Abstract
Phytophthora sojae causes Phytophthora root and stem rot disease of soybean (Glycine max), leading to huge annual yield loss worldwide, but resistance to Phytophthora sojae (Rps) genes remains elusive. Soybean cultivar “Yudou 29” is resistant to P. sojae strain PsMC1, and this study aimed to clone, identify, and characterize the Rps gene in Yudou 29 (RpsYD29) and clarify its functional mechanism. We map-based cloned RpsYD29 (ZINC FINGER PROTEIN03, GmZFP03) using the families of a cross between Yudou 29 and a P. sojae-susceptible soybean cultivar “Jikedou 2”. P. sojae resistance of GmZFP03 was functionally validated by stable soybean genetic transformation and allele-phenotype association analysis. GmZFP03 was identified as a C2H2-type zinc finger protein transcription factor, showing 4 amino acid residue polymorphisms (V79F, G122-, G123-, and D125V) and remarkably different expression patterns between resistant and susceptible soybeans. Notably boosted activity and gene expression of superoxide dismutase (SOD) in resistant-type GmZFP03-expressed transgenic soybean, substantial enhancement of P. sojae resistance of wild-type soybean by exogenous SOD treatment, and GmZFP03 binding to and activation of 2 SOD1 (Glyma.03g242900 and Glyma.19g240400) promoters demonstrated the involvement of SOD1s in GmZFP03-mediated resistance to P. sojae strain PsMC1. Thus, this study cloned the soybean P. sojae-resistant GmZFP03, the product of which specifically targets 2 SOD1 promoters. GmZFP03 can be directly used for precise P. sojae-resistance soybean breeding.
The soybean transcription factor ZINC FINGER PROTEIN03 targets and activates two SUPEROXIDE DISMUTASE1 promoters to confer resistance to Phytophthora sojae.
Introduction
The oomycete Phytophthora sojae is a severely damaging pathogen of soybean [Glycine max (L.) Merr.], causing Phytophthora root and stem rot (PRSR) disease underlying plant wilt with $1–2 billion of annual yield loss globally (Tyler 2007). Planting resistant soybean varieties is the most effective, economical, and environmentally friendly means to manage this pathogen. Many genetically resistant soybean resources have been identified and show 2 completely different types of resistance against P. sojae. One is controlled by single genes with strong resistance to specific races of P. sojae, and the other is controlled by multigenes, which are quantitative trait loci (QTL), with broad spectrum but partial resistance to most races of P. sojae (Sugimoto et al. 2012). Some PRSR-resistant soybeans may carry more than 1 resistant gene or 2 different types of resistant genes (strong and partial resistance) (Gordon et al. 2007a, 2007b; Zhang et al. 2014a). The single genes can usually maintain resistance for only about 8–15 yr, whereas the resistance of a QTL can remain much longer and is more stable (Dorrance 2018). To elucidate the nature of soybean resistance to PRSR for long-term and effective control, identification of the P. sojae-resistant genes in soybean is primarily required, but challenging.
Since the first P. sojae-resistance locus (resistance to Phytophthora sojae, Rps) was identified in 1957 (Bernard et al. 1957), more than 40 genes (loci) underlying complete resistance to P. sojae have been mapped in soybean. Recently, a total of 75 novel Rps loci were identified from 16 panels of plant introductions (PIs) (Van et al. 2021). These loci are distributed on chromosomes 02, 03, 07, 10, 13, 16, 17, 18, and 19. Among these, over half the loci (Rps1a, Rps1b, Rps1c, Rps1d, Rps1k, Rps7, Rps9, Rps14, RpsYu25, RpsYD29, RpsUN1, RpsAH, RpsWY, RpsQ, RpsHN, RpsHC18, RpsT1, RpsT2, RpsT3, RpsX, and 2 unnamed loci) are located on chromosome 03 (chr. 03) (Sun et al. 2011; Wu et al. 2011; Sugimoto et al. 2012; Lin et al. 2013; Zhang et al. 2013; Guo et al. 2015; Cheng et al. 2017; Li et al. 2017; Niu et al. 2017; Zhong et al. 2018a, 2019; Jang et al. 2020; Chen et al. 2021; Matsuoka et al. 2021). Other Rps loci are also linked. For example, Rps3 and Rps8 are linked on chromosome 13; Rps4 and Rps6 co-segregate and are linked to Rps12 and Rps13 on chromosome 18 (Sugimoto et al. 2012; Sahoo et al. 2021). Many nucleotide-binding site-leucine-rich repeat (NBS-LRR)-type genes were detected in the Rps loci intervals and estimated to likely have the functions of P. sojae resistance (Graham et al. 2002; Sandhu et al. 2004; Zhang et al. 2013, 2014b; Sun et al. 2014; Zhong et al. 2018a, 2019; Jang et al. 2020). Previously, Rps1k-1 and Rps1k-2 at the Rps1k locus were isolated among all the identified Rps loci (Bhattacharyya et al. 2005; Gao et al. 2005). The complementation analyses showed that the susceptible soybean transformed with the bacterial artificial chromosome (BAC) clones carrying either Rps1k-1 or Rps1k-2 restored the PRSR resistance (Gao et al. 2005). Recently, a cluster containing 12 NBS-LRR genes at the 348 kb genomic region of Rps11 locus on chromosome 07 was demonstrated to confer resistance to P. sojae. Of them, 1 giant gene with 27.7 kb was identified as Rps11 by its responsibility for the resistance (Wang et al. 2021). However, to date, most of the Rps genes have neither been cloned nor functionally validated.
Soybean cultivar “Yudou 29” was developed for resistance against P. sojae in China. The P. sojae strain MC1 (PsMC1)-resistant locus (RpsYD29) of this cultivar has been mapped on chr. 03 using 214 F2:3 families derived from a cross between Yudou 29 and a susceptible soybean cultivar, “Jikedou 2” (Zhang et al. 2013). The main aims of this study were to clone and functionally validate RpsYD29 gene(s) using those crossed families and to decipher the possible molecular mode of action of the identified RpsYD29 gene(s) in soybean. In this study, we identified a ZINC FINGER PROTEIN03 (GmZFP03, Glyma.03g033600) as the RpsYD29 gene, which will promote research and application in precise resistance breeding and control of soybean PRSR disease.
Results
Map-based cloning of the soybean RpsYD29 gene GmZFP03
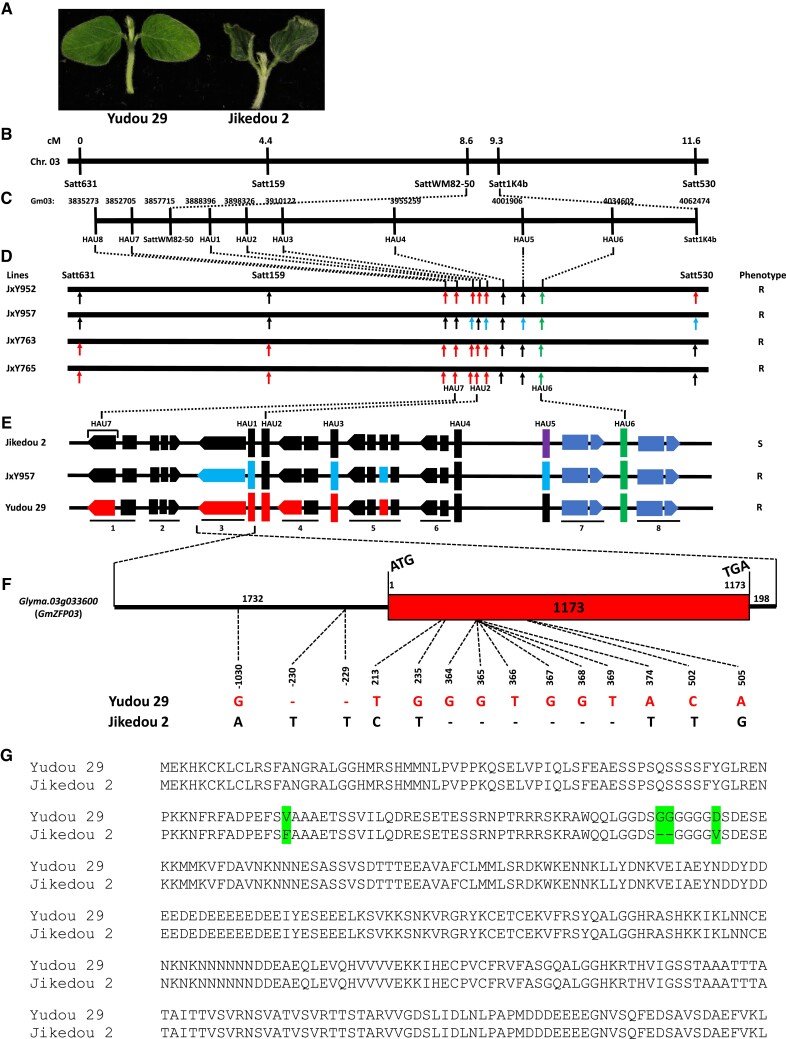
The PRSR resistance of soybean cv. Yudou 29 is controlled by 1 single gene, RpsYD29 (Zhang et al. 2013). In this study, Yudou 29 and Jikedou 2 were confirmed to be resistant and susceptible to P. sojae strain PsMC1, respectively (Fig. 1A). RpsYD29 was mapped at the positions 3857715 to 4062474 (∼204.8 kb) on chr. 03, including 11 potential genes (Fig. 1, B and C; Zhang et al. 2013). Eight more molecular markers, including 6 polymorphic molecular markers, were further developed to construct a high-density genetic map of this interval, and the recombination events and chromosome breakpoints were analyzed. Finally, the best resistant candidate gene(s) was (were) mapped on the genomic region between markers HAU7 and HAU4 (Gm03: 3833920 to 3955259) using 4 families (JxY952, JxY957, JxY763, and JxY765) (Fig. 1, B–E). This region contains 6 genes from Glyma.03g033400 (Gene 1) to Glyma.03g033900 (Gene 6) (http://www.soybase.org, Wm82.a4.v1). Because the resistance of Yudou 29 to P. sojae strain PsMC1 is different from Williams 82 (Zhang et al. 2013; Fig. 5, B and C), the region(s) carrying corresponding Williams 82 genomic sequence does/do not exhibit the resistance. There was no difference in genomic sequences at Gene 2 and Gene 6 between Yudou 29 and Jikedou 2, and Gene 1 and Gene 4 in JxY957, which was resistant (Fig. 1, D and E), showed a Williams 82 genotype. As a result, Gene 1, Gene 2, Gene 4, and Gene 6 could be directly excluded as candidate genes. No amino acid residues were changed in the predicted protein sequence of Gene 5 (Glyma.03g033800) between Yudou 29 and Jikedou 2: there was 1 nucleotide polymorphism in Exon 2, but the corresponding amino acid residue L86 was not changed (L86=) (Supplemental Fig. S1). Therefore, Glyma.03g033800 could also be eliminated. Finally, only Gene 3 (Glyma.03g033600) remained as the candidate resistance gene. Glyma.03g033600 (named GmZFP03 hereafter) was cloned from cDNA of Yudou 29 and Jikedou 2, both containing only 1 exon, and the Yudou 29 GmZFP03 exon had 1,173 bp in length. There were 5 single nucleotide polymorphisms (SNPs, T213C, G235T, A374T, C502T, and A505G) and 6 consequent insertions and deletions (InDels, G364-, G365-, T366-, G367-, G368-, and T369-) between Yudou 29 and Jikedou 2, resulting in 4 amino acid alterations (V79F, G122-, G123-, D125V) in the predicted protein sequences (Fig. 1, F and G; Supplemental Fig. S2). The entire gene of GmZFP03 was also cloned using genomic DNA, containing 1,732 bp of promoter region, 1,173 bp of gene-encoding region, and 198 bp of 3′-terminal UTR sequence. There were 1 SNP (−1,030) and 2 InDels (−229 and −230) in the promoter region of Yudou 29 and Jikedou 2 (Fig. 1F). GmZFP03, thus, was identified as the sole RpsYD29 candidate gene conferring resistance to PsMC1. Because GmZFP03 was heterozygous in JxY957 that showed resistance (Fig. 1, D and E), it can be deduced that GmZFP03 is a dominant candidate gene against PsMC1.
Figure 1.
Map-based cloning of soybean RpsYD29 gene conferring resistance to P. sojae strain PsMC1. A) Phenotypes of Yudou 29 and Jikedou 2 at 5 dpi of P. sojae strain PsMC1. B) The genetic map of RpsYD29 locus according to Zhang et al. (2013). C) Molecular markers (SNPs and InDels) developed at RpsYD29 locus in this study and their locations on chr. 03 (Gm03). D) High density genetic mapping of RpsYD29 locus of 4 families. E) Distribution and structure of genes at mapped PRSR-resistant region flanking markers HAU7 to HAU6. In D and E, Red: Unique Yudou 29 genotype; Purple: Unique Jikedou 2 genotype; Black: Williams 82 genotype; Sky-blue: heterozygote; Green: Yudou 29 and Jikedou 2 show the same genotype but different from Williams 82 genotype; Light-blue: not sequenced. F) Cloning and structure of whole resistant gene, GmZFP03 (Glyma.03g033600), including 1,732 bp of promoter region, 1,173 bp of gene-encoding sequence (just 1 exon) and 198 bp of 3′-terminal UTR sequence. There are 1 SNP and 2 InDels in promoter, 5 SNPs and 6 InDels in gene-encoding sequence, of GmZFP03 between Yudou 29 and Jikedou 2. G) Alignment of predicted protein sequences of GmZFP03 of Yudou 29 and Jikedou 2. There are 4 amino acid differences highlighted in green.
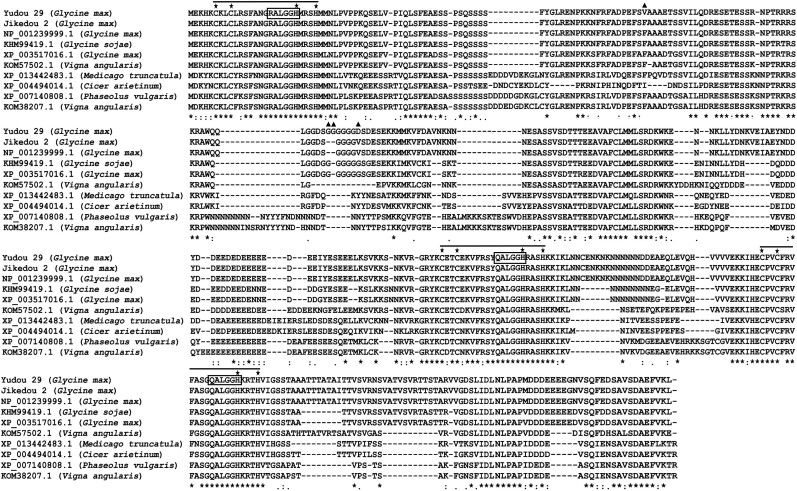
Figure 5.
Alignment of the protein sequences of GmZFP03. Sequences of 10 proteins classified in the clade of GmZFP03 were aligned. The triple C2H2-type zinc finger domains, conserved C2H2 amino acid residues and Q/RALGGH motifs are labeled with lines, five-stars, and rectangles, respectively. The changed amino acids between Yudou29 and Jikedou2 are labeled with triangles. The asterisks (*) indicate positions that are 100% conserved in the alignment; The colons (:) indicate conserved positions that are strongly similar in the alignment. The periods (.) indicate conserved positions that are weekly similar in the alignment.
GmZFP03 alleles genetically link P. sojae resistance
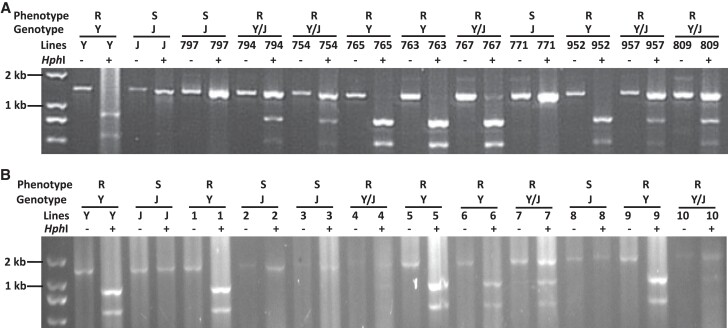
On the basis of the genomic sequences of GmZFP03 (Supplemental Fig. S2), a specific cleaved amplified polymorphic sequences (CAPS) marker was developed. The GmZFP03 PCR product of Jikedou 2 digested with restriction enzyme HphI showed just 1 band, while those of Yudou 29 digested with HphI exhibited 2 smaller-size bands, indicating that HphI cut Yudou 29-GmZFP03 PCR product into 2 pieces (Fig. 2A).
Figure 2.
Genetic relationships between GmZFP03 alleles and P. sojae strain PsMC1-infection phenotypes of soybean plants. A) Digestion patterns of soybean parents Yudou 29 and Jikedou 2 and families of their cross. B) Digestion patterns of progeny of F2:3 family JxY957. The PCR product was separated by 1.5% agarose gel after complete digestion with HphI in A and B. R, Resistant; S, Susceptible; Y, Yudou 29 or Yudou 29 genotype; J, Jikedou 2 or Jikedou 2 genotype; Y/J, Heterozygote.
The same experiments were performed using the families derived from the cross of Yudou 29 and Jikedou 2. The link analysis of gel-separation patterns with their phenotypes (Fig. 2A) showed that the soybean lines presented a resistant phenotype when the digestions showed 3 or 2 bands (heterozygous and homozygous Yudou 29 genotypes, respectively). On the other hand, the soybean lines displayed a susceptible phenotype when the digestion only showed 1 band (homozygous Jikedou 2 genotype), completely consistent with the genetic mapping results (Fig. 1). Next, the genotypes using the developed CAPS marker and phenotypes of the progeny of F2:3 family JxY957 were analyzed, indicating that there was also a link between genotypes and phenotypes that both homozygous and heterozygous Yudou 29 alleles showed resistance while homozygous Jikedou 2 alleles displayed susceptibility (Fig. 2B). All these results show that the GmZFP03 alleles genetically linked the resistance of soybean to P. sojae strain PsMC1.
Yudou 29-GmZFP03 enhances resistance to P. sojae
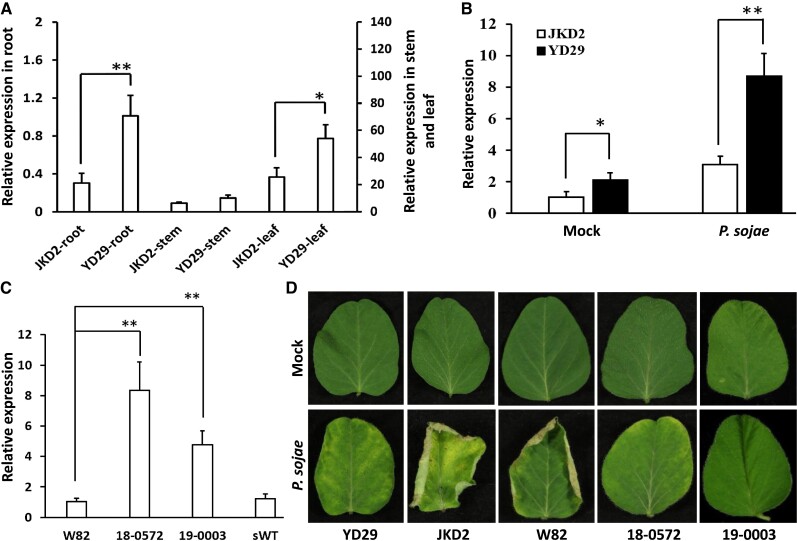
We first analyzed the expression patterns of GmZFP03 in different organs of Yudou 29 and Jikedou 2. GmZFP03 was expressed much higher in stems and leaves relative to roots (Fig. 3A). Expression of GmZFP03 in roots and leaves of Yudou 29 was significantly higher than that of Jikedou 2, while there was no significant difference of expression in stems between Yudou 29 and Jikedou 2 (Fig. 3A). Subsequently, expression of GmZFP03 in Yudou 29 and Jikedou 2 leaves with and without infection of PsMC1 was compared. At 2 d post inoculation (dpi), GmZFP03 was induced in both Yudou 29 and Jikedou 2, but the expression level of GmZFP03 in Yudou 29 was significantly higher than in Jikedou 2 (Fig. 3B). We proposed that the expression level of GmZFP03 might also contribute to the resistance of soybean to PsMC1.
Figure 3.
Expression of Yudou 29-GmZFP03 by stable genetic transformation substantially enhanced resistance of Williams 82 to P. sojae strain PsMC1. A) Relative expression of GmZFP03 (Glyma.03g033600) in different organs of soybean Yudou 29 and Jikedou 2 without infection of P. sojae strain PsMC1. B) Relative expression of GmZFP03 in leaves of Yudou 29 and Jikedou 2 with and without infection of P. sojae strain PsMC1. JKD2, Jikedou 2; YD29, Yudou 29; Mock, without infection of P. sojae; P. sojae, with infection of P. sojae strain PsMC1. C) Expression of GmZFP03 in the transgenic Williams 82 plants. 18-0572 and 19-0003 represent the identified transgenic Williams 82 lines expressing Yudou 29-GmZFP03; sWT (i.e. segregated wild-type) represents an identified nontransgenic Williams 82 line. D) Phenotypes of the transgenic Williams 82 plants (18-0572 and 19-0003) expressing Yudou 29-GmZFP03 at 5 dpi of P. sojae strain PsMC1 or not. GmTubulin was used as the internal control. Data are given as mean ± Sd of 3 technical replicates. The asterisks represent the significant difference by Student's t-test (*P < 0.05; **P < 0.01). All the experiments were performed thrice with similar trends.
Subsequently, to validate the resistance of GmZFP03, driven by CaMV 35S promoter (pCaMV35S), the Yudou 29-GmZFP03 was stably transformed into soybean Williams 82 that carries the susceptible Jikedou 2-type GmZFP03 (Fig. 1E). Two identified homozygous transgenic lines expressed with Yudou 29-GmZFP03 (“18-0572” and “19-0003”) were selected for next analyses (Fig. 3C and Supplemental Fig. S3A). Compared to wild-type Williams 82, the growth of transgenic plants was not impacted, and similar seeds were harvested (Supplemental Fig. S3B). Both 18-0572 and 19-0003 were employed to conduct the following PsMC1-infection phenotyping experiments. At 5 dpi, the wild-type Williams 82 and Jikedou 2 leaves were fairly wilted, whereas the inoculated wild-type Yudou 29 seedlings grew well, whose leaves were similar to those of the noninoculated plants (Fig. 3D). These results further verified the susceptibilities of both Williams 82 and Jikedou 2 and the resistance of Yudou 29 to PsMC1. Above all, the transgenic Williams 82 lines expressing Yudou 29-GmZFP03 (both 18-0572 and 19-0003) did not show any susceptibility symptoms to the infection of PsMC1 (Fig. 3D). These results clearly indicate that expression of Yudou 29-GmZFP03 substantially enhanced the resistance of Williams 82 to PsMC1. Hence, GmZFP03 is functionally verified as the RpsYD29 gene conferring resistance to PsMC1.
Two SOD1s are involved in GmZFP03-mediated resistance
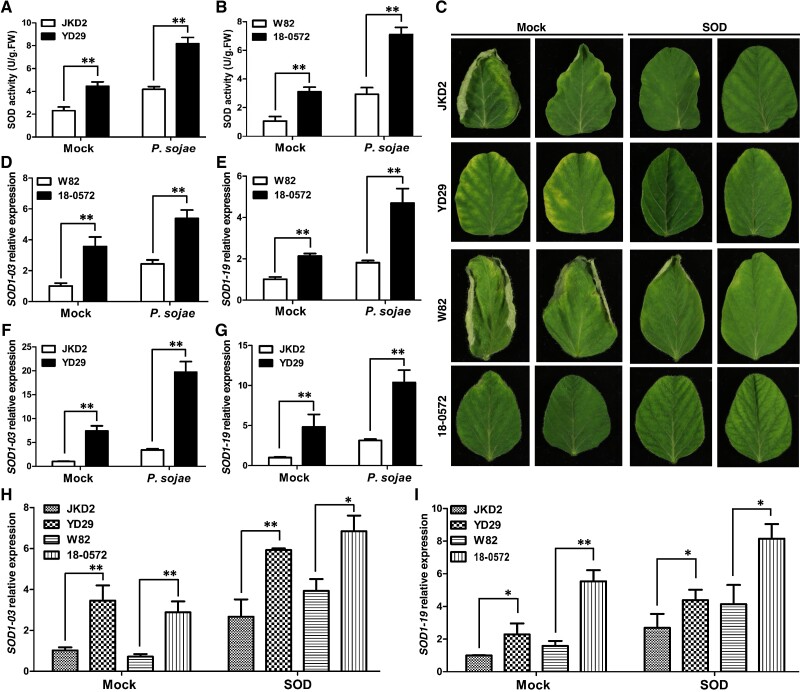
Interestingly, superoxide dismutase (SOD) enzymatic activity in Yudou 29 was much higher than in Jikedou 2 no matter with or without infection of PsMC1 (Fig. 4A). We therefore also measured the SOD activity between transgenic 18-0572 expressing Yudou 29-GmZFP03 and wild-type Williams 82 with and without infection of the pathogen. The results indicated that the SOD activity in resistant 18-0572 also exhibited a remarkable increase compared to that in susceptible Williams 82 (Fig. 4B), similar to the trends between resistant Yudou 29 and susceptible Jikedou 2. These results suggest that SOD might be associated with the resistance of GmZFP03 to PsMC1. We subsequently employed exogenous SOD to treat soybean roots. After treating the roots with 100 mg L−1 SOD, both Williams 82 and Jikedou 2 displayed resistance to P. sojae strain PsMC1 (Fig. 4C). Also, with and without treatment of SOD, both transgenics 18-0572 and Yudou 29 still showed high resistance to the pathogen (Fig. 4C). These results indicate that exogenous SOD substantially enhanced the resistance of Williams 82 and Jikedou2 to PsMC1, further indicating that SOD is likely associated with the resistance of GmZFP03 to PsMC1.
Figure 4.
Two SOD1 genes are involved in GmZFP03-mediated resistance to P. sojae strain PsMC1. A and B) Enzymatic activity of SOD in soybeans with and without the infection of P. sojae strain PsMC1. C) Phenotypes of soybeans infected with P. sojae strain PsMC1 after treatment of exogenous SOD. D–G) Expression of SOD1-03 and SOD1-19 in soybeans with and without P. sojae strain PsMC1 infection. H and I) Expression of SOD1-03 and SOD1-19 in soybeans treated with exogenous SOD. JKD2, YD29, and W82 are short for Jikedou 2, Yudou 29, and Williams 82, respectively. “Mock” in A, B, and D–G means “without P. sojae strain PsMC1 infection”. “Mock” in C, H, and I means “without SOD treatment”. And 100 mg L−1 SOD was used for treatment. U and FW are short for Unit and Fresh weight, respectively. Data are given as mean ± Sd of 3 technical replicates. The asterisks represent the significant difference by Student's t-test (*P < 0.05; **P < 0.01). All the experiments were performed thrice with similar trends.
Subsequently, we identified which SOD genes might be involved in the resistance of GmZFP03 to PsMC1. We first obtained 18 SOD genes from the Williams 82 reference genome (http://www.soybase.org) (Supplemental Table S1). Afterwards, we measured the expression patterns of these SOD genes in the transgenic 18-0572 and wild-type Williams 82 without and with infection of PsMC1. Of them, Glyma.20g196900 (a Fe-SOD2 gene) was not detectable by RT-qPCR. In total, we acquired the expression patterns of 17 SOD genes. Among them, only 2 Cu/Zn-SOD1s (SOD1-03: Glyma.03g242900 and SOD1-19: Glyma.19g240400) were expressed much more in the transgenic 18-0572 than in wild-type Williams 82 no matter without or with pathogen infection (Supplemental Fig. S4; Fig. 4, D and E). We successively measured the expression levels of these 2 SOD1s in resistant Yudou 29 and susceptible Jikedou 2. The results also displayed similar expression trends as in the resistant transgenic 18-0572 and susceptible Williams 82 (Fig. 4, F and G). After treatment with exogenous SOD, the expression of both SOD1-03 and SOD1-19 was substantially increased in all 4 susceptible and resistant soybeans (Fig. 4, H and I). In particular, the expression of both SOD1-03 and SOD1-19 in Williams 82 and Jikedou 2 with exogenous SOD treatment reached up to or close to the level in the resistant transgenic 18-0572 and Yudou 29 without exogenous SOD treatment (Mock) (Fig. 4, H and I). We propose, according to these data, that a “threshold” of SOD1-03 and SOD1-19 expression is likely required to confer resistance. In the mock treatments of Yudou 29 and 18-0572, expression levels of SOD1-03 and SOD1-19 were close to the threshold. Upon inoculation of PsMC1, expression of SOD1-03 and SOD1-19 increased above the threshold and plants showed the resistance phenotype. However, expression levels of SOD1-03 and SOD1-19 in Jikedou 2 and Williams 82 were not close to the threshold, and even though their expression increased when inoculated with PsMC1, their expression did not reach the threshold needed for resistance. Therefore, both Williams 82 and Jikedou 2 could show resistance to PsMC1 with the treatment of exogenous SOD (Fig. 4C). All these results indicate that both SOD1-03 and SOD1-19 are involved in the resistance of GmZFP03 to PsMC1.
GmZFP03 is a transcription factor binding to and activating 2 SOD1 promoters
While blasting the predicted GmZFP03 protein sequence on the NCBI (http://www.ncbi.nlm.nih.gov) database, 100 hits were gained. The phylogenetic analyses employing the predicted Yudou 29- and Jikedou 2-GmZFP03 protein sequences and the protein sequences of those 100 blasted hits (Supplemental Fig. S5) displayed that Yudou 29-GmZFP03, Jikedou 2-GmZFP03, NP_001239999.1, KHM99419.1, XP_003517016.1, KOM57502.1, XP_007140808.1, KOM38207.1, XP_0013442483.1, and XP_004494014.1 were clustered in 1 clade. Of which, NP_001239999.1 and XP_003517016.1 are the proteins encoded by Williams 82 Glyma.03g033600 and Glyma.01g01g134200, respectively. KHM99419.1 is a ZFP in wild soybean (Glycine sojae) and a combination of NP_001239999.1 and XP_003517016.1, where KHM99419.1 is identical to NP_001239999.1 from 1st to 112th amino acid residues and to XP_003517016.1 after the 112th amino acid residue. These results suggest that the wild soybean GmZFP might have duplicated and diverged into Glyma.03g033600 (GmZFP03) and Glyma.01g134200. All the proteins in this clade are from legumes and classified as the classical C2H2-type zinc finger protein (C2H2-ZFP) family, each containing the conserved triple C2H2 zinc finger domains and 2 QALGGH motifs plus 1 RALGGH motif (Fig. 5).
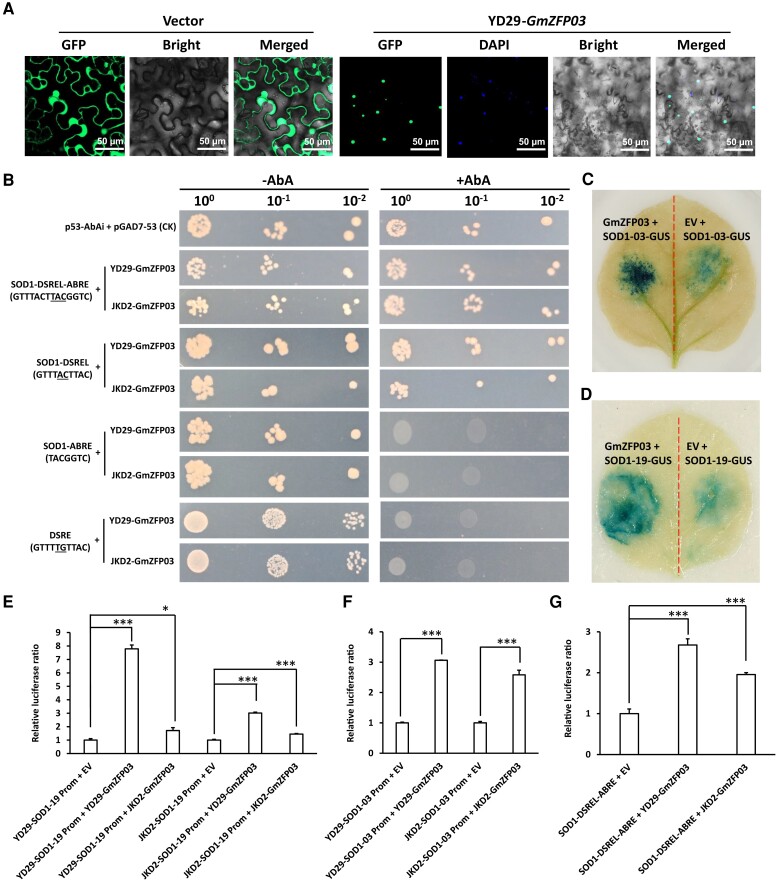
As a predicted transcription factor, we examined the subcellular localization of GmZFP03. GmZFP03 was localized in the cell nucleus of Nicotiana benthamiana leaves (Fig. 6A). Because ZFPs localized in the nucleus usually act as transcription factors to bind to and activate gene promoters (Pabo et al. 2001), we, therefore, analyzed the targets of GmZFP03. On the basis of the involvement of 2 SOD1s in resistance of GmZFP03 to PsMC1 (Fig. 4), the promoters of SOD1-03 and SOD1-19 were cloned from Yudou 29 and Jikedou 2, and aligned (Supplemental Figs. S6 and S7). Analyses of the promoters of these 2 genes (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) showed that there was a special sequence (GTTTACTTACGGTC) in the promoters which was shared between SOD1-03 and SOD1-19 (Supplemental Figs. S6 and S7). This sequence contained 2 motifs with 3 nucleotides (TAC, underlined) overlapped in the middle: one was a new motif, a cis-acting element involved in defense and stress responsiveness-like motif (named DSREL hereafter, GTTTACTTAC, positioned from −327 to −318), which showed a 2-nucleotide difference (underlined) from the known cis-acting element involved in defense and stress responsiveness motif (DSRE, GTTTTGTTAC) in the middle, and the other was a known cis-acting element involved in abscisic acid responsiveness motif (ABRE, TACGGTC, positioned from −320 to −314). So, we named this sequence (GTTTACTTACGGTC) as GmZFP03 promoter DSREL-ABRE motif. Furthermore, there was 1 SNP in ABRE of SOD1-03 between this DSREL-ABRE motif of Yudou 29 and Jikedou 2 (Supplemental Fig. S6), while this DSREL-ABRE motif was identical between Yudou 29- and Jikedou 2-SOD1-19 (Supplemental Fig. S7). Subsequently, using DSRE as the mutant, the binding of GmZFP03 to the DSREL-ABRE of SOD1-19 and SOD1-03 was tested by yeast one hybrid. The results showed that GmZFP03 (both Yudou 29- and Jikedou2-GmZFP03) could interact with DSREL-ABRE and DSREL, but did not interact with ABRE, or with DSRE (Fig. 6B). These results clearly indicate that GmZFP03 specifically binds to the novel DSREL motif of the promoters of 2 SOD1s in soybean.
Figure 6.
GmZFP03 binds to a cis-acting element involved in defense and stress responsiveness-like motif and activates the promoters of 2 SOD1s. A) Subcellular localization of GmZFP03 in N. benthamiana leaf cells. B) Yeast one-hybrid analyses of GmZFP03 binding to the motifs of the promoters of SOD1s. DSREL (GTTTACTTAC), cis-acting element involved in defense and stress responsiveness-like motif; ABRE (TACGGTC), cis-acting element involved in abscisic acid responsiveness motif; DSRE (GTTTTGTTAC), cis-acting element involved in defense and stress responsiveness motif, acting as the mutant with 2 different nucleotides (labelled with underline) from DSREL; YD29, Yudou 29; JKD2, Jikedou 2; AbA, aureobasidin A. C and D) Activation of the SOD1s promoters by GmZFP03 through GUS-staining. GUS was detected at 60 hpi. EV, empty vector. E–G) Activation of the SOD1 promoters by GmZFP03 using the dual-luciferases system. Prom, promoter; EV, empty vector; Relative luciferase ratio, Relative firefly/Renilla luciferase activity ratio. Data are given as mean ± Sd of 3 technical replicates. The asterisks represent the significant difference by Student's t-test (*P < 0.05; ***P < 0.001). All the experiments were performed thrice with similar trends.
Successively, we validated the activation of the promoters of 2 SOD1s by GmZFP03. On one hand, Yudou 29-GmZFP03 and the promoters of either SOD1-03 or SOD1-19 fused with GUS (beta-glucuronidase) were co-infiltrated into N. benthamiana leaves for transient expression (Supplemental Fig. S8). Substantially stronger blue GUS signal was shown in the leaves co-infiltrated with GmZFP03 and the promoter of either GUS-fused SOD1-03 or GUS-fused SOD1-19 when compared to the co-infiltration of an empty vector with either GUS-fused SOD1-03 promoter or GUS-fused SOD1-19 promoter (Fig. 6, C and D), indicating the activation of the promoters of SOD1-03 and SOD1-19 by GmZFP03. On the other hand, in the dual-luciferases system, as shown in Fig. 6, E–G, compared to the control without the presence of GmZFP03 (empty vector), the relative firefly/Renilla luciferase ratio was significantly increased regardless in the entire promoters of SOD1-19 and SOD1-03 or in the DSREL-ABRE with the presence of GmZFP03 (both Yudou 29's and Jikedou 2's), indicating that both Yudou 29- and Jikedou 2-GmZFP03 directly activated the DSREL-ABRE of the promoters of SOD1-19 and SOD1-03. However, the changes in firefly/Renilla luciferase ratios were much larger in the presence of Yudou 29-GmZFP03 than in the presence of Jikedou 2-GmZFP03. The relative firefly/Renilla luciferase ratio of SOD1-19 and SOD1-03 promoters of Yudou 29 plus Yudou 29-GmZFP03 reached 7.8 and 3.1, while that of SOD1-19 and SOD1-03 promoters of Jikedou 2 plus Jikedou2-GmZFP03 was only 1.4 and 2.6, respectively (Fig. 6, E and F). The large difference in the promoter sequences of SOD1-19 and SOD1-03 (Supplemental Fig. S9) might cause such a significant difference. Furthermore, the relative firefly/Renilla luciferase ratio of DSREL-ABRE plus Yudou 29-GmZFP03 reached up to 2.7, while that of DSREL-ABRE plus Jikedou2-GmZFP03 was only 2.0 (Figure 6G). All these results indicate that Yudou 29-GmZFP03 activated the promoters of SOD1 (both SOD1-19 and SOD1-03) much more than Jikedou 2-GmZFP03, causing accumulation of substantially more transcripts of the 2 SOD1s in resistant Yudou 29 (Fig. 2B).
Taken together, we demonstrated that GmZFP03 functions as a transcription factor specifically binding to the new DSREL motif and then activating the promoters of 2 SOD1s (SOD1-03 and SOD1-19) for their transcription in soybean.
Discussion
In this study, GmZFP03 was positionally cloned as the RpsYD29 gene conferring resistance to P. sojae strain PsMC1 using the families derived from the cross between Yudou 29 and Jikedou 2 by high-density genetic mapping and genomic sequence analyses (Fig. 1). The resistance of GmZFP03 was functionally validated by stable soybean genetic transformation (Fig. 3D), and was further supported by the analyses of the genetic link between the alleles and the PsMC1-infection phenotypes of soybean lines (Fig. 2). Previously, 2 genes, Rps1k-1 and Rps1k-2, were identified on the Rps1k locus, and the PRSR-susceptible soybean transformed with the BAC clones carrying them restored the resistance (Bhattacharyya et al. 2005; Gao et al. 2005). However, the exact physical location of Rps1k still remained unclear (Lin et al. 2013; Zhong et al. 2018a, 2018b, 2019). Recently, 1 giant NBS-LRR gene of 27.7 kb was identified to be responsible for the P. sojae resistance of Rps11 (Wang et al. 2021). GmZFP03 encodes a classical C2H2-type zinc finger protein (Fig. 5). Clearly, RpsYD29 GmZFP03 is completely different from Rps1k-1, Rps1k-2, the giant NBS-LRR Rps11 gene, and other predicted candidate genes, all of which belong to the NBS-LRR gene family (Graham et al. 2002; Sandhu et al. 2004; Bhattacharyya et al. 2005; Gao et al. 2005; Zhang et al. 2013, 2014b; Sun et al. 2014; Zhong et al. 2018a, 2019; Jang et al. 2020; Wang et al. 2021). Therefore, GmZFP03 is a cloned P. sojae-resistance gene in soybean. Furthermore, GmZFP03 is a dominant resistant gene against PsMC1 due to the resistance of heterozygous JxY957 (Fig. 1, D and E). There are many species of P. sojae (Zhang et al. 2013). However, in this study, only the P. sojae strain PsMC1 was tested, so it is better to test whether GmZFP03 is still resistant to the other P. sojae species in the future. However, discovery of the GmZFP03 conferring P. sojae resistance in this study, undoubtedly, will facilitate the research on the resistance nature of soybean and the accurate resistance breeding for management of PRSR disease in soybean.
In this study, 4 amino acid residue polymorphisms were shown in the predicted protein sequences of GmZFP03 between resistant Yudou 29 and susceptible Jikedou 2 (Fig. 1G). The expression of Yudou 29 GmZFP03 driven by pCaMV35S substantially enhanced the resistance of Williams 82 to PsMC1 (Fig. 3D). These results indicate the contribution of the 4 amino acid residue polymorphisms of GmZFP03 to the resistance against PsMC1. Furthermore, higher expression levels of SOD1s in pCaMV35S driven-Yudou 29 GmZFP03-expressing transgenic plants and resistant Yudou 29 plants than in susceptible Williams 82 and Jikedou 2 plants (Fig. 4), and the different binding patterns of GmZFP03 from Yudou 29 and Jikedou 2 to the new motif DSREL of 2 SOD1s promoters (Fig. 6, E–G) suggest that the amino acid residue polymorphisms in GmZFP03 are mostly responsible for the promoter motif binding, causing different SOD1s expression patterns in resistant and susceptible soybeans. But this requires additional soybean transformation experiments using the construct Yudou 29 GmZFP03 promoter:Jikedou 2 GmZFP03 and the construct Jiekdou 2 GmZFP03 promoter:Yudou 29 GmZFP03 for confirmation in the future. On the other hand, GmZFP03 was substantially induced by the infection of pathogenic PsMC1, and expressed significantly higher in resistant Yudou 29 than in susceptible Jikedou 2 no matter without or with infection of this pathogen (Fig. 3, A and B), suggesting that the expression level of GmZFP03 may also contribute to the resistance to PsMC1. Meanwhile, these results also suggest that GmZFP03 may be mediated by the other regulator(s), which is worthy of further investigation.
Several transcription factors have been revealed to mediate the resistance of soybean against pathogen P. sojae. In this study, the P. sojae-resistant GmZFP03 (Fig. 3) was identified as a C2H2-type transcription factor (Figs. 5 and 6) specifically binding to a new DSREL motif (GTTTACTTAC) and activating the promoters of 2 Cu/Zn SOD1 genes: SOD1-03 and SOD1-19 (Fig. 6) for highly enhanced SOD1 expression and SOD enzymatic activity (Fig. 4). A soybean basic helix-loop-helix (bHLH)-type transcription factor GmPIB1 (P. sojae-inducible bHLH transcription factor) modulated the resistance to P. sojae by binding to an E-box of the promoter of GmSPOD1, a peroxidase gene, and interacting with a 26S proteasome regulatory subunit GmPSMD to reduce ROS accumulation (Cheng et al. 2018; Liu et al. 2021). A soybean BTB/POZ domain-containing nuclear protein, GmBTB/POZ, promoted the ubiquitination and degradation of soybean LIKE HETEROCHROMATIN PROTEIN1 (GmLHP1), which directly bound to the promoter of GmWRKY40 and impaired the salicylic acid (SA) accumulation, to regulate the response of soybean to P. sojae (Zhang et al. 2021a). GmbZIP15 (basic leucine Zipper Protein15) positively mediated the resistance of soybean to P. sojae and Sclerotinia sclerotiorum but also depended on the phytohormone signaling (Zhang et al. 2021b). Obviously, GmZFP03 is a transcription factor underlying different molecular modes of action from those above-mentioned transcription factors against PsMC1 by activating the Cu/Zn-SOD1 pathway. SOD1s are 1 type of 3 radical scavengers, playing a very important role in plant tolerance against diverse abiotic stresses. However, the involvement of SOD1s in plant resistance to pathogens has been rarely reported (Fernansez-Ocana et al. 2011; Lightfoot et al. 2017; Verma et al. 2019). Our study expands the functional scope of SOD1s in plant disease defense.
Conclusion
The P. sojae PsMC1-resistant gene GmZFP03 in resistant soybean cultivar Yudou 29 was cloned and functionally identified in this study. GmZFP03 encodes a zinc finger transcription factor, which is a type of pathogen-resistance gene with an innovative molecular mode of action by specifically targeting a novel motif and activating the promoters of 2 SOD1 genes to confer resistance against P. sojae.
Materials and methods
Soybean and microbial strains
Soybean (Glycine max) cvs. Yudou 29 and Jikedou 2 are resistant and susceptible to P. sojae strain PsMC1, respectively (Zhang et al. 2013). The 214 F2:3 families of a cross between Yudou 29 and Jikedou 2 were developed in the Chinese Academy of Agricultural Sciences, P. R. China (Zhang et al. 2013). P. sojae strain PsMCl with virulence pathotype to Rps1a, Rps1c, Rps1k, Rps2, Rps3b, Rps3c, Rps4, Rps5, Rps6, Rps7, and Rps8 (Zhang et al. 2013) was used for phenotyping in this study.
Extraction of genomic DNA
The first true leaves on the top of seedlings grown in nutrient soil with 12-h light/12-h dark were harvested to extract genomic DNA. The extraction of DNA was carried out using the Qiagen DNeasy Plant Mini kit (Qiagen Sciences, USA) per the manufacturer's instructions. The concentration and quality of extracted DNA were quantified using a nanodrop microvolume spectrophotometer and fluorometer (Thermo Scientific, USA).
Development of molecular markers and high-density genetic mapping
Based on the previously constructed genetic map of RpsYD29 locus (Zhang et al. 2013), the genomic sequence of Williams 82 was employed to design a series of primers (Supplemental Table S2, 1 pair each about 10–30 kb distance) at the region flanking markers SattWM82-50 to Satt1k4b for the development of molecular markers (SNPs and InDels). Finally, 6 polymorphic markers, including HAU1, HAU2, HAU3, HAU5, HAU7, and HAU8, and 2 nonpolymorphic markers (HAU4 and HAU6) were developed. The 10 F2:3 families (JxY754, JxY763, JxY765, JxY767, JxY771, JxY794, JxY797, JxY809, JxY952, and JxY957) were first screened using 3 SSR markers, Satt530, Satt159, and Satt631, of which, 4 families, JxY952, JxY957, JxY763, and JxY765 that were all resistant to PsMC1, were screened to facilitate fine mapping of the resistant region. Then, the above-mentioned molecular markers were used to genotype these 4 families for the high-density genetic mapping.
The PCR was performed with a denaturation at 94 °C for 4 min, followed by 35 cycles of 94 °C for 30 s, 50–55 °C for 30 s, and 72 °C for 1 min with a final extension at 72 °C for 7 min. The PCR products were separated on a 1.5%–4% (w/v) agarose gel. For SNP detection, the PCR products were purified following separation by agarose gel and then sequenced at Sangon Biotech Co. Ltd. (Shanghai, P. R. China).
Extraction of RNA and synthesis of cDNA
The young leaf, root, and stem tissues at 4th trifoliate leaf stage were harvested to extract RNA using the general Trizol method. The cDNA was then synthesized employing the TransScript One-Step gDNA removal and cDNA Synthesis SuperMix kit (TransGen Biotech, P. R. China) per the instructions from the manufacturer.
Isolation of GmZFP03 genomic DNA and cDNA
The genomic DNA and synthesized cDNA were used to amplify the GmZFP03 using the corresponding primers (Supplemental Table S2) and high-fidelity pfu enzyme by PCR. The purified PCR products were ligated into the pGEM-T easy vector. The ligated plasmid was transformed into Escherichia coli DH5α competent cells by electroporation. The transformed E. coli was recovered and cultured on the LB solid plate with ampicillin antibiotic. After screening and culture, the plasmids were extracted and sequenced to obtain the GmZFP03 genomic DNA and cDNA sequences.
Development of a CAPS marker
According to the GmZFP03 genomic sequences (Supplemental Fig. S2), HphI was selected to distinguish GmZFP03 from Yudou 29 and Jikedou 2. Thus, the primers (Supplemental Table S2) were used to amplify the GmZFP03 genomic sequence, and then the PCR products were separated by 1.5% agarose gel after complete digestion by HphI at 37 °C for 6 h.
Reverse transcription quantitative PCR
The synthesized cDNA was used to conduct reverse transcription quantitative PCR (RT-qPCR) for the transcription level analysis of GmZFP03 and other genes using a fluorescence quantitative PCR machine (Applied Biosystems 7500). The primers are listed in Supplemental Table S2. GmTubulin was used as the internal reference gene. The experiments were conducted thrice, and each sample was triplicated each time.
Soybean transformation
Binary vector pCAMBIA3301 was used for constructing plant expression vector p187001. The Yudou 29-GmZFP03 gene was amplified using primers listed in Supplemental Table S2, and then cloned into pCAMBIA3301 vector by replacing the GUS gene, during which both Yudou 29-GmZFP03 gene DNA fragment and plasmid pCAMBIA3301 were digested with NcoI and BstEII, respectively. Target fragments were then extracted and linked with T4 ligase and transformed into E. coli. The vector carried T-DNA containing the Yudou 29-GmZFP03 gene driven by pCaMV35S and the Bar gene as an herbicide resistance marker.
The soybean transformation was carried out using the protocol from Wimi Biotechnology Co., Ltd, P. R. China. Briefly, the sterilized Williams 82 seeds were germinated in the dark at 26 °C for 24 h. The Agrobacterium tumefaciens strain EHA105, containing binary vector p187001, was used for transformation. The seed coat was removed to expose the cotyledons. The shoot apex was wounded using a sterile scalpel under a dissecting scope. The wounded cotyledon explants were placed into the Agrobacterium suspension for 60 min. Then the explants were placed on the filter paper overlying the co-cultivation medium containing 2.2 mg L−1 Murashige and Skoog basal salt mixture, 1 g L−1 MES, 30 g L−1 sugar, 1 mL L−1 Gamborg’s vitamin solution, 40 mg L−1 AS, 1 mg L−1 6-benzylaminopurine (6-BA) and 7 g L−1 agar, pH 5.6. Following co-cultivation in the dark at 23 °C for 3 d, the explants were recovered on the recovery medium which contained 3.1 g L−1 Gamborgs basal salt mixture, 1 g L−1 MES, 30 g L−1 sucrose, 1 mL L−1 Gamborg’s vitamin solution, 200 mg L−1 cefotaxime, 1 mg L−1 6-BA, and 7 g L−1 agar, pH 5.8, and cultured at 26 °C with 60 µEm−2 s−1 light intensity and 16/8-h light/dark photoperiod for 7 d. Then, the explants were transferred to the regeneration medium containing 3.1 g L−1 Gamborg’s basal salt mixture, 1 g L−1 MES, 30 g L−1 sucrose, 1 mL L−1 Gamborg’s vitamin solution, 200 mg L−1 cefotaxime, 1 mg L−1 6-BA, 7.5 g L−1 agar, and 8 mg L−1 glufosinate, pH 5.8 and cultured at 26 °C with 60 µEm−2 s−1 light intensity and 16/8-h light/dark photoperiod for about 2–3 wk. Afterwards, the cotyledon and other dead tissues were removed from each explant. The remaining tissues were inserted into the elongation medium containing 4.3 g L−1 Murashige and Skoog basal salt mixture, 0.6 g L−1 MES, 30 g L−1 sucrose, 1 mL L−1 Gamborg’s vitamin solution, 200 mg L−1 cefotaxime, 0.1 mg L−1 3-indoleacetic acid, 0.5 mg L−1 gibberellic acid, 1 mg L−1 zeatin, 7.5 g L−1 agar, and 8 mg L−1 glufosinate, pH 5.8, and cultured at 26 °C with 60 µEm−2 s−1 light intensity and 16/8-h light/dark photoperiod. The tissues were subcultured every 3 wk. Elongated shoots were transferred to the rooting medium containing 2.2 g L−1 Murashige and Skoog basal salt mixture, 20 g L−1 sucrose, 1 mL L−1 Gamborg’s vitamin solution, and 7.5 g L−1 agar, pH 5.8, and then grown in the greenhouse. The seeds from T0 plants were harvested for further analyses. The Bar gene detection in transgenic plants was demonstrated by PCR amplification of a 269 bp fragment using the primer pair listed in Supplemental Table S2.
Phenotyping of soybean
The PsMC1-infection phenotyping was adapted from the previous method by Lebreton et al. (2018). Briefly, PsMC1 was transferred from PDA medium to PDB liquid medium and grew at 26 °C for 7 d, then the spores were collected and the concentration of spores was adjusted to 5 × 105/mL in PDB liquid medium. Meanwhile, the 7-d old soybean seedlings were gently transferred from soil to 50 mL tubes containing 20 mL of PsMC1spores or mock (PDB liquid medium), and then the plants were kept in the growth chamber at 25 °C to analyze phenotypes.
Measurement of SOD activity
Weighed plant leaves of 0.4 g were fully ground with 5 mL of phosphate buffer (pH 7.8) and then centrifuged at 8,000 r/min, 4 °C for 15 min. The obtained supernatant was collected and diluted with phosphate buffer to 1/10–1/100 based on the concentration of SOD in the supernatant. The supernatant was used to measure the SOD activity. The measurement of plant SOD activity proceeded as described previously by Lombard et al. (2000). The experiments were conducted thrice, and each sample was triplicated each time.
Exogenous SOD treatment of plants
The 7-d old soybean seedlings were gently transferred from soil to 50 mL tubes containing 100 mg L−1 SOD (Yuanye Biotechnology Co., Ltd, Shanghai, Cat. 9054-89-1) or mock (water). The plants were kept in the growth chamber at 25 °C for 24 h and then harvested the samples to extract RNA for RT-qPCR or inoculated with PsMC1 to analyze phenotypes.
Phylogenetic analyses
The predicted GmZFP03 protein sequence of Yudou 29 was used to blast on GenBank, and 100 hits were obtained. Then, all these protein sequences were employed to perform phylogenetic analyses using MEGA-X software (http://www.megasoftware.net). The phylogenetic tree was constructed with the Neighbor-Joining method. Finally, all the protein sequences in the same clade of Yudou 29 GmZFP03 were aligned using the MAFFT program (http://www.mafft.cbrc.jp/alignment/software) to search zinc finger types, and conserved zinc finger domains and sequences.
Subcellular localization of GmZFP03
The Yudou 29-GmZFP03 cDNA was cloned into pGDG and introduced into A. tumefaciens GV3101, then the cultured Agrobacterium (OD600 value = 0.4) was infiltrated into the leaf back of N. benthamiana for transient expression. At 2 dpi, the subcellular localization was observed on a Carl Zeiss LSM880-type confocal laser fluorescence scanning microscopy (Germany). GFP fluorescence was excited with 488 nm blue light, and a signal was acquired at 492–555 nm (gain 50).
Yeast one-hybrid assay
The sequences containing 3 copies of the DSREL-ABRE motifs, DSREL motif, and ABRE motif of SOD1, and the mutant DSRE (Supplemental Table S2) were cloned into pAbAi, and the cDNAs of Jikedou 2-GmZFP03 and Yudou 29-GmZFP03 were cloned into pGADT7. Then, they were transformed into the Y1H Gold Saccharomyces cerevisiae strain using the Yeastmaker Yeast Transformation System 2 (Takara, Mountain View, USA). The yeast one-hybrid experiments were carried out according to the recommendations of the manufacturer (Clontech, http://www.clontech.com). The p53-AbAi plus pGAD7-53 was used as the positive control, and DSRE was used as the mutant.
GUS-staining
Yudou 29-GmZFP03 cDNA was cloned into pCAMBIA1307 vector. The SOD1-03 promoter (−722 to −1 bp) and SOD1-19 promoter (−695 to −1 bp) were cloned into T35S-HPT-MCS-GUS vector. These recombinant plasmids were transformed into A. tumefaciens strain GV3101, respectively. The Agrobacterium cultures were centrifuged and re-suspended in the infiltration buffer (10 mM MES, 0.15 mM Acetosyringone, and 10 mM MgCl2) to a concentration of OD600 value = 0.6. Equal volumes of different bacterial suspensions as demanded were mixed, and then the mixed suspension was infiltrated into the leaf back of N. benthamiana for transient expression. After infiltration, plants were cultured in the dark for 12 h and followed by 48 h under 16-h light/8-h dark at 24 °C. The infiltrated leaves were cut and soaked in the GUS-staining buffer (Coolaber, P. R. China, SL7160) under vacuum using a vacuum pump at a relative vacuum degree −20 kPa for 5 min. Subsequently, the samples were incubated at 37 °C in the dark for 8 to 16 h and then moved into 70% ethanol at 90 °C for destaining with ethanol changes of 2 to 3 times till the background was clear. In addition, the DNA of the infiltrated location of leaves was extracted, and the corresponding genes were detected by PCR for confirmation of the success Agrobacterium infiltration.
Measurement of dual luciferase activity
The luciferase activity was measured using the Dual-Glo Luciferase Assay System (Promega, Madison, WI, USA) per the instructions. The Jikedou 2-GmZFP03 and Yudou 29-GmZFP03 were cloned into pYBA1143 to generate pYBA1143:Jikedou 2-GmZFP03, and pYBA1143:Yudou 29-GmZFP03. The promoter sequences of SOD1-19 and SOD1-03 of Yudou 29 and Jikedou 2 were cloned into the pGreenII 0800-LUC. Simultaneously, the sequence containing 3 copies of SOD1 DSREL-ABRE motifs was also cloned into the pGreenII 0800-LUC. The constructs were transformed into A. tumefaciens GV3101 to prepare Agrobacterium suspensions, which were then infiltrated into N. benthamiana leaves. The leaves were collected at 2 d post infiltration. The collected leaves were ground into powder with liquid nitrogen and lysed in 1 × passive lysis buffer (Promega, Madison, WI, USA) on ice for 30 min. The lysed leaves were centrifuged at 4 °C for 15 min, and the supernatant was used for the luciferase activity measurement.
Subsequently, 50 µL of the obtained supernatant and 50 µL of Dual-Glo Luciferase Reagent were added to each well of 96-well plates and mixed. After at least 10 min, the firefly luminescence was measured using a GloMax 96 microplate luminometer (Promega). Afterwards, 50 µL of Dual-Glo Stop & Glo Reagent was added to each well and mixed. After at least 10 min, the Renilla luminescence was measured. The ratio of firefly luminescence and Renilla luminescence was calculated. The experiments were conducted thrice, and each sample was triplicated each time.
Statistical analysis
All the enzymatic activity and RT-qPCR data were analyzed for the variance by Student's t-test mean comparison using JMP Pro V12 software.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: GmZFP03 (NM_001253070), GmSOD1-03 (NM_001249007), and GmSOD1-19 (NM_001248369).
Supplementary Material
Acknowledgments
We thank the graduate students Ruicheng Zhang and Tian Qiu at Hunan Agricultural University for their assistance in the experiments.
Contributor Information
Wei Li, Hunan Provincial Key Laboratory for Biology and Control of Plant Diseases and Insect Pests, College of Plant Protection, Hunan Agricultural University, Changsha 410128, P. R. China.
Xiang Zheng, Hunan Provincial Key Laboratory for Biology and Control of Plant Diseases and Insect Pests, College of Plant Protection, Hunan Agricultural University, Changsha 410128, P. R. China.
Rong Cheng, Hunan Provincial Key Laboratory for Biology and Control of Plant Diseases and Insect Pests, College of Plant Protection, Hunan Agricultural University, Changsha 410128, P. R. China.
Chanjuan Zhong, Hunan Provincial Key Laboratory for Biology and Control of Plant Diseases and Insect Pests, College of Plant Protection, Hunan Agricultural University, Changsha 410128, P. R. China.
Jie Zhao, State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, P. R. China.
Tyler H Liu, College of Letters and Science, University of Wisconsin, Madison, WI 53706, USA.
Tuyong Yi, Hunan Provincial Key Laboratory for Biology and Control of Plant Diseases and Insect Pests, College of Plant Protection, Hunan Agricultural University, Changsha 410128, P. R. China.
Zhendong Zhu, Institute of Crop Sciences, Chinese Academy of Agricultural Sciences, Beijing 100081, P. R. China.
Jieting Xu, Wimi Biotechnology Co., Ltd, Changzhou 213000, P. R. China.
Khalid Meksem, Department of Plant, Soil and Agricultural Systems, Southern Illinois University, Carbondale, IL 62901, USA.
Liangying Dai, Hunan Provincial Key Laboratory for Biology and Control of Plant Diseases and Insect Pests, College of Plant Protection, Hunan Agricultural University, Changsha 410128, P. R. China.
Shiming Liu, Hunan Provincial Key Laboratory for Biology and Control of Plant Diseases and Insect Pests, College of Plant Protection, Hunan Agricultural University, Changsha 410128, P. R. China; State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing 100193, P. R. China.
Author contributions
W.L. and R.C. conducted the genetic mapping and CAPS marker experiments. S.L. designed the DNA markers, constructed the genetic map, developed the CAPS marker, and conducted the phylogenetic analyses. W.L. and X.Z. performed the RT-qPCR and transient expression binding analyses. C.Z., X.Z., and W.L. performed the phenotyping experiments. J.Z. carried out subcellular localization, yeast one hybrid, and luciferase activity measurement experiments. J.X. performed the soybean transformation. S.L., W.L., and T.H.L. analyzed the data. L.D., T.Y., and Z.Z. provided the experimental materials. S.L. conceived the project, designed the experiments, and wrote the manuscript. T.H.L. W.L., and K.M. edited the manuscript. S.L. and L.D. coordinated the project. All the authors commented on and approved the manuscript.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1 . Sequence alignment of genomic DNA, cDNA, and predicted protein and analyses of nucleotide polymorphism and amino acid change effects of Glyma.03g033800 of Yudou 29 and Jikedou 2.
Supplemental Figure S2 . Sequence alignment of genomic DNA, cDNA, and predicted protein of GmZFP03 (Glyma.03g033600) of Yudou 29 and Jikedou 2.
Supplemental Figure S3 . Identification of the transgenic Williams 82 plants (lines) expressing Yudou 29-GmZFP03 and the harvested seeds.
Supplemental Figure S4 . Relative expression of SOD genes in soybean with and without infection of P. sojae strain PsMC1.
Supplemental Figure S5 . Phylogenetic analyses of GmZFPs.
Supplemental Figure S6 . Alignment of the sequences of promoter of SOD1-03 (Glyma.03g242900) of Yudou 29 and Jikedou 2.
Supplemental Figure S7 . Alignment of the sequences of promoter of SOD1-19 (Glyma.19g240400) of Yudou 29 and Jikedou 2.
Supplemental Figure S8 . PCR identification of the expression of GmZFP03, SOD1-03, and SOD1-19 in N. benthamiana leaves after co-infiltration.
Supplemental Figure S9 . Alignment of the promoter sequences of SOD1-19 (Glyma.19g240400) and SOD1-03 (Glyma.03g242900).
Supplemental Table S1 . List of SOD genes in soybean.
Supplemental Table S2 . List of the primers used in this study.
Funding
This work is financially supported by the Shennong Scholar Program of Hunan Agricultural University, the National Natural Science Foundation of China (31972248, 31300250), the Crop Germplasm Conservation, and Utilization Program from the Ministry of Agriculture of China (2017NWB030-12), the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (ASTIP-02-IPP-04), and the Science and Technology Project of Hunan Province (2021NK1040, 2021JJ30010).
References
- Bernard RL, Smith PE, Kaufmann MJ, Schmitthenner AF. Inheritance of resistance to Phytophthora root and stem rot in soybean. Agron J. 1957:49(7):391. 10.2134/agronj1957.00021962004900070016x [DOI] [Google Scholar]
- Bhattacharyya MK, Narayanan NN, Gao H, Santra DK, Salimath SS, Kasuga T, Liu Y, Espinosa B, Ellison L, Marek L, et al. Identification of a large cluster of coiled coil-nucleotide binding site-leucine rich repeat-type genes from the Rps1 region containing Phytophthora resistance genes in soybean. Theor Appl Genet. 2005:111(1):75–86. 10.1007/s00122-005-1993-9 [DOI] [PubMed] [Google Scholar]
- Chen L, Wang W, Ping J, Fitzgerald JC, Cai G, Clark CB, Aggarwal R, Ma J. Identification and molecular mapping of Rps14, a gene conferring broad spectrum resistance to Phytophthora sojae in soybean. Theor Appl Genet. 2021:134(12):3863–3872. 10.1007/s00122-021-03933-9 [DOI] [PubMed] [Google Scholar]
- Cheng Q, Dong L, Gao T, Liu T, Li N, Wang L, Chang X, Wu J, Xu P, Zhang S. The bHLH transcription factor GmPIB1 facilitates resistance to Phytophthora sojae in Glycine max. J Exp Bot. 2018:69(10):2527–2541. 10.1093/jxb/ery103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Ma Q, Ren H, Xia Q, Sone E, Tan Z, Li S, Zhang G, Nian H. Fine mapping of a Phytophthora-resistance gene RpsWY in soybean (Glycine max L) by high-throughput genome-wide sequencing. Theor Appl Genet. 2017:130(5):1041–1051. 10.1007/s00122-017-2869-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrance AE. Management of Phytophthora sojae of soybean: a review and future perspectives. Can J Plant Pathol. 2018:40(2):210–219. 10.1080/07060661.2018.1445127 [DOI] [Google Scholar]
- Fernandez-Ocana A, Chaki M, Luque F, Gómez-Rodríguez MV, Carreras A, Valderrama R, Begara-Morales JC, Hernandez LE, Corpas FJ, Barroso JB. Functional analysis of superoxide dismutases (SODs) in sunflower under biotic and abiotic stress conditions. Identification of two new genes of mitochondrial Mn-SOD. J Plant Pathol. 2011:136(11):1303–1308. 10.1016/j.jplph.2011.01.020 [DOI] [PubMed] [Google Scholar]
- Gao H, Narayanan NN, Ellison L, Bhattacharyya MK. Two classes of highly similar coiled coil-nucleotide binding-leucine rich repeat genes isolated from the Rps1-k locus encode Phytophthora resistance in soybean. Mol Plant Microbe Interact. 2005:18(10):1035–1045. 10.1094/MPMI-18-1035 [DOI] [PubMed] [Google Scholar]
- Gordon SG, Berry SA, St Martin SK, Dorrance AE. Genetic analysis of soybean plant introductions with resistance to Phytophthora sojae. Phytopathology. 2007a:97(1):106–112. 10.1094/PHYTO-97-0106 [DOI] [PubMed] [Google Scholar]
- Gordon SG, Kowitwanich K, Pipatpongpinyo W, Martin SKS, Dorrance AE. Molecular marker analysis of soybean plant introductions with resistance to Phytophthora sojae. Phytopathology. 2007b:97(1):113–118. 10.1094/PHYTO-97-0113 [DOI] [PubMed] [Google Scholar]
- Graham MA, Marek LF, Shoemaker RC. PCR Sampling of disease resistance-like sequences from a disease resistance gene cluster in soybean. Theor Appl Genet. 2002:105(1):50–57. 10.1007/s00122-001-0846-4 [DOI] [PubMed] [Google Scholar]
- Guo N, Hu G, Zhao J, Huang J, Sun J, Li L, Gai J, Xing H. Genetic analysis and mapping of a single dominant Phytophthora sojae resistance gene in soybean. J Nanjing Agric Univ. 2015:38(4):532–537. 10.7685/j.issn.1000-2030.2015.04.002 [DOI] [Google Scholar]
- Jang IH, Kang IJ, Kim JM, Kang ST, Jang YE, Lee S. Genetic mapping of a resistance locus to Phytophthora sojae in the Korean soybean cultivar Daewon. Plant Pathol J. 2020:36(6):591–599. 10.5423/PPJ.OA.09.2020.0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton A, Labbe C, De Ronne M, Xue AG, Marchand G, Belanger RR. Development of a simple hydroponic assay to study vertical and horizontal resistance of soybean and pathotypes of Phytophthora sojae. Plant Dis. 2018:102(1):114–123. 10.1094/PDIS-04-17-0586-RE [DOI] [PubMed] [Google Scholar]
- Li Y, Sun S, Zhong C, Wang X, Wu X, Zhu Z. Genetic mapping and development of co-segregating markers of RpsQ, which provides resistance to Phytophthora sojae in soybean. Theor Appl Genet. 2017:130(6):1223–1233. 10.1007/s00122-017-2883-7 [DOI] [PubMed] [Google Scholar]
- Lightfoot DJ, McGrann GRD, Able AJ. The role of a cytosolic superoxide dismutase in barley–pathogen interactions. Mol Plant Pathol. 2017:18(3):323–335. 10.1111/mpp.12399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Zhao M, Ping J, Johnson A, Zhang B, Abney TS, Hughes TJ, Ma J. Molecular mapping of two genes conferring resistance to Phytophthora sojae in a soybean landrace PI 567139B. Theor Appl Genet. 2013:126(8):2177–2185. 10.1007/s00122-013-2127-4 [DOI] [PubMed] [Google Scholar]
- Liu T, Wang H, Liu Z, Pang Z, Zhang C, Zhao M, Ning B, Song B, Liu S, He Z, et al. The 26S proteasome regulatory subunit GmPSMD promotes resistance to Phytophthora sojae in soybean. Front Plant Sci. 2021:12:513388. 10.3389/fpls.2021.513388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard M, Fontecave M, Touati D, Nivière V. Reaction of the desulfoferrodoxin from Desulfoarculus baarsii with superoxide anion. Evidence for a superoxide reductase activity. J Biol Chem. 2000:275(1):115–121. 10.1074/jbc.275.1.115 [DOI] [PubMed] [Google Scholar]
- Matsuoka JI, Takahashi M, Yamada T, Kono Y, Yamada N, Takahashi K, Moriwaki J, Akamatsu H. Identification of three closely linked loci conferring broad-spectrum Phytophthora sojae in soybean variety Tosan-231. Theor Appl Genet. 2021:134(7):2151–2165. 10.1007/s00122-021-03813-2 [DOI] [PubMed] [Google Scholar]
- Niu J, Guo N, Sun J, Li L, Cao Y, Li S, Huang J, Zhao J, Zhao T, Han X. Fine mapping of a resistance gene RpsHN that controls Phytophthora sojae using recombinant inbred lines and secondary populations. Front Plant Sci. 2017:8:538. 10.3389/fpls.2017.00538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo CO, Peisach E, Grant RA. Design and selection of novel Cys2His2 zinc finger proteins. Annu Rev Biochem. 2001:70(1):313–340. 10.1146/annurev.biochem.70.1.313 [DOI] [PubMed] [Google Scholar]
- Sahoo DK, Das A, Huang X, Cianzio S, Bhattacharyya MK. Tightly linked Rps12 and Rps13 genes provide broad-spectrum Phytophthora resistance in soybean. Sci Rep. 2021:11(1):16907. 10.1038/s41598-021-96425-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu D, Gao H, Cianzio S, Bhattacharyya MK. Deletion of a disease resistance nucleotide-binding-site leucine-rich- repeat-like sequence is associated with the loss of the Phytophthora resistance gene Rps4 in soybean. Genetics. 2004:168(4):2157–2167. 10.1534/genetics.104.032037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T, Kato M, Yoshida S, Matsumoto I, Kobayashi T, Kaga A, Hajika M, Yamamoto R, Watanabe K, Aino M, et al. Pathogenic diversity of Phytophthora sojae and breeding strategies to develop Phytophthora-resistant soybeans. Breed Sci. 2012:61(5):511–522. 10.1270/jsbbs.61.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Li LH, Zhao J, Huang J, Yann Q, Xing H, Guo N. Genetic analysis and fine mapping of RpsJS, a novel resistance gene to Phytophthora sojae in soybean [Glycine max (L) Merr]. Theor Appl Genet. 2014:127(4):913–919. 10.1007/s00122-014-2266-2 [DOI] [PubMed] [Google Scholar]
- Sun S, Zhao SL, Wang JM, Tang QH, Yu DY, Gai JY, Xing H. Characterization and mapping of RpsYu25, a novel resistance gene to Phytophthora sojae. Plant Breed. 2011:130(2):139–143. 10.1111/j.1439-0523.2010.01794.x [DOI] [Google Scholar]
- Tyler BM. Phytophthora sojae: root rot pathogen of soybean and model oomycete. Mol Plant Pathol. 2007:8(1):1–8. 10.1111/j.1364-3703.2006.00373.x [DOI] [PubMed] [Google Scholar]
- Van K, Rolling W, Biyashev RM, Matthiesen RL, Abeysekara NS, Robertson AE, Veney DJ, Dorrance AE, McHale LK, Saghai Maroof MA. Mining germplasm panels and phenotypic datasets to identify loci for resistance to Phytophthora sojae in soybean. Plant Genome. 2021:14(1):e20063. 10.1002/tpg2.20063 [DOI] [PubMed] [Google Scholar]
- Verma D, Neha Lakhanpal N, Singh K. Genome-wide identification and characterization of abiotic-stress responsive SOD (superoxide dismutase) gene family in Brassica juncea and B. rapa. BMC Genomics. 2019:20(1):227. 10.1186/s12864-019-5593-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Chen L, Fengler K, Bolar J, Llaca V, Wang X, Clark CB, Fleury TJ, Myrvold J, Oneal D, et al. A giant NLR gene confers broad-spectrum resistance to Phytophthora sojae in soybean. Nat Commun. 2021:12(1):6263. 10.1038/s41467-021-26554-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhang B, Sun S, Zhao J, Yang F, Guo N, Gai JY, Xing H. Identification, genetic analysis and mapping of resistance to Phytophthora sojae of Pm28 in soybean. Sci Agric Sin. 2011:10(10):1506–1511. 10.1016/S1671-2927(11)60145-4 [DOI] [Google Scholar]
- Zhang C, Cheng Q, Wang H, Gao H, Fang X, Chen X, Zhao M, Wei W, Song B, Liu S, et al. GmBTB/POZ promotes the ubiquitination and degradation of LHP1 to regulate the response of soybean to Phytophthora sojae. Commun Biol. 2021a:4(1):372. 10.1038/s42003-021-01907-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Liu Y, Li Z, She Z, Chai M, Aslam M, He Q, Huang Y, Chen F, Chen H, et al. The bZIP transcription factor GmbZIP15 facilitates resistance against Sclerotinia sclerotiorum and Phytophthora sojae infection in soybean. iScience. 2021b:24(6):102642. 10.1016/j.isci.2021.102642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Sun S, Wang G, Duan C, Wang X, Wu X, Zhu Z. Characterization of Phytophthora resistance in soybean cultivars/lines bred in Henan province. Euphytica. 2014a:196(3):375–384. 10.1007/s10681-013-1040-x [DOI] [Google Scholar]
- Zhang J, Xia C, Duan C, Sun S, Wang X, Wu X, Zhu Z. Identification and candidate gene analysis of a novel Phytophthora resistance gene Rps10 in a Chinese soybean cultivar. PLoS One. 2014b:8(7):e69799. 10.1371/journal.pone.0069799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xia C, Wang X, Duan C, Sun S, Wu X, Zhu Z. Genetic characterization and fine mapping of the novel Phytophthora resistance gene in a Chinese soybean cultivar. Theor Appl Genet. 2013:126(6):1555–1561. 10.1007/s00122-013-2073-1 [DOI] [PubMed] [Google Scholar]
- Zhong C, Li Y, Sun S, Duan C, Zhu Z. Genetic mapping and molecular characterization of a broad-spectrum Phytophthora sojae resistance gene in Chinese soybean. Int J Mol Sci. 2019:20(8):e1809. 10.3390/ijms20081809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Sun S, Li Y, Duan C, Zhu Z. Next-generation sequencing to identify candidate genes and develop diagnostic markers for a novel Phytophthora resistance gene, RpsHC18, in soybean. Theor Appl Genet. 2018a:131(3):525–538. 10.1007/s00122-017-3016-z [DOI] [PubMed] [Google Scholar]
- Zhong C, Sun S, Yao L, Ding J, Duan C, Zhu Z. Fine mapping and identification of a novel Phytophthora root rot resistance locus RpsZS18 on chromosome 2 in soybean. Front Plant Sci. 2018b:9: 44. 10.3389/fpls.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.