Abstract
Background:
The literature is inconsistent for the role of neutrophil-to-lymphocyte ratio (NLR) obtained before neoadjuvant therapy (pre-NLR) in predicting pathological response to neoadjuvant chemoradiation (neoCRT) in patients with locally advanced rectal cancer (LARC). In the present cohort study, we explored the predictive role of pre-NLR in this setting.
Methods:
We prospectively included patients with LARC who were candidates for neoCRT at the Shohada-e-Hafte Tir Hospital (Tehran, Iran) between Mar 2018 and Feb 2020. The pre-NLR was obtained through a peripheral blood smear before CRT. We used the AJCC system for evaluating tumor regression grade (TRG). The TRGs were categorized into: response-group 1 (TRG 0-1 vs. 2-3), response-group 2 (TRG 0 vs. 1-3), and response-group 3 (TRG 0-2 vs. 3). We applied receiver operating characteristic (ROC) analysis to assess the predictive value of pre-NLR.
Results:
Of the 86 screened patients with rectal cancer, 30 patients who fulfilled the inclusion criteria were included in the study. In total, 63.3% were responsive, and 23.3% had complete pathologic response. Pre-NLR could not predict the pathologic response in response-group 1 (area under the ROC curve [AUC]: 0.45, 95%CI 0.23-0.66) and response-group 2 (AUC: 0.36, 95%CI 0.13-0.59). Nevertheless, it had a poor predictive value in response-group 3 (AUC: 0.55, CI%95 0.33-0.75) with an optimal NLR cutoff value of 2.94.
Conclusions:
Pre-NLR could not predict the pathological response to neoCRT in our cohort of patients with LARC.
Key Words: Neoadjuvant therapy- neutrophil-to-lymphocyte ratio- pathologic response- rectal cancer
Introduction
Rectal cancer is the seventh most common malignancy and the tenth leading cause of cancer-related death worldwide (Sung et al., 2021). Approximately half of the rectal cancers are diagnosed at the locally advanced stage (Gerard et al., 2006; Siavashpour et al., 2020). Neoadjuvant chemoradiotherapy (neoCRT) is the standard of care in locally advanced rectal cancer (LARC). However, nearly one-half of cases do not respond to neoCRT (Novin et al., 2021; Park et al., 2012). This issue has demanded the march toward exploring the relevant predictive factors. So far, numerous predictive markers have been introduced in this setting, among which systemic inflammatory response markers are of great interest (García-Flórez et al., 2015). Cancer-associated systemic inflammation is considered a key determinant of outcome (Fazilat-Panah et al., 2020). So far, several biomarkers of the systemic inflammatory response to cancer are proposed, such as neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), lymphocyte-monocyte ratio (LMR), and C-reactive protein (CRP) (Ameri et al., 2016; Hashemi-Bahremani et al., 2019; Kumarasamy et al., 2019).
NLR has been suggested as the most potent systemic inflammation marker of survival in patients with cancer, including LARC (Chantharakhit et al., 2020; Zhang et al., 2020). It is the ratio of absolute neutrophil to absolute lymphocyte count in a blood sample. In 2005, Walsh et al. firstly reported the prognostic role of NLR in colorectal cancer (Walsh et al., 2005). Since then, numerous researches have been conducted in this context. Many studies have indicated the predictive value of elevated NLR on poor survival of patients with LARC (Dong et al., 2016; Ke et al., 2020; Ozdemir et al., 2014). And, several others have found the association between NLR taken after neoCRT (henceforth called post-NLR) and pathologic response to CRT (Ishikawa et al., 2020; Jeon et al., 2019). Notwithstanding, the predictive biomarkers before initiation of neoCRT would be of great interest; since they can guide to spare the nonresponders from treatment-related side effects and choose an alternative therapeutic option.
The value of NLR taken before neoCRT (henceforth called pre-NLR) in predicting the pathologic response in LARC is still a matter of debate; several studies assigned a predictive role for it (Braun et al., 2019; Kim et al., 2014; Shin et al., 2016; X Zhang et al., 2019); however, several others did not (Ergen et al., 2021; Ishikawa et al., 2020; Jeon et al., 2019; Lai et al., 2020; Picardo et al., 2016). This discrepancy might originate from the retrospective nature of studies available in the literature with their inherent limitations, such as selection bias and miss to control the effect of confounding factors. So that, studies with a higher level of evidence are required to delineate this association. The present prospective cohort study was therefore designed to clarify the value of pre-NLR in predicting pathologic response to neoCRT in patients with LARC.
Materials and Methods
Study Design and Participants
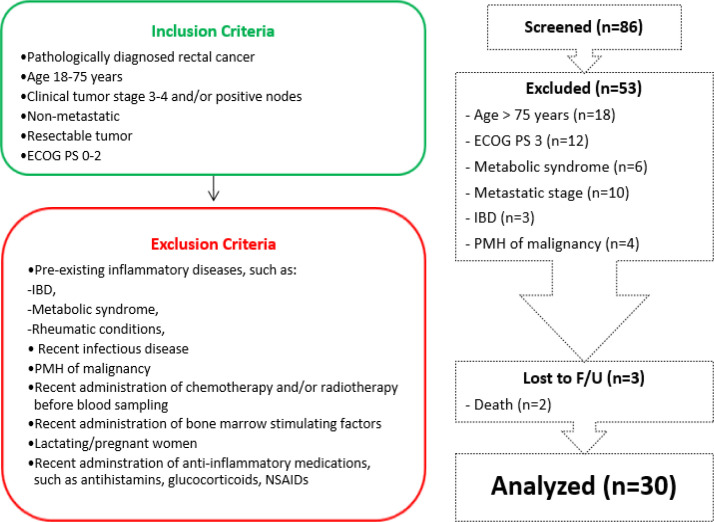
This prospective cohort study included patients with pathologically diagnosed, nonmetastatic, and resectable LARC (defined as T3/T4 or lymph node involvement). The participants were candidates to receive neoCRT before curative rectal surgery at the Shohada-e-Hafte Tir Hospital (Tehran, Iran) between March 21, 2018, and February 20, 2020. Clinical staging was based on complete history taking and physical examination –including digital rectal exam (DRE), colonoscopy, thoracic and abdominal computed tomography (CT) scan, pelvic magnetic resonance imaging (MRI), or endoscopic ultrasound (EUS) per the American Joint Committee on Cancer (AJCC) 8th edition. The inclusion and exclusion criteria are presented in Figure 1. By considering the correlation coefficient (r) between NLR and response rate to be 0.56, type 1 error (zα) 5%, and type 2 error (zβ) 20%, the sample size was calculated as 30, using the following formula:
Figure 1.
The Inclusion and Exclusion Criteria, and Flowchart of Patient Allocation in this Study. ECOG PS, Eastern Cooperative Oncology Group performance status; F/U, follow-up; IBD, inflammatory bowel disease; NSAIDs, nonsteroidal anti-inflammatory drugs; PMH, past medical history
We documented the following data for all subjects before the treatment: age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, body mass index (BMI), histology, tumor location, clinical tumor stage, and blood neutrophils and lymphocytes numbers. The study protocol was approved by the institution IRB, and all participants declared written and verbal informed consent. The ethical approval was provided by the ethical committee of the Iran University of Medical Sciences (IR.IUMS.FMD.REC.1398.231), and the study was conducted per the principles of the Declaration of Helsinki and current ethical guidelines.
Treatment and Assessment
To determine the pretreatment absolute neutrophil and lymphocyte count, at least two slides of peripheral blood smears were obtained from eligible participants before initiating neoCRT. The amounts were averaged for the final NLR. We advised the included patients not to smoke, exercise vigorously, or take anti-inflammatory drugs at least 24 hours prior to blood sampling. If the patient had an acute infection, blood sampling was delayed until complete recovery of symptoms (Higuchi et al., 2016; Neves et al., 2015). In addition, we applied manual cell counting by an expert pathologist –instead of automated cell analyzers– to enhance the accuracy of results (Martín et al., 2021). Following initial tumor staging, patients received neoCRT per long-course protocol (whole pelvis 45 Gy, then boost to the tumor bed with a 2-cm margin to a total prescribed dose of 50.4 Gy). Radiotherapy (RT) was delivered five days per week at a 1.8 Gy daily dose with concurrent oral administration of capecitabine 825 mg/m2 BID on each RT day. Patients were evaluated for toxicities weekly during CRT by physical examination for performance status, vital signs, body weight, and stomatitis and checking complete blood count and liver function tests. Treatment toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 (Freites-Martinez et al., 2021). The concurrent chemotherapy was held until recovery if (i) the absolute neutrophil count was less than 1,500 cells per microliter, (ii) the platelet count was less than 75,000 per microliter, or (iii) patients developed grade 2-3 hand-foot syndrome or grade 2-4 stomatitis, vomiting, or diarrhea Four weeks after CRT, patients were re-evaluated with DRE, flexible sigmoidoscopy, and pelvic MRI. The clinical response was based on Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 (Eisenhauer et al., 2009). Four to six weeks after completion of neoCRT, all patients underwent total mesorectal excision (TME) with sphincter preservation (whenever feasible). An experienced pathologist –who was blinded to the patients’ clinical outcomes– evaluated the tumor response using the four-point tumor regression grade (TRG) introduced in AJCC 8th edition, in the following order: (i) TRG 0: no viable cancer cells (complete response), (ii) TRG 1: single or small groups of tumor cells (moderate response), (iii) TRG 2: residual cancer outgrown by fibrosis (minimal response), and (iv) TRG 3: minimal or no tumor cells killed (poor response)(Weiser, 2018). The same pathologist re-evaluated the specimens to enhance the reliability of the results. For analysis, we categorized the TRG records into three groups: response-group 1 (TRG 0-1 vs. 2-3), response-group 2 (TRG 0 vs. 1-3), and response-group 3 (TRG 0-2 vs. 3).
Statistical analysis
We used IBM SPSS Statistics® (ver.26) for statistical analysis. Categorical variables were summarized as numbers and percentages and were compared using Fisher’s exact test. Continuous variables were summarized using mean and standard deviation, and intergroup values were compared using the Mann-Whitney U test (if non-parametric) or independent t-test (if parametric). Normality was analyzed using the Shapiro-Wilk test. We applied Kruskal-Wallis H test to evaluate the association between pre-NLR and demographic-clinical factors. We employed receiver operating characteristic (ROC) curve analysis (i) to evaluate the predictive value of pre-NLR in tumor response and (ii) to determine the dichotomization thresholds for the response to neoCRT, as described by Youden (Youden, 1950). Then, we applied univariable analysis –using Fisher’s exact test and Mann-Whitney U test (or independent t-test if applicable)– and multivariable logistic regression using backward elimination (Wald test) to identify independent predictors of pathologic response to neoCRT (Heinze et al., 2017). The statistical significance level was set to 0.05, except for including covariates into multivariate analysis that p-value was set to 0.25 to impede miss the possible potential predictive factors (Bursac et al., 2008).
Results
During the study period, 86 patients with rectal cancer were evaluated. Of them, 33 cases who fulfilled the criteria were enrolled (Figure 1). A total of 3 patients were missed for analysis: one patient refused surgery due to significant symptom relief, one patient died of metastasis, and another one died of local tumor progression during neoCRT. The treatment was well-tolerated with few adverse effects, and no grade 4-5 toxicity was reported. The baseline characteristics –including age, sex, clinical stage, location of tumor– are demonstrated in Table 1. The study population had a mean age of 54.8 ± 9.1 years, of which 40% were female. Adenocarcinoma was the only pathology diagnosis that was well- or moderate-differentiated in 86.7%. The tumor stage was clinical T2 in 2 patients (6.7%), T3 in 22 patients (73.3%), and T4 in 6 patients (20%), and nodal involvement was reported in 26 patients (86.7%). Of the total 30 patients, 19 cases (63.3%) were responsive, and 7 cases (23.3%) showed complete pathologic response (pCR).
Table 1.
Baseline Characteristics and Treatment Results of the Study Population
| Characteristics | Total | Response-group 1 a, g | Response-group 2 b, g | Response-group 3 c, g | |||
|---|---|---|---|---|---|---|---|
| (n = 30) | GR | PR | pCR | Non-pCR | Responsive | Non-responsive | |
| (n = 11) | (n = 19) | (n=7) | (n=23) | (n=19) | (n=11) | ||
| Age at diagnosis, mean (SD), years | 54.8 (9.1) | 52.8 (11.1) | 56.1 (7.9) | 52.8 (11.9) | 55.4 (8.4) | 55.1 (10.0) | 54.3 (7.9) |
| ≤ 54 years d, n (%) | 15 (50.0) | 5 (45.5) | 10 (52.6) | 3 (42.9) | 12 (52.2) | 7 (36.9) | 8 (72.7) |
| > 54 years, n (%) | 15 (50.0) | 6 (54.5) | 9 (47.4) | 4 (57.1) | 11 (47.8) | 12 (63.1) | 3 (27.3) |
| Sex, n (%) | |||||||
| Female | 12 (40.0) | 5 (45.5) | 7 (36.8) | 4 (57.1) | 8 (34.8) | 9 (47.4) | 3 (27.3) |
| Male | 18 (60.0) | 6 (54.5) | 12 (63.2) | 3 (42.9) | 15 (65.2) | 10 (52.6) | 8 (72.7) |
| Clinical tumor stage, n (%) | |||||||
| T2 | 2 (6.7) | 1 (9.1) | 1 (5.3) | 0 | 2 (8.7) | 1 (5.3) | 1 (9.1) |
| T3 | 22 (73.3) | 9 (81.8) | 13 (68.4) | 7 (100) | 15 (65.2) | 16 (84.2) | 6 (54.5) |
| T4 | 6 (20.0) | 1 (9.1) | 5 (26.3) | 0 | 6 (26.1) | 2 (10.5) | 4 (36.4) |
| Clinical nodal status, n (%) | |||||||
| Negative | 4 (13.3) | 1 (9.1) | 3 (15.8) | 0 | 4 (17.4) | 2 (10.5) | 2 (18.2) |
| Positive | 26 (86.7) | 10 (90.9) | 16 (84.2) | 7 (100) | 19 (82.6) | 17 (89.5) | 9 (81.8) |
| Distance from AV (cm), mean (SD) | 6.5 (3.6) | 5.7 (2.6) | 7.1 (4.1) | 5.5 (2.8) | 6.9 (3.8) | 6.1 (3.0) | 7.3 (4.6) |
| Upper third | 8 (26.7) | 2 (18.1) | 6 (31.6) | 1 (14.2) | 7 (30.4) | 5 (26.3) | 3 (27.3) |
| Middle third | 12 (40.0) | 5 (45.5) | 7 (36.8) | 3 (42.9) | 9 (39.2) | 8 (42.1) | 4 (36.4) |
| Lower third | 10 (33.3) | 4 (36.4) | 6 (31.6) | 3 (42.9) | 7 (30.4) | 6 (31.6) | 4 (36.4) |
| Pretherapy NLR, mean (SD) | 2.5 (1.2) | 2.7 (1.6) | 2.4 (1.1) | 3.1 (1.8) | 2.4 (1.0) | 2.5 (1.3) | 2.7 (1.2) |
| ≤ 2.24 d | 15 (50.0) | 6 (54.5) | 9 (47.4) | 3 (42.9) | 12 (52.2) | 10 (52.6) | 5 (45.5) |
| > 2.24 | 15 (50.0) | 5 (45.5) | 10 (52.6) | 4 (57.1) | 11 (47.8) | 9 (47.4) | 6 (54.5) |
| ≤ 2.94 e | 21 (70.0) | - | - | - | - | 15 (78.9) | 6 (54.5) |
| > 2.94 | 9 (30.0) | - | - | - | - | 4 (21.1) | 5 (45.5) |
| Clinical response f | |||||||
| Complete response | 7 (23.4) | 7 (63.6) | 0 | 7 (100) | 0 | 7 (36.9) | 0 |
| Partial response | 18 (60.0) | 3 (27.3) | 15 (78.9) | 0 | 18 (78.2) | 10 (52.6) | 8 (72.7) |
| Stable disease | 4 (13.3) | 1 (9.1) | 3 (15.8) | 0 | 4 (17.4) | 2 (10.5) | 2 (18.2) |
| Progressive disease | 1 (3.3) | 0 | 1 (5.3) | 0 | 1 (4.4) | 0 | 1 (9.1) |
Abbreviations: AV, anal verge; GR, good response; NLR, neutrophil-to-lymphocyte ratio; pCR, complete pathologic response; PR, poor response; SD, standard deviation; TRG, tumor regression grade; a, GR (TRG 0-1), PR (TRG 2-3); b, pCR (TRG 0), non-pCR (TRG 1-3); c,. responsive (TRG 0-2), non-responsive (TRG 3); d, The cutoff is the median value; e, The cutoff value is defined based on Receiver operating characteristic (ROC) analysis, which was only significant for response-group 3; f, Based on Response Evaluation Criteria In Solid Tumors (RECIST) 1.1; g, Based on American Joint Committee on Cancer (AJCC) 8th edition
The mean pre-treatment NLR was 2.5 ± 1.2, which was statistically similar in each response-group (response-group 1: 2.7 vs. 2.4, p=0.70; response-group 2: 3.1 vs. 2.4, p=0.66, and response-group 3: 2.5 vs. 2.7, p=0.16) (Table 1). The Fisher’s exact test showed a significant relationship between clinical response to neoCRT and response-groups 1 and 2 (p=0.001, p=0.000, respectively), but, non-significant results for response-group 3 (p=0.08).
Among the evaluated demographic and clinical factors, the elevated pre-NLR tended to be associated with younger age and male gender (p=0.07 and p=0.06, respectively) (Table 2).
Table 2.
The Association between Pretherapy Neutrophil-to-Lymphocyte Ration and Demographic or Clinical Factors (Using Kruskal-Wallis H Test)
| Characteristics | Age | Sex | Tumor stage | Nodal status | Location | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean rank | P | Mean rank | P | Mean rank | P | Mean rank | p | Mean rank | P | ||||||
| NLR | ≤ 54 y | 18.4 | 0.07 | M | 18.1 | 0.06 | T2 | 11 | 0.47 | N+ve | 15.5 | 1 | upper | 14.8 | 0.87 |
| T3 | 15.2 | middle | 15 | ||||||||||||
| > 54 y | 12.6 | F | 12.1 | T4 | 17.1 | N-ve | 15.5 | lower | 16.6 | ||||||
NLR, neutrophil-to-lymphocyte ratio; y, years old.
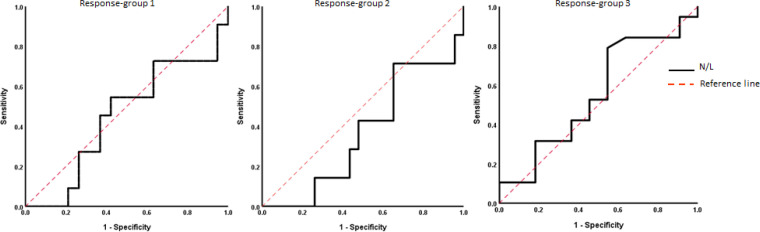
The ROC analysis identified an inability of NLR to predict good response (i.e., TRG 0-1) and pCR (i.e., TRG 0) to neoCRT with area under the ROC curve (AUC)s of 0.45 (95%CI 0.23-0.66) and 0.36 (95%CI 0.13-0.59), respectively. Nevertheless, it had a poor value to discriminate non-responsive group (AUC: 0.55, CI%95 0.33-0.75) with an optimal NLR cutoff value of 2.94 with a sensitivity of 78.9% and specificity of 45.5%. Considering this cutoff value, 71.4% of patients with NLR ≤ 2.94 were responsive to neoCRT compared to 44.4% of patients with more values of NLR. Figure 2 demonstrates the ROC curve plots for the three response-groups.
Figure 2.
Receiver Operating Characteristic (ROC) Curve of Three Response-Groups –using Wilson/Brown method– showing the predictive value of neutrophil-to-lymphocyte ratio for pathologic response to neoCRT in locally advanced rectal cancer. (AUC response-group 1 = 0.450, AUC response-group 2 = 0.366, and AUC response-group 3 = 0.553)
Then, we analyzed the association between clinicopathological profile and pathologic response to neoCRT (Table 3). In response-group 1, the univariable analysis did not demonstrate an association between age, sex, disease stage, tumor site, or pretherapy NLR (pre-NLR) and good pathologic response. In response-groups 2, nodal status was the only significant predictor; therefore, multivariable logistic regression analysis was not practical. In response-group 3, the univariable analysis showed that age, clinical tumor stage, and pre-NLR were associated with the outcome. However, the multivariable analysis did not confirm it.
Table 3.
Univariable and Multivariable Analysis of Response to Neoadjuvant Chemoradiation (Using Binary Logistic Regression)
| Characteristics | Response-group 1 a, e | Response-group 2 b, e | Response-group 3 c, e | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Uni | Multi | Uni | Multi | Uni | Multi | ||||
| P | P | OR | P | P | OR | P | P | OR | |
| Age | 0.7 | - | - | 0.66 | - | - | 0.06 | 0.96 | 1.002 |
| Sex (M vs. F) | 0.64 | - | - | 0.29 | - | - | 0.27 | - | - |
| Clinical tumor stage | 0.31 | - | - | 0.34 | - | - | 0.19 | 1.0g | 0.00g |
| 0.12h | 0.22h | ||||||||
| Clinical nodal status (N+ve vs. N-ve) | 0.74 | - | - | 0.23f | - | - | 0.61 | - | - |
| Distance from AV | 0.48 | - | - | 0.65 | - | - | 0.94 | - | - |
| Pretherapy NLR d | 0.70 | - | - | 0.66 | - | - | 0.16 | 0.82 | 1.08 |
Abbreviations: AV, anal verge; NLR, neutrophil-to-lymphocyte ratio, OR, odds ratio; TRG, tumor regression grade; a, GR (TRG 0-1), PR (TRG 2-3); b, pCR (TRG 0), non-pCR (TRG 1-3); c, responsive (TRG 0-2), non-responsive (TRG 3); d, The cutoff is the median value for response-groups 1 and 2 and based on receiver operating characteristic (ROC) analysis for response-groups 3; e, Based on American Joint Committee on Cancer (AJCC) 8th edition; f, Not entered to multivariable analysis due to single significant variable in the univariable analysis; g, T3 vs. T2; h, T4 vs. T3
Discussion
To our knowledge, this is the first prospective studies evaluating the predictive role of pre-NLR in determining pathologic response to neoCRT in patients with LARC. We primarily demonstrated that pre-NLR –as a biomarker of systemic inflammatory response– could not predict pathologic response to neoCRT in our cohort of patients with locally advanced adenocarcinoma of the rectum. Nevertheless, pre-NLR had a limited value to discriminate nonresponders to neoCRT (AUC 0.55, CI%95 0.33-0.75) with a cutoff value of 2.94. Next, the multivariate logistic regression analysis did not demonstrate the significant effect of age, sex, clinical stage, tumor location, or pre-NLR on the likelihood of pathologic response to neoCRT.
Previous evidence has demonstrated conflicting results regarding the association between pre-NLR and pathologic response to neoCRT in LARC; some studies assigned a predictive role (Braun et al., 2019; Kim et al., 2014; Shin et al., 2016; Zhang et al., 2019); however, others did not (Ergen et al., 2021; Ishikawa et al., 2020; Jeon et al., 2019; Lai et al., 2020; Picardo et al., 2016).
The following studies showed that pre-NLR can predict the response to neoCRT. A small, retrospective cohort noted that a pre-NLR < 2.0 is associated with pCR to neoCRT in patients with LARC (X Zhang et al., 2019). Another small, single-center retrospective study found similar findings, and authors concluded that pre-NLR ≥ 3 is a significant predictor of poor pathologic response (Kim et al., 2014). Likewise, a team in Germany realized that patients with a good pathologic response to neoCRT (Dworak regression grades 3 and 4) have a lower pre-NLR than patients with less pronounced tumor regression (Braun et al., 2019). These findings were in line with a large, single-centered retrospective study, in which pre-NLR ≥ 5 was significantly associated with a lower rate of pCR (Shin et al., 2016).
However, on the other side of the coin, the following retrospective studies drew contrast conclusions. A unicentric survey conducted in South Korea did not find a correlation between pre-NLR and pCR to neoCRT in LARC; however, post-NLR –with a cutoff value of 3.23– was significantly associated with pCR (Jeon et al., 2019). These findings were confirmed in a large, single-center retrospective study conducted in China, in which pre-NLR was not correlated with pCR; however, investigators showed that > 21.5% increase in NLR after CRT is a negative predictor of pathologic response (Lai et al., 2020). A multicentric, retrospective cohort study based in the U.S. and Ireland noted that pre-NLR cannot predict the tumor response (Picardo et al., 2016). Recently, other teams from Turkey and Japan reported similar findings and concluded that pre-NLR is not a predictor for pathologic tumor response (Ergen et al., 2021; Ishikawa et al., 2020).
This inconsistency might originate from the retrospective nature of these studies with its inherent drawbacks that would adversely affect the results (e.g., selection bias, missed confounders, etc.). The prospective design of present study allowed us to consider the following issues to enhance the validity of the results: (i) uniform treatment schedule without induction chemotherapy that would interfere with the immune cell counts, (ii) uniform interval to evaluate the treatment response, (iii) uniform assessment protocol, (iv) exclusion of patients with a history of inflammatory disorders or administration of anti-inflammatory medications, (v) considering the bias effect of smoking and strenuous exercise on blood cell counts, (vi) manual cell counting instead of automated cell analyzers, (vii) re-evaluation of pathology specimens by the same pathologist to impede the inter-observer bias (Lino-Silva et al., 2020), and (viii) applying AJCC-TRG to evaluate the pathologic response that is considered better than other systems (Trakarnsanga et al., 2014). Another strength of our study was the robust and standard follow-up data with limited loss to follow-up.
The present study had several limitations. First, our cohort was limited in sample size and comprised cases with LARC enrolled in a unicentric setting. Increasing sample size by driving a multicentric study would enhance the power of the survey. Second, the enrolled patients represented a selected cohort (i.e., ones who received neoCRT); thus, the findings might not represent all patients diagnosed with LARC (e.g., those who receive induction chemotherapy before neoCRT). Third, several factors –that might interfere with the response to neoCRT– were not analyzed; for example, histology subtype, tumor differentiation, and pretreatment carcinoembryonic antigen (CEA) (Huang et al., 2019; Moureau-Zabotto et al., 2011; Tan et al., 2019). Fourth, in this cohort, we just evaluated the pre-NLR as a systemic inflammatory biomarker. Including other relevant biomarkers (e.g., PLR, LMR, and CRP) can enhance the results. Despite these limitations, compared to the previous retrospective researches, the prospective nature of the current study provides a higher level of evidence that can serve as a basis for future larger-scale prospective cohorts.
In conclusions, despite extensive research on the association between pre-NLR and response to neoCRT in patients with LARC, its role is still unclear. This might originate from the retrospective nature of the previous studies. In summary, the results of our prospective study showed that pre-NLR may not a reliable marker to predict pathologic response to neoCRT in patients with locally advanced adenocarcinoma of the rectum. Larger-scale prospective studies are warranted to confirm this finding.
Author Contribution Statement
Conceptualization: P.F, K.N., Methodology: M.Sh., F.T.H., Software: F.T.H., Validation: P.F., Formal analysis: F.T.H. , Investigation: P.F., M.Sh., Resources: M.Sh., P.F., Data Curation: M.Sh., Writing-original draft: F.T.H., Writing-review & editing: M.Sh., P.F., K.N., Visualization: N.M., M.S., H.Kh., M.B., R.N., S.H., Supervision: P.F., Project administration: P.F., K.N.., Funding acquisition: N/A.
Acknowledgments
Scientific body approval
The study protocol was approved by the Institutional Review Board of Iran University of Medical Sciences
Ethical Approval
In the act provided by Iran University of Medical Sciences, the ethical regulations dictated were approved to review of the medical records for the purposes of our study (ethical code: IR.IUMS.FMD.REC.1398.231).
Availability of data (if apply to your research)
Data are available upon official request from the corresponding author
Conflict of interest
There is no conflict of interest to be declared.
References
- Ameri A, Mortazavi N, Khoshbakht Ahmadi H, et al. Ercc1 expression can predict response to platinum-based induction chemotherapy in head and neck cancer cases. Asian Pac J Cancer Prev. 2016;17:87–91. doi: 10.7314/apjcp.2016.17.s3.87. [DOI] [PubMed] [Google Scholar]
- Braun LH, Baumann D, Zwirner K, et al. Neutrophil-to-Lymphocyte Ratio in Rectal Cancer—Novel Biomarker of Tumor Immunogenicity During Radiotherapy or Confounding Variable? Int J Mol Sci. 2019;20:2448. doi: 10.3390/ijms20102448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:1–8. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantharakhit C, Sujaritvanichpong N. Pretreatment absolute neutrophil-to-lymphocyte ratio (NLR) predict the risk for febrile neutropenia in the first cycle adjuvant chemotherapy for breast cancer. Asian Pac J Cancer Biol. 2020;5:81–7. [Google Scholar]
- Dong Y-w, Shi Y-q, He L-w, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in rectal cancer: a meta-analysis. Onco Targets Ther. 2016;9:3127. doi: 10.2147/OTT.S103031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1). Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Ergen ŞA, Barlas C, Yıldırım C, et al. Prognostic Role of Peripheral Neutrophil-Lymphocyte Ratio (NLR) and Platelet-Lymphocyte Ratio (PLR) in Patients with Rectal Cancer Undergoing Neoadjuvant Chemoradiotherapy. J Gastrointest Cancer. 2021;2021:1–10. doi: 10.1007/s12029-020-00578-7. [DOI] [PubMed] [Google Scholar]
- Fazilat-Panah D, Vakili Ahrari Roudi S, Keramati A, et al. Changes in Cytokeratin 18 during Neoadjuvant Chemotherapy of Breast Cancer: A Prospective Study. Iran J Pathol. 2020;15:117–26. doi: 10.30699/ijp.2020.116238.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5 0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl Ed) 2021;112:90–2. doi: 10.1016/j.ad.2019.05.009. [DOI] [PubMed] [Google Scholar]
- García-Flórez LJ, Gómez-Álvarez G, Frunza AM, et al. Predictive markers of response to neoadjuvant therapy in rectal cancer. J Surg Res. 2015;194:120–6. doi: 10.1016/j.jss.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Gerard J-P, Arefpour A, Ortholan C, et al. Sphincter and rectal preservation approaches for early stage distal rectal cancers. Curr Colorectal Cancer Rep. 2006;2:161–67. [Google Scholar]
- Hashemi-Bahremani M, Mortazavi N, Novin K, et al. Blood neutrophil-to-lymphocyte ratio as a predictor of response to chemotherapy in head-and-neck cancers. J Head Neck Phys Surg. 2019;7:20. [Google Scholar]
- Heinze G, Dunkler D. Five myths about variable selection. Transpl Int. 2017;30:6–10. doi: 10.1111/tri.12895. [DOI] [PubMed] [Google Scholar]
- Higuchi T, Omata F, Tsuchihashi K, et al. Current cigarette smoking is a reversible cause of elevated white blood cell count: Cross-sectional and longitudinal studies. Prev Med Rep. 2016;4:417–22. doi: 10.1016/j.pmedr.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Qin H, Xiao J, et al. Association of tumor differentiation and prognosis in patients with rectal cancer undergoing neoadjuvant chemoradiation therapy. Gastroenterol Rep. 2019;7:283–90. doi: 10.1093/gastro/goy045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa D, Nishi M, Takasu C, et al. The role of neutrophil-to-lymphocyte ratio on the effect of CRT for patients with rectal cancer. In Vivo. 2020;34:863–8. doi: 10.21873/invivo.11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BH, Shin US, Moon SM, et al. Neutrophil to lymphocyte ratio: a predictive marker for treatment outcomes in patients with rectal cancer who underwent neoadjuvant chemoradiation followed by surgery. Ann Coloproctol. 2019;35:100. doi: 10.3393/ac.2018.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke T-M, Lin L-C, Huang C-C, et al. High neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predict poor survival in rectal cancer patients receiving neoadjuvant concurrent chemoradiotherapy. Medicine. 2020:99. doi: 10.1097/MD.0000000000019877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IY, You SH, Kim YW. Neutrophil-lymphocyte ratio predicts pathologic tumor response and survival after preoperative chemoradiation for rectal cancer. BMC Surg. 2014;14:1–7. doi: 10.1186/1471-2482-14-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasamy C, Sabarimurugan S, Madurantakam RM, et al. Prognostic significance of blood inflammatory biomarkers NLR, PLR, and LMR in cancer—A protocol for systematic review and meta-analysis. Medicine. 2019:98. doi: 10.1097/MD.0000000000014834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S, Huang L, Luo S, et al. Systemic inflammatory indices predict tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Oncol Lett. 2020;20:2763–70. doi: 10.3892/ol.2020.11812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lino-Silva LS, Guzmán-López JC, Salazar-García JA, et al. Interobserver Variability in Assessing Pathologic Response to Preoperative Treatment in Rectal Cancer: Standardization of an Evaluation Method and Comparisons Between Published Scales. J Gastrointest Cancer. 2020;51:709–13. doi: 10.1007/s12029-019-00331-9. [DOI] [PubMed] [Google Scholar]
- Moureau-Zabotto L, Farnault B, de Chaisemartin C, et al. Predictive factors of tumor response after neoadjuvant chemoradiation for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2011;80:483–91. doi: 10.1016/j.ijrobp.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Neves PRDS, Tenório TRDS, Lins TA, et al. Acute effects of high-and low-intensity exercise bouts on leukocyte counts. J Exerc Sci Fit. 2015;13:24–8. doi: 10.1016/j.jesf.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novin K, Saneii M, Noori R, et al. Association Between Pathological Complete Response and Tumor Location in Patients with Rectal Cancer After Neoadjuvant Chemoradiotherapy, a Prospective Cohort Study. Int J Cancer Manag. 2021:14. [Google Scholar]
- Ozdemir Y, Akin ML, Sucullu I, et al. Pretreatment neutrophil/lymphocyte ratio as a prognostic aid in colorectal cancer. Asian Pac J Cancer Prev. 2014;15:2647–50. doi: 10.7314/apjcp.2014.15.6.2647. [DOI] [PubMed] [Google Scholar]
- Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picardo SL, Teo M, Abdul Jalil KI, et al. Correlation between platelet/lymphocyte ratio, neutrophil/lymphocyte ratio and response to neoadjuvant chemoradiation therapy in rectal cancer. Am J Clin Oncol. 2016:2016. [Google Scholar]
- Raffone A, Travaglino A, Cerbone M, et al. Diagnostic Accuracy of Immunohistochemistry for Mismatch Repair Proteins as Surrogate of Microsatellite Instability Molecular Testing in Endometrial Cancer. Pathol Oncol Res. 2020;26:1417–27. doi: 10.1007/s12253-020-00811-5. [DOI] [PubMed] [Google Scholar]
- Shin US, You YN, Price BA, et al. Is the neutrophil-lymphocyte ratio (NLR) a predictive and prognostic factor in rectal cancer patients treated with neoadjuvant chemoradiation (nCRT)? Am J Clin Oncol. 2016:2016. [Google Scholar]
- Siavashpour Z, Taghizadeh-Hesary F, Rakhsha A. Recommendations on management of locally advanced rectal cancer during the COVID-19 pandemic: an Iranian Consensus. J Gastrointest Cancer. 2020;2020:1–5. doi: 10.1007/s12029-020-00454-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Tan Y, Fu D, Li D, et al. Predictors and Risk Factors of Pathologic Complete Response Following Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Population-Based Analysis. Front Oncol. 2019:9. doi: 10.3389/fonc.2019.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trakarnsanga A, Gönen M, Shia J, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst. 2014;106:dju248. doi: 10.1093/jnci/dju248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S, Cook E, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–4. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- Weiser MR. AJCC 8th edition: colorectal cancer. Ann Surg Oncol. 2018;25:1454–55. doi: 10.1245/s10434-018-6462-1. [DOI] [PubMed] [Google Scholar]
- Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li J, Peng Q, et al. Association of markers of systemic and local inflammation with prognosis of patients with rectal cancer who received neoadjuvant radiotherapy. Cancer Manag Res. 2019;11:191. doi: 10.2147/CMAR.S187559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu X, Xu M, et al. Prognostic value of pretreatment systemic inflammatory markers in patients with locally advanced rectal cancer following neoadjuvant chemoradiotherapy. Sci Rep. 2020;10:1–11. doi: 10.1038/s41598-020-64684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]