Abstract
Background and Objectives:
To review clinical outcomes and toxicities in locally advanced differentiated thyroid cancer patients treated with external beam radiotherapy (EBRT) with or without concurrent chemotherapy (CCRT).
Methods:
Between 1990 and 2012, 66 patients with gross residual/unresectable non-anaplastic non-medullary thyroid cancer were treated with EBRT.
Results:
The median overall survival was 42.0 months. The overall locoregional progression-free survival (LPFS) at 3 years was 77.3%. CCRT resulted in a non-significant improvement in LPFS (90.0% vs. 73.0%, P = 0.347). Poorly differentiated histology had significantly improved LPFS (89.4% vs. 66.1%, P = 0.020), despite a significantly worse distant metastasis-free survival (43.9% vs. 82.5%, P = 0.023).
Acute treatment-related toxicity included dermatitis, mucositis, and dysphagia with grade three rates of 12.1%, 19.7%, and 16.7%, respectively. The incidence of late toxicity was low. CCRT was only associated with a significant greater rate of acute grade 3 hoarseness (10.0% vs. 0.0%, P = 0.033), but with no difference in the rate of grade 2 late toxicity.
Conclusions:
EBRT is a safe and effective treatment modality with 90% LPFS at 3 years in patients with gross residual or unresectable nonanaplastic, non-medullary thyroid carcinoma treated with CCRT. Further incorporation of EBRT with concurrent chemotherapy may result in improved disease control.
Keywords: IMRT, thyroid cancer, chemoradiation, radiation therapy
INTRODUCTION
An estimated 60,220 new cases of thyroid cancer were diagnosed in the United States in 2013 (45,310 women and 14,910 men), the majority of which were differentiated (follicular or papillary) cancers [1]. The 10-year overall survival (OS) for papillary and follicular cancer is approximately 95% and 85%, respectively [2]. Despite excellent survival, up to one third of patients recur, two-thirds of whom recur locally [3].
As the primary treatment modality for differentiated thyroid cancer is surgical resection, the role of external beam radiotherapy (EBRT) is unknown. The only prospective randomized trial conducted failed to accrue patients because of the reluctance of multiple centers to adopt EBRT [4]. In the absence of prospective trials, the current indications for EBRT have largely been based on retrospective series [5]. Multiple single-institution experiences have shown EBRT to improve locoregional control in high-risk patients (i.e., microscopic/gross residual disease after surgical resection and unresectable disease) [6–18]. The American Thyroid Association Task Force and National Comprehensive Cancer Network recommend EBRT in papillary and follicular carcinomas only in patients with unresectable disease not amenable to RAI therapy, whereas they recommend the consideration of EBRT in patients over the age of 45 years with grossly visible extrathyroid extension and a high likelihood of microscopic residual disease, and in patients of any age with gross residual disease or unresectable bulky disease not amendable to RAI therapy [19,20].
Locoregional disease control is an important endpoint when examining outcomes after EBRT for thyroid cancer, as locally recurrent/locally advanced disease in the head and neck is associated with significant morbidity secondary to the proximity of many critical organs including the esophagus, larynx, brachial plexus, and spinal cord. The addition of EBRT can potentially limit the associated morbidity from uncontrolled locoregional disease such as obstruction of the esophagus and/or trachea, need for a laryngectomy, neurovascular compromise, pain, hemorrhage, and repeated surgical procedures. We sought to review the clinical outcomes and acute/late toxicities in locally advanced non-anaplastic thyroid cancer patients with gross residual or unresectable disease treated with EBRT with or without concurrent chemotherapy (CCRT).
MATERIALS AND METHODS
The institutional review board approved this retrospective study with a waiver of informed consent. Between July 1990 and February 2012, 66 patients with pathologically confirmed gross residual or unresectable non-anaplastic thyroid cancer were treated with definitive-intent EBRT to the primary site at a large tertiary cancer center.
The T and N category was determined from the disease extent at the initial presentation (pathologically based in 90.0% of patients). At the time of radiation all patients had locally advanced unresectable or gross residual disease (i.e., T4c or R2 disease). The M category was recorded at time of EBRT start. Staging workup included a complete history and physical examination, focused head and neck evaluation, complete blood counts, liver function tests, and chest X-ray [21].
Pathological Findings
Non-anaplastic thyroid carcinoma was histologically confirmed by internal pathological review. Poorly differentiated thyroid carcinomas were defined on the basis of ≥5 mitosis/10 high-power microscopic fields (400×) and/or the presence of tumor necrosis. As previously reported this definition identifies patients with an intermediate prognosis between those with well-differentiated and anaplastic thyroid carcinoma [7,22,23].
Radiotherapy Technique
The radiation technique, dose, and fractionation varied according to physician preference and clinical scenario. Fifty-one patients (77.3%) underwent intensity-modulated radiation therapy (IMRT). Our current radiation technique has been previously described [24]. Briefly, we treated (1) low-risk areas, including the upper and lower paratracheal nodal levels and cervical lymph node levels II–VI to 54Gy, (2) high-risk areas, including the operative or tumor bed, operative thyroid gland volume, tracheoesophageal grooves, and central nodal compartment, of microscopic disease, to 60Gy, (3) close or microscopically positive margins to 66Gy, and (4) areas of gross disease to 70Gy [24]. The gross tumor volume was defined as the gross extent of tumor visible by imaging studies and clinical examination. The clinical target volume was defined as the gross tumor volume plus a margin for potential microscopic spread, including high-risk lymph node areas. The clinical target volume was expanded to a planning target volume to account for intrafractional patient motion and interfractional setup error. All patients were immobilized in the treatment position using a three- or five-point Aquaplast mask (Orfit Industries, Wijnegem, Belgium) to control the movement of the head and neck.
Organs at risk (OAR) such as the parotid glands, larynx, lungs, esophagus, brachial plexus, and spinal cord were contoured. A dose-volume histogram was constructed to evaluate target coverage and the doses to the surrounding organs at risk. The dose to the OAR was limited to <26Gy mean parotid dose, <70Gy maximum point and <45Gy mean larynx dose, <21Gy mean lung dose, <34Gy mean esophagus dose, <65Gy maximum brachial plexus point dose, and <45Gy maximum spinal cord point dose. In addition, the volume of lung receiving 20Gy or more was limited to 37%.
Toxicity and Response Assessment
Patients were assessed jointly by radiation oncology, medical oncology, endocrinology, and/or head and neck surgery weekly during the radiation course and at approximate intervals of 4, 8, and 12 weeks after completion of treatment, then every 3 months for 2 years, followed by every 6–12 months thereafter. In the early 2000s a standardized toxicity form was implemented to help improve the accuracy and reproducibility in recording treatment toxicities for dermatitis, nausea, vomiting, mucositis, xerostomia, dysphagia, hoarseness, fatigue, and need for a feeding tube. The incidence of the worst-grade toxicity sustained by a patient up to 90 days after the start of radiation therapy was recorded as an acute toxicity event based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 [25]. All late toxicity (>90 days post-treatment completion) was scored with the Radiation Therapy Oncology Group (RTOG) late radiation morbidity scoring system [26].
Statistical Analysis
Locoregional progression, distant metastasis, and death were recorded from the end of radiotherapy. Locoregional progression was defined as local (i.e., thyroid bed) or nodal (i.e., central compartment or cervical and superior mediastinal lymph nodes) disease progression (i.e., new or enlarging disease in the thyroid bed, central compartment, or lymph nodes on ultrasound, diagnostic RAI, positron-emission tomography (PET)/computed tomography (CT), CT, and/or magnetic resonance imaging (MRI)). Biopsy confirmation was not required. Stable disease with no evidence of progression was classified as “controlled.” If systemic therapy or adjuvant RAI was initiated after EBRT for clinical suspicion of progressive disease in the neck, these patients were classified as having locoregional progression. Patients without evidence of metastatic disease at time of EBRT were followed for the development of distant metastases; patients with M1 disease prior to EBRT were excluded from this subset-analysis.
The Kaplan–Meier method was used to calculate locoregional progression-free survival (LPFS), distant metastasis-free survival (DMFS), and OS [27]. The log-rank test was used to compare survival curves when indicated. Comparisons between cohorts were performed using either the Chi-square test or two-tailed log-rank test. A probability value of <0.05 was considered statistically significant for all analyses. All analyses were performed in SPSS statistics version 21 (IBM, Armonk, New York).
RESULTS
Patient and Tumor Characteristics
The median age was 65.8 years (interquartile range, 56.2–72.6) with an overall median follow-up of 35.0 months (interquartile range; 22.1–74.4) among surviving patients and 24.5 months (interquartile range; 13.6–54.1) for all patients.
Thirty-four patients (51.5%) had poorly differentiated histology. An additional 13 patients (19.7%) had high-risk pathology (12 tall cell variant papillary, 1 Hurthle cell). Of the 19 remaining patients, 9 had well-differentiated papillary carcinoma, 4 had moderately differentiated papillary carcinoma, and 7 had papillary thyroid carcinoma with no differentiation stated in the pathology report. There was no difference in age, sex, T category, N category, or the presence of distant metastasis between patients with well/moderately differentiated versus poorly differentiated histology (Table I).
TABLE I.
Patient, Tumor, and Treatment Characteristics
| N (%) | N (%) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Characteristics | Overall (N = 66) | EBRT (N = 45) | CCRT (N = 21) | P-Value | WD/MD Histology (N = 32) | PD histology (N = 34) | P-Value |
|
| |||||||
| Median follow-up (months) | 24.5 | 30.0 | 21.9 | 0.067 | 35.3 | 23.0 | 0.335 |
| Age | |||||||
| <45 years | 5 (7.6%) | 4 (8.9%) | 1 (4.8%) | 3 (9.4%) | 2 (5.9%) | ||
| ≥45 years | 61 (92.4%) | 41 (91.1%) | 20 (95.2%) | 0.555 | 29 (90.6%) | 32 (94.1%) | 0.592 |
| Gender | |||||||
| Male | 34 (51.5%) | 24 (53.3%) | 10 (47.6%) | 18 (56.3%) | 16 (47.1%) | ||
| Female | 32 (48.5%) | 21 (46.7%) | 11 (52.4%) | 0.665 | 14 (43.8%) | 18 (52.9%) | 0.455 |
| Tumor stage | |||||||
| T1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||
| T2 | 5 (7.6%) | 3 (6.7%) | 2 (9.5%) | 3 (9.4%) | 2 (5.9%) | ||
| T3 | 4 (6.1%) | 4 (8.9%) | 0 (0%) | 1 (3.1%) | 3 (8.8%) | ||
| T4 | 57 (86.4%) | 38 (84.4%) | 19 (90.5%) | 0.353 | 28 (87.5%) | 29 (85.3%) | 0.560 |
| Regional node stage | |||||||
| N0 | 11 (16.7%) | 8 (17.8%) | 3 (14.3%) | 5 (15.6%) | 6 (17.6%) | ||
| N1 | 55 (83.0%) | 37 (82.2%) | 18 (85.7%) | 0.723 | 27 (84.4%) | 28 (82.4) | 0.826 |
| Distant metastasis | |||||||
| No | 34 (51.5%) | 21 (46.7%) | 13 (61.9%) | 15 (46.9%) | 19 (55.9%) | ||
| Yes | 32 (48.5%) | 24 (53.3%) | 8 (38.1%) | 0.249 | 17 (53.1%) | 15 (44.1%) | 0.464 |
| Histology | |||||||
| WD/MD | 32 (48.5%) | 26 (57.8%) | 6 (28.6%) | — | — | ||
| PD | 34 (51.5%) | 19 (42.2%) | 15 (71.4%) | 0.027 | — | — | |
| Concurrent chemotherapy | |||||||
| EBRT | 45 (68.2%) | — | — | 26 (81.3%) | 19 (55.9%) | ||
| CCRT | 21 (32.3%) | — | — | 6 (18.8%) | 15 (44.1%) | 0.027 | |
| Radiation technique | |||||||
| EBRT | 15 (22.7%) | 12 (26.7%) | 3 (14.3%) | 0.264 | 7 (21.9%) | 8 (23.5%) | 0.873 |
| IMRT | 51 (77.3%) | 33 (73.3%) | 25 (78.1%) | 26 (76.5%) | |||
EBRT, external beam radiation therapy; CCRT, concurrent chemoradiation therapy; WD/MD histology, well-differentiated/moderately differentiated histology; PD histology, poorly differentiated histology; IMRT, intensity-modulated radiation therapy.
Treatment Characteristics
Pre-EBRT treatment characteristics are depicted in Figure 1. EBRT was administered to all 66 patients to a median dose of 66.3Gy (interquartile range, 60.0–70.0) in a median of 33 fractions (interquartile range, 33–35). CCRT was administered to 21 (31.8%) patients: doxorubicin (10mg/m2) was administered to 19 patients, and cisplatin (100mg/m2), cisplatin (60mg/m2) with etoposide (120mg/m2), and doxorubicin (10mg/m2) followed by paclitaxel (40mg/m2) were each administered to 1 patient. There was no difference in age, sex, T category, N category, presence of distant metastasis, radiation technique, or radiation dose between CCRT and EBRT patients (Table I). There was a significantly greater percentage of patients with poorly differentiated histology who underwent CCRT (71.4% vs. 42.2%, P = 0.027).
Fig. 1.
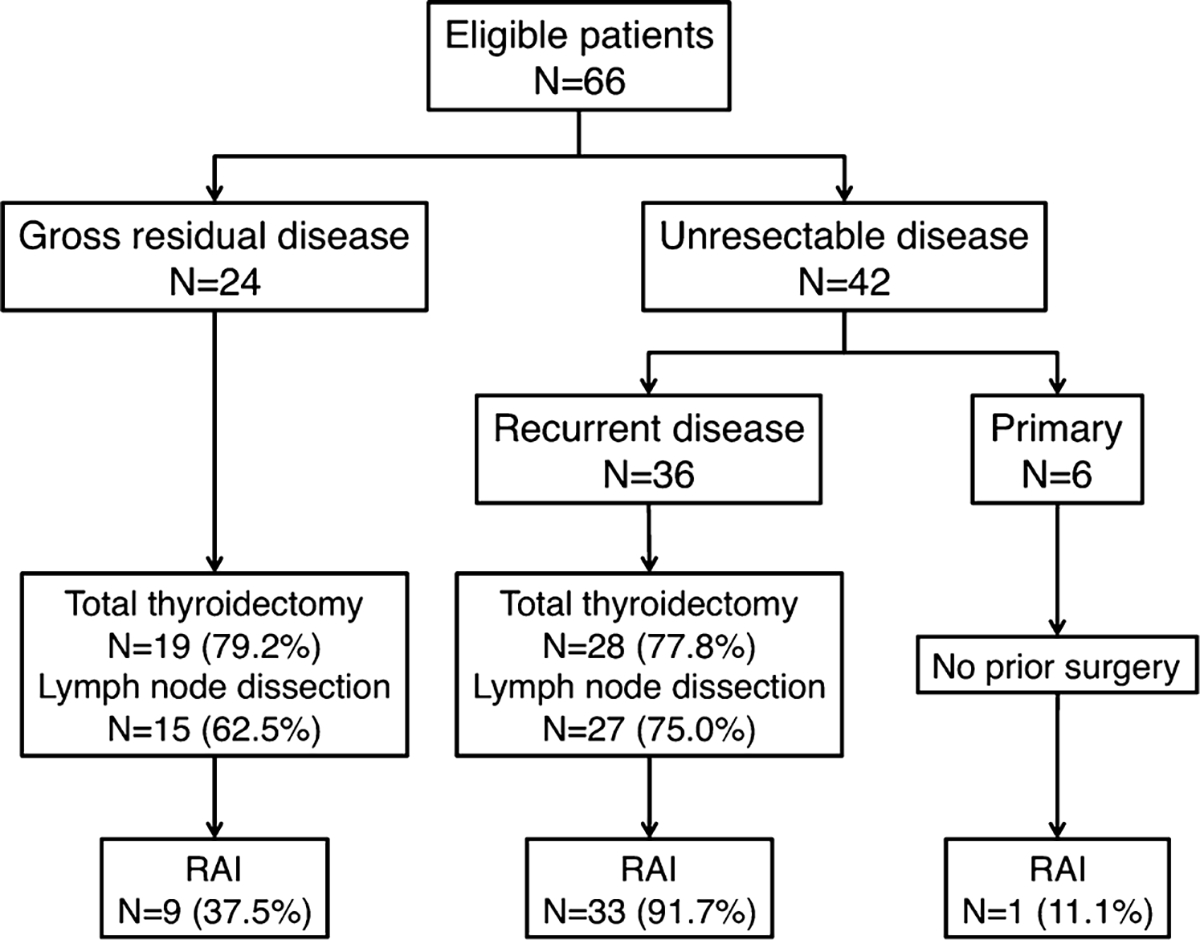
Pre-external beam radiation therapy treatment characteristics. RAI, radioiodine.
Locoregional Progression-Free Survival
The 3-year actuarial LPFS was 77.3% (Fig. 2A). Twelve patients (18.2%) developed locoregional progression at a median of 11.4 months (interquartile range, 4.6–18.4). Four patients failed in the thyroid bed/central compartment alone, 5 in the lateral cervical neck alone, and 3 simultaneously in the thyroid bed/central compartment and lateral neck.
Fig. 2.
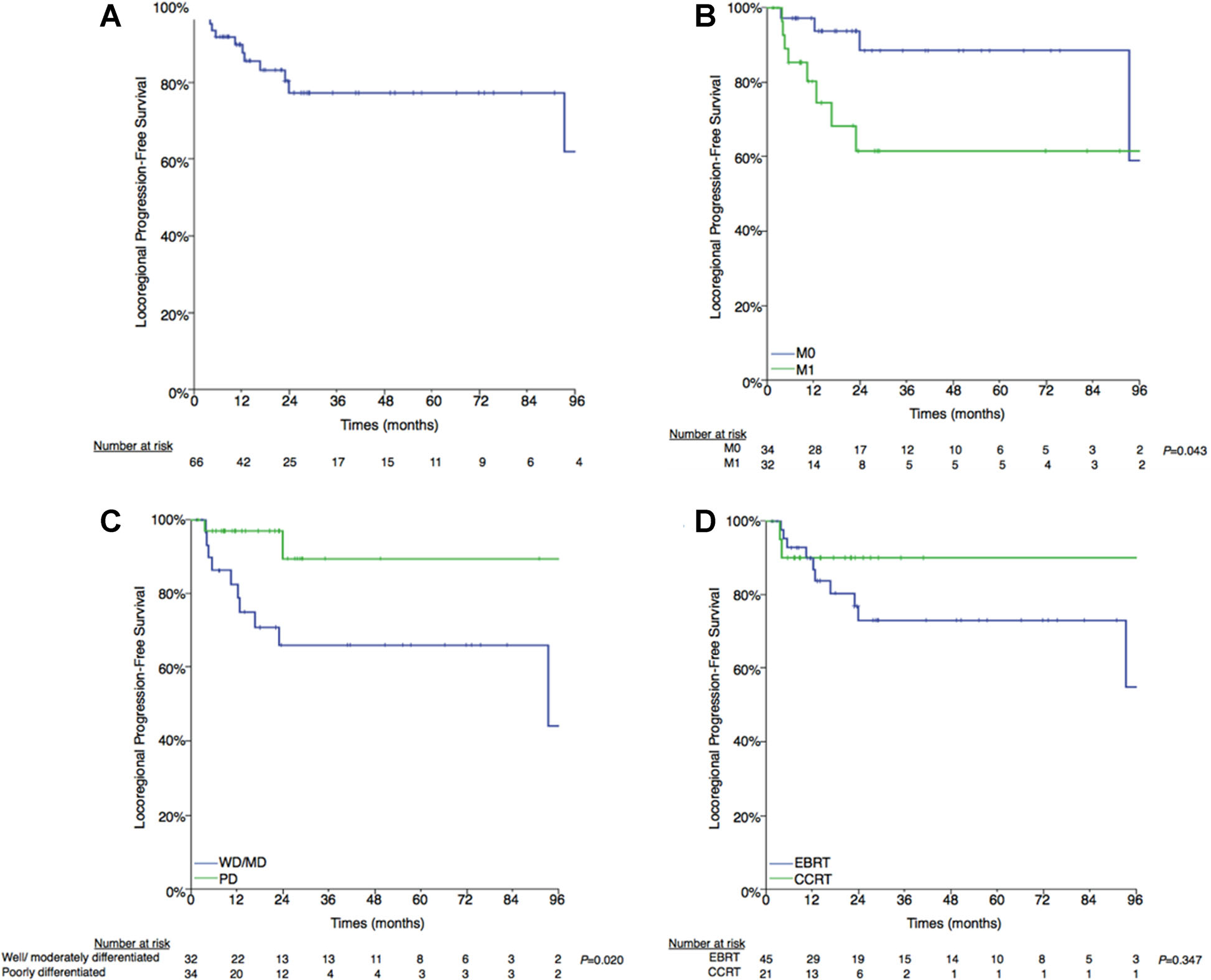
A: Locoregional progression-free survival. B:M0 versus M1. C: Well-/moderately differentiated versus poorly differentiated histology. D:External beam radiation therapy (EBRT) versus concurrent chemoradiation therapy (CCRT).
Patients with metastatic disease at time of EBRT had a significantly worse LPFS compared with those with localized disease (3-year actuarial LPFS, 61.4% vs. 88.4%; P = 0.043) (Fig. 2B). Poorly differentiated histology had significantly improved LPFS compared with well/moderately-differentiated histology (89.4% vs. 66.1%, P = 0.020) (Fig. 2C). CCRT resulted in a non-significant improvement in the actuarial 3-year LPFS (90.0% vs. 73.0%, P = 0.347) (Fig. 2D). There was no difference in the LPFS between patients with gross residual and unresectable disease (70.8% vs. 81.1%, P = 0.393).
Distant Metastases
Of the 34 patients without evidence of metastatic disease prior to EBRT, 16 (47.1%) developed distant metastases at a median of 15.6 months (interquartile range, 10.3–36.0) resulting in a 3-year actuarial DMFS of 59.4% (Fig. 3A).
Fig. 3.
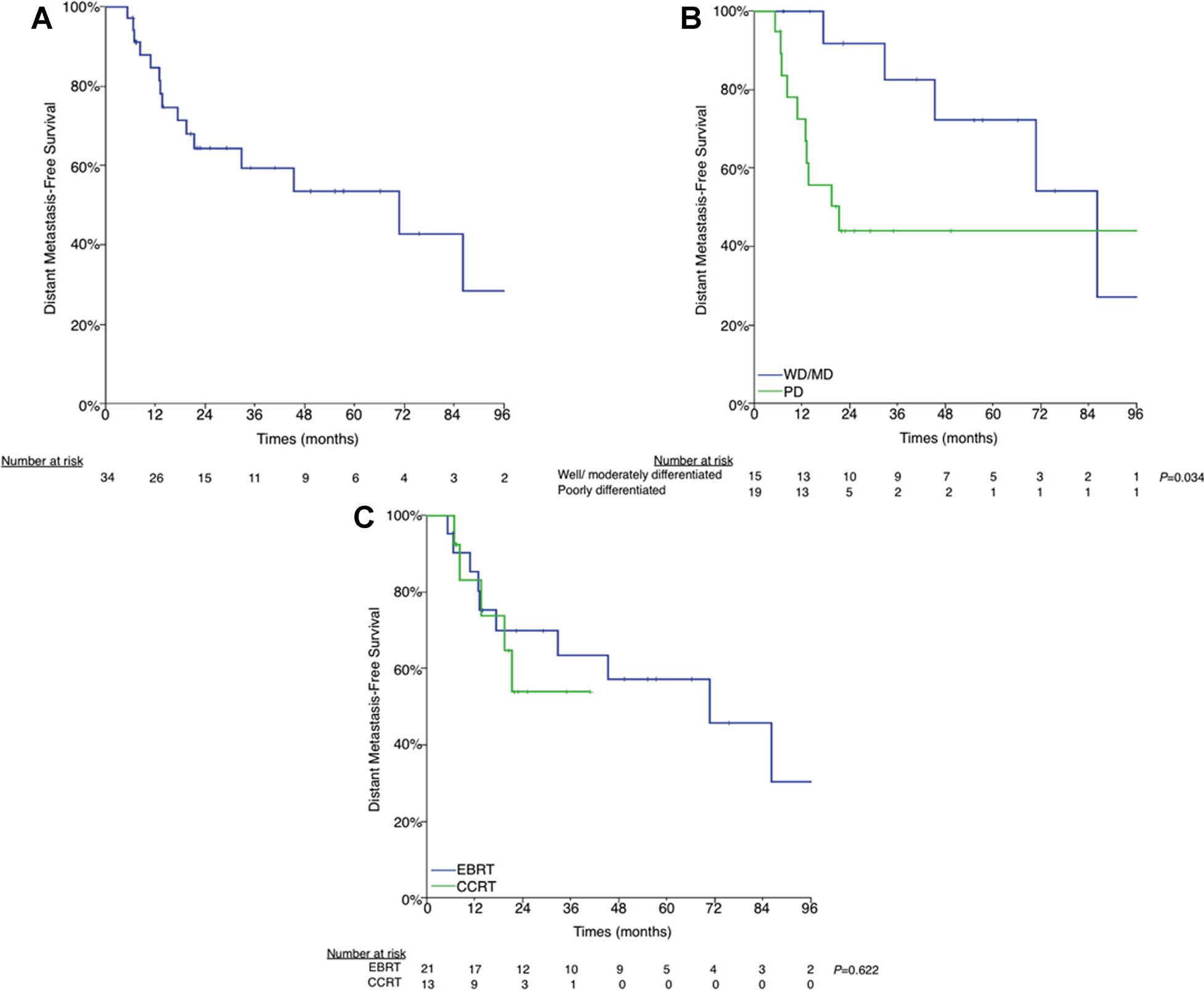
A: Distant metastasis-free survival. B: Well-/moderately differentiated versus poorly differentiated histology. C: External beam radiation therapy (EBRT) versus concurrent chemoradiation therapy (CCRT).
Patients with poorly differentiated histology had significantly worse DMFS (43.9% vs. 82.5%, P = 0.023) (Fig. 3B). There was no difference in the DMFS between patients treated with CCRT compared with EBRT alone (53.8% vs. 63.5%, P = 0.622) (Fig. 3C).
Overall Survival
The median OS was 42.0 months and the actuarial 3-year OS was 54.4% (Fig. 4A). Thirty-nine patients (59.1%) died at a median of 19.4 months post-EBRT completion (interquartile range, 11.3–42.6). The median survival for patients with metastatic disease at the time of EBRT was significantly shorter than those without evidence of metastatic disease (25.9 vs. 77.7 months, P = 0.003, respectively) (Fig. 4B).
Fig. 4.
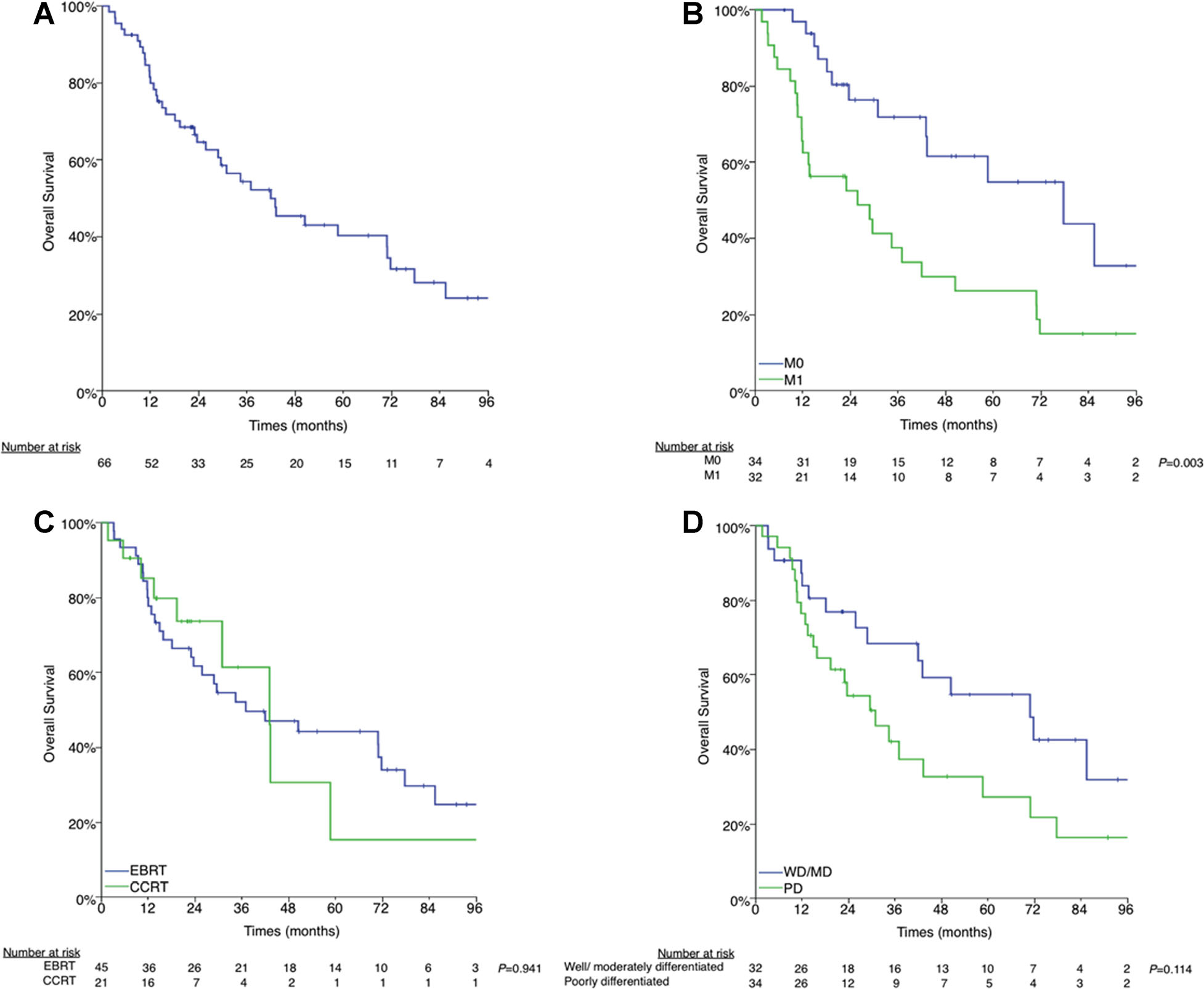
A: Overall survival. B: M0 versus M1. C: External beam radiation therapy (EBRT) versus concurrent chemoradiation therapy (CCRT). D: Well-/moderately differentiated versus poorly differentiated histology Presented in part as an oral presentation at the 2nd World Congress on Thyroid Cancer on July 10–14th, 2013 in Toronto, Canada and as a poster at the Multidisciplinary Head and Neck Cancer Symposium on February 20–22, 2014 in Scottsdale, Arizona.
Patients with poorly differentiated histology had a non-significantly shorter median OS compared with patients with well-differentiated or moderately differentiated histology (40.0 vs. 70.9 months, P = 0.114) (Fig. 4C). There was no difference in the median OS between patients treated with CCRT versus EBRT alone (43.1 vs. 37.1 months, P = 0.941) (Fig. 4D).
Toxicity
Acute treatment-related toxicity included dermatitis, mucositis, and dysphagia with grade 3 rates of 12.1%, 19.7%, and 16.7%, respectively. Seven EBRT-alone (15.6%) and 5 CCRT (23.8%) patients required treatment breaks for a median of 3 and 1 days, respectively (P = 0.395). Concurrent chemotherapy was only associated with a significant greater rate of acute grade 3 hoarseness (10.0% vs. 0.0%, P = 0.033) (Table II), but with no difference in the rate of grade 2 or greater late toxicity, including hoarseness (5.0% versus 0.0%, P = 0.159) (Table III). Seven (10.6%) patients required a tracheostomy: 5 (7.6%) patients had a tracheostomy tube placed prior to RT, 1 (1.6%) patient required a tracheostomy during EBRT, and 1 (1.6%) patient required a tracheostomy post-EBRT.
TABLE II.
Acute Grade 3 or Greater Toxicity
| Toxicity | EBRT | CCRT | P-Value |
|---|---|---|---|
|
| |||
| Dermatitis | 4 (9.1%) | 4 (20.0%) | 0.221 |
| Nausea | 1 (2.3%) | 0 (0%) | 0.497 |
| Vomiting | 1 (2.3%) | 0 (0%) | 0.497 |
| Mucositis | 10 (22.7%) | 3 (15.0%) | 0.476 |
| Xerostomia | 1 (2.3%) | 0 (0%) | 0.497 |
| Dysphagia | 5 (11.4%) | 6 (30.0%) | 0.067 |
| Hoarseness | 0 (0%) | 2 (10.0%) | 0.033 |
| Fatigue | 2 (4.5%) | 1 (5.0%) | 0.936 |
Analysis limited to 44 and 20 patients, respectively. CCRT, concurrent chemoradiation therapy; EBRT, external beam radiation therapy.
TABLE III.
Late Grade 2 or Greater Toxicity
| Toxicity | EBRT | CCRT | P-Value |
|---|---|---|---|
|
| |||
| Dermatitis | 1 (2.6%) | 0 (0%) | 0.470 |
| Xerostomia | 8 (20.5%) | 4 (20.0%) | 0.963 |
| Dysphagia | 7 (17.9%) | 4 (20.0%) | 0.848 |
| Hoarseness | 0 (0%) | 1 (5.0%) | 0.159 |
| Fatigue | 2 (5.1%) | 2 (10.0%) | 0.481 |
Analysis limited to 39 and 20 patients, respectively. CCRT, concurrent chemoradiation therapy; EBRT, external beam radiation therapy.
Twenty-two patients (33.3%) required a gastrostomy tube (G-tube): 15 (22.7%) patients had a G-tube in place prior to initiation of EBRT, 6 (11.8%) patients required a reactive G-tube (defined as G-tube placement during EBRT or within 90 days of completing EBRT), and 1 (2.2%) patient required a late G-tube (placed 3 months after completion of EBRT). There was no significant difference in the rate of reactive G-tube between the EBRT-alone and CCRT cohorts (8.3% vs. 20.0% patients, P = 0.239). Grade 3 or greater late dysphagia was 10.3% (n = 4) in EBRT alone and 5.0% (n = 1) in CCRT patients (P = 0.097) secondary to G-tube dependence in four patients, of which two underwent G-tube placement prior to starting EBRT, and cricopharyngeus stenosis requiring dilatation in 1 EBRT-alone patient. No tracheoesophageal fistulas were noted.
DISCUSSION
Differentiated thyroid cancers respond well to surgical resection and appropriately selected adjuvant therapy, which may include RAI with or without TSH suppression. Nonetheless a subset of these patients will have a locoregional recurrence. Salvage therapies include additional surgery, RAI, and/or EBRT. The use of EBRT in the salvage setting remains controversial given a lack of prospective studies and concern for unnecessary treatment-related toxicity. Similar concerns exist when considering EBRT for the definitive treatment of unresectable disease.
EBRT with or without concurrent chemotherapy is an effective treatment for patients with gross residual or unresectable non-anaplastic thyroid cancer with greater than an 85% locoregional control rate in patients with non-metastatic disease at time of EBRT. EBRT was well tolerated, as the majority of patients had grade2 or less acute toxicity and minimal late toxicity. While this is not the first study reporting on the efficacy of EBRT for patients with gross residual/unresectable nonanaplastic thyroid cancer, we uniquely report on a cohort of patients who were predominantly treated with a modern RT technique, as 77.3% of patients were treated with IMRT.
Chow et al. [6] reported on the role of EBRT in papillary thyroid carcinoma in 124 patients with gross residual disease of which 69 underwent EBRT at Queen Elizabeth Hospital in Hong Kong from 1960 to 1997. At 10 years those who underwent EBRT had significantly improved 10-year rates of locoregional control (56.2% vs. 24%, P = 0.0019, respectively). In a subsequent update Chow et al. reported on the outcomes of 217 patients with gross residual disease after primary surgery. In this updated report they found that EBRT with or without RAI improved locoregional control (63.4% vs. 24%, P<0.0001) and cancer-specific survival (74.1% vs. 49.7%, P = 0.001) [28]. None of the patients were treated with modern radiotherapy techniques, such as IMRT.
The Princess Margaret Cancer Center experience reported the outcomes of 46 patients with postoperative macroscopic residual disease, of which 13 received postoperative RT alone and 20 received postoperative EBRT and RAI [12]. No patient was treated with IMRT. The 5-year local relapse free rate was 62%, which is considerably lower than our 3-year rate of 90.0% in patients treated with CCRT. For unclear reasons the RT dose had a considerable range, from 5 to 65Gy.
In a more modern report, M.D. Anderson reported on their experience with postoperative EBRT for differentiated thyroid cancer with conformal treatment [8]. Schwartz et al. reported on 131 patients, of which only 15 had gross residual or unresectable disease, treated with IMRT (43.5%) or conventional EBRT. Patients were treated to a median RT dose of 60 Gy in 30 fractions; 82% of patients received RAI prior to EBRT, and all patients received TSH suppression. Of the 15 patients (11%) with gross residual or unresectable disease at time of EBRT, 4 had a complete response, 3 had a partial response, 6 had stable disease, and 2 progressed through treatment. All four patients with a complete response remained disease free at a median of 21.5 months.Four of the six patients with stable disease ultimately progressed within 3–21 months.
A Phase I study demonstrating the safety of IMRT in locally advanced thyroid cancer reported a 31% grade 3 dysphagia rate and a 38.5% grade 3 dermatitis rate [29]. Four of the 13 patients (30%) developed L’Hermitte’s syndrome of which all cases resolved spontaneously. Comparably, our 16.7% grade 3 dysphagia rate and 12.1% grade 3 dermatitis rate were considerably lower despite 30% of our patients being treated with CCRT.
Treatment efficacy is highly dependent on tumor localization. Significant scarring and anatomical variation from previous and often multiple head and neck surgeries (median, 2; range, 0–8 in this series) make neck ultrasonography and CT scans with or without contrast difficult to interpret. Multiple studies have reported on the utility of fluorodeoxyglucose (FDG)-PET in the detection of local recurrence, cervical lymph node metastases, and distant metastases in patients with well-differentiated thyroid cancer, particularly in those with RAI-refractory disease as FDG uptake is inversely correlated with RAI uptake [30–33]. As such, we now routinely use FDG-PET-CT to aid in tumor delineation. Accurate tumor delineation will help improve treatment outcomes and reduce treatment-related toxicity. Further incorporation of MRI may also result in improved tumor delineation and should be considered if the anatomy is particularly difficult to interpret.
Chemoradiation appeared to correlate with improved LPFS, but statistical significance was not met. Nevertheless, these data are concordant with what is known for other head and neck sites; concurrent chemoradiation results in superior locoregional disease control [34,35]. Interestingly, tumors with poorly differentiated histology had significantly improved locoregional progression-free survival despite a significantly greater rate of distant metastases compared with patients with well-/moderately differentiated histology. As patients with poorly differentiated histology were more likely to receive concurrent chemoradiation, this is a plausible explanation that merits further study. Our data did not suggest any benefit in the prevention of distant metastatic disease from the use of low-dose concurrent radiosensitizing chemotherapy.
New and effective systemic agents such as multikinase inhibitors, selective kinase inhibitors, and histone deacetylase inhibitors have dramatically changed our clinical armamentarium for patients with metastatic disease [5,36,37]. Of particular interest is the report of enhanced RAI uptake in patients treated with selumetinib, a mitogen-activated protein kinase (MAPK) inhibitor, which given the short course of drug therapy would allow a kinase inhibitor free interval during which EBRT could be considered [38]. While promising, studies are needed to determine the timing and sequencing of systemic therapies, low-dose radiation sensitizing chemotherapy, and EBRT.
While this study supports EBRT as a safe and effective treatment for patients with gross residual/unresectable non-medullary, non-anaplastic thyroid carcinoma, certain study limitations merit discussion. We included patients treated over a quarter century and as such, unaccountable variation may exist in pathological classification, radiation techniques, and sensitivity in detecting disease recurrence. Given a lack of robust tumor mutation analysis (i.e., BRAF, PIK3CA, AKT, PTEN, TP53), we were unable to account for biological differences between tumors, which have been associated with RAI resistance and a higher rate of tumor recurrence and cancer death [5]. We acknowledge that there was variability in the choice to use concurrent chemotherapy, which could not be accounted for by patient or tumor characteristics, though tumors with poorly differentiated histology were more likely to be treated with CCRT. As with any retrospective review the accurate classification of acute and late toxicities is dependent on accurate and meticulous documentation. Fortunately, since the year 2000 our institution adopted a systematic system to document treatment-related toxicities.
CONCLUSION
Surgery and RAI with or without TSH suppression is the standard of care for patients with differentiated thyroid carcinoma. In patients with gross residual or unresectable disease, EBRT is a safe and effective treatment modality with greater than 85% locoregional control in patients with non-metastatic disease and 90% locoregional control in patients treated with CCRT. The addition of EBRT can potentially limit the associated morbidity with uncontrolled locoregional disease, even in patients with widely metastatic disease. Further incorporation of EBRT with low-dose radiation-sensitizing concurrent chemotherapy including modern targeted therapies may result in improved treatment outcomes. Prospective studies are needed to validate the safety and efficacy of radiation and chemoradiation.
Footnotes
The authors declared that they have no conflict of interests.
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures 2013. InAtlanta, GA: American Cancer Society. [Google Scholar]
- 2.Hundahl SA, Fleming ID, Fremgen AM, et al. : A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985–1995 [see comments]. Cancer 1998;83:2638–2648. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferri EL, Kloos RT: Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab 2001;86:1447–1463. [DOI] [PubMed] [Google Scholar]
- 4.Biermann M, Pixberg MK, Schuck A, et al. : Multicenter study differentiated thyroid carcinoma (MSDS). Diminished acceptance of adjuvant external beam radiotherapy. Nuklearmedizin 2003;42:244–250. [PubMed] [Google Scholar]
- 5.Brierley J, Sherman E: The role of external beam radiation and targeted therapy in thyroid cancer. Semin Radiat Oncol 2012;22:254–262. [DOI] [PubMed] [Google Scholar]
- 6.Chow SM, Law SC, Mendenhall WM, et al. : Papillary thyroid carcinoma: Prognostic factors and the role of radioiodine and external radiotherapy. Int J Radiat Oncol Biol Phys 2002;52:784–795. [DOI] [PubMed] [Google Scholar]
- 7.Terezakis SA, Lee KS, Ghossein RA, et al. : Role of external beam radiotherapy in patients with advanced or recurrent nonanaplastic thyroid cancer: Memorial Sloan-kettering Cancer Center experience. Int J Radiat Oncol Biol Phys 2009;73:795–801. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz DL, Lobo MJ, Ang KK, et al. : Postoperative external beam radiotherapy for differentiated thyroid cancer: Outcomes and morbidity with conformal treatment. Int J Radiat Oncol Biol Phys 2009;74:1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azrif M, Slevin NJ, Sykes AJ, et al. : Patterns of relapse following radiotherapy for differentiated thyroid cancer: Implication for target volume delineation. Radiother Oncol 2008;89:105–113. [DOI] [PubMed] [Google Scholar]
- 10.Keum KC, Suh YG, Koom WS, et al. : The role of postoperative external-beam radiotherapy in the management of patients with papillary thyroid cancer invading the trachea. Int J Radiat Oncol Biol Phys 2006;65:474–480. [DOI] [PubMed] [Google Scholar]
- 11.Kim TH, Yang DS, Jung KY, et al. : Value of external irradiation for locally advanced papillary thyroid cancer. Int J Radiat Oncol Biol Phys 2003;55:1006–1012. [DOI] [PubMed] [Google Scholar]
- 12.Tsang RW, Brierley JD, Simpson WJ, et al. : The effects of surgery, radioiodine, and external radiation therapy on the clinical outcome of patients with differentiated thyroid carcinoma. Cancer 1998;82:375–388. [PubMed] [Google Scholar]
- 13.Farahati J,Reiners C,Stuschke M,et al. :Differentiated thyroid cancer. Impact of adjuvant external radiotherapy in patients with perithyroidal tumor infiltration (stage pT4). Cancer 1996;77:172–180. [DOI] [PubMed] [Google Scholar]
- 14.Meadows KM, Amdur RJ, Morris CG, et al. : External beam radiotherapy for differentiated thyroid cancer. Am J Otolaryngol 2006;27:24–28. [DOI] [PubMed] [Google Scholar]
- 15.Brierley JD, Tsang RW: External-beam radiation therapy in the treatment of differentiated thyroid cancer. Semin Surg Oncol 1999; 16:42–49. [DOI] [PubMed] [Google Scholar]
- 16.Phlips P, Hanzen C, Andry G, et al. : Postoperative irradiation for thyroid cancer. Eur J Surg Oncol 1993;19:399–404. [PubMed] [Google Scholar]
- 17.O’Connell ME, A’Hern RP, Harmer CL: Results of external beam radiotherapy in differentiated thyroid carcinoma: A retrospective study from the Royal Marsden Hospital. Eur J Cancer 1994;30A:733–739. [DOI] [PubMed] [Google Scholar]
- 18.Sheline GE, Galante M, Lindsay S: Radiation therapy in the control of persistent thyroid cancer. Am J Roentgenol Radium Ther Nucl Med 1966;97:923–930. [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Thyroid Carcinoma Version 2. In, 2013.
- 20.Cooper DS, Doherty GM, Haugen BR, et al. : Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 21.Edge SB, Byrd DR, Compton, et al. , editors: AJCC cancer staging manual 7th edition. Chicago, IL: Springer; 2010. [Google Scholar]
- 22.Hiltzik D, Carlson DL, Tuttle RM, et al. : Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: A clinicopathologic study of 58 patients. Cancer 2006;106:1286–1295. [DOI] [PubMed] [Google Scholar]
- 23.Volante M, Landolfi S, Chiusa L, et al. : Poorly differentiated carcinomas of the thyroid with trabecular, insular, and solid patterns: A clinicopathologic study of 183 patients. Cancer 2004;100:950–957. [DOI] [PubMed] [Google Scholar]
- 24.Lee NY, Lu JJ, editors: Target volume delineation and field setup: A practical guide for conformal and intensity-modulated radiation therapy. New York: Springer; 2012. [Google Scholar]
- 25.Trotti A, Colevas AD, Setser A, et al. : CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–181. [DOI] [PubMed] [Google Scholar]
- 26.Cox JD, Stetz J, Pajak TF: Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–1346. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P: Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:25. [Google Scholar]
- 28.Chow SM, Yau S, Kwan CK, et al. : Local and regional control in patients with papillary thyroid carcinoma: Specific indications of external radiotherapy and radioactive iodine according to T and N categories in AJCC 6th edition. Endocr Relat Cancer 2006;13:1159–1172. [DOI] [PubMed] [Google Scholar]
- 29.Urbano TG, Clark CH, Hansen VN, et al. : Intensity Modulated Radiotherapy (IMRT) in locally advanced thyroid cancer: Acute toxicity results of a phase I study. Radiother Oncol 2007;85:58–63. [DOI] [PubMed] [Google Scholar]
- 30.Khan N, Oriuchi N, Higuchi T, et al. : PET in the follow-up of differentiated thyroid cancer. Br J Radiol 2003;76:690–695. [DOI] [PubMed] [Google Scholar]
- 31.Grunwald F, Menzel C, Bender H, et al. : Comparison of 18FDG-PET with 131iodine and 99mTc-sestamibi scintigraphy in differentiated thyroid cancer. Thyroid 1997;7:327–335. [DOI] [PubMed] [Google Scholar]
- 32.Grunwald F, Kalicke T, Feine U, et al. : Fluorine-18 fluorodeoxyglucose positron emission tomography in thyroid cancer: Results of a multicentre study. Eur J Nucl Med 1999;26:1547–1552. [DOI] [PubMed] [Google Scholar]
- 33.Wang W, Larson SM, Tuttle RM, et al. : Resistance of [18f]-fluorodeoxyglucose-avid metastatic thyroid cancer lesions to treatment with high-dose radioactive iodine. Thyroid 2001,11:1169–1175. [DOI] [PubMed] [Google Scholar]
- 34.Pignon JP, Bourhis J, Domenge C, et al. : Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: Three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 2000;355:949–955. [PubMed] [Google Scholar]
- 35.Blanchard P, Baujat B, Holostenco V, et al. : Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): A comprehensive analysis by tumour site. Radiother Oncol 2011;100:33–40. [DOI] [PubMed] [Google Scholar]
- 36.Haugen BR, Sherman SI: Evolving approaches to patients with advanced differentiated thyroid cancer. Endocr Rev 2013;34: 439–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherman EJ, Su YB, Lyall A, et al. : Evaluation of romidepsin for clinical activity and radioactive iodine reuptake in radioactive iodine-refractory thyroid carcinoma. Thyroid 2013;23:593–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho AL, Grewal RK, Leboeuf R, et al. : Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 2013;368:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]


