Abstract
Background:
Cancer remains a challenging target to cure, with present therapeutic methods unable to exhibit restorative outcomes without causing severe negative effects. Molecular hydrogen (H2) has been reported to be a promising adjunctive therapy for cancer treatment, having the capability to induce anti-proliferative, anti-oxidative, pro-apoptotic and anti-tumoural effects. This review summarises the findings from various articles on the mechanism, treatment outcomes, and overall effectiveness of H2 therapy on cancer management.
Methods:
Using Cochrane, PubMed, and Google Scholar as the search engines, full-text articles in the scope of the study, written in English and within 10 years of publication were selected.
Results:
Out of the 677 articles, 27 articles fulfilled the eligibility criteria, where data was compiled into a table, outlining the general characteristics and findings. Throughout the different forms of H2 administration, study design and types of cancers reported, outcomes were found to be consistent.
Conclusion:
From our analysis, H2 plays a promising therapeutic role as an independent therapy as well as an adjuvant in combination therapy, resulting in an overall improvement in survivability, quality of life, blood parameters, and tumour reduction. Although more comprehensive research is needed, given the promising outcomes, H2 is worth considering for use as a complement to current cancer therapy.
Key Words: Molecular hydrogen, cancer, oxidative stress, complementary therapy, anti-tumour
Introduction
In the last few decades, the burden of cancer continues to rise while simultaneously becoming less curable with existing therapeutic options such as chemotherapy, radiation, and surgery. Reports on current therapies have shown its complications of increasing circulating tumour cells, distant metastasis and enhancing cancer growth (Arumugam, 2021). With oxidative stress being linked to cancer’s aetiology, whereby a redox imbalance decreases the availability of antioxidants, it is proven hard to find antioxidative treatments that can avoid present-day challenges. Hydrogen, the lightest element on the earth, is an effective antioxidant that has been shown to selectively reduce harmful reactive oxygen species (ROS) in tissues (Yang et al., 2018).
Over recent years since the first publication on the potential therapeutic effects of molecular hydrogen (H2) in cancer management (1975), many studies have emerged to further advance and reinforce its practice in clinical settings. While the first two studies revolved around hydrogen’s effects on skin squamous carcinoma, researchers have gradually expanded its scope from its application on different types of cancer to various routes of administration to achieve its efficacy (Dole et al., 1975; Roberts et al., 1978. In the 1980s, studies were focused on hydrogen gas being used as a clearance method (Kiyotaki, 1988; Lee et al., 1989), however, no updates were provided from 1990 onwards.
A novel study by Ohsawa et al., (2007) was published on hydrogen’s newfound ability to selectively reduce cytotoxic oxygen radicals. This was considered a turning point in cancer therapy research, eventually increasing the frequency of studies on H2 as a potential therapeutic agent. Ohsawa et al., (2007) discussed hydrogen’s capability in reducing and neutralising strong oxidants such as hydroxyl ( ٜ OH) radicals, and to protect nuclear DNA, mitochondria, tissues, and cells from oxidative stress by acting as a scavenger for ( ٜ OH). Despite the expanding scope of cancer research, studies remain scarce. There were no clinical trial studies on cancer prior to 2011. However, seven clinical trials on cancer have been conducted since then, with four of them occurring in the last two years (Akagi and Baba, 2020; Akagi and Baba, 2018; Chen et al., 2020; Chen et al., 2020). From 2017 until the present, the number of papers on hydrogen therapy and its applications in cancer treatment has steadily increased, indicating that the topic is an emerging area of research.
Although H2 has demonstrated significant anti-tumoural effects, the underlying mechanisms have not yet been elucidated. Many studies have shown that H2 therapy can reduce oxidative stress. This, however, contradicts radiation therapy and chemotherapy, in which ROS are required to induce apoptosis and combat cancer. Therefore, many questions remain on how H2 can act as a therapeutic agent for cancer. In this article, studies involving the effects of H2 on cancer were systematically reviewed.
Materials and Methods
The review protocol
This systematic review was done in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021).
Selection criteria and process
Two reviewers independently conducted the review by screening through relevant titles and abstracts from each electronic database, followed by a discussion on discrepancies until agreement was reached. Full text articles were reviewed independently and duplicated articles and studies that are out of scope were excluded. The inclusion criteria for this review are full-text research studies focusing on H2 therapy and its effects on cancer, published within the past 10 years (2011 to 2021) and written in English. The corresponding author guided in making the final decision on which articles were used for this systematic review.
Systematic searching strategy
The systematic search from the 22 November 2021 to 8 December 2021, using three well-known search engines, i.e. Cochrane, PubMed, and Google Scholar. The keywords that were implemented across the three search engines are (“molecular hydrogen” OR “hydrogen therap*” OR “hydrogen gas” OR “hydrogen water” OR “hydrogen inhalation” OR “hydrogen-rich”) AND (“cancer” OR “carcinoma” OR “tumour” OR “tumour”), using appropriate boolean operators suited for each database as shown in Table 1.
Table 1.
Keyword Applied during the Identification Phase
| Database | Search String |
|---|---|
| Cochrane | (“molecular hydrogen” OR “hydrogen therapy” OR “hydrogen treatment” OR “hydrogen gas” OR “hydrogen water” OR “hydrogen inhalation” OR “hydrogen-rich”) AND (“cancer” OR “carcinoma” OR “tumor” OR “tumour”) |
| PubMed | (“molecular hydrogen” OR “hydrogen therap*” OR “hydrogen treatment” OR “hydrogen gas” OR “hydrogen water” OR “hydrogen inhalation” OR “hydrogen-rich”) AND (“cancer” OR “carcinoma” OR “tumor” OR “tumour”) |
| Google Scholar | (“molecular hydrogen” OR “hydrogen therapy” OR “hydrogen therapeutic” OR “hydrogen treatment” OR “hydrogen gas” OR “hydrogen water” OR “hydrogen inhalation” OR “hydrogen-rich”) AND (“cancer” OR “carcinoma” OR “tumor” OR “tumour”) |
Quality appraisal
The Mixed Method Appraisal Tool (MMAT) was used in determining and appraising the quality of the included studies, consisting of a wide range of study designs; qualitative, randomised controlled trials, non-randomized studies, quantitative descriptive studies, and mixed method studies (Hong et al., 2018). A standardised checklist was used to rate the criteria of the chosen category in each study. After assessing each study individually by two reviewers, a quality score was issued to it. All 27 articles utilised in this systematic review had good quality hence data from each article were extracted, analysed and deduced for the systematic review.
Data extraction and analysis
Data were obtained from fully eligible research articles into a predefined data gathering Excel file. Extraction of data was based on the baseline characteristics of the eligible research reports following the selection criteria, i.e., general details of the study (author, country, study design), H2 form of administration, duration of therapy along with their general findings and conclusions.
Results
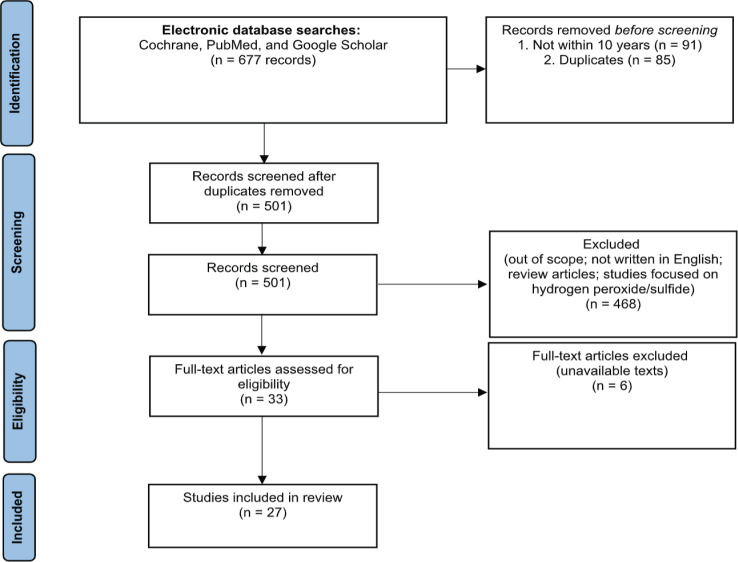
A total of 677 research articles were retrieved from the preliminary search (Figure 1). A screening was done by two independent individuals to filter out and remove the duplicated or unrelated reports to maintain the search quality and accuracy. Inclusion and exclusion criteria were established to further exclude studies not related to the area of focus. As a result, a total of 27 articles met the inclusion criteria, therefore collated for this systematic review.
Figure 1.
PRISMA Flow Diagram for the Systematic Review of Molecular Hydrogen Research on Cancer in the Past 10 Years
Table 2 shows the list of included studies as well as the basic information and methodologies used for the experiments. The table was split into Table 2a and Table 2b. Table 2a includes studies focusing on in vitro and in vivo models. Studies in both tables are listed according to the type(s) of cancer being studied, nature of the study (e.g. independent H2 therapy or combination therapy), study design (e.g. in vitro, in vivo, clinical trial, etc.), H2 forms and concentration as well as duration of H2 treatment. Results of the studies were presented as treatment outcomes.
Table 2a.
General Characteristics of Included Studies. List of in vitro and in vivo studies
| Author, Year (Ref.) | Type(s) of Cancer | Nature of Study | Study Design | H2 Form (Concentration) | Duration of H2 Treatment | Treatment Outcomes |
|---|---|---|---|---|---|---|
| Yu Jiang et al., 2018 | Non-small cell lung cancer (NSCLC) | Combination of H2 with LY294002, PI3K inhibitor | in vitro | Hydrogen saline (Concentration maintained at >0.6 mmol/l) | 24 hours | - Antiproliferation and apoptotic effectiveness was increased after hydrogen saline treatment on the NSCLC A549 cell lines. |
| Yuanren Gao et al., 2021 | Liver cell injury and liver cancer | Independent H2 study | in vitro | Hydrogen-rich water (HRW) (Concentration not stated) | 24 hours | - HRW provided a protective impact against liver cell injury as well as an anti-proliferative effect on liver cancer cells. - It promotes normal liver cell proliferation in a co-culture system to resist the invasion of normal cells by liver cancer cells. |
| Ye Yang et al., 2020 | Endometrial cancer | Independent H2 study | in vitro | HRW (0.7 ppm) | 24 hours | - TNF/NF- κB signaling pathways, as well as apoptotic pathways, were activated by HRW treatment. |
| Ye Yang et al., 2020 | Endometrial cancer | Independent H2 study | in vitro & in vivo | HRW (1.0 ppm) | 24 days | - H2 upregulated ROS generation in association with upregulated expression of NLPR3 inflammasome. - HRW inhibits xenograft volume and weight of endometrial cancers via the pyroptotic pathway. There is a significant difference in tumour volume and weight between hydrogen culture media and normal culture media. |
| Runtuwene J. et al., 2015 | Colon cancer | Combination of H2 and 5-fluorouracil treatment (FU) | in vitro & in vivo | HRW (0.8mM) | 10 days | - HRW treatment showed strong anti-oxidative effects and increased survival rate of the treated mice for 16 days on hydrogen water alone. - The treatment enhanced 5-FU effectiveness through increased anticancer activity and cell death with a survival rate of 25 days. |
| Daisuke Kawai et al., 2012 | Nonalcoholic steatohepatitis and accompanying hepatocarcinoge-nesis | Independent H2 study | in vivo | HRW (Concentration not stated) | 8 weeks | - The number of hepatic tumours was much lower (from 20 to 5), with smaller maximum tumour size (from 3mm to 2mm) in the HRW group than the control group. |
| Fang-Yin Li et al., 2013 | Ferric nitrilotriacetate-induced nephrotoxicity (renal injury/cancer) | Combination of H2 and ferric nitrilotriacetate treatment | in vivo | HRW (Concentration maintained at more than 0.8mg/l) | 12 weeks | - Fe-NTA-induced inflammation, oxidative stress and renal mitochondrial dysfunction were reduced significantly after HRW therapy. - Reduced renal damage and suppressed early tumour promoting events. |
| Leyuan Liu et al., 2020 | Lung cancer | Independent H2 study | in vitro | Hydrogen gas (20%, 40%, 60%) | 48 hours | - Molecular hydrogen lowered STAT3/Bcl2 signaling, hence promoting lung cancer cell death and autophagy. - Autophagy suppression improves H2 involvement in inducing lung cancer cell death. |
| Yayoi Murakami et al., 2017 | Neuroblastoma cells | Independent H2 study | in vitro | Hydrogen gas (50% H2) | - | - Pretreatment inhibited H2O2-induced cell death. - Hydrogen gas enhanced mitochondrial membrane potential and cellular ATP levels. - Treatment with hydrogen gas induced weak oxidative stress and the system's anti-oxidative defense mechanism. |
| Meng-yu Liu et al., 2019 | Glioblastoma growth | Independent H2 study | in vitro & in vivo | Hydrogen gas (67% H2) | - | - Glioma growth was inhibited while glioma stem-like cell development was reduced by hydrogen gas therapy. - Glioma cell migration, invasion, and colony formation were inhibited after the hydrogen gas treatment. |
| Baocheng Zhu et al., 2021 | Gastric cancer cells | Independent H2 study | in vitro & in vivo | Hydrogen gas (67% H2) | 5 weeks | - Hydrogen treatment greatly reduced gastric tumour development in vivo. - Cell proliferation, migration, and lncRNA MALAT1 and EZH2 expression were inhibited by hydrogen gas while miR-124-3p expression was upregulated. |
| Jing Chu et al., 2021 | Cervical cancer | Independent H2 study | in vitro & in vivo | Hydrogen gas (67% H2) | 7 days | - Treatment with hydrogen promoted apoptosis while decreasing cell growth and oxidative stress. - Tumour growth and cell proliferation was reduced, along with increased cell death. |
| Dongchang Wang et al., 2018 | Lung cancer | Independent H2 study | in vitro & in vivo | Hydrogen gas (20%, 40%, 60%, 80% H2) | - | - Cell viability, migration, and invasion were reduced by hydrogen treatment, while cell apoptosis was accelerated via the downregulation of SMC3 expression. - Reduction in tumour weight and protein expression were also observed. |
| Jinghong Meng et al., 2020 | Lung cancer | Independent H2 study | in vitro & in vivo | Hydrogen gas (20%, 40%, 60% H2) | 4 weeks | - Hydrogen treatment increased apoptosis of cancer cells while suppressing cell proliferation, invasion, and migration. - Macrophage-mediated phagocytosis and the overall role of H2 in lung cancer inhibition was enhanced via down-regulating CD47. |
| Lei Shang et al., 2018 | Ovarian cancer | Independent H2 study | in vitro & in vivo | Hydrogen gas (66.7% H2) | 6 weeks | - Growth of tumour was inhibited, as are cancer cell proliferation, invasion, migration, and colony formation after hydrogen treatment. - PA-1 and Hs38.T cells' capacity to form spheres was significantly inhibited, along with reduced CD34 expression, showing anti-angiogenesis actions from the hydrogen gas. |
Table 2b.
Clinical Trials and Patient-based Studies
| Author, Year (Ref.) | Type (s) of Cancer | Nature of Study | Study Design | H2 Form/ Administration Method (Concentration) | Duration of H2 Therapy | Treatment Outcomes |
|---|---|---|---|---|---|---|
| Qingxi Yang et al., 2017 | Liver function in colorectal cancer patients | Combination of H2 and mFOLFOX6 chemotherapy (5-fluorouracil, oxaliplatin and calcium folinate) | Randomized Clinical Trial | HRW/Ingestion (0.27–0.4 ppm) | 4 days | - HRW lowered the incidence and severity of liver injury, (P<0.05). - No significant variations were shown in liver function before and after therapy, uncompromising to original chemotherapy. |
| Ki-Mun Kang et al., 2011 | Quality of life of patients treated with radiotherapy for liver tumours | Combination of H2 and radiotherapy | Randomized Clinical Trial | HRW/Ingestion (0.55-0.65 mM) | 6 weeks | - Significantly less appetite loss and fewer taste problems were observed after treatment. - Hydrogen reduced oxidative stress and did not alter liver function or blood composition. - There was no influence on the anti-tumour effects of radiation with 48% of patients showing completed or partial response and no patients with progressive disease during the 3 months follow-up period. |
| Junji Akagi, Hideo Baba, 2020 | Lung cancer | Combination of H2 and Nivolumab | Clinical Trial | Hydrogen gas/Inhalation (680,000 ppm H2 gas generated) | 2 weeks | - Combination H2 therapy showed a substantially longer overall survival (OS), with a median survival time of 28 months, compared to the 9 months of those who received Nivolumab solely. - An improved prognosis was obtained by decreasing PDT+ cells. |
| Junji Akagi, Hideo Baba, 2019 | Advanced colorectal cancer | Combination of H2 and XELOX (CapeOX) chemotherapy (capecitabine, oxaliplatin and bevacizumab) | Clinical Trial | Hydrogen gas/Inhalation (680,000 ppm H2 gas generated) | 3 months | - Treatment enhanced OS times and progression-free survival (PFS). - Improved prognosis was obtained by reversing imbalances toward PD1+ CD8+ T cells. |
| Ji-Bing Chen et al., 2020 | Advanced NSCLC | Independent H2 study with combined-therapy groups as comparison | Clinical Trial | Hydrogen gas/Inhalation (66.7% H2) | 5 months or stopped when cancer recurrence | - Cancer symptoms were significantly reduced, and the control group's PFS were lower (4.4 ± 1.2 months) than in other H2 treated groups (more than 7.9 ± 2.2 months). - Most drug-related side effects gradually diminished or were fully eliminated in the combined-therapy groups. |
| Ji-Bing Chen et al., 2020 | Advanced NSCLC | Independent H2 study | Clinical Trial | Hydrogen gas/Inhalation (66.7% H2) | 2 weeks | - All peripheral blood lymphocyte subsets increased to within the normal range after hydrogen gas inhalation. - The fraction of regulatory T cells in the blood also reduced as the number of exhausted and senescent cytotoxic T cells decreased to within the normal range. |
| Ji-Bing Chen et al., 2019 | Metastatic gallbladder cancer | Independent H2 study | Case Report | Hydrogen gas/Inhalation (67% H2) | 3 months | - H2 gas caused a gradual decrease in tumour markers and intestinal obstruction - Improvement in hematological indications were improved after H2 therapy and when patient was able to resume normal life. - Patients experienced drowsiness or agitation after H2 inhalation. There were no other adverse events identified. |
| Ji-Bing Chen et al., 2019 | Gallbladder carcinoma | Independent H2 study | Case Report | Hydrogen gas/Inhalation (Concentration not stated) | 10 months | - H2 gas restored patient's blood parameters to normal and alleviated duodenal blockage. - H2 has subsequently lowered the expression of tumour markers. |
| Ji-Bing Chen et al., 2019 | Nasopharyngeal cancer induced hearing loss | Combination of H2 and radiotherapy | Case Report | Hydrogen gas/Inhalation (67% H2) | 1 to 2 months | - Subjects inhaling H2 gas exhibited improved pure-tone audiograms and the ear pus removal. |
| Ji-Bing Chen et al., 2019 | NSCLC | Independent H2 study | Case Report | Hydrogen gas/Inhalation (67% H2) | 1 year | - H2 gas was able to gain significant effective control of the brain metastases from NSCLC and extended survival time. |
| Ji-Bing Chen et al., 2019 | Advanced (stage III and IV) cancers | Independent H2 study | Observational study | Hydrogen gas/Inhalation (66.7% H2) | 3 to 46 months | - Patients' quality of life (QOL) has been improved after inhaling H2, along with their physical and mental health. - H2 gas could prevent cancer progression by lowering tumour markers. |
| Shi-ichi Hirano et al., 2021 | Radiation-induced bone marrow damage in cancer patients | Combination of H2 and radiotherapy | Observational study | Hydrogen gas/Inhalation (5% H2) | 1 to 4 weeks | - Inhalation of H2 gas improved IMRT-induced bone marrow damage without affecting IMRT's anti-tumour efficacy and patient’s QOL. - There was a reduction in radiation-induced apoptosis, OH levels, oxidative stress, apoptosis, and inflammation. |
The study designs included in this review consists of in vitro, in vivo, and patient-based studies. Almost all the research articles conducted both in vitro and in vivo study together. Only two studies focused solely on in vitro, and another two solely on in vivo. Patient-based studies include clinical trials, case reports and observational studies.
It was observed that three main methods of administration were employed throughout all the included studies, namely hydrogen saline, hydrogen-rich water, and hydrogen gas. These methods are further divided by their study design with only one in vitro study utilising hydrogen saline (Jiang et al., 2018). 28% of the studies used HRW as a method of administration including studies that are in vitro, in vivo and two clinical trials. The most popular mode of administration, used in 69% of the included studies, is hydrogen gas. However, almost half of the studies focusing on hydrogen gas were by Ji-Bing et al., from 2019 to 2021. Similar to HRW, hydrogen gas was investigated in the form of in vitro and in vivo studies, as well as patient-based studies. The research articles collectively showed that hydrogen gas can reduce tumour growth, increase apoptosis rate, and improve patients’ quality of life (QOL) such as breathing, appetite and physical fitness. There are varying concentrations used among the included studies, however, the most employed concentration of hydrogen gas seems to be 67%, in combination with 33.3% oxygen, commonly performed using a nebulizer/atomizer.
Among all the studies, gastrointestinal and respiratory system related cancers were highly investigated, both in independent hydrogen therapy and combination therapy. The remaining group of cancers are related to reproductive, CNS and renal system. Interestingly, the one study on renal cancer was a combination therapy of hydrogen and ferric nitrilotriacetate treatment. The last two studies under ‘unspecified’ were observational studies involving cancers in general. Without specifying the type of cancer, one studied independent H2 therapy while the other reported on combination therapy.
Discussion
H2 forms of administration
This systematic review is the first to summarise effects associated with the use of molecular hydrogen for cancer. Based on the included studies for this systematic review, the methods used for administering H2 are injecting hydrogen-rich saline, ingesting hydrogen-rich water (HRW), or inhaling hydrogen gas.
Among the three methods used to administer H2, reduced oxidative stress, anti-proliferation and apoptosis-inducing effects are common effects seen throughout the included studies, suggesting that the effects of hydrogen on cancer are similar and consistent no matter the method. A review by Tian et al., (2021) indicated different administration methods may have different effects by their hydrogen peaks and sustaining time. A direct comparison in the method’s elevation of H2 concentration cannot be deduced due to many differing factors, nevertheless inhaling hydrogen appears to be the most straightforward means of achieving rapid elevations in hydrogen concentration across tissues. Patients are required to inhale hydrogen gas daily for about 3 hours or more, at their own homes through a cannula or mask. It does, however, need the use of equipment, whereas HRW can be delivered far more easily while still having a good impact and efficacy (Shimouchi et al, 2013).
Retention of hydrogen in the dilution is the key issue in the manufacture of hydrogen water since the amount of hydrogen in the HRW is naturally inclined to decrease (Kajiyama et al., 2008). Several papers employing HRW overcome this issue by replacing and providing hydrogen water daily. The patients were instructed to drink 100-300 mL of hydrogen-rich water at least 10 times a day, with each day using the magnesium sticks given in a new bottle of water (Kang et al., 2011). Jiang et al., (2018)’s study employed hydrogen saline whose concentration was maintained at more than 0.6 mmol/l eventuating in reduced oxidative stress, anti-proliferation and apoptosis-inducing effects. It was mentioned that hydrogen gas is found to be dangerous and difficult for clinical usage, thus hydrogen saline is used as an alternate model to administer H2. Since the use of saline solutions is common in the hospital environment, hydrogen saline could be a suitable technique of H2 delivery, however more studies in animal models and humans would be required.
There are also no major adverse effects seen from all the types of the H2 forms. Although drowsiness and agitation have been reported for patients inhaling hydrogen gas for long hours of the day, there has been no evidence of harm in either animals or people from molecular hydrogen therapies as supported in past review papers (Shin, 2014; Wilson et al., 2017). Since hydrogen can readily diffuse across the plasma, HRW or hydrogen-rich saline is becoming more widely acknowledged as a possible treatment method, owing to the lack of side effects (Chen et al., 2019).
Study design
Research articles involving both animal and cellular models were included in this systematic review, focusing on H2’s effects on cancer. There is a noticeable consistency across all the articles from the three databases, whereby most articles implied that H2 has anti-proliferative, anti-tumour, antioxidant, and pro-apoptotic properties, all of which are desirable properties in cancer therapy. Albeit being able to see its effects in vitro, using animal models would give a better insight into how H2 induces its effects whilst including other physiological factors. After all, what was observed in a cell culture might not be the same as observed in a living model with various parameters involved.
Most in vitro studies focused on H2’s effects on a molecular level. A study has shown that 67% hydrogen gas is able to act on specific protein expressions, such as lncRNA MALAT1 and enhancer of zeste homolog 2 (EZH2) (Zhu et al., 2021), to inhibit the proliferation and migration of gastric cancer cells, MGC-083 and BGC-823. In the context of gastric cancer, lncRNA MALAT1 is a relevant protein as it has been quoted to be a contributor to gastric cancer cells’ chemoresistance capability towards cisplatin (DDP) and oxaliplatin (OXA), as well as upregulating the expression of autophagy-related genes and reducing apoptosis (Li et al., 2021). EZH2 stimulates the overexpression of tumour protein p53 (Jiang et al., 2016). Hence, these two proteins became appropriate targets for H2 to act on. In Zhu et al., (2021)’s study, hydrogen was able to inhibit the overexpression of EZH2 and suppress lncRNA MALAT1 whilst upregulating miRNA-124-2p which resulted in the inhibition of gastric cancer’s proliferation and migration.
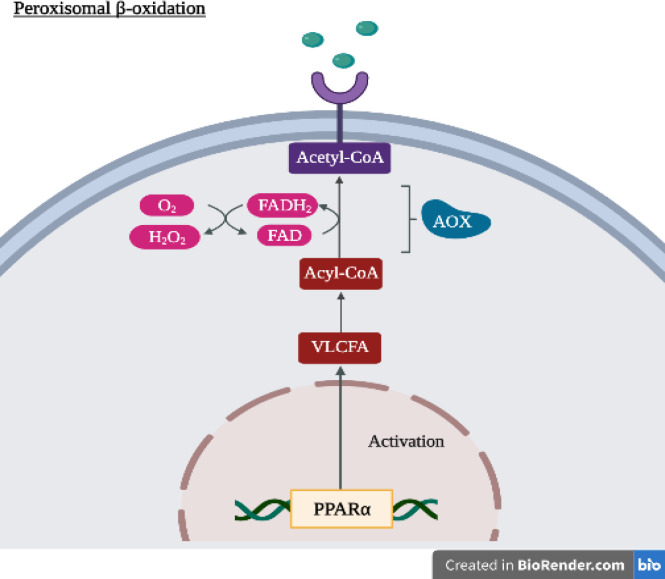
Most of the findings in in vivo studies crossover with those in the in vitro studies, showing that H2 could lower the severity of various types of cancer. A study on cervical cancer demonstrates how H2 had a much lower ROS level compared to the control group (p < 0.01). This suggests the treatment’s role in reducing the oxidative stress level even in animal studies (Chu et al., 2021). A common effect also seen in animal models is the suppression of tumour growth through various antitumor mechanisms. Kawai et al., (2012) noticed that hydrogen water could induce great antioxidative and anti-hepatic carcinogenic effects. In their study, H2 reduces the expression of key genes involved in lipid metabolism to control the hepatic expression of fatty acid synthesis and fatty acid oxidation genes by interacting with the peroxisome proliferator-activated receptors (PPAR) pathway (Figure 2). Overall, these in vivo studies give affirmation that H2 can be a reliable alternative to current cancer treatment.
Figure 2.
Activation of PPAR pathway inducing fatty acid oxidation (diagram created using BioRender software). Under normal circumstances, PPARɑ up-regulates the peroxisomal β-oxidation by increasing the expression of the enzyme AOX, which facilitates the conversion of Acyl-CoA. However, when subjected to HRW, the hydrogen group lowers the activation of PPARɑ, resulting in the reduced expression of AOX. VLCFA very-long-chain fatty acid, HW hydrogen water, PPARα peroxisome proliferator-activated receptor alpha, AOX acyl-coenzyme A oxidase, FAD flavin adenine dinucleotide, O2 oxygen, H2O2 hydrogen peroxide
Clinical trials are a crucial next step after in vivo and in vitro studies. It provides a wider context on H2’s effect in cancer patients which ensures the usefulness and effectiveness of H2 on patients. A general trend can be seen across the results, with improvements in a patient’s QOL, blood parameters, expression of tumour markers, and tangible changes such as tumour weight and volume. With regards to tumour weight, H2 seemed to act on tumour-progression genes. Wang et al., (2018) showcased the inhibition of cancer cell proliferation, invasion, and migration by downregulating SMC3 expression in the presence of H2 which resulted in a decline in tumorigenesis in animal studies (Yang et al, 2018). Chen et al. have done extensive studies on various case reports on the efficacy of H2 towards cancer which can be deduced that hydrogen may play a role in modulating leukocyte count as it has been reported that increasing levels of leukocyte may participate in cancer cell invasion, hence enhancing its severity in patients (Madsen and Sahai, 2010). Considering these patient-based studies done by Chen et al., it provides sufficient evidence that H2 can indeed be a novel approach to prolong a cancer patient’s survivability.
Effects of H2 based on types of cancer
This systematic review provided an overview of the overall effects of complementary hydrogen therapy on a wide range of cancer types. Since Ohsawa’s study, H2 has been thoroughly investigated in various contexts; being the types of cancer and nature of the study (independent hydrogen study and combination therapy). Combining two treatment strategies has been used in cancer therapy to achieve an improved therapeutic response and longer control of tumour modalities control.
Respiratory cancer types were the most abundant among the included studies, covering NSCLC and lung cancer, with only one on nasopharyngeal cancer. The study on 58 adults with advanced NSCLC as subjects reported that cancer symptoms were significantly reduced after H2 treatment where the control group’s PFS was 4.4 ± 1.2 months and then the other H2 treated groups reported survival of more than 7.9 ± 2.2 months up to 10.1 ± 2.6 months (Chen et al., 2020). These studies highlighted a general tumour-repressive phenomenon and increased apoptosis activity. In the context of NSCLC, Chen et al. established that there is an overall improvement in progression-free survival (PFS) and overall survival (OS) with the duration of therapy ranging from 2 weeks to a year, indicating hydrogen’s anti-tumour abilities. This is in line with Terasaki et al., (2019)’s study whereby H2-rich water improved the overall survivability rate when in combination with other medications such as gefitinib and naphthalene. Considering this fact, Terasaki et al., (2019)’s study was also in agreement with our findings regarding combination therapy with hydrogen whereby the combinative use of 5-FU with 0.8mM of hydrogen in water could enhance the effects of 5-FU without compromising its effectiveness (Runtuwene et al., 2015).
It was found that the downregulation of CD47 can induce decreased density of tumour tissues and cell atypia after hydrogen treatment, ultimately inhibiting the development of lung cancer (Meng et al., 2020). This further proves the treatment’s anti-tumour effects as CD47 is a molecule that if blocked, its action results in tumour cell phagocytosis and elimination (Willingham et al., 2012). Furthermore, studies collated for this review highlighted the fact that H2 treatment can attenuate the side effects of radiotherapy such as Cis-platinum since the anti-cancer medications can give rise to serious adverse effects such as severe kidney problems, decreased immunity and hearing loss (Dasari and Bernard, 2014). This is supported by our findings that hydrogen possesses the ability to improve radiotherapy-induced hearing loss through the removal of ear-pus and enhancement of pure-tone audiograms (Chen et al., 2019). It has also been highlighted before by the significant reduction in fluorescence intensity in irradiated C57BL/6 mice (Chuai et al., 2012). Additionally, Chuai et al., (2012) outline how consuming H2 daily for 6 weeks can minimise the presence of reactive oxygen metabolites in blood and provide an overall improvement in a patient’s quality of life during radiotherapy. Therefore, it can be agreed that H2 can be used as an adjuvant to either enhance the effectiveness of anti-cancer medications or improve its adverse effects.
Promising effects of H2 can be found in patients suffering from gastrointestinal (GI)-related cancers. These GI-related cancers cover studies on patients suffering from colorectal, gallbladder, liver, gastric and colon cancer. Studies were able to showcase the protective effects of H2 on liver function of colorectal cancer patients. Yang et al., (2017) included 136 adult human subjects who were subjected to hydrogen-rich water with a concentration of 0.27 - 0.4 ppm and were shown to have no significant differences in their liver function indicators such as AST, ALT, ALP, IBIL or DBIL.
Furthermore, research on GI-related cancers reported the positive impact of H2 on a patient’s QOL such as appetite, taste buds (Kang et al., 2011), and physical and mental health (Chen et al., 2019). A past review further supported the potential of H2 to alleviate patients’ quality of life, especially when undergoing radiotherapy or chemotherapy as well. It was highlighted in a liver cancer study that H2 administration could prevent patients from suffering a drop in quality of life of 4.0 units after radiotherapy treatment (Ostojic, 2015). Yet, Hirano et al. exhibit no difference in QOL, despite being subjected to H2. It can be speculated that the reason for this varying response could be due to duration of H2 administration since Hirano et al. admitted that long-term inhalation may be required to observe such improvement.
A clinical trial discovered hydrogen’s ability to restore clusters of differentiation 8 (CD8+) T cells in colorectal cancer patients which improves the patient’s prognosis (Akagi and Baba, 2018). In this trial, Akagi subjected 55 patients with colorectal carcinoma, all aged between 28 to 96, to a hydrogen-oxygen gas mixture consisting of 680,000 ppm and 320,000 ppm respectively. The subjects undertook hydrogen gas treatment for 3 hours daily and subsequently received chemotherapy as well. The outcome of his clinical trial resulted in a significant PFS and longer but non-significant OS. The improvement in patient prognosis has been a common finding in many clinical trials that uses hydrogen as a form of treatment against cancer. Chen et al., (2020)’s review highlighted case studies that showed a significant improvement in patient’s prognosis after hydrogen therapy. These case studies showcased the pseudo-progression of gallbladder cancer, significant reduction in tumour size in lung cancer as well as difference in binaural hearing. Furthermore, hydrogen therapy has also been shown to improve patient prognosis in an emergency context. Sano et al. described a similar finding with ours in which the neurological prognosis of rat models with cardiac arrest was significantly improved at 24 hours after the return of spontaneous circulation after H2 inhalation treatment (Sano et al., 2018).
There have been few studies that highlighted hydrogen’s capability to cooperate synonymously with current cancer therapy. Runtuwene et al. demonstrated the enhancement of 5-FU induced inhibition of colon cancer by utilising hydrogen. As mentioned before, it was found that the combination of 5-FU with 0.8mM of hydrogen in water induced a more effective cell apoptosis compared to 5-FU treatment, whilst treatment with hydrogen alone showed almost no significant difference in HepG2 cells. According to Yang et al., hydrogen can in fact alleviate the adverse effects of mFOLFOX-6 cancer therapy by inducing a protective effect on the liver (Chen et al., 2019). Great improvements in blood parameters and reduction in tumour markers were found in a couple of case reports by Chen et al., (2019) prolonging the survival of the patient. Therefore, it can be deduced that hydrogen can be used in various approaches when it comes to cancer treatment, either as an adjuvant or as a sole independent treatment.
Cancers affecting the female reproductive system can also be alleviated with hydrogen treatment. There are a total of 4 studies on the several types of reproductive cancer: one on cervical cancer, two on endometrial cancer and one on ovarian cancer. Although no clinical trials have been included, there are enough studies that do showcase hydrogen’s effect on reproductive cancer types. It was found that hydrogen has the ability to shrink tumour growth through minimal cell proliferation which in turns promote cell apoptosis in in vitro studies using HeLa cells (Chu et al., 2021). Yang et al., (2020) did an RNA sequencing analysis to reveal hydrogen can inducing apoptosis through TNF and NF-ϰB pathways in endometrial cancer cells. Gene ontology (GO) pathway enrichment analysis showed that 30 pathways were upregulated in hydrogen-treated endometrial cell lines, HEC1A and ANC3A cells, many of which include TNF and NF-κB pathways. Upon 24 hours of hydrogen treatment, HEC1A and Ishikawa cells exhibited a significant increase in apoptotic rate (p < 0.05).
It was also discovered that hydrogen-treated culture medium and irradiated HEC1A cells displayed a significant apoptosis rate compared to a normal culture medium (Yang et al., 2020). H2 plays a role in radiotherapy-induced apoptosis in HEC1A cells by increasing its rate 39% in hydrogen-cultured media, further proving itself as an effective enhancer to radiotherapy. Besides that, H2 could also induce protective effects against oxidative stress by downregulating NF-KB p65 and HO-1 expressions. Although HO-1 has anti-oxidative properties itself, it still played a role in stimulating cell growth which would enhance the severity of cancer due to excessive proliferation (Lien et al., 2014; Nitti et al., 2017).
Further studies that support the fact that hydrogen could play a role in minimising the severity of cancers affecting the female reproductive system are limited, hence it can only be deduced from the 4 articles found. In addition, Shang et al., (2018) displayed the therapeutic potential of hydrogen in ovarian cancer. The in vivo studies showed a similar result to Chu et al., (2021)’s study in which tumour growth was suppressed after 6 weeks of hydrogen inhalation. This can be explained through their in vitro studies in which hydrogen can inhibit cancer cell proliferation, invasion, and colony formation.
For cancers under the central nervous system, glioblastoma and neuroblastoma, studies found primarily focus on hydrogen’s effects alone. According to Meng-yu Liu et al., (2019)’s in vitro study on glioblastoma (GBM), H2 therapy inhibits glioma cell motility and colony formation due to its small molecular size, allowing it to easily pass the blood-brain barrier. Its ability to pass through the blood-brain barrier is a crucial fact when it comes to searching for novel treatments for glioblastoma. The reason is due to the fact that very few molecules can permeate through the blood-brain barrier; even if some do make it through, efflux pumps would only export the remainders out (Shergalis et al, 2018). Hence, from Liu et al., (2019) we were able to confirm the therapeutic concentration needed to reach the target site through hydrogen inhalation treatment. Through this, It was also proven that hydrogen could induce glioma stem-cell differentiation and reduce glioma cell stemness through immunohistochemistry staining. The capacity of glioma cells to form spheres was likewise inhibited by hydrogen treatment.
In this review, only one in vivo study focused on hydrogen’s effects on renal cell carcinoma (Li et al., 2013). According to the study, hydrogen can protect rats from Fe-NTA-induced tubular injury. From this finding, it can be deduced that hydrogen has a similar effect on other types of cancer whereby it could act on molecules to inhibit early tumour promotional events; in this case, suppressing ornithine decarboxylase (ODC) activity and lowering the incorporation of 3H-thymidine into renal DNA during long-term consumption. Furthermore, H2 could lessen mitochondrial dysfunction in kidneys and emphasise hydrogen’s behaviour towards various molecules and pathways such as the signal transducer and activator of the transcription 3 (STAT3) signalling pathway. Despite not having extensive studies regarding hydrogen’s effects on renal cell carcinoma, Fang-Yin Li et al. were able to elaborate on its effects in their in vivo study.
Limitations
Some limitations were observed in this review, which may affect the accuracy of the findings. One of them was that there were relatively fewer clinical trials when compared to other types of studies. Secondly, because of the diverse focus of studies, the comparison of the outcomes between papers was not as accurate as studies with a specific focus on the types of cancer and its investigated pathway. There is also a difference in number between the studies, with most on respiratory and gastrointestinal cancers, leaving other types of cancer with inadequate or unknown data to report on. Moreover, the majority of the papers originate from East-Asian countries, which may not have similar outcomes as in other populations.
In conclusion, the findings of this systematic review have shown that there is sufficient evidence of H2’s therapeutic effects on cancer. The therapy can be applied through various administration methods while generally demonstrating similar outcomes with no major adverse effects. However, it is worth noting the context of how each administration method was employed. When analysing in terms of study design, in vitro studies have shown that H2 can induce its effect via its specific interaction on a molecular level, whilst in vivo and patient-based studies give us more concrete evidence of hydrogen’s effects on cancer. Through combination therapy studies, it is shown how hydrogen can amplify the use and effectiveness of radiation and chemotherapy treatment, in addition to alleviating the adverse effect without interfering with the primary cancer treatment. This eventually raises an overall improvement towards cancer treatment and prognosis. As a future recommendation, studies should be conducted with more focus on in vivo and patient-based studies, to further investigate the safety, effectiveness, and long-term effects of H2 therapy in cancer.
Author Contribution Statement
Muhammad Nooraiman Zufayri Mohd Noor and Adlin Sofea Alaud din performed the literature search, data analysis and written the first draft of the manuscript. Chai Hong Yeong contributed to the idea of this review article. Chai Hong Yeong, Yin How Wong, Chung Yeng Looi, Eng Hwa Wong and Priya Madhavan critically revised, edited and proofread the work. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Adrinna Yee Weng Lum and Sylvia Chong Hongli for their assistance in literature search.
Funding Statement
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Ethics Declaration
Ethics approval was not requested since the data collected from this review was retrieved and synthesised from secondary sources (published articles and clinical trials).
Data Availability
Not applicable.
Conflict of Interest
The authors declare no conflict of interest in this study.
References
- Arumugam T. More than 66,000 Malaysians to be diagnosed with cancer annually by 2030. New Straits Times. 2021. Available from: https://www.nst.com.my/news/nation/2021/10/736871/more-66000-malaysians-be-diagnosed-cancer-annually-2030.
- Akagi J, Baba H. [Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis]; Oncol Rep. 2018 41:301–11. doi: 10.3892/or.2018.6841. [DOI] [PubMed] [Google Scholar]
- Akagi J, Baba H. [Hydrogen gas activates coenzyme Q10 to restore exhausted CD8+ T cells, especially PD 1+Tim3+terminal CD8+ T cells, leading to better nivolumab outcomes in patients with lung cancer]; Oncol Lett. 2020 20:258. doi: 10.3892/ol.2020.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte PM, Caicedo A. [Stemness in cancer: Stem cells, cancer stem cells, and their microenvironment]; Stem Cells Int. 2017 doi: 10.1155/2017/5619472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhou C, Xie K, et al. [Hydrogen-rich saline alleviated the hyperpathia and microglia activation via autophagy mediated inflammasome inactivation in neuropathic pain rats]; Neuroscience. 2019 421:17–30. doi: 10.1016/j.neuroscience.2019.10.046. [DOI] [PubMed] [Google Scholar]
- Chen JB, Kong XF, Lv YY, et al. [“Real world survey” of hydrogen-controlled cancer: a follow-up report of 82 advanced cancer patients]; Med Gas Res. 2019 9:115–21. doi: 10.4103/2045-9912.266985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JB, Kong XF, Mu F, et al. [Hydrogen–oxygen therapy can alleviate radiotherapy-induced hearing loss in patients with nasopharyngeal cancer]; Ann Palliat Med. 2019 8:746–51. doi: 10.21037/apm.2019.11.18. [DOI] [PubMed] [Google Scholar]
- Chen JB, Kong XF, Mu F, et al. [Hydrogen therapy can be used to control tumor progression and alleviate the adverse events of medications in patients with advanced non-small cell lung cancer]; Med Gas Res. 2020 10:75–80. doi: 10.4103/2045-9912.285560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JB, Kong XF, Qian W, et al. [Two weeks of hydrogen inhalation can significantly reverse adaptive and innate immune system senescence patients with advanced non-small cell lung cancer: a self-controlled study]; Med Gas Res. 2020 10:149–54. doi: 10.4103/2045-9912.304221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JB, Lu YY, Xu KC. A narrative review of hydrogen oncology: from real world survey to real world evidence. Med Gas Res. 2020;10:130–3. doi: 10.4103/2045-9912.296044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JB, Mu F, Lu T, Du DM, Xu KC. [Brain metastases completely disappear in non-small cell lung cancer using hydrogen gas inhalation: A case report]; Onco Targets Ther. 2019 12:11145–51. doi: 10.2147/OTT.S235195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JB, Mu F, Lu T, et al. [A gallbladder carcinoma patient with pseudo-progressive remission after hydrogen inhalation]; Onco Targets Ther. 2019 12:8645–51. doi: 10.2147/OTT.S227217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JB, Pan ZB, Du DM, et al. [Hydrogen gas therapy induced shrinkage of metastatic gallbladder cancer: A case report]; World J Clin Cases. 2019 7:2065–74. doi: 10.12998/wjcc.v7.i15.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Gao J, Wang J, et al. [Mechanism of hydrogen on cervical cancer suppression revealed by high throughput RNA sequencing]; Oncol Rep. 2021 46:141. doi: 10.3892/or.2021.8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuai Y, Qian L, Sun X, Cai J. [Molecular hydrogen and radiation protection]; Free Radic Res. 2012 doi: 10.3109/10715762.2012.689429. [DOI] [PubMed] [Google Scholar]
- Dasari S, Bernard Tchounwou P. [Cisplatin in cancer therapy: Molecular mechanisms of action]; Eur J Pharmacolo. 2014 740:364–78. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weille J. [On the genesis of neuroblastoma and glioma]; Int J Brain Sci. 2014 [Google Scholar]
- Dole M, Wilson FR, Fife WP. [Hyperbaric hydrogen therapy: a possible treatment for cancer]; Science. 1975 190:152–4. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- Gao Y, Jiang S, Xing Y, et al. Investigating the effect of hydrogen-rich water on liver cell injury and liver cancer by regulating GP73/ TGF-β pathway. 2021 DOI:10.21203/rs.3.rs-201468/v1. [Google Scholar]
- Hirano S, Aoki Y, Li XK, et al. [Protective effects of hydrogen gas inhalation on radiation-induced bone marrow damage in cancer patients: a retrospective observational study]; Med Gas Res. 2021 11:104–9. doi: 10.4103/2045-9912.314329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Wang Y, Zhou F, et al. [Prognostic value of high EZH2 expression in patients with different types of cancer: a systematic review with meta-analysis]; Oncotarget. 2016 7:4584–97. doi: 10.18632/oncotarget.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Liu G, Zhang L, et al. [Therapeutic efficacy of hydrogen rich saline alone and in combination with PI3K inhibitor in non small cell lung cancer]; Mol Med Rep. 2018 18:2182–90. doi: 10.3892/mmr.2018.9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama S, Hasegawa G, Asano M, et al. [Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance]; Nutr Res. 2008 28:137–43. doi: 10.1016/j.nutres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Kang KM, Kang YN, Choi IB, et al. [Effects of drinking hydrogen-rich water on the quality of life of patients treated with radiotherapy for liver tumors]; Med Gas Res. 2011 1:11. doi: 10.1186/2045-9912-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai D, Takaki A, Nakatsuka A, et al. [Hydrogen-rich water prevents progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice]; Hepatology. 2012 56:912–21. doi: 10.1002/hep.25782. [DOI] [PubMed] [Google Scholar]
- Kiyotaki S. [An experimental study of the tissue blood flows under hyperthermia in normal rat bladder and bladder tumor]; Jpn J Urol. 1988 79:287–96. doi: 10.5980/jpnjurol1928.79.2_287. [DOI] [PubMed] [Google Scholar]
- Lee SW, Nakajima K, Uchibayashi T, Yamamoto H, Hisazumi H. A study of local blood flow in renal cancer and normal tissue using a hydrogen gas clearance method. Thermal Medicine. 1989;5:375–9. [Google Scholar]
- Li FY, Zhu SX, Wang ZP, et al. Consumption of hydrogen-rich water protects against ferric nitrilotriacetate-induced nephrotoxicity and early tumor promotional events in rats. Food Chem Toxico. 2013;61:248–54. doi: 10.1016/j.fct.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Li Z, Lü M, Zhou Y, et al. Role of long non-coding RNAs in the chemoresistance of gastric cancer: A systematic review. Onco Targets Ther. 2021;14:503–18. doi: 10.2147/OTT.S294378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien GS, Wu MS, Bien MY, et al. Epidermal growth factor stimulates nuclear factor-κB activation and heme oxygenase-1 expression via c-Src, NADPH oxidase, PI3K, and Akt in human colon cancer cells. PLoS One. 2014;9:e104891. doi: 10.1371/journal.pone.0104891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yan Z, Wang Y, Meng J, Chen G. Suppression of autophagy facilitates hydrogen gas mediated lung cancer cell apoptosis. Oncol Lett. 2020;20:112. doi: 10.3892/ol.2020.11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MY, Xie F, Zhang Y, et al. Molecular hydrogen suppresses glioblastoma growth via inducing the glioma stem-like cell differentiation. Stem Cell Res Ther. 2019;10:145. doi: 10.1186/s13287-019-1241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen CD, Sahai E. Cancer dissemination—Lessons from leukocytes. Dev Cell. 2010;19:13–26. doi: 10.1016/j.devcel.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Meng J, Liu L, Wang D, Yan Z, Chen G. Hydrogen gas represses the progression of lung cancer via down-regulating CD47. Biosci Rep. 2020:40. doi: 10.1042/BSR20192761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Ito M, Ohsawa I. Molecular hydrogen protects against oxidative stress-induced SH-SY5Y neuroblastoma cell death through the process of mitohormesis. PLoS One. 2017;12:e0176992. doi: 10.1371/journal.pone.0176992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitti M, Piras S, Marinari U, et al. HO-1 induction in cancer progression: A matter of cell adaptation. Antioxidants. 2017;6:29. doi: 10.3390/antiox6020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Ito M, Ichihara M, Ito M. Molecular hydrogen as an emerging therapeutic medical gas for neurodegenerative and other diseases. Oxid Med Cell Longev. 2012:2012. doi: 10.1155/2012/353152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–94. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- Ostojic SM. Molecular hydrogen: An inert gas turns clinically effective. Ann Med. 2015;47:301–4. doi: 10.3109/07853890.2015.1034765. [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BJ, Fife WP, Corbett TH, Schabel FM. Response of five established solid transplantable mouse tumors and one mouse leukemia to hyperbaric hydrogen. Cancer Treat Rep. 1978;62:1077–79. [PubMed] [Google Scholar]
- Runtuwene J, Amitani H, Amitani M, et al. Hydrogen–water enhances 5-fluorouracil-induced inhibition of colon cancer. Peer J. 2015;3:e859. doi: 10.7717/peerj.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L, Xie F, Li J, et al. Therapeutic potential of molecular hydrogen in ovarian cancer. Transl Cancer Res. 2018;7:988–95. [Google Scholar]
- Shergalis A, Bankhead III A, Luesakul U, Nongnuj M, Neamati N. Current challenges and opportunities in treating glioblastoma. Pharmacol Rev. 2018;70:412–45. doi: 10.1124/pr.117.014944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimouchi A, Nose K, Mizukami T, Che D-C, Shirai M. Molecular hydrogen consumption in the human body during the inhalation of hydrogen gas. Adv Exp Med Biol. 2013;789:315–21. doi: 10.1007/978-1-4614-7411-1_42. [DOI] [PubMed] [Google Scholar]
- Shin W. Medical applications of breath hydrogen measurements. Anal Bioanal Chem. 2014;406:3931–9. doi: 10.1007/s00216-013-7606-6. [DOI] [PubMed] [Google Scholar]
- Sano M, Suzuki M, Homma K, et al. Promising novel therapy with hydrogen as for emergency and critical care medicine. Acute Med Surg. 2018;5:113–8. doi: 10.1002/ams2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki Y, Suzuki T, Tonaki K, et al. Molecular hydrogen attenuates gefitinib-induced exacerbation of naphthalene-evoked acute lung injury through a reduction in oxidative stress and inflammation. Lab Invest. 2019;99:793–806. doi: 10.1038/s41374-019-0187-z. [DOI] [PubMed] [Google Scholar]
- Tian Y, Zhang Y, Wang Y, et al. Hydrogen, a novel therapeutic molecule, regulates oxidative stress, inflammation, and apoptosis. Front Physiol. 2021;12:789507. doi: 10.3389/fphys.2021.789507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi S, Sharma S, Gupta P, Saini A, Kaushal C. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J Adv Pharm Technol Res. 2011;2:236–40. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Wang L, Zhang Y, Zhao Y, Chen G. Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed Pharmacother. 2018;104:788–97. doi: 10.1016/j.biopha.2018.05.055. [DOI] [PubMed] [Google Scholar]
- Willingham SB, Volkmer J-P, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–7. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR, Veal D, Whiteman M, Hancock JT. Hydrogen gas and its role in cell signalling. CABI Review. 2017;12:1–3. [Google Scholar]
- Yang Q, Ji G, Pan R, Zhao Y, Yan P. Protective effect of hydrogen-rich water on liver function of colorectal cancer patients treated with mFOLFOX6 chemotherapy. Mol Clin Oncol. 2017;7:891–6. doi: 10.3892/mco.2017.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu PY, Bao W, et al. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer. 2020;20:28. doi: 10.1186/s12885-019-6491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liu YP, Bao W, Chen JS, Xi XW. RNA sequencing analysis reveals apoptosis induction by hydrogen treatment in endometrial cancer via TNF and NF-κB pathways. Transl Cancer Res. 2020;9:3468–82. doi: 10.21037/tcr.2020.03.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Zhu Y, Xi X. Anti inflammatory and antitumor action of hydrogen via reactive oxygen species (review) Oncol Lett. 2018;16:2771–6. doi: 10.3892/ol.2018.9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B, Cui H, Xu W. Hydrogen inhibits the proliferation and migration of gastric cancer cells by modulating lncRNA MALAT1/miR-124-3p/EZH2 axis. Cancer Cell Inter. 2021;21:70. doi: 10.1186/s12935-020-01743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.