Abstract
Background:
Angiosarcoma (AS) of the urinary bladder is a very rare and aggressive malignancy with a dismal outcome.
Case report:
Here, we report a primary epithelioid angiosarcoma (EAS) of the urinary bladder in a forty-nine-year-old male patient who presented with severe hematuria. Cystoscopic examination revealed hemorrhagic ulcerated bladder mucosa but no definite mass lesions. Intractable hematuria raised the initial clinical impression of idiopathic hemorrhagic cystitis. Analysis of the cystoscopic biopsy revealed features of old bilharzial cystitis, markedly atypical epithelioid endothelial cells arranged as primitive anastomosing vascular structures and expressing vascular markers. The diagnosis of EAS was established. The patient developed intractable severe hematuria, and a radical cystoprostatectomy was performed. The patient was started on chemotherapy but suddenly developed widespread distant metastasis (liver, lung, suprarenal glands, and lymph nodes) and succumbed to death two months after the surgery.
Conclusion:
To the best of these authors’ knowledge, we presented the first report of primary EAS arising in a bilharzial bladder. The relevant studies were discussed.
Key Words: Epithelioid, angiosarcoma, bilharzial, urinary bladder
Introduction
It was in 1907 when angiosarcoma (AS) of the urinary bladder was first reported in the English literature (Jungano, 1907). AS is a sporadic tumor representing about 2% of soft tissue sarcomas of the genitourinary tract. It can be primary or secondary. To date, only 6 cases of primary epithelioid angiosarcoma (EAS) of the urinary bladder have been reported in the English literature (Kulaga et al., 2007; Abbasov et al., 2011; Wang et al., 2016; Tynski et al., 2017; Nizam et al., 2018; Panwar et al., 2022 ). AS of the urinary bladder usually affects males in their fifth through seventh decades. It is associated with exposure to polyvinyl chlorides, thorium dioxide, or chemotherapeutic agents and a remote history of pelvic radiation and cigarette smoking (Matoso and Epstein, 2015). Its clinical presentations include hematuria, dysuria, lower abdominal pain, vaginal bleeding, weight loss, or metastasis (Abbasov et al., 2011). The treatment modalities include cystoprostatectomy, radiotherapy, and chemotherapy. At the molecular level, AS overexpress genes involved in the process of angiogenesis, including genes for vascular-specific receptor tyrosine kinases (Antonescu et al., 2009). Here, we presented a fatal case of primary EAS arising in a bilharzial bladder. The relevant literature was reviewed.
Case report
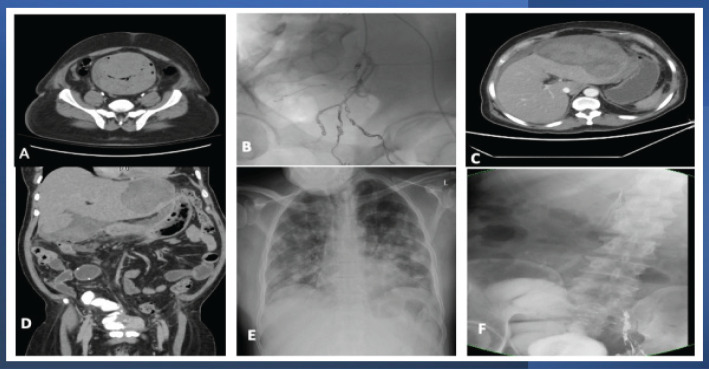
Clinical findings: Forty-nine years old male patient presented to the emergency room with a chief complaint of total, painless hematuria with blood clots and recurrent clot urinary retention of one-month duration. His history was unremarkable, i.e., no history of medical or surgical interventions. His laboratory investigations were unremarkable except for a low Hgb level (5.9 gm/dl) that was dropping continuously even after the blood transfusion. CT scan revealed a urinary bladder markedly distended with blood clots (Figure 1-a) but no definite bladder mass.
Figure 1.
Radiological Findings of the Epithelioid Angiosarcoma of the Urinary Bladder. A), CT scan findings include a marked thickening of the wall of the urinary bladder and distension of its lumen by blood clots; B), Selective Angioembolization of left vesical vessels and angioembolization of the anterior branch of the left internal iliac artery; C-D), CT scan with IV contrast post radical cystoprostatectomy and studor ileal neobladder revealing a huge hepatic subcapsular hematoma; E), Follow-up CXR, CT chest revealed the development of metastasis in the lungs; F), post radical cystoprostatectomy follow up with pouchogram, the placement of IVC filter and angioembolization
Cystoscopy revealed hemorrhagic bladder mucosa and a large urinary bladder hematoma occupying the whole bladder but no bladder mass. The blood clots were evacuated, and transurethral resection (TUR) biopsies were taken. The clinical impression was that of idiopathic hemorrhagic cystitis.
Irrigation and instillation therapy
A 24-Fr triple-way urethral catheter was inserted, and the bladder was continuously irrigated using normal saline for two days without improvement. Alum (aluminum ammonium sulphate, an astringent and vasoconstrictor that precipitate protein) irriga-tion was also used to stop hematuria, but without any improvement. The severe, intractable hematuria was associated with the repeated formation of blood clots obstructing the catheters and causing a marked progressive drop in the Hgb levels. The cystoscopic examination was repeated to evacuate the rapidly accumulating blood clots. The bladder was irrigated with 4% formalin solution (intravesical hemostatic agent) with slight improvement. The cystoscopic biopsy results revealed atypical cells suspicious for sarcomatoid carcinoma arising in a background of schistosomiasis.
Embolization therapy
Repeated angioembolization was performed to control severe hematuria. Initially, we performed embolization of small arterial bleeder and venous malformation of left urinary bladder wall, then selective embolization of left vesical vessels and finally angioembolization of the anterior branch of the left internal iliac artery (Figure 1-b) with temporary improvement in hematuria. Although the patient received 45 units of packed RBCs (+ Fresh frozen plasma units) over 45 days admission period, there was a continuous drop in the Hgb levels. Therefore, radical cystoprostatectomy with ileal neobladder (Studer’s pouch) was performed to control the persistent, life-threatening hematuria, and to remove this suspiciously malignant bladder. The postoperative period was uneventful.
Pathological findings
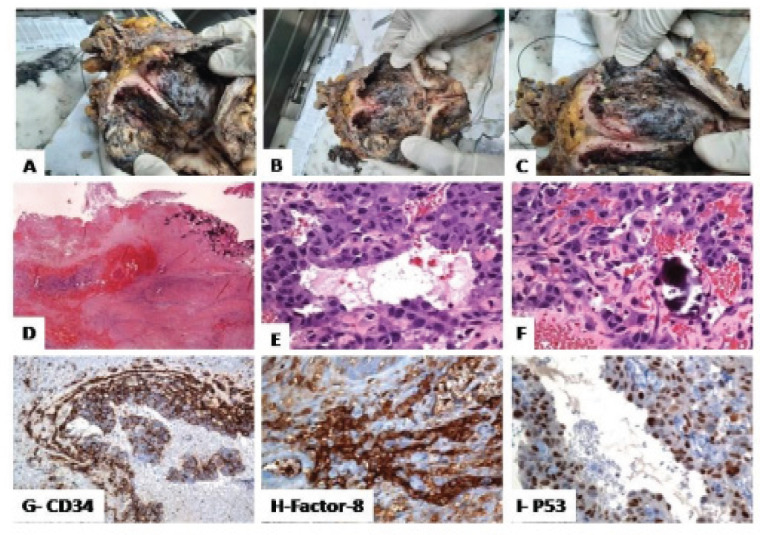
A gross examination of the urinary bladder revealed irregularly ulcerated, hemorrhagic, necrotic diffusely thickened bladder mucosa but no definite mass (Figure 2: a-b-c). The prostate, ureters, urethra, and seminal vesicles were grossly unremarkable. Histologically, there was extensively hemorrhagic and ulcerated urothelium with extravasation of the red blood. Within the lamina propria, there were multifocal areas composed of atypical variable-sized vascular channels (primitive, cystic, and anastomosing vaso-formative structures) lined by highly atypical endothelial cells, extending into the muscularis propria. The atypical cells exhibit nuclear pleomorphism, irregular nuclear membranes, and clumped chromatin. Mitotic figures are frequent. Focally, the atypical cells have an epithelioid appearance with plump vesicular nuclei. Areas with. Numerous calcified Schistosoma eggs are noted (Figure 2: D-E-F)). Further immunohistochemical analysis revealed that the malignant epithelioid cells were reactive for CD31, CD34, factor VIII, FLI1, and p53 (Fig 2 G-H-I). Negative stains included pan-cytokeratin (AE1/AE3), CK5/6, CK34betaE12, CK7, CK20, S100, SMA, Desmin, CD117, Ki-67 proliferation index is high. The overall morphologic and immunophenotypic features are consistent with a diagnosis of “muscle-invasive EAS with extensive schistosomiasis”.
Figure 2.
Histological Findings of the Epithelioid Angiosarcoma of the Urinary Bladder. A-B-C, Urinary bladder with ulcerated, hemorrhagic, necrotic mucosa and numerous calcified bilharzial eggs; D-E-F, Within the lamina propria, there is a vasoformative neoplasm composed of vascular channels lined by atypical endothelial cells (nuclear pleomorphism, and hyperchromatism); G-H-I, The malignant epithelioid cells were positive for CD34, and factor VIII
Clinical outcome and follow-up
On day ten, post-cystectomy, the patient developed tachycardia and a rapid, severe drop in the Hgb level (about 4 grams). He was resuscitated, and a CT scan with IV contrast showed a massive hepatic subcapsular hematoma (Figure 1-C-D) which was successfully managed conservatively (ICU admission, complete bed rest, blood transfusion, and stopping anticoagulation with the placement of IVC filter (Figure 1-F). A Follow-up CT scan revealed the development of multiple metastatic deposits in the liver (with spontaneous bleeding, Figure 1-F), regional and para-aortic lymph nodes, lungs (Figure 1- E), and left suprarenal gland. The patient received supportive medical care and rapidly succumbed to death after one month due to respiratory failure from extensive pulmonary metastasis.
Review of literature
A database search of PubMed, Embase, Google scholar, and Scopus (up to October 2022) to identify and report all studies reported the angiosarcoma of the urinary bladder.
Discussion
To the best of our knowledge, this study reports the first case of primary EAS arising in association with Schistosomiasis of the urinary bladder. AS is a rare and aggressive malignant mesenchymal neoplasm with vascular endothelial differentiation. It develops along the differentiation from mesenchymal stem cell to endothelial cells. AS usually arises from the skin, soft tissues, and rarely from the viscera (lung, liver, bone, spleen, and breast). EAS is a variant of AS, composed primarily of malignant epithelioid endothelial cells (Nizam et al., 2018). In agreement with previous reports, the clinical presentations of the case reported here were non-specific. Our initial clinical impression was hemorrhagic cystitis was based on the presence of an intractable bladder hemorrhage with no definite urinary bladder mass. This diagnosis was questioned for two reasons. The presence of diffusely hemorrhagic ulcerated bladder mucosa which is unusual in hemorrhagic cystitis where the mucosa usually shows edema and multiple punctate hemorrhagic spots rather than diffuse hemorrhage. Also, the patient was not responding to therapeutic modalities of hemorrhagic cystitis (clot evacuation, irrigations, instillation and emobilization therapies) (deVries and Freiha, 1990).
We analyzed the previously published 40 cases of AS of the urinary bladder (Tables, 1, 2 and 3) and found several observations. The AS of the urinary bladder usually affects males with male: female ratio of 5:1. Several patients (55% of cases) had a history of radio-therapy with a median of 9.5 (range from 8 months to 22) years’ time interval to develop AS of the bladder. Some patients (12.5% of cases) had a history of Tobacco smoking or expo-sure to chemicals (5% of cases). The clinical symptoms were non-specific, but hematuria was the main presentation. Cystoscopic examination revealed the presence of mass lesions (92.5% of cases) or ulcers (10% of cases). The most common sites for metastasis were the liver, lungs, and peritoneum, followed by bone, spine, inguinal and para-aortic lymph nodes. Other metastatic sites included the suprarenal glands, and colon. EAS was reported in 7 cases, including the current case. The median survival was 12 weeks (ranging between 5 to 20), while the median survival time for bladder AS was 5 months, ranging between 5 weeks and 6 years.
Table 1.
Epithelioid Angiosarcoma of the Urinary Bladder: Previous Case Reports
| Study | Age/sex | Risk factors | Symptoms | Treatment | Site of metastasis |
Outcome/ weeks |
|---|---|---|---|---|---|---|
| Kulaga et al., 2007 | 83/F | RT/14 years | micro- hematuria | TURB | Peritoneum | DOD/12 |
| Abbasov et al., 2011 | 51/M | NA | Hematuria | RCP | Peritoneum | DOD/5 |
| Wang et al., 2016 | 79/M | RT/6 years | Hematuria | TURB + RCP | NA | DOD/20 |
| Tynski et al., 2017 | 69/M | RT/ 5 years | Ascites | chemotherapy (docetaxel plus gemcitabine) |
Ascites | DOD/6 |
| Nizam et al., 2018 | 57/M | NA | Hematuria, and painful voiding | PC | Rectus abdominis muscle, and bone | DOD/12 |
| Panwar et al., 2022 | 70/M | RT/10 | NA | NA | NA | NA |
RCP, radical cystoprostatectomy; RT, radiotherapy; TURB, transurethral resection of the bladder; NA, not available, PC, Partial cystectomy, DOD, dead of disease.
Table 2.
Angiosarcoma of the Urinary Bladder: Previous Case Reports
| Study | Age/sex | Risk fac-tors | Symptoms | Treatment | Site of me-tastasis | Outcome/ months |
|---|---|---|---|---|---|---|
| Jungano et al., 1907 | 54 /M | NA | Hematuria | TURB | NA | NA |
| Casal et al., 1970 | 85/F | CHEMO | Dysuria, hematuria | PC | NA | Died,/MI after 3 days |
| Schwartz et al., 1983 | 46/M | chemi-cals | hematuria | CHEMO | Colon, lung, brain, and scrotum | DOD/23 |
| Stroup and Chang, 1987 | 68/M | smooking | Hematuria | PC and RCP | Liver and lung | DOD/ 8 |
| Morgan et al., 1989 | 72/F | RT | Hematuria/ vaginal bleeding |
Doxorubicin | NA | DOD/ 7 |
| Aragona et al., 1991 | 78/M | smoking | Dysuria, hematuria | Diverticulectomy | NA | DOD/ 2 |
| Ravi, 1993 | 55/M | smoking | Hematuria | PC, and RT | NA | Alive/8 |
| Ravi, 1993 | 78/M | RT | Hematuria | RCP | NA | Alive/30 |
| Engel et al., 1998 | 47/M | smoking | Hematuria | RCP, CHEMO, RT | Groin LNs | Died 6 years/MI |
| Schindler et al., 1999 | 47/M | NA | Dysuria, hematuria | RCP | RT inguinal LN | NA |
| Seethala et al., 2006 | 66/M | RT | Hematuria | RCP,CHEMO | peritoneum | Alive/19 |
| Williams et al., 2008 | 71/M | RT | Hematuria | RCP, CHEMO, RT | Metastases | DOD/3 |
| Warne et al., 2011 | 32/F | NA | Hematuria/ pain | TURB, CHEMO, RT | Lung | DOD/19 |
| Beyazal et al., 2014 | 20/M | NA | Hematuria | PC and RT | NA | Alive/12 |
| Bahouth et at., 2015 | 89/M | RT | Hematuria | TURB and RT | Spinal | DOD/ 3 |
| Ojerholm et al., 2015 | 61/M | RT | Hematuria | RCP | NA | Alive/4 |
| Gerbaud et al., 2017 | 72/M | Smoking, chemical | Hematuria | RT, radical pel-vectomy |
Liver, lung, peritoneoum |
DOD/5 |
RCP, radical cystoprostatectomy; RT, radiotherapy; TURB, transurethral resection of the bladder; NA, not available, PC, Partial cystectomy, DOD, dead of disease.
Table 3.
Angiosarcoma of the Urinary Bladder: Previous Case Reports
| Study | Age/sex | Risk factors | Symptoms | Treatment | Site of metastasis | Outcome/ months |
|---|---|---|---|---|---|---|
| Tavora et al., 2008 | 73/F | RT/ 17 months | Hematuria | RCP | NA | DOD/ 2 |
| Tavora et al., 2008 | 77/M | NA | Hematuria | TUR biopsy | NA | DOD/5 |
| Tavora et al., 2008 | 71/M | RT/ 8 months | Hematuria | TUR biopsy | NA | DOD/4 |
| Tavora et al., 2008 | 63/F | NA | Hematuria | TUR biopsy | NA | DOD/ 3 |
| Matoso and Epstein, 2015 | 73/F | RT/10 years | Hematuria | TURB, PC | Lung and bone | DOD/ 6 |
| Matoso and Epstein, 2015 | 77/M | RT/ 9 years | Hematuria | TURB | NA | DOD/14 |
| Matoso and Epstein, 2015 | 71/M | RT/ 10 years | Hematuria | TURB, RCP | NA | DOD/ 7 |
| Matoso and Epstein, 2015 | 85/M | RT/ 15 years | Hematuria | TURB | NA | DOD/ 6 |
| Matoso and Epstein, 2015 | 39/M | NA | Hematuria | TURB and RCP | NA | DOD/ 13 |
| Matoso and Epstein, 2015 | 64/M | RT/ 6 years | Hematuria | TURB, RCP | NA | Alive/12 |
| Matoso and Epstein, 2015 | 43/M | NA | Hematuria | TURB | NA | Alive/6 |
| Matoso and Epstein, 2015 | 73/M | NA | Hematuria | TURB | NA | DOD/ 3 |
| Matoso and Epstein, 2015 | 64/M | RT/ 15 years | Hematuria | TURB | NA | Alive/ 3 |
| Navon et al., 1997 | 78/M | RT/ 13 years | Hematuria | RCP | NA | Alive/30 |
| Rallabandi et al., 2016 | 65/F | RT/ 22 years | Hematuria | TURB | NA | NA |
| Cito et., 2021 | 78/M | RT/ 8 years | Incidental finding | TURB, RCP | NA | Early post-operative death(sepsis) |
| Gupta and Erickson, 2022 | 70/M | RT | Hematuria | NA | NA | NA |
RCP, radical cystoprostatectomy; RT, radiotherapy; TURB, transurethral resection of the bladder; NA, not available, PC, Partial cystectomy, DOD, dead of disease.
The patient with EAS reported here had a long history of urinary schistosomiasis. This association between EAS and bladder schistosomiasis may be similar to the development of EAS of the liver in a background of Schistosomiasis previously reported by other studies (Pimentel and Menezes, 1977; El-Zayadi, 2004). Some authorities indicated relationship between the toxic effects of K antimony tartrate (tartar-emetic is a heavy metal used for treatment of bilharziasis) and the development of hepatic AS (El-Zayadi, 2004). Moreover, the exposure to copper sulfate (a spray used in canals to combat the snail) has been proposed to contribute to the development of hepatic AS (Pimentel and Menezes, 1977). The pathogenesis of AS is poorly understood. The development of widespread distant metastasis in our case may be reasoned to alterations in the angiogenic pathways involved in the development of this aggressive tumor such as p16 pathway, RAS/RAF/MEK/Erk -pathway, and PI3K/AKT/mTOR-pathway (Weidema et al., 2019).
To conclude, EAS of the urinary bladder has a nonspecific clinical presentation. Therefore, its diagnosis is challenging and can be easily missed. Its diagnosis is established based on the constellation of the clinical, cystoscopic examination and the results of the immunohistological studies.
Author Contribution Statement
AE, MH, OS, AA, OS, MA, SS, AE, SA, NA, , MA, MB, AA are the authors who are solely responsible for the design and implementation of the research and analyzed and interpreted the patient data, performing protocol/project development, manuscript writing/editing, and data analysis. Authors read and approved the final manuscript.
Acknowledgements
Funding Statement
No financial support was received by the authors for the research, author-ship and/or publication of this article.
Ethical Declaration
Ethical approval to report our case was obtained from the ethical and moral committee of Armed forced Hospital Southern Region Hospital, KSA (Reg. AFHSRM-REC/2021/urology/487). All methods were carried out following relevant guidelines and regulations (Declaration of Helsinki).
Data Availability
The datasets used during the current study are available from the corresponding author upon request.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Abbasov B, Munguia G, Mazal PR, et al. Epithelioid angiosarcoma of the bladder: report of a new case with immunohistochemical profile and review of the literature. Pathology. 2011;43:290–3. doi: 10.1097/PAT.0b013e328344e2fb. [DOI] [PubMed] [Google Scholar]
- Antonescu CR, Yoshida A, Guo T, et al. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res. 2009;69:7175–9. doi: 10.1158/0008-5472.CAN-09-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona F, Ostardo E, Prayer-Galetti T, Piazza R, Capitanio G. Angiosarcoma of the bladder: a case report with regard to histologic and immunohistochemical findings. Eur Urol. 1991;20:161–3. doi: 10.1159/000471688. [DOI] [PubMed] [Google Scholar]
- Bahouth Z, Masarwa I, Halachmi S, Nativ O. Primary angiosarcoma of urinary bladder: 13th reported patient. Case Rep Oncol Med. 2015;2015:652870. doi: 10.1155/2015/652870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyazal M, Pirincci N, Yavuz A, Ozkacmaz S, Bulut G. Computed tomography and magnetic resonance imaging findings of primary bladder angiosarcoma: a case report. Clin Imaging. 2014;38:212–4. doi: 10.1016/j.clinimag.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Casal J, Singer ED, Monserrat JM. Angiosarcoma of the bladder. Rev Argent Urol Nefrol. 1970;39:53–5. [PubMed] [Google Scholar]
- Cito G, Santi R, Gemma L, et al. Angiosarcoma of the Urinary Bladder Following Radiotherapy: Report of a Case and Review of the Literature. Medicina (Kaunas) 2021;57:329. doi: 10.3390/medicina57040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries CR, Freiha FS. Hemorrhagic cystitis: a review. J Urol. 1990;143:1–9. doi: 10.1016/s0022-5347(17)39848-8. [DOI] [PubMed] [Google Scholar]
- Pimentel JC, Menezes AP. Liver disease in vineyard sprayers. Gastroenterology. 1977;72:275–83. [PubMed] [Google Scholar]
- El-Zayadi AR. Curse of schistosomiasis on Egyptian liver. World J Gastroenterol. 2004;10:1079–81. doi: 10.3748/wjg.v10.i8.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel JD, Kuzel TM, Moceanu MC, Oefelein MG, Schaeffer AJ. Angiosarcoma of the bladder: a review. Urology. 1998;52:778–84. doi: 10.1016/s0090-4295(98)00286-6. [DOI] [PubMed] [Google Scholar]
- Gerbaud F, Ingels A, Ferlicot S, Irani J. Angiosarcoma of the Bladder: Review of the Literature and Discussion About a Clinical Case. Urol Case Rep. 2017;13:97–100. doi: 10.1016/j.eucr.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Erickson LA. Postirradiation Angiosarcoma of the Urinary Bladder. Mayo Clinic Proceedings. 2022;97:1406–8. doi: 10.1016/j.mayocp.2022.05.019. [DOI] [PubMed] [Google Scholar]
- Jungano F. Sur un cas d’angiosarcome de la vessie, Ann. Mal. Organes Genitourinaires. 1907;25:1451–64. [Google Scholar]
- Kulaga A, Yilmaz A, Wilkin RP, Trpkov K. Epithelioid angiosarcoma of the bladder after irradiation for endometrioid adenocarcinoma. Virchows Arch. 2007;450:245–6. doi: 10.1007/s00428-006-0336-9. [DOI] [PubMed] [Google Scholar]
- Matoso A, Epstein JI. Epithelioid Angiosarcoma of the Bladder: A Series of 9 Cases. Am J Surg Pathol. 2015;39:1377–82. doi: 10.1097/PAS.0000000000000444. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Moutos DM, Pippitt CH, et al. Vaginal and bladder angiosarcoma after therapeutic irradiation. South Med J. 1989;82:1434–6. doi: 10.1097/00007611-198911000-00025. [DOI] [PubMed] [Google Scholar]
- Navon J.D, Rahimzadeh M, Wong AK, Carpenter PM, Ahlering TE. Angiosarcoma of the bladder after therapeutic irradiation for prostate cancer. J Urol. 1997;157:1359–60. [PubMed] [Google Scholar]
- Nizam A, Paquette EL, Wang BG, Aragon-Ching JB. Epithelioid Angiosarcoma of the Bladder: A Case Report and Review of the Literature. Clin Genitourin Cancer. 2018;16:1091–e5. doi: 10.1016/j.clgc.2018.07.010. [DOI] [PubMed] [Google Scholar]
- Ojerholm E, Stripp D, Mamtani R, Van Arsdalen K, Tripp P. Angiosarcoma of the bladder following prostate radiotherapy. Am J Med. 2015;128:e11–2. doi: 10.1016/j.amjmed.2014.11.022. [DOI] [PubMed] [Google Scholar]
- Panwar V, Tintle SJ, Koorse GS, Koduru P, Jia L. MYC Amplification in Epithelioid Angiosarcoma of the Urinary Bladder and Prostate Following Prostate Radiotherapy: A Case Report with a Novel Molecular Alteration. Int J Surg Pathol. 2022;2022:10668969221081740. doi: 10.1177/10668969221081740. [DOI] [PubMed] [Google Scholar]
- Rallabandi HB, Swain M, Gowrishankar S, Sinha S. Postradiation angiosarcoma of bladder with extensive osseous metaplasia. Indian J Pathol Microbiol. 2016;59:78–80. doi: 10.4103/0377-4929.178234. [DOI] [PubMed] [Google Scholar]
- Ravi R. Primary angiosarcoma of the urinary bladder. Arch Esp Urol. 1993;46:351–3. [PubMed] [Google Scholar]
- Schindler S, De Frias DV, Yu GH. Primary angiosarcoma of the bladder: cytomorphology and differential diagnosis. Cytopathology. 1999;10:137–43. doi: 10.1046/j.1365-2303.1999.00119.x. [DOI] [PubMed] [Google Scholar]
- Schwartz RA, Kardashian JF, McNutt NS, et al. Cutaneous angiosarcoma resembling anaplastic Kaposi’s sarcoma in a homosexual man. Cancer. 1983;51:721–6. doi: 10.1002/1097-0142(19830215)51:4<721::aid-cncr2820510428>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Seethala RR, Gomez JA, Vakar-Lopez F. Primary angiosarcoma of the bladder. Arch Pathol Lab Med. 2006;130:1543–7. doi: 10.5858/2006-130-1543-PAOTB. [DOI] [PubMed] [Google Scholar]
- Stroup RM, Chang YC. Angiosarcoma of the bladder: a case report. J Urol. 1987;137:984–5. doi: 10.1016/s0022-5347(17)44323-0. [DOI] [PubMed] [Google Scholar]
- Tavora F, Montgomery E, Epstein JI. A series of vascular tumors and tumorlike lesions of the bladder. Am J Surg Pathol. 2008;32:1213–9. doi: 10.1097/PAS.0b013e31816293c5. [DOI] [PubMed] [Google Scholar]
- Tynski Z, Barrett AJ, Bastacky SI. Primary urinary bladder angiosarcoma with ascites. Hum Pathol Case Rep. 2017;10:5–9. [Google Scholar]
- Wang G, Black PC, Skinnider BF, Hayes MM, Jones EC. Post-radiation epithelioid angiosarcoma of the urinary bladder and prostate. Can Urol Assoc J. 2016;10:197–200. doi: 10.5489/cuaj.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne RR, Ong JS, Snowball B, Vivian JB. Primary angiosarcoma of the bladder in a young female. BMJ Case Rep. 2011;2011:bcr1120103484. doi: 10.1136/bcr.11.2010.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: A challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019;138:120–31. doi: 10.1016/j.critrevonc.2019.04.010. [DOI] [PubMed] [Google Scholar]
- Williams S, Romaguera R, Kava B. Angiosarcoma of the bladder: case report and review of the literature. Sci World J. 2008;8:508–11. doi: 10.1100/tsw.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author upon request.