Abstract
The metabolic changes occurring after ischaemic stroke were measured to investigate the functional anatomy of clinical motor recovery. Positron emission tomography (PET) and the steady-state 15O technique was used to compare resting relative metabolic distributions at the onset of functional deficit with those following recovery. Ten patients were studied with repeat scans. Motor recovery was associated in some patients with an increase of relative oxygen metabolism in anatomical structures normally involved in motor function in the affected hemisphere, particularly in the cortical motor areas. In those patients without such metabolic changes in the cortex of the diseased hemisphere, relative increases in cortical metabolism in the contralateral hemisphere were associated with better motor recovery than in patients with no relative cortical metabolic increase in either hemisphere. There was no correlation between the degree of improvement in motor function and the severity of motor deficit at onset, the size and site of the lesion and the metabolic changes in the infarcted zone. No particular pattern of global metabolic changes was observed after recovery. Thus different relative patterns of metabolic recovery were seen in patients with different lesions and evidence was found for the participation of contralateral structures in the recovery process in some patients.
Full text
PDF
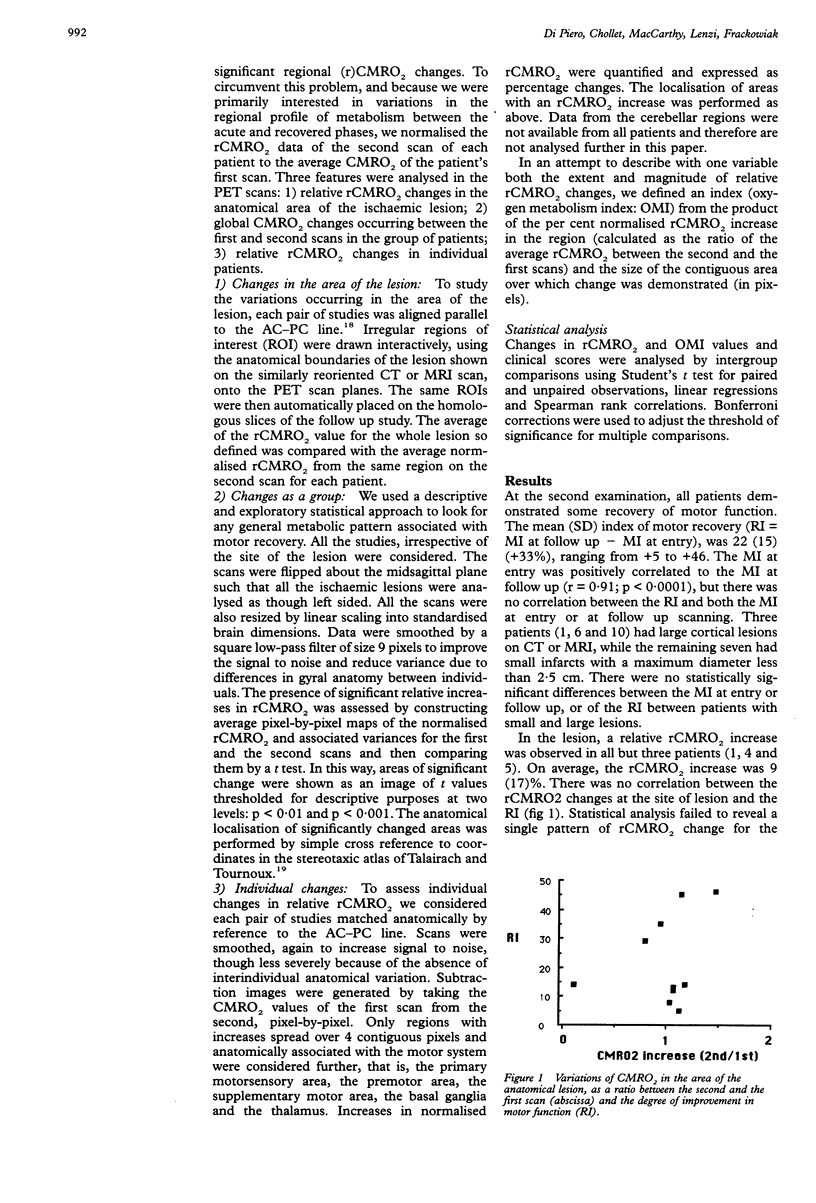
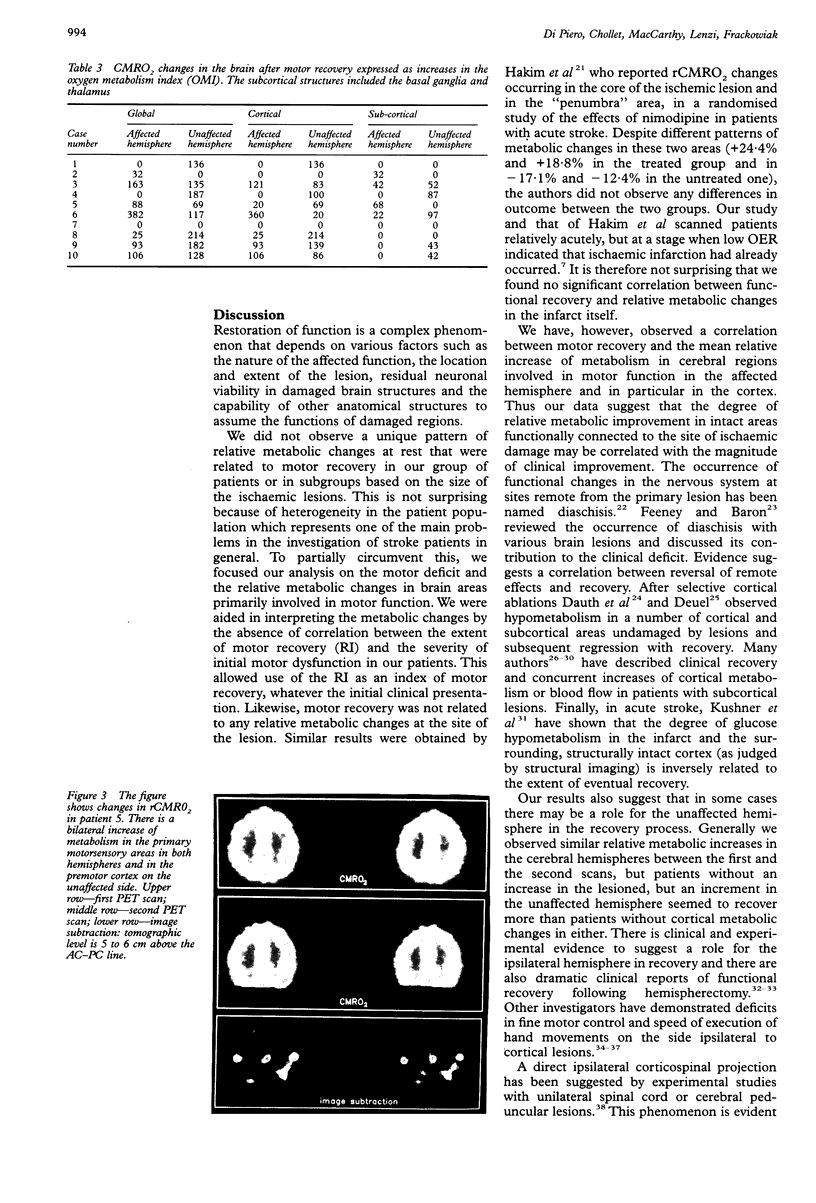


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron J. C., D'Antona R., Pantano P., Serdaru M., Samson Y., Bousser M. G. Effects of thalamic stroke on energy metabolism of the cerebral cortex. A positron tomography study in man. Brain. 1986 Dec;109(Pt 6):1243–1259. doi: 10.1093/brain/109.6.1243. [DOI] [PubMed] [Google Scholar]
- Brinkman J., Kuypers H. G. Cerebral control of contralateral and ipsilateral arm, hand and finger movements in the split-brain rhesus monkey. Brain. 1973 Dec;96(4):653–674. doi: 10.1093/brain/96.4.653. [DOI] [PubMed] [Google Scholar]
- Brodal A. Self-observations and neuro-anatomical considerations after a stroke. Brain. 1973 Dec;96(4):675–694. doi: 10.1093/brain/96.4.675. [DOI] [PubMed] [Google Scholar]
- Bucy P. C., Ladpli R., Ehrlich A. Destruction of the pyramidal tract in the monkey. The effects of bilateral section of the cerebral peduncles. J Neurosurg. 1966 Jul;25(1):1–23. doi: 10.3171/jns.1966.25.1.0001. [DOI] [PubMed] [Google Scholar]
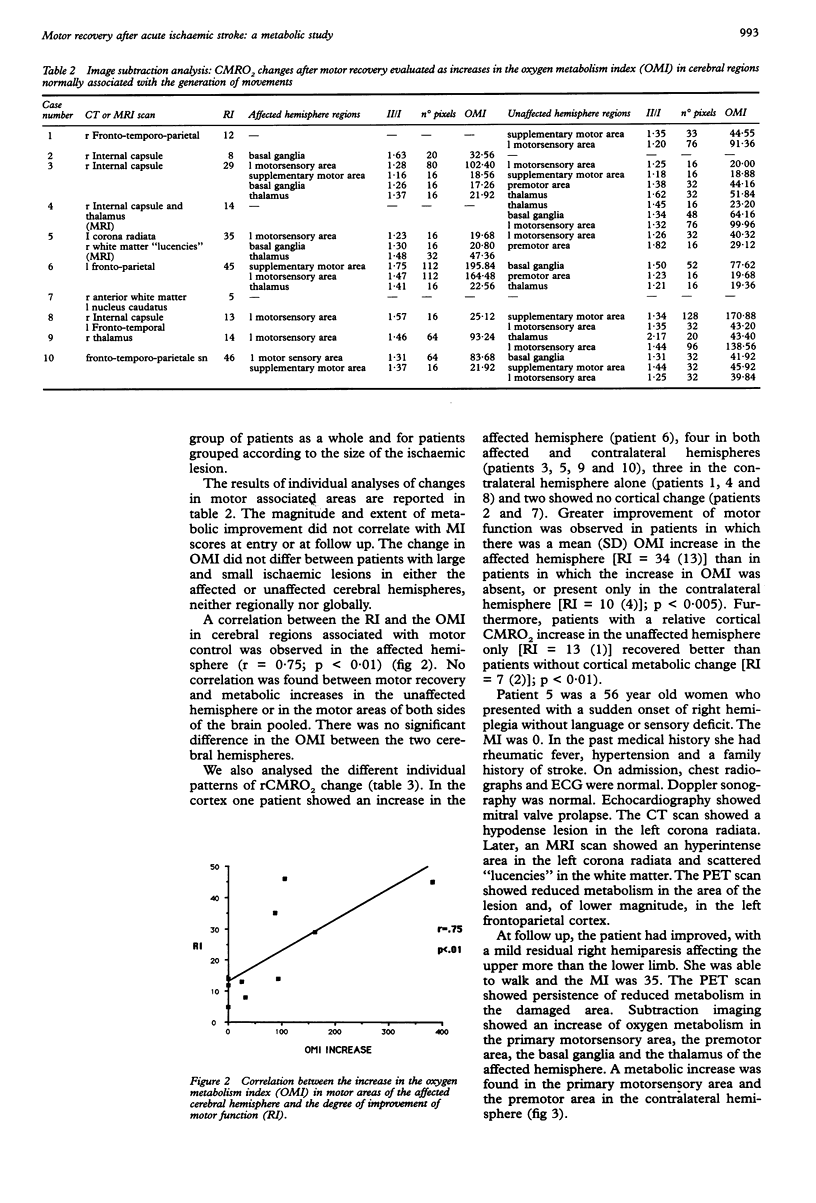
- Burke A. M., Younkin D., Gordon J., Goldberg H., Graham T., Kushner M., Obrist W., Jaggi J., Rosen M., Reivich M. Changes in cerebral blood flow and recovery from acute stroke. Stroke. 1986 Mar-Apr;17(2):173–178. doi: 10.1161/01.str.17.2.173. [DOI] [PubMed] [Google Scholar]
- Chollet F., DiPiero V., Wise R. J., Brooks D. J., Dolan R. J., Frackowiak R. S. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991 Jan;29(1):63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Dauth G. W., Gilman S., Frey K. A., Penney J. B., Jr Basal ganglia glucose utilization after recent precentral ablation in the monkey. Ann Neurol. 1985 May;17(5):431–438. doi: 10.1002/ana.410170503. [DOI] [PubMed] [Google Scholar]
- Demeurisse G., Demol O., Robaye E. Motor evaluation in vascular hemiplegia. Eur Neurol. 1980;19(6):382–389. doi: 10.1159/000115178. [DOI] [PubMed] [Google Scholar]
- Dombovy M. L., Bach-y-Rita P. Clinical observations on recovery from stroke. Adv Neurol. 1988;47:265–276. [PubMed] [Google Scholar]
- Feeney D. M., Baron J. C. Diaschisis. Stroke. 1986 Sep-Oct;17(5):817–830. doi: 10.1161/01.str.17.5.817. [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Raichle M. E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackowiak R. S., Lenzi G. L., Jones T., Heather J. D. Quantitative measurement of regional cerebral blood flow and oxygen metabolism in man using 15O and positron emission tomography: theory, procedure, and normal values. J Comput Assist Tomogr. 1980 Dec;4(6):727–736. doi: 10.1097/00004728-198012000-00001. [DOI] [PubMed] [Google Scholar]
- Friston K. J., Passingham R. E., Nutt J. G., Heather J. D., Sawle G. V., Frackowiak R. S. Localisation in PET images: direct fitting of the intercommissural (AC-PC) line. J Cereb Blood Flow Metab. 1989 Oct;9(5):690–695. doi: 10.1038/jcbfm.1989.97. [DOI] [PubMed] [Google Scholar]
- Gazzaniga M. S., Bogen J. E., Sperry R. W. Dyspraxia following division of the cerebral commissures. Arch Neurol. 1967 Jun;16(6):606–612. doi: 10.1001/archneur.1967.00470240044005. [DOI] [PubMed] [Google Scholar]
- Giubilei F., Lenzi G. L., Di Piero V., Pozzilli C., Pantano P., Bastianello S., Argentino C., Fieschi C. Predictive value of brain perfusion single-photon emission computed tomography in acute ischemic stroke. Stroke. 1990 Jun;21(6):895–900. doi: 10.1161/01.str.21.6.895. [DOI] [PubMed] [Google Scholar]
- Hakim A. M., Evans A. C., Berger L., Kuwabara H., Worsley K., Marchal G., Biel C., Pokrupa R., Diksic M., Meyer E. The effect of nimodipine on the evolution of human cerebral infarction studied by PET. J Cereb Blood Flow Metab. 1989 Aug;9(4):523–534. doi: 10.1038/jcbfm.1989.76. [DOI] [PubMed] [Google Scholar]
- Hier D. B., Mondlock J., Caplan L. R. Recovery of behavioral abnormalities after right hemisphere stroke. Neurology. 1983 Mar;33(3):345–350. doi: 10.1212/wnl.33.3.345. [DOI] [PubMed] [Google Scholar]
- Jones R. D., Donaldson I. M., Parkin P. J. Impairment and recovery of ipsilateral sensory-motor function following unilateral cerebral infarction. Brain. 1989 Feb;112(Pt 1):113–132. doi: 10.1093/brain/112.1.113. [DOI] [PubMed] [Google Scholar]
- Knopman D. S., Rubens A. B., Selnes O. A., Klassen A. C., Meyer M. W. Mechanisms of recovery from aphasia: evidence from serial xenon 133 cerebral blood flow studies. Ann Neurol. 1984 Jun;15(6):530–535. doi: 10.1002/ana.410150604. [DOI] [PubMed] [Google Scholar]
- Knopman D. S., Rubens A. B. The validity of computed tomographic scan findings for the localization of cerebral functions. The relationship between computed tomography and hemiparesis. Arch Neurol. 1986 Apr;43(4):328–332. doi: 10.1001/archneur.1986.00520040016011. [DOI] [PubMed] [Google Scholar]
- Kushner M., Reivich M., Fieschi C., Silver F., Chawluk J., Rosen M., Greenberg J., Burke A., Alavi A. Metabolic and clinical correlates of acute ischemic infarction. Neurology. 1987 Jul;37(7):1103–1110. doi: 10.1212/wnl.37.7.1103. [DOI] [PubMed] [Google Scholar]
- Lammertsma A. A., Jones T. Correction for the presence of intravascular oxygen-15 in the steady-state technique for measuring regional oxygen extraction ratio in the brain: 1. Description of the method. J Cereb Blood Flow Metab. 1983 Dec;3(4):416–424. doi: 10.1038/jcbfm.1983.67. [DOI] [PubMed] [Google Scholar]
- Lassen N. A. The luxury-perfusion syndrome and its possible relation to acute metabolic acidosis localised within the brain. Lancet. 1966 Nov 19;2(7473):1113–1115. doi: 10.1016/s0140-6736(66)92199-4. [DOI] [PubMed] [Google Scholar]
- Lenzi G. L., Frackowiak R. S., Jones T., Heather J. D., Lammertsma A. A., Rhodes C. G., Pozzilli C. CMRO2 and CBF by the oxygen-15 inhalation technique. Results in normal volunteers and cerebrovascular patients. Eur Neurol. 1981;20(3):285–290. doi: 10.1159/000115248. [DOI] [PubMed] [Google Scholar]
- Levine D. N., Warach J. D., Benowitz L., Calvanio R. Left spatial neglect: effects of lesion size and premorbid brain atrophy on severity and recovery following right cerebral infarction. Neurology. 1986 Mar;36(3):362–366. doi: 10.1212/wnl.36.3.362. [DOI] [PubMed] [Google Scholar]
- MAHONEY F. I., BARTHEL D. W. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J. 1965 Feb;14:61–65. [PubMed] [Google Scholar]
- Perani D., Di Piero V., Lucignani G., Gilardi M. C., Pantano P., Rossetti C., Pozzilli C., Gerundini P., Fazio F., Lenzi G. L. Remote effects of subcortical cerebrovascular lesions: a SPECT cerebral perfusion study. J Cereb Blood Flow Metab. 1988 Aug;8(4):560–567. doi: 10.1038/jcbfm.1988.97. [DOI] [PubMed] [Google Scholar]
- Schenkman M., Butler R. B., Naeser M. A., Kleefield J. Cerebral hemisphere asymmetry in CT and functional recovery from hemiplegia. Neurology. 1983 Apr;33(4):473–477. doi: 10.1212/wnl.33.4.473. [DOI] [PubMed] [Google Scholar]
- Spinks T. J., Guzzardi R., Bellina C. R. Performance characteristics of a whole-body positron tomograph. J Nucl Med. 1988 Nov;29(11):1833–1841. [PubMed] [Google Scholar]
- Steiner I., Melamed E. Conjugate eye deviation after acute hemispheric stroke: delayed recovery after previous contralateral frontal lobe damage. Ann Neurol. 1984 Oct;16(4):509–511. doi: 10.1002/ana.410160413. [DOI] [PubMed] [Google Scholar]
- Vallar G., Perani D., Cappa S. F., Messa C., Lenzi G. L., Fazio F. Recovery from aphasia and neglect after subcortical stroke: neuropsychological and cerebral perfusion study. J Neurol Neurosurg Psychiatry. 1988 Oct;51(10):1269–1276. doi: 10.1136/jnnp.51.10.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. J., Bernardi S., Frackowiak R. S., Legg N. J., Jones T. Serial observations on the pathophysiology of acute stroke. The transition from ischaemia to infarction as reflected in regional oxygen extraction. Brain. 1983 Mar;106(Pt 1):197–222. doi: 10.1093/brain/106.1.197. [DOI] [PubMed] [Google Scholar]
- Yamaguchi F., Meyer J. S., Sakai F., Yamamoto J. Case reports of three dysphasic patients to illustrate rCMF responses during behavioral activation. Brain Lang. 1980 Jan;9(1):145–148. doi: 10.1016/0093-934x(80)90080-2. [DOI] [PubMed] [Google Scholar]