Abstract
Tumor necrosis factor alpha (TNF-α) is associated with malarial pathology in both humans and mice. In Plasmodium chabaudi chabaudi (AS) infections, the production of TNF-α and reactive metabolites from macrophages are also thought to play a role in controlling acute parasitemia. Since many of the biological functions of TNF-α are effected through the p55 receptor (p55R), mice made defective in this receptor via a targeted gene disruption (p55R−/−) have been used to study its involvement in the immune response against P. chabaudi chabaudi and in the pathology associated with this infection. In the absence of the p55R, mice could overcome their primary infection, although higher acute-blood-stage parasitemias and more significant recrudescences were observed. Hypoglycemia, hypothermia, loss of erythrocytes, and loss of body weight, which occur transiently in this infection, were exacerbated by the lack of the p55R, but the differences were small, suggesting that other factors affect these symptoms. In contrast to wild-type (WT) mice, a second challenge infection in p55R−/− mice resulted in a course of infection similar to a primary infection. The malaria-specific immunoglobulin G antibody response of p55R−/− mice was lower than that of WT mice and was not increased by the second challenge infection. These data suggest that p55R−/− mice do not develop an efficient memory B-cell response against malarial infection and that this antibody response is important in immunity to reinfection.
Tumor necrosis factor alpha (TNF-α) is thought to play a role in the development of immunity and pathology in malaria infections in experimental models and in humans (11). High levels of TNF-α in the spleen correlate with resistance to Plasmodium chabaudi chabaudi (AS) infections (23). Inflammatory cytokines, including TNF-α, may contribute to the clearance of acute stage infections of P. chabaudi, P. yoelii, and P. vinckei, possibly through the induction of mediators such as nitric oxide (NO) and reactive oxygen intermediates (42). However, it appears that TNF-α may not be a critical cytokine for these early protective responses, since mice deficient in the receptors through which the biological effects of TNF-α are mediated (p55R and p75R) are able to control and reduce parasitemia (45). TNF-α can bring about killing of intraerythrocytic stages of P. falciparum and P. vivax in vitro through the action of intermediaries such as NO (42, 43). The level of NO in the blood, which is a downstream product of TNF-α activity, is correlated with resistance in young children infected with P. falciparum (1).
On the other hand, TNF-α is clearly implicated in the pathology of malaria (11, 17, 18, 25). It has been shown to be crucial for the development of an experimental form of cerebral malaria induced by P. berghei in mice (17), and high plasma TNF-α levels in humans infected with P. falciparum are associated with a poor prognosis in cases of cerebral malaria (18, 25). Treatment of P. falciparum-infected children with anti-TNF-α antibodies reduces fever (5, 26). In other experimental models of malaria, TNF-α is associated with hypoglycemia and loss of body weight (31).
TNF-α acts through two receptors, p55R and p75R, which it shares with the functionally related TNF-β (3, 4, 30). The majority of the biological functions of TNF-α/β, such as those associated with inflammatory pathology and endotoxic shock, however, are attributed to signaling through p55R (13, 39, 44, 56). p75R preferentially binds membrane bound TNF-α (38), and its role in inflammation is unclear. However, in the absence of p55R, TNF-p75R interactions can eventually lead to macrophage activation and NO production (16). TNF-p55R interactions have been shown to be important not only for inflammatory responses and for host immunity against a variety of pathogens (7, 13, 15, 16, 39, 44, 58) but also for the architecture of lymphoid organs and the correct localization of B cells to the follicles (33, 55). On the other hand, TNF-p75R interactions have been shown to be involved in lymphocyte proliferation (19) and the migration of Langerhans cells (57).
We have investigated here a P. chabaudi chabaudi infection in p55R knockout (KO) mice (p55R−/−) to determine whether signaling through this receptor plays a role in the development of pathology associated with an acute primary infection and also whether TNF-p55R interactions have any impact on the acquisition of protective immunity. In agreement with previous observations in p55R-p75R double-KO mice (23), p55R−/− mice can overcome a primary infection of P. chabaudi chabaudi (AS) with little obvious alteration in accompanying acute-phase pathology. However, a secondary challenge infection of these mice results in a course of infection indistinguishable from that of a primary infection and little development of a malaria-specific immunoglobulin G (IgG) antibody response. These experiments suggest that TNF-p55R interactions are essential for an effective memory response and underline the requirement for antibody and B cells in protective immunity to reinfection.
MATERIALS AND METHODS
Mice.
p55R−/− and wild-type (WT) mice (44) on a mixed background of 129sv and C57BL/6 mice were a kind gift from H. Blüthmann (Hoffmann-La Roche, Basel, Switzerland) and were maintained by interbreeding homozygous males and females in the animal facilities at Imperial College, London, United Kingdom. All mice were maintained with sterile bedding, food, and water. The genotype of all experimental animals was confirmed by PCR before infection. The defective TNF-α p55R gene was detected by PCR of tail DNA using the following specific primers: sense, 5′-CTC TCT TGT GAT CAG CAC TG-3′; antisense, 5′-CTG GAA GTG TGT CTC AC-3′; and neo-34, 5′-TCC CGC TTC AGC AAC GTC-3′. The combination of a sense and antisense primer set amplified the WT p55R gene and gave a PCR product of 1.4 kb, whereas the sense and neo-34 primer combination detected the mutate p55R gene at 1.0 kb (H. Blüthmann, personal communication).
Infection with P. chabaudi chabaudi (AS) parasites.
P. chabaudi chabaudi (AS) parasites were maintained as described previously (51). Mice aged 6 to 12 weeks were infected by injecting 105 parasitized erythrocytes intraperitoneally (i.p.). The course of infection was monitored by examination of Giemsa-stained (Fluka) thin blood films every 2 days throughout the experimental period. Two months after the primary infection, surviving p55R−/− and WT mice were rechallenged with 105 P. chabaudi chabaudi (AS) parasites i.p. Naive p55R−/− and WT mice were infected at the same time as the controls.
Malaria-specific antibody responses.
Plasma samples were collected from at least eight female p55R−/− and WT mice before infection, weekly for 6 weeks after the primary infection, and weekly for 4 weeks after the secondary infection. The amounts of malaria-specific antibodies were measured by using a direct enzyme-linked immunosorbent assay (ELISA) as described previously (27). Briefly, a lysate of P. chabaudi chabaudi blood-stage parasites was used to capture the specific antibody present in plasma samples. The isotype of bound specific antibody was revealed by using anti-mouse isotype antibodies conjugated with alkaline phosphatase (Southern Biotechnology, Cambridge, England). A pooled immune plasma sample obtained from mice that had recovered from more than five challenge infections of P. chabaudi chabaudi was used as a standard and was given an arbitrary value of 1,000 U/ml for each of the isotypes. The concentration of each specific isotype was calculated according to the standard curve generated from the immune plasma. Plasma from uninfected p55R−/− and WT mice were also included as controls.
IFN-γ and TNF-α in the plasma of infected mice.
Plasma samples were taken using heparinized sterile pipettes from at least four mice daily from day 5 to day 10 and then weekly until week 4 of the infection. The samples were collected at the same time each day to ensure that the time of schizogony did not differentially affect the level of cytokines in the mice. Plasma gamma interferon (IFN-γ) levels were measured using a sandwich ELISA described previously (47). Briefly, R4-6A2 (48) was used as the capture antibody, and biotin-labeled AN-18 (41) was used as the detection antibody. Plasma TNF-α levels were measured using a TNF-α ELISA kit (PharMingen/Becton Dickinson). The sensitivities of the IFN-γ and TNF-α ELISAs were 20 pg/ml and 40 pg/ml, respectively.
Malaria-associated pathology.
Erythrocyte counts, blood glucose levels, body temperature, and weight changes were measured as described previously (31) every 2 days during the acute primary infection. Uninfected female p55R−/− and WT mice were also included during the experiments to control for the variation in these parameters that were not associated with the malaria infection. The number of erythrocytes was counted using hemocytometer. The blood glucose level was measured using a commercial glucose machine and glucose strips (BM-40; Boehringer Mannheim, East Sussex, United Kingdom). Body temperature was measured using a rectal thermoprobe. Body weight was measured using a top-pan electronic balance.
Cytokine expression at the mRNA level using competitive PCR.
The inducible nitric oxide synthase (iNOS) mRNA level was measured using a competitive PCR as described previously (6, 31). mRNA was extracted from splenocytes of male and female p55R−/− and WT mice before and at weekly intervals during the primary infection. cDNA from reverse transcription of mRNA was competed with an internal competitive fragment, pNIL (46). The concentration of sample cDNA was calculated when the log10 ratio of band intensity equaled zero (i.e., those bands giving equal intensity). The concentration equaled the number of molecules of the competitive fragment. Levels of measured cytokines were then normalized against 106 β2 microglobulin molecules.
Statistical analysis.
The Student's t test was used to calculate the significance of the differences seen in the course of infection in p55R−/− and WT mice. The Mann-Whitney U test was carried out to analyze the difference in pathology parameters, malaria-specific antibody, and cytokine levels between the p55R−/− and the WT mice.
RESULTS
Secondary P. chabaudi chabaudi infections in p55R−/− mice are more pronounced than in WT mice.
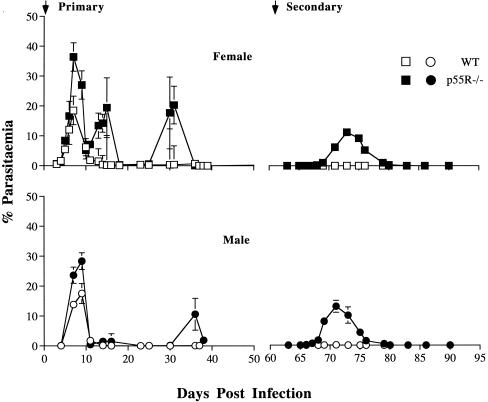
Twenty female p55R−/− and littermate WT mice and fifteen male p55R−/− and littermate WT mice were infected with 105 infected erythrocytes by i.p. injection (Fig. 1). Both male and female WT control mice had a typical P. chabaudi chabaudi (AS) infection (51). Parasites were first detectable at day 3 of infection, with a mean peak parasitemia of 19% between days 7 and 9. Parasites were then cleared rapidly from the circulation and, by day 18, parasitemia decreased to <0.1%. Two small patent recrudescences with parasitemias of approximately 1% occurred at days 12 and 36 of infection in female WT mice; these recrudescences became undetectable after day 40 in both male and female WT mice. In contrast, male and female p55R−/− mice had significantly higher peak levels of parasitemia (30 and 37%, respectively) compared to WT mice (P < 0.001, Student's t test). Two significantly higher recrudescences with parasitemias of 20% occurred at days 15 and 32 in female p55R−/− mice (P < 0.001, Student t test), whereas in male p55R−/− mice, two recrudescences with parasitemias of lesser magnitude were also measured at day 15 and day 35 postinfection. All mice survived the primary infection.
FIG. 1.
The primary and secondary course of a P. chabaudi chabaudi (AS) infection in p55R−/− and WT mice (BL6 × 129sv). Male and female p55R−/− (● and ■, respectively) and WT mice (○ and □, respectively) were infected with 105 parasites i.p. The course of infection was monitored by examination of Giemsa-stained blood films. Two months after primary infection, p55R−/− and WT mice were rechallenged with 105 parasites i.p. The graphs represent the means of 15 male and 20 female p55R−/− and WT mice (from three independent experiments). The error bars represent the standard error of the geometric mean of parasitemia (SEM). For clarity, the SEM values of <10% of the mean are not shown.
p55R−/− and WT mice were given a second P. chabaudi chabaudi infection 2 months after clearance of the primary infection. A parasitemia of <0.5% was detected in WT mice. Surprisingly, the second challenge infection of p55R−/− mice resulted in a significantly higher level of parasitemia than seen in the WT mice. Parasites were detectable in the blood 7 to 8 days after injection of 105 infected erythrocytes and resulted in peak parasitemias of 12 to 15% in females and males, respectively, between days 11 and 12. Parasitemia became subpatent by day 20 postchallenge. All animals recovered from the challenge infection.
Malaria-associated pathology in p55R−/− and WT mice during the acute primary infection.
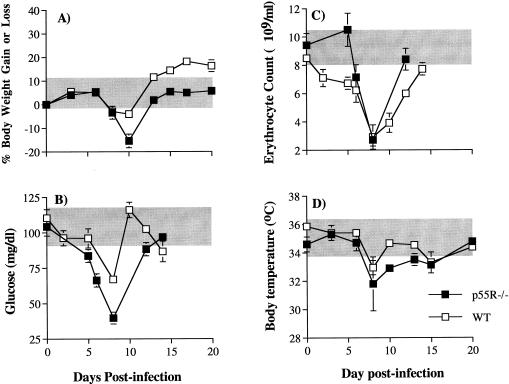
Four common parameters of pathology (anemia, hypothermia, hypoglycemia, and weight loss) were measured. All p55R−/− and WT mice displayed these pathological signs during the acute phase of infection (the data for female mice are shown in Fig. 2). Weight loss in p55R−/− and WT mice occurred between days 8 and 12 of infection (Fig. 2A). At day 10 postinfection, p55R−/− mice had lost 15% of their body weight compared to 5% in WT mice (P < 0.01, Mann-Whitney U test). By day 12, body weight in p55R−/− mice had recovered to normal uninfected levels. WT mice also regained their lost body weight during this time.
FIG. 2.
Body weight (A), blood glucose levels (B), erythrocyte counts (C), and body temperature (D) in female p55R−/− (■) and WT (□) mice during the primary P. chabaudi chabaudi (AS) infection. Mice were infected with 105 parasites i.p. The graphs represent the mean of eight p55R−/− and WT mice (from two independent experiments). The error bars indicate the SEM. SEM values of <10% of the mean are not shown. The shaded areas represent the range of weight, glucose level, and body temperature changes in uninfected animals measured during the infection period.
Plasma glucose levels in both p55R−/− and WT mice diminished within 2 days of infection. However, the decrease was more marked in the p55R−/− mice (Fig. 2B), which became significantly more hypoglycemic than WT mice from day 6 postinfection throughout the measurement period (P < 0.01, Mann-Whitney U test).
Erythrocyte counts decreased to a similar minimum level in both WT and p55R−/− mice at day 8 of the primary infection (Fig. 2C). However, anemia was of a shorter duration in the p55R−/− mice, where lower erythrocyte counts were not observed until day 6 postinfection (P < 0.01, Mann-Whitney U test).
Both p55R−/− and WT mice experienced hypothermia. Although there was no significant difference in the lowest temperature between p55R−/− and WT mice at day 8 postinfection (Fig. 2D), between days 10 and 12 p55R−/− mice had significantly lower body temperatures (P < 0.01, Mann-Whitney U test).
The differences between p55R−/− and WT male mice were similar to those shown for female mice and are not shown.
IgG antibody responses in p55−/− and WT mice during a second challenge infection.
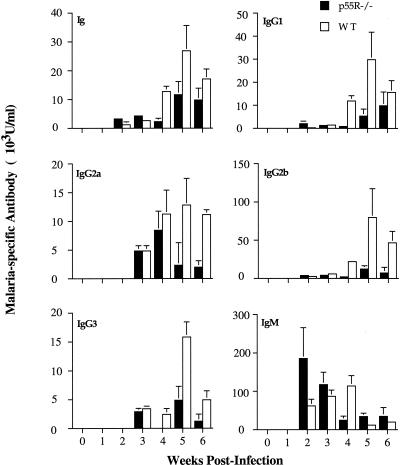
Plasma samples were taken from eight female p55R−/− mice and WT littermate controls once before and weekly during a primary and secondary infection and were tested for the presence of malaria-specific antibodies. The level of malaria-specific immunoglobulin (Fig. 3) was significantly higher in the plasma of WT mice compared with p55R−/− mice after 4 weeks of infection (P < 0.01, Mann-Whitney U test). When the isotype composition of the malaria-specific antibody response was analyzed, it was apparent that this difference was due to a lower amount of IgG antibodies. Initially (weeks 2 and 3), there was no significant difference in the amount of IgG antibody between p55R−/− and WT mice. However, at week 4 of the infection the levels of specific IgG1, IgG2b, and IgG3 were significantly lower in p55R−/− mice compared with WT controls (P < 0.01, Mann-Whitney U test), and at 5 weeks the levels of IgG2a, IgG2b, and IgG3 were significantly lower (P < 0.01, Mann-Whitney U test). This suggests that the p55R−/− mice were unable to maintain an IgG response.
FIG. 3.
Isotypes of malaria-specific antibodies during a primary infection in female p55R−/− (■) and WT (□) mice. Mice were infected with 105 P. chabaudi chabaudi (AS) parasites i.p. Plasma samples were collected before and for 4 weeks postinfection. Data are shown as arbitrary units of antibody of a given isotype as calculated from a standard curve of hyperimmune serum (see Materials and Methods). The histogram bars represent the geometric mean of the concentration of antibody, and the vertical lines are the SEM values for eight individual mice. For clarity, SEM values of <10% of the mean are not shown.
The kinetics of the IgM antibody response of p55R−/− and WT mice was different, but the amount of antibody present in the plasma was similar. IgM antibody levels were maximal at 2 weeks postinfection in the p55R−/− mice and significantly greater than in WT mice (P < 0.01, Mann-Whitney U test), whereas overall the greatest response was seen in WT mice at week 4.
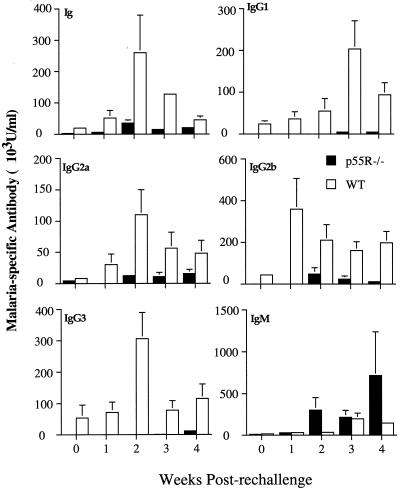
Mice received a second infection 2 months after the primary infection. At the time of challenge, the antibody levels of all isotypes, IgG2a and IgM, were substantially lower in the p55R−/− mice than in the WT mice (day 0, Fig. 4). By 2 weeks postchallenge, a striking difference in the amounts of malaria-specific antibodies was observed (Fig. 4). Specific IgG1, IgG2a, IgG2b, and IgG3 could not be detected until week 2 of the second infection in the p55R−/− mice and did not exceed the amounts measured during the primary infection. In contrast, the levels of all the IgG isotypes in the WT mice increased at least 10-fold compared to those observed in the primary infection. These results, together with a significantly higher IgM response in the p55R−/− mice, suggest that p55R−/− mice are not able to make an effective secondary antibody response to P. chabaudi chabaudi.
FIG. 4.
Isotypes of the malaria-specific antibody response in female p55R−/− (■) and WT (□) mice after a secondary infection of P. chabaudi chabaudi (AS). Mice were challenged with 105 parasites i.p. 8 weeks after the primary infection. Plasma samples were collected before challenge and at weekly intervals for 4 weeks. Data are shown as arbitrary units of the antibody of a given isotype as calculated from a standard curve of hyperimmune serum (see Materials and Methods). The histogram bars represent the geometric mean of the concentration of antibody, and the vertical lines are the SEM values for eight individual mice. For clarity, SEM values of <10% of the mean are not shown.
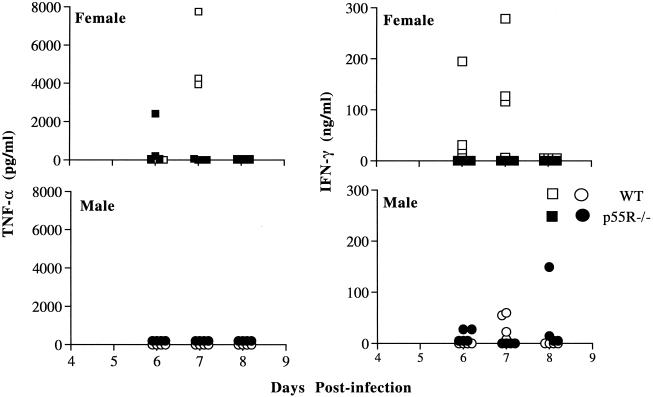
Decreased IFN-γ and TNF-α in plasma of p55R−/− and WT mice.
IFN-γ and TNF-α were detectable in the plasma of male and female WT mice on days 6 and 7 following a primary infection (Fig. 5). The plasma IFN-γ levels of female mice were greater than those in male mice and were at concentrations similar to those described previously for C57BL/6 mice infected with P. chabaudi chabaudi (AS) (47). In contrast, IFN-γ and TNF-α were only detectable in a small proportion of p55R−/− mice at these times (one out of eight was positive for TNF-α, and two out of eight were positive for IFN-γ). We were unable to detect nitric oxide in the blood in plasma during infection. However, the levels of iNOS mRNA in the spleens of p55R−/− and WT mice were comparable during weeks 1 and 2 postinfection (data not shown), suggesting that this enzyme can be induced in the absence of the p55R.
FIG. 5.
Plasma TNF-α and IFN-γ levels during the primary P. chabaudi chabaudi (AS) infection in male and female p55R−/− (● and ■, respectively) and WT (○ and □, respectively) mice. Four mice per group were bled before and during the infection. The concentration of cytokines were measured by ELISA as described in Materials and Methods. Each symbol represents the amount of cytokine in an individual plasma sample.
DISCUSSION
TNF-α is implicated in the pathology of malaria in both humans and animals (11, 17, 18, 25). Most of the biological functions of TNF-α induced during infections are considered to be the result of its interaction with the p55R (13, 39, 44, 56). Therefore, we have used mice lacking this receptor to investigate the development of disease and protective immunity during the course of a P. chabaudi chabaudi (AS) infection.
Despite the reports linking TNF-α with changes in body temperature and hypoglycemia in experimental and human malaria (18, 25), the inability to signal through the p55R did not ameliorate these symptoms in mice infected with P. chabaudi chabaudi (AS). In contrast, loss of body weight and hypoglycemia were generally significantly more pronounced in the gene-targeted mice. As described previously in resistant and susceptible mice infected with P. chabaudi chabaudi (AS) (12), these symptoms were strongly associated more with the level of parasitemia than with the amount of TNF-α produced. The lack of effect of p55R inactivation on the development of hypoglycemia supports the view that hypoglycemia in mouse malaria infections is not caused by increased levels of TNF-α but rather is an event secondary to hyperinsulinemia (14), which may be stimulated directly by parasite products (53).
The inability to signal through the p55R did have some effect on the erythrocyte count in peripheral blood in a P. chabaudi chabaudi (AS) infection. Reduction in the number of red cells occurred in both p55R−/− and WT mice during the acute infection. However, in the absence of the p55R, the erythrocyte counts in the p55R−/− mice corresponded to those in uninfected WT mice during the first 5 days of infection. At the maximum level of parasitemia, when erythrocyte loss was greatest, the erythrocyte count in the p55R−/− mice was equivalent to that of the infected WT mice despite a significantly higher parasitemia level during the acute infection. The causes of severe anemia in mouse malaria models and in human P. falciparum infections are probably multifactorial (9, 20, 34, 35, 40, 54) and may be the result of red cell destruction (hemolysis and phagocytosis) and decreased erythropoesis (9, 34). TNF-α may be involved in one or more of these processes. In this regard, dyserythropoesis and enhanced erythrophagocytosis associated with anemia can be induced in different rodent models by the administration of TNF-α (9). Our data suggest that signaling through the p55R may play a minor role in reducing the number of erythrocytes in this model of malaria but that other factors are clearly involved as well.
Although the lack of a functioning p55R had little impact on the development of the various disease parameters during a P. chabaudi chabaudi (AS) infection, TNF-α or TNF-β signaling through this receptor was involved in controlling parasitemia in the primary and challenge infections. Our data agree in part with previous findings using TNFp55Rp75R−/− double-KO mice infected with P. chabaudi chabaudi (AS), where a significantly higher peak of parasitemia was observed only in female KO mice, and there were no differences between double KO and WT mice in subsequent recrudescent parasitemias (45). Although in our studies the differences between KO and WT mice were greater in female mice, parasitemias were also significantly elevated in male mice. The discrepancies may lie in differences in the genetic backgrounds of the mice used in the two studies. We have shown previously that there are small differences in the course of P. chabaudi chabaudi (AS) infection in the C57BL/6 and 129sv strains of mice (31). In addition, the mixture of C57BL/6 and 129sv genes in the two experiments is unlikely to be the same.
The experiments described here support previous studies showing an effect of TNF-α on the parasitemia in transgenic mice expressing human TNF-α, which can only bind to the p55R in mice (52), and in mice given recombinant TNF-α in vivo (49, 50). The most striking difference in parasitemia between p55R−/− and WT mice was observed after a second challenge infection given 2 months after the initial infection. Unlike the WT mice, which developed a peak parasitemia level of <0.1%, p55R−/− mice had a secondary infection with a peak parasitemia level of 12 to 15%, but with a delay in the day of maximum parasitemia and no patent recrudescence. This suggests that there is some partial immunity existing at the time of challenge, but compared with WT mice it is impaired. In spite of the lack of a TNF-p55R interaction, these deficient mice could clear their parasites from the bloodstream. This result supports the idea that several mechanisms are involved in parasite clearance and that either TNF is eventually able to signal through p75R or mechanisms unrelated to TNF and TNF receptor are able to mediate the downstream events necessary to eliminate the parasite. Although a role for NO and O2− in the control of Plasmodium infections in vivo is controversial (8, 10), there are several studies showing that NO can kill parasites in vitro (21, 43). The induction of NO and O2− in vivo is mainly due to signaling through the p55R. However, TNF-TNFp75R interaction has been shown to induce iNOS after some delay in mycobacterial (16) and other parasitic infections (7, 36, 45, 58), and NO has been detected in p55Rp75R−/− mice with P. chabaudi chabaudi (AS) infection (45). In line with these reports, the detection of mRNA for iNOS in the spleens of p55R−/− mice at 1 and 2 weeks of infection does not therefore rule out the possibility of a parasiticidal role for NO at this time.
There are few studies which have investigated memory responses or immune mechanisms in secondary infections with malaria parasites in rodent models. It is widely accepted that immunity following the acute phase of infection is maintained by specific antibodies (29). Passive transfer of immune serum into naive mice can protect them from malaria infection (24), and mice depleted of CD4+T cells after production of parasite-specific antibodies are able to control parasites as long as a high specific antibody titer is maintained (28). Exacerbated secondary infections observed in B-cell-deficient mice after drug treatment to eliminate the primary infection (29) and in interleukin-4 KO mice (L. Packwood and J. Langhorne, manuscript in preparation) also indicate the importance of B cells and antibody in immunity to reinfection. These data suggest that the significant secondary infection seen in p55R−/− mice may be related to low levels of specific antibody production. The amount of specific IgG was significantly lower in p55R−/− mice than in WT mice, and the difference was most striking just prior to and early during secondary infection, when the titer and the kinetics of specific IgG response in p55R−/− mice were comparable with those in the primary infection.
Defects in antibody responses in p55R−/− mice have previously been reported (7) and have been ascribed to the essential role of the p55R in germinal center formation within lymphoid follicles (33), where activated B cells undergo hypermutation to produce high-affinity antibodies and become memory B cells. The interaction between TNF-β (lymphotoxin) and p55R is responsible for the migration of B cells into the follicles in the spleen (33), and the presence of p55R is crucial for maintaining the structure of the spleen (33, 55). Upon activation, naive B cells can produce low-affinity antigen-specific antibodies of both the IgM and the IgG isotypes in the extrafollicular environment (22). However, migration to germinal centers is necessary for a long-lived memory B-cell response producing antibodies of high affinity (32, 37). Malaria-specific IgG was produced during the primary P. chabaudi chabaudi (AS) infection. However, the antibody response was of relatively low titer and of short duration. Upon rechallenge, an antibody response of the same composition, magnitude, and duration as that seen in the primary infection was elicited. This suggests that the defect in p55R signaling may have an effect on the development of memory B cells in the infection. B-cell development, germinal-center formation, and the relative affinity of the antibodies produced during a P. chabaudi chabaudi (AS) infection in p55R−/− mice is currently under investigation.
These results strongly support an important role for specific antibodies in the development and maintenance of immunity against P. chabaudi chabaudi (AS) infections and may have relevance to human malaria infections. Natural immunity in humans is generally achieved through multiple infection and is thought to be short-lived in the absence of continuous reinfection (2). Although antigenic variation may be partly responsible for this phenomenon, it is also possible that generation of immunological memory is impaired during an infection, thus affecting both the longevity of the antibody response and its affinity.
ACKNOWLEDGMENTS
We thank Mike Blackman, Stuart Quin, Latifu Sanni, and Ariel Achtman for their helpful discussions and critical reading of the manuscript and Horst Blüthmann, Hoffmann-La Roche, for the kind gift of the p55R−/− mice.
This work was funded by the Medical Research Council (United Kingdom) and The Wellcome Trust (United Kingdom).
REFERENCES
- 1.Al Yaman F, Awburn M M, Clark I A. Serum creatinine levels and reactive nitrogen intermediates in children with cerebral malaria in Papua New Guinea. Trans R Soc Trop Med Hyg. 1997;91:303–305. doi: 10.1016/s0035-9203(97)90085-7. [DOI] [PubMed] [Google Scholar]
- 2.Baird J K. Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today. 1995;11:105–111. doi: 10.1016/0169-4758(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 3.Bazzoni F, Beutler B. The tumor necrosis factor ligand and receptor families. N Eng J Med. 1996;334:1717–1725. doi: 10.1056/NEJM199606273342607. [DOI] [PubMed] [Google Scholar]
- 4.Beutler B, van Huffle C. Unraveling function in the TNF ligand and receptor families. Science. 1994;264:667–668. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- 5.Van Hensbroek M B, Palmer A, Onyiorah E, Schneider G, Jaffar S, Dolan G, Memming H, Frenkel J, Enwere G, Bennett S, Kwiatkowski D, Greenwood B. The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. J Infect Dis. 1996;174:1091–1097. doi: 10.1093/infdis/174.5.1091. [DOI] [PubMed] [Google Scholar]
- 6.Bouaboula M, Legoux P, Pessague B, Delpech B, Dumont X, Pichaczyk M, Casellas P, Shire D. Standardization of mRNA titration using a polymerase chain reaction method involving co-amplification with a multispecific internal control. J Biol Chem. 1992;267:21830–21838. [PubMed] [Google Scholar]
- 7.Castanos-Velez E, Maerlan S, Osorio L M, Aberg F, Biberfeld P, Orn A, Rottenberg M E. Trypanosoma cruzi infection in tumor necrosis factor receptor p55-deficient mice. Infect Immun. 1998;66:2960–2968. doi: 10.1128/iai.66.6.2960-2968.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavacini L A, Guidotti M, Parke L A, Melancon-Kaplan J, Weidanz W P. Reassessment of the role of splenic leukocyte oxidative activity and macrophage activation in expression of immunity to malaria. Infect Immun. 1989;57:3677–3682. doi: 10.1128/iai.57.12.3677-3682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark I A, Chaudhri G. Tumour necrosis factor may contribute to the anemia of malaria by causing dyserythropoiesis and erythrophagocytosis. Br J Haematol. 1988;70:99–103. doi: 10.1111/j.1365-2141.1988.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 10.Clark I A, Hunt N H, Butcher G A, Cowden W B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987;139:3493–3496. [PubMed] [Google Scholar]
- 11.Clark I A, Al Yaman F M, Jacobson L S. The biological basis of malarial disease. Int J Parasitol. 1997;27:1237–1249. doi: 10.1016/s0020-7519(97)00121-5. [DOI] [PubMed] [Google Scholar]
- 12.Cross C E, Langhorne J. Plasmodium chabaudi chabaudi (AS): inflammatory sytokines and pathology in an erythrocytic-stage infection in mice. Exp Parasitol. 1998;90:220–229. doi: 10.1006/expr.1998.4335. [DOI] [PubMed] [Google Scholar]
- 13.Deckert-Schluter M, Bluethmann H, Rang A, Hof H, Schluter D. Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75), in murine toxoplasmosis. J Immunol. 1998;160:3427–3436. [PubMed] [Google Scholar]
- 14.Elased K, Playfair J H. Hypoglycemia and hyperinsulinemia in rodent models of severe malaria infection. Infect Immun. 1994;62:5157–5160. doi: 10.1128/iai.62.11.5157-5160.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everest P, Roberts M, Dougan G. Susceptibility to Salmonella typhimurium infection and effectiveness of vaccination in mice deficient in the tumor necrosis factor alpha p55 receptor. Infect Immun. 1998;66:3355–3364. doi: 10.1128/iai.66.7.3355-3364.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 17.Grau G E, Fajardo L F, Piquet P F, Allet B, Lambert P H, Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987;237:1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- 18.Grau G, Taylor T, Molyneux M, Wirima J, Vassalli P, Hommel M, Lambert P. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 19.Grell M, Becke F M, Wajant H, Mannel D N, Scheurich P. TNF receptor type 2 mediates thymocyte proliferation independently of TNF receptor type 1. Eur J Immunol. 1998;28:257–263. doi: 10.1002/(SICI)1521-4141(199801)28:01<257::AID-IMMU257>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Gupta G M. Red cell membrane alternations in malaria. Ind J Biochem Biophys. 1988;25:20–24. [PubMed] [Google Scholar]
- 21.Gyan B, Troy-Blomberg M, Perlmann P, Bjorkman A. Human monocytes culture with and without interferon-gamma inhibit Plasmodium falciparum parasites growth in vitro via secretion of reactive nitrogen intermediates. Parasite Immunol. 1994;16:371–375. doi: 10.1111/j.1365-3024.1994.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 22.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl I. The architecture and dynamics of responding cell population. J Exp Med. 1991;173:1165–1172. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs P, Radzioch D, Stevenson M M. A Th1-associated increase in tumor necrosis factor alpha expression in the spleen correlates with resistance to blood-stage malaria in mice. Infect Immun. 1996;64:535–541. doi: 10.1128/iai.64.2.535-541.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarra W, Hills L A, March J C, Brown K N. Protective immunity to malaria. Studies with cloned lines of Plasmodium chabaudi chabaudi and P. berghei in CBA/Ca mice. II. The effectiveness and inter- or intra-species specificity of the passive transfer of immunity with serum. Parasite Immunol. 1986;8:239–254. doi: 10.1111/j.1365-3024.1986.tb01036.x. [DOI] [PubMed] [Google Scholar]
- 25.Kwiatkowski D, Hill A V, Sambou I, Twumasi P, Castracane J, Manogue K R, Cerami A, Brewster D R, Greenwood B M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 26.Kwiatkowski D, Molyneux M E, Stephens S, Curtis N, Klein N, Pointaire P, Smith M, Allan R, Brewster D R, Grau G E, Greenwood B M. Anti-TNF therapy inhibits fever in cerebral malaria. Q J Med. 1993;86:91–98. [PubMed] [Google Scholar]
- 27.Langhorne J, Simon B. Limiting dilution analysis of the T cell response to Plasmodium chabaudi chabaudi in mice. Parasite Immunol. 1989;11:545–559. doi: 10.1111/j.1365-3024.1989.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 28.Langhorne J, Simon-Haarhaus B, Meding S J. The role of CD4+ T cells in the protective immune response to Plasmodium chabaudi in vivo. Immunol Lett. 1990;25:101–107. doi: 10.1016/0165-2478(90)90099-c. [DOI] [PubMed] [Google Scholar]
- 29.Langhorne J, Cross C, Seixas E, Li C, von der Weid T. A role for B cells in the development of T cell helper function in a malaria infection in mice. Proc Natl Acad Sci USA. 1998;95:1730–1734. doi: 10.1073/pnas.95.4.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis M, Tartaglia L, Lee A, Bennett G, Rice G, Wong G, Chen E, Goeddlel D. Cloning and expression of cDNAs for two distinct tumor necrosis factor (TNF) receptors demonstrate one receptor is species specific. Proc Natl Acad Sci USA. 1991;88:2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Corraliza I, Langhorne J. A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi (AS) infection in mice. Infect Immun. 1999;67:4435–4442. doi: 10.1128/iai.67.9.4435-4442.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLennan I C. Germinal centers. Annu Rev Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto M, Mariathasan S, Nahm M H, Baranyay F, Peschon J J, Chaplin D D. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science. 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 34.Miller K L, Silverman P H, Kullgren B, Mahlmann L J. Tumor necrosis factor alpha and the anemia associated with murine malaria. Infect Immun. 1989;57:1542–1546. doi: 10.1128/iai.57.5.1542-1546.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moll G N, Vial H J, Bevers E M, Ancelin M L, Roelofsen B, Slotboom A J, Zwaal R F, Op den Kamp J A, van Deenen L L. Phospholipid asymmetry in the plasma membrane of malaria infected erythrocytes. Biochem Cell Biol. 1990;68:579–585. doi: 10.1139/o90-083. [DOI] [PubMed] [Google Scholar]
- 36.Nashleanas M, Kanaly S, Scott P. Control of Leishmania major infection in mice lacking TNF receptors. J Immunol. 1998;160:5506–5513. [PubMed] [Google Scholar]
- 37.Nossal G J. The molecular and cellular basis of affinity maturation in antibody response. Cell. 1992;68:1–2. doi: 10.1016/0092-8674(92)90198-l. [DOI] [PubMed] [Google Scholar]
- 38.Peschon J J, Torrance D S, Stocking K L, Glaccum M B, Otten C, Willis C R, Charrier K, Morrissey P J, Ware C B, Mohler K M. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 39.Pfeffer K, Matsuyama T, Kundig T, Wakeham A, Kishihara K, Shaninian A, Wiegmann K, Ohashi P, Kronke M, Mak T. Mice deficient for the 55kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 40.Phillips R E, Looareesuwan S, Warrell D A, Lee H, Karbwang J, Warrell M J, White N J, Swasdichai C, Weatherall D J. The importance of anemia in cerebral and uncomplicated falciparum malaria: role of complications, dyserythropoiesis and iron sequestration. Q J Med. 1986;58:305–323. [PubMed] [Google Scholar]
- 41.Prat M, Gribaudo G, Comoglio P M, Cavallo G, Landolfo S. Monoclonal antibodies against murine γ interferon. Proc Natl Acad Sci USA. 1984;81:4515–4519. doi: 10.1073/pnas.81.14.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rocket K A, Awburn M M, Aggarwal B B, Cowden W B, Clark I A. In vivo induction of nitrite and nitrate by tumor necrosis factor, lymphotoxin, and interleukin-1: possible roles in malaria. Infect Immun. 1992;60:3725–3730. doi: 10.1128/iai.60.9.3725-3730.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rocket K A, Awburn M M, Cowden W B, Clark I A. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991;59:3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor I are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 45.Sam H, Su Z, Stevenson M M. Deficiency in tumor necrosis factor alpha activity does not impair early Th1 responses against blood-stage mamaria. Infect Immun. 1999;67:2660–2664. doi: 10.1128/iai.67.5.2660-2664.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shakhov A N. New derivative of pMUS for quantitation of mouse IL-12 (p35, p40), IL-10 and IFN-γ receptor mRNA. Eur Cytokine Network. 1994;5:337–338. [PubMed] [Google Scholar]
- 47.Slade S J, Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989;179:353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- 48.Spitalny G L, Havell E A. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984;159:1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevenson M M, Ghadirian E. Human recombinant tumor necrosis factor alpha protects susceptible A/J mice against lethal Plasmodium chabaudi AS infection. Infect Immun. 1989;57:3936–3939. doi: 10.1128/iai.57.12.3936-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevenson M M, Tam M F, Belosevic M, van der Meide P H, Podoba J E. Role of endogenous gamma interferon in host response to infection with blood-stage Plasmodium chabaudi AS. Infect Immun. 1990;58:3225–3232. doi: 10.1128/iai.58.10.3225-3232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Süss G, Eichmann K, Kury E, Linke A, Langhorne J. Roles of CD4- and CD8-bearing T lymphocytes in the immune response to the erythrocytic stages of Plasmodium chabaudi. Infect Immun. 1988;56:3081–3080. doi: 10.1128/iai.56.12.3081-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taverne J, Sheikh N, de Souza J B, Playfair J H, Probert L, Kollias G. Anemia and resistance to malaria in transgenic mice expressing human tumour necrosis factor. Immunology. 1994;82:397–403. [PMC free article] [PubMed] [Google Scholar]
- 53.Taylor K, Bate C A, Carr R E, Butcher G A, Taverne J, Playfair J H. Phospholipid-containing toxic malaria antigens induce hypoglycaemia. Clin Exp Immunol. 1992;90:1–5. doi: 10.1111/j.1365-2249.1992.tb05822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ternynck T, Falanga P B, Uterkirscher C, Gregoire J S L P, Avrameas S. Induction of high levels of autoantibodies in mice infected with Plasmodium chabaudi. Int Immunol. 1991;3:29–37. doi: 10.1093/intimm/3.1.29. [DOI] [PubMed] [Google Scholar]
- 55.Tkachuk M, Bolliger S, Ryffel B, Pluschke G, Banks T A, Herren S, Gisler R H, Kosco-Vilbois M H. Crucial role of tumor necrosis factor receptor 1 expression on nonhematopoietic cells for B cell localization within the splenic white pulp. J Exp Med. 1998;187:469–477. doi: 10.1084/jem.187.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vieira L Q, Goldschmidt M, Nashleanas M, Pfeffer K, Mak T, Scott P. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J Immunol. 1996;157:827–835. [PubMed] [Google Scholar]
- 57.Wang B, Fujisawa H, Zhuang L, Kondo S, Shivji G M, Kim C S, Mak T W, Sauder D N. Depressed Langerhans cell migration and reduced contact hypersensitivity response in mice lacking TNF receptor p75. J Immunol. 1997;159:6148–6155. [PubMed] [Google Scholar]
- 58.Yap G S, Scharton-Kersten T, Charest H, Sher A. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J Immunol. 1998;160:1340–1345. [PubMed] [Google Scholar]