Abstract
Background:
A rare yet severe neoplasia called malignant pleural mesothelioma (MPM) typically manifests itself in advanced stages. Despite some improvements in the treatment of patients with MPM, this malignancy continues to have a detrimental prognosis . The primary goal of the present study was to assess the associatin between ERCC1, RRM1, and thymidylate synthase (TS) expression and disease outcome in patients with malignant pleural mesothelioma (MPM) treated with either pemetrexed plus cisplatin or gemcitabine plus cisplatin.
Methods:
This prospective cohort study was done on ninety-one advanced MPM patients. The patients received either pemetrexed plus platinum (55 of 91) or gemcitabine plus platinum chemotherapy (36 of 91). Tissue biopsy was taken at time of diagnosis. Immunohistochemistry was used to assess the levels of ERCC1, RRM1, and TS transcription in tissue biopsies (paraffin embedded).
Results:
Based on the findings, 70% of patients with low expression of TS had low expression of ERCC1, and 68% of patients with high expression of TS had high expression of ERCC1, suggesting a significat association between ERCC1 expression and TS expression (p=0.005). However, there was no relation between ERCC1 and RRM1 expression patterns (p= 0.296). In patients underwen platinum-based theraphy (n 91), a significant correlation was detected between low ERCC1 median High-scoring and longer progression time (TTP;9.6 v 5.3 months;P< .001) or overall survival (OS) (OS;18.1 v 9.1 months; P<0.001). A significant correlation was found between low TS protein expression and longer time progression (TTP; 11.8 v 5.4 months; P< .001) or OS (OS; 19.8 v 8.5 months; P <0.001) in patients undergoing pemetrexed plus platinum chemotherapy (n 55). Low RRM1 expression was accompanied by high progression free survival (TTP; 10.6 v 3.8 months) and OS (OS; 20.6 v 8.6 months) in patients receiving gemcitabine plus platinum chemotherapy. Based on the multivariate test results, the independent variables significantly affecting OS were tumor stage (HR: 2.3; 95% CI: 1.1-4.9; p= 0.021) and ERCC1 (HR: 2.7; 95% CI: 1.7-4.3; p < 0.001).
Conclusion:
Decreased TS protein expression can be indicative of greater responsivness to pemtrexed and of longer TTP and OS in individuals with advanced MPM (locally progressed or metastatic) who are receiving pemetrexed-based chemotheraphy. Low ERCC1 expressions in individuals with advanced MPM can predict increased PFS and OS, as well as a better responsivness to platinum-based chemotherapy. In patients with progressed MPM receiving gemcitabine plus cisplatin chemotherapy, lower RRM1 expression was associated with a better prognosis, longer PFS, and longer OS.
Key Words: RRM1, ERCC1, immunohistochemistry, biomarkers, mesothelioma
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive tumor characterised with difficulty of diagnosis in early stage, poor prognosis, and no effective treatment. MPM has a growing tendency worldwide (Cihan et al., 2014), and its peak incidence is expected to occur between 2015 and 2030 (Neumann et al., 2013). Apart from cisplatin and the antifolates, most single-agent chemotherapy regimens? demonstrated low mean survival durations and low response rates. On the other hand, combined chemotherapy regimens frequently showed higher efficacy. Pemetrexed, as a a novel multitargeted antifolate, inhibits multiple targets of folic acid metabolic pathway, especially thymidylate synthase (TS) (Shih et al., 1997). Increased baseline TS expression in non-small-cell lung cancer (NSCLC) cell lines can predict poor response to pemetrexed, in a way that TS rate is connected to pemetrexed effectiveness in a range of solid tumours (Gomez et al., 2006).
Excision repair cross-complementation group 1 (ERCC1), as a key element in the nucleotide excision repair (NER) pathway, can eliminate cisplatin-induced DNA adducts (Friedberg 2001). ERCC1 mRNA concentrations and treatment response to cisplatin-based therapy have been demonstrated to be inversely correlated in multiple retrospective studies, in which tumour specimens from ovarian cancer clinical trials (Weberpals et al., 2009), colorectal cancer (Kim et al, 2009), and NSCLC (Olaussen et al., 2006) and (Vilmar et al., 2010) were investigated.
An enzyme called ribonucleotide reductases transforms ribonucleotides triphosphates into deoxyribonucleotides diphosphates, which is necessary for the production of DNA. The holoenzyme of ribonucleotide reductases is composed of a large subunit, namely RRM1, and a small subunit, namely RRM2. The cytoplasmic proteins are present during the entire cell division process (Pontarin et al., 2008).
This study aimed to evaluate the prognostic and predictive significance of ERCC1, RRM1, and TS in patients with advanced mesothelioma (patients with locally advanced and metastatic cancers).
Materials and Methods
Study Population
Ninety-one chemotherapy-naive patients with advanced MPM (either locally advanced or metastatic MPM) were prospectively selected amomg those who were treated at the National Cancer Institute, Cairo, Egypt. All patients signed a written consent form before participating in the study. The ethical committee at national cancer institute approved the study.
The patients were diagnosed with MPM based on clinical data and pathological assessment using immunohistochemistry. They had inoperable tumors. Patients’ clinical and pathological information, such as gender, age, histopathological findings, medical history, and physical examination results, and Karnofsky functioning condition were gathered. Chemotherapy-naive patients with ECOG performance status 0-2 were considred as inclusion citeri. involved in the study.
Eligible patients were classified into 2 groups
First group
Patients received Pemetrexed 500mg/m2 plus cisplatin 75mg/m2 on day1 (n= 55).The treatment cycle was repeated every 21 days. ERCC1 and TS were assessed at the time of diagnosis and treatment management of participants. Treatment responsiveness was assesed after 4 treatment cycles by using chest CT with contrast.
Second group
Patients received Gemcitabine 1000mg/m2 on day1 and day 8 plus cisplatin 75 mg/m2 on day 1 (number of patients 36). The treatment cycle was repeated every 21 days. ERCC1 and RRM1 were studied at the time of diagnosis. If there was a satisfactory treatment response following four cycles, chemotherapy was resumed every 3 weeks for a total of 6 rounds. Dexamethasone prophylaxis, along with vitamins supplements, were given to all patients who received pemetrexed. Folic acid and vitamin B12 supplementations began 7–14 days before the first chemotherapy treatment session. On the day before every chemotherapy dosage, the day of the dosage, and the day following the dosages, dexamethasone (4 mg p.o. twice daily) was given. Folic acid was prescibed for an additional 21 days following the last chemotherapy treatment session.
Majority of patients with regressive diseases underwent surgical resection. Four to eight weeks after the final chemotherapy treatment session, surgery was carried out. The patients with resectable conditions underwent pleurectomy/decortication. PFS was the main outcome. PFS was described as the duration between the intiation of treatment and the first clinically or radiologically obvious sign of progression of the disease, such as a pleural effusion or metastasis. Patients who had no evidence of disease progresion were assessed at the time of the last follow-up . OS served as the secondarily ending point. The time from diagnosis to mortality or the last follow-up was considered as the mean survival rate.
Pathological assessment of the markers
Specific monoclonal mouse antihuman antibodies for ERCC1 (clone 8F1, dilution 1:50, Santa Cruz Biotechnology) and TS were used in immunohistochemistry examinations (clone 106, dilution 1:100, DAKO). The samples were paraffin-embedded and formalin-fixed and then cut into 2-mm-thick cell slices.
Antigen retrievals were carried out for both ERCC1 and TS for thirty minutes at room temperature in W-CAP pH 8.0 buffers (Bio-Optica) in order to improve the immunoreactivity. After blocking endogenous peroxidase activities for thirty minutes using three percent hydrogen peroxide, the antibodies were incubated for an hour at room temperature. The immunoreactions were detected using a biotin-free detecting method (EnVision, Dako Cytomation) based on the activities of the peroxidase enzymes and a dextran chains coupled to the secondarily antibodies while diaminobenzidine (Dako Cytomation) was served as the chromogens.
The samples were fixed, dehydrated, and counterstained with hematoxylins. Two physicians who were blinded to the patients’ identities and clinical outcomes read and evaluated the portions. A method used the staining strength and quantity of cells to analyze the findings. Tumor cells were deemed positively for ERCC1 staining when they had nuclear reactivity, although TS positivity was determined by both nuclear and cytoplasmic reactions.
A semiquantitative histological scoring was used to determine the proportion of tumour cells that were positive with intens staining (H-score). In specifically, the proportion of positive neoplastic cells was multiplied by the stained intensity of the tumor, which ranged from low (score 1) to moderately and higher (scories 2 and 3). (Giving values from 0 to 300). Regarding RRM1, every biopsy sample was sliced into thin (2 m) pieces and placed on glass plates. Deparaffinization of the sections was followed by heat-induced antigen retrieval in the DAKO-PT-link modules using a pH 9 target retrievals solutions for twenty minutes at 97°C.
After blocking endogenous peroxidase, slices were treated with rabbits polyclonal anti-human RRM1 (Proteins Tech Europe) mixed 1:50 at room temperature for twenty minutes. Using EnVision Flex+ kit from DAKO and diaminobenzidine as the chromophore, staining was done. Mostly, cytoplasm marking was seen, but lesser nuclear stains were also present. The bigger vessels’ fibrous connective tissue acted as negative controls, and no stains could be seen there. In order to categorise the specimens into positive (H-scoring upper≥quartiles) and negative (H-scoring upper<quartiles) groups, the upper quartile level of the H-score was predetermined.
Statistical Analysis
IBM SPSS® Statistical edition 22 (IBM® Corp., Armonk, NY, USA) was used for data analysis. Data were presented using means, standard deviations, median, and range. Frequencies and percentages were also used to represent qualitative data. To investigate the relationship between various markers and qualitative factors, chi-squared test or Fisher’s exacting test was applied. Kaplan-Meier methodology was used to do survival analysis, and the log-rank testing was used to compare two survival curves. To investigate variables influencing mortality on a univariate analysis, a Cox-proportional hazards regression modelling was used. For estimating risk, the hazard ratios (HR) and 95% confidence level (CI) were considered. A p-value less than 0.05 was regarded as significant.
Results
The current study was done on 91 patients suffering from mesothelioma. In terms of gender, there were 51 males (56%) and 40 females (44%). The patients’ mean age was 54.4±10.9 years. The patients’sdemographic and clinical characteristics are shown in Table 1.
Table 1.
Relation of ERCC1 Expression with Demographic Characteristics of the Studied Group
| ERCC1 Expression | ||||
|---|---|---|---|---|
| Low | High | p-value | ||
| Age (years) | ≤ 55 | 27 (54.0%) | 23 (46.0%) | 0.974 |
| > 55 | 22 (53.7%) | 19 (46.3%) | ||
| Sex | Male | 25 (49.0%) | 26 (51.0%) | 0.297 |
| Female | 24 (60.0%) | 16 (40.0%) | ||
| Residence | Shobra | 13 (61.9%) | 8 (38.1%) | 0.556 |
| Helwan | 10 (45.5%) | 12 (54.5%) | ||
| Others | 26 (54.2%) | 22 (45.8%) | ||
| Smoking | Non- or Ex-smoker | 30 (58.8%) | 21 (41.2%) | 0.282 |
| Current Smoker | 19 (47.5%) | 21 (52.5%) | ||
| Pathology | Epithelioid | 44 (57.1%) | 33 (42.9%) | 0.139 |
| Sarcomatoid/biphasic | 5 (35.7%) | 9 (64.3%) | ||
| Treatment Protocol | GEM | 20 (55.6%) | 16 (44.4%) | 0.791 |
| Alimta | 29 (52.7%) | 26 (47.3%) | ||
| Response | Regressive | 24 (68.6%) | 11 (31.4%) | 0.019 |
| Progressive | 13 (36.1%) | 23 (63.9%) | ||
| Stable | 12 (60.0%) | 8 (40.0%) | ||
ERCC1 expression was tested in all the patients (n 91). RRM1 expression was tested in patients treated with Gemcitabine + cisplatin (n 36) and TS expression was asssed in those treated with Pemetrexed + cisplatin (n 55).
There was a positive association between ERCC1 expression and TS expression (p=0.005). It was found that 70% of patients with low expression of TS had low expression of ERCC1, and 68% of patients with high expression of TS had high expression of ERCC1. Howver, there was no relation between ERCC1 expression and RRM1 expression (Table 3).
Table 3.
Relation of the Expression of ERCC1 with that of RRM1 and TS
| ERCC1 Expression | ||||
|---|---|---|---|---|
| Low | High | p-value | ||
| RRM1 Expression |
Low | 11 (64.7%) | 6 (35.3%) | 0.296 |
| High | 9 (47.4%) | 10 (52.6%) | ||
| TS Expression |
Low | 21 (70.0%) | 9 (30.0%) | 0.005 |
| High | 8 (32.0%) | 17 (68.0%) | ||
Survival analysis
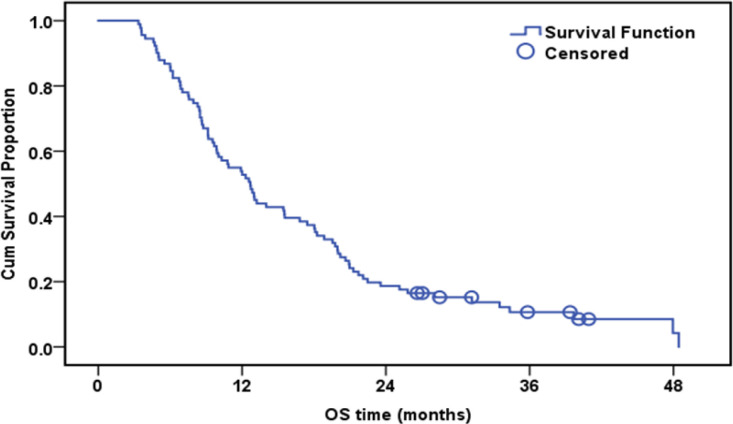
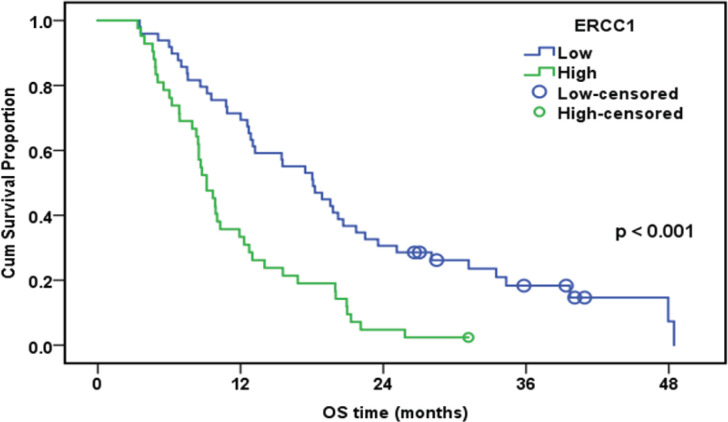
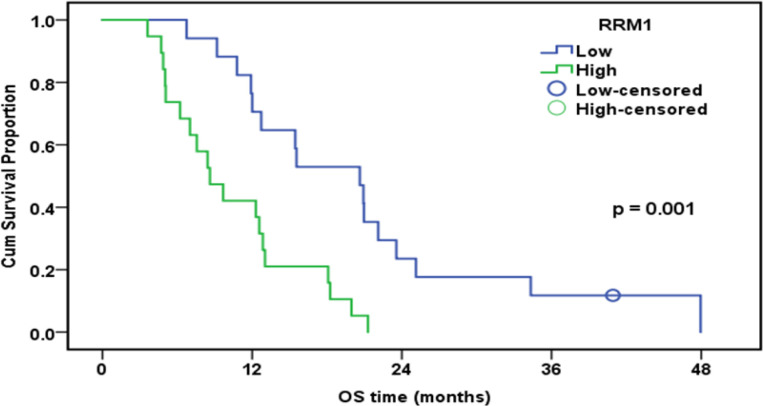
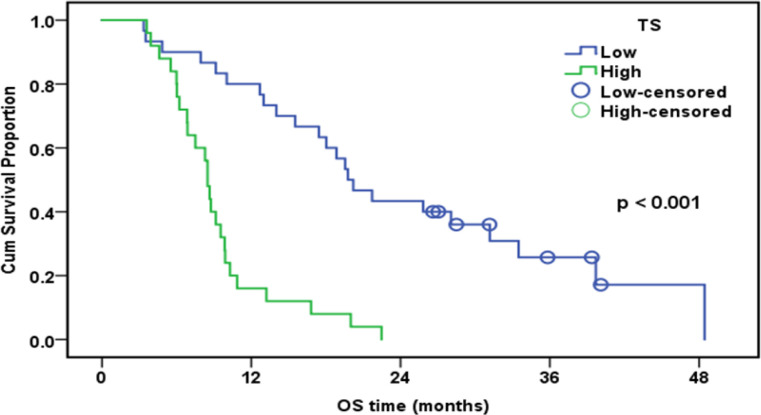
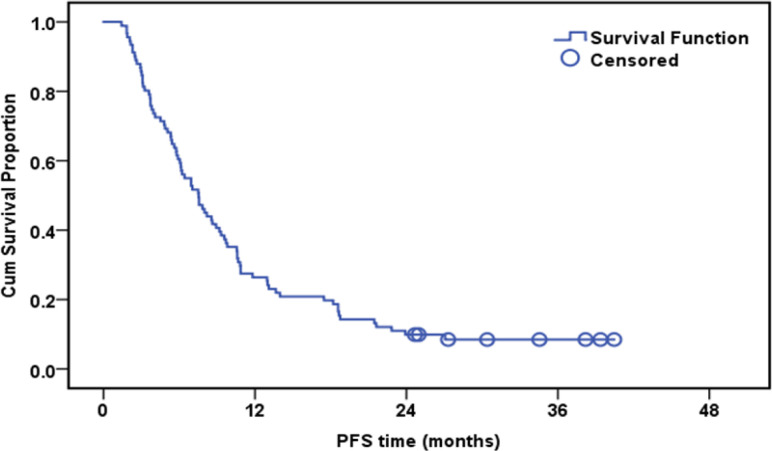
The median follow-up duration was 12.7 months (range: 3.4-48.5). By the end of the follow-up, only eight patients were alive. The cumulative one-year overall survival (OS) of the patinets in both groups was 53.8%. The median survival was 12.7 months. High expression of ERCC1, RRM1, and TS was associated with worsen OS (Table 5).
Table 5.
Overall Survival and Its Relation to the Three Markers ERCC1, RRM1, and TS
| N | No of events | Cumulative survival at one year (%) | Median survival (months) | p-value | |
|---|---|---|---|---|---|
| Whole group | 91 | 83 | 53.90% | 12.7 | |
| ERCC1 | < 0.001 | ||||
| Low | 49 | 42 | 71.40% | 18.1 | |
| High | 42 | 41 | 33.30% | 9.1 | |
| RRM1 (n=36) | 0.001 | ||||
| Low | 17 | 16 | 76.50% | 20.6 | |
| High | 19 | 19 | 42.10% | 8.6 | |
| TS (n=55) | < 0.001 | ||||
| Low | 30 | 23 | 80.00% | 19.8 | |
| High | 25 | 25 | 16.00% | 8.5 |
There was a positive association betweeen ERCC1 expression and TS expression (p=0.005); 70% of patients with low TS had low ERCC1, and 68% of high TS was associated with high ERCC1. On the other hand, there was no relation between ERCC1 and RRM1 expression pattern (p= 0.296). In patients underwen platinum-based theraphy (n 91), a significant correlation was detected between low ERCC1 median High-scoring and longer progression time to (TTP;9.6 v 5.3 months;P< 0.001) or OS (OS;18.1 v 9.1 months; P<0.001). A significant correlation was found between low TS protein expression and longer progression time (TTP; 11.8 v 5.4 months; P< 0.001) or OS (OS; 19.8 v 8.5 months; P <0.001) in patients undergoing pemetrexed plus platinum chemotherapy (n 55) .
Low RRM1 expression was accompanied by high progression free survival (TTP; 10.6 v 3.8 months) and OS (OS; 20.6 v 8.6 months)in patients receiving gemcitabine plus platinum chemotherapy. Based on the multivariate Cox regression analysis, the independent factors significantly affecting OS were tumor stage (HR: 2.3; 95% CI: 1.1-4.9; p= 0.021) and ERCC1 (HR: 2.7; 95% CI:1.7-4.3; p < 0.001).
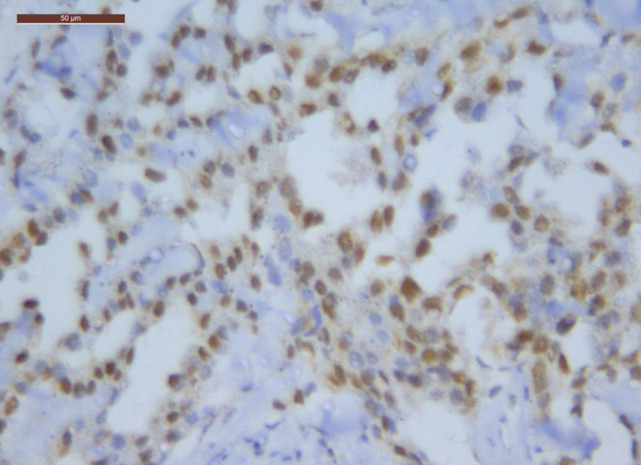
Figure 1.
ERCC1 Positive Expression in MPM (40 HPF)
Figure 2.
ERCC1 Positive Staining in MPM (20 HPF)
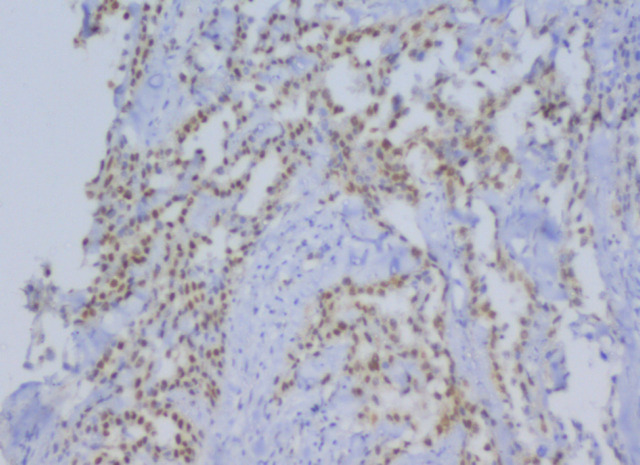
Figure 3.
Ribonucleotide-Reductase Subunit 1 (RRM1) Staining in MPM (40 HPF)
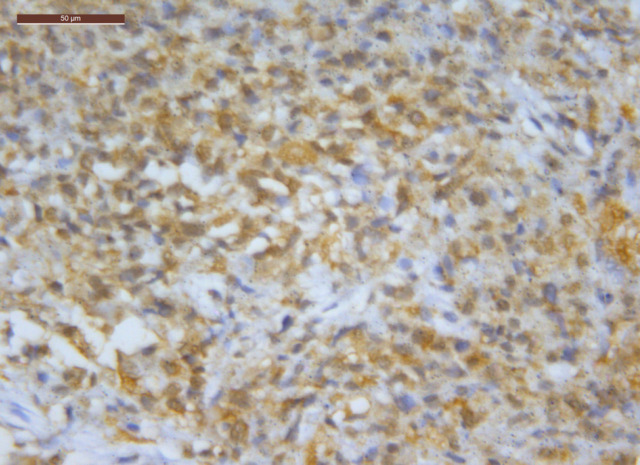
Figure 4.
RRM1 Positive Staining in MPM (20 HPF)
Table 2.
Expression Level of ERCC1, RRM1, and TS in the Studied Group
| Number | Percentage | |
|---|---|---|
| ERCC1 | ||
| Low | 49 | 53.8 |
| High | 42 | 46.2 |
| RRM1 (n=36) | ||
| Low | 17 | 47.2 |
| High | 19 | 52.8 |
| TS (n=55) | ||
| Low | 30 | 54.5 |
| High | 25 | 45.5 |
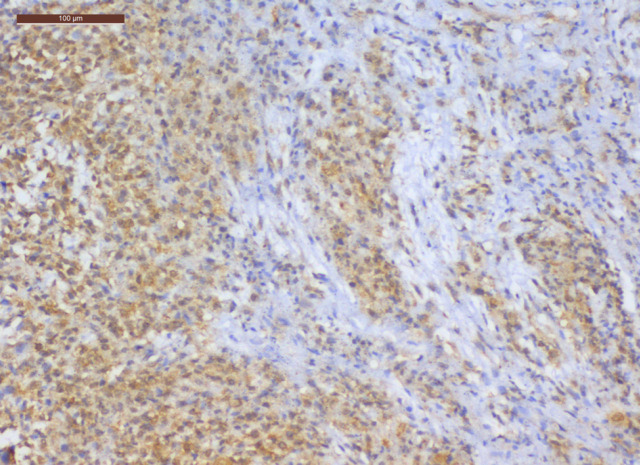
Figure 5.
TS Positive Staining in Malignant Pleural Mesothelioma
Figure 6.
Overall Survival of the Whole Studied Group (n=91)
Figure 7.
Overall Survival of the Whole Studied Group in Relation to ERCC1 Expression (n=91)
Figure 8.
Overall Survival of Patients Treated with GEM in Relation to RRM1 Expression (n=36)
Table 4.
Relation of ERCC1, TS and RRM1 Expression with Response to Treatment of the Studied Group
| ERCC1 Expression | |||
|---|---|---|---|
| Low | High | p-value | |
| Response | |||
| Regressive | 24 (68.6%) | 11 (31.4%) | |
| Progressive | 13 (36.1%) | 23 (63.9%) | 0.019 |
| Stable | 12 (60.0%) | 8 (40.0%) | |
| RRM1 Expression | |||
| Low | High | p-value | |
| Response | |||
| Regressive | 7 (87.5%) | 1 (12.5%) | < 0.001 |
| Progressive | 2 (11.1%) | 16 (88.9%) | |
| Stable | 8 (80.0%) | 2 (20.0%) | |
| TS Expression | |||
| Low | High | p-value | |
| Response | |||
| Regressive | 16 (59.3%) | 11 (40.7%) | 0.047 |
| Progressive | 6 (33.3%) | 12 (66.7%) | |
| Stable | 8 (80.0%) | 2 (20.0%) | |
Figure 9.
Overall Survival of Patients Treated with Alimta in Relation to TS Expression (n=55)
Table 6.
Overall Survival and Its Relation to the Prognostic Factors
| n | No of events | Cumulative survival at 1 year (%) | Median survival (months) | p-value | |
|---|---|---|---|---|---|
| Whole group | 91 | 83 | 53.90% | 12.7 | |
| Age (years) | 0.76 | ||||
| ≤ 55 | 50 | 45 | 52.00% | 12 | |
| > 55 | 41 | 38 | 56.10% | 14 | |
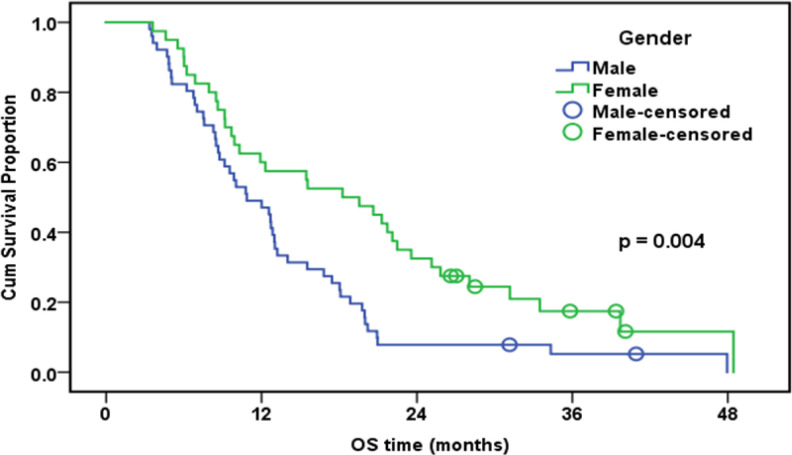
| Sex | 0.004 | ||||
| Male | 51 | 49 | 49.00% | 10.9 | |
| Female | 40 | 34 | 60.00% | 18.3 | |
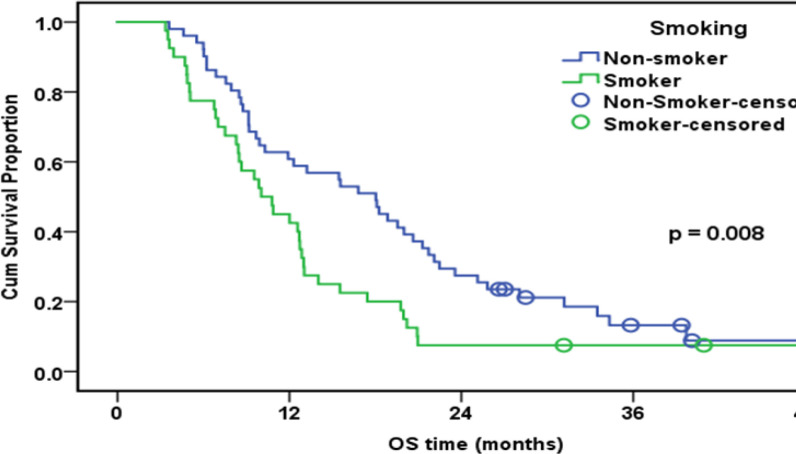
| Smoking | 0.008 | ||||
| No or Ex-smoker | 51 | 45 | 60.80% | 18 | |
| Yes | 40 | 38 | 45.00% | 10 | |
| Pathology | 0.24 | ||||
| Epithelioid | 77 | 69 | 57.10% | 12.9 | |
| Sarco/biphasic | 14 | 14 | 35.70% | 8.6 | |
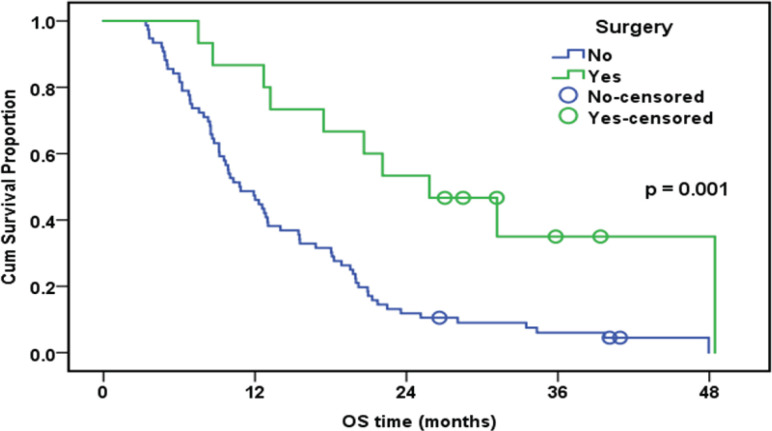
| Surgery | 0.001 | ||||
| No | 76 | 73 | 47.40% | 10.8 | |
| Yes | 15 | 10 | 86.70% | 25.8 |
Table 7.
Progression-Free Survival and Its Relation to the Three Markers ERCC1, RRM1, and TS
| N | No of events | Cumulative survival at one year (%) | Median survival (months) | p-value | |
|---|---|---|---|---|---|
| Whole group | 91 | 83 | 26.40% | 7.5 | |
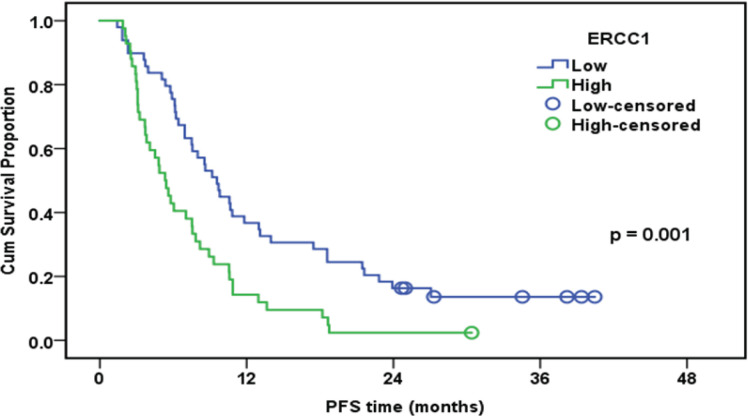
| ERCC1 | 0.001 | ||||
| Low | 49 | 42 | 36.70% | 9.6 | |
| High | 42 | 41 | 14.30% | 5.3 | |
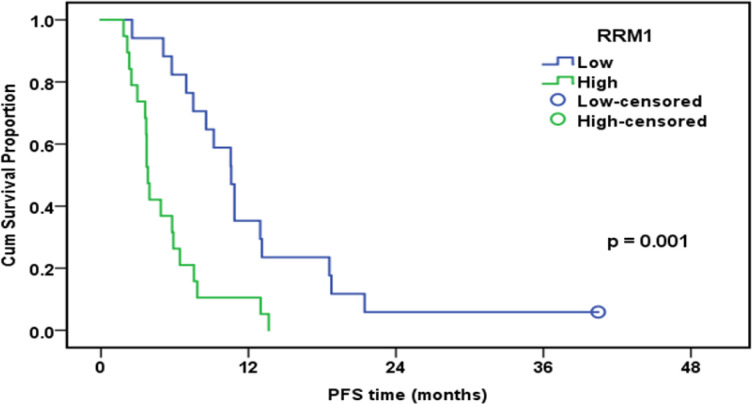
| RRM1 | 0.001 | ||||
| Low | 17 | 16 | 35.30% | 10.6 | |
| High | 19 | 19 | 10.50% | 3.8 | |
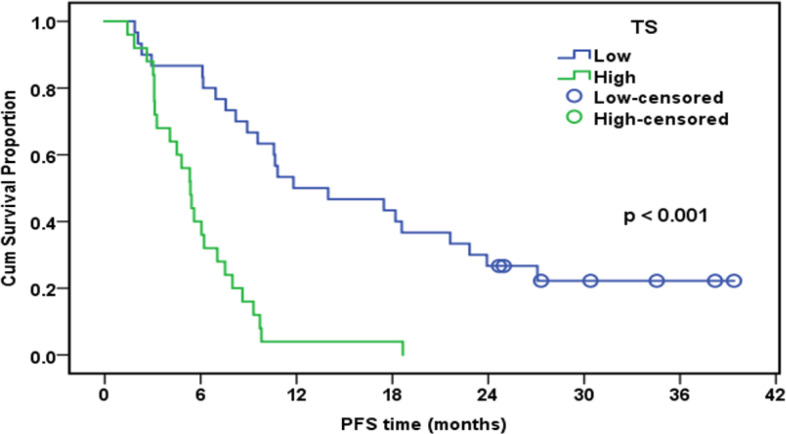
| TS | < 0.001 | ||||
| Low | 30 | 23 | 50.00% | 11.8 | |
| High | 25 | 25 | 4.00% | 5.4 |
Figure 10.
Overall Survival of the Whole Studied Group in Relation to sex (n=91)
Figure 11.
Overall Survival of the whole Studied Group in Relation to Smoking Status (n=91)
Figure 12.
Overall Survival of the Whole Studied Group in Relation to Surgical Treatment (n=91)
Table 8.
Factors Independently Affecting Progression-Free Survival Using Multivariate Analysis
| p-value | HR | 95.0% CI for HR | ||
|---|---|---|---|---|
| Lower | Upper | |||
| ERCC1 | 0.001 | 2.25 | 1.42 | 3.56 |
| Tumor stage | <0.001 | 6.77 | 3.02 | 15.21 |
| Surgery | <0.001 | 4.53 | 2.13 | 9.66 |
| Residence | 0.002 | |||
| Residence (Helwan vs Shobra) | <0.001 | 3.71 | 1.8 | 7.65 |
| Residence ((Others vs Shobra) | 0.075 | 1.7 | 0.95 | 3.04 |
Figure 13.
Progression-Free Survival of the whole Studied Group (n=91)
Figure 14.
Progression-Free Survival of the whole Studied Group in Relation to ERCC1 Expression (n=91)
Figure 15.
Progression-Free Survival of Patients Treated with GEM in Relation to RRM1 Expression (n=36)
Figure 16.
Progression-Free Survival of Patients Treated with Alimta in Relation to TS Expression (n=55)
Discussion
In the present study, immunohistochemistry was used to detect ERCC1 expression in 91 MPM patients. It was found that decreased expression of ERCC1 proteins was associated with improved chemotherapeutic responsiveness, prolonged PFS, and extended OS. High expressions of ERCC1 was shown to be associated with disease progression (p = 0.019). ERCC1- negative tumors had a median PFS of 9.6 months, whereas ERCC1-positive tumors had a median PFS of 5.3 months (p=0.001). Multivariate Cox regressions results revealed thatpatients with ERCC1-positive tumors had a considerably lesser disease progression than those with ERCC1-negative tumors (HR, 2.25; 95 percent CI, 1.42 - 3.56; p =0.001).
According to findings reported by Zimling et al., MPM cases, who received cisplatin-vinorelbin treatment and had low ERCC1 expressions, had a greater OS. PS and OS were significantly correlated in this study according to multivariable regression analysis (HR, 2.15; 95%ci, 1.04 - 4.42; p= 0.03). In ERCC1-negative tumors group, the median PFS was 10.9 months (95%CI, 5.6 -16.7 months), while it was 6.7 months in ERCC1-positive tumor group (95% CI, 4.4 -7.2 months) (p=0.053). In comparison to to ERCC1-negative tumor group, multivariable Cox modelling revealed that ERCC1-positive patients had a substantially lesser disease progression or lower mortality rate (HR, 2.24; 95% CI, 1.16 - 4.34; p=0.0163) (Zimling et al, 2012).
In the current research, based on multivariable Cox logistic regression results, tumor staging (HR: 2.3; 95 % CI:1.1-4.9; p=0.021) and ERCC1 (HR: 2.7; 95 percent CI:1.7-4.3; p<0.001) were found as independent predictors substantially affecting OS. The median follow-up time in this research was 12.7 months (range: 3.4-48.5). Exactly eight of the patients were alive at the end of the follow-up. Collectively, the group’s one-year OS rate was 53.8%. Poorer OS was correlated with high expression of ERCC1. According to our findings, OS was significantly higher in women, non- or ex-smokers, and patients who underwent surgery. In a nutshell, it seems that patients who have long-term disease survival and are responsive to chemotherapy are more probably to have negative ERCC1 expression.
Similarlary, Cihan et al., (2014) reported that 36% of patients with negative ERCC1 expression and 54% of patients with positive ERCC1 expression had disease progression . With respect to the course of treatment, the mean OS was determined to be 11.7 in patients who had positive ERCC1 expression, while it was 19.2 in patients with negative ERCC1 expression, suggesting an association between ERCC1 expression and disease prognosis.
Immunohistochemistry was used in Zucali et al., (2011)’s study to identify ERCC1 expression in 67 MPM patients who received pemetrexed and carboplatin treatments. They discovered no correlation between the presence of the ERCC1 proteins and PFS and OS.
Righi et al., (2010) also used immunohistochemistry to identify ERCC1 expression in 45 MPM cases who received various platinum-based treatments. According to their findings, increased ERCC1 expression could be a prognostic factor regardless of the chemotherapy regimen. Neverthelese, they reported no correlation between ERCC1 expression and treatment responsivness. Furthermore, they yielded that patients in the lowest tertile had considerably shorter survival rate. They did not detect a relationship between survival rate and ERCC1 median-H scoring (HR: 3.06; % CI: 1.08-8.69; p=0.035).
Low TS protein expresion in MPM cases were found to be an indicator of extended TTP and OS by Righi et al. It was discovered that individuals with reduced TS expression had greater OS and DFS. On the other hand, they did not discover a significant association between TS expression and outcomes in MPM cases who did not receive pemetrexed treatment (Righi et al, 2010).
In the current research, disease progression was noted in 33.3% of patients with low TS protein expressions and in 66.7% of paients with elevated TS protein expression (p= 0.047), revealing a significant association between low TS expression and a better treatment response with pemetrexed cisplatin (in 55 individuals).The mean OS in the current research was 12.7 months. The median PFS (11.8 months vs 5.4 months) and OS (19.8 months vs 8.9 months) were both significantly longer in patients with low TS expression level (p< 0.001). Lower TS protein expression was significantly correlated with DCR given that disease progression was observed in 33.3% of cases with low TS expression (p <0.001). The median OS was 10.9 months in men 18.3 months in women (p =0.004), suggesting gender a significant predictor of OS and PFS.
MPM was managed in 85 patients by using pemetrexed. out of these 85 patients, 75 received pemetrexed together with platinum and 10 received just pemetrexed. Gender did not have any significant effect on TTP or OS in this study. Reduced survivability was associated with non-epithelioid histology, but not with reduced TTP (HR 3.03; 95% CI - 1.64 - 5.62; p< 0.001). IHC was used to determine the degree of TS expression. The findings revealed no difference between higher and lower TS concentrations in terms of disease management ratios (p = 0.73). Even though patients with poor TS ratings had statistically higher median TTP (8 months vs. 6 months; p=0.93) and OS (14 mo vs. 11.25 mo; p=0.59) compared to those with greater TS ratings , this difference was not statistically significant (Lustgarten et al., 2013).
Overexpression of RRM1 has been linked to resistance to gemcitabine, a powerful ribonucleotide reductase inhibitor used in chemotherapy. Higher nuclear RRM1 protein level had a predictive relevance for extended FFR in MPM individuals after platinum/gemcitabine and platinum/pemetrexed standard chemotherapy accompanied by EPP (Jordheim et al., 2011).
Nuclear RRM1 plays a more significant predictive role in the subset of individuals undergoing platinum/gemcitabine induction chemotherapy. Prolonged FFR and OS were both substantially correlated with higher nuclear RRM1 expressions (Frischknecht et al, 2015).
The current study evaluated the prognostic and predictive values of RRM1in thirty six advanced MPM patients treated with six cycles of gemcitabine and cisplatin. The findings showed a significant correlation between decreased RRM1 expression and improved therapeutic responses. Additionally, decreased RRM1 expression was associatedwith prolonged PFS (p= 0.001). Lower RRM1 expression was observed to be significantly correlated with prolonged OS (p =0.001).
In conclusion, Lower TS protein expression can be indicative of greater responsivness to pemtrexed and of longer TTP and OS in individuals with advanced MPM (locally progressed or metastatic) who are receiving pemetrexed-based chemotheraphy. Low ERCC1 expressions in individuals with advanced MPM can predict increased PFS and OS, as well as a better responsivness to platinum-based chemotherapy. In patients with progressed MPM receiving gemcitabine plus cisplatin chemotherapy, lower RRM1 expression was associated with a better prognosis, longer PFS, and longer OS.
Recommendation
Analysis of ERCC1 protein expression should be done in MPM patients before starting platinum-based chemotherapy. It is recommend to avoid platinum-based regimens if there is high expression of ERRC1 to avoid resistance to treatment or early disease progression.In these cases, immunotherapy is suggested. Analysis of TS protein expression should be done in MPM patients before starting pemetrexed- based chemotherapy. It is suggested to avoid pemetrexed-based regimens if high expression of TS was detected to avoid resistance to treatment or early disease progression.
Analysis of RRM1 expression should be done before gemcitabine-base chemotherapy administration. In case of high expression of RRM1, gemcitabine should be avoided.
Author Contribution Statement
Nagwa Ismail collected the clinical data and tissue specimens and wrote the manuscript. Ola Khorshid supervised overall research, provided, and interpreted data. Fatma abu Alkassem helped in supervision, data analysis, and writing of the manuscript. Abeer Bahnassy provided the pathological assessment and results. Rabab Gaafar designed the research plan and supervised overall research.
Acknowledgments
Funding statement
This work was part of a thesis for MD degree in medical oncology and was funded by national cancer institute Cairo University, Egypt.
Ethical statement
This study was approved by the ethical committee of national cancer institute Cairo University, Egypt.
Availability of data
The data supporting this study results are available from the corresponding author upon reasonable request.
Conflicts of interest
All authors declare that there is no conflict of interest.
References
- Cihan Y, Öztürk A, Arslan A, et al. ERCC1 as a biological marker guiding management in malignant pleural mesothelioma. Asian Pac J Cancer Prev, 2014;15 doi: 10.7314/apjcp.2014.15.10.4117. [DOI] [PubMed] [Google Scholar]
- Friedberg EC. How nucleotide excision repair protects against cancer. Nat Rev Cancer, 2001 22;1 doi: 10.1038/35094000. [DOI] [PubMed] [Google Scholar]
- Frischknecht L, Meerang M, Soltermann A, et al. Importance of excision repair cross-complementation group 1 and ribonucleotide reductase M1 as prognostic biomarkers in malignant pleural mesothelioma treated with platinum-based induction chemotherapy followed by surgery. J Thorac Cardiov Sur. 2015;149:1539–47. doi: 10.1016/j.jtcvs.2015.01.065. [DOI] [PubMed] [Google Scholar]
- Gomez HL, Santillana SL, Vallejos CS, et al. 2006) A phase II trial of pemetrexed in advanced breast cancer: clinical response and association with molecular target expression. Clin Cancer Res. 12:832–8. doi: 10.1158/1078-0432.CCR-05-0295. [DOI] [PubMed] [Google Scholar]
- Jordheim LP, Sève P, Trédan O, et al. The ribonucleotide reductase large subunit (RRM1) as a predictive factor in patients with cancer. Lancet Oncol. 2011;12:693–702. doi: 10.1016/S1470-2045(10)70244-8. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kwon HC, Oh SY, et al. 2009) Prognostic value of ERCC1, thymidylate synthase, and glutathione S-transferase π for 5-FU/oxaliplatin chemotherapy in advanced colorectal cancer. Am J Clin Oncol. 32:38–43. doi: 10.1097/COC.0b013e31817be58e. [DOI] [PubMed] [Google Scholar]
- Lustgarten DS, Deshpande C, Aggarwal C, et al. Thymidylate synthase and folyl-polyglutamate synthase are not clinically useful markers of response to pemetrexed in patients with malignant pleural mesothelioma. J Thorac Oncol. 2013;8:469–77. doi: 10.1097/JTO.0b013e318283da3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non–small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355:983–91. doi: 10.1056/NEJMoa060570. [DOI] [PubMed] [Google Scholar]
- Pontarin G, Fijolek A, Pizzo P, et al. Ribonucleotide reduction is a cytosolic process in mammalian cells independently of DNA damage. Natl Acad Sci Proc U S A. 2008;105:17801–6. doi: 10.1073/pnas.0808198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi L, Papotti MG, Ceppi P, et al. Thymidylate synthase but not excision repair cross-complementation group 1 tumor expression predicts outcome in patients with malignant pleural mesothelioma treated with pemetrexed-based chemotherapy. J Clin Oncol. 2010;28:1534–9. doi: 10.1200/JCO.2009.25.9275. [DOI] [PubMed] [Google Scholar]
- Shih C, Chen VJ, Gossett LS, et al. LY231514, a pyrrolo [2, 3-d] pyrimidine-based antifolate that inhibits multiple folate-requiring enzymes. Cancer Res. 1997;57:1116–23. [PubMed] [Google Scholar]
- Vilmar A, Santoni-Rugiu E, Sørensen J, et al. ERCC1 and histopathology in advanced NSCLC patients randomized in a large multicenter phase III trial. Ann Oncol. 2010;21:1817–24. doi: 10.1093/annonc/mdq053. [DOI] [PubMed] [Google Scholar]
- Weberpals J, Garbuio K, O’Brien A, et al. The DNA repair proteins BRCA1 and ERCC1 as predictive markers in sporadic ovarian cancer. Int J Cancer. 2009;124:806–15. doi: 10.1002/ijc.23987. [DOI] [PubMed] [Google Scholar]
- Zimling ZG, Sørensen JB, Gerds TA, et al. Low ERCC1 expression in malignant pleural mesotheliomas treated with cisplatin and vinorelbine predicts prolonged progression-free survival. J Thorac Oncol. 2012;7:249–56. doi: 10.1097/JTO.0b013e318233d6a9. [DOI] [PubMed] [Google Scholar]
- Zucali PA, Giovannetti E, Destro A, et al. Thymidylate synthase and excision repair cross-complementing group-1 as predictors of responsiveness in mesothelioma patients treated with pemetrexed/carboplatints and ERCCL expression as predictors of outcome in mpm. Clin Cancer Res. 2011;17:2581–90. doi: 10.1158/1078-0432.CCR-10-2873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study results are available from the corresponding author upon reasonable request.