Abstract
Background:
Cognitive impairment occurs in 40%–70% of persons with multiple sclerosis (MS).
Objective:
To examine the effectiveness of natalizumab compared with other disease-modifying treatments (DMTs) on improving cognition as measured by the Symbol Digit Modalities Test (SDMT).
Methods:
Data were collected as part of Swedish nationwide phase IV surveillance studies (2007–2020). An increase in SDMT score by ⩾10% of the difference between maximum score possible (110) and the baseline value was defined as cognitive improvement. The likelihood of improvement was compared between natalizumab-treated individuals and individuals treated with other DMTs using mixed effect logistic regression. Trend in odds of improvement was investigated using slope analyses.
Results:
We included 2100 persons with relapsing-remitting MS treated with natalizumab and 2622 persons treated with other DMTs. At 6 months, 45% reached improvement. The natalizumab group showed largest odds of improvement during follow-up (odds ratio: 2.3, 95% confidence interval (CI): 1.5–3.5). The odds of improvement increased by 7% (95% CI: 6–7) per month of natalizumab treatment. The equivalent estimate was 4% (95% CI: 2–5) for other monoclonal antibodies and nonsignificant for oral or platform therapies.
Conclusion:
Treatment with natalizumab or other monoclonal antibodies is associated with a significantly faster likelihood of cognitive improvement than platform or oral DMTs.
Keywords: Cognition, natalizumab, comparative effectiveness
Introduction
Cognitive decline occurs in 40–70% of persons with multiple sclerosis (MS). 1 It is associated with stress, decline in standards of living, loss of employment, and withdrawal from social activities.2,3 Cognitive impairment occurs in all stages of the disease4,5 often independent of physical disability. 6
Natalizumab is a humanized monoclonal antibody 7 used as a highly potent disease-modifying treatment (DMT) in persons with active MS. Natalizumab has been shown to efficiently reduce the risk of sustained progression of physical disability and the rate of clinical relapses in relapsing-remitting multiple sclerosis (RRMS). 8
Studies have also shown that natalizumab decreases the risk of cognitive decline and is associated with clinically meaningful improvement in cognition in individuals with MS.9–12 However, the findings are mainly reported in non-randomized observational studies with short-term follow-ups and small sample sizes. In general, evidence on the effect of DMTs on cognitive improvement in MS is lacking, particularly from randomized controlled trials. 13 Furthermore, no study has investigated the comparative effectiveness of natalizumab on cognition with other DMTs. Hence, the objective of this study was to determine and compare the long-term effectiveness of natalizumab on cognition as measured by the Symbol Digit Modalities Test (SDMT)14,15 in a large population-based cohort of individuals with MS. The SDMT has been shown to be associated with several clinical and patient-centered outcomes in MS, such as income, employment, and daily activity.3,16,17 In MS, performance on the SDMT is shown to be predictive of future cognitive decline. 18 The SDMT has been found to be the most sensitive individual cognitive measure for use in MS.19,20 In this work, we examined the effectiveness of natalizumab on the improvement of cognition and compared its effect to the effects seen by using platform therapies, other monoclonal antibodies, and oral DMTs.
Method
Data source
Data for this study were collected from a Swedish post-market surveillance study of the long-term effectiveness and safety of DMTs, which started in 2006, the Immunomodulation and MS Epidemiology (IMSE) study.21,22 The IMSE cohorts are prospective recruitments of all individuals with MS throughout Sweden who start on natalizumab, fingolimod, alemtuzumab, teriflunomid, dimetyl fumarate, peginterferon beta-1a, rituximab, daclizumab, ocrelizumab, or cladribin. Based on total sold doses, it has been estimated that more than 95% of the Swedish MS patients who started on natalizumab are part of the IMSE cohort. 21 In IMSE, patients are evaluated at baseline (treatment initiation), 6 months and annually thereafter until treatment discontinuation. A neurologist performs clinical evaluation at each time point, which includes recording of number of relapses, Expanded Disability Status Scale (EDSS) score, and possible adverse events. A trained MS nurse performs the SDMT as well as performing the blood sampling. Data collection, storage, and reports of adverse events are performed using the infrastructure provided by the Swedish MS Registry (SMSreg) web platform. 23
Data availability
Data related to this article are available from Tomas Olsson, Karolinska Institutet. To share data from the IMSE cohorts and Swedish MS registry, a data transfer agreement must be completed between Karolinska Institutet and the institution requesting data access. This is in accordance with the data protection legislation in Europe (General Data Protection Regulation [GDPR]). Persons interested in obtaining access to the data should contact Ali Manouchehrinia (ali.manouchehrinia@ki.se).
Study population
Included in this study were RRMS participants from the IMSE natalizumab-treated cohort, treated with natalizumab for minimum of 6 months and naïve to treatment with other monoclonal antibodies and a randomly selected population of natalizumab-naïve individuals with MS who had received other therapies (natalizumab naïve) identified from the Swedish MS registry for the comparative effectiveness analysis. Treatment categories were defined in accordance with categorization used previously 24 as other monoclonal antibodies (rituximab (or biosimilars), ocrelizumab, alemtuzumab, daclizumab, and ofatumumab), oral DMTs (teriflunomide, fingolimod, cladribine, dimethyl fumarate, siponimod, and ozanimod), and platform therapies (interferon beta-1a, interferon beta-1b, peginterferon beta-1a, glatiramer acetate, and peginterferon). All included individuals were required to have (1) no SDMT test performed prior to the baseline SDMT test to avoid learning effects, (2) baseline SDMT test performed at most 90 days prior to respective treatment initiation, (3) baseline SDMT score ⩾24 and ⩽90 (mean ± 2 standard deviations) to reduce potential reporting errors caused by outliers, and (4) at least three SDMT tests performed during treatment. All included participants had MS fulfilling the McDonald criteria23–25 as judged by their neurologist.
Study outcome
We evaluated cognition by investigating the changes in SDMT performance over time, specifically likelihood of cognitive improvement at each follow-up visit. The SDMT ranges from 0 to 110, with a higher score indicating better cognitive performance. SDMTs are completed at baseline, at 6th month, and on an approximately annual basis during treatment with natalizumab or other therapies (natalizumab naïve). Because MS centers performed the SDMT test as either an oral or a written test, giving rise to some inconsistency in the score, we included follow-up periods of only one SDMT type (either oral or written tests) and all statistical analyses were controlled for the type of test that each participant completed. Cognitive improvement was defined as an increase in the SDMT score by ⩾10% of the difference between the maximum possible score (110) and the baseline SDMT score (at the time of treatment initiation). That is ⩾6 SDMT score improvement for a person with a baseline score of 50 and ⩾5 SDMT score improvement for a person with a baseline score of 60. The choice of relative cut-off for measuring improvement (as opposed to a fixed ⩾4- or ⩾8-point improvement) prevented ceiling effect, obscuring the improvement in persons with relatively high SDMT scores at baseline.
Statistical analysis
We assessed the impact of natalizumab and other therapies on the likelihood of cognitive improvement using two methods. First, we started by investigating the proportion of patients treated with natalizumab who reached cognitive improvement (see above) at 6 months up to 126 months post-treatment initiation. We compared the basic clinical and demographic characteristics of patients who reached cognitive improvement at 6 months post-treatment initiation compared to those who did not show improvement using parametric or non-parametric tests.
We then proceeded to evaluate the effect of natalizumab on likelihood of cognitive improvement by fitting (1) a mixed-effects logistic regression model to obtain the likelihood (in terms of odds ratios) of cognitive improvement over the follow-up time and (2) simple slope analyses with treatment and time interaction to calculate the odds of cognitive improvement per each follow-up month for natalizumab-treated persons and persons treated with other treatments. All models were adjusted for sex, age at onset of MS, age at treatment initiation, baseline SDMT score, presence of relapse within 120 days of test, number of tests performed during follow-up, time for follow-up, and type of SDMT test performed.
We finally investigated the differences in the proportion of persons with cognitive improvement at each follow-up time between treatment groups using the chi-square tests.
To ensure the robustness of the findings, we conducted three sensitivity analyses. In the first analysis, similar models but on a propensity score-matched population was fitted. The second sensitivity analysis was performed on a subset of individuals from the original study population who were classified as cognitively impaired at baseline (the baseline SDMT score <1.5 standard deviations of sample mean). The third sensitivity analysis was performed on those who performed only oral version of the SDMT test.
All statistical analyses were performed using R version 4.0. Ethical approval was obtained from the Stockholm ethical committee (EPN) at Karolinska Institutet.
Results
A summary of the demographic and clinical characteristics of the study population is presented in Table 1. In total, 3538 persons with MS had ever been treated with natalizumab in Sweden between 2007 and 2020 and were part of the IMSE cohort. We had to exclude 1438 patients as they did not meet the criteria to be included. From the 1438 excluded patients, 83 were excluded as they had been exposed to other monoclonal antibodies before natalizumab initiation, 277 had no SDMT performed during treatment period, 342 had previously performed SDMT before natalizumab initiation, 374 had performed <3 SDMT during follow-up, for 10 the type of SDMT test could not be determined, 225 were not RRMS, 72 had missing onset date, and 55 had their baseline SDMT score <24 or >90. This left us with a final study population comprised of 2100 patients recruited from 47 MS specialist clinics throughout Sweden. These patients had performed 17,387 SDMT tests over an average follow-up time of 54 (range: 6–156) months.
Table 1.
Clinical and demographic characteristics of the natalizumab-treated individuals.
| Overall (N = 2100) | |
|---|---|
| Sex | |
| Female | 1504 (71.6%) |
| Male | 596 (28.4%) |
| Age at MS onset | |
| Mean (SD) | 28.30 (8.79) |
| First SDMT score | |
| Median (Q1, Q3) | 51 (44, 58) |
| Age at baseline | |
| Mean (SD) | 34.97 (9.73) |
| Duration of treatment (follow-up), months | |
| Median (Q1, Q3) | 54 (30, 78) |
| Number of SDMT score performed during treatment | |
| Median (Q1, Q3) | 7 (5, 9) |
| Type of SDMT test performed | |
| Oral only | 1613 (76.8%) |
| Written only | 487 (23.2%) |
| Duration of exposure to platform DMTs before study entry (months)* | |
| Median (Q1, Q3) | 15.5 (0, 48) |
| EDSS score at baseline | |
| N-Miss | 594 |
| Median (Q1, Q3) | 2.50 (1.5, 3.5) |
SDMT: Symbol Digit Modalities Test; SD: standard deviation.
Includes interferon beta-1a, interferon beta-1b, glatiramer acetate and peginterferon.
The effect of natalizumab on SDMT score
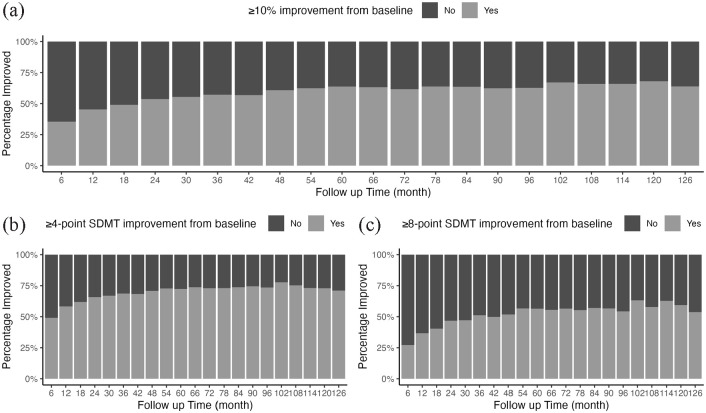
Natalizumab treatment was associated with a significant improvement in the SDMT performance. Approximately 45% of patients (806 persons of 1779 with SDMT score at 6 month) reached improvement (increase in the SDMT score by ⩾10% of the difference between maximum possible score and the baseline score) at 6 months after treatment initiation (Figure 1(a)). Figure 1(b) and (c) illustrate percentage of natalizumab-treated patients reaching ⩾4-point and ⩾8-point SDMT improvement over the treatment with natalizumab, respectively. General demographic and baseline characteristics of those with and without cognitive improvement at 6-month post-natalizumab treatment are described in Table 2.
Figure 1.
Proportion of patients with (a): 10% improvement (an increase of SDMT score by ⩾10% of the difference between the maximum possible score (110) and the baseline value), (b): ⩾4-point SDMT improvement from baseline value and (c): ⩾8-point improvement from the baseline value after treatment initiation with natalizumab.
Table 2.
Comparison of general baseline demographic and clinical characteristics of patients, 6 months post-natalizumab treatment stratified by cognitive improvement status.
| Did not improve (N = 973) | Improved (N = 806) | P value | |
|---|---|---|---|
| Sex | 0.310 | ||
| Female | 680 (69.9%) | 581 (72.1%) | |
| Male | 293 (30.1%) | 225 (27.9%) | |
| Age at MS onset | 0.002 | ||
| Mean (SD) | 29.20 (9.13) | 27.74 (8.49) | |
| Age at baseline | <0.001 | ||
| Mean (SD) | 36.24 (9.92) | 33.73 (9.55) | |
| Number of SDMT score performed during treatment | 0.718 | ||
| Median (Q1, Q3) | 7 (5, 10) | 7 (5, 10) | |
| First SDMT score | 0.54 | ||
| Median (Q1, Q3) | 51 (44, 59) | 51 (44, 58) | |
| Duration of treatment (follow-up), months | <0.001 | ||
| Median (Q1, Q3) | 48 (24, 78) | 60 (30, 78) | |
| Duration of exposure to platform DMTs before study entry (months)* | 0.053 | ||
| Median (Q1, Q3) | 17 (0, 51) | 15 (0, 45) | |
| EDSS score at baseline | 0.001 | ||
| Number of missing | 226 | 275 | |
| Median (Q1, Q3) | 2.5 (1.5, 3.5) | 2.0 (1.5, 3.0) |
SDMT: Symbol Digit Modalities Test; SD: standard deviation.
Improvement: An increase of SDMT score by ⩾10% of the difference between the maximum score possible (110) and the baseline value.
Includes interferon beta-1a, interferon beta-1b, glatiramer acetate and peginterferon.
Comparative effectiveness
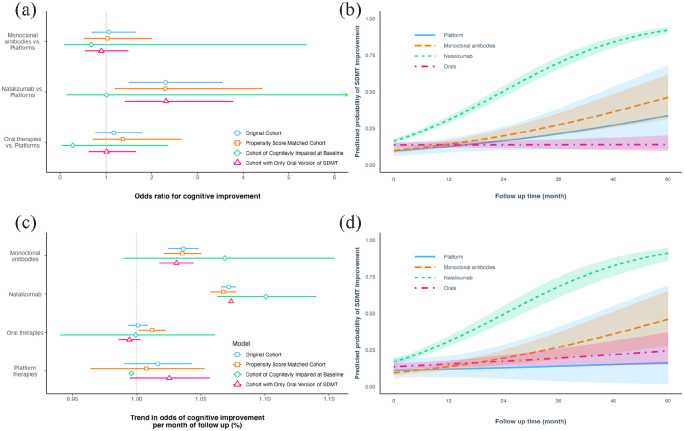
Characteristics of the natalizumab-treated and natalizumab-naïve individuals, before and after matching, are presented in Table 3. On average, the odds of cognitive improvement was 2.3 times (95% CI: 1.5–3.5) higher in natalizumab-treated individuals compared to individuals treated with platform DMTs (the reference category). The corresponding odds ratios were 1.06 (95% CI: 0.7–1.6) for patients treated with other monoclonal antibodies and 1.2 (95% CI: 0.7–1.8) for patients on oral therapies, respectively (compared to platform DMTs) (Figure 2(a) and (b)). Younger age at MS onset and younger age at SDMT performance were positively associated with an increased probability of cognitive improvement. Inversely, the presence of a relapse within 120 days of SDMT test, and a higher baseline SDMT score decreased the probability of improvement.
Table 3.
Clinical characteristics of the natalizumab-treated and natalizumab-naïve populations before and after matching.
| Natalizumab treated (N = 2100) | Never exposed to natalizumab (N = 2622) | Standardized mean difference | Natalizumab treated (N = 1077) | Never exposed to natalizumab (N = 1524) | Standardized mean difference | |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 1504 (71.6%) | 1797 (68.5%) | 0.07 | 755 (70.1%) | 1067 (70.0%) | 0 |
| Male | 596 (28.4%) | 825 (31.5%) | −0.07 | 322 (29.9%) | 457 (30.0%) | 0 |
| Age at MS onset | ||||||
| Mean (SD) | 28.30 (8.79) | 32.17 (9.91) | −0.44 | 29.68 (9.19) | 30.07 (9.33) | 0.03 |
| Age at baseline | ||||||
| Mean (SD) | 34.97 (9.73) | 40.04 (10.47) | −0.52 | 36.46 (10.12) | 37.11 (10.05) | 0.02 |
| Duration of treatment (follow-up), months | ||||||
| Median (Q1, Q3) | 48 (30, 60) | 30 (24, 48) | 0.51 | 36 (18, 54) | 30 (24, 48) | −0.01 |
| Type of SDMT test performed | ||||||
| Oral only | 1613 (76.8%) | 2148 (81.9%) | −0.12 | 856 (79.5%) | 1234 (81.0%) | −0.03 |
| Written only | 487 (23.2%) | 474 (18.1%) | 0.12 | 221 (20.5%) | 290 (19.0%) | 0.03 |
| First SDMT score | ||||||
| Median (Q1, Q3) | 51 (44, 58) | 52 (45, 59) | −0.08 | 51 (44, 59) | 51.5 (45, 58) | 0.01 |
| Number of SDMT score performed during treatment | ||||||
| Median (Q1, Q3) | 7 (5, 9) | 4 (3, 5) | 0.95 | 4 (3, 6) | 7 (5, 9) | 0.01 |
| Treatment type (n) | ||||||
| Natalizumab | 2100 | 0 | 1077 | 0 | ||
| Monoclonal antibodies a | 0 | 892 | 0 | 589 | ||
| Oral DMTs b | 0 | 1541 | 0 | 865 | ||
| Platform DMTs c | 0 | 189 | 0 | 70 | ||
SDMT: Symbol Digit Modalities Test, SD: standard deviation.
Rituximab (or biosimilars), ocrelizumab, alemtuzumab, daclizumab, and ofatumumab.
Teriflunomide, fingolimod, cladribine, dimethyl fumarate, siponimod, and ozanimod.
Interferon beta-1a, interferon beta-1b, glatiramer, and peginterferon beta-1a.
Figure 2.
(a) Forest plot of odds ratios for cognitive improvement obtained from multivariable adjusted logistic mixed-effect models. (b) Predicted odds of reaching cognitive improvement in different treatment categories before propensity score matching. (c) Trend in odds of cognitive improvement per month of follow-up (slope analysis). Estimates indicate the percentage increase in the probability of improvement per month. (d) Predicted odds of reaching cognitive improvement after propensity score matching.
Cognitive improvement was defined as an increase of SDMT score by ⩾10% of the difference between the maximum possible score (110) and the baseline value. SDMT = Symbol Digit Modalities Test.
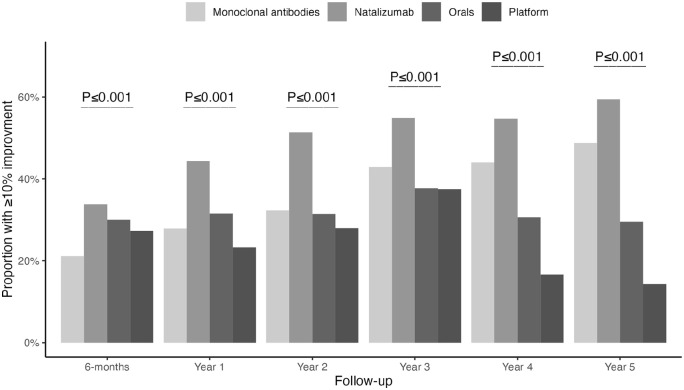
The odds of cognitive improvement (slope analysis) for natalizumab-treated individuals increased by 7% (95% CI: 6–7) per month of therapy (follow-up). The equivalent estimates were 4% (95% CI: 2–5) for other monoclonal antibodies, 2% (95% CI: –1 to 4) for platform DMTs, and 1% (95% CI: –1 to 1) in those receiving oral DMTs (Figure 2(c)). Progression with time in proportion of individuals with cognitive improvement at each year of follow-up and its comparison between groups are shown in Figure 3.
Figure 3.
Comparison of the proportion of patients with cognitive improvement at each year of follow-up between treatment groups using the chi-square test.
One hundred and seventy-eight natalizumab-treated individuals, 58 individuals treated with other monoclonal antibodies, 64 treated with oral DMTs, and 9 with platform therapies (n = 309) were classified as being cognitively impaired at baseline (baseline SDMT score < 1.5 standard deviation of cohort mean). While we did not observe an overall difference between treatments in odds of improvement over the follow-up time (most likely due to small sample size) (Figure 2(a)), the odds of cognitive improvement in the natalizumab group increased by 10% (95% CI: 6–13) per month of therapy. The equivalent estimates were 7% (95% CI: –1 to 14) for other monoclonal antibodies, 0% (95% CI: 0–0) for platform DMTs and 0% (95% CI: –6 to 6) in those receiving oral therapies (Figure 2(c)). We did not see an effect of SDMT test type (oral vs written test) on the overall findings (Figure 2(a)).
Propensity score matching analysis
In summary, similar results to those obtained before matching were found after propensity score matching (Figure 2(a) and (d)). For 1077 of the 2100 natalizumab-treated individuals, we could find 1524 propensity score-matched individuals treated with other therapies but never with natalizumab (Table 3). The two groups were matched for sex, age at MS onset, age at baseline SDMT test, baseline SDMT score, type of SDMT test (oral or written), total number of SDMTs performed, and duration of follow-up time. The odds of cognitive improvement for natalizumab-treated patients increased by 7% (95% CI: 6–7) per each month of follow-up and were 4% (95% CI: 2–5) for other monoclonal antibodies, 1% (95% CI: –6 to 5) for platform therapies and 1% (95% CI: 0–2) for oral DTMs (Figure 2(c)).
Discussion
Using a large, nationwide population-based cohort of persons with RRMS treated with natalizumab, we found that treatment with natalizumab or other monoclonal antibodies was associated with a significantly better rate of improvement in cognition. Natalizumab and other monoclonal antibodies showed superior performance in improving cognition compared to platform therapies or oral DMTs during the follow-up time. While the trend in progression of odds of cognitive improvement was significantly higher in those receiving natalizumab and other monoclonal antibodies compared to those on platform or oral therapies, we observed an overall greater odds of having cognitive improvement in the natalizumab-treated individuals. These results remained mainly unchanged after a rigorous propensity score matching analysis. We also found beneficial effects of natalizumab and other monoclonal antibodies in improving cognition in those with major cognitive impairments, albeit with smaller effect sizes.
Despite the availability of DMTs for more than two decades, their effect on cognitive decline in MS is mostly unknown. A recent systematic review and meta-analysis of the effect of DMTs on cognitive performance in RRMS showed a minimal positive effect of DMTs on SDMT score. 25 Even though the study included 17 (out of 44 included studies) randomized control trials, no statistically significant difference between platform and highly potent DMTs was observed. A similar conclusion has been reached in a recent systematic review of studies investigating the effects of DMT on cognition in MS. 13 Contrary to a meta-analysis which may carry forward the limitation of included studies, 26 the results from this study suggest that monoclonal antibodies can improve cognition in MS compared with the platform or oral therapies. Our result is consistent with results of smaller previous observational studies showing significant improvements in cognitive function after natalizumab treatment.27–29
The majority of patients with MS show a similar progressive decline of cognition 1 as seen for physical disability, and both physical and cognitive deteriorations exhibit marked between and inter-patient variability. Hence, evaluating the effect of different treatments on cognition is important to improve the possibility of choosing a favorable treatment for each patient.
Former studies have used an improvement of ⩾4 SDMT score from baseline as a definition of reaching clinically meaningful improvement. We found that this approach gives individuals with a lower baseline SDMT artificially higher chance of reaching clinically meaningful improvement as it is easier to improve ⩾4 when they score lower at baseline. To handle this problem, we used the definition of cognitive improvement as an increase in SDMT by ⩾10% of the difference between maximum possible score and the baseline score which makes the impact of baseline SDMT score on cognitive improvement less significant. The learning effect, caused by repeated testing, could influence the performance on SDMT score. However, in a comparative study design, this should affect all groups equally to bias the estimates. As the models and the case–control propensity score matching were controlled for number of tests performed and duration of follow-up (i.e. treatment duration), we therefore do not think that the learning effect has significantly affected the results of our analyses.
Strengths of this study include a large nationwide population-based cohort of MS patients treated with natalizumab, longitudinal follow-up, multiple analysis methods with similar results and a large, population-based, matched control cohort of MS patients never treated with natalizumab. However, this study has some limitations. Due to cohort matching, we had to exclude many participants. We did not have information and therefore did not control for the effect of educational level, socioeconomic factors and other factors that could potentially influence cognition, which could impact the SDMT performance. However, given the Swedish population’s socioeconomic and educational homogeneity, we do not think these factors have substantially confounded our results. Furthermore, this study examined the effect of natalizumab on cognition in persons with MS as one homogeneous population and compared to patients on other DMTs. Although all patients had relapsing-remitting course and active disease, it is possible that type and severity of cognitive dysfunction may have been unevenly distributed in this cohort. It is quite likely that the characteristics of the cognitive problems affected the choice of therapy in the first place which could interfere with the results of this study beyond what our analyses could be adjusted for. Therefore, additional studies and ideally randomized trials are needed to examine further the impact of different DMTs on cognition in MS.
In conclusion, we observed that treatment with natalizumab and other monoclonal antibodies is associated with significant improvement in cognitive performance in persons with RRMS as measured by the SDMT score. The effect of these treatments on cognitive improvement was superior to the platform or oral therapies.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.M. is supported by the Margaretha af Ugglas Foundation. M.E.K. is supported by the Michael Smith Foundation for Health Research Scholar award, BC Support Unit’s Real-World Clinical Trials Methods Cluster, Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant and Discovery Accelerator Supplements. Over the past 3 years, he has received consulting fees from Biogen Inc. for consulting. J.L. has received travel support and/or lecture honoraria from Biogen, Novartis, Teva, Sanofi, Merck, BMS, Axelion and Roche; has served on scientific advisory boards for Biogen, Novartis, Teva, Sanofi, Merck, BMS, Axelion, and Roche; serves on the editorial board of the Acta Neurologica Scandinavica; and has received unconditional research grants from Biogen, Novartis, and Teva. I.K. has support in the form of research grants from Swedish Brain Foundation, Swedish research council (2020-01638), EU Horizon 2020 (MultipleMS, project nr 733161and EU-STANDS4PM, project no. 825843) and Region Stockholm. T.O. has grant support from the Swedish research council, the Swedish Brain Foundation, and the Wallenberg Foundation. T.O. has received honoraria for advisory boards/lectures and unrestricted MS research grants from Biogen, Novartis, Merck, Sanofi, and Roche. The remaining author declares no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The IMSE project on natalizumab, dimethyl fumarate and peginterferon was supported by Biogen. The IMSE project on fingolimod was supported by Novartis. The IMSE project on cladribine was supported by Biogen. The IMSE projects on teriflunomid and lemtrada was supported by Sanofi.
ORCID iDs: Ali Manouchehrinia  https://orcid.org/0000-0003-4857-5762
https://orcid.org/0000-0003-4857-5762
Mohammad Ehsanul Karim  https://orcid.org/0000-0002-0346-2871
https://orcid.org/0000-0002-0346-2871
Jan Lycke  https://orcid.org/0000-0002-7891-8466
https://orcid.org/0000-0002-7891-8466
Ingrid Kockum  https://orcid.org/0000-0002-0867-4726
https://orcid.org/0000-0002-0867-4726
Contributor Information
Ali Manouchehrinia, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden/Centre for Molecular Medicine, Karolinska University Hospital, Stockholm, Sweden/The Karolinska Neuroimmunology & Multiple Sclerosis Centre, Department of Clinical Neurosciences, Karolinska Institutet, Centre for Molecular Medicine, Stockholm, Sweden.
Hanna Larsson, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden/Centre for Molecular Medicine, Karolinska University Hospital, Stockholm, Sweden.
Mohammad Ehsanul Karim, School of Population and Public Health, The University of British Columbia, Vancouver, BC, Canada/Centre for Health Evaluation and Outcome Sciences, University of British Columbia, Vancouver, BC, Canada.
Jan Lycke, Department of Clinical Neuroscience, Institute of Neuroscience and Physiology, Sahlgrenska Academy, Gothenburg University, Gothenburg, Sweden/Department of Neurology, Sahlgrenska University Hospital, Gothenburg, Sweden.
Tomas Olsson, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden/Centre for Molecular Medicine, Karolinska University Hospital, Stockholm, Sweden.
Ingrid Kockum, Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden/Centre for Molecular Medicine, Karolinska University Hospital, Stockholm, Sweden.
References
- 1.Benedict RHB, Amato MP, DeLuca J, et al. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol 2020; 19(10): 860–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hakim EA, Bakheit AMO, Bryant TN, et al. The social impact of multiple sclerosis—A study of 305 patients and their relatives. Disabil Rehabil 2000; 22(6): 288–293. [DOI] [PubMed] [Google Scholar]
- 3.Kavaliunas A, Danylaite Karrenbauer V, Gyllensten H, et al. Cognitive function is a major determinant of income among multiple sclerosis patients in Sweden acting independently from physical disability. Mult Scler 2019; 25: 104–112. [DOI] [PubMed] [Google Scholar]
- 4.Koutsouraki E, Kalatha T, Grosi E, et al. Cognitive decline in Multiple Sclerosis patients. Hell J Nucl Med 2019; 22: 75–81. [PubMed] [Google Scholar]
- 5.Penner IK.Cognition in multiple sclerosis. Neurodegener Dis Manag 2017; 7(6): 19–21. [DOI] [PubMed] [Google Scholar]
- 6.Kavaliunas A, Tinghög P, Friberg E, et al. Cognitive function predicts work disability among multiple sclerosis patients. Mult Scler J Exp Transl Clin 2019; 5(1): 2055217318822134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stüve O, Bennett JL.Pharmacological properties, toxicology and scientific rationale for the use of natalizumab (Tysabri®) in inflammatory diseases. CNS Drug Rev 2007; 13(1): 79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Eng J Med 2006; 354(9): 899–910. [DOI] [PubMed] [Google Scholar]
- 9.Iaffaldano P, Viterbo RG, Trojano M.Natalizumab discontinuation is associated with a rebound of cognitive impairment in multiple sclerosis patients. J Neurol 2016; 263(8): 1620–1625. [DOI] [PubMed] [Google Scholar]
- 10.Iaffaldano P, Viterbo RG, Paolicelli D, et al. Impact of natalizumab on cognitive performances and fatigue in relapsing multiple sclerosis: A prospective, open-label, two years observational study. PLoS ONE 2012; 7(4): e35843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunkel A, Fischer M, Faiss J, et al. Impact of natalizumab treatment on fatigue, mood, and aspects of cognition in relapsing-remitting multiple sclerosis. Front Neurol 2015; 6: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rorsman I, Petersen C, Nilsson PC.Cognitive functioning following one-year natalizumab treatment: A non-randomized clinical trial. Acta Neurol Scand 2018; 137(1): 117–124. [DOI] [PubMed] [Google Scholar]
- 13.Chen MH, Goverover Y, Genova HM, et al. Cognitive efficacy of pharmacologic treatments in multiple sclerosis: A systematic review. CNS Drugs 2020; 34(6): 599–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith A.Symbol digit modalities test. Los Angeles, CA: Western Psychological Services, 1973. [Google Scholar]
- 15.Smith A.Symbol digit modalities test (Manual, Revised). Los Angeles, CA: Western Psychological Services, 1982. [Google Scholar]
- 16.Strober L, Chiaravalloti N, Moore N, et al. Unemployment in multiple sclerosis (MS): Utility of the MS Functional Composite and cognitive testing. Mult Scler 2014; 20(1): 112–115. [DOI] [PubMed] [Google Scholar]
- 17.Goverover Y, Strober L, Chiaravalloti N, et al. Factors that moderate activity limitation and participation restriction in people with multiple sclerosis. Am J Occup Ther 2015; 69(2): 1–9. [DOI] [PubMed] [Google Scholar]
- 18.Amato MP, Portaccio E, Goretti B, et al. Relevance of cognitive deterioration in early relapsing-remitting MS: A 3-year follow-up study. Mult Scler 2010; 16(12): 1474–1482. [DOI] [PubMed] [Google Scholar]
- 19.Strober L, DeLuca J, Benedict RHB, et al. Symbol Digit Modalities Test: A valid clinical trial endpoint for measuring cognition in multiple sclerosis. Mult Scler 2018; 25(13): 1781–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa SL, Genova HM, Deluca J, et al. Information processing speed in multiple sclerosis: Past, present, and future. Mult Scler 2017; 23(6): 772–789. [DOI] [PubMed] [Google Scholar]
- 21.Holmén C, Piehl F, Hillert J, et al. A Swedish national post-marketing surveillance study of natalizumab treatment in multiple sclerosis. Mult Scler 2011; 17(6): 708–719. [DOI] [PubMed] [Google Scholar]
- 22.Piehl F, Holmén C, Hillert J, et al. Swedish natalizumab (Tysabri) multiple sclerosis surveillance study. Neurol Sci 2011; 31(Suppl. 3): 289–293. [DOI] [PubMed] [Google Scholar]
- 23.Hillert J, Stawiarz L.The Swedish MS registry—clinical support tool and scientific resource. Acta Neurol Scand 2015; 132(199): 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benkert P, Meier S, Schaedelin S, et al. Serum neurofilament light chain for individual prognostication of disease activity in people with multiple sclerosis: A retrospective modelling and validation study. Lancet Neurol 2022; 21(3): 246–257. [DOI] [PubMed] [Google Scholar]
- 25.Landmeyer NC, Bürkner PC, Wiendl H, et al. Disease-modifying treatments and cognition in relapsing-remitting multiple sclerosis: A meta-analysis. Neurology 2020; 94(22): e2373–e2383. [DOI] [PubMed] [Google Scholar]
- 26.Amato MP, Krupp LB.Disease-modifying therapy aids cognition in multiple sclerosis. Nat Rev Neurol 2020; 16(10): 525–526. [DOI] [PubMed] [Google Scholar]
- 27.Morrow SA, O’Connor PW, Polman CH, et al. Evaluation of the Symbol Digit Modalities Test (SDMT) and MS neuropsychological screening questionnaire (MSNQ) in natalizumab-treated MS patients over 48 weeks. Mult Scler 2010; 16(11): 1385–1392. [DOI] [PubMed] [Google Scholar]
- 28.Mattioli F, Stampatori C, Capra R.The effect of natalizumab on cognitive function in patients with relapsing-remitting multiple sclerosis: Preliminary results of a 1-year follow-up study. Neurol Sci 2011; 32(1): 83–88. [DOI] [PubMed] [Google Scholar]
- 29.Lang C, Reiss C, Mäurer M.Natalizumab may improve cognition and mood in multiple sclerosis. Eur Neurol 2012; 67(3): 162–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data related to this article are available from Tomas Olsson, Karolinska Institutet. To share data from the IMSE cohorts and Swedish MS registry, a data transfer agreement must be completed between Karolinska Institutet and the institution requesting data access. This is in accordance with the data protection legislation in Europe (General Data Protection Regulation [GDPR]). Persons interested in obtaining access to the data should contact Ali Manouchehrinia (ali.manouchehrinia@ki.se).