of 713) at age 35–44 years, and 6% (40 of 673) at age ≥45 years.
HPV16-positive anal HSIL+ includes only HSIL+ with simultaneous presence of HPV16 positivity in swabs; overall prevalence of HPV16-positive anal HSIL+ in women with HIV was 3% (48 of 1852); prevalence was 3% (12 of 466) at age15–34 years, 2% (17 of 713) at age 35–44 years, and 3% (19 of 673) at age ≥45 years.
DISCUSSION
This collaborative pooled analysis of individual-level data from 7900 HIV-negative women and 3277 WWH is the first to comprehensively describe age-specific epidemiology of anal HPV infection among women by HIV status. Building upon our previous pooled analysis of anal HPV infection in women [8], by updating with newer studies and focusing on those not selected on cervical abnormalities, it allows standardized comparisons with that in male risk groups [7] and with corresponding cervical HPV infection in a population-based approach. With a focus on HPV16, our data showed that, whereas anal HPV prevalence decreases with age in HIV-negative women (albeit to a lesser extent than the concomitant decrease in cervical HPV infection), this is not the case in WWH, in whom anal HPV infection remains high among those aged ≥55, despite a concomitant age-specific decrease in cervical HPV infection. These data provide important evidence of shifts in HPV16 prevalence from cervix to anus with increasing age, a phenomenon most apparent in WWH, but to a lesser degree even in HIV-negative women.
It is unfortunate that the cross-sectional nature of this pooled analysis cannot provide evidence on HPV transmission between cervix and anus. Nevertheless, when considered with other evidence on the directionality of cervical/anal HPV transmission in women, age-specific shifts in HPV16 prevalence suggest infections may persist in the anus despite clearance from the cervix. Indeed, multiple lines of evidence suggest that anal HPV exposure in females may occur from the cervix/external genital region via auto- or partner-assisted inoculation. These include studies reporting associations between anal sexual intercourse and anal HPV that are either nonsignificant or less important than number of sexual partners per se [2, 8–11], studies showing that a majority of women with anal cancer report no history of anal sexual intercourse [12], and studies reporting a link between front-to-back wiping or partner anal touching using fingers or mouth and anal HPV infection [13, 14]. Finally, longitudinal studies have shown that anal HPV incidence is higher among women with previous cervical HPV infection of the same type, and that this association is stronger than that for sequential transmission in the other direction [15, 16]. Alternative explanations for observed age-specific shifts in prevalence from cervix to anus include age-specific differences in tissue or immune susceptibility [17, 18], or sexual behavior [19].
Correlation of cervical and anal HPV infection was also observed to decrease with age. In our previous pooled analysis [8], we reported cervical and anal HPV to be strongly correlated, with approximately 40% of women with cervical HPV16 infection having concurrent anal HPV16 infection, irrespective of HIV infection. In the current study, we show this correlation to be contributed predominantly by younger women, in whom cervical and anal HPV prevalence are the highest. This is consistent with longitudinal evidence that rates of sequential acquisition of cervical-to-anal, as well as anal-to-cervical, HPV infection, decrease with age [15].
We were able to collate abundant data on anal HPV16 infection in HIV-negative women. These data are important to informing ASCC prevention programs because, although ASCC incidence rate is lower than that in WWH [6], HIV-negative women contribute to the majority of ASCC at a population level, at least in high-income settings such as the United States [20]. Indeed, the recognition that ASCC burden in HIV-negative women is almost entirely HPV16-related [2] has led to speculation about whether modern HPV-based cervical screening programs might contribute to ASCC prevention in older, unvaccinated generations of women, by referring women testing cervical HPV16-positive for secondary ASCC prevention measures [8]. Given the significant decreases in anal HPV16 infection observed at older ages in HIV-negative women, the number of anal HPV16 infections observed in women aged ≥55 years in this analysis remained very limited, highlighting the need for more studies on anal HPV infection in older HIV-negative women. Indeed, the fact that only 5 of 13 anal HPV16-positive women aged ≥45 years were concurrently positive for cervical HPV16 already raises questions about the population-level feasibility of using cervical HPV16 infection to identify HIV-negative women at high risk of HPV16-related ASCC.
Consistent with pooled analyses of anal HPV infection in men [7], as well as with evidence of elevated anal cancer incidence in WWH [6], HIV positivity was a strong determinant of anal HPV infection among women, in all age groups. In WWH, HPV16 prevalence was constant across all age groups in the anus, whereas cervical HPV16 prevalence declined with age. The underlying reasons for such age-specific differences are unclear but may be due to differences in age-related immune surveillance or susceptibility between the 2 anatomical sites, exacerbated in the presence of HIV-related immunosuppression. Of note, we have previously shown severity of concurrent markers of HIV-positive immunosuppression (eg, lower current CD4 cell count or higher current HIV viral load) to be relatively weak determinants of anal HPV16 and HSIL+ prevalence in WWH [8], as well as in MSMWH [7], and findings on age-specific trends of anal and cervical HPV16 prevalence in the current study were essentially unchanged after adjustment for current CD4.
Such high anal (and cervical) HPV prevalence observed already in young WWH highlights the advantages of prophylactic HPV vaccination in 10- to 14-year-olds [21], before sexual debut and acquisition of HIV and HPV infection, to prevent HPV-related cancer in this high-risk group. Given lack of vaccine efficacy against existing HPV infection, and high HPV exposure in women aged 15–24, the cost-benefit ratio of HPV vaccination in WWH is expected to fall quickly with increasing age. Indeed, although a recent clinical trial has reported significant effectiveness of HPV vaccination in MSMWH under 26 [22], 2 other trials reported lack of effectiveness against anal HPV infection and lesions in HIV-infected adults older than 26 years [23, 24].
In the meantime, generations of unvaccinated WWH remain at elevated ASCC risk and may benefit from secondary prevention. Certain guidelines for HIV management already make specific recommendations, tending to focus on WWH with cervical lesions [24-27]. Although no data yet exist on ASCC risk in WWH with cervical HSIL+, evidence from our previous pooled analysis of elevated prevalence of surrogate markers of anal cancer (prevalence of anal HPV16, anal HSIL+, and their combination) among WWH with cervical HSIL supports their higher risk [8]. By restricting to women unselected for cervical abnormalities, the current analysis describes the relationship of anal and cervical cytopathology in the opposite direction, namely, cervical status of women with anal HSIL+. This revealed that only 7% of anal HSIL+ were detected in WWH with concurrent cervical HSIL+, and that less than half had any concurrent cervical abnormality or any cervical HR-HPV infection, as reported previously [28]. Upon restriction to women with HPV16-positive anal HSIL+, the most severe known surrogate for anal cancer risk, only 10% had evidence of concurrent cervical HSIL+ and, surprisingly, only one fifth had evidence of concurrent cervical HPV16 infection, even among women aged ≥45 years. These data suggest that not only anal HPV16 infection, but even HPV16-positive anal HSIL, may persist at the anus of WWH after HPV16 infection has been cleared from the cervix. If this is the case, the discriminating power of cervical HSIL or HPV16 infection to identify WWH at high risk for ASCC may be suboptimal and may limit the utility of triaging WWH for anal cancer screening based on cervical screening results, particularly in settings where the population of WWH is relatively small and might be universally referred for secondary ASCC prevention measures.
Certain limitations of our analysis should be noted. First, women with unknown HIV status were defined as HIV negative. We believe this is valid because most of these women were from population-based studies in China, United States, and Costa Rica, where HIV prevalence is very low, and because we had previously shown equivalence in their anal and cervical HPV prevalence with that in HIV-negative women [8]. Second, although we could not analyze data by HPV vaccination status, we excluded the few women who were known to be HPV vaccinated, and the combination of study years, country, and age group of included women suggested almost all were ineligible for HPV vaccination programs. Thus, these data can be considered to represent the epidemiological picture before HPV vaccine impact. We recognize that most of our findings come from high-income settings, leading to an underrepresentation of many world regions, even for WWH.
It is unfortunate that small sample sizes in this pooled analysis precluded analyses of anal and cervical cytopathology in HIV-negative women. Furthermore, even for WWH, important heterogeneity in detection of anal HSIL between studies [7] precluded analyses of cervical results using classic screening performance indicators (sensitivity, specificity, and negative and positive predictive values); rather, we focused on a description of cervical status in known anal HSIL+ cases only. Although overall prevalence of anal HSIL+ (6%) and HPV16-positive HSIL+ (3%) in WWH was lower than in similarly collated data from MSMWH (22%; 10%) [7], or even HIV-negative MSM (11%; 5%) [7], we cannot exclude heterogeneity in anal HSIL+ detection across different studies.
We are more confident, however, in the comparability of age-stratified anal HPV prevalence data across male and female risk groups [7], because of greater objectivity in HPV testing compared with cytopathological ascertainment. By focusing on women aged ≥45 years (below which anal cancer rarely occurs), we reveal that the patterns in anal HPV16 prevalence in male and female risk groups are broadly similar to patterns of ASCC incidence rates among these same risk groups [6]. For example, anal HPV16 prevalence in WWH was lower than that of MSMWH, but it was similar to that of MSWWH and HIV-negative MSM, broadly mirroring ASCC incidence rates. This comparison suggests that anal HPV16 prevalence does have some utility as a surrogate of anal cancer risk, even if the differences between these risk groups are much smaller for anal HPV16 infection than for anal cancer risk. These age-specific data can inform prioritization of risk groups for secondary ASCC screening initiatives, and they provide an estimate of the proportion of women (and men) in different age groups that could be expected to test positive in an anal cancer screening program based upon detection of prevalent anal HPV16 or HR-HPV infection.
Supplementary Material
Notes
Acknowledgments. We thank Catharina J. Alberts for her comments on manuscript, Susan Gamon for her help on text editing, and the other colleagues involved in original studies: Yurii Shvetsov and Lynne Wilkens ([17] in the appendix); Arati Mane ([23] in the appendix); and Nittaya Phanuphak and Stephen Kerr ([13] in the appendix).
Disclaimer. The authors alone are responsible for the views expressed in this paper and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Contributor Information
Feixue Wei, Early Detection, Prevention and Infections Branch, International Agency for Research on Cancer/World Health Organization, Lyon, France.
Ningshao Xia, State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Institute of Diagnostics and Vaccine Development in Infectious Diseases, School of Public Health, Xiamen University, Xiamen, Fujian, China.
Rebeca Ocampo, Agencia Costarricense de Investigaciones Biomédicas-Fundación INCIENSA, San José, Costa Rica.
Marc T Goodman, Cancer Prevention and Control Program, Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Medical Center, Los Angeles, California, USA.
Nancy A Hessol, Department of Clinical Pharmacy, University of California, San Francisco, California, USA; Department of Medicine, University of California, San Francisco, California, USA.
Beatriz Grinsztejn, Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil.
Ana P Ortiz, Puerto Rico Cancer Control and Population Sciences Division, University of Puerto Rico Comprehensive Cancer Center, San Juan, Puerto Rico; Department of Biostatistics and Epidemiology, Graduate School of Public Health, Medical Sciences Campus, University of Puerto Rico, San Juan, Puerto Rico.
Fanghui Zhao, Department of Cancer Epidemiology, National Cancer Center & Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China.
Erna M Kojic, Division of Infectious Diseases, Department of Medicine, Mount Sinai West and Morningside, New York, New York, USA.
Rupert Kaul, Department of Medicine, University of Toronto and University Health Network, Toronto, Ontario, Canada.
Isabelle Heard, Department of Endocrinology and Reproductive Medicine, Institut Endocrinologie, Maladies Métaboliques et Médecine Interne, Assistance Publique - Hôpitaux de Paris, Groupe Hospitalier Pitié- Salpêtrière, Paris, France.
Imran O Morhason-Bello, Department of Obstetrics and Gynaecology, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria; Institute of Advanced Medical Research and Training, College of Medicine, University of Ibadan, Ibadan, Oyo State, Nigeria.
Anna-Barbara Moscicki, Department of Pediatrics, University of California, Los Angeles, California, USA.
Alexandra de Pokomandy, McGill University Department of Family Medicine and McGill University Health Centre, Montreal, Quebec, Canada.
Joel M Palefsky, Department of Medicine, University of California, San Francisco, California, USA.
Luana L S Rodrigues, Programa de Pós-Graduação em Ciências da Saúde, Instituto de Saúde Coletiva, Universidade Federal do Oeste do Pará, Santarém, Pará, Brazil; Laboratório de AIDS e Imunologia Molecular, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Rio de Janeiro, Brazil.
Racheal S Dube Mandishora, Early Detection, Prevention and Infections Branch, International Agency for Research on Cancer/World Health Organization, Lyon, France; Medical Microbiology Unit, University of Zimbabwe Faculty of Health Sciences, Harare, Zimbabwe.
Reshmie A Ramautarsing, Institute of HIV Research and Innovation, Bangkok, Thailand.
Silvia Franceschi, Centro di Riferimento Oncologico, Istituto di Ricovero e Cura a Carattere Scientifico, Aviano, Italy.
Sheela V Godbole, Division of Epidemiology and Biostatistics, Indian Council of Medical Research, National AIDS Research Institute, Pune, India.
Fernanda K Tso, Department of Gynecology, Federal University of São Paulo, São Paulo, Brazil.
Lynette J Menezes, Division of Infectious Disease and International Medicine, University of South Florida, Tampa, Florida, USA.
Chunqing Lin, National Cancer Center, National Clinical Research Center for Cancer, and Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Gary M Clifford, Early Detection, Prevention and Infections Branch, International Agency for Research on Cancer/World Health Organization, Lyon, France.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
References
- 1. Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine 2006; 24 (Suppl 3):S3/42–51. [DOI] [PubMed] [Google Scholar]
- 2. Lin C, Franceschi S, Clifford GM.. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect Dis 2018; 18:198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans . Biological agents. Volume 100B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 2012; 100: 1–475. [PMC free article] [PubMed] [Google Scholar]
- 4. Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A.. International trends in anal cancer incidence rates. Int J Epidemiol 2017; 46:924–38. [DOI] [PubMed] [Google Scholar]
- 5. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM.. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 2020; 8:e180–90. [DOI] [PubMed] [Google Scholar]
- 6. Clifford GM, Georges D, Shiels MS, et al. A meta-analysis of anal cancer incidence by risk group: toward a unified anal cancer risk scale. Int J Cancer 2021; 148:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wei F, Gaisa MM, D’Souza G, et al. Epidemiology of anal human papillomavirus infection and high-grade squamous intraepithelial lesions in 29 900 men according to HIV status, sexuality, and age: a collaborative pooled analysis of 64 studies. Lancet HIV 2021; 8:e531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin C, Slama J, Gonzalez P, et al. Cervical determinants of anal HPV infection and high-grade anal lesions in women: a collaborative pooled analysis. Lancet Infect Dis 2019; 19:880–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Volpini LPB, Boldrini NAT, de Freitas LB, Miranda AE, Spano LC.. The high prevalence of HPV and HPV16 European variants in cervical and anal samples of HIV-seropositive women with normal Pap test results. PLoS One 2017; 12:e0176422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kojic EM, Cu-Uvin S, Conley L, et al. Human papillomavirus infection and cytologic abnormalities of the anus and cervix among HIV-infected women in the study to understand the natural history of HIV/AIDS in the era of effective therapy (the SUN study). Sex Transm Dis 2011; 38:253–9. [DOI] [PubMed] [Google Scholar]
- 11. Stier EA, Sebring MC, Mendez AE, Ba FS, Trimble DD, Chiao EY.. Prevalence of anal human papillomavirus infection and anal HPV-related disorders in women: a systematic review. Am J Obstet Gynecol 2015; 213:278–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daling JR, Madeleine MM, Johnson LG, et al. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004; 101:270–80. [DOI] [PubMed] [Google Scholar]
- 13. Simpson S Jr., Blomfield P, Cornall A, Tabrizi SN, Blizzard L, Turner R.. Front-to-back & dabbing wiping behaviour post-toilet associated with anal neoplasia & HR-HPV carriage in women with previous HPV-mediated gynaecological neoplasia. Cancer Epidemiol 2016; 42:124–32. [DOI] [PubMed] [Google Scholar]
- 14. Moscicki AB, Ma Y, Farhat S, et al. Natural history of anal human papillomavirus infection in heterosexual women and risks associated with persistence. Clin Infect Dis 2014; 58:804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goodman MT, Shvetsov YB, McDuffie K, et al. Sequential acquisition of human papillomavirus (HPV) infection of the anus and cervix: the Hawaii HPV Cohort Study. J Infect Dis 2010; 201:1331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei F, Su Y, Cui X, et al. Sequential acquisition of human papillomavirus infection at genital and anal sites, Liuzhou, China. Emerg Infect Dis 2020; 26:2387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Sanjose S, Palefsky J.. Cervical and anal HPV infections in HIV positive women and men. Virus Res 2002; 89:201–11. [DOI] [PubMed] [Google Scholar]
- 18. Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst 2005; 97:577–86. [DOI] [PubMed] [Google Scholar]
- 19. Roberts H, Clark A, Sherman C, Heitzeg MM, Hicks BM.. Age, sex, and other demographic trends in sexual behavior in the United States: initial findings of the sexual behaviors, internet use, and psychological adjustment survey. PLoS One 2021; 16:e0255371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shiels MS, Pfeiffer RM, Chaturvedi AK, Kreimer AR, Engels EA.. Impact of the HIV epidemic on the incidence rates of anal cancer in the United States. J Natl Cancer Inst 2012; 104:1591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Human papillomavirus vaccines: WHO position paper, May 2017. Wkly Epidemiol Rec 2017; 92:241–68. [PubMed] [Google Scholar]
- 22. Palefsky JM, Lensing SY, Belzer M, et al. High prevalence of anal high-grade squamous intraepithelial lesions, and prevention through human papillomavirus vaccination, in young men who have sex with men living with HIV. Clin Infect Dis 2021; 73:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilkin TJ, Chen H, Cespedes MS, et al. A randomized, placebo-controlled trial of the quadrivalent human papillomavirus vaccine in human immunodeficiency virus-infected adults aged 27 years or older: AIDS Clinical Trials Group Protocol A5298. Clin Infect Dis 2018; 67:1339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hidalgo-Tenorio C, Pasquau J, Omar-Mohamed M, et al. Effectiveness of the quadrivalent HPV vaccine in preventing anal ≥ HSILs in a Spanish population of HIV+ MSM aged > 26 years. Viruses 2021; 13:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. European AIDS Clinical Society. EACS guidelines version 9.1. Available at: http://www.eacsociety.org/files/2018_guidelines-9.1-english.pdf. Accessed 31 July 2021. [Google Scholar]
- 26. Morlat P. [Prise en charge médicale des personnes vivant avec le VIH. Recommandations du groupe d’expert. Cancers. Conseil national du sida et des hépatites virales/Agence nationale de recherches sur le sida et les hépatites virales]. Available at: https://cns.sante.fr/wp-content/uploads/2017/10/experts-vih_cancers.pdf. Accessed 31 July 2021.
- 27. National AIDS Treatment Advocacy Project. NYS guidelines recommendations on anal pap smears. Available at: http://www.natap.org/2010/HIV/032510_01.htm. Accessed 31 July 2021.
- 28. Chiao EY, Lensing SY, Wiley DJ, et al. Screening strategies for the detection of anal high-grade squamous intraepithelial lesions in women living with HIV. AIDS 2020; 34:2249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.