Abstract
Background:
Bladder cancer (BC) is one of the most prevalent malignancies worldwide, 70% of patients initially diagnosed with superficial BC. In addition, 20% of BC patients with recurrence experience disease progression. Thus, identification of novel biomarkers for diagnosis, prognosis and therapeutic targets of BC will help to advance clinical diagnosis and treatment of this disease. MicroRNAs (miRNAs) are single stranded, non coding RNAs that are hypothesized to regulate gene expression at the post transcriptional level. This study aimed to assess the urine and tissue expression levels of miR-200, miR-145 and miR-21 in BC patients o evaluate their potential as noninvasive biomarkers.
Subjects and methods:
Urine and their corresponding tissue samples were collected from 111 BC patients and from 25 healthy controls. A quantitative real-time polymerase chain reaction method based on a TaqMan probe was used to evaluate the expression levels of miR200, miR145 and miR-21, the correlations between these miRNA expression levels in urine and tissues and certain clinicopathological parameters were investigated.
Results:
The expression of the 3 studied miRNAs was significantly higher in urine of low and high tumor grade BC patients compared to the controls and the expression were increased in BC tissues compared with those in normal bladder tissues, the results proved that the 3 miRNAs function as oncogenes. A marked positive correlation was observed between the mRNA expression of miR-200 and miR 21, with a coefficient of 0.511 and P value of 0.02.
Conclusion:
The results of the present study indicated that miR-200, miR-145 and miR-21 may function as oncogenes and have a potential to serve as an early noninvasive diagnostic biomarkers and therapeutic targets for treatment of BC.
Key Words: Bladder cancer, gene expression, non-invasive diagnosis
Introduction
Bladder cancer is an important public health problem and is considered the fourth most common malignant neoplasm in men and the eighth in women where about 81,180 new cases of BC were estimated in the USA for 2022 by the American Cancer Society accounting for about 61,700 in men and 19,480 in women. It also estimated about 17,100 deaths from BC, about 12,120 in men and 4,980 in women (American Cancer Society, 2022). Bladder cancer is the most common neoplasm of the urinary system and urothelial carcinoma (UC) is the most common histologic type of BC (approximately 90%) (Kaseb and Aeddula, 2022).
In recent years, the rates of new bladder cancers and deaths linked to this type of cancer have been dropping slightly in women. In men, incidence rates have been decreasing, but death rates have been stable (Cancer Stat Facts, 2018). Bladder cancer is highly heterogeneous, and its two major subsets are non-muscle-invasive BC (NMIBC) and muscle-invasive BC (MIBC). In Egypt, most of the diagnosed cases are non-muscle invasive bladder cancer and about 20% of those cases develop muscle-invasive during five years based on histopathological conditions. For that reason, early diagnosis of superficial stages of bladder cancer is extremely essential (Mahmoud et al., 2022).
Cystoscopyguided biopsy with histological evaluation has high diagnostic accuracy, but it is invasive, expensive, and inconvenient for general cancer screening. Therefore, new noninvasive diagnostic and prognostic markers and more effective treatment strategies are required. One approach to achieve these goals is through the analysis of RNA networks (Hany et al., 2021).
Lee et al., (1993) discovered in Caenorhabditis elegans that the lin-4 gene (currently known as lin-4 microRNA [miRNA]) could decrease the levels of lin-14 protein through antisense complementary binding of the RNA transcripts. Later, other miRNA genes with the same mechanism of action were found in different species, including humans. Thus, miRNAs were established as novel, small regulatory RNA molecules.
MicroRNAs (miRNAs) are non-coding, single-stranded RNA molecules (20–25 nucleotides), which can regulate gene expression by inhibiting mRNA translation or reducing the stability of mRNA (Gharib et al., 2022). An abnormal miRNA expression profile was found to exist widely in cancer cell, which induces limitless replicative potential and evading apoptosis. MiRNAs function as oncogenes (oncomiRs) or tumor suppressors during tumor development and progression. It was shown that regulation of specific miRNA alterations using miRNA mimics or antagomirs can normalize the gene regulatory network and signaling pathways, and reverse the phenotypes in cancer cells (Fu et al., 2021).
The miR-200 family is a critical regulator of the EMT (Epithelial-mesenchymal transition) phenotype in breast cancer. The miR-200 family includes five miRNAs classified into two clusters: miR-200a, miR-200b, and miR-429 on human chromosome 1; and miR-200c and miR-141 on human chromosome 12 (Ryou and Takahiro, 2019).
Notably, miR-200 family is downregulated in certain types of cancer, such as hepatocellular carcinoma and renal cell carcinoma, while being overexpressed in others, including melanoma, and ovarian and bladder cancer (Chang et al., 2015).
miR-145 is located on chromosome 5q32 and is widely established as a tumor suppressor. It exerts a regulatory effect on multiple types of human tumors, including colorectal cancer, non small cell lung cancer, pancreatic cancer, oral cancer and bladder cancer (Zhang et al., 2018).
miRNA-21 is among the most abundant and highly conserved miRNAs recognized. It is expressed in essentially all cells where it performs vital regulatory roles in health and disease. It is located within the Vacuole Membrane Protein 1 (VMP1) locus on chromosome 17. It has been implicated in both neoplastic and non-neoplastic pathologies through many of its gene targets (Jenike and Halushka, 2021).
Our aim is to estimate the expression profile of miR-200, miR-145 and miR-21 in urine and corresponding tissue of low and high grade BC patients, identify their fingerprint, speculating on its potential role as a non invasive prognostic or diagnostic tool to detect urinary BC and differentiation of tumor tissue and to investigate the relationship of miRNA expression with the clinicopathological characteristics of patients.
Materials and Methods
Patients and samples
In the current study, urine and their corresponding tissue samples were collected from 111 BC patients with age ranging from 45.5 to 72.5 years, and from 25 healthy controls, they matched to sex and age, their ages ranged from 42 to 65 years. All BC patients were recruited from the Urology Department of Theodor Bilharz Research Institute (TBRI).
The patient inclusion criteria were those who diagnosed BC and did not receive any type of therapy, and the diagnosis was confirmed by histo-pathological examination of the removed tumor tissues by 2 independent pathologists and stored at -80°C and used for molecular studies. The remaining tissue of all cases were fixed in 10% formalin, processed routinely into paraffin sections and pathologically diagnosed for tumor stage and grade. The exclusion criteria included previous cancer, metastasized cancer from other or unknown origins and previous radiotherapy or chemotherapy.
The research protocol was conducted according to the guidelines of the ethical principles outlined in the declaration of Helsinki and was approved by the institutional review board of the Ethics Committee of TBRI. (Approval number: FWA 00010609). Informed consent was obtained from all the eligible patients.
Specimens of 39 low-grade and 72 high-grade urothelial carcinomas obtained from patients who underwent transurethral resection or radical cystectomy were the subject of the study. A normal bladder tissue from 25 patients who underwent retropubic prostatectomy to treat benign prostatic hyperplasia were included as a control.
Twenty ml of urine samples were collected from all patients and 25 healthy volunteers as controls, samples were centrifuged at 3000 rpm for 20 min, the supernatants were decanted and the pellets were stored at −80°C until the RNA extraction.
miRNA extraction
MiR-200, miR-145 and miR-21 were isolated from the urine pellets and from 100 mg of BC tissue using a mirVana Kit® (Applied Biosystems, CA, USA) according to the manufacturer’s instructions, and the concentration was determined by 260/280 nm absorbance using a Nanodrop® ND-1000 spectrophotometer (Thermo Scientific). The spike in C. elegans miR-39 as a synthetic RNA oligo nucleotide (Qiagen, Hilden, Germany) was added to all samples before extraction.
Quantitative Real-Time Reverse-Transcription Assay (qRT-PCR)
TaqMan miR-200, miR-145, miR-21 and miR-39 ready-made primers and probes (Applied Biosystems, San Diego, CA) were used to perform qRT-PCR assays according to the manufacturer’s instructions. Briefly, 5 μl aliquot of the RNAs extracted from the urine and tissue samples were reverse transcribed using the microRNA reverse transcription kit (Applied Biosystems, San Diego, CA). Five μl of the cDNA were used for the real time PCR amplification step. All reactions were run in duplicate. The ΔΔCT method was used for the relative quantification of miRNAs in all samples (Akin et al., 2012).
Statistical analysis
The data were analyzed using Microsoft Excel 2016 and statistical package for social science ‘IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA)’. Continuous normally distributed variables were represented as mean±SD. with 95% confidence interval, while non-normal variables were summarized as median with 25 and 75 percentile, and using the frequencies and percentage for categorical variables; a P value < 0.05 was considered statistically significant. To compare the means of normally distributed variables between groups, the Student’s t test was performed, and Mann-Whitney U test was used in non-normal variables. χ2 test or Fisher’s exact test was used to determine the distribution of categorical variables between groups. The diagnostic performance of the studied markers was assessed by receiver operating characteristic (ROC) curves. The area under the ROC (AUC) was calculated as an accuracy index for prognostic performance of selected tests. Univariate analysis was conducted to determine the prognostic performance of each studied biomarker. Pearson Correlation was done to correlate between the studied parameters with the studied biomarkers.
Results
Patient and control characteristics
A total of 111 BC participants with mean age of (59.5±7.6) were enrolled in this study, including 99 males (89.2%) and 12 females (10.8%) and 25 healthy individuals with no history of bladder disease were included as a control group with 18 male (72%) and 7 female (28%) with mean age of (55.4±6.8). Thirty nine patients with low grade and with mean age of (58.9±6.9 years) and 72 with high grade and with mean age of (60.2±8.3 years).
Smoking information were collected through face-to-face interviewed by trained interviewers and those who had smoked less than an average of one cigarette per day and < 1 year in their lifetime were defined as nonsmokers which and they were 21 (18.9%) patients, while others were considered as smokers and they were 90 (81.1%) patients. Fifty seven (51.0%) were positive for bilharziasis and 54 (49.0%) were negative (Table 1).
Table 1.
Descriptive Statistics of the Studied Parameters
| Total patients | Urine | ||||
|---|---|---|---|---|---|
| N=111 | Low grade BC | High grade BC | P- value | ||
| N=39 | N=72 | ||||
| Age | 59.5±7.6 | 58.9±6.9 | 60.2±8.3 | 0.8 | |
| Sex | Female | 12 (10.8%) | 6 (15.4%) | 6 (8.3%) | 0.03* |
| Male | 99 (89.2%) | 33 (84.6%) | 66 (91.7%) | ||
| Smoking | No | 21 (18.9%) | 9 (23.1%) | 12 (16.7%) | 0.09 |
| Yes | 90 (81.1%) | 30 (76.9%) | 60 (83.3%) | ||
| Bilharziasis | Negative | 54 (49.0%) | 21 (53.8%) | 15 (20.8%) | 0.001** |
| Positive | 57 (51.0%) | 18 (46.2%) | 57 (79.2%) | ||
| Serum creatinine | Normal | 93 (83.8%) | 39 (100.0%) | 54 (75.0%) | 0.001** |
| High | 18 (16.2%) | 0 (0.0%) | 18 (25.0%) | ||
| Clinical staging | T1 | 27 (24.3%) | 21 (53.8%) | 6 (8.3%) | 0.001** |
| T2 | 36 (32.4%) | 3 (7.7%) | 33 (45.8%) | 0.001** | |
| T3 | 30 (27.0%) | 0 (0.0%) | 30 (41.7%) | 0.001** | |
| T4 | 18 (16.2%) | 15 (38.5%) | 3 (4.2%) | 0.001** | |
| Cytology | Negative | 54 (48.6%) | 36 (92.3%) | 18 (25.0%) | 0.001** |
| Positive | 57 (51.4%) | 3 (7.7%) | 54 (75.0%) | ||
| Lymph node | Negative | 51 (45.8%) | 39 (100.0%) | 33 (45.8%) | 0.001** |
| Positive | 60 (54.2%) | 0 (0.0%) | 39 (54.2%) | ||
| Histopathological type of the Tumor | Squamous cell carcinoma | 15 (13.5%) | 0 (0.0%) | 15 (20.8%) | 0.08 |
| Urothelial carcinoma | 96 (86.5%) | 39 (100.0%) | 57 (79.2%) | ||
| Histopathological Stage of the Tumor | Negative | 27 (24.3%) | 21 (53.8%) | 6 (8.3%) | 0.001** |
| Non muscle invasive | 15 (13.5%) | 0 (0.0%) | 15 (20.8%) | 0.01* | |
| Muscle invasive | 48 (43.2%) | 0 (0.0%) | 48 (66.7%) | 0.001** | |
| Superficial BC | 21 (18.9%) | 18 (46.2%) | 3 (4.2%) | 0.001** | |
| Grade | GI | 39 (35.1%) | 36 (92.3%) | 3 (4.2%) | 0.001** |
| GII | 21 (18.9%) | 0 (0.0%) | 21 (29.2%) | 0.001** | |
| GIII | 51 (45.9%) | 3 (7.7%) | 48 (66.7%) | 0.001** | |
Age is represented as Mean ± SD; the data were analyzed by student t test. While Sex, Smoking, Bilharziasis, Serum creatinine, Clinical Staging., Cytology, Lymph node, Histopathological type of the tumor, Stage of Tumor, and Grade were represented as F (%) frequency and percent; the data were analyzed by X2 test. * P value <0.05 is significant, ** P value <0.01 is highly significant.
As regards the demographic data, for urine samples, there was no significant difference between patients with low grade and others with high grade BC in terms of age with (P= 0.8). For sex, there was a statistically significant difference between the low and high grade patients (P=0.03) with male predominance in patients with high grade BC. A significant difference was observed regarding bilharzial infection and serum creatinine level with (P= 0.001).
Regarding patients with T1 and T4 stage, a highly significant difference was observed in low grade patients with (P=0.001) while those with T2, T3 stage, positive urine cytology and positive lymph node, the statistical significant difference was observed in high grade patients with the same (P= 0.001). For patients with GI, a highly significant difference was observed in low grade patients with (P=0.001) while those with GII and GIII, the statistical significant difference was observed in high grade patients with the same (P= 0.001). Individual demographic and clinical data patients are shown in (Table 1).
Expression of the studied miRNAs in urine samples of low and high grades of BC patients
Using miR-39 as reference for miRNA, we determine that the three miRNAs expression levels were significantly upregulated in the urine of BC patients compared to controls and the expression levels increased with tumor grade.
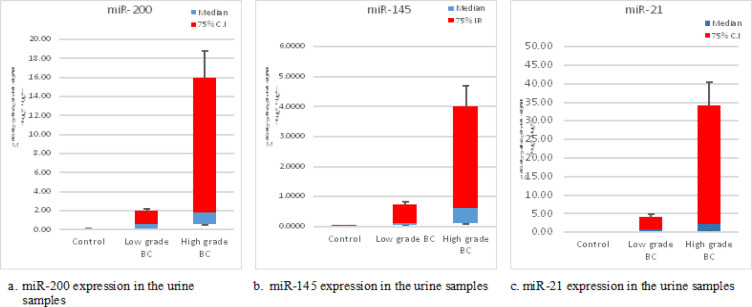
Statistical analysis showed that the expression of miR-200, miR-145 and miR-21 in urine samples of patients with low grade versus (Vs) controls is significant with (P = 0.02, 0.01 and 0.05) respectively, while the expression of the miR-200 and miR-145 in patients with high grade Vs controls is highly significant with (P = 0.001, 0.001) and for miR-21, (P = 0.01), finally, the expression of high grade Vs low grade patients is significant with (P = 0.04, 0.04 and 0.01) respectively (Table 2, Figure 1 a, b, and c).
Table 2.
Urine Expression Level of miR-200, miR-145 and miR-21 among Bladder Cancer Patients and Controls
| Urine | Control N=25 | Low grade BC N=39 | High grade BC N=72 | P. value | ||
|---|---|---|---|---|---|---|
| Low grade & Control |
High grade & Control |
High grade & Low grade |
||||
| miR-200 | 0.034 (0.008 - 0.08) | 0.53 (0.043 - 2.19) | 1.72 (0.49 - 18.35) | 0.02* | 0.001** | 0.04* |
| miR-145 | 0.006 (0.002 - 0.03) | 0.09 (0.013 - 0.92) | 0.59 (0.09 - 4.37) | 0.01* | 0.001** | 0.04* |
| miR-21 | 0.02 (0.02- 0.04) | 0.70 (0.05- 8.74) | 2.21 (0.24- 38.17) | 0.05* | 0.01* | 0.01* |
The fold change results depend on the fold change low: Fold-Change (2-∆∆CT); All parameters are represented as Median with Interquartile range (25% -75%) of the fold change of the studied groups, the data were analyzed by Mann-Whitney U test; * P value <0.05 is significant, ** P value <0.01 is highly significant.
Figure 1.
Box Plot Showed the Median of Urine Expression of the Studied miRNAs
Expression of the studied miRNAs in tissue samples
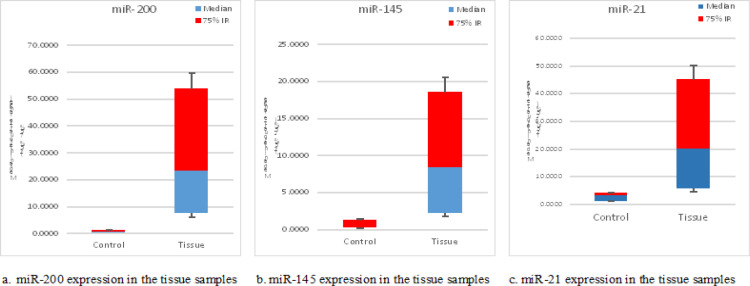
The statistical analysis showed that the expression levels of miR-200, miR-145 and miR-21 in bladder cancer tissue were higher when compared to normal bladder tissue samples with significant (P = 0.002, 0.008 and 0.05) respectively (Table 3, Figure 2 a, b, and c).
Table 3.
Tissue Expression Level of miR-200, miR-145 and miR-21 among Bladder Cancer Patients and Controls
| Tissue | Control N=25 | Tissue N= 111 | P. value |
|---|---|---|---|
| miR-200 | 0.62 (0.26 - 1.70) | 23.43 (6.02 - 59.71) | 0.002** |
| miR-145 | 0.29 (0.18 - 1.31) | 8.40 (1.74 - 21.26) | 0.008* |
| miR-21 | 3.06 (1.05- 4.28) | 19.97 (3.92- 48.50) | 0.05* |
The fold change results depend on the fold change low: Fold-Change (2-∆∆CT); All parameters are represented as Median with Interquartile range (25% -75%) of the fold change of the studied groups, the data were analyzed by Mann-Whitney U test; * P value <0.05 is significant, ** P value <0.01 is highly significant.
Figure 2.
Box Plot Showed the Median of Tissue Expression of the Studied miRNAs
Association analysis between the expression of the studied miRNAs and the clinicopathological factors among BC patients
Regarding the association analysis between the studied miRNAs and the parameters among BC patients. For miR-200, an association was observed between its expression in urine and patients with non-muscle invasive tumor stage with median (25%-75%) C.I of 250.732(7.592- 859.968) and (P = 0.03), while no association was observed between expression level in tissue samples and the studied parameters of BC patients (Table 4).
Table 4.
The Association between miR-200 Level with the Studied Parameters
| miR-200 | Urine N=111 | Tissue N=111 | |||
|---|---|---|---|---|---|
| Median (25% - 75%) C.I |
P- value | Median (25% - 75%) C.I |
P- value | ||
| Serum creatinine | Normal | 0.973 (0.088 - 2.89) | 0.6 | 11.472 (4.563 - 37.014) | 0.08 |
| High | 1.717 (0.634 - 188.74) | 59.922 (26.682 - 80.602) | |||
| Clinical Staging | T1 | 1.214 (0.337- 2.578) | 0.2 | 2.362 (2.362 - 2.362) | 0.1 |
| T2 | 0.656 (0.040 - 191.729) | 40.141 (14.460 - 80.602) | |||
| T3 | 2.215 (0.831 - 20.447) | 10.846 (3.464 - 42.689) | |||
| T4 | 0.287 (0.017 - 1.811) | - | |||
| Cytology | Negative | 1.093 (0.044- 2.704) | 0.3 | 31.246 (2.362 – 35.124) | 0.9 |
| Positive | 1.717 (0.382 - 14.723) | 23.425 (7.702 - 53.775) | |||
| Lymph node | Negative | 1.349 (0.183- 2.863) | 0.6 | 17.449 (4.928- 35.872) | 0.2 |
| Positive | 0.841 (0.056- 4.338) | 59.714 (9.383- 87.427) | |||
| Histopathological type of the Tumor | Squamous cell carcinoma | 1.717 (0.465- 488.609) | 0.7 | 23.425 (15.671- 25.854) | 0.7 |
| Urothelial carcinoma | 1.093 (0.077- 3.352) | 21.959 (4.928- 57.056) | |||
| Histopathological Stage of the Tumor | Negative | 1.214 (0.347- 2.578) | 0.03* | 17.404 (2.362- 21.324) | 0.7 |
| Non muscle invasive | 250.732 (7.592- 859.968) | 23.425 (11.472- 28.324) | |||
| Muscle invasive | 1.285 (0.162- 4.754) | 26.342 (5.657- 66.954) | |||
| Superficial BC | 0.045 (0.023 - 1.485) | - | |||
| Grade | GI | 0.973 (0.043 - 2.578) | 0.4 | 11.472 (11.472 - 11.472) | 0.3 |
| GII | 1.717 (0.382 - 744.434) | 12.527 (1.813 - 32.601) | |||
| GIII | 1.485 (0.275 - 11.534) | 42.425 (12.627 - 80.498) | |||
All parameters are represented as Median with Interquartile range (25% -75%) of the fold change of the studied groups, the data were analyzed by Mann-Whitney U test and multigrade parameters were analyzed by Kruskal-Wallis Test; * P value <0.05 is significant, **P value <0.01 is highly significant.
For miR-145, the clinicopathological factors which were significantly associated with the overall expression in urine samples included, high levels of serum creatinine with median (25%-75%) C.I = 3.530 (0.658- 90.194) and (P = 0.01), positive lymph node with median (25%-75%) C.I = 0.742(0.174- 9.318) and (P = 0.05), Squamous cell carcinoma with median (25%-75%) C.I = 3.811 (1.699- 62.829) and (P = 0.03) and finally a statistical significant association was observed between miR-145 expression in urine samples and patients with GIII with median (25%-75%) C.I = 1.181(0.114 - 8.679) and (P = 0.04). But the expression of miR-145 in tissue samples was significantly associated only with negative lymph node patients with median (25%-75%) C.I = 14.854 (7.794- 109.627) and (P = 0.05) (Table 5).
Table 5.
The Association between miR-145 Level with the Studied Parameters
| miR-145 | Urine N=111 | Tissue N=111 | |||
|---|---|---|---|---|---|
| Median (25% - 75%) C.I | P. value | Median (25% - 75%) C.I | P. value | ||
| Serum creatinine | Normal | 0.155 (0.042 - 1.404) | 0.01* | 10.853 (1.741- 24.084) | 0.8 |
| High | 3.530 (0.658- 90.194) | 5.812 (1.662 - 18.044) | |||
| Clinical Staging | T1 | 0.092 (0.013 - 0.707) | 0.2 | 0.379 (0.379 - 0.379) | 0.3 |
| T2 | 0.962 (0.144 - 3.947) | 9.625 (3.632 - 23.378) | |||
| T3 | 1.724 (0.099 - 38.604) | 8.578 (0.890 - 55.852) | |||
| T4 | 0.168 (0.020 - 1.542) | - | |||
| Cytology | Negative | 0.225 (0.024 - 1.375) | 0.2 | 4.388 (0.379 – 7.231) | 0.4 |
| Positive | 0.429 (0.079 - 4.563) | 10.853 (2.163 - 22.671) | |||
| Lymph node | Negative | 0.124 (0.030- 1.349) | 0.05* | 14.854 (7.794- 109.627) | 0.05* |
| Positive | 0.742 (0.174- 9.318) | 2.585 (1.141- 8.398) | |||
| Histopathological type of the Tumor | Squamous cell carcinoma | 3.811 (1.699- 62.829) | 0.03* | 3.227 (1.141- 5.624) | 0.8 |
| Urothelial carcinoma | 0.247 (0.045- 1.349) | 9.625 (1.952- 19.889) | |||
| Histopathological Stage of the Tumor | Negative | 0.155 (0.049- 1.056) | 0.2 | 69.260 (0.379- 75.234) | 0.7 |
| Non muscle invasive | 2.099 (0.030- 42.228) | 10.853 (6.774- 18.326) | |||
| Muscle invasive | 0.586 (0.116- 11.114) | 5.812 (1.591- 17.150) | |||
| Superficial BC | 0.042 (0.006- 1.133) | - | |||
| Grade | GI | 0.057 (0.013 - 0.707) | 0.04* | 6.774 (6.774 - 6.774) | 0.2 |
| GII | 0.742 (0.105 - 4.563) | 2.906 (0.319 - 12.319) | |||
| GIII | 1.181 (0.114 - 8.679) | 14.854 (4.019 - 108.921) | |||
All parameters are represented as Median with Interquartile range (25% -75%) of the fold change of the studied groups, the data were analyzed by Mann-Whitney U test and multigrade parameters were analyzed by Kruskal-Wallis Test. * P value <0.05 is significant, ** P value <0.01 is highly significant.
No significant association was observed between the expression of miR-21 and the clinicopathological factors of the BC patients neither in urine nor in tissue samples (Table 6).
Table 6.
The Association between miR-21 level with the Studied Parameters
| miR-21 | Urine N=111 | Tissue N=111 | |||
|---|---|---|---|---|---|
| Median (25% - 75%) C.I | P. value | Median (25% - 75%) C.I | P. value | ||
| Serum creatinine | Normal | 1.87 (0.18 - 17.39) | 0.6 | 7.01 (0.12 - 67.18) | 0.1 |
| High | 32.64 (0.21 - 66.49) | 20.33 (11.54 - 38.71) | |||
| Clinical Staging | T1 | 0.28 (0.04- 8.57) | 0.2 | 20.68 (16.00- 41.93) | 0.3 |
| T2 | 1.69 (0.22-15.14) | 10.06 (10.06- 10.06) | |||
| T3 | 9.74 (0.34- 65.47) | - | |||
| T4 | 10.57 (0.29- 80.44) | - | |||
| Cytology | Negative | 2.24 (0.09 - 46.73) | 0.8 | 20.68 (16.00- 41.93) | 0.5 |
| Positive | 1.87 (0.23 - 17.63) | 10.06 (10.06- 10.06) | |||
| Lymph node | Negative | 1.69 (0.19- 17.30) | 0.7 | 20.33 (11.54- 38.71) | - |
| Positive | 2.08 (0.21- 48.45) | - | |||
| Histopathological type of the Tumor | Squamous cell carcinoma | 1.87 (0.26- 66.04) | 0.9 | 20.33 (11.54- 38.71) | - |
| Urothelial carcinoma | 1.87 (0.20- 17.57) | - | |||
| Histopathological Stage of the Tumor | Negative | 0.70 (0.05- 8.57) | 0.2 | 7.01 (0.12- 67.18) | 0.4 |
| Non muscle invasive | 7.67 (1.05- 541.59) | 19.97 (10.06- 29.04) | |||
| Muscle invasive | 1.87 (0.20- 28.41) | - | |||
| Superficial BC | 4.11 (0.08- 75.58) | 41.93 (41.93- 41.93) | |||
| Grade | GI | 0.70 (0.05 - 10.52) | 0.4 | 7.01 (0.12- 67.18) | 0.3 |
| GII | 2.08 (0.28 - 66.26) | 20.68 (16.00- 41.93) | |||
| GIII | 1.66 (0.21 - 36.11) | 10.06 (10.06- 10.06) | |||
All parameters are represented as Median with Interquartile range (25% -75%) of the fold change of the studied groups, the data were analyzed by Mann-Whitney U test and multigrade parameters were analyzed by Kruskal-Wallis Test; * P value <0.05 is significant, ** P value <0.01 is highly significant.
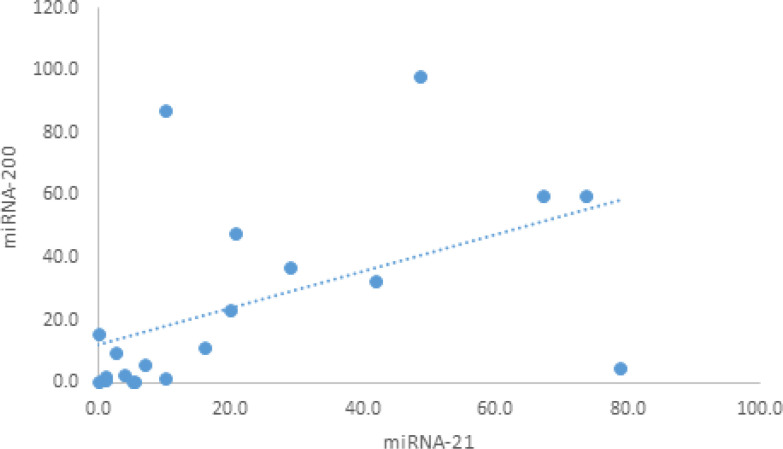
Based on the Pearson correlation analysis to calculate the P value, the statistical study showed a positive correlation between miR-200 and miR-21 in tissue samples with a correlation coefficient of 0.511 and P value of 0.02 while no correlation was observed between the 3 studied miRNAs in urine (Table 7, Figure 3).
Table 7.
Correlation Study
| Urine | Tissue | |||||||
|---|---|---|---|---|---|---|---|---|
| miR-145 | miR-21 | miR-145 | miR-21 | |||||
| r | P | r | P | r | P | r | P | |
| miR-200 | 0.225 | 0.1 | 0.201 | 0.1 | -0.027 | 0.9 | 0.511* | 0.02 |
| miR-145 | 0.039 | 0.8 | 0.05 | 0.8 | ||||
r, Correlation; P, P value; P value calculated depend on Pearson correlation analysis; * P value: Correlation is significant at the 0.05 level (2-tailed); ** P value, Correlation is significant at the 0.01 level (2-tailed).
Figure 3.
The Correlation between miR-200 and miR-21 among the Tissue Samples
Diagnostic performances of miR-200, miR-145 and miR-21 regarding urine samples
Receiver Operating characteristic (ROC) analysis is a useful and fundamental tool for evaluating the performance of diagnostic tests and more generally for evaluating the accuracy of a statistical model.
In our statistical analysis, ROC Curve was established to assess the diagnostic performance of miR-200, miR-145 and miR-21 in urine of BC patients and to evaluate the specificity and sensitivity of BC prediction and also to evaluate their discriminatory properties of the patients and healthy individuals.
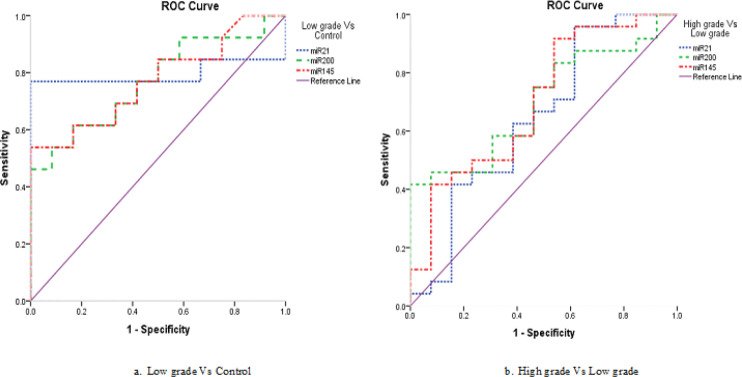
For discrimination of patients with low grade Vs controls, it was found that, miR-200 was at the cut-off value of 0.796, with sensitivity of 46.2% and specificity of 100.0%, with an area under curve (AUC) was 0.769 and accuracy of 72.0% (P = 0.004). While miR-145 was at the cut-off value of 0.090, with sensitivity of 53.8% and specificity of 100.0%, with an AUC was 0.772 and accuracy of 76.0% (P =0.004), and for miR-21 was at the cut-off value of 0.095, with sensitivity of 61.5% and specificity of 100.0%, with an AUC was 0.712 and accuracy of 80.0% (P = 0.08) (Table 8, Figure 4a).
Table 8.
Diagnostic Performances of miR-200, miR-145 and miR-21 in Discrimination the Disease Progression Regarding the Urine Samples
| Urine | Cut-off | Sn. | Sp. | PPV | NPV | Accuracy | AUC | 95% C.I | P. value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||||
| Low grade Vs Control |
miR-200 | 0.796 | 46.20% | 100.00% | 100.00% | 63.20% | 72.00% | 0.769 | 0.584 | 0.955 | 0.004** |
| miR-145 | 0.090 | 53.80% | 100.00% | 100.00% | 66.70% | 76.00% | 0.772 | 0.588 | 0.957 | 0.004** | |
| miR-21 | 0.095 | 61.50% | 100.00% | 100.00% | 70.60% | 80.00% | 0.712 | 0.470 | 0.953 | 0.08 | |
| High grade Vs Low grade |
miR-200 | 2.789 | 41.70% | 100.00% | 100.00% | 48.10% | 62.20% | 0.699 | 0.530 | 0.867 | 0.02* |
| miR-145 | 0.042 | 91.70% | 46.20% | 75.90% | 75.00% | 75.70% | 0.702 | 0.520 | 0.884 | 0.03* | |
| miR-21 | 0.083 | 95.80% | 38.50% | 74.20% | 83.30% | 75.70% | 0.647 | 0.444 | 0.851 | 0.2 | |
Sn, Sensitivity; Sp, Specificity; PPV, Positive predictive value; NPV, Negative predictive value; AUC, Area under curve; C.I, 95% Confidence Interval; * P value <0.05 is significant, ** P value <0.01 is highly significant.
Figure 4.
ROC Curve of the Studied miRNAs in the Urine Samples Regarding Tumor Grades
For discrimination of patients with high grade Vs others with low grade, it was found that, miR-200 was at the cut-off value of 2.789, with sensitivity of 41.7% and specificity of 100.0%, with AUC was 0.699 and accuracy of 62.2% (P = 0.02). While miR-145 was at the cut-off value of 0.042, with sensitivity of 91.7% and specificity of 46.2%, with an AUC was 0.702 and accuracy of 75.7% (P =0.03), and miR-21 was at the cut-off value of 0.083, with sensitivity of 95.8% and specificity of 38.5%, with an AUC was 0.647 and accuracy of 75.7% (P = 0.2) (Table 8, Figure 4b).
Diagnostic performances of miR-200, miR-145 and miR-21 regarding urine and tissue samples
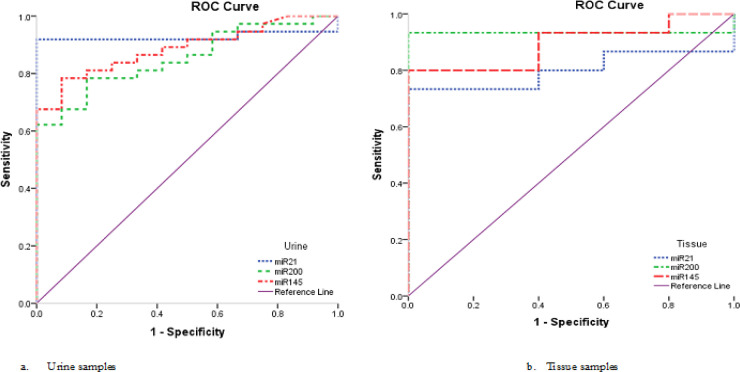
In urine samples, the diagnostic performance for miR-200 was found at cut off value of 0.796, with sensitivity of 62.2% and specificity of 100.0% with an AUC of 0.854 (P < 0.0001, 95% C.I: 0.750 - 0.957) and accuracy 71.4%, for miR-145, it was at cut-off value of 0.049, with sensitivity of 78.4% and specificity of 91.7% with an AUC of 0.886 (P < 0.0001, 95% C.I: 0.798 - 0.975) and accuracy 81.6% and finally for miR-21 was at cut-off value of 0.095, with sensitivity of 83.8% and specificity of 100.0% with an AUC of 0.890 (P < 0.0001, 95% C.I: 0.794 - 0.985) and accuracy 87.8% (Table 9, Figure 5a).
Table 9.
Diagnostic Performances of miR-200, miR-145 and miR-21 in Discrimination the Disease Progression
| Cut-off | Sn. | Sp. | PPV | NPV | Accuracy | AUC | 95% C.I | P- value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||||
| Urine | miR-200 | 0.796 | 62.20% | 100.00% | 100.00% | 46.20% | 71.40% | 0.854 | 0.75 | 0.957 | < 0.0001** |
| miR-145 | 0.049 | 78.40% | 91.70% | 96.70% | 57.90% | 81.60% | 0.886 | 0.798 | 0.975 | < 0.0001** | |
| miR-21 | 0.095 | 83.80% | 100.00% | 100.00% | 66.70% | 87.80% | 0.89 | 0.794 | 0.985 | < 0.0001** | |
| Tissue | miR-200 | 2.071 | 93.30% | 100.00% | 100.00% | 83.30% | 95.00% | 0.933 | 0.803 | 1 | < 0.0001** |
| miR-145 | 1.385 | 80.00% | 100.00% | 100.00% | 62.50% | 85.00% | 0.893 | 0.759 | 1 | < 0.0001** | |
| miR-21 | 4.28 | 73.30% | 80.00% | 75.00% | 54.50% | 84.00% | 0.8 | 0.608 | 0.992 | 0.05* | |
Sn, Sensitivity; Sp, Specificity; PPV, Positive predictive value; NPV, Negative predictive value; AUC Area under curve and C.I, 95% Confidence Interval; * P value <0.05 is significant, ** P value <0.01 is highly significant.
Figure 5.
ROC Curve of the Studied miRNAs in Urine and Tissue Samples
In tissue samples, The diagnostic performance for miR-200 was at cut off value of 2.071, with sensitivity of 93.3% and specificity of 100.0% with an AUC of 0.933 (P < 0.0001, 95% C.I: 0.803 – 1,000) and accuracy 95.0%, while the diagnostic performance for miR-145 showed that it was at cut-off value of 1.385, with sensitivity of 80.0% and specificity of 100.0% with an AUC of 0.893 (P < 0.0001, 95% C.I: 0.759 – 1,000) and accuracy 85.0%, and for miR-21, it was at cut-off value of 4.28, with sensitivity of 73.3% and specificity of 80.0% with an AUC of 0.800 (P = 0.05, 95% C.I: 0.608 – 0.992) and accuracy 84.0% (Table 9, Figure 5b).
The above data indicate that the 3 studied miRNAs may be used as diagnostic biomarkers and could differentiate between urine of BC patients and that of controls and also could differentiate between BC tissue and normal bladder tissue. But only miR-200 and miR-145 which could differentiate between urine of low grade BC patients and controls and could also differentiate between urine of low and high grade BC patients.
Prognostic performances of miR-200, miR-145 and miR-21in urine and tissue samples
Logistic regression analysis of the 3 studied miRNAs was carried out to evaluate their efficiency to use as a predictor and/or prognostic parameter, the analysis revealed no statistical association with BC.
In urine samples, the 3 miRNAs can not be used as prognostic biomarker in low grade patients Vs controls and also in high grade Vs low grade patients (Table 10).
Table 10.
Univariate Analysis of the Studied miRNAs in Urine Samples
| Urine | OR | 95% C.I | P. value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Low grade Vs Control |
miR-200 | 3.823 | 0.736 | 19.804 | 0.1 |
| miR-145 | 4.661 | 0.216 | 35.84 | 0.3 | |
| miR-21 | 3.3 | 1.8 | 9.4 | 0.3 | |
| High grade Vs Low grade |
miR-200 | 1.299 | 0.891 | 1.892 | 0.2 |
| miR-145 | 1.026 | 0.986 | 1.069 | 0.2 | |
| miR-21 | 1.01 | 0.99 | 1.03 | 0.6 | |
OR, Odd Ratio; C.I, Confidence Interval; P value calculated depend on log linear regression analysis; * P value <0.05 is significant, ** P value <0.01 is highly significant.
While the Logistic regression analysis of the 3 miRNAs showed a statistical significance in urine samples. An increase in 1 degree of miR-200, increased the odds by a factor of 11.05 with P value <0.02. For miR-145, an increase in 1 degree of its level increased the odds by a factor of 44.11 with P = 0.04 and for miR-21, the odds was increased by a factor of 11.9 with P = 0.05. This means that the 3 miRNAs may be used as predictor and/or prognostic parameters for BC. While there was no significance for BC prediction concerning tissue samples (Table 11).
Table 11.
Univariate Analysis of the Studied miRNAs in Urine and Tissue Samples
| OR | 95% C.I | P- value | |||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Urine | miR-200 | 11.05 | 1.414 | 86.368 | 0.02* |
| miR-145 | 44.11 | 2.14 | 90.9 | 0.04* | |
| miR-21 | 11.9 | 0.661 | 212.5 | 0.05* | |
| Tissue | miR-200 | 1.42 | 0.864 | 2.337 | 0.2 |
| miR-145 | 1.81 | 0.777 | 4.208 | 0.2 | |
| miR-21 | 1.25 | 0.890 | 1.74 | 0.2 | |
OR, Odd Ratio; C.I, Confidence Interval; P value calculated depend on log linear regression analysis; * P value <0.05 is significant, ** P value <0.01 is highly significant.
Discussion
Bladder cancer in Egypt is still a major problem where patients presented with advanced stage of the disease. Early detection is needed to detect the disease at early stage. Many advances have been made to discover a reliable marker that may detect the disease in its early stages (Samah et al., 2015).
MicroRNAs are class of non-coding RNAs, about 18–22 nucleotides long that may possibly serve as novel non-invasive molecular biomarkers for cancer diagnosis (Jang et al., 2021). They are small endogenous RNAs molecules account for only 1% of the human genome, involved in translation repression by posttranscriptional regulation of gene expression (Yerukala et al., 2022).
Several studies have examined their role in regulation of different biological processes such as: protein synthesis, energy production, apoptosis, and differentiation (Annese et al., 2020). This study focused on three miRNAs; miR-200, miR-145 and miR-21 and aims to estimate their expression profile in urine samples of BC patients with low and high grades, identify their fingerprint, speculating on its potential role as a non invasive prognostic or diagnostic tool to detect urinary BC and differentiation of tumor tissue and to investigate the relationship of miRNA expression with the clinicopathological characteristics of patients.
Firstly, miRNAs were examined in 111 urine and their corresponding tissue samples of BC patients. All the three miRNAs were increased in urine and tissue samples of BC patients compared to normal samples of healthy volunteers. Secondly, the expression results were correlated to the clinicopathological characteristics of patients, for miR-200, the increased expression in BC urine samples was significantly associated with patients with non-muscle invasive tumor stage with P= 0.03. Our results of expression are in agreement with those of Yang et al., (2020) who stated that miR-200a expression was upregulated in both human and mouse invasive BC tissues. Braicu et al., (2019) have compared miRNAs profile between BC tissues and nearby non-cancerous tissues using microarray technique, they have shown up-regulation of 187 miRNAs, among them was miR-200 which validated by qPCR method and their findings support our results.
But, our results of miR-200 expression are in contrast to those of Wang et al., (2012) who reported that the patients with bladder cancer had a lower expression of the miR-200 in the urinary sediment compared to controls. Our results do not agree with those of Yun et al., (2012) who reported that the levels of miR-145 and miR-200a were measured in urine of 207 patients with primary transitional cell carcinoma of the urinary bladder and 144 healthy controls, their findings contradict ours as they reported that the levels of miR-200 and miR-145 were significantly decreased in NMIBC and MIBC patients compared to controls (P<0.001).
Regarding miR-145, the increased expression in BC urine samples was significantly associated with high levels of serum creatinine with (P = 0.01), positive lymph node with (P = 0.05), Squamous cell carcinoma with (P = 0.03) and patients with GIII and (P = 0.04). But the increased expression of miRNA-145 in tissue samples was significantly associated only with negative lymph node patients with (P = 0.05). Our results of expression are considered unconventional and contradict most of the published results.
Zaravinos et al., (2012) have reported down-regulation of miR-145 in BC tissue samples compared with neighboring normal urothelium and their results were supported by Avgeris et al., (2015) who aimed to evaluate the unexplored clinical potential of the urological cancer-related miR-145 in a total of 279 bladder tissue specimens were included in their study (133 BC, 107 adjacent normal and 39 healthy samples), they stated that the expression level of miR-145 was significantly decreased.
Kutwin et al., (2021) collected urine and serum samples from patients with bladder cancer, they assessed the expression of 4 miRNAs (106b-3p, 130b-3, 145 and 199a-5p) using real-time PCR, the statistical analysis was performed with the Mann-Whitney U test which revealed that miRNA-145 was significantly under-expressed in urine (P=0.0111) compared with control group, whereas in serum, they did not find relevant differences between groups (P=0.0903).
Regarding mirR-21, the expression level was significantly upregulated in the urine of BC patients compared to controls and its expression was also significantly higher in tissue of BC patients compared to normal bladder with (P= 0.05). No significant association was observed between the expression of miRNA-21 and the clinicopathological factors of the BC patients neither in urine nor in tissue samples.
Zaravinos et al., (2012) analyzed the expression profile of a group of miRNAs involved in 77 bladder cancer cases and their associated normal samples, regarding the expression of miR-21, they reported that no significant difference was observed between the 2 tissue types. Zhang et al., (2015) presented results unlike Zaravinos’ s et al., (2012) results, they demonstrated that miR 21 expression was increased in BC tissues compared with those in normal bladder tissues, these results are consistent with ours regarding miR-21 expression.
The statistical analysis of the current study concerning the diagnostic performance using ROC curve indicates that miR-200, miR-145 could be used as diagnostic biomarkers to differentiate between urine of low grade BC patients and that of controls and could differentiate between urine of low grade BC patients and high grade, these results are promising because they expressing that the discriminatory properties of miR-200, miR-145 is very powerful and could help patients to detect the disease at early stage.
Univariate logistic regression analysis of the 3 targeted miRNAs was carried out to evaluate their efficiency to use as prognostic biomarkers, the analysis revealed that all of the studied miRNAs were statistically associated with BC, this means that the 3 miRNAs may be used as predictor and/or prognostic parameters for BC.
In conclusion, we have investigated the expression of miR-200, miR-145 and miR-21 in urine and tissues of BC patients, and found that all the three miRNAs were up-regulated in the patient’s urine and tissues, suggesting that miR-200, miR-145 and miR-21 may function as diagnostic and prognostic markers as well a possible therapeutic targets for treatment of BC.
Author Contribution Statement
All authors contributed equally in this study.
Acknowledgements
None.
References
- AkinY , Hacer I, Ebru A, Sevda M. Real-time PCR for gene expression analysis in’ polymerase chain reaction’ Eds Hernandez P and Gomez A. In Tech J. 2012;Chapter 12: 229–54. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures. Atlanta, Ga: American Cancer Society; 2022. [Google Scholar]
- Annese T, Tamma R, De Giorgis Mand Ribatti D. microRNAs Biogenesis, Functions and Role in Tumor Angiogenesis. Front Oncol. 2020;10:581007. doi: 10.3389/fonc.2020.581007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgeris M, Mavridis K, Tokas T, et al. Uncovering the clinical utility of miR-143, miR-145 and miR-224 for predicting the survival of bladder cancer patients following treatment. Carcinogenesis. 2015;36:528–37. doi: 10.1093/carcin/bgv024. [DOI] [PubMed] [Google Scholar]
- Braicu C, Buiga R, Cojocneanu R, et al. Connecting the dots between different networks: miRNAs associated with bladder cancer risk and progression. J Exp Clin Cancer Res. 2019;38:1–17. doi: 10.1186/s13046-019-1406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Guo F, Huo B, et al. Expression and clinical significance of the microRNA-200 family in gastric cancer. Oncol Lett. 2015;9:2317–24. doi: 10.3892/ol.2015.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z, Wang L, Li S, et al. MicroRNA as an Important Target for Anticancer Drug Development. Front Pharmacol. 2021;12:736323. doi: 10.3389/fphar.2021.736323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharib AF, Eed EM, Khalifa AS, et al. Value of Serum miRNA-96-5p and miRNA-99a-5p as Diagnostic Biomarkers for Hepatocellular Carcinoma. Int J Gen Med. 2022;15:2427–36. doi: 10.2147/IJGM.S354842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany N, Bahgat A, Youssef O, et al. Circulating miR210 and miR23b in bladder cancer. Urol Sci. 2021;32:64–70. [Google Scholar]
- Jang JY, Kim YS, Kang KN, et al. Multiple microRNAs as biomarkers for early breast cancer diagnosis. Mol Clin Oncol. 2021;14:31. doi: 10.3892/mco.2020.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenike AE, Halushka MK. miR-21: a non-specific biomarker of all maladies. Biomark Res. 2021;9:18. doi: 10.1186/s40364-021-00272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutwin P, Borkowska EM, Bogucka P, Jabłonowski Z. Expression profile of microRNAs (106b-3p, 130b-3, 145-3p, 199a-5p) in urine and serum samples from patients with the diagnosis of bladder cancer. Pol Merkur Lekarski. 2021;49:103–7. [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V. The C elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Mahmoud ES, Fathia ES, Marwa S, Hussein K. Exosomal caveolin-1 as a biomarker of bladder cancer in Egypt. Int J Adv Res. 2022;10:227–35. [Google Scholar]
- Ryou-u T, Takahiro O. Chapter 2 - Small Interfering RNA-Mediated Silencing of the Ribophorin II Gene: Advances in the Treatment of Malignant Breast Cancer, Editor(s): Marco Filice, Jesús Ruiz-Cabello, In Micro and Nano Technologies, Nucleic Acid Nanotheranostics. Elsevier; 2019. pp. 27–41. [Google Scholar]
- Samah M, Ayman M, Ahmed M, et al. Bat-26 is associated with clinical stage and lymph node status in schistosomiasis associated bladder cancer. Am J Biochem. 2015;5:15–21. [Google Scholar]
- Wang G, Chan ES, Kwan BC, et al. Expression of microRNAs in the urine of patients with bladder cancer. Clin Genitourin Cancer. 2012;10:106–13. doi: 10.1016/j.clgc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Yang R, Xu J, Hua X, et al. Overexpressed miR-200a promotes bladder cancer invasion through direct regulating Dicer/miR-16/JNK2/MMP-2 axis. Oncogene. 2020;39:1983–96. doi: 10.1038/s41388-019-1120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerukala Sathipati S, Tsai MJ, Shukla SK, et al. MicroRNA signature for estimating the survival time in patients with bladder urothelial carcinoma. Sci Rep. 2022;12:4141. doi: 10.1038/s41598-022-08082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SJ, Jeong P, Kim W, et al. Cell-free microRNAs in urine as diagnostic and prognostic biomarkers of bladder cancer. Int J Oncol. 2012;41:1871–8. doi: 10.3892/ijo.2012.1622. [DOI] [PubMed] [Google Scholar]
- Zaravinos A, Radojicic J, Lambrou G I, et al. Expression of miRNAs involved in angiogenesis, tumor cell proliferation, tumor suppressor inhibition, epithelial-mesenchymal transition and activation of metastasis in bladder cancer. J Urol. 2012;188:615–23. doi: 10.1016/j.juro.2012.03.122. [DOI] [PubMed] [Google Scholar]
- Zhang HH, Qi F, Cao YH, Zu XB, Chen MF. Expression and clinical significance of microRNA-21, maspin and vascular endothelial growth factor-C in bladder cancer. Oncol Lett. 2015;10:2610–16. doi: 10.3892/ol.2015.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang X, Chang Z, Wu C, Guo H. microRNA 145 modulates migration and invasion of bladder cancer cells by targeting N cadherin. Mol Med Rep. 2018;17:8450–56. doi: 10.3892/mmr.2018.8910. [DOI] [PubMed] [Google Scholar]