Abstract
The enteric pathogen Salmonella enterica serotype Typhimurium induces apoptosis in infected macrophages. This process is rapid, specific, and depends on the type III protein secretion system encoded within Salmonella pathogenicity island 1 (SPI1). Here, we demonstrate that serotype Typhimurium can activate programmed macrophage cell death independently of SPI1. SPI1 independent induction of apoptosis in infected macrophages is observed as early as 12 to 13 h postinfection, even in the absence of intracellular bacterial replication. Delayed activation of programmed macrophage cell death is not observed with serotype Typhimurium strains mutated in ompR or SPI2. Even though SPI2 mutants have a defect in intracellular proliferation, our results indicate that long-term intracellular survival and growth are not required for delayed macrophage killing per se, since Salmonella mutants that are severely defective in intracellular growth still induce delayed apoptosis. Inactivation of genes required for either rapid or delayed induction of apoptosis results in a conditional noncytotoxic phenotype, whereas simultaneous inactivation of genes required for both rapid and delayed induction of apoptosis renders serotype Typhimurium noncytotoxic under all conditions tested. Our hypothesis is that differential activation of programmed macrophage cell death by serotype Typhimurium occurs under discrete physiological conditions at distinct locations within an infected host.
Salmonella enterica serotype Typhimurium is a facultative intracellular pathogen that causes a typhoid like disease in mice. Following oral infection, bacteria actively invade the intestinal mucosa and enter the bloodstream via the gut-associated lymphoid tissue (GALT). Subsequent residence within professional phagocytes of the liver and spleen is required for a persistent infection, which ultimately leads to the death of the mouse. Growth and survival of Salmonella within macrophages is supported by numerous studies, including the direct observation of Salmonella within hepatic phagocytes (45), comparative infection studies in genetic strains of mice that produce macrophages with varying resistance to Salmonella (38, 40), and the persistence of infection in mice treated with gentamicin, an antibiotic that primarily kills extracellular bacteria (10, 18). Finally, genetic studies indicate that Salmonella mutants that are attenuated for intramacrophage survival are also attenuated for systemic infection in mice (20). While all of these studies demonstrate that Salmonella survives and replicates within macrophages, several groups have recently shown that Salmonella is also able to kill these host cells (3, 13, 35, 39).
Contradictory results have been reported for Salmonella genes required for the induction of apoptosis as well as the timing at which it takes place. One study showed that serotype Typhimurium kills macrophages as late as 18 h postinfection (35). This process depends on the two-component regulatory system ompR-envZ, as ompR was the only gene identified in a stringent selection to find Salmonella mutants that are unable to kill macrophages. InvA is an essential structural component of the Salmonella pathogenicity island 1 (SPI1)-encoded type III export apparatus, whereas SipB is a SPI1-secreted effector molecule (22, 30). Null mutations in either invA or sipB, two genes within SPI1, had no effect on the ability of serotype Typhimurium to kill infected macrophages in this study (35). However, other studies appear to contradict these observations and demonstrate that within a few hours upon contact, serotype Typhimurium induces apoptosis in infected macrophages in an invA (and thus SPI1)-dependent process (13, 36, 39). SipB is both necessary and sufficient for the rapid activation of this apoptotic pathway (29).
Here, we resolve this apparent controversy by demonstrating that serotype Typhimurium kills macrophages via two independent processes. It is demonstrated that SPI1 gene expression accounts for rapid induction of apoptosis, whereas SPI1-independent, delayed induction of apoptosis is abrogated in strains mutated in ompR and SPI2. These results have important implications for understanding Salmonella pathogenesis, which are discussed.
MATERIALS AND METHODS
Bacterial strains, bacteriophages, and recombinant DNA techniques.
Bacteria were grown overnight in Luria-Bertani (LB) broth at 37°C. Antibiotics, when required, were used at the following concentrations: nalidixic acid (Nal), 50 μg/ml; chloramphenicol (Cam), 30 μg/ml; kanamycin (Kan), 60 μg/ml; and ampicillin (Amp), 100 μg/ml. Recombinant DNA techniques and Southern hybridizations were performed using standard protocols (4, 37). Analytical-grade chemicals were purchased from Sigma (St. Louis, Mo.) or Roche Biochemicals/Boehringer Mannheim (Indianapolis, Ind.).
Mutations in the ompR, invA, spiB, and prc genes have been described previously (20, 23, 35, 49) and were used to construct a set of isogenic serotype Typhimurium mutants (Table 1). Bacteriophage KB1int was used to transduce the ompR::MudJ allele of SWL350 (35) into SR-11 χ3041 (wild type [wt]), yielding strain AWM405 (ompR). Bacteriophage P22HTint was used to transduce the invA::TnphoA allele of AJB75 (7) into AWM501 (sipB, see below) and AWM527 (ssrB, see below), yielding AWM544 (invA sipB) and AWM545 (ssrB invA), respectively. Bacteriophage P22HTint was used to transduce the sipB::mTn5 allele of STN119 (49) into SR-11 χ3041 (wt), yielding strain AWM568 (sipB). Bacteriophage P22HTint was also used to transduce the prc::Tn10 allele of MS4290 (20) into SR-11 χ3041 (wt), yielding strain AWM664 (prc).
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| E. coli | ||
| DH5α | endA1 hsdR17 supE44 thi-1 recA1 gyrA relA1 Δ(lacZYA-argF)U169 deoR [φ80 dlac Δ(lacZ)M15] | Laboratory collection |
| S17λpir | pro thi recA hsdR::chromosomal RP4-2 (Tn1::ISR1 tet::Mu Km::Tn7); λpir | 31 |
| S. enterica serotype Typhimurium | ||
| AJB3 | SR-11 χ4252 (Nalr) | 51 |
| AJB75 | ATCC 14028 invA::TnphoA (Kanr) | 7 |
| ATCC 14028 | wt | ATCC |
| BA715 | ATCC 14028 rpsL (Strr) | 1 |
| MJW129 | ATCC 14028 ssrB::cat (Camr) | This study |
| MS4290 | ATCC 14028 prc::Tn10 (Tetr) | 20 |
| SR-11 χ3041 | wt | R. Curtiss III |
| STN119 | IR715 spiB::mTn5 (Kanr) | 49 |
| SWL350 | SR-11 ompR::MudJ (Kanr) | 35 |
| SWL2020 | SR-11 invA::cat | This study |
| SWL2025 | SR-11 sipB::cat | This study |
| AWM405 | SR-11 χ3041 ompR::MudJ | This study |
| AWM472 | SR-11 χ3041 invA::cat | This study |
| AWM501 | SR-11 χ3041 sipB::cat | This study |
| AWM498 | SR-11 χ3041 ompR::MudJ invA::cat | This study |
| AWM499 | SR-11 χ3041 ompR::MudJ sipB::cat | This study |
| AWM527 | SR-11 χ3041 ssrB::cat | This study |
| AWM543 | SR-11 χ3041 ompR::MudJ ssrB::cat | This study |
| AWM544 | SR-11 χ3041 sipB::cat invA::TnphoA | This study |
| AWM545 | SR-11 χ3041 ssrB::cat invA::TnphoA | This study |
| AWM568 | SR-11 χ3041 spiB::mTn5 | This study |
| AWM664 | SR-11 χ3041 prc::Tn10 | This study |
Allelic exchange was performed to disrupt the serotype Typhimurium invA gene. An internal fragment of the invA gene was amplified from serotype Typhimurium ATCC14028 (wt) using primers 5′-GCATGAATTCGCAGAACAGCGTCG-3′ and 5′-GTTGTCTAGATCTTTTCCTTAATTAAGCC-3′, which generated a PCR fragment with unique 5′ EcoRI and 3′ XbaI sites, respectively. This PCR product was cloned into the EcoRV site of pBluescript II SK(+) and sequenced. Subsequently, the invA allele was inactivated by insertion of a chloramphenicol resistance gene (a 1.2-kb SmaI fragment from pCMXX [7]) into a unique internal SnaBI site and cloned into suicide plasmid pKAS32 (48). The resulting plasmid was electroporated into Escherichia coli SM10λpir and conjugated to serotype Typhimurium ATCC 14028 derivative BA715 (rpsL) (1). A double crossover at the invA allele was obtained via homologous recombination. A chloramphenicol- and streptomycin-resistant exconjugant was selected and named SWL2020 (invA). Bacteriophage KB1int was used to transduce the invA::cat mutation into SR-11 χ3041 (wt), yielding strain AWM472 (invA).
Allelic exchange was performed to disrupt the serotype Typhimurium sipB gene. A fragment of the sipB gene was amplified from serotype Typhimurium SR-11 (wt) using primers 5′-GAAGGTACCGAAGATGAGTCTCTGCGG-3′ and 5′-GAGCTCTTCTCAACAGAATGAT-3′, which generated a PCR fragment with unique 5′ KpnI and 3′ SacI sites, respectively. The resulting PCR product was blunt-end ligated into the EcoRV site of pBluescript SK(+) and sequenced to verify its accuracy. Subsequently, the sipB allele was inactivated by insertion of a chloramphenicol resistance gene (a 1.2-kb SmaI fragment from pCMXX [7]) into a unique SmaI site. This plasmid was restricted with KpnI and SacI, and the insertionally mutagenized sipB::cat allele was cloned into suicide plasmid pJP5603 (42). The resulting plasmid was electroporated into E. coli S17λpir (31) and conjugated to AJB3, a nalidixic acid-resistant derivative of serotype Typhimurium SR-11 (51). A chloramphenicol- and nalidixic acid-resistant exconjugant was selected and named SWL2025 (sipB). Bacteriophage KB1int was used to transduce the sipB::cat mutant allele into SR-11 χ3041 (wt) and AWM405 (ompR), yielding strains AWM501 (sipB) and AWM499 (ompR sipB), respectively.
Allelic exchange was performed to disrupt the serotype Typhimurium ssrB gene. An 853-bp fragment of the ssrB allele was amplified from serotype Typhimurium ATCC14028 (wt) using primers 5′-CTTAATTTTCGCGAGGGCAGC-3′ and 5′-TAGAATACGACATGGTAAAGCCCG-3′. This PCR product was cloned into pCR-Blunt (Invitrogen, Carlsbad, Calif.). The ssrB allele was inactivated upon insertion of a chloramphenicol resistance gene (a 1.2-kb SmaI fragment from pCMXX [7]) into a unique SspI site. This plasmid was digested with EcoRI, and the disrupted ssrB allele was ligated into suicide vector pKAS32 (48). The resulting plasmid (pMJW99) was transformed into E. coli SM10λpir and conjugated to serotype Typhimurium ATCC 14028 derivative BA715 (rpsL) (1). A double crossover at the ssrB allele was obtained via homologous recombination. A chloramphenicol- and streptomycin-resistant exconjugant was selected and named MJW129 (ssrB). Bacteriophage P22HTint was used to transduce the ssrB::cat mutant allele into SR-11 χ3041 (wt) and AWM405 (ompR), yielding strains AWM527 (ssrB) and AWM543 (ompR ssrB), respectively.
Macrophage assays.
The murine derived macrophage cell lines J774 (American Type Culture Collection [ATCC], Manassas, Va.) and RAW264.7 (ATCC) were cultured (37°C, 5% CO2) in Dulbecco modified Eagle medium (DMEM; Gibco-BRL, Rockville, Md.), supplemented with 10% fetal bovine serum (FBS; Gibco-BRL), glutamine (Gibco-BRL), sodium pyruvate (Gibco-BRL), and nonessential amino acids (Gibco-BRL). Bone marrow-derived macrophages were isolated from C57BL/6 mice (Jackson Laboratories, Bar Harbor, Maine) and cultured for 6 days (37°C, 5% CO2) in DMEM supplemented with 10% FBS, 20% L929 supernatant (a generous gift from H. G. A. Bouwer, Immunology Research, VAMC, Portland, Oreg.), and glutamine and sodium pyruvate (Gibco-BRL).
Macrophage survival assays (gentamicin protection assays) were performed as described by Fields et al. (20). In brief, 105 J774 macrophages were infected with stationary-phase cultures (below) at a multiplicity of infection (MOI) of ≤1. At 18 h postinfection, monolayers were washed three times with phosphate-buffered saline (PBS) and lysed with Triton X-100 (Sigma). Bacterial viability was determined by plating for CFU at various times postinfection. Similar results were obtained using RAW264.7 macrophages (data not shown).
The percentage of macrophage cytotoxicity was determined by measuring the release of host cytoplasmic lactate dehydrogenase (LDH). J774 and RAW264.7 macrophages were infected with bacterial cultures grown to either late-log phase or stationary phase (below) at an input MOI of ∼60. At 1 h postinfection, infected monolayers were washed three times with PBS and lysed with Triton X-100 (Sigma), after which bacterial uptake was determined by plating for viable intracellular CFU. Differences between strains were observed and taken into account by normalizing to the number of internalized bacteria (approximately 1% of input bacteria). At 6 and 18 h postinfection, the release of LDH was quantified colorimetrically using the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, Wis.). The absorbance (A490) was determined on a microplate reader (Dynatech Laboratories, Inc., Chantilly, Va.), after which the percentage of cytotoxicity was calculated using the following formula: 100 × [(experimental release − spontaneous release)/(maximum release − spontaneous release)]. The spontaneous release is the amount of LDH released from the cytoplasm of uninfected macrophages, whereas the maximum release is the amount of LDH present in whole-cell lysates from uninfected macrophages.
In addition to measuring the release of LDH, quantitative macrophage cytotoxicity assays were performed as described by Lindgren et al. (35; data not shown). In brief, to determine the MOI at which 50% of the infected macrophages are killed (MOICD50), 105 J774 macrophages were infected with twofold serial dilutions of bacterial cultures (31 ≤ MOI ≤ 1,000, the limits of detection), as verified by plating for CFU. At 6 and 18 h postinfection, the remaining viable macrophages were fixed in a 10% formalin solution (10 to 15 min) and stained in a 0.13% crystal violet solution (>2 h). The absorbance (A595) was determined on a microplate reader (Dynatech Laboratories); the MOI for the well that gave 50% of the absorbance recorded for uninfected wells was considered the MOICD50 (i.e., 50% of the cytotoxic dose). Similar results were obtained using RAW264.7 macrophages (data not shown).
The Cell Death Detection ELISAPLUS Assay (Roche Diagnostics Corp.) was used to determine whether serotype Typhimurium-infected macrophages were undergoing apoptosis. This assay has been used successfully to study Pseudomonas aeruginosa-induced apoptosis in eukaryotic cells (26). Macrophages were infected with bacterial cultures grown to either late-log phase (data not shown) or stationary phase (below) at an infection rate of 1.5 bacteria per macrophage. The amount of cytoplasmically located histones bound to fragmented DNA was quantified colorimetrically at 18 h postinfection, after which the absorbance (A410nm) was determined on a microtiter plate reader. An enrichment factor indicative of apoptosis was calculated using the following formula: (A410[experimental])/(A410[uninfected]).
Bacterial cultures were grown under various conditions. To obtain stationary-phase cultures, bacteria were grown aerobically in LB broth (3 ml) for 15 h at 37°C. To obtain late-log phase cultures, bacteria were grown overnight (aerobically, 15 h at 37°C) in LB broth (3 ml), subcultured 1:20 in LB broth (3 ml), and grown to late-log phase (3 h) under the same culture conditions. Using a MudJ transcriptional fusion to sipB, optimal transcription of SPI1 genes in late-log phase cultures was confirmed since under these culture conditions high levels of β-galactosidase were produced (data not shown).
RESULTS
Serovar Typhimurium kills macrophages independently of SPI1.
Conflicting reports on macrophage killing (13, 35, 39) prompted us to investigate the effect of bacterial growth phase on the ability of serotype Typhimurium to kill macrophages. Throughout this study, two complementary methods were used to determine Salmonella-induced cell death in both J774 and RAW264.7 macrophages. In addition to measuring the release of cytoplasmic LDH, macrophage killing was calculated using a quantified macrophage cytotoxicity assay (data not shown) (35). Strikingly similar results were obtained with these two independent assays. Salmonella-induced macrophage cell death was determined by measuring the release of LDH at infection rates of about 0.7 and 1.5 bacteria per macrophage. Other MOIs were also tested, with identical results (data not shown).
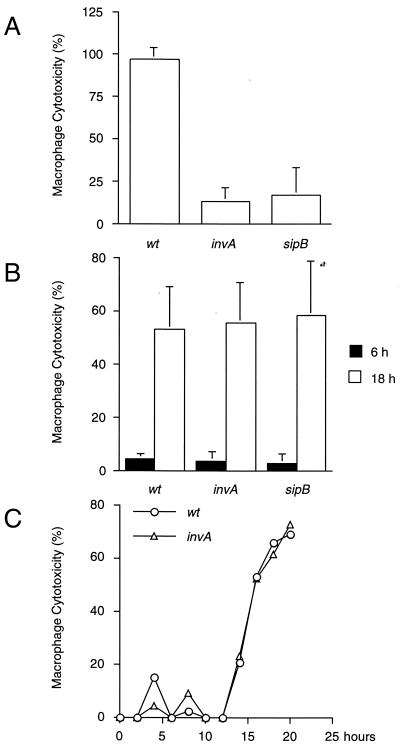
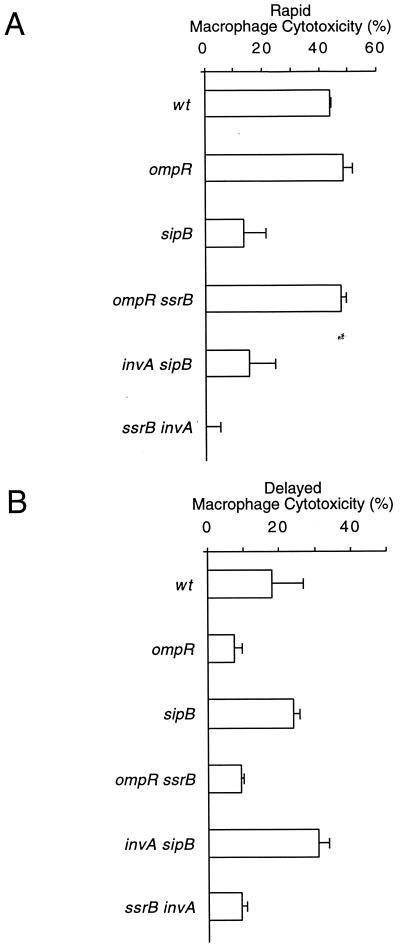
Under SPI1-inducing conditions (see Materials and Methods) (13), rapid, SPI1-dependent macrophage killing was observed (Fig. 1A). In contrast, bacterial cultures grown to stationary phase, while unable to rapidly kill infected macrophages, induced a delayed cytotoxic effect (Fig. 1B). Delayed induction of macrophage cell death required neither invA nor sipB (Fig. 1B) and was observed as early as 12 to 13 h postinfection (Fig. 1C). These results suggest that serotype Typhimurium induces delayed macrophage cell death independently of SPI1.
FIG. 1.
Serovar Typhimurium kills macrophages independently of SPI1. J774 macrophages were infected with late-log-phase (A) or stationary-phase (B) cultures of wt serotype Typhimurium or strains carrying null mutations in invA or sipB. Bacterial growth was monitored by measuring optical density at 600 nm (see Materials and Methods; also, data not shown). (A and B) Macrophage cell death was quantitated at 6 h (A) and 18 h (B) postinfection by measuring the release of LDH. (C) Using stationary-phase cultures of either wild-type serotype Typhimurium or an invA-deficient strain, macrophage cytotoxicity was monitored for 20 h and quantitated at 2-h intervals by measuring the release of LDH. Data from the graphs in panels A and B are arithmetic means of at least three independent experiments. Error bars indicate the standard deviations of the mean. The data from graph C are representative of two independent experiments.
SPI2 and ompR are required for delayed macrophage killing.
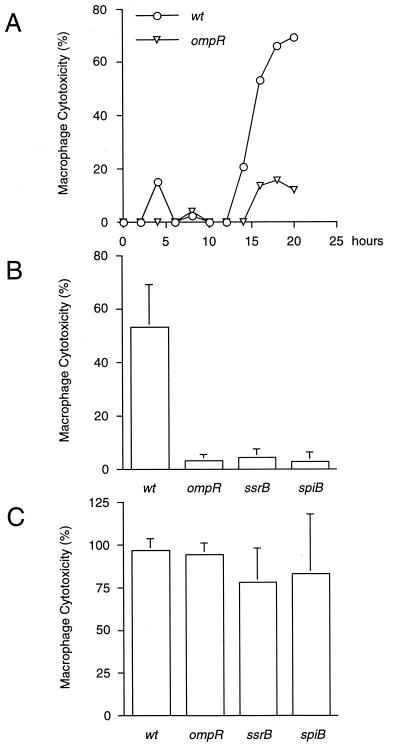
Delayed cytotoxicity was dependent on a functional ompR locus, since ompR mutant bacteria were unable to kill infected macrophages (Fig. 2A). Recent evidence suggests that OmpR activates transcription of the SPI2 encoded regulon ssrAB (32). This operon is essential for the transcription of SPI2 genes (14), which are highly induced inside macrophages (16, 50). To test whether, in addition to ompR, SPI2 is required for delayed induction of macrophage cell death, serotype Typhimurium strains mutated in ssrB and spiB were tested. These genes encode a transcriptional activator and a structural component of the SPI2 encoded type III protein export apparatus, respectively (41). As shown in Fig. 2B, serotype Typhimurium strains mutated in ompR, ssrB, or spiB were unable to kill infected macrophages when grown to stationary phase prior to infection. However, these strains were fully cytotoxic under SPI1 inducing conditions (Fig. 2C), indicating that ompR and SPI2 are not required for rapid induction of macrophage cell death. Cumulatively, these results suggest that delayed, SPI1-independent cytotoxic effects are masked under conditions that turn on SPI1 gene expression.
FIG. 2.
SPI2 and ompR are required for delayed macrophage killing. (A) J774 macrophages were infected with stationary-phase cultures of either wt serotype Typhimurium or an ompR-deficient strain, after which macrophage cytotoxicity was monitored for 20 h and quantitated at 2-h intervals by measuring the release of LDH. (B and C) In addition, J774 macrophages were infected with stationary-phase (B) or late-log-phase (C) cultures of wild-type serotype Typhimurium or strains carrying null mutations in either ompR, ssrB, or spiB. Macrophage cell death was quantitated at 18 h (B) and 6 h (C) postinfection by measuring the release of LDH. Data from the graph in panel A are representative of two independent experiments. The data from the graphs in panels B and C are arithmetic means of at least three independent experiments. The error bars indicate the standard deviations of the mean.
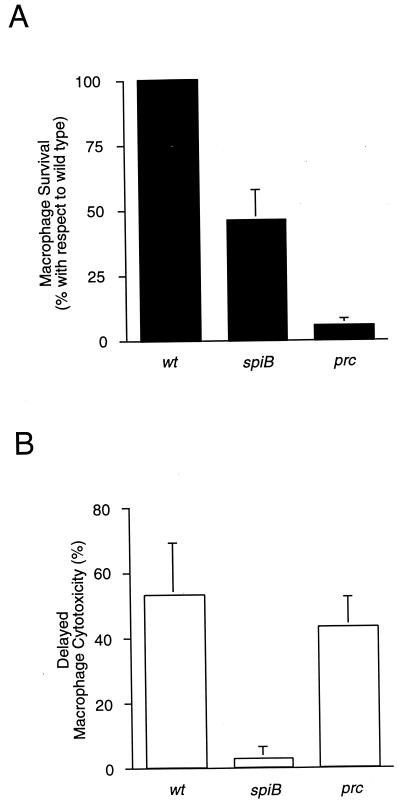
In agreement with the literature, we observed a defect (2- to 10-fold) in intracellular proliferation for SPI2 mutant strains at 15 h postinfection (14, 27, 28, 41, 46). However, long-term intracellular survival and proliferation is not required for delayed macrophage killing per se, since a prc mutant, encoding a periplasmic protease (6, 20) required for intracellular survival and growth (Fig. 3A) (11, 21), kills infected macrophages as efficiently as the wild type (Fig. 3B). Thus, despite a profound macrophage survival defect, the prc mutant was fully cytotoxic. In fact, the prc mutant strain was representative of a large panel of serotype Typhimurium mutants that are defective in intramacrophage survival and yet were still cytotoxic (data not shown). Collectively, these observations suggest that long-term intramacrophage survival and growth are not required for delayed, ompR- and SPI2-dependent macrophage killing. However, an indirect effect can not be ruled out until we have identified the SPI2 secreted effector(s) involved.
FIG. 3.
Long-term intracellular survival and growth is not required for delayed macrophage killing. (A) J774 macrophages were infected with stationary-phase cultures (conditions shown to turn off SPI1-dependent rapid induction of macrophage cell death) of either wild-type serotype Typhimurium, a spiB mutant strain, or a macrophage-sensitive prc-deficient strain, after which macrophage survival was determined at 15 and 18 h postinfection (three times each) by measuring the viable intracellular CFU. (B) Macrophage cytotoxicity was quantitated at these times by measuring the release of cytoplasmic LDH. The data are arithmetic means of at least three independent experiments from 15-h time points. The error bars indicate the standard deviations of the mean.
Rapid and delayed macrophage killing processes are independent.
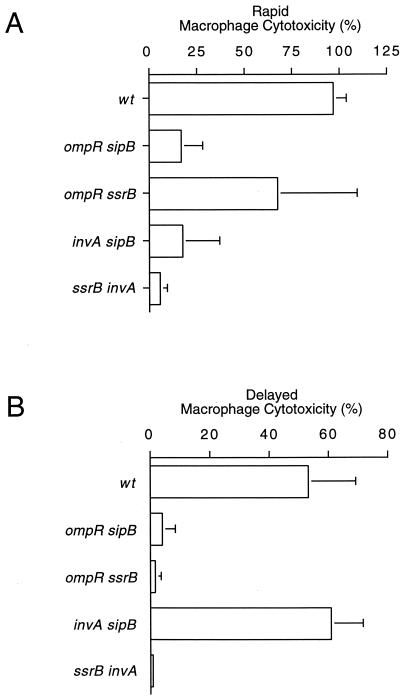
To determine whether rapid and delayed macrophage killing were independent of one another, doubly deficient mutant strains were constructed. Double mutants carried null mutations in genes required for either rapid macrophage killing only (invA sipB), delayed macrophage killing only (ompR ssrB), or genes required for both rapid and delayed macrophage killing (ompR sipB and ssrB invA). Under SPI1 inducing conditions, ompR sipB, invA sipB, and ssrB invA doubly deficient mutants were noncytotoxic, whereas an ompR ssrB double mutant was as cytotoxic as the wt (Fig. 4A). Under conditions that favored delayed macrophage killing, an invA sipB doubly deficient strain was fully cytotoxic, whereas ompR sipB, ompR ssrB, and ssrB invA double mutants were unable to kill infected macrophages (Fig. 4B). To demonstrate that these observations were not specific to J774 macrophages, these results were confirmed using RAW264.7 macrophages (data not shown) and bone marrow-derived macrophages (Fig. 5).
FIG. 4.
Rapid and delayed macrophage killing processes are independent. J774 macrophages were infected with wt serotype Typhimurium or ompR sipB, ompR ssrB, invA sipB, or ssrB invA double mutants. (A and B) Bacterial cultures were grown to either late-log phase (A) or stationary phase (B) prior to infection. Macrophage cell death was quantitated at 6 h (A) and 18 h (B) postinfection by measuring the release of LDH. The data from each graph are arithmetic means of at least three independent experiments. The error bars indicate the standard deviations of the mean.
FIG. 5.
S. typhimurium induces rapid and delayed macrophage cell death in bone marrow-derived macrophages. To demonstrate that serotype Typhimurium induced rapid and that delayed macrophage cell death was not specific to J774 macrophages, these results were repeated in RAW264.7 macrophages (data not shown). In addition, bone marrow-derived macrophages were established from C57BL/6 mice and infected with mutant strains defective in inducing either rapid macrophage cell death (sipB, invA sipB) or delayed macrophage cell death (ompR, ompR ssrB) or with a mutant strain defective in both rapid and delayed macrophage killing (ssrB invA). (A and B) Bacterial strains were grown to either late-log phase (A) or stationary phase (B) prior to infection. Macrophage cell death was quantitated at 6 h (A) and 30 h (B) postinfection by measuring the release of LDH. The data from each graph are arithmetic means of three independent experiments. The error bars indicate the standard deviations of the mean.
Collectively, these results indicate that bacterial strains mutated in genes required for either rapid or delayed induction of macrophage cell death are noncytotoxic only under specific pregrowth conditions. However, bacterial strains mutated in loci that affect both rapid and delayed macrophage killing are noncytotoxic under all conditions tested. These observations are evidence that rapid and delayed macrophage killing processes act independently of one another.
ompR and SPI2, but not SPI1, are required for delayed induction of apoptosis in infected macrophages.
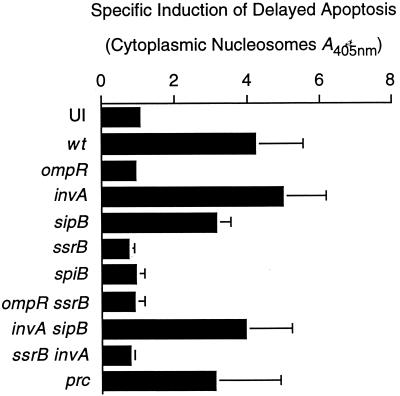
Next, we investigated the nature of serotype Typhimurium-induced rapid and delayed macrophage cell death. Thus far, a nonspecific method, measuring the release of host cytoplasmic LDH, was used to calculate macrophage cytotoxicity. To determine whether macrophages were undergoing apoptosis upon infection with serotype Typhimurium, the amount of cytoplasmically located histones bound to fragmented DNA was quantified. Under SPI1 inducing conditions, serotype Typhimurium rapidly induced apoptosis via an SPI1-dependent process (data not shown). Under conditions that favored delayed macrophage cytotoxicity, killing was independent of SPI1 (Fig. 6). Delayed induction of apoptosis was abrogated in strains defective in either ompR or SPI2 (Fig. 6). These results indicate that serotype Typhimurium induces either rapid or delayed apoptosis in infected macrophages. Rapid activation of programmed cell death depends on SPI1, whereas delayed induction of apoptosis is SPI1 independent. Furthermore, our observations suggest that ompR and SPI2 are required for delayed activation of programmed macrophage cell death.
FIG. 6.
ompR and SPI2, but not SPI1, are required for delayed induction of apoptosis in infected macrophages. J774 macrophages were infected with wt serotype Typhimurium or with mutant strains defective in either rapid killing (sipB, invA, invA sipB) or delayed killing (ompR, ssrB, spiB, ompR ssrB) with a mutant strain defective in both rapid and delayed macrophage killing (ssrB invA) or with a strain defective in macrophage survival (prc). The ability of these strains to induce apoptosis was determined at 18 h postinfection by measuring the amount of cytoplasmically located histones bound to fragmented DNA. The data from this graph are the arithmetic means of three independent experiments. The error bars indicate the standard deviations of the mean.
DISCUSSION
In this study, we demonstrate that macrophages undergo either rapid or delayed apoptosis upon infection with serotype Typhimurium. Delayed activation of programmed cell death is masked when SPI1 genes are expressed. Mutations that affect either rapid or delayed induction of apoptosis result in noncytotoxic phenotypes only under specific growth conditions. However, mutants defective in both rapid and delayed macrophage killing are unable to induce apoptosis under any condition tested, even at a high MOI (data not shown). Rapid activation of programmed macrophage cell death depends on SipB and the SPI1 encoded type III protein export machinery, whereas delayed induction of apoptosis is SPI1 independent. Our results indicate that ompR and a functional SPI2 encoded type III protein secretion system are required for delayed induction of apoptosis. However, a nonspecific effect cannot be excluded until we have identified an SPI2 effector(s) that is both necessary and sufficient for the activation of delayed programmed macrophage cell death.
In agreement with the literature, we observed a defect (2- to 10-fold) in intracellular proliferation for SPI2 mutants at 15 and 18 h postinfection (14, 27, 28, 41, 46). However, prc, htrA, and 11 other macrophage-sensitive mutants tested are fully cytotoxic and yet are more severely defective in their ability to survive and grow inside phagocytic cells (Fig. 3A) (11, 20). In fact, MS4290 (prc) was the most sensitive mutant isolated in an extensive search for Salmonella mutants that cannot survive inside macrophages (11, 20). Despite this substantial defect, prc mutant bacteria, as well as a large panel of other macrophage-sensitive serotype Typhimurium mutants, induced both rapid (data not shown) and delayed apoptosis in infected macrophages (Fig. 3B and Fig. 6). These results strongly support an additional role for SPI2 in delayed induction of apoptosis in infected macrophages.
Our observations indicate that rapid and delayed activation of programmed macrophage cell death are independent of one another, since mutations in SPI1 do not affect delayed induction of apoptosis and mutations in SPI2 do not affect rapid induction of apoptosis. Recent studies support this view by demonstrating that these two specialized protein secretion systems are controlled by distinct regulatory circuits. For example, substrates for the SPI1 encoded type III protein export apparatus are secreted under mildly alkaline conditions (15), whereas substrates for the type III protein export system encoded within SPI2 are secreted at pH 5.0 (9). Furthermore, numerous studies suggest that, once inside a phagocytic host, serotype Typhimurium represses SPI1 gene expression and turns on genes that are important for long-term residence, growth, and survival inside these host cells (2, 5, 8, 14, 16, 19, 24, 25, 33, 34, 43, 44, 50). It is therefore unlikely that substrates for SPI1 and SPI2 encoded type III protein export systems are secreted simultaneously.
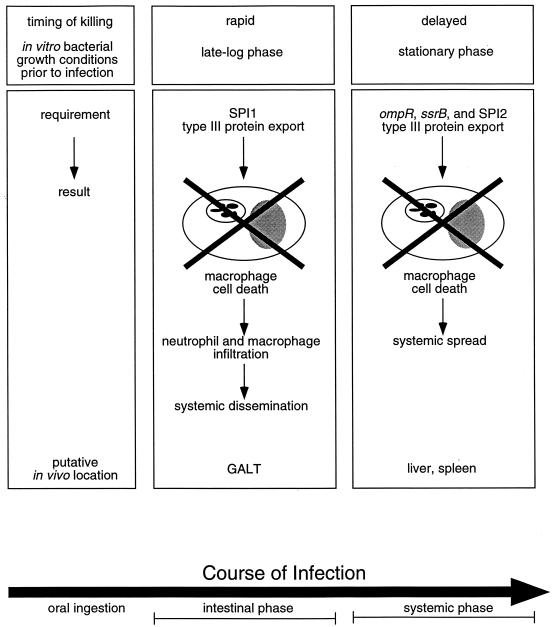
Our hypothesis is that serotype Typhimurium induces rapid and delayed apoptosis in infected macrophages under discrete physiological conditions at distinct times and locations during the natural course of infection in the host (Fig. 7). Accumulating evidence suggests that the SPI1 encoded type III protein secretion system is important primarily during the intestinal phase of infection, since SPI1 mutants are significantly attenuated only when administered to mice orally (reference 22 and references therein and reference 23). In contrast, ompR and SPI2 are absolutely required during the systemic phase of infection (12, 16, 17, 41, 47, 50). In fact, SPI2 has been implicated in growth inside phagocytic cells at systemic sites of infection (12, 16, 17, 41, 47, 50). A possible consequence of the rapid, SPI1-dependent induction of apoptosis in macrophages of the GALT is that additional phagocytic cells are attracted to the site of inflammation. Our model suggests that Salmonella represses the SPI1-dependent killing mechanism upon internalization by macrophages, allowing continued proliferation and systemic spread prior to ompR- and SPI2-dependent induction of delayed apoptosis at systemic sites of infection. Because apoptotic cells are ingested by neighboring phagocytes, we propose that delayed induction of apoptosis in infected macrophages may allow Salmonella to spread intercellularly within apoptotic bodies. This model is supported by a recent study in which it was demonstrated that serotype Typhimurium is transported from the intestine, via the bloodstream, to the liver and spleen by CD18-expressing monocytes in an SPI1-independent process (52), as well as by studies in which it was demonstrated that Salmonella virulence was unaffected by treatment with antibiotics that kill extracellular bacteria (10, 18).
FIG. 7.
Model of serotype Typhimurium-induced apoptosis in vivo. We propose that serotype Typhimurium induces rapid and delayed apoptosis in infected macrophages under discrete physiological conditions at distinct times and locations during the natural course of infection. Because the SPI1 encoded type III protein secretion system is important primarily during the intestinal phase of infection (23), we propose that rapid, SPI1-dependent induction of apoptosis in macrophages of the GALT results in increased inflammation and recruitment of phagocytes that may be required for systemic dissemination. Our model predicts that Salmonella represses the rapid macrophage killing mechanism upon internalization, permitting extensive intracellular proliferation and systemic spread prior to delayed, ompR- and SPI2-dependent induction of apoptosis at systemic sites of infection. In support of this view, ompR and SPI2, unlike SPI1, are required during the systemic phase of infection (12, 16, 17, 41, 47, 50). This model predicts that Salmonella induces delayed apoptosis in infected macrophages to spread intercellularly within apoptotic bodies.
ACKNOWLEDGMENTS
We thank Renée Tsolis for providing strain STN119 prior to publication and Joanne Engel for helpful discussions. L929 supernatant was generously provided by Archie Bouwer. We thank members of the Heffron and So laboratories for critical comments on the manuscript.
This work was supported by Public Health Service grant AI37201 to F.H. from the National Institutes of Health.
REFERENCES
- 1.Ahmer B M, van Reeuwijk J, Watson P R, Wallis T S, Heffron F. Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol Microbiol. 1999;31:971–982. doi: 10.1046/j.1365-2958.1999.01244.x. [DOI] [PubMed] [Google Scholar]
- 2.Alpuche-Aranda C M, Swanson J A, Loomis W P, Miller S I. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci USA. 1992;89:10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai T, Hiromatsu K, Nishimura H, Kimura Y, Kobayashi N, Ishida H, Nimura Y, Yoshikai Y. Endogenous interleukin 10 prevents apoptosis in macrophages during Salmonella infection. Biochem Biophys Res Commun. 1995;123:600–607. doi: 10.1006/bbrc.1995.2174. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 5.Bajaj V, Lucas R L, Hwang C, Lee C A. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental factors is mediated by control of hilA expression. Mol Microbiol. 1996;22:703–714. doi: 10.1046/j.1365-2958.1996.d01-1718.x. [DOI] [PubMed] [Google Scholar]
- 6.Bäumler A J, Kusters J G, Stojiljkovic I, Heffron F. Salmonella typhimurium loci involved in survival within macrophages. Infect Immun. 1994;62:1623–1630. doi: 10.1128/iai.62.5.1623-1630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bäumler A J, Tsolis R M, Valentine P J, Ficht T A, Heffron F. Synergistic effect of mutations in invA and lpfC on the ability of Salmonella typhimurium to cause murine typhoid. Infect Immun. 1997;65:2254–2259. doi: 10.1128/iai.65.6.2254-2259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behlau I, Miller S J. A PhoP repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beuzon C R, Banks G, Deiwick J, Hensel M, Holden D W. pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol Microbiol. 1999;33:806–816. doi: 10.1046/j.1365-2958.1999.01527.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonina L, Costa G B, Mastroeni P. Comparative effect of gentamycin an pefloxacin treatment on the late stages of mouse typhoid. New Microbiol. 1998;21:9–14. [PubMed] [Google Scholar]
- 11.Buchmeier N A, Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989;57:1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatfield S N, Dorman C J, Hayward C, Dougan G. Role of ompR-dependent genes in Salmonella typhimurium virulence: mutants deficient in both OmpC and OmpF are attenuated in vivo. Infect Immun. 1991;59:449–452. doi: 10.1128/iai.59.1.449-452.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L M, Kaniga K, Galan J E. Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 14.Cirillo D M, Valdivia R H, Monack D M, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 15.Daefler S. Type III secretion by Salmonella typhimurium does not require contact with a eukaryotic host. Mol Microbiol. 1999;31:45–51. doi: 10.1046/j.1365-2958.1999.01141.x. [DOI] [PubMed] [Google Scholar]
- 16.Deiwick J, Nikolaus T, Erdogan S, Hensel M. Environmental regulation of Salmonella pathogenicity island 2 gene expression. Mol Microbiol. 1999;31:1759–1773. doi: 10.1046/j.1365-2958.1999.01312.x. [DOI] [PubMed] [Google Scholar]
- 17.Dorman C J, Chatfield S, Higgins C F, Hayward C, Dougan G. Characterization of porin and ompR mutants of a virulent strain of Salmonella typhimurium: ompR mutants are attenuated in vivo. Infect Immun. 1989;57:2136–2140. doi: 10.1128/iai.57.7.2136-2140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunlap N E, Benjamin W H, Jr, Berry A K, Eldridge J H, Briles D E. A ‘safe-site’ for Salmonella typhimurium is within splenic polymorphonuclear cells. Microb Pathog. 1991;13:181–190. doi: 10.1016/0882-4010(92)90019-k. [DOI] [PubMed] [Google Scholar]
- 19.Ernst R K, Dombroski D M, Merrick J M. Anaerobiosis, type 1 fimbriae, and growth phase are factors that affect invasion of HEp-2 cells by Salmonella typhimurium. Infect Immun. 1990;58:2014–2016. doi: 10.1128/iai.58.6.2014-2016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gahring L C, Heffron F, Finlay B B, Falkow S. Invasion and replication of Salmonella typhimurium in animal cells. Infect Immun. 1990;58:443–448. doi: 10.1128/iai.58.2.443-448.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galán J E. Molecular genetic basis of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 23.Galán J E, Curtiss R., III Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galán J E, Curtiss R., III Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun. 1990;58:1879–1885. doi: 10.1128/iai.58.6.1879-1885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Vescovi E, Soncini F C, Groisman E A. Mg2+ as an extracellular signal: environmental regulation of Salmonella virulence. Cell. 1996;84:165–174. doi: 10.1016/s0092-8674(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 26.Hauser A R, Engel J N. Pseudomonas aeruginosa induces type III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect Immun. 1999;67:5530–5537. doi: 10.1128/iai.67.10.5530-5537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hensel M, Shea J E, Rapauch B, Monack D, Falkow S, Gleeson C, Kubo T, Holden D W. Functional analysis of ssaJ and the ssaK/U operon, 13 genes encoding components of the type III secretion apparatus of Salmonella pathogenicity island 2. Mol Microbiol. 1997;24:155–167. doi: 10.1046/j.1365-2958.1997.3271699.x. [DOI] [PubMed] [Google Scholar]
- 28.Hensel M, Shea J E, Waterman S R, Mundy R, Nikolaus T, Banks G, Vazquez-Torres A, Gleeson C, Fang F C, Holden D W. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 29.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaniga K, Tucker S, Trollinger D, Galán J E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinder S A, Badger J L, Bryant G O, Pepe J C, Miller V L. Cloning of the Yenl restriction endonuclease and methyltransferase from Yersinia enterocolitica serotype O:8 and construction of a transformable R−M+ mutant. Gene. 1993;136:271–275. doi: 10.1016/0378-1119(93)90478-l. [DOI] [PubMed] [Google Scholar]
- 32.Lee A K, Detweiler C S, Falkow S. OmpR/EnvZ regulates the two-component system SsrA-SsrB in Salmonella pathogenicity island 2. J Bacteriol. 2000;182:771–781. doi: 10.1128/jb.182.3.771-781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee C A, Falkow S. The ability of Salmonella typhimurium to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci USA. 1990;87:4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee C A, Jones B D, Falkow S. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci USA. 1992;89:1847–1851. doi: 10.1073/pnas.89.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindgren S W, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundberg U, Vinatzer U, Berdnik D, Gabain A v, Baccarini M. Growth phase-regulated induction of Salmonella-induced macrophage apoptosis correlates with transient expression of SPI-1 genes. J Bacteriol. 1999;181:3433–3437. doi: 10.1128/jb.181.11.3433-3437.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maniatis T, Sambrook J, Fritsch E F. Molecular cloning. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 38.Mock B A, Holiday D L, Cerretti D P, Darnell S C, O'Brien A D, Potter M. Construction of a series of congenic mice with recombinant chromosome 1 regions surrounding the genetic loci for resistance to intracellular parasites (Ity, Lsh, and Bcg), DNA responses (Rep-1), and cytoskeletal protein villin (Vil) Infect Immun. 1994;62:325–328. doi: 10.1128/iai.62.1.325-328.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monack D M, Raupach B, Hromockyj A E, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proc Natl Acad Sci USA. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Brien A D. Influence of host genes on resistance of inbred mice to lethal infection with Salmonella typhimurium. Curr Top Microbiol Immunol. 1986;124:37–48. [PubMed] [Google Scholar]
- 41.Ochman H, Soncini F C, Solomon F, Groisman E A. Identification of a pathogenicity island for Salmonella survival in host cells. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Penfold R J, Pembert J M. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene. 1992;118:145–146. doi: 10.1016/0378-1119(92)90263-o. [DOI] [PubMed] [Google Scholar]
- 43.Pfeifer C G, Marcus S L, Steele-Mortimer O, Knodler L A, Finlay B B. Salmonella typhimurium virulence genes are induced upon bacterial invasion into phagocytic and nonphagocytic cells. Infect Immun. 1999;67:5690–5698. doi: 10.1128/iai.67.11.5690-5698.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pratt L A, Silhavy T J. Porin regulon of Escherichia coli. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. pp. 105–27. [Google Scholar]
- 45.Richter-Dahlfors A, Buchan A M J, Finlay B B. Murine salmonellosis studied by confocal microscopy: Salmonella typhimurium resides intracellularly inside macrophages and exerts a cytotoxic effect on phagocytes in vivo. J Exp Med. 1997;186:569–580. doi: 10.1084/jem.186.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shea J E, Beuzon C R, Gleeson C, Mundy R, Holden D W. Influence of the Salmonella typhimurium pathogenicity island 2 type III secretion system on bacterial growth in the mouse. Infect Immun. 1999;67:213–219. doi: 10.1128/iai.67.1.213-219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shea J E, Hensel M, Gleeson C, Holden D W. Identification of a virulence locus encoding a second type III secretion system in Salmonella typhimurium. Proc Natl Acad Sci USA. 1996;93:2593–2597. doi: 10.1073/pnas.93.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skorupski K, Taylor R K. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 49.Tsolis R M, Townsend S M, Ficht T A, Adams L G, Bäumler A J. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect Immun. 1999;67:4879–4885. doi: 10.1128/iai.67.9.4879-4885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valdivia R H, Falkow S. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol Microbiol. 1996;22:367–378. doi: 10.1046/j.1365-2958.1996.00120.x. [DOI] [PubMed] [Google Scholar]
- 51.van der Velden A W M, Bäumler A J, Tsolis R M, Heffron F. Multiple fimbrial adhesins are required for full virulence of Salmonella typhimurium in mice. Infect Immun. 1998;66:2803–2808. doi: 10.1128/iai.66.6.2803-2808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasquez-Torres A, Jones-Carson J, Bäumler A J, Falkow S, Valdivia R, Brown W, Le M, Berggren R, Parks W T, Fang F C. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature. 1999;401:804–808. doi: 10.1038/44593. [DOI] [PubMed] [Google Scholar]