Abstract
Cancer is a leading cause of death worldwide and involves an oxidative stress mechanism. The transcription factor Nrf2 has a crucial role in cytoprotective response against oxidative stress, including cancer growth and progression and therapy resistance. For this reason, inhibitors of Nrf2 are new targets to be studied. Traditional plant-based remedies rich in phytochemicals have been used against human cancers and phenolic compounds are known for their chemopreventive properties. This comprehensive review offers an updated review of the role of phenolic compounds as anticancer agents due to their action on Nrf2 inhibition. In addition, the role of naturally-occurring bioactive anticancer agents are covered in the clinical applications of polyphenols as Nrf2 inhibitors.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-023-01109-0.
Keywords: Phenolic compounds, Cancer, Nrf2, Oxidative stress, Cytotoxicity, Apoptosis
Introduction
Each year millions of people suffer from different types of cancer and almost half of them die due to the progression of this to an uncontrollable condition [1]. Cancer is the second leading cause of mortality globally, with an estimated 19 million new cases and 10 million deaths yearly in 2020 [2].
Tumors are composed of heterogeneous cells with the capacity to adapt dynamically their microenvironment by genetic/epigenetic changes and metabolic reprogramming [3, 4]. The tumour cells are formed in the body due to metastasis, uncontrolled proliferation, lower immunity in the body, and resistance to apoptosis [5]. Apart from this, a higher level of oxidative stress exerted by reactive oxygen species (ROS) and/or reactive nitrogen species (RNS) is one of the major features of cancerous cells [6–8]. In humans, complex protective machinery defends against the attacks of ROS and RNS, which are regularly produced in the human body as a result of cellular metabolism and environmental exposure [9, 10]. The metabolic rewiring promotes cancer cell proliferation and tumor growth. Moreover, an increased ROS production is counterbalanced by an increased endogenous antioxidant capacity as part of the adaptive response by cancer cells to face adverse conditions [11]. Cancer cells during malignant progression can often develop resistance to treatment [12, 13]. The increased antioxidant capacity of cancer cells is a crucial determinant of resistance to therapy [14]. In this sense, inhibiting the metabolic and antioxidant circuits that support the redox balance in cancer cells may be a promising anticancer therapy. Especially considering that conventional anticancer therapies generate cytotoxicity dependent on the efficient accumulation of ROS [14, 15]. The nuclear factor E2-related factor 2 (Nrf2)/Kelch-like ECH-associated protein 1 (Keap1) signalling pathway is one of the most important for cell defence against xenobiotic and oxidative stress [16, 17].
In normal cells, Nrf2 plays a crucial role in the cellular defense mechanism that protects cells and promotes cell survival under stress conditions [18]. Additionally, it is a tumor suppressor that can remove ROS and carcinogenic agents [19]. However, it has been suggested that Nrf2 also protects cells from radiotherapy, chemotherapeutic agents, and anticancer drugs through its antioxidant defense mechanism [20, 21]. Elevation of Nrf2 levels has been shown in clinical studies in cancer such as lung, ovarian, melanoma, colorectal cancer, endometrial carcinoma, breast cancer, kidney cancer, pancreatic cancer, endometrial carcinoma, and hepatocellular carcinoma [22]. Moreover, an increase in Nrf2 levels has been associated with therapeutic resistance and metastatic invasion in cancer cells [20]. The pharmacological role of Nrf2 has been demonstrated in studies with mice deficient in Nrf2 and single nucleotide polymorphism in the NRF2 gene NFE2L2 [23]. These and other findings have suggested that targeting the Nrf2 pathway may be a new cancer therapy. Researchers have tried to identify molecules activators of NrF2 as chemoprevention ROS-dependent carcinogenesis, while others have focused on identifying NrF2 inhibitors to increase sensitivity of cancer cells to chemotherapy.
Due to the dual effect of Nrf2 as defends normal cells under oxidative stress and their role in the redox adaptation of the cancer cells. Therefore it is logical that both activators and inhibitors of Nrf2 could act in anticancer therapy [24]. It is widely accepted that chemoprevention in cancer includes the activity of detoxifying and cytoprotective enzymes. Using compounds capable of activating the NRF2 could be a possible strategy in cancer prevention and therapy [6]. However, due to the modulatory effects of Nrf2 in the detoxification process, the potential use of activators in cancer prevention and therapy needs to be further elucidated. In addition, a high expression of Nrf2 has been associated with a low response from radiation and some anticancer drugs therapy such as carboplatin, cisplatin, 5-fluorouracil, and doxorubicin [25, 26]. Considering the oncogenic nature of Nrf2, using Nrf2 inhibitors is an exciting approach for treating cancers with elevated Nrf2 levels and could be a reasonable alternative to control tumor growth. In this regard, Nrf2 inhibitors decrease drug-detoxifying enzymes, inducing an increase in chemotherapeutic sensitization [17]. The transcription factor Nrf2, which has long been thought to be the master regulator of cytoprotective responses against electrophilic/xenobiotic and oxidative stress, has recently been discovered to promote cancer growth, progression, and therapy resistance [27]. More research on Nrf2 has observed its function in various types of tumours and possible therapeutic approaches to prevent or reverse its activation. Nrf2 pharmacological regulation tends to be context-dependent in cancer [28].
Secondary metabolites in plants with a typical aromatic ring bearing one or more hydroxyl groups are known as phenolic compounds [29]. Simple phenols, flavonoids, lignins and lignans, tannins, xanthones, and coumarins are examples of phenolic compounds isolated from plant sources. Some of these phenolic compounds have been shown to have potent anti-cancer properties as well as the ability to fight oxidative stress-related diseases. Phenolic and sulfur-containing compounds are two main groups of natural compounds that show promise as chemopreventive agents [30, 31]. Phenolic compounds have a variety of biological properties, including anticarcinogenic activity [32]. One of the most significant molecular mechanisms for polyphenols' cancer chemo-preventive effects is the induction of phase II detoxifying and antioxidant protection enzymes via the Nrf2/Keap1 signalling pathway [22]. Curcumin and resveratrol have been investigated extensively as natural compounds with anticancer properties in a variety of cancers. Via interactions with multiple cell signalling proteins, various cancers' proliferation, invasion, angiogenesis, and metastasis are inhibited [6]. This comprehensive review aims to offer updates on the role of phenolic compounds as anticancer agents due to their inhibitory action on Nrf2 inhibition.
Review methodology
The relevant information about the role of phenolic compounds as potential anticancer agents with inhibitory effects on Nrf2 signaling pathway were collected from electronic scientific databases such as PubMed/MedLine, Web of Science, Scopus, Google Scholar, ScienceDirect and SciFinder. For searching we used the next MeSH terms: “Antineoplastic Agents/pharmacology”, “Antioxidant Response Elements /drug effect”, “Drug Resistance”, “Neoplasm/drug effect”, “Drug Resistance, Neoplasm/genetics”, “NF-E2-Related Factor 2/antagonists & inhibitors”, “NF-E2-Related Factor 2/genetics”, “NF-E2-Related Factor 2/metabolism”, “Neoplasms/drug therapy”, “Neoplasms/genetics”, “Neoplasms/metabolism”, “Neoplasms/pathology”, “Oxidative Stress/drug effects”, “Proto-Oncogenes”, “Signal Transduction”. The scientific names of the plants have been validate according to the World Flora Online, and the chemical structures according to the PubChem [33, 34].The most representative data and mechanisms of actions were summarized in tables and figures.
Phytochemistry of phenolic compounds
Medicinal plants used in folk medicine are known to be small pharmaceutical factories, which are capable of producing compounds with interesting biological properties, such as antioxidant, anti-inflammatory, antimicrobial, antihypertensive, anticancer, etc. [35–38]. To check the activity of biological molecules, present in medicinal plants, in the first, a step they have to be extracted and then characterized [39]. Water [40–44], ethanol [45–48], methanol [49–54], methanol: dichloromethane [43], n-hexane [41], chloroform [41], ethyl acetate [41], butanol [41] are mainly used as solvents for the extraction of phenolics with anticancer activities. Phenolic acids can be found in a variety of plant components, including roots, leaves, fruits, and vegetables. Caffeic acid is the most common type of phenolic acid contained in fruits, while ferulic acid is found in an esterified form in the cell walls of the seed coat, bran, and fruits [55, 56]. Plant leaves and stems have higher levels of phenolic acids, but there are major differences between species [57]. Complex polyphenols, for example, can be found in cell vacuoles, tissues in the leaf, epidermis, flowers, and fruits. Tannins are abundant in bark, wood, and fruit pods, while flavonoids are abundant in flowers. There are different varieties of phenolic compounds (flavonoids, hydroxybenzoate, coumarins, hydroxycinnamates, xanthones, chalcones, stilbenes, lignins, and lignans) and their metabolic pathways have been reported in several review articles [58, 59]. Phenolic compounds are generally divided into two groups:
-
i)
simple phenols (phenolic acids and coumarins);
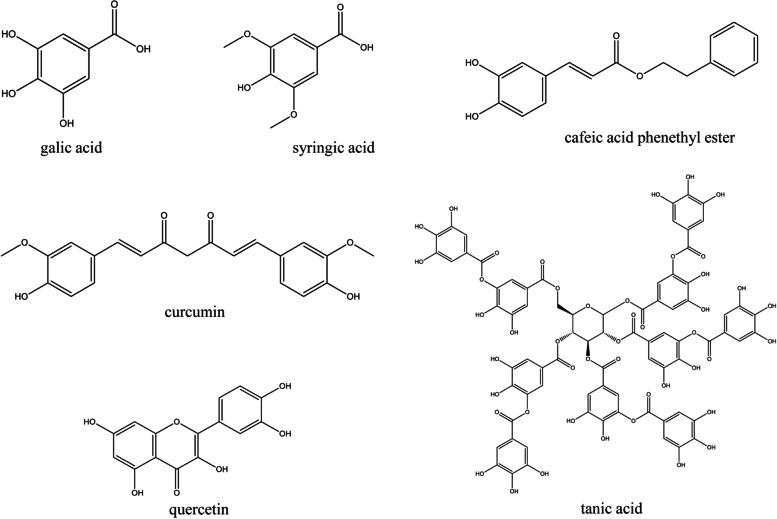
Phenolic acids include hydroxybenzoic acids (e.g. gallic acid, p-hydroxybenzoic acid, protocatechuic acid, vanillic acid and syringic acid) and hydroxycinnamic acids (e.g., ferulic acid, caffeic acid, p-coumaric acid, chlorogenic acid and sinapic acid) [31]. The basic phenolic compounds with 6- and 9-carbon skeletons, benzoic acids, and cinnamic acids are found in nature. A carboxylic group is added to the benzene ring, which is followed by one or more hydroxyl or methoxys groups. Gallic acid, for example, has three hydroxy (-OH) groups attached to the third (meta), fourth (para), and fifth (meta) carbons, while syringic acid has two methoxys (-OCH3) groups at the third and fifth (meta) carbons, and one -OH group at the fourth carbon (para). Compounds with a higher molecular weight are known as complex phenolics. These phenolic acids are mostly present in the vacuoles of cells. Complex phenolics found in fruits and vegetables are best represented by tannins and flavonoids.
-
ii)
polyphenols (flavonoids and non-flavonoids like tannins, lignans, and stilbenes).
The flavonoids are categorized mainly into flavones (e.g. apigenin, luteolin), flavonols (e.g. quercetin, rutin, kaempferol), flavanones (e.g. naringenin, hesperetin), flavanols (e.g. catechin, epicatechin, gallocatechin, epigallocatechin), anthocyanins (e.g. cyanidin, malvidin, petunidin), chalcones (e.g. arbutin, phloretin), isoflavonoids (e.g. genistein, daidzein) [31, 60].
Flavonoids are composed of two phenolic rings joined by an oxygenated heterocyclic pyran ring [61]. Flavonoids are further divided into anthocyanins, flavones, and flavanols based on the oxygenated state of the pyran ring. Beyond their main substitutions with hydroxyl or methoxy groups, these molecules are acetylated or glycosylated to achieve higher complexity [62]. Some of the naturally-occurring bioactive phenolics compounds which are used as anticancer agents are summarized in Fig. 1.
Fig. 1.
Chemical structures of bioactive phenolics compounds which are used as potential anticancer agents
Naturally-occurring polyphenolic compounds as anticancer agents
Ethnomedicinal and traditional importance
Herbal medicine, also known as botanical medicine, phytomedicine or phytotherapy is one of the oldest and continues to be of great interest [63, 64]. Folk medicinal applications of traditional remedies are popular in many countries all over the world [65]. This natural medicine is usually used by people who want to improve their health and do not have access to conventional medical services [66, 67]. Most often plant-derived products such as vegetables, herbs, spices, and fruits are used as natural remedies or food additives [68] Usually, these plants are applied in the form of poultice, decoction, infusion or concoction, but the most commonly used form is water extracts [49, 69, 70]. Traditional medicine is a collection of traditional, natural recipes based on beliefs and experiences indigenous to different cultures [71, 72]. A wide variety of fruits, vegetables, herbs, species, flowers, trees, and bushes is known in folk medicine as a source of phytochemicals, which are used to treat health-related ailments and their associated symptoms [73, 74]. It is well known that cancer diseases are linked to an imbalanced diet poor in fruit and vegetables [75–77]. They have been used for centuries as natural remedies all over the world Recently, dietary phenolics have aroused great interest in the prevention and treatment of cancer [31, 60, 78–80]. Phenolic compounds are known to be responsible for their chemopreventive properties (e.g., antioxidant, antimutagenic and anti-inflammatory effects) [31, 51, 81, 82]. Aumeeruddy, Mahomoodally [83] in their documentation of medicinal plants, traditionally used in cancer management (performed for years1980-2019), showed that in a total of 62 countries, 948 plant species from 153 families and 628 genera were reported to be used against cancer. The most popular were vegetables – onion (Allium cepa L.), spices – ginger (Zingiber officinale Roscoe), turmeric (Curcuma longa L.) and black cumin (Nigella sativa L.), herbs – nettle (Urtica dioica L.) and black calla (Arum palaestinum Boiss.), fruits – pomegranate (Punica granatum L.) and graviola (Annona muricata L.) and shrubby succulent plant (Aloe vera (L.) Burm.f.). In the literature, there are numerous reports from in vitro, but also in vivo experiments confirming the effectiveness of traditional plant-based remedies rich in phytochemicals in the prevention or mitigation of human cancers (mainly due to anti-proliferative and apoptotic effects). Allium genus vegetables, such as garlic (Allium sativum L.), A. cepa, shallot (Allium hirtifolium Boiss.), leek (Allium tuberosum Rottler ex Spreng.) and chives (Allium schoenoprasum L.) have been exploited in folk medicine for centuries and are proposed to prevent cancers [84]. Vegetables from Cruciferous family like cauliflower, cabbage, Chinese cabbage, broccoli, Brussels sprouts, Pak choi are a popular component of the diet, consumed in large quantities. They possess strong antioxidant potential due to secondary metabolites like phenolics [85]. Moringa (Moringa oleifera Lam.) is another common vegetable, used in many countries since ancient times, due to a wide array of biological activities attributed to phenolic compounds [41]. According to a recent study, one of the most popular in cancer management is U. dioica and A. palaestinum. The cancer chemopreventive potential of nettle (especially in the case of breast cancer) was described in detail in the review of Esposito et al. [86] and Hodroj et al. [42]. The authors presented numerous examples of the use of U. dioica extracts (obtained with water, ethanol, methanol, and dichloromethane) in the in vitro treatment of breast, cervical, epidermoid, colon, gastric, lung, and prostate cancer cells. A. palaestinum is traditionally used in Palestinian herbal medicine for the treatment of diverse diseases like stomach acidity, atherosclerosis and also cancer. Its effectiveness is attributed, inter alia, to polyphenols – mainly flavonoids [43, 45].
Spices like Z. officinale, C. longa, N. sativa and many others contain polyphenols and can act as anticancer agents. Ginger is a common spice in food (used as a vegetable), beverages (e.g. tea) and herbal medicine, which is rich in bioactive phenolics, such as gingerols, paradols and shogaols. Is proposed to be used in the treatment of breast, colorectal, and prostate cancer cells [87]. Turmeric – an aromatic and nutraceutical plant, intensively used in Indian traditional medicine (Ayurveda), contains curcumin – a polyphenolic compound, which can be used in the treatment of nasopharyngeal, lung, hepatobiliary, breast, gastric, colorectal, uterus, prostate cancer and hematopoietic tumor [47, 87]. An effective therapeutic potential of black cumin to suppress tumor development, reduce tumor incidence and ameliorate carcinogenesis was described by Majdalawieh, Fayyad [88]. As shown in this review, black cumin seed extract induces cytotoxic effects against human hepatoma, adenocarcinoma, breast cancer, Dalton's Lymphoma Ascites, Ehrlich Ascites Carcinoma etc. Another spice that is widely used in many dishes is black pepper (Piper nigrum L.), which exhibits anticancer properties against several cell lines like breast, cervical, prostate and colon [89]. Polyphenols derived from cinnamon (Cinnamomum cassia (L.) J.Presl) – a traditional oriental medicinal herb, are examined for the treatment of melanoma, leukemia, etc. [87]. Besides vegetables, herbs and species, also fruits can be a rich source of antioxidants in the daily diet and can exert chemopreventive and/or chemotherapeutic potential [90]. According to Aumeeruddy, Mahomoodally [83], the most often examined fruits, in terms of anticancer properties are P. granatum and A. muricata. The anticancer activity of pomegranate is generally attributed to the high polyphenols content [91]. In the comprehensive review of Turrini et al. [91], prevention and treatment methods for several types of cancer cells (breast, prostate, lung, colon, leukemia, bladder cancers, and brain tumors) were described. The second popular fruit is graviola, a fruit tree with a long history of traditional use in folk medicine, especially in Africa and South America [92]. The authors in their review presented plenty of studies showing anti-proliferative effects of different extracts (water, ethanol, ethyl acetate) obtained from A. muricata towards various cancer cell lines, like as example lung A549 cancer cells, colon HT-29 cancer cells, K562 chronic myeloid leukemia cells, metastatic breast cancer. Fruits such as blueberries, strawberries, kiwi, and apples can also contain another polyphenolic compound with potent antioxidant properties – catechins (flavanols of the flavonoid family). But the most commonly consumed source of catechins in green tea – natural health beverage. Catechins extracted from green tea (Camellia sinensis (L.) Kuntze) are proposed to be efficient in the prevention of lung, breast, esophageal, stomach, liver and prostate cancer [80]. Biomedical potential in cancer prevention, control and reduction of development may also have seaweeds (also known as macroalgae), which are widely used in Asia, especially in traditional Chinese medicine and Japanese folk medicine, as well as a component of the daily diet [52, 53, 93]. Polyphenol-rich seaweeds in cancer research can exert antioxidant, antiangiogenesis effects, anti-proliferative efficacy (breast cancer cell lines), as well as can induce apoptosis [52, 53]. Shrubs and trees, traditionally used in folk medicine, can also constitute candidates for the isolation of novel bioactive compounds with anticancer properties [51, 94]. Phenolic compounds can be found in all parts of plants like roots, bark, lignified parts, leaves, flowers, fruits and seeds [94]. For example, Ficus is one of the largest genera of medicinal plants all over the world in tropical and subtropical regions – there are around 750 species of woody plants, trees and shrubs rich in polyphenols [51]. Trees and shrubs growing in a temperate climatic zone – pine, spruce, fir, beech, oak, walnut, willow, rowan, hawthorn, and bird cherry also offer a wide variety of phenolic compounds like phenols, phenolic acids, phenylpropanoids, flavonoids, coumarins, tannins [94]. Shrubby succulent plant – A. vera is one of the few botanical medications, which is in widespread domestic use. This plant is considered a good natural source of antioxidants. In folk medicine, aloe juice, as well as fresh leaf gel, are used in many nutraceutical and cosmetics formulations [95].
From medicinal plants to potential anticancer bioactive compounds
The secondary metabolites, extracted from plants may be responsible for antioxidant and anticancer properties [96, 97]. Phenolic compounds are effective scavengers of ROS and RNS including the scavenging of superoxide anion, hydrogen peroxide, nitric oxide, free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) radical cation (ABTS) [89]. Free radicals are known to be potential carcinogens [50], therefore, antioxidants used by cancer patients can help maintain the balance between the free radicals formed and the trapping abilities of radicals [50]. Natural compounds derived from dietary vegetables and fruits receive considerable attention for the prevention and treatment of cancer. Allium genus vegetables like A. sativum, A. cepa, A. tuberosum, A. schoenoprasum and A. hirtifolium are one of the most interesting antioxidants in the prevention of cancer. Asemani et al. [84] in their review summarized the anticancer activities of biologically active compounds derived from Allium genus vegetables. Phenolic acids like gallic and ferulic acid, as well as flavonoids (flavonols – quercetin, kaempferol, myricetin; flavones – apigenin, luteolin; flavanols – catechin, epicatechin) and tannins are predominant phenolics found in Allium vegetables. All of the listed vegetables are strong antioxidants, participate in ROS scavenging and exert cytotoxicity and apoptosis‐inducing effects – for example, garlic – induction of apoptosis in human leukemia cell line (HL‐60), human erythroleukemic cell line (OCIM‐1), chronic myeloid leukaemia cells (CML), liver cancer cell lines – human hepatoma (HepG2) and Hep3B cells. Onion showed cytotoxic and anti-proliferative effects on a breast cancer cell line (MCF‐7), human breast adenocarcinoma cell line (MDA‐MB231), HL‐60, HepG2, human colon cancer cell line (HT29) and prostate cancer (PC3) cells. Popular M. oleifera, used as a vegetable and a popular plant in traditional herbal medicine, contains several phytochemicals including phenolic compounds like flavonoids – flavonols (e.g. quercetin), phenolic acids (e.g. gallic acid, sinapic acid, vanillic acid, caffeic acid and syringic acid), which exhibit various biological activities such as antioxidant, antimicrobial, antiviral, anti-inflammatory, immune-boosting, antidiabetic, antiatherosclerotic and anticancer (e.g. cervical carcinoma) [41, 98]. Herbs are known to be a rich source of phenolic compounds in traditional medicine. For example, U. dioica owes its biological properties (e.g. antioxidant, antimutagenic and anti-proliferative) to phytonutrients such as phenolic compounds, including coumarins and polyphenols – flavonoids (flavonols – myricetin and rutin), tannins and lignans [86]. Hodroj et al. [42] also showed that the chemical composition of U. dioica aqueous extract indicated the presence of flavonoids, mainly patuletin, rutin, quercetin, apigenin and phenolic acids as caffeic acid, m/p-hydroxybenzoic acid, gallic acid, syringic acid to which the pro-apoptotic and antitumor properties can be attributed. Fern’s (Asplenium nidus L.) bioactive compounds, especially flavonoids, exhibit antioxidant, antibacterial (against multidrug-resistant pathogens like Pseudomonas aeruginosa, Proteus vulgaris and Proteus mirabilis) and anticancer properties (e.g. human hepatoma and human carcinoma) [54]. Spices commonly used in many cuisines all over the world can easily provide phenolic compounds in the diet. Black cumin seed extract, containing polyphenols like flavonoids (quercetin, an important flavonol) exerts numerous activities such as anti-proliferative and pro-apoptotic effects (reduction in serum levels of total sialic acid, lipid-bound sialic acid, α-fetoprotein, tumor necrosis factor (TNF-α), interleukin-6 (IL-6), prolactin, estradiol, progesterone, tissue caspase-3, -8, -9 activity), antioxidative and cytotoxic effects (reduced serum levels of malondialdehyde (MDA), nitric oxide, ROS, reduction in lipid peroxides, increased level of glutathione (GSH)), antimutagenic effect (increase in detoxifying enzymes, degradation of mutagens, DNA repair) [88]. Phenolic compounds (e.g. 3,4-dihydroxyphenyl ethanol glucoside, 3,4-dihydroxy-6-(N-ethylamino)benzamide and phenolic acid glycosides) extracted from medicinal plants like P. nigrum can scavenge DPPH radicals [99]. C. cassia extract due to the content of polyphenols (e.g. tannins), as well as essential oils (e.g. cinnamic and cinnamyl aldehyde) and carbohydrates, shows various biological functions including antioxidant, antimicrobial, anti-inflammatory, antidiabetic, anti-apoptotic and antitumor activity (e.g. cervical carcinoma and epithelial colorectal adenocarcinoma) [40, 87]. Besides vegetables, also fruits contain natural substances, such as antioxidants that have cancer-protective effects. P. granatum is one of the fruits, which has been used for the prevention and treatment of many diseases for centuries. Due to its strong antioxidant activity, it was proposed as a promising chemopreventive/chemotherapeutic agent. The anticancer activity of pomegranate is generally attributed to the high content of polyphenols such as ellagitannins, ellagic acid, and other flavonoids (flavonols – quercetin, kaempferol, flavones – luteolin) [100]. Phytochemical evaluation of A. muricate showed the presence of various phytochemicals such for example phenolics – hydroxycinnamic acids (e.g. p-coumaric acid, caffeic acid) and flavonol triglycosides, which can be responsible for antiparasitic, antimalarial, anticonvulsant, anti-arthritic, hepatoprotective, antidiabetic and anticancer activities [92]. A Brazilian berry – jaboticaba (Plinia cauliflora (Mart.) Kausel) is traditionally used in folk medicine. Phenolic compounds such as flavonoids, anthocyanins, ellagitannins are responsible for their antioxidant, anti-inflammatory, acetylcholinesterase, antifungal and chemo-preventive activities [48]. Drinking green tea is suggested to have many beneficial health effects, including cancer prevention. Catechins (flavan-3-ol) like epicatechin, epigallocatechin, epicatechin gallate and epigallocatechin gallate, belonging to the group of flavonoids from C. sinesis strongly neutralize ROS and RNS, inhibit the formation of free radicals and lipid peroxidation [80]. Seaweeds (brown, green and red macroalgae) are known as a rich source of bioactive phenolic compounds such as phlorotannins (found only in brown seaweeds), bromophenols, flavonoids, phenolic terpenoids and mycosporine-like amino acids, which exhibit a wide range of activities such as antioxidant, anti-inflammatory, anti-allergic, antibacterial, antiviral, antidiabetic, hepatoprotective, hypotension, neuroprotective and anticancer (e.g. breast cancer) [52, 53, 93]. Lopez et al. [95] examined the phenolic profile of extracts derived from the A. vera leaf skin and flowers. Catechin, sinapic acid, quercetin, quercitrin, rutin, myricetin and epicatechin were the main compounds found in leaves, whereas gentisic acid, epicatechin and quercitrin, sinapic acid, gallic acid and rutin were the most prominent phenolic compounds in flowers. Additionally, both extracts exhibited strong antioxidant activity. Senegalia macrostachya (Rchb. ex DC.) Kyal. & Boatwr., due to the presence of polyphenols like tannins, as well as triterpenes, steroids may be responsible for antioxidant and anticancer properties (e.g. acute and chronic myeloid leukemia) [50]. Ficus carica L. tree is known to contain phenolic acids, flavones and flavonols (quercetin) and inhibit the growth of human cervical carcinoma, liver and breast cancer cells [101]. Biologically active compounds derived from medicinal plants may be an alternative in the search for new anticancer molecules. The examples of medicinal plants used in folk medicine and their biologically active phenolic compounds with anticancer properties (cytotoxic effect) are presented in Table 1.
Table 1.
Examples of medicinal plants as a source of phenolic compounds with anticancer properties
| Medicinal plants | Bioactive compound-phenolics/type of tested extract | Experimental Model | Results | Ref |
|---|---|---|---|---|
| Moringa oleifera (moringa, Moringaceae) | Quercetin, phenolic acids: gallic, sinapic, vanillic, 4-hydroxy-3-methoxy benzoic, p-coumaric, m-coumaric, 4-hydroxy-3-methoxy cinnamic, caffeic, syringic n-hexane extract |
in vitro Hela cervical cancer cells |
↓ cancer cell viability IC50 = 416 μg/ml |
[41] |
| Capsicum annuum (sweet pepper, Solanaceae) |
Phenolics – flavonoids dihydroxycinnamic acids methanolic and ethanolic extract |
in vitro PC3 prostate cancer cells L929 normal fibroblasts HCT116 colon carcinoma cells |
↑cytotoxicity IC50 = 51 μg/ml no toxicity on L929 IC50 = 94 μg/ml ethanolic extract which contains capsianoside, and its fractions – water, 40% methanol in water and 70% methanol in water were less cytotoxic for HCT116 |
[46] |
| Urtica dioica leaves (nettle, Urticaceae) |
Flavonoids and phenolics, mainly patuletin, m/p-hydroxybenzoic acid, caffeic acid aqueous extract |
in vitro U937, KG-1 acute myeloid leukemia cells |
↓proliferation IC50 = 40 μM–100 μM/ml |
[42] |
| Arum palaestinum (black calla, Araceae) |
Polyphenols—flavonoids ethanolic extract |
in vitro Hela cancer cells |
↑cytotoxicity, ↓proliferation IC50 = 256 -512 μg/ml |
[43] |
| Arum palaestinum (black calla, Araceae), Urtica pilulifera (nettle, Urticaceae) |
Phenols—flavonoids ethanolic extract |
in vitro MCF-7 breast cancer cells |
↑cytotoxicity antioxidant, free radical scavenging U. pilulifera extract, IC50= 63 μg/ml A. palaestinum extract, IC50= 500–600 μg/ml |
[45] |
| Asplenium nidus (fern, Aspleniaceae) | Flavonoids (gliricidin7-O-hexoside, quercetin-7-O-rutinoside, keampferol-3-O-rutinoside, myricetin-3-O-rhamnoside) ethanolic extract |
in vitro HepG2 human hepatoma cells Hela cervical carcinoma cells |
↑cytotoxicity antioxidant free radical scavenging |
[54] |
| Curcuma longa (turmeric) and Zingiber officinale (ginger) (Zingiberaceae) |
Polyphenols ethanolic extract |
in vitro B164A5 murine melanoma cells |
↑antioxidant capacity and amount of polyphenols in ethanolic extract from C. longa rhizome than from Z. officinale rhizome; inhibition index for the B164A5 cell line two times was higher for Curcuma compared with Zingiber extract |
[47] |
| Cinnamomum cassia (cinnamon, Lauraceae) |
Polyphenols—tannins ethanolic extract |
in vitro B16F10, clone M3 mouse melanoma cells Hela cervical carcinoma cells Caco2 human epithelial colorectal adenocarcinoma cells |
↓proliferation IC50 = 0.5 mg/ml |
[40] |
| Senegalia macrostachya (Fabaceae) |
Flavonoids—tannins dichloromethane and methanolic extract from the root and steam bark |
in vitro U937, K562 myeloid leukemia cancer cells |
antioxidant the root bark dichloromethane and methanolic extracts demonstrated higher cytotoxicity on U937 and K562 the stem bark methanolic extract selectively affected U937 |
[50] |
| Plinia cauliflora (berry, Myrtaceae) |
Flavonoids, anthocyanins, ellagitannins ethanolic extract |
in vitro L929 murine non-cancer cells MDA-MB-231 estrogenic receptor-negative breast cells |
antioxidant non-toxic effect on L929, ↑ cytotoxicity ↓viability of cells IC50 = 500–1000 μg/ml |
[48] |
| Green tea |
Catechins—epigallocatechin gallate, epigallocatechin, epicatechin, epicatechin gallate ethanolic extract |
in vitro MCF-7 breast cancer cells HepG2 liver cancer Hela cervical cancer cells PC3 prostate cancer cells A549 lung cancer cells |
↑ cytotoxicity IC50 = 292 mg/ml for MCF-7 IC50 = 327 mg/ml for HepG2 IC50 = 331 mg/ml for Hela IC50 = 351 mg/ml for PC3 IC50 = 384 mg/ml for A549 |
[79] |
| Eucheuma cottonii (red seaweed, Rhodophyta) |
Phenolics—catechin, rutin, quercetin |
in vitro MCF-7 estrogen-dependent human breast cancer cells MB-MDA-231 estrogen-independent human breast cancer cells |
↓proliferation IC50 = 20 μg/ml for MCF-7 IC50 = 42 μg/ml for MB-MDA-231 no toxicity on normal cell lines |
[52] |
| Sargassum muticum (brown seaweed, Ochrophyta) |
Phenolics – catechin, phlorotannin, quercetin methanolic extract |
in vitro MCF-7 estrogen-dependent human breast cancer cells MB-MDA-231 estrogen-independent human breast cancer cells |
↑ cytotoxicity ↑apoptosis IC50 = 22 μg/ml for MCF-7 IC50 = 55 μg/ml for MDA-MB-231 |
[53] |
| Acanthospermum hispidum (bristly starbur, Asteraceae) |
Polyphenolics methanolic extract |
in vitro MCF-7 breast cancer cells CORL2 human large cell lung carcinoma cells SVK-14 normal human keratinocytes |
↑ cytotoxicity IC50 ≤ 50 μg/ml |
[49] |
| Diospyros kaki (persimmon) |
Polyphenols Kaki tannin water extract |
in vitro Molt 4B human lymphoid leukemia cells |
↓ growth of cancer cells ↑ apoptosis |
[44] |
| Ficus carica (fig tree, Moraceae) |
Polyphenols – flavonoids Tannins methanolic extract |
in vitro Huh7it liver cancer cells |
antioxidant ↓ growth of cancer cells IC50 = 1000 μg/ml |
[51] |
Phenolic compounds in cancer prevention and treatment: impact on Nrf2
The potential of phenolic compounds within natural extracts and on their own as new chemopreventive agents and/or cancer treatments is discussed below. The most important mechanisms of action are summarized in Table 2 and Fig. 2.
Table 2.
Summarized data of the most representative results from preclinical studies regarding inhibitory effects of phenolic compounds on Nrf2 signaling pathways
| Tested extracts/ polyphenolic compounds |
Experimental Model | Main results | Ref |
|---|---|---|---|
| Cinnamomi Cortex extract |
A549 human lung cancer cells |
↓Nrf2 overexpression ↓IGF-1R ↓anticancer drug resistance |
[102, 103] |
|
Chrysanthemum naktongense Nakai licorice Glycyrrhiza uralensis Fisch |
HT-29 human colon cancer cells HepG C8 human hepatoma cells |
antioxidant ↑detoxifying enzymes induction mediated by ↓Nrf2 ↓NF-κB, ↓pro-inflammatory markers ↑NQO1, ↑Nrf2, ↑UGT1A1, ↑Nrf2-ARE |
[104] |
|
Castanea crenata Siebold & Zucc extract |
MCF-7 breast cancer stem cells |
↓Nrf2 ↑susceptibility of cancerc cells to anticancer drugs |
[105] |
| rosemary extract |
HT-29 human colon cancer cells |
↓Nrf2, ↑ ROS ↑apoptosis, ↑cytotoxicity |
[106] |
| strawberry tree honey |
HT-29 human colon cancer cells |
↓NF-kB, ↓P-IkBa, ↓Nrf2 | [107] |
|
B Bergenia pacumbis (Buch.-Ham. ex D.Don) C.Y.Wu & J.T.Pan extract |
PC3 prostate cancer cells PC3 tumor xenograft mice |
↓Nrf2 ↑ apoptosis, ↓MAO-A, ↑ROS ↓tumor growth |
[108] |
| Rhododendron luteum Sweet extract |
HeLa human cervical cancer cells |
↓Nrf2, ↓ mRNA ↑antiproliferative effect |
[109] |
| Luteolin |
NHBE normal human bronchial epithelial NHBE cells exposed to cigarette smoke |
IC50 = 5–40 μM ↓toxic effects of cigarette smoke ↑cell viability, ↓oxidative stress, ↓apoptosis ↓Nrf2, ↓NADPH, ↓NQO1, ↓HO-1 |
[110] |
|
Wedelolactone isolated from Eclipta prostrate Lour |
IC50 = 2.5–20 μM ↑ NHBE cell viability ↓COX-2, ↓ICAM-1 ↓NQO1, ↓HO-1 |
[111] | |
|
Procyanidins Isolated from Cinnamomi cortex |
PC3 prostate cancer cells |
IC50 = 2.5 μg/ml ↓Nrf2 ↑antiproliferative effect |
[102, 112] |
|
neferine isolated from Nelumbo nucifera Gaertn. leaves |
KYSE30, KYSE150 and KYSE510 esophageal squamous cells carcinoma |
IC50 = 0–20 μM ↓Nrf2, ↑ ROS, ↑JNK ↑antiproliferative effect |
[113] |
|
schisantherin A isolated from Fructus schisandrae |
MKN45, SGC-7901 gastric cancer cell lines |
IC50 = 2.5 μM ↓cell viability, ↓Nrf2 ↑cell cycle arrest, ↑apoptosis ↓migration, ↑ ROS/JNK |
[114] |
|
galloyl glucoses—1,2,3,4,6-penta-O-galloyl-β-D-glucose 1,3,6 -tri-O-galloyl-β-d-glucose isolated from Excoecaria formosana |
Huh7 cancer liver cells |
↓Nrf2 ↑antiproliferative effect |
[115] |
|
agrimoniin isolated from Agrimonia pilosa Aitch |
PANC-1, CFPAC-1 pancreatic cancer cells |
IC50 = 100, 200, 300 μM ↓Nrf2, ↑ ROS ↑apoptosis |
[116] |
|
pterostilbene a natural dimethoxylated analog of resveratrol |
Tumour xenograft mice with human melanomas |
↓Nrf2 ↓tumor growth of melanomas |
[117] |
Abbreviations and symbols: ↑increase, ↓decrease, Nrf2 Nuclear factor erythroid 2-related factor, IGF-1R Insulin-like growth factor 1 receptor, NF-κB Nuclear factor-kappa B, NQO1 (NAD(P)H quinone oxidoreductase 1, NADPH Nicotinamide adenine dinucleotide phosphate, HO-1 Heme oxygenase 1, ROS Reactive oxygen species, JNK Jun N-terminal kinase, ICAM-1 Intercellular adhesion molecule
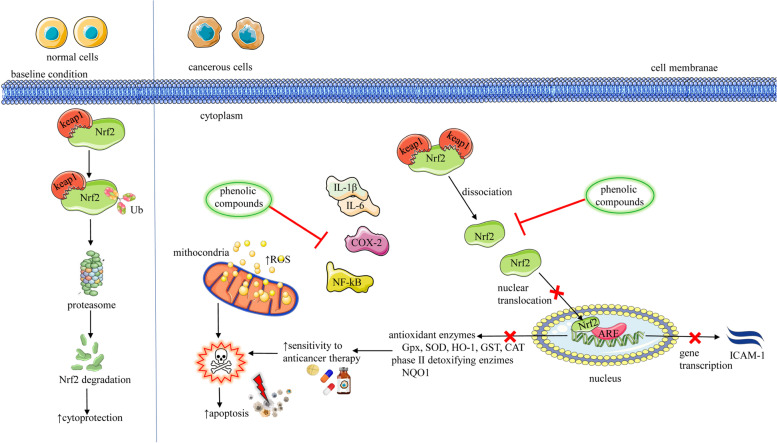
Fig. 2.
Summarized scheme regarding the inhibitory effect of phenolic compounds on Nrf2. Nrf2 inhibition by phenolic compounds leads to increasing the sensitivity of cancer cells to conventional anticancer therapy, reducing tumor growth and cancer cells death. [105] Abbreviations and symbols: ↑ increase, ↓ decrease, Nrf2 (nuclear transcription factor), ROS (Reactive oxygen species), Nrf2-Keap 1 (Kelch-like ECH-associated protein 1), Ub (Ubiquinone), NF-κB Nuclear factor kappa-light-chain-enhancer of activated B cells, Cox-2 (Cyclooxygenase-2), NQO1 (NAD(P)H quinone oxidoreductase 1), (GPx) Glutathione peroxidase, JNK Jun N-terminal kinases, ICAM-1 (Intercellular Adhesion Molecule 1)
Phenolic compounds-rich matrices with inhibitory effects on Nrf2
Cinnamomi cortex extract (CCE) has been shown to inhibit the nuclear translocation of Nrf2, thereby reducing the resistance of tumour cells to conventional chemotherapeutic drugs. Also, the results of this in vitro study performed on human A549 lung cancer cells showed that by inhibiting Nrf2 at the molecular level, CCE potently inhibits the enzyme NQO1 [102] (Fig. 2). In 2017, a novel mechanism by which CCE procyanidin was investigated in A549 cells, this study found that treatment can promote the degradation of nuclear Nrf2 through insulin-line grow factor-1 receptor (IGF-1R). Research results showed that the natural flavonoids contained in CCE suppress Nrf2 activity by inhibiting its nuclear translocation and Nrf2 mRNA expression, thus leading to increased sensitivity of cancer cells to the action of conventional cytostatic drugs [103] (Fig. 2). Chrysanthemum naktongense Nakai and licorice Glycyrrhiza uralensis Fisch. extracts presented an induction to antioxidant and detoxifying enzymes induction mediated by Nrf2. Both extracts inhibited NF-kB and proinflammatory markers such as COX-2, interleukins IL-6, IL-1β having antioxidant and anti-inflammatory effects; they also induced Nrf2 mRNA transcription, ARE expression, NQO1, UGT1A1 genes and phase II detoxifying enzymes leading to apoptotic death of cancer cells. Therefore, the extracts contribute to promoting pharmacological effects against diseases, like cancer [104].
Moreover, it has been observed that the extracts of Castanea crenata Siebold & Zucc. (chestnut) could rise, through the inhibition of the Nrf2 signaling pathway, the susceptibility of breast cancer stem cells (CSCs) to an anticancer drugs, which could be used as an adjuvant for chemotherapy. The mechanisms of action were to increase intracellular ROS levels, inhibit Nrf2-mediated intracellular antioxidant systems, thereby inducing apoptosis of cancer cells [105].
The antitumor activity of a rosemary extract (RE) in colon cancer cells has been investigated, which was obtained through supercritical fluid extraction. Treatment with RE caused an increment of intracellular ROS, leading to apoptosis of tumor cells. So, the cytotoxic effects of RE were increased by NRF2 gene silencing [106]. These results show that the ROS intracellular increment can target colon cancer cells and decrease cell survival mechanisms, which could be a potential treatment in colon cancer by the combination of chemotherapeutic drugs and rosemary compounds. It has been observed that strawberry tree honey (STH) can suppress the expression of pro-inflammatory markers such as NF-kB and P-IkBα (nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha), and inhibition of Nrf2 in colon cancer cells. Glycolysis and mitochondrial respiration processes were also affected by STH, leading to apoptotic death of tumor cancer cells. So, STH could be helpful as a potential treatment for cancer prevention [107]. Another study evaluated the sensitizing effects of the polyphenol-rich fraction of B Bergenia pacumbis (Buch.-Ham. ex D.Don) C.Y.Wu & J.T.Pan in prostate cancer cells [108]. The treatment with the extract-induced apoptosis in PC3 cells both in vitro and in vivo on PC3-tumor xenograft mice involves in a process mediated by activation of monoamine oxidase A (MAO-A) catalytic activity, inactivation of Nrf2 pathway and increased ROS production. The combined treatment of the extract with Paclitaxel led to synergistic effects in reducing tumor growth and apoptosis of tumor cells. The effects of Rhododendron luteum Sweet extract (RLE) have been evaluated on human cervical cancer (HeLa) cells, and the results indicate that can inhibit the mRNA expression levels of Nrf2 showing an antiproliferative effect of RLE [109].
Phenolic compounds with Nrf2 inhibitory effects
The effects of luteolin have been investigated in normal human bronchial epithelial (NHBE) cells exposed to cigarette smoke extract as the main causative agent of lung cancer [110]. Treatment with luteolin (5–40 μM) significantly reduced the toxic effects of cigarette smoke, improving cell viability and, reducing oxidative stress and inducing apoptosis. In addition, luteolin attenuated the protein expressions of Nrf2 and some of the downstream target proteins such as phase II enzymes NAD(P)H dehydrogenase: Quinone 1(NQO1) and heme oxygenase-1 (HO-1) (Fig. 2). Also, the effects of luteolin on Nrf2 were blocked when using a NRF2 gene knockdown model, evidencing the implication of the Nrf2 pathway in cigarette smoke toxicity. In a recent study using cigarette smoke, the treatment with wedelolactone (2.5–20 μM), isolated from Eclipta prostrate Lour., improved NHBE cell viability, recovered the antioxidant defence systems and reduced the levels of proinflammatory mediators such as cyclooxygenase 2 (COX-2) and intercellular adhesion molecule 1 (ICAM-1) [111] (Fig. 2). Moreover, wedelolactone also reduced the expression of NQO1 and HO-1, and this expression was blocked by treatment with the Nrf2 inhibitor all-trans-retinoic acid (ATRA). Procyanidins from Cinnamomi cortex (2.5 μg/ml) were found to exert anti-proliferative activity in lung and prostate cancer cells that overexpress Nrf2 and suppress the elevated Nrf2 expression and the Nrf2-regulated enzyme activity [102, 112] (Fig. 2). Further transfection assays demonstrated that procyanidins had a selective capability to inhibit the excessive activation of Nrf2. Another study reported that neferine (10–20 μM) from Nelumbo nucifera Gaertn. leaves induced apoptosis in esophageal squamous cell carcinoma by increasing ROS production and activating the c-Jun N-terminal kinase (JNK) pathway [113]. In addition, neferine inhibited the expression of Nrf2 favouring the production of ROS. The involvement of the Nrf2 pathway was confirmed by the pre-treatment of cells with the Nrf2 activator, tert‑butylhydroquinone (tBHQ), observing a partial compensation of the proliferative inhibition effect of neferine. The anti-proliferative effects of schisantherin A, a lignan isolated from Fructus schisandrae were investigated in gastric cancer cell lines MKN45 and SGC-7901 [114]. The treatment of cells with schisantherin A (2.5 μM) induced cell cycle arrest and apoptosis and inhibited cell migration. The pro-apoptotic effects were associated with the activations of ROS/JNK and the inhibition of Nrf2 pathways. In addition, treatment of cells with tBHQ counteracted the inhibitory effect of schisantherin A on cell viability activating the Nrf2 pathway. In a study where a total of 44 compounds were isolated from Excoecaria formosana Hayata & Kawak. ex Hayata was analysed, it was observed that two galloyl glucoses—1,2,3,4,6-penta-O-galloyl-β-D-glucose and 1,3,6 -tri-O-galloyl-β-d-glucose—significantly inhibited Nrf2 activity in cancer liver Huh7 cells [115]. Agrimoniin, a dimeric hydrolysable tannin isolated from Agrimonia pilosa Aitch., was also found to sensitize pancreatic PANC-1 and CFPAC-1 cells to apoptosis [116]. Agrimoniin treatment (100, 200, and 300 μM) increased the ROS production in pancreatic cells by suppressing the Nrf2 antioxidant signalling pathway, and altered energy metabolism leading to apoptosis. Regarding studies in animal models, the available data are still scarce and only one report was found. In that study, pterostilbene, a natural dimethoxylated analog of resveratrol, was investigated in tumour xenograft mice using different human melanomas [117]. Intravenous administration of pterostilbene decreased the growth of melanomas and downregulated the Nrf2-dependent signalling pathway after 35 days. In addition, a genetically induced Nrf2 overexpression in the melanoma cells blocked the inhibition of tumour growth.
Synergistic effects of phenolic compounds associated with other anticancer agents on Nrf2
Promising studies have shown the ability of some phenolic compounds to sensitize cancer cells when combined with anticancer agents (Table 3). In this sense, chrysin (5,7-dihydroxy flavone) was investigated against doxorubicin-resistant BEL-7402 hepatocellular carcinoma cells [118]. The treatment with chrysin (10, 20 μM) significantly reduced the mRNA and protein levels of Nrf2 expression via down-regulation of the PI3K-Akt and ERK signalling pathways. The combined treatment with adriamycin and chrysin (10 μM) significantly enhanced the cytotoxicity when compared with adriamycin alone. Luteolin (3,4,5,7-tetrahydroxy flavone) was found to sensitize two resistant colorectal cancer cell lines (HCT116-OX and SW620-OX) to oxaliplatin [119]. Pre-treatment with luteolin (1, 5 and 10 μM) inhibited the Nrf2 pathway in a dose-dependent manner and also inhibited the expression of downstream target genes (HO-1, NQO1, GSTα1/2). In addition, luteolin when combined with chemotherapeutic drugs (doxorubicin, and cisplatin) resulted in a greater anticancer effect, indicating a synergistic effect. An interesting study revealed the capability of 3',4',5',5,7-pentamethoxyflavone, a natural flavonoid extracted from Rutaceae plants, to sensitize cisplatin-resistant A549 cells to cisplatin [120]. The treatment with the flavone (10–400 μM) ameliorated the elevated expression of Nrf2 and its downstream genes HO-1, NQO1 and cysteine ligase catalytic subunit (GCLC) in cisplatin-resistant A549 cells. Moreover, co-exposure of 3',4',5',5,7-pentamethoxyflavone with cisplatin-induced apoptosis to a greater extent than cisplatin alone and this effect was downregulated by siRNA specific for Nrf2. Another study reported the sensitizing capability of resveratrol (15 μM) when combined with clofarabine in mesothelioma MSTO-211H cells [121]. The combined treatment induced a noticeable growth-inhibitory effect, associated with the suppression of the Nrf2 pathway and decreased expression of HO-1. The role of Nrf2 was confirmed through overexpression of Nrf2 which conferred protection to cells and reduced the apoptosis rate. An interesting line of research is the one that deals with the sensitizing capacity of polyphenols in radiotherapy. There is evidence showing the radiosensitizing capacity of some polyphenols such as genistein, ferulic acid or alpinumisoflavone, among others (Wang et al. [122]). The mechanism of action in some of them is related to an increase in ROS production due to the inhibition of Nrf2-mediated cytoprotective gene expression.
Table 3.
The Synergistic effects on Nrf2 of phenolic compounds associated with other anticancer agents
| Type of anticancer agent/treatment associated with phenolic compounds | Experimental Model | Potential Mechanisms | Synergistic effect | Ref |
|---|---|---|---|---|
|
Chrysin (5,7-dihydroxy flavone) |
in vitro BEL-7402 doxorubicin resistant hepatocellular carcinoma cells IC50 = 10, 20 μM |
↓ Nrf2 ↓mRNA ↓PI3K-Akt ↓ERK |
sensitize cancer cells to doxorubicin ↑cytotoxicity |
[118] |
|
Luteolin (3,4,5,7-tetrahydroxy flavone) |
in vitro HCT116-OX SW620-OX oxaliplatin-resistant colorectal cancer cells IC50 = 1, 5—10 μM |
↓ Nrf2 ↓HO-1 ↓NQO1 ↓GSTα1/2 |
sensitize cancer cells to oxaliplatin ↑cytotoxicity |
[119] |
| 3',4',5',5,7-pentamethoxyflavone |
in vitro A549 cisplatin-resistant lung cancer cells IC50 = 10–400 μM |
↓ Nrf2 ↓siRNA ↓HO-1 ↓NQO1 ↓GCLC |
sensitize cancer cells to cisplatin ↑apoptosis |
[120] |
|
resveratrol and clofarabine |
in vitro MSTO-211H mesothelioma cells IC50 = 15 μM |
↓ Nrf2 ↓HO-1 |
↓cancer cells growth ↑apoptosis |
[121] |
| radiotherapy | - |
↓ Nrf2 ↑ROS |
radiosensitivity of cancer cells | Wang et al. [122] |
Therapeutic perspectives, challenges and limitations
Plant-derived phenolic compounds have great anticancer potential. They are responsible for chemopreventive properties such as antioxidant, antimutagenic and anti-inflammatory and the action of phenolic compounds can be considered on several levels, through different mechanisms [123, 124]. Phenolic compounds act as scavengers of ROS/RNS, which are potential carcinogens and induce the cellular defence antioxidant/detoxifying enzymes [125, 126] These compounds can induce antioxidant enzymes such as superoxide dismutase, glutathione-S-transferase, glutathione peroxidase and reductase (e.g., nordihydroguaiaretic in Larrea tridentata (DC.) Coville – [127]; green tea polyphenols – [80]). Polyphenols activate the Nrf2, which is responsible for the control of gene expression and regulation of antioxidant and detoxifying enzymes [80, 127]. As shown in Table 1, numerous plant-derived extracts exert a cytotoxic effect on different types of cancer cell lines. The lack of selectivity in the cytotoxic activity between cancerous and non-cancerous cell lines minimizes the perspective of using active compounds of plant origin as novel anti-cancer drugs [49].
Apoptosis induction is one of the strategies in cancer therapies. In the literature, it was shown that phenolic compounds can induce apoptosis by arresting the cell cycle, regulating carcinogen metabolism and ontogenesis expression, inhibiting DNA binding and reducing cell adhesion, migration, proliferation or differentiation, and blocking signaling pathways [128]. Such properties were noted for example for black pepper phenolics [89], catechin, phlorotannin, quercetin extracted from brown seaweed Sargassum muticum [53], nordihydroguaiaretic acid – a phenolic lignan from the leaves of the evergreen desert shrub (L. tridentate) [127], green tea polyphenols [79], persimmon (Diospyros kaki L.f.) extract in apoptosis of leukemia cells [44], cinnamon extract, which strongly inhibited tumor cell proliferation, induced active tumor cells death by up-regulating pro-apoptotic molecules and inhibiting nuclear factor κB (NF-κB) and activator protein 1 (AP-1) and their target genes such as BCL-2 and BCL-XL – key regulators of apoptosis [40]. Cancer cell lines treated with phenolics-rich plant extracts can exhibit apoptotic morphological changes such as cell shrinkage, DNA fragmentations, a bulge of the plasma membrane of a cell, reduction or absence of microvilli, chromatin condensation and caspase cascade activation—major effector of apoptosis [52]. The main goal of the development of new therapeutic anticancer techniques is to elaborate a strategy to selectively induce apoptosis of cancer cells without altering healthy cells [44]. Based on potential applications in cancer treatment, several polyphenols have been characterized as inhibitors of Nrf2 circuits. For example, luteolin, and a 4-methoxy-chalcone derivative, have been promising in improving the sensitivity of tumor cells to neoplastic drugs [129, 130]. On the other hand, studies in cancer cells line showed that Camptothecin, another Nrf2 inhibitor, could be used in combination with other anticancer drugs to increase its efficacy in cancers with high Nrf2 levels [131].
Although the in vitro results of the use of Nrf2 inhibitors are promising, especially concerning sensitivity to chemotherapy of Nrf2-addicted cancers, the using systemic Nrf2 inhibitors may have pharmacological activity is unsatisfactory due to side effects caused by the essential roles of Nrf2 in cytoprotection. This aspect must be resolved before the use of Nrf2 inhibitors as drugs applied in cancer therapy. Moreover, challenges regarding efficacy, safety, pharmacodynamic properties, and target specificity remain. In this regard, still is under investigation therapeutic new molecules beside Nrf2 for tumors with high Nrf2 levels. In conclusion, the drug development that targets Nrf2 is moving slowly due to the dual effects of NRF2 in cancer.
In the case of medicinal plants, there is still a long way from folk medicine to clinical use. The observation of the natural environment and selection of the active substances that can alleviate disease symptoms should be verified and confirmed in the toxicological studies, which are associated with a detailed phytochemical analysis. It is important to evaluate the potentially toxic effects that may reduce the therapeutic value of natural remedies [132]. It is worth emphasizing that medicinal plants with anticancer molecules may support preventive and chemotherapeutic effects, but they cannot replace pharmacological treatment [80]. Scientific research conducted around the world on the cytotoxicity of extracts from medical plants on cancer cell lines confirms their effectiveness and supports their use in traditional medicine in cancer treatment. Also, experimental studies showed that the activity of plant extracts is usually attributed to the mixture of biologically active compounds (phenolic compounds, polysaccharides, proteins, amino acids, vitamins, minerals, phytosterols, essential oils, etc.), no single compounds. Therefore, the combined anticancer mechanism and effects of the various compounds, including phenolics on cancer cell lines have still to be explored.
Conclusions
Natural products obtained from medicinal plants can serve as a rich source of compounds, for example, phenolic compounds with health-promoting properties [46, 50]. Due to antioxidant, anti-inflammatory and immunomodulatory properties, these compounds can be used in the prevention and treatment of several diseases, including cancer [87, 88]. Literature data have already confirmed the effectiveness of plant-derived products, including their cytotoxic effect on the cancer cell lines, which to a certain extent supports their traditional inclusion in cancer prevention and treatment [49]. The effect of natural products on the health maintenance and prevention of diseases is usually not confirmed by their exact chemical composition or the explained mechanism of action. Therefore, there is a need to find a relationship between the effectiveness of natural products and their composition and biological activity, confirmed in scientific research (in vitro and in vivo studies). Despite the enormous advances in conventional medicine and drug discovery, the use of herbal remedies is still extremely widespread around the world [88]. Nrf2 has a significant part in cellular defense against oxidative stress and exogenous toxic materials. Moreover, it plays a crucial role in tumorigenesis and drug sensitivity. Due to the critical role of Nrf2, it has emerged as a therapeutic target for the prevention and therapy of cancer. However, since Nrf2 has paradoxical roles in cancer biology, it is necessary to understand the molecular pathway leading to tumor suppress or the oncogenic effect of Nrf2 for the development of drugs with high specific and limited side effects. Natural products, including phenolic compounds, mediate the Nrf2/ARE pathway and may act as chemopreventive or chemotherapeutic agents. New phytodrugs need to be studied and developed to better understand the role of phenolic compounds in cancer.
Acknowledgements
A. Sureda was supported by Instituto de Salud Carlos III through the Fondo de Investigación para la Salud (CIBEROBN CB12/03/30038). M. Martorell wants to thank ANID CENTROS BASALES ACE210012.
Authors’ contributions
Conceptualization and design were performed by D.C., J.S.-R., and W.C.C.; investigation, data curation, and writing were performed by V.S., M.I., M.M.-M., V.O., F.A.Z., S.S.M., R.P., A.Y., G.T.; validation, review and editing were performed by D.C., M.M., A.S., J.S.-R., and W.C.C.; supervision D.C., M.M., A.S., J.S.-R., and W.C.C.; All the authors contributed equally, read and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
No Funding received.
Availability of data and materials
Not Applicable.
Declarations
Ethics approval and consent to participate
Not Applicable
Consent for publication
Not Applicable
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Veronique Seidel, Email: veronique.seidel@strath.ac.uk.
Michalak Izabela, Email: izabela.michalak@pwr.edu.pl.
Margalida Monserrat-Mequida, Email: margalida.monserrat@uib.es.
Antoni Sureda, Email: antoni.sureda@uib.es.
Valeska Ormazabal, Email: vormazabal@udec.cl.
Felipe A. Zuniga, Email: fzuniga@udec.cl
Shivaprasad Shetty Mangalpady, Email: shivaprasad@nitte.edu.in.
Raffaele Pezzani, Email: raffaele.pezzani@unipd.it.
Alibek Ydyrys, Email: alibek.ydyrys@kaznu.kz.
Gulmira Tussupbekova, Email: gulmira.tussupbekova@kaznu.edu.kz.
Miquel Martorell, Email: mmartorell@udec.cl.
Daniela Calina, Email: calinadaniela@gmail.com.
William C. Cho, Email: chocs@ha.org.hk
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. (https://gcoiarc.fr/today). Accesed 20 Sept 2022. 2020.
- 3.McGranahan N, Swanton C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell. 2017;168(4):613–628. doi: 10.1016/j.cell.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Dhyani P, Quispe C, Sharma E, Bahukhandi A, Sati P, Attri DC, et al. Anticancer potential of alkaloids: a key emphasis to colchicine, vinblastine, vincristine, vindesine, vinorelbine and vincamine. Cancer Cell Int. 2022;22(1):206. doi: 10.1186/s12935-022-02624-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozkan G, Günal-Köroğlu D, Karadag A, Capanoglu E, Cardoso SM, Al-Omari B, et al. A mechanistic updated overview on lycopene as potential anticancer agent. Biomed Pharmacother. 2023;161:114428. doi: 10.1016/j.biopha.2023.114428. [DOI] [PubMed] [Google Scholar]
- 6.Sova M, Saso L. Design and development of Nrf2 modulators for cancer chemoprevention and therapy: a review. Drug Des Devel Ther. 2018;12:3181–3197. doi: 10.2147/DDDT.S172612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anantharaju PG, Gowda PC, Vimalambike MG, Madhunapantula SV. An overview on the role of dietary phenolics for the treatment of cancers. Nutr J. 2016;15(1):99. doi: 10.1186/s12937-016-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prasher P, Sharma M, Sharma AK, Sharifi-Rad J, Calina D, Hano C, et al. Key oncologic pathways inhibited by Erinacine A: A perspective for its development as an anticancer molecule. Biomedicine & Pharmacotherapy. 2023;160:114332. doi: 10.1016/j.biopha.2023.114332. [DOI] [PubMed] [Google Scholar]
- 9.Mititelu RR, Padureanu R, Bacanoiu M, Padureanu V, Docea AO, Calina D, et al. Inflammatory and Oxidative Stress Markers-Mirror Tools in Rheumatoid Arthritis. Biomedicines. 2020;8(5). 10.3390/biomedicines8050125. [DOI] [PMC free article] [PubMed]
- 10.Kostoff RN, Kanduc D, Porter AL, Shoenfeld Y, Calina D, Briggs MB, et al. Vaccine- and natural infection-induced mechanisms that could modulate vaccine safety. Toxicol Rep. 2020;7:1448–1458. doi: 10.1016/j.toxrep.2020.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K, Jiang J, Lei Y, Zhou S, Wei Y, Huang C. Targeting Metabolic-Redox Circuits for Cancer Therapy. Trends Biochem Sci. 2019;44(5):401–414. doi: 10.1016/j.tibs.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Pezzani R, Jiménez-Garcia M, Capó X, Sönmez Gürer E, Sharopov F, Rachel TYL, et al. Anticancer properties of bromelain: State-of-the-art and recent trends. Front Oncol. 2023;12. 10.3389/fonc.2022.1068778. [DOI] [PMC free article] [PubMed]
- 13.Garzoli S, Alarcón-Zapata P, Seitimova G, Alarcón-Zapata B, Martorell M, Sharopov F, et al. Natural essential oils as a new therapeutic tool in colorectal cancer. Cancer Cell Int. 2022;22(1):407. doi: 10.1186/s12935-022-02806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ju HQ, Gocho T, Aguilar M, Wu M, Zhuang ZN, Fu J, et al. Mechanisms of Overcoming Intrinsic Resistance to Gemcitabine in Pancreatic Ductal Adenocarcinoma through the Redox Modulation. Mol Cancer Ther. 2015;14(3):788–798. doi: 10.1158/1535-7163.MCT-14-0420. [DOI] [PubMed] [Google Scholar]
- 15.Sharifi-Rad J, Bahukhandi A, Dhyani P, Sati P, Capanoglu E, Docea AO, et al. Therapeutic Potential of Neoechinulins and Their Derivatives: An Overview of the Molecular Mechanisms Behind Pharmacological Activities. Front Nutr. 2021;8:664197. doi: 10.3389/fnut.2021.664197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Tian Z, Guo R, Ren F. Nrf2 Inhibitor, Brusatol in Combination with Trastuzumab Exerts Synergistic Antitumor Activity in HER2-Positive Cancers by Inhibiting Nrf2/HO-1 and HER2-AKT/ERK1/2 Pathways. Oxid Med Cell Longev. 2020;2020:9867595. doi: 10.1155/2020/9867595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robledinos-Anton N, Fernandez-Gines R, Manda G, Cuadrado A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid Med Cell Longev. 2019;2019:9372182. doi: 10.1155/2019/9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600–Mutant Anaplastic Thyroid Cancer. J Clin Oncol. 2018;36(1):7–13. doi: 10.1200/jco.2017.73.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milkovic L, Zarkovic N, Saso L. Controversy about pharmacological modulation of Nrf2 for cancer therapy. Redox Biol. 2017;12:727–732. doi: 10.1016/j.redox.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisek K, Campaner E, Ciani Y, Walerych D, Del Sal G. Mutant p53 tunes the NRF2-dependent antioxidant response to support survival of cancer cells. Oncotarget. 2018;9(29):20508–20523. doi: 10.18632/oncotarget.24974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 22.Telkoparan-Akillilar P, Suzen S, Saso L. Pharmacological Applications of Nrf2 Inhibitors as Potential Antineoplastic Drugs. Int J Mol Sci. 2019;20(8):2025. doi: 10.3390/ijms20082025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339(1):79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 24.Sporn MB, Liby KT. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer. 2012;12(8):564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cescon DW, She D, Sakashita S, Zhu CQ, Pintilie M, Shepherd FA, et al. NRF2 Pathway Activation and Adjuvant Chemotherapy Benefit in Lung Squamous Cell Carcinoma. Clin Cancer Res. 2015;21(11):2499–2505. doi: 10.1158/1078-0432.CCR-14-2206. [DOI] [PubMed] [Google Scholar]
- 26.Cho HY, Kwak MK, Pi J. Nrf2 in host defense: over the rainbow. Oxid Med Cell Longev. 2013;2013:975839. doi: 10.1155/2013/975839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sajadimajd S, Khazaei M. Oxidative Stress and Cancer: The Role of Nrf2. Curr Cancer Drug Targets. 2018;18(6):538–557. doi: 10.2174/1568009617666171002144228. [DOI] [PubMed] [Google Scholar]
- 28.Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the Hallmarks of Cancer. Cancer Cell. 2018;34(1):21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coêlho ML, Islam MT, Laylson da Silva Oliveira G, Oliveira Barros de Alencar MV, Victor de Oliveira Santos J, Campinho dos Reis A, et al. Cytotoxic and Antioxidant Properties of Natural Bioactive Monoterpenes Nerol, Estragole, and 3,7-Dimethyl-1-Octanol. Adv Pharmacol Pharm Sci. 2022;2022:8002766. doi: 10.1155/2022/8002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Jiang Z, Lu H, Xu Z, Tong R, Shi J, et al. Recent Advances of Natural Polyphenols Activators for Keap1-Nrf2 Signaling Pathway. Chem Biodivers. 2019;16(11):e1900400. doi: 10.1002/cbdv.201900400. [DOI] [PubMed] [Google Scholar]
- 31.Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer. 2010;62(1):1–20. doi: 10.1080/01635580903191585. [DOI] [PubMed] [Google Scholar]
- 32.Subramaniam S, Selvaduray KR, Radhakrishnan AK. Bioactive Compounds: Natural Defense Against Cancer? Biomolecules. 2019;9(12):758. doi: 10.3390/biom9120758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.WFO: WFO The World Flora Online. http://www.worldfloraonline.org/. (2021). Accessed.
- 34.PubChem: Explore Chemistry. https://pubchem.ncbi.nlm.nih.gov/. Accessed.
- 35.Sharifi-Rad J, Quispe C, Herrera-Bravo J, Martorell M, Sharopov F, Tumer TB, et al. A Pharmacological Perspective on Plant-derived Bioactive Molecules for Epilepsy. Neurochem Res. 2021;46(9):2205–2225. doi: 10.1007/s11064-021-03376-0. [DOI] [PubMed] [Google Scholar]
- 36.Sharifi-Rad J, Kamiloglu S, Yeskaliyeva B, Beyatli A, Alfred MA, Salehi B, et al. Pharmacological Activities of Psoralidin: A Comprehensive Review of the Molecular Mechanisms of Action. Front Pharmacol. 2020;11:11. doi: 10.3389/fphar.2020.571459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharifi-Rad J, Cruz-Martins N, López-Jornet P, Lopez EP-F, Harun N, Yeskaliyeva B, et al. Natural Coumarins: Exploring the Pharmacological Complexity and Underlying Molecular Mechanisms. Oxidative Med Cell Longevity. 2021;2021:6492346. doi: 10.1155/2021/6492346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharifi-Rad ZMAJ, Adetunji CO, Samuel Michael O, Chandran D, Radha R, Sharma N, Kumar M, Calina D. Neuroprotective effect of curcumin and curcumin-integrated nanocarriers in stroke: from mechanisms to therapeutic opportunities. Minerva Biotechnol Biomol Res. 2022;34(4):153–69. doi: 10.23736/S2724-542X.22.02946-7. [DOI] [Google Scholar]
- 39.Scheau C, Caruntu C, Badarau IA, Scheau AE, Docea AO, Calina D, et al. Cannabinoids and Inflammations of the Gut-Lung-Skin Barrier. J Pers Med. 2021;11(6). 10.3390/jpm11060494. [DOI] [PMC free article] [PubMed]
- 40.Kwon HK, Hwang JS, So JS, Lee CG, Sahoo A, Ryu JH, et al. Cinnamon extract induces tumor cell death through inhibition of NFkappaB and AP1. BMC Cancer. 2010;10:392. doi: 10.1186/1471-2407-10-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mumtaz MZ, Kausar F, Hassan M, Javaid S, Malik A. Anticancer activities of phenolic compounds from Moringa oleifera leaves: in vitro and in silico mechanistic study. Beni-Suef Univ J Basic Appl Sci. 2021;10(1):12. 10.1186/s43088-021-00101-2.
- 42.Hodroj MH, Al Bast NAH, Taleb RI, Borjac J, Rizk S. Nettle tea inhibits growth of acute myeloid leukemia cells in vitro by promoting apoptosis. Nutrients. 2020;12(9). 10.3390/nu12092629. [DOI] [PMC free article] [PubMed]
- 43.Naseef Shtaya H, Qadadha H, Asfour Y, Sabri I, Al-Rimawi F, Abu-Qatouseh L, et al. Anticancer, antibacterial, and antifungal activities of Arum palaestinum plant extracts. World J Pharm Res. 2017;6(15):31–43. doi: 10.20959/wjpr201715-10091. [DOI] [Google Scholar]
- 44.Achiwa Y, Hibasami H, Katsuzaki H, Imai K, Komiya T. Inhibitory effects of persimmon (Diospyros kaki) extract and related polyphenol compounds on growth of human lymphoid leukemia cells. Biosci Biotechnol Biochem. 1997;61(7):1099–1101. doi: 10.1271/bbb.61.1099. [DOI] [PubMed] [Google Scholar]
- 45.Husein AI, Ali-Shtayeh MS, Jondi WJ, Zatar NA, Abu-Reidah IM, Jamous RM. In vitro antioxidant and antitumor activities of six selected plants used in the traditional Arabic Palestinian herbal medicine. Pharm Biol. 2014;52(10):1249–55. 10.3109/13880209.2014.886274. [DOI] [PubMed]
- 46.Chilczuk B, Marciniak B, Stochmal A, Pecio L, Kontek R, Jackowska I, et al. Anticancer potential and capsianosides identification in lipophilic fraction of sweet pepper (Capsicum annuum L.). Molecules. 2020;25(13). 10.3390/molecules25133097. [DOI] [PMC free article] [PubMed]
- 47.Danciu C, Vlaia L, Fetea F, Hancianu M, Coricovac DE, Ciurlea SA, et al. Evaluation of phenolic profile, antioxidant and anticancer potential of two main representants of Zingiberaceae family against B164A5 murine melanoma cells. Biol Res. 2015;48(1):1. doi: 10.1186/0717-6287-48-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendonca de Assis P, Cypriano Dutra R, Amarante CBD, Afonso Miranda Chaves MDG, Moreira CPS, Brandao MAF, et al. Plinia cauliflora (Mart.) Kausel: toxicological assays, biological activities, and elemental analysis of organic compounds. Nat Prod Res. 2021;35(10):1727–31. doi: 10.1080/14786419.2019.1633642. [DOI] [PubMed] [Google Scholar]
- 49.Ashidi JS, Houghton PJ, Hylands PJ, Efferth T. Ethnobotanical survey and cytotoxicity testing of plants of South-western Nigeria used to treat cancer, with isolation of cytotoxic constituents from Cajanus cajan Millsp. leaves. J Ethnopharmacol. 2010;128(2):501–12. doi: 10.1016/j.jep.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 50.Ganame HT, Karanga Y, Tapsoba I, Dicato M, Diederich MF, Cerella C, et al. Phytochemical screening and antioxidant and cytotoxic effects of acacia macrostachya. plants (Basel). 2021;10(7). 10.3390/plants10071353. [DOI] [PMC free article] [PubMed]
- 51.Purnamasari R, Winarni D, Permanasari AA, Agustina E, Hayaza S, Darmanto W. Anticancer Activity of Methanol Extract of Ficus carica Leaves and Fruits Against Proliferation, Apoptosis, and Necrosis in Huh7it Cells. Cancer Inform. 2019;18:1176935119842576. doi: 10.1177/1176935119842576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Namvar F, Mohamed S, Fard SG, Behravan J, Mustapha NM, Alitheen NBM, et al. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012;130(2):376–382. doi: 10.1016/j.foodchem.2011.07.054. [DOI] [Google Scholar]
- 53.Namvar F, Mohamad R, Baharara J, Zafar-Balanejad S, Fargahi F, Rahman HS. Antioxidant, antiproliferative, and antiangiogenesis effects of polyphenol-rich seaweed (Sargassum muticum) Biomed Res Int. 2013;2013:604787. doi: 10.1155/2013/604787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jarial R, Thakur S, Sakinah M, Zularisam AW, Sharad A, Kanwar SS, et al. Potent anticancer, antioxidant and antibacterial activities of isolated flavonoids from Asplenium nidus. J King Saud Univ Sci. 2018;30(2):185–192. doi: 10.1016/j.jksus.2016.11.006. [DOI] [Google Scholar]
- 55.Custódio L, Vizetto-Duarte C, Cebeci F, Özçelik B, Sharopov F, Gürer ES, et al. Natural products of relevance in the management of attention deficit hyperactivity disorder. eFood. 2023;4(1):e57. doi: 10.1002/efd2.57. [DOI] [Google Scholar]
- 56.Amin R, Thalluri C, Docea AO, Sharifi-Rad J, Calina D. Therapeutic potential of cranberry for kidney health and diseases. eFood. 2022;3(5):e33. doi: 10.1002/efd2.33. [DOI] [Google Scholar]
- 57.Quetglas-Llabrés MM, Quispe C, Herrera-Bravo J, Catarino MD, Pereira OR, Cardoso SM, et al. Pharmacological Properties of Bergapten: Mechanistic and Therapeutic Aspects. Oxid Med Cell Longev. 2022;2022:8615242. doi: 10.1155/2022/8615242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Briguglio G, Costa C, Pollicino M, Giambò F, Catania S, Fenga C. Polyphenols in cancer prevention: New insights (Review) Int J Funct Nutr. 2020;1(2):9. doi: 10.3892/ijfn.2020.9. [DOI] [Google Scholar]
- 59.Haslam E. Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J Nat Prod. 1996;59(2):205–215. doi: 10.1021/np960040+. [DOI] [PubMed] [Google Scholar]
- 60.Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines (Basel). 2018;5(3). 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed]
- 61.Carocho M, Ferreira IC. The role of phenolic compounds in the fight against cancer–a review. Anticancer Agents Med Chem. 2013;13(8):1236–1258. doi: 10.2174/18715206113139990301. [DOI] [PubMed] [Google Scholar]
- 62.Batra P, Sharma AK. Anti-cancer potential of flavonoids: recent trends and future perspectives. 3 Biotech. 2013;3(6):439–59. doi: 10.1007/s13205-013-0117-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsoukalas D, Fragkiadaki P, Docea AO, Alegakis AK, Sarandi E, Vakonaki E, et al. Association of nutraceutical supplements with longer telomere length. Int J Mol Med. 2019;44(1):218–226. doi: 10.3892/ijmm.2019.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsoukalas D, Zlatian O, Mitroi M, Renieri E, Tsatsakis A, Izotov BN, et al. A Novel Nutraceutical Formulation Can Improve Motor Activity and Decrease the Stress Level in a Murine Model of Middle-Age Animals. J Clin Med. 2021;10(4). 10.3390/jcm10040624. [DOI] [PMC free article] [PubMed]
- 65.Popović-Djordjević J, Quispe C, Giordo R, Kostić A, Katanić Stanković JS, Tsouh Fokou PV, et al. Natural products and synthetic analogues against HIV: A perspective to develop new potential anti-HIV drugs. Eur J Med Chem. 2022;233:114217. doi: 10.1016/j.ejmech.2022.114217. [DOI] [PubMed] [Google Scholar]
- 66.Tsoukalas D, Fragoulakis V, Sarandi E, Docea AO, Papakonstaninou E, Tsilimidos G, et al. Targeted metabolomic analysis of serum fatty acids for the prediction of autoimmune diseases. Front Mol Biosci. 2019;6(120). 10.3389/fmolb.2019.00120. [DOI] [PMC free article] [PubMed]
- 67.Calina D, Buga AM, Mitroi M, Buha A, Caruntu C, Scheau C, et al. The treatment of cognitive, behavioural and motor impairments from brain injury and neurodegenerative diseases through cannabinoid system modulation-evidence from in vivo studies. J Clin Med. 2020;9(8):28. 10.3390/jcm9082395. [DOI] [PMC free article] [PubMed]
- 68.Islam MS, Quispe C, Hossain R, Islam MT, Al-Harrasi A, Al-Rawahi A, et al. Neuropharmacological effects of quercetin: a literature-based review. Front Pharmacol. 2021;12(1533). 10.3389/fphar.2021.665031. [DOI] [PMC free article] [PubMed]
- 69.Sharifi-Rad J, Quispe C, Shaheen S, El Haouari M, Azzini E, Butnariu M, et al. Flavonoids as potential anti-platelet aggregation agents: from biochemistry to health promoting abilities. Crit Rev Food Sci Nutr. 2022;62(29):8045–8058. doi: 10.1080/10408398.2021.1924612. [DOI] [PubMed] [Google Scholar]
- 70.Sharifi-Rad J, Quispe C, Bouyahya A, El Menyiy N, El Omari N, Shahinozzaman M, et al. Ethnobotany, phytochemistry, biological activities, and health-promoting effects of the genus Bulbophyllum. Evid Based Complement Alternat Med. 2022;2022:6727609. doi: 10.1155/2022/6727609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharifi-Rad J, Dey A, Koirala N, Shaheen S, El Omari N, Salehi B, et al. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front Pharm. 2021;12:600139. doi: 10.3389/fphar.2021.600139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salehi B, Sestito S, Rapposelli S, Peron G, Calina D, Sharifi-Rad M, et al. Epibatidine: A Promising Natural Alkaloid in Health. Biomolecules. 2019;9(1):6. doi: 10.3390/biom9010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsoukalas D, Buga AM, Docea AO, Sarandi E, Mitrut R, Renieri E, et al. Reversal of brain aging by targeting telomerase: A nutraceutical approach. Int J Mol Med. 2021;48(5). 10.3892/ijmm.2021.5032. [DOI] [PMC free article] [PubMed]
- 74.Tsoukalas D, Fragkiadaki P, Docea AO, Alegakis AK, Sarandi E, Thanasoula M, et al. Discovery of potent telomerase activators: Unfolding new therapeutic and anti-aging perspectives. Mol Med Rep. 2019;20(4):3701–3708. doi: 10.3892/mmr.2019.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rahaman MM, Hossain R, Herrera-Bravo J, Islam MT, Atolani O, Adeyemi OS, et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci Nutr. 10.1002/fsn3.3217. [DOI] [PMC free article] [PubMed]
- 76.Sharma E, Attri DC, Sati P, Dhyani P, Szopa A, Sharifi-Rad J, et al. Recent updates on anticancer mechanisms of polyphenols. Front Cell Dev Biol. 2022;10. 10.3389/fcell.2022.1005910. [DOI] [PMC free article] [PubMed]
- 77.Sharifi-Rad J, Quispe C, Durazzo A, Lucarini M, Souto EB, Santini A, et al. Resveratrol’ biotechnological applications: Enlightening its antimicrobial and antioxidant properties. J Herbal Med. 2022;32:100550. doi: 10.1016/j.hermed.2022.100550. [DOI] [Google Scholar]
- 78.Lee JH, Khor TO, Shu L, Su ZY, Fuentes F, Kong AN. Dietary phytochemicals and cancer prevention: Nrf2 signaling, epigenetics, and cell death mechanisms in blocking cancer initiation and progression. Pharmacol Ther. 2013;137(2):153–171. doi: 10.1016/j.pharmthera.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu SM, Ou SY, Huang HH. Green tea polyphenols induce cell death in breast cancer MCF-7 cells through induction of cell cycle arrest and mitochondrial-mediated apoptosis. J Zhejiang Univ Sci B. 2017;18(2):89–98. doi: 10.1631/jzus.B1600022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Musial C, Kuban-Jankowska A, Gorska-Ponikowska M. Beneficial properties of green Tea catechins. Int J Mol Sci. 2020;21(5). 10.3390/ijms21051744. [DOI] [PMC free article] [PubMed]
- 81.Thyagarajan A, Forino AS, Konger RL, Sahu RP. Dietary polyphenols in cancer Chemoprevention: implications in pancreatic cancer. Antioxidants (Basel). 2020;9(8). 10.3390/antiox9080651. [DOI] [PMC free article] [PubMed]
- 82.Asgharian P, Tazekand AP, Hosseini K, Forouhandeh H, Ghasemnejad T, Ranjbar M, et al. Potential mechanisms of quercetin in cancer prevention: focus on cellular and molecular targets. Cancer Cell Int. 2022;22(1):257. doi: 10.1186/s12935-022-02677-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aumeeruddy MZ, Mahomoodally MF. Global documentation of traditionally used medicinal plants in cancer management: A systematic review. S Afr J Bot. 2021;138:424–494. doi: 10.1016/j.sajb.2021.01.006. [DOI] [Google Scholar]
- 84.Asemani Y, Zamani N, Bayat M, Amirghofran Z. Allium vegetables for possible future of cancer treatment. Phytother Res. 2019;33(12):3019–3039. doi: 10.1002/ptr.6490. [DOI] [PubMed] [Google Scholar]
- 85.Ahmad JS. Antioxidant and anticancer activities of Brassica rapa: a review. MOJ Biol Med. 2018;3(4):175–178. doi: 10.15406/mojbm.2018.03.00094. [DOI] [Google Scholar]
- 86.Esposito S, Bianco A, Russo R, Di Maro A, Isernia C, Pedone PV. Therapeutic Perspectives of Molecules from Urtica dioica Extracts for Cancer Treatment. Molecules. 2019;24(15). doi: 10.3390/molecules24152753. [DOI] [PMC free article] [PubMed]
- 87.Zheng J, Zhou Y, Li Y, Xu DP, Li S, Li HB. Spices for Prevention and Treatment of Cancers. Nutrients. 2016;8(8). doi: 10.3390/nu8080495. [DOI] [PMC free article] [PubMed]
- 88.Majdalawieh AF, Fayyad MW. Recent advances on the anti-cancer properties of Nigella sativa, a widely used food additive. J Ayurveda Integr Med. 2016;7(3):173–180. doi: 10.1016/j.jaim.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takooree H, Aumeeruddy MZ, Rengasamy KRR, Venugopala KN, Jeewon R, Zengin G, et al. A systematic review on black pepper (Piper nigrum L.): from folk uses to pharmacological applications. Crit Rev Food Sci Nutr. 2019;59(sup1):S210–S43. doi: 10.1080/10408398.2019.1565489. [DOI] [PubMed] [Google Scholar]
- 90.Hossain R, Sarkar C, Hassan SMH, Khan RA, Arman M, Ray P, et al. In silico screening of natural products as potential inhibitors of SARS-CoV-2 using molecular docking simulation. Chin J Integr Med. 2021:1–8. 10.1007/s11655-021-3504-5. [DOI] [PMC free article] [PubMed]
- 91.Turrini E, Ferruzzi L, Fimognari C. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxid Med Cell Longev. 2015;2015:938475. doi: 10.1155/2015/938475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moghadamtousi SZ, Fadaeinasab M, Nikzad S, Mohan G, Ali HM, Kadir HA. Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci. 2015;16(7):15625–58. doi: 10.3390/ijms160715625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cotas J, Pacheco D, Gonçalves AMM, Silva P, Carvalho LG, Pereira L. Seaweeds’ nutraceutical and biomedical potential in cancer therapy: a concise review. J Cancer Metastasis Treat. 2021;2021. 10.20517/2394-4722.2020.134.
- 94.Szwajkowska-Michałek LP-BARTS-SKPCiT, Shrubs of Central E. Applied Sciences. 2020 10.3390/app10196907.
- 95.Lopez A, de Tangil MS, Vega-Orellana O, Ramirez AS, Rico M. Phenolic constituents, antioxidant and preliminary antimycoplasmic activities of leaf skin and flowers of Aloe vera (L.) Burm. f. (syn. A. barbadensis Mill.) from the Canary Islands (Spain) Molecules. 2013;18(5):4942–54. doi: 10.3390/molecules18054942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hossain R, Quispe C, Saikat ASM, Jain D, Habib A, Janmeda P, et al. Biosynthesis of Secondary Metabolites Based on the Regulation of MicroRNAs. Biomed Res Int. 2022;2022:9349897. doi: 10.1155/2022/9349897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alshehri MM, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Tutuncu S, Aydar EF, et al. A Review of Recent Studies on the Antioxidant and Anti-Infectious Properties of Senna Plants. Oxid Med Cell Longev. 2022;2022:6025900. doi: 10.1155/2022/6025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bagheri G, Martorell M, Ramírez-Alarcón K, Salehi B, Sharifi-Rad J. Phytochemical screening of Moringa oleifera leaf extracts and their antimicrobial activities. Cell Mol Biol (Noisy-le-grand). 2020;66(1):20–6. doi: 10.14715/cmb/2019.66.1.3. [DOI] [PubMed] [Google Scholar]
- 99.Chatterjee S, Niaz Z, Gautam S, Adhikari S, Variyar PS, Sharma A. Antioxidant activity of some phenolic constituents from green pepper (Piper nigrum L.) and fresh nutmeg mace (Myristica fragrans) Food Chem. 2007;101(2):515–23. doi: 10.1016/j.foodchem.2006.02.008. [DOI] [Google Scholar]
- 100.Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in Pomegranate juice. J Nutr Biochem. 2005;16(6):360–7. 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed]
- 101.Salehi B, Prakash Mishra A, Nigam M, Karazhan N, Shukla I, Kiełtyka-Dadasiewicz A, et al. Ficus plants: State of the art from a phytochemical, pharmacological, and toxicological perspective. Phytother Res. 2021;35(3):1187–1217. doi: 10.1002/ptr.6884. [DOI] [PubMed] [Google Scholar]
- 102.Ohnuma T, Matsumoto T, Itoi A, Kawana A, Nishiyama T, Ogura K, et al. Enhanced sensitivity of A549 cells to the cytotoxic action of anticancer drugs via suppression of Nrf2 by procyanidins from Cinnamomi Cortex extract. Biochem Biophys Res Commun. 2011;413(4):623–629. doi: 10.1016/j.bbrc.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 103.Ohnuma T, Sakamoto K, Shinoda A, Takagi C, Ohno S, Nishiyama T, et al. Procyanidins from Cinnamomi Cortex promote proteasome-independent degradation of nuclear Nrf2 through phosphorylation of insulin-like growth factor-1 receptor in A549 cells. Arch Biochem Biophys. 2017;635:66–73. doi: 10.1016/j.abb.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 104.Wu TY, Khor TO, Saw CL, Loh SC, Chen AI, Lim SS, et al. Anti-inflammatory/Anti-oxidative stress activities and differential regulation of Nrf2-mediated genes by non-polar fractions of tea Chrysanthemum zawadskii and licorice Glycyrrhiza uralensis. AAPS J. 2011;13(1):1–13. doi: 10.1208/s12248-010-9239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Woo Y, Oh J, Kim JS. Suppression of Nrf2 activity by chestnut leaf extract increases chemosensitivity of breast cancer stem cells to paclitaxel. Nutrients. 2017;9(7). 10.3390/nu9070760. [DOI] [PMC free article] [PubMed]
- 106.Perez-Sanchez A, Barrajon-Catalan E, Ruiz-Torres V, Agullo-Chazarra L, Herranz-Lopez M, Valdes A, et al. Rosemary (Rosmarinus officinalis) extract causes ROS-induced necrotic cell death and inhibits tumor growth in vivo. Sci Rep. 2019;9(1):808. doi: 10.1038/s41598-018-37173-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Afrin S, Forbes-Hernández TY, Cianciosi D, Pistollato F, Zhang J, Pacetti M, et al. Strawberry tree honey as a new potential functional food. Part 2: Strawberry tree honey increases ROS generation by suppressing Nrf2-ARE and NF-кB signaling pathways and decreases metabolic phenotypes and metastatic activity in colon cancer cells. J Funct Foods. 2019;57:477–87. doi: 10.1016/j.jff.2019.04.037. [DOI] [Google Scholar]
- 108.Ghosh S, Dutta N, Banerjee P, Gajbhiye RL, Sareng HR, Kapse P, et al. Induction of monoamine oxidase A-mediated oxidative stress and impairment of NRF2-antioxidant defence response by polyphenol-rich fraction of Bergenia ligulata sensitizes prostate cancer cells in vitro and in vivo. Free Radic Biol Med. 2021;172:136–151. doi: 10.1016/j.freeradbiomed.2021.05.037. [DOI] [PubMed] [Google Scholar]
- 109.Turan I, Demir S, Yaman SO, Canbolat D, Mentese A, Aliyazicioglu Y. An Investigation of the Antiproliferative Effect of Rhododendron luteum Extract on Cervical Cancer (HeLa) Cells via Nrf2 Signaling Pathway. Nutr Cancer. 2022;74(5):1882–1893. doi: 10.1080/01635581.2021.1955287. [DOI] [PubMed] [Google Scholar]
- 110.Tan X, Jin P, Feng L, Song J, Sun E, Liu W, et al. Protective effect of luteolin on cigarette smoke extract-induced cellular toxicity and apoptosis in normal human bronchial epithelial cells via the Nrf2 pathway. Oncol Rep. 2014;31(4):1855–1862. doi: 10.3892/or.2014.3007. [DOI] [PubMed] [Google Scholar]
- 111.Ding S, Hou X, Yuan J, Tan X, Chen J, Yang N, et al. Wedelolactone protects human bronchial epithelial cell injury against cigarette smoke extract-induced oxidant stress and inflammation responses through Nrf2 pathway. Int Immunopharmacol. 2015;29(2):648–655. doi: 10.1016/j.intimp.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 112.Ohnuma T, Anzai E, Suzuki Y, Shimoda M, Saito S, Nishiyama T, et al. Selective antagonization of activated Nrf2 and inhibition of cancer cell proliferation by procyanidins from Cinnamomi Cortex extract. Arch Biochem Biophys. 2015;585:17–24. doi: 10.1016/j.abb.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 113.An K, Zhang Y, Liu Y, Yan S, Hou Z, Cao M, et al. Neferine induces apoptosis by modulating the ROSmediated JNK pathway in esophageal squamous cell carcinoma. Oncol Rep. 2020;44(3):1116–1126. doi: 10.3892/or.2020.7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Z, Yu K, Hu Y, Su F, Gao Z, Hu T, et al. Schisantherin A induces cell apoptosis through ROS/JNK signaling pathway in human gastric cancer cells. Biochem Pharmacol. 2020;173:113673. doi: 10.1016/j.bcp.2019.113673. [DOI] [PubMed] [Google Scholar]
- 115.Wu HC, Cheng MJ, Yen CH, Chen YA, Chen YS, Chen IS, et al. Chemical Constituents with GNMT-Promoter-Enhancing and NRF2-Reduction Activities from Taiwan Agarwood Excoecaria formosana. Molecules. 2020;25(7):1746. doi: 10.3390/molecules25071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhu X, Zhang Y, Wang Y, Zhang H, Wang X, Tang H, et al. Agrimoniin sensitizes pancreatic cancer to apoptosis through ROS-mediated energy metabolism dysfunction. Phytomedicine. 2022;96:153807. doi: 10.1016/j.phymed.2021.153807. [DOI] [PubMed] [Google Scholar]
- 117.Benlloch M, Obrador E, Valles SL, Rodriguez ML, Sirerol JA, Alcacer J, et al. Pterostilbene decreases the antioxidant defenses of aggressive cancer cells in vivo: a physiological glucocorticoids- and Nrf2-dependent mechanism. Antioxid Redox Signal. 2016;24(17):974–90. 10.1089/ars.2015.6437. [DOI] [PMC free article] [PubMed]
- 118.Gao AM, Ke ZP, Shi F, Sun GC, Chen H. Chrysin enhances sensitivity of BEL-7402/ADM cells to doxorubicin by suppressing PI3K/Akt/Nrf2 and ERK/Nrf2 pathway. Chem Biol Interact. 2013;206(1):100–108. doi: 10.1016/j.cbi.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 119.Chian S, Li YY, Wang XJ, Tang XW. Luteolin sensitizes two oxaliplatin-resistant colorectal cancer cell lines to chemotherapeutic drugs via inhibition of the Nrf2 pathway. Asian Pac J Cancer Prev. 2014;15(6):2911–2916. doi: 10.7314/apjcp.2014.15.6.2911. [DOI] [PubMed] [Google Scholar]
- 120.Hou X, Bai X, Gou X, Zeng H, Xia C, Zhuang W, et al. 3',4',5',5,7-pentamethoxyflavone sensitizes Cisplatin-resistant A549 cells to Cisplatin by inhibition of Nrf2 pathway. Mol Cells. 2015;38(5):396–401. doi: 10.14348/molcells.2015.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee YJ, Im JH, Lee DM, Park JS, Won SY, Cho MK, et al. Synergistic inhibition of mesothelioma cell growth by the combination of clofarabine and resveratrol involves Nrf2 downregulation. BMB Rep. 2012;45(11):647–652. doi: 10.5483/bmbrep.2012.45.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang P, Long F, Lin H, Wang S, Wang T. Dietary Phytochemicals Targeting Nrf2 to Enhance the Radiosensitivity of Cancer. Oxid Med Cell Longev. 2022;2022:7848811. doi: 10.1155/2022/7848811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Islam MT, Quispe C, Martorell M, Docea AO, Salehi B, Calina D, et al. Dietary supplements, vitamins and minerals as potential interventions against viruses: Perspectives for COVID-19. Int J Vitam Nutr Res. 2022;92(1):49–66. doi: 10.1024/0300-9831/a000694. [DOI] [PubMed] [Google Scholar]
- 124.Hadaya O, Landau SY, Muklada H, Deutch-Traubmann T, Glasser T, Bransi-Nicola R, et al. Direct effects of phenolic compounds on the mammary gland: In vivo and ex vivo evidence. Food Chem (Oxf). 2021;3:100034. 10.1016/j.fochms.2021.100034. [DOI] [PMC free article] [PubMed]
- 125.Semwal P, Painuli S, Abu-Izneid T, Rauf A, Sharma A, Daştan SD, et al. Diosgenin: An Updated Pharmacological Review and Therapeutic Perspectives. Oxid Med Cell Longev. 2022;2022:1035441. doi: 10.1155/2022/1035441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Butnariu M, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Singh L, Aborehab NM, et al. The pharmacological activities of Crocus sativus L.: a review based on the mechanisms and therapeutic opportunities of its phytoconstituents. Oxid Med Cell Longev. 2022;2022:8214821. 10.1155/2022/8214821. [DOI] [PMC free article] [PubMed]
- 127.Hernandez-Damian J, Anderica-Romero AC, Pedraza-Chaverri J. Paradoxical cellular effects and biological role of the multifaceted compound nordihydroguaiaretic acid. Arch Pharm (Weinheim) 2014;347(10):685–697. doi: 10.1002/ardp.201400159. [DOI] [PubMed] [Google Scholar]
- 128.Jain D, Chaudhary P, Varshney N, Bin Razzak KS, Verma D, Zahra TRK, et al. Tobacco Smoking and Liver Cancer Risk: Potential Avenues for Carcinogenesis. J Oncol. 2021;2021. 10.1155/2021/5905357. [DOI] [PMC free article] [PubMed]
- 129.Tang X, Wang H, Fan L, Wu X, Xin A, Ren H, et al. Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic Biol Med. 2011;50(11):1599–1609. doi: 10.1016/j.freeradbiomed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 130.Lim J, Lee SH, Cho S, Lee IS, Kang BY, Choi HJ. 4-methoxychalcone enhances cisplatin-induced oxidative stress and cytotoxicity by inhibiting the Nrf2/ARE-mediated defense mechanism in A549 lung cancer cells. Mol Cells. 2013;36(4):340–346. doi: 10.1007/s10059-013-0123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen F, Wang H, Zhu J, Zhao R, Xue P, Zhang Q, et al. Camptothecin suppresses NRF2-ARE activity and sensitises hepatocellular carcinoma cells to anticancer drugs. Br J Cancer. 2017;117(10):1495–1506. doi: 10.1038/bjc.2017.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Felix-Silva J, Giordani RB, da Silva AA, Jr, Zucolotto SM, Fernandes-Pedrosa Mde F. Jatropha gossypiifolia L. (Euphorbiaceae): a review of traditional uses, phytochemistry, pharmacology, and toxicology of this medicinal plant. Evid Based Complement Alternat Med. 2014;2014:369204. 10.1155/2014/369204. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.