Abstract
Purpose—
The aim of this statement is to summarize data on stroke risk factors that are unique to and more common in women than men and to expand on the data provided in prior stroke guidelines and cardiovascular prevention guidelines for women. This guideline focuses on the risk factors unique to women, such as reproductive factors, and those that are more common in women, including migraine with aura, obesity, metabolic syndrome, and atrial fibrillation.
Methods—
Writing group members were nominated by the committee chair on the basis of their previous work in relevant topic areas and were approved by the American Heart Association (AHA) Stroke Council’s Scientific Statement Oversight Committee and the AHA’s Manuscript Oversight Committee. The panel reviewed relevant articles on adults using computerized searches of the medical literature through May 15, 2013. The evidence is organized within the context of the AHA framework and is classified according to the joint AHA/American College of Cardiology and supplementary AHA Stroke Council methods of classifying the level of certainty and the class and level of evidence. The document underwent extensive AHA internal peer review, Stroke Council Leadership review, and Scientific Statements Oversight Committee review before consideration and approval by the AHA Science Advisory and Coordinating Committee.
Results—
We provide current evidence, research gaps, and recommendations on risk of stroke related to preeclampsia, oral contraceptives, menopause, and hormone replacement, as well as those risk factors more common in women, such as obesity/metabolic syndrome, atrial fibrillation, and migraine with aura.
Conclusions—
To more accurately reflect the risk of stroke in women across the lifespan, as well as the clear gaps in current risk scores, we believe a female-specific stroke risk score is warranted.
Keywords: AHA Scientific Statements, atrial fibrillation, hormone replacement therapy, menopause, metabolic syndrome X, preeclampsia/eclampsia, sex differences, stroke
Stroke has a large negative impact on society, with women disproportionately affected. An estimated 6.8 million (2.8%) of people in the United States are living after having had a stroke, including 3.8 million women and 3 million men.1 Stroke is the fifth-leading cause of death for men, but the third leading cause for women.2 By 2030, there will be an estimated 72 million people >65 years old (19% of the population), and women will increasingly outnumber men.3 These demographics suggest an anticipated increase of the burden of stroke in women.4 Nearly half of stroke survivors have residual deficits, including weakness or cognitive dysfunction, 6 months after stroke,5 which translates into ≈200 000 more disabled women with stroke than men. Some of the impact is explained by the fact that women live longer, and thus the lifetime risk of stroke in those aged 55 to 75 years is higher in women (20%) than men (17%).6 Women are more likely to be living alone and widowed before stroke, are more often institutionalized after stroke, and have poorer recovery from stroke than men.7–13 Therefore, women are more adversely affected by stroke than men. How our society adapts to the anticipated increase in stroke prevalence in women is vitally important. Now more than ever, it is critical to identify women at higher risk for stroke and initiate the appropriate prevention strategies.
Despite the importance of stroke in women, there has never been an American Heart Association (AHA)/American Stroke Association guideline dedicated to stroke risk and prevention in women. This endeavor is important because women differ from men in a multitude of ways, including genetic differences in immunity,14,15 coagulation,16,17 hormonal factors,18 reproductive factors including pregnancy and childbirth, and social factors,5,9 all of which can influence risk for stroke and impact stroke outcomes. This document provides a new stroke prevention guideline that covers topics specific to women in more detail than has been included in current primary and secondary stroke prevention guidelines19,20 and provides more emphasis on stroke-specific issues in women than are included in the current cardiovascular prevention guideline for women.21
Writing group members were nominated by the committee chair on the basis of their previous work in relevant topic areas and were approved by the AHA Stroke Council’s Scientific Statement Oversight Committee and the AHA’s Manuscript Oversight Committee. Multiple disciplines are represented, including neurology, neuroscience research, internal medicine, obstetrics/gynecology, cardiology, pharmacology, nursing, epidemiology, and public policy. The panel reviewed relevant articles on adults using computerized searches of the medical literature through May 15, 2013. The evidence is organized within the context of the AHA framework and is classified according to the joint AHA/American College of Cardiology and supplementary AHA Stroke Council methods of classifying the level of certainty and the class and level of evidence (Tables 1 and 2). The document underwent extensive AHA internal peer review, Stroke Council Leadership review, and Scientific Statements Oversight Committee review before consideration and approval by the AHA Science Advisory and Coordinating Committee. Each topic was assigned to a primary author and a secondary reviewer. In this guideline, we focus on the risk factors unique to women, such as reproductive factors, and those that are more common in women, including migraine with aura, obesity, metabolic syndrome, and atrial fibrillation (AF). Topics that are not covered in detail include management of diabetes mellitus and cholesterol, because there are no recommendations for these risk factors that are specific to women. We therefore direct readers to the most recent primary and secondary prevention guidelines for specific detailed recommendations.19,20
Table 1.
Applying Classification of Recommendation and Level of Evidence
| ESTIMATE OF CERTAINTY (PRECISION) OF TRETMENT EFFECT | SIZE OF TREATMENT EFFECT | ||||||
|
CLASS I Benefit >>> Risk Procedure/Treatment SHOULD be performed/administered |
CLASS IIa Benefit > > Risk Additional studies with focused objectives needed IT IS REASONABLE to per-form procedure/administer treatment |
CLASS lib Benefit ≥ Risk Additional studies with broad objectives needed; additional registry data would be helpful Procedure/Treatment MAY BE CONSIDERED |
CLASS III No Benefit or CLASS III Harm | ||||
| Procedure/Test | Treatment | ||||||
| COR III: No benefit | Not Helpful | No Proven Benefit | |||||
| COR III: Harm | Excess Cost w/o Benefit or Harmful | Harmful to Patients | |||||
|
LEVEL A Multiple populations evaluated* Data derived from multiple randomized clinical trials or meta-analyses |
|
|
|
|
|||
|
LEVEL B Limited populations evaluated* Data derived from a single randomized trial or nonrandomized studies |
|
|
|
|
|||
|
LEVEL C Very limited populations evaluated* Only consensus opinion of experts, case studies, or standard of care |
|
|
|
|
|||
| Suggested phrases for writing recommendations | should is recommended is indicated is useful/effective/beneficial |
is reasonable can be useful/effective/beneficial is probably recommended or indicated |
may/might be considered may/might be reasonable usefulness/effectiveness is unknown/unclear/uncertain or not well established |
COR III: No Benefit | COR III: Harm | ||
| is not recommended is not indicated should not be performed/administered/other is not useful/beneficial/effective |
potentially harmful causes harm associated with excess morbidity/mortality should not be performed/administered/other |
||||||
| Comparative effectiveness phrases† | treatment/strategy A is recommended/indicated in preference to treatment B treatment A should be chosen over treatment B | treatment/strategy A is probably recommended/indicated in preference to treatment B it is reasonable to choose treatment A over treatment B |
|||||
A recommendation with Level of Evidence B or C does not imply that the recommendation is weak. Many important clinical questions addressed in the guidelines do not lend themselves to clinical trials. Although randomized trials are unavailable, there may be a very clear clinical consensus that a particular test or therapy is useful or effective.
Data available from clinical trials or registries about the usefulness/efficacy in different subpopulations, such as sex, age, history of diabetes, history of prior myocardial infarction, history of heart failure, and prior aspirin use.
For comparative effectiveness recommendations (Class I and IIa; Level of Evidence A and B only), studies that support the use of comparator verbs should involve direct comparisons of the treatments or strategies being evaluated.
Table 2.
Definition of Classes and Levels of Evidence Used in AHA/ASA Recommendations
| Class I | Conditions for which there is evidence for and/or general agreement that the procedure or treatment is useful and effective. |
| Class II | Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a procedure or treatment. |
| Class IIa | The weight of evidence or opinion is in favor of the procedure or treatment. |
| Class IIb | Usefulness/efficacy is less well established by evidence or opinion. |
| Class III | Conditions for which there is evidence and/or general agreement that the procedure or treatment is not useful/effective and in some cases may be harmful. |
| Therapeutic recommendations | |
| Level of Evidence A | Data derived from multiple randomized clinical trials or meta-analyses |
| Level of Evidence B | Data derived from a single randomized trial or nonrandomized studies |
| Level of Evidence C Diagnostic recommendations | Consensus opinion of experts, case studies, or standard of care |
| Level of Evidence A | Data derived from multiple prospective cohort studies using a reference standard applied by a masked evaluator |
| Level of Evidence B | Data derived from a single grade A study or 1 or more case-control studies, or studies using a reference standard applied by an unmasked evaluator |
| Level of Evidence C | Consensus opinion of experts |
AHA/ASA indicates American Heart Association/American Stroke Association.
One of the writing group’s goals was to review risk factors that are unique to women or might affect women’s risk of stroke differentially, as well as to determine whether there is a need for a stroke risk score for women that incorporates female-specific factors such as reproductive and menopausal factors (Table 3). Recommendations that are unique to women are included, as well as gaps in knowledge where additional research is needed to inform risk identification and thus improve stroke prevention in women. To demonstrate the importance of enhancing stroke risk scores for women, we have reviewed existing stroke risk scores and assessed their relevance on the basis of our summary of the literature on specific risk factors. Evidence from this guideline will inform providers and researchers of the current understanding of stroke risk and prevention in women. More importantly, this guideline may empower women and their families to understand their own risk and how they can minimize the chances of having a stroke.
Table 3.
Stroke Risk Factors, Categorized by Those That Are Sex-Specific, Stronger or More Prevalent in Women, or Similar Between Women and Men
| Risk Factor | Sex-Specific Risk Factors | Risk Factors That Are Stronger or More Prevalent in Women | Risk Factors With Similar Prevalence in Men and Women but Unknown Difference in Impact |
|---|---|---|---|
| Pregnancy | X | ||
| Preeclampsia | X | ||
| Gestational diabetes | X | ||
| Oral contraceptive use | X | ||
| Postmenopausal hormone use | X | ||
| Changes in hormonal status | X | ||
| Migraine with aura | X | ||
| Atrial fibrillation | X | ||
| Diabetes mellitus | X | ||
| Hypertension | X | ||
| Physical inactivity | X | ||
| Age | X | ||
| Prior cardiovascular disease | X | ||
| Obesity | X | ||
| Diet | X | ||
| Smoking | X | ||
| Metabolic syndrome | X | ||
| Depression | X | ||
| Psychosocial stress | X |
Epidemiology of Ischemic and Hemorrhagic Stroke in Women
Overview
In the United States, more than half (53.5%) of the estimated 795 000 new or recurrent strokes occur among women annually, resulting in ≈550 00 more stroke events in women than men.1 Results from the Framingham cohort show that women have a higher lifetime risk of stroke than men.6,12 Although stroke incidence rates have declined, data suggest that the decline may be smaller for women than men.22–24 Data from epidemiological studies demonstrate that the majority (87%) of strokes are ischemic (IS), with the remainder hemorrhagic (10% intracerebral [ICH] and 3% subarachnoid [SAH]).1 With an anticipated increase in the aging population, the prevalence of stroke survivors is projected to increase, particularly among elderly women.4 Because the United States lacks a national surveillance system for cardiovascular disease (CVD),25 and sex-specific or age- and sex-specific stroke incidence data have not been routinely reported in published studies, there are important gaps in our understanding of sex differences in incident and recurrent stroke events, temporal patterns of stroke events, and outcomes after stroke. Most of what is known about the epidemiology of stroke comes from mortality data. As noted previously, the higher stroke mortality for women is often attributed to the longer life expectancy of women. Of 128 842 deaths related to stroke in 2009, 76 769 (59.6%) occurred in women.1
Incidence
Ischemic Stroke
Within most age strata, women have a lower IS incidence than men, and as such, the overall age-adjusted incidence of IS is lower for women than men4,24,26–31; however, sex differences in IS incidence rates differ across the age strata. In the oldest age groups (generally >85 years of age), women tend to have higher12,24,27–30 or similar incidence of IS as men.4,26 Because women tend to be older when they have their stroke events, and women have a longer life expectancy than men, age-adjusted rates can be misleading and may underestimate the total burden of stroke in women. Differences by race/ethnicity have also been noted, with higher rates among blacks and Hispanics31 than among whites for both women and men.1,28–31
Hemorrhagic Stroke (SAH and ICH)
The majority of studies show that women have higher rates of SAH incidence than men26,32–43; however, sex differences are modified by age such that SAH rates are higher in men at younger ages but higher in women relative to men beginning at ≈55 years of age.44,45 Data reported from non-US populations have shown differing sex-related patterns across countries, with higher SAH incidence among men in Finland and eastern Europe, possibly because of regional differences in risk factor prevalence in men and women.46 The incidence of ICH has been reported to be lower in women than men in most26,39–41,47 but not all42 studies. Differences by race/ethnicity have been noted, with higher ICH incidence rates in blacks than whites30,31,48 and in Hispanics than whites for both women and men.31
Increased Prevalence of SAH in Women: Risks Related to Cerebral Aneurysms
There has been significant debate about the potential cause of the increased risk of SAH in women. Autopsy and angiographic studies have documented a higher prevalence of cerebral aneurysms in women,49 as well as a higher risk of rupture.50 These findings are in agreement with results of a recent study from the Nationwide Inpatient Sample, which claimed that more than twice as many women as men were discharged with both ruptured and unruptured cerebral aneurysms.51 There is also a difference in the distribution of aneurysm locations in women versus men, and this may convey a higher hemorrhagic risk, especially with greater prevalence of aneurysms at the posterior communicating artery.52 Other studies have suggested similar trigger factors for aneurysm rupture in men and women.53 There is also no convincing evidence of increased risk of aneurysmal SAH in pregnancy or the puerperium,54 and before age 50 years, aneurysmal SAH is more common in men.55 A population-based case-control study showed that the risk of SAH was lower in women with first pregnancy after 23 years of age and in those who had ever used hormone therapy (HT).56 The literature certainly confirmed a higher incidence of SAH and a higher prevalence of cerebral aneurysms in women, but not necessarily a higher risk for rupture of aneurysms with similar characteristics.
Prevalence
On the basis of self-report data from the US 2010 National Health Interview Survey, it is estimated that just more than half (51.8%, 3.223 million) of the 6.226 million adults (3%) in the United States who have been told they had a stroke were women.57 Data from the Behavioral Risk Factor Surveillance System for the time period 2006 to 2010 showed that the age-adjusted self-reported prevalence of stroke survivors did not change significantly for women (2.5%–2.6%), whereas it did for men, with prevalence declining from 2.8% in 2006 to 2.5% in 2009 and then increasing to 2.7% in 2010.58
Mortality
In the United States, ≈60% of deaths related to stroke in 2010 occurred in women (77 109 of 129 476 deaths).1,2,59 Age-specific stroke mortality is higher for men than women for all age groups except ≥85 years, and this pattern is consistent across all racial/ethnic groups (Figures 1 and 2).1,2,59 In 2010, age-adjusted stroke mortality (based on International Classification of Diseases, 10th Revision, codes I60–I69) for women was 38.3 per 100 000 compared with 39.3 per 100 000 for men (relative risk [RR], 0.97).59 For most of the past century, age-adjusted stroke mortality rates declined dramatically in the United States,60 and between 1996 and 2005, these declines were marginally greater for men (−28.2%) than women (−23.9%).1,61 Stroke is a major cause of death worldwide, accounting for an estimated 10% of all deaths in 2002. Similar to the United States, women worldwide have lower stroke mortality than men except in the older age groups,62–65 and IS mortality has declined for both men and women, with some acceleration in the rate of decline in the 1990s for certain age-sex groups.66
Figure 1.
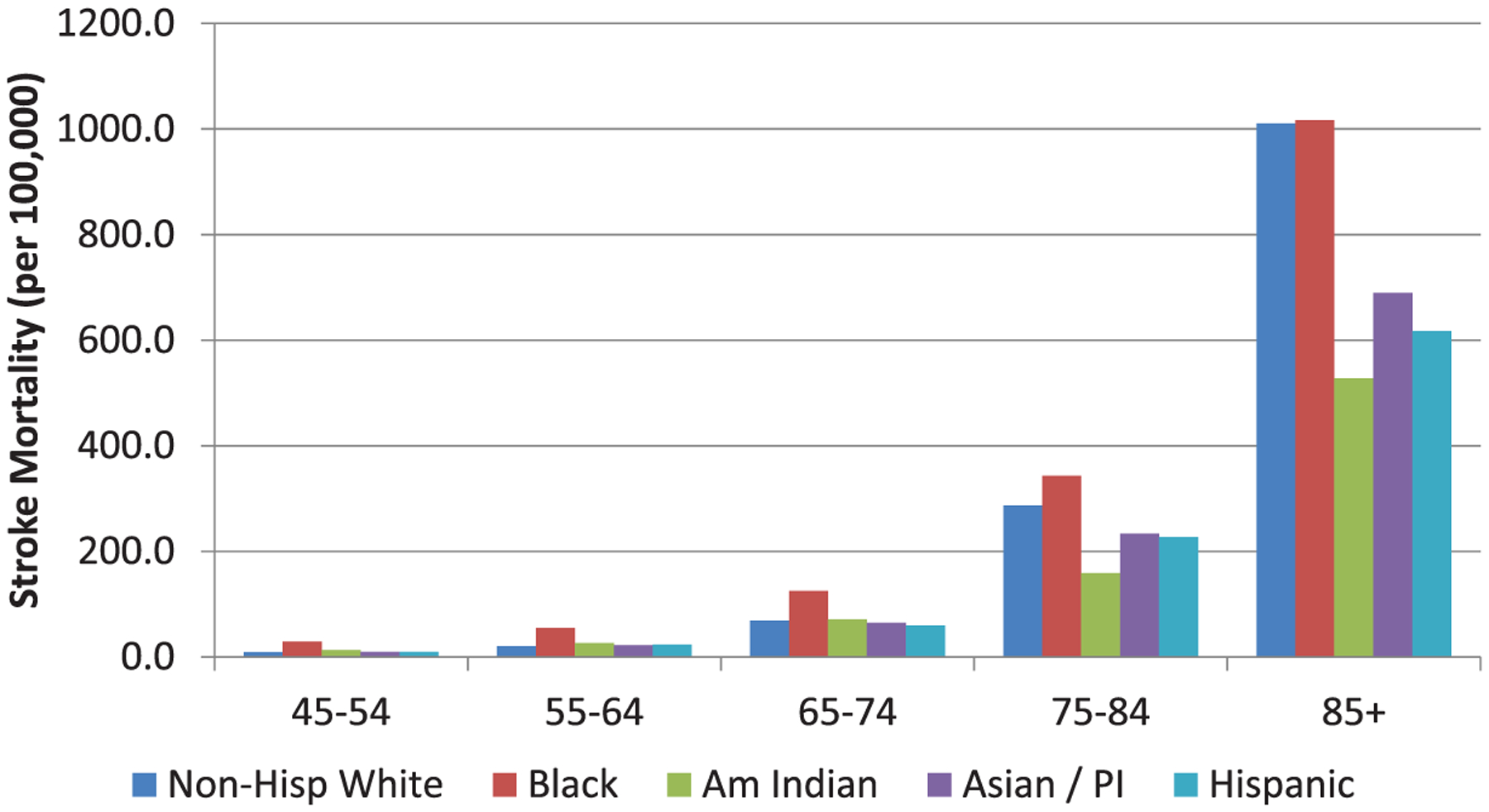
US stroke mortality rates for women, 2009. Am Indian indicates American Indian; Non-Hisp, non-Hispanic; and PI, Pacific Islander.
Figure 2.
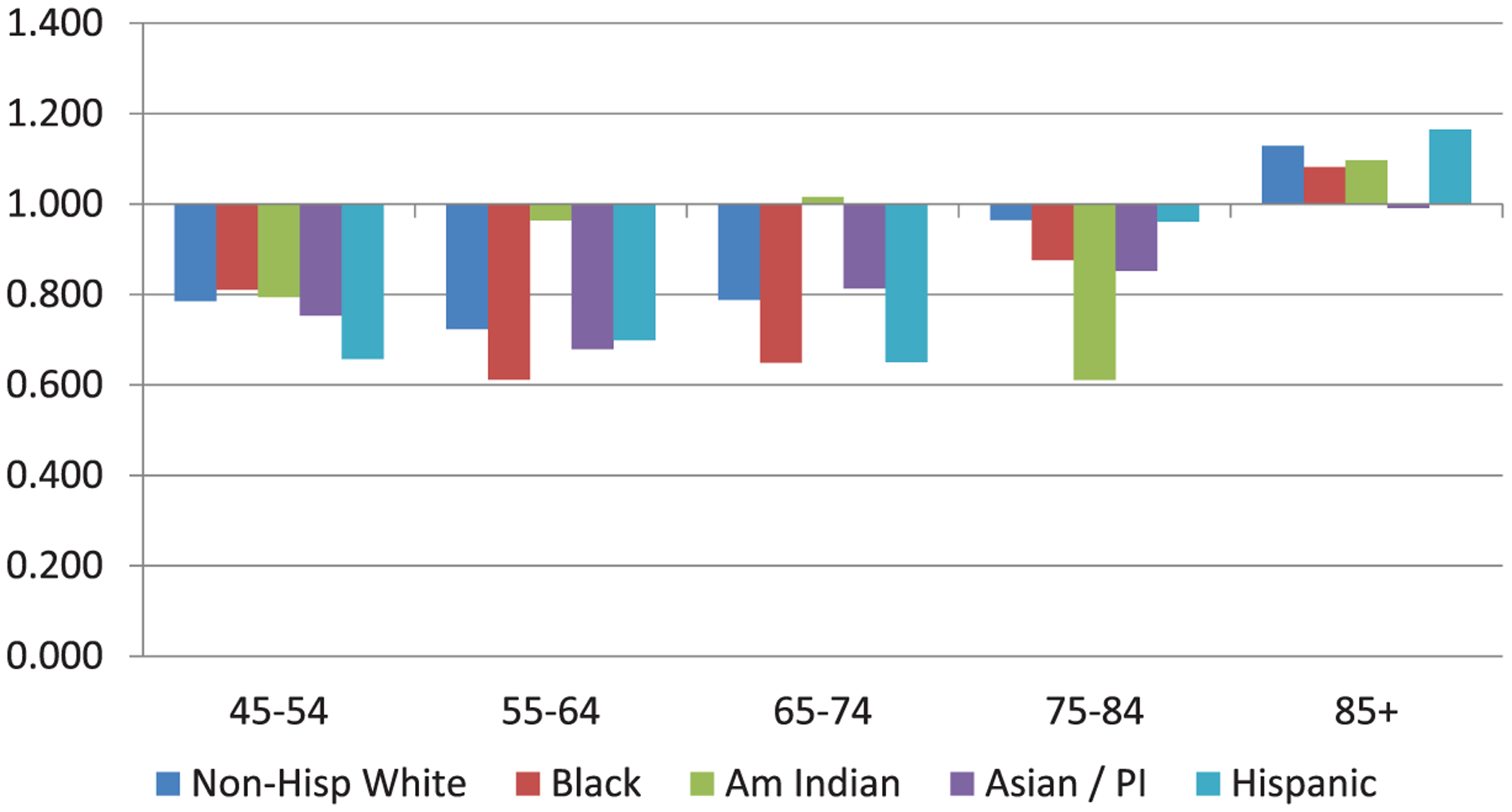
Female-male US stroke mortality ratio, 2009. Am Indian Indicates American Indian; Non-Hisp, non-Hispanic; and PI, Pacific Islander.
Ischemic Stroke
An analysis of US death certificate data from 1995 to 1998 found that IS constitutes a larger percentage of stroke mortality overall in women than men (82% of stroke deaths in women versus 78% in men), with the greatest difference seen for older women.67 The overall age-adjusted IS death rate in women is slightly lower (74.3 per 100 000 compared with 78.8 per 100 000 for men; RR, 0.94; 95% confidence interval [CI], 0.93–0.95). Younger women have lower age-specific IS mortality than men, but there is a crossover at ≈65 years of age, at which point older women have higher age-specific IS mortality than men.67 This study also reported that the age-adjusted death rate for IS was higher for white women than white men (RR, 1.21; 95% CI, 1.21–1.22), but for all other racial/ethnic groups, the age-adjusted death rate for IS was lower or similar for women and men.67
Hemorrhagic Stroke
Women have higher age-adjusted SAH mortality than men (4.9 versus 3.1 per 100 000; RR, 1.59; 95% CI, 1.54–1.62).67 Sex differences persisted across racial/ethnic groups and were highest among Asian Americans. In addition, the risk ratio of mortality in women versus men increased with age.67,68 In contrast to SAH, women have lower age-adjusted ICH mortality rates than men (13.3 per 100 000 for women and 16.2 per 100 000 for men; RR, 0.82; 95% CI, 0.81–0.83). Mortality was lower for women aged <65 years, but there was no sex difference in ICH mortality risk for adults ≥65 years of age.67
Total Stroke Case Fatality
The findings of studies that have examined sex differences in short-term case-fatality rates (commonly defined as within 30 days of onset and inclusive of all strokes) have been quite variable and are complicated by a lack of age adjustment. Some studies have reported that women have higher case fatality than men,26,27,30,69,70 whereas others have not.9,13,42,71 Although a recent systematic review found that short-term case fatality was higher in women than men in 26 of 31 studies (with a pooled rate of 24.7% versus 19.7%),26 these results were based on crude unadjusted data. Much of the higher case fatality in women is likely to be attributable to the fact that women tend to be older at the time of their stroke.4 Studies that have adjusted for age (as well as other characteristics) show that the sex difference in short-term mortality can actually reverse, with women having lower mortality after adjustment.72,73 A study of temporal trends (1950–2004) in the US Framingham Study found that age-adjusted 30-day fatality decreased significantly for men but not women.22 Non-US populations have also reported mixed results in terms of sex differences in stroke case fatality over time,69 which may be attributable to differences in the time periods studied, underlying demographics, lack of age adjustment, and other factors. Case-fatality studies for IS have shown either no sex differences or higher rates in men.27,30 A study from the Netherlands that examined trends in IS 30-day case fatality for the period 1997 to 2005 showed that in all age-sex groups, the case fatality declined significantly; the largest decline for men was from 12.5% to 6.9% (−0.42 change) in the 65- to 74-year-old age group, and the largest decline for women was from 6.4% to 3.5% (−0.45) in the 35- to 64-year-old age group.66 Data are limited to assess case fatality for hemorrhagic strokes. A study restricted to a younger population (20–44 years of age) reported lower 30-day case fatality after SAH in women than in men (9% versus 17%).41 Studies have shown differing patterns of ICH case fatality by sex. The Atherosclerosis Risk in Communities study (ARIC) reported a lower 30-day ICH case fatality for women than for men (30.4% versus 34.5%),30 but the Northern Manhattan Stroke Study found slightly higher 1-month case fatality for women than men (40% versus 35%).41 Temporal trends in case fatality for hemorrhagic stroke are largely unreported. A Finnish study found similar declines in 28-day case fatality for women and men over a 12-year period from 1991 to 2002.74
Sex Differences in Stroke Awareness (Delay, Warning Signs, Risk Factors)
Delayed hospital arrival is the single most important reason for the failure to administer thrombolytic treatment within the eligible time window of 3 or 4.5 hours. Most studies have not found important sex differences in delayed hospital arrival,4,75 but a few found women have longer prehospital delay than men.76–80 Most studies that have explored knowledge and awareness of stroke symptoms in either stroke patients or at-risk populations have not compared results by sex; however, several population-based studies have shown that knowledge and awareness of stroke warning signs and symptoms are somewhat higher in women than men.81–83 One study reported that although women were more likely than men to have heard of tissue-type plasminogen activator therapy for stroke, they were less likely to know that it must be administered within 3 hours.84 Population-based surveys of women conducted by the AHA have identified an overall poor level of knowledge about CVD and stroke, particularly in minority women85,86; however, the studies excluded men and were therefore unable to report on sex differences.
Epidemiology of Ischemic and Hemorrhagic Stroke in Women: Summary and Gaps
Stroke epidemiology research predominantly describes IS events. Additional research is needed to understand sex differences for hemorrhagic stroke events.
Data are limited in terms of sex-, race-, and age-specific rates of stroke incidence, mortality, and case fatality. This represents an important gap, because disease patterns and outcomes have been shown to vary by these characteristics. Future studies should report data separately for men and women, stratify by age when examining sex differences in disease rates, and clarify whether first-ever stroke events, recurrent events, or both are being reported. In addition to reporting by sex and age, for each stroke subtype, the incidence, mortality, and case fatality should be reported by race/ethnicity. In general, stroke event rates are lower in women than men, but sex comparisons based on age-adjusted rates mask important differences by age. There is a higher lifetime risk of stroke in women than men and a greater number of stroke deaths in women than men.
Vascular Differences in Stroke Risk: Sex and Hypertension
Hypertension is the most common modifiable risk factor for stroke in both men and women and has the highest population-attributable risk.2,19 There are a number of important sex differences in the prevalence, treatment, and pathophysiology of hypertension that should be highlighted to improve awareness and treatment of this risk factor in women.
Sex Differences in Stroke Risk With Hypertension
Among stroke patients, some studies,9,13,71,72,88,89 but not all,90,91 have shown that women are more likely to have hypertension than men. Similarly, women may have a higher risk of first stroke with hypertension. For example, the INTERSTROKE study showed that women had a higher risk of stroke with self-reported blood pressures (BPs) of 160/90 mm Hg (odds ratio [OR], 4.89; 95% CI, 3.79–6.32) than men (OR, 3.88; 95% CI, 3.22–4.68), although the CIs overlapped.92 In addition, older women (mean age 63 years) with prehypertension had a 93% increased risk of stroke compared with normotensive women in the Women’s Health Initiative (WHI) cohort, which implies that early and sustained treatment of hypertension is critical.93
Efficacy of Hypertension Treatment and Reduction of Stroke in Women
The effects of pharmacological intervention to lower BP and thereby reduce the risk of stroke on cardiovascular outcomes and surrogate cardiovascular end points have been studied extensively,94–107 and women have been well represented in large clinical trials of antihypertensive therapy; however, no trials have specifically examined a differential effect of pharmacological BP treatment in men and women on stroke events. Similarly, post hoc analyses and meta-analyses of clinical trial data have not reported sex differences in response to treatment or stroke events. In a recent meta-analysis of 31 large, randomized BP trials, treatment of hypertension in women aged >55 years (90% of whom were white) was associated with a 38% risk reduction in fatal and nonfatal cerebrovascular events (95% CI, 27%–47%). A reduction of 25% in fatal and nonfatal cardiovascular events (95% CI, 17%–33%) was also reported, together with a 17% reduction in cardiovascular mortality (95% CI, 3%–29%).108 Therefore, women benefit significantly from these interventions, as do men, and the type of medication used to lower the BP may be less relevant than the achievement of target BP goals.
Analyses of women of different racial/ethnic and age groups have suggested particular benefit of BP reduction in younger and black women. In 1 large systematic review of prospective studies, BP treatment in those aged 30 to 54 years (of whom 79% were white) yielded a reduction in risk of fatal and nonfatal cerebrovascular events of 41% (95% CI, 8%–63%), as well as a 27% reduction in fatal and nonfatal cardiovascular events (95% CI, 4%–44%).109 In this same study, when black women were considered as a separate group, BP treatment reduced the risk of fatal and nonfatal cerebrovascular events by 53% (95% CI, 29%–69%,) and all-cause mortality by 34% (95% CI, 14%–9%,).109
Sex, BP, Antihypertensive Treatment, and Achieving BP Goals
Numerous studies have shown that females have lower BP levels over much of their life span than their age-matched male counterparts,110 but this changes with age. For example, the prevalence of hypertension in adults <45 years of age is lower in women than men, but hypertension becomes increasingly prevalent and is higher in postmenopausal women than men after the age of 55 years, which suggests an important role of sex hormones in the regulation of BP.1 The lifetime risk of developing hypertension in the United States is ≈29% for women and 31% for men1; however, ≈75% of women >60 years of age become hypertensive.2 Age-adjusted hypertension prevalence, both diagnosed and undiagnosed, from 1999 to 2002 was 78% for older women and only 64% for older men.111
Sex differences in the pattern of prescribed antihypertensive medications have been seen across several large studies. For example, in the Framingham Heart Study, 38% of women but only 23% of men were prescribed thiazide diuretics,112 and similar rates were seen in the National Health and Nutrition Examination Survey (NHANES) cohorts, with higher diuretic (31.6% versus 22.3%) and angiotensin receptor blocker (11.3% versus 8.7%) use in women.113
Currently, there is no compelling evidence that there are differences in the response to BP medications between the sexes111; however, in large-scale reviews that examined the efficacy of β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics,114 there is no mention that sex-specific efficacy end points were evaluated or even considered. The possibility of differences in efficacy of BP medications therefore exists.
Some studies have suggested that antihypertensive medication use is significantly higher among women than men (61.4% versus 56.8%). Among treated hypertensive people, the proportion taking ≥3 antihypertensive drugs was lower among women than men, especially among older people (60–69 years old: 12.3% versus 19.8%; 70–79 years old: 18.6% versus 21.2%; and ≥80 years old: 18.8% versus 22.8%). Only 44.8% of treated women achieved BP control versus 51.1% of treated men.113 Notably, hypertensive women are significantly more likely to be treated than men but less likely to have achieved BP control. This may be because of unknown physiological mechanisms (ie, arterial stiffness, overactivation of the reninangiotensin system) or poorer compliance in women. The recent PARITE study, which examined 3440 patients, found that in French office-based cardiology practices, the antihypertensive regimen is adjusted as often in female as in male patients. Hypertension was uncontrolled in 76% of both men and women, and 69% were at high global cardiovascular risk (75% of men, 62% of women; P<0.001).113,115
Unfortunately, control of hypertension is poor in high-risk elderly women. Data from the Framingham Heart Study showed an age-related decrease in BP control rates that was more pronounced in women than men.112 Among participants >80 years of age with hypertension, only 23% of women (versus 38% of men) had BP <140/90 mm Hg.112
In analyses from the NHANES III and IV cohorts, the age-adjusted prevalence of uncontrolled BP was 50.8±2.1% in men and 55.9±1.5% in women, which was not significantly different; women had a higher prevalence of other concomitant cardiovascular risk factors,110 which likely contributed to poorer BP control in elderly women. These included central obesity, elevated total cholesterol, and low high-density lipoprotein cholesterol levels.110 Among adults with hyper‐tension in NHANES from 1999 to 2004, women were at higher risk of cardiovascular events than men, such that 53% of women but only 41% of men had >3 of the 6 risk factors studied (P<0.001).
Sex differences in hypertension and BP regulation are complex, because ovarian hormones influence BP considerably. Therefore, studies that examine vascular function and BP must take hormonal status into account.111,116 Sex differences in sympathetic activity, vascular reactivity, water regulation (arginine vasopressin signaling), and autonomic control have been well documented,116 but most of these studies were performed in young women. Efforts to assess the effects of hormonal effects on the vasculature have examined specific points in the menstrual cycle or suppressed ovarian function using gonadotropin-releasing hormone agonists or antagonists. In addition to hormone-dependent effects, these investigations have demonstrated hormone-independent sex differences in the vasculature.116 Hormone-independent approaches to BP regulation may be more relevant to older, postmenopausal women and may provide important information that will inform future clinical trials of different BP reduction strategies.
Several nonpharmacological recommendations for BP management are relevant to both men and women. A recent meta-analysis showed that even a modest reduction in salt intake for ≥4 weeks led to significant and important decreases in BP in both hypertensive and normotensive individuals, irrespective of sex and ethnic group. This was accompanied by a small physiological increase in plasma renin activity, aldosterone, and noradrenaline. Therefore, reductions in salt intake from 9 to 12 g/d to 3 g/d have been recommended.117
Side effects of antihypertensive therapy tend to be encountered with a higher degree of frequency in women than men. Diuretic-induced disturbances of electrolyte concentration are seen more frequently in women,118,119 as is angiotensin-converting enzyme inhibitor-induced cough and calcium channel blocker (CCB)-related dependent edema.120
Hypertension in Women of Childbearing Age
Prepregnancy hypertension increases the risk for preeclampsia/eclampsia and stroke during pregnancy. The choice of BP-lowering medications before pregnancy should be made based on a woman’s intentions for future pregnancy, because some categories of medications are associated with various risks if continued during pregnancy (Table 4).120a,121*
Table 4.
Summary of Antihypertensive Drugs Used During Pregnancy
| Category | Maternal Side Effects | Teratogenicity or Fetal-Neonatal Adverse Effects | Class/Level of Evidence (see Table 2) |
|---|---|---|---|
| Centrally acting α2-adrenerglc agonist (eg, methyldopa) | Sedation, elevated LFTs, depression | No | IIa/C |
| Diuretics (thiazide) | Hypokalemia | No | III/B |
| β-Blockers (atenolol) | Headache | Associated with fetal growth restriction | III/B |
| β-Blockers (pindolol, metoprolol) | Headache | Possible fetal growth restriction, neonatal bradycardia | IIa/B |
| Calcium channel blockers (eg, nifedipine) | Headache; possible interaction with magnesium sulfate; may interfere with labor | No | I/A |
| Combined α-β blockers (labetalol) | May provoke asthma exacerbation | Possible neonatal bradycardia | IIa/B |
| Hydralazine | Reflex tachycardia, delayed hypotension | Neonatal thrombocytopenia, fetal bradycardia | III/B |
| ACE inhibitors, angiotensin receptor blockers, renin inhibitors | Skeletal and cardiovascular abnormalities, renal dysgenesis, pulmonary hypoplasia | III/C |
ACE indicates angiotensin-converting enzyme; and LFTs, liver function tests. Modified from Umans et al120a with permission from Elsevier, Copyright © 2009.
α-Blockers, β-blockers, CCBs, hydralazine, and thiazide diuretics have been used in pregnancy; all transfer across the placenta. There are no data from large, well-controlled, randomized controlled trials directly comparing specific antihypertensive agents in pregnancy. Methyldopa has been extensively used in pregnancy and appears to be safe,122–127 including for neonates in a long-term pediatric study.128 A Cochrane review of the use of β-blockers in pregnancy noted that these drugs decreased the risk of progression to severe hypertension but may have increased risk for fetal growth restriction (n=1346; RR, 1.36; 95% CI, 1.02–1.82),125,126 although this may have been confounded in part by the inclusion of trials that used atenolol, which is not recommended in pregnancy because of its known association with fetal growth restriction.129,130 Pindolol and metoprolol appear safe for use in pregnancy.131 CCBs appear to be safe in pregnancy, with the most commonly used CCB being nifedipine.132,133 A 2007 Cochrane review indicated that there was a small increase in the risk for preeclampsia with the use of CCBs versus no therapy (725 women; RR, 1.40; 95% CI, 1.06–1.86).132 Diuretics, predominantly thiazide-type, have been indicated to be safe in pregnancy,124,134 and women taking thiazides before pregnancy do not need to discontinue them; however, a 2007 Cochrane review examined the use of diuretics to prevent preeclampsia.135 For thiazides, the reviewers noted that several studies were of uncertain quality and that there was insufficient evidence for any differences between treatment and control groups (4 trials, 1391 women; RR, 0.68; 95% CI, 0.45–1.03).135
Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and direct renin inhibitors are contraindicated at all stages of pregnancy because of teratogenicity and adverse fetal outcomes.136–139
Sex and Hypertension in Relation to Prevention of Stroke: Summary and Gaps
There is insufficient evidence to warrant a different approach to BP treatment in women from that used for men; as such, the existing guidelines for measurement, identification, and management of BP in adults should be followed. Existing guidelines for nonpharmacological intervention (predominantly dietary modification) to lower BP and to reduce stroke risk in adults should be followed.19,140 It is unclear whether the age-related decline in BP control among women is related to inadequate intensity of treatment, inappropriate drug choices, lack of compliance, true treatment resistance, biological factors, or other factors. Further research to resolve these questions is needed. In addition, hormone-dependent and -independent approaches to BP treatment require further study.
Sex and Hypertension in Relation to Prevention of Stroke: Recommendations
The recommendations for BP treatment to prevent stroke are currently the same for women as for men and can be found in the AHA/American Stroke Association “Guidelines for the Primary Prevention of Stroke,”19 the European Society of Hypertension/European Society of Cardiology guidelines,141 and the “Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.”142
Sex-Specific Risk Factors
Pregnancy and Stroke
Pregnancy is a condition unique to women. Although stroke is uncommon in pregnancy (34 strokes per 100 000 deliveries),143 the risk for stroke is higher in pregnant than in non-pregnant young women (21 per 100 000144), with the highest stroke risk occurring in the third trimester and post partum. The physiological changes of pregnancy, specifically venous stasis, edema, and hypercoagulability caused by activated protein C resistance, lower levels of protein S, and increased fibrinogen, combine to make pregnancy and the postpartum period a time of increased risk for stroke. Pregnancy-related hypertension is the leading cause of both hemorrhagic stroke and IS in pregnant and postpartum women.145–147
Hypertensive Disorders of Pregnancy
Preeclampsia/eclampsia and pregnancy-induced hypertension are the 2 most important hypertensive disorders of pregnancy. Preeclampsia is defined as progressively worsening high BP in pregnancy that occurs in the setting of proteinuria (≥300 mg of protein in a 24- hour urine specimen).148 Preeclampsia may be of early onset (before 37 weeks’, gestation) or late onset (after 37 weeks). Eclampsia is preeclampsia that progresses to seizures. Preeclampsia is a multisystem disorder, and abnormalities such as HELLP (hemolysis, elevated liver enzymes, or low platelets), disseminated intravascular coagulation, acute renal failure, myocardial infarction (MI), pulmonary edema, and stroke may occur. Preeclampsia is hypothesized to be caused by as-yet-unnamed placental factors that enter the maternal circulation, provoking the signs, symptoms, and laboratory findings associated with this disorder.149
Pregnancy-induced (sometimes called gestational) hypertension is defined as an elevation in BP, usually near term, that occurs without the other signs and symptoms of preeclampsia. Although gestational hypertension may or may not progress to preeclampsia, it can result in markedly elevated BPs. By definition, gestational hypertension usually resolves by 12 weeks post partum.150
Recognized risk factors for pregnancy-induced hypertension include obesity, age (>40 years), chronic hypertension, personal or family history of preeclampsia or gestational hypertension, nulliparity, multiple pregnancy, preexisting vascular disease, collagen vascular disease, diabetes mellitus, and renal disease.131 By far the most important predisposing factor is chronic hypertension, because superimposed preeclampsia develops in ≈25% of pregnant women with this condition. Regardless of its origin, high BP during pregnancy is associated with risk to both mother and baby, and BP-related complications remain a leading cause of maternal morbidity and mortality, as well as preterm birth, fetal growth restriction, and stillbirth.121,151
Women with high BP during pregnancy who have given birth continue to be at risk for preeclampsia and stroke. Although less common than preeclampsia during pregnancy, postpartum preeclampsia is more insidious and potentially more dangerous, because women may be unaware of its development and are no longer being seen regularly, as they were for prenatal care. Postpartum preeclampsia is associated with a high risk for stroke and may be the underlying cause of severe postpartum headaches.152 Transient elevations in BP are common post partum because of volume redistribution, iatrogenic administration of fluid, alterations in vascular tone, and use of nonsteroidal anti-inflammatory drugs,153–155 but persistently elevated BP should be categorized and treated according to the adult guidelines.140
A 2010 Cochrane review noted that the RR of hypertension in pregnancy was decreased with calcium supplementation of ≥1 g/d (RR, 0.65; 95% CI, 0.53–0.81).156 A reduction in preeclampsia/eclampsia was also noted (RR, 0.45; 95% CI, 0.31–0.65). Low-dose aspirin can also lower the risk for preeclampsia, on the basis of a meta-analysis of 46 trials and 32 891 women (RR, 0.83; 95% CI, 0.77–0.89; number needed to treat, 72).157 Recent research suggests that vitamin D3 deficiency may be associated with increased risk for preeclampsia,158 but there are insufficient data to support a recommendation.
Treatment of Elevated BP During Pregnancy, Including Preeclampsia
The central autoregulatory plateau in pregnancy is estimated at 120 mm Hg, and women with moderate to severe high BP in pregnancy, especially those with preeclampsia, are at risk for loss of central cerebral vascular autoregulation. The association between high BP and stroke risk in women with preeclampsia is not linear, such that stroke can occur at moderately elevated BPs, which suggests that current thresholds for treatment may not be sufficiently stringent.159 Pharmacological treatment to lower BP during pregnancy should be chosen after consideration of tolerability, preexisting therapy, and risk of teratogenicity, because all agents cross the placenta. (Table 4).
High BP during pregnancy may be defined as mild (diastolic BP 90–99 mm Hg or systolic BP 140–149 mm Hg), moderate (diastolic BP 100–109 mm Hg or systolic BP 150–159 mm Hg), or severe (diastolic BP ≥110 mm Hg or systolic BP ≥160 mm Hg). The goal of BP management in pregnancy is to maintain systolic BP between 130 and 155 mm Hg and diastolic BP between 80 and 105 mm Hg, with lower target ranges in the context of comorbidity; however, the treatment rationale for women with mild to moderate high BP in pregnancy is not as clear-cut as for severe high BP in pregnancy because maternal and fetal risk-benefit ratios have not been established.125 For example, a meta-analysis that examined the association between reduction in maternal BP and fetal growth found that a 10-mm Hg decrement in maternal mean arterial pressure was associated with a 176-g decrease in neonatal birth weight, regardless of the antihypertensive agent used.160 In addition, Abalos et al132 performed a meta-analysis of randomized controlled trials of treatment versus no treatment of mild to moderate high BP in pregnancy. Although the risk for development of severe hypertension in pregnancy was reduced by 50% in the treatment group (19 trials, 2409 women; RR, 0.50; 95% CI, 0.41–0.61; number needed to treat, 10), there was no statistically significant difference in risk for preeclampsia (22 trials, 3081 women; RR, 0.73; CI 0.50–1.08) and no evidence for benefit or harm to the fetus.
Severe hypertension in pregnancy is categorized with the same criteria as for stage 2 hypertension in nonpregnant adults according to the “Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure” (BP ≥160/110 mm Hg) and is associated with high risk for stroke and eclampsia.131,161 The American College of Obstetricians and Gynecologists recommends treatment of severe hypertension and suggests labetalol as first-line therapy,121 and it recommends avoidance of atenolol, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.
In addition to pharmacological control of hypertension, the use of magnesium sulfate for seizure prophylaxis is well established and has been demonstrated in randomized trials to decrease risk of stroke in women with severe high BP in pregnancy and eclampsia. A Cochrane review showed a >50% reduction in eclampsia with the use of magnesium sulfate versus placebo (6 trials, 11 444 women; RR, 0.41; 95% CI, 0.29–0.58; number needed to treat for additional benefit, 100), with a nonsignificant decrease in maternal death (RR, 0.54; 95% CI, 0.26–1.10).162 Although modest decrements in BP can be observed with magnesium sulfate alone, the latter has not been shown to effectively decrease BP in moderate to severe high BP in pregnancy, and there is no evidence to support its use as monotherapy.125
Pregnancy Complications and the Long-term Risk of Stroke
An expanding body of research has shown that complications of pregnancy (preeclampsia, gestational diabetes, and pregnancy-induced hypertension) are associated with higher risk for future CVD and stroke beyond the childbearing years than among women without these disorders163 (Tables 5 and 6). For example, women with a history of preeclampsia have a markedly increased risk for developing renal disease and a 2- to 10-fold increase in risk for development of chronic hypertension, a major risk factor for stroke. In addition, 50% of women with gestational diabetes will develop type 2 diabetes mellitus, a major risk factor for stroke, within 5 to 10 years of their pregnancy (although only 1 study has suggested an increased risk for CVD after a pregnancy complicated by gestational diabetes; CVD was defined as a composite outcome of admission to hospital for acute MI, coronary bypass, coronary angioplasty, stroke, or carotid endarterectomy [CEA]).180–182 A 2012 study of long-term risk for CVD reported that 18.2% of women with a history of preeclampsia versus 1.7% of women with uncomplicated pregnancies had a CVD event in 10 years (OR, 13.08; 95% CI, 3.38–85.5). Likewise, the 30-year risk (OR, 8.43; 95% CI, 3.48–23.2) and lifetime risk (OR, 3.25; 95% CI, 1.76–6.11) for CVD for women who formerly had preeclampsia were significantly increased compared with women with uncomplicated pregnancies.183 A 2008 systematic review and meta-analysis by McDonald et al181 noted that women with a history of preeclampsia/eclampsia had twice the risk of cerebrovascular disease (not further defined) as women without these disorders (RR, 2.03; 95% CI, 1.54–2.67). Another meta-analysis by Bellamy et al180 combined 4 cohort studies and reported a cumulative OR of 1.81 for any stroke (OR, 1.81; 95% CI, 1.37–2.33) in women with a history of preeclampsia, whereas Brown et al184 noted an OR of 1.76 for cerebrovascular disease (95% CI, 1.43–2.21) for women with a history of pregnancies with preeclampsia. In one study, the mean age at stroke onset was ≤50 years in women with these disorders, which suggests an accelerated time course to severe CVD or cerebrovascular disease, as well as loss or attenuation of women’s premenopausal cardiovascular advantage.185 Early-onset preeclampsia (before 32 weeks’ gestation) in particular has been noted to increase risk for stroke 5-fold compared with later-onset preeclampsia.186 Early-onset preeclampsia is also associated with an increase in white matter lesions independent of hypertension in women years after pregnancies complicated by preeclampsia or eclampsia, which suggests a vulnerability to future events.187
Table 5.
Adverse Pregnancy Outcomes and Future Hypertension
| Study Date and First Author | Total No. of Subjects | Study Design | Pregnancy Outcome | Mean Follow-up, y | RR or OR of Hypertension (95% CI) |
|---|---|---|---|---|---|
| Sibai 1986164 | 815 | Prospective cohort | Preeclampsia and eclampsia | 7.3 | 2.64 (1.66–4.17) |
| Nisell 1995165 | 138 | Retrospective cohort | Preeclampsia | 7 | 8.8(1.16–66.59) |
| North 1996166 | 100 | Retrospective cohort | Preeclampsia | 5 | 20.0(2.79–143.38) |
| Hannaford 1997167 | 23000 | Prospective cohort | Preeclampsia | 12.5 | 2.35 (2.08–2.65) |
| Marin 2000168 | 359 | Prospective and retrospective cohort | Preeclampsia | 14.2 | 3.70 (1.72–7.97) |
| Hubel 2000169 | 60 | Retrospective cohort | Preeclampsia and eclampsia | 32.7 | 5.00 (1.19–20.92) |
| Wilson 2003170 | 1312 | Retrospective cohort | Preeclampsia | 32 | 2.62 (1.77–3.86) |
| Sattar 2003171 | 80 | Retrospective cohort | Preeclampsia | 19 | 3.50(0,77–15.83) |
| Diehl 2008172 | 202 | Retrospective cohort | Preeclampsia | 27.4 | 2.2(1.45–3.36) |
CI indicates confidence interval; OR, odds ratio; and RR, risk ratio.
Adapted from Garovic et al149 with kind permission from Springer Science+Business Media. Authorization for this adaptation has been obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
Table 6.
Adverse Pregnancy Outcomes and Risk for Stroke
| Study Date and Author | Total No. of Subjects | Study Design | Pregnancy Outcome | Cerebrovascular Outcome | Follow-up, y | HR or OR for Outcome (95% CI) |
|---|---|---|---|---|---|---|
| Mannistö et al, 2013173 | 10314 | Prospective cohort study | Gestational hypertension | Ischemic cerebrovascular disease | 40 | 1.67(1.13–2.45) |
| Bonamy et al, 2011174 | 923686 | Retrospective cohort study | Preterm birth; SGA | Cerebrovascular events (infarction, hemorrhage, subarachnoid hemorrhage, TIA, other stroke) | Preterm birth 2.41 (1.4–4.17); SGA birth 1.68 (1.46–2.06); preterm and SGA birth 3.11 (1.91–5.09) | |
| Irgens et al, 2001175 | 626272 | Retrospective cohort study | Preeclampsia | Stroke mortality | Term preeclampsia 0.98 (0.5–1.91); preterm preeclampsia* 5.08 (2.09–12.35) | |
| Wilson et al, 2003170 | 1312 | Retrospective cohort study | Preeclampsia | Stroke mortality | 32 | 3.59 (1.04–12.4) |
| Ray et al, 2005176 | 1026265 | Retrospective cohort study | Maternal placental syndrome | Cerebrovascular disease | 1.90 (1.42–2.54) | |
| Funai et al, 2005177 | 37061 | Retrospective cohort study | Preeclampsia | Stroke | 3.07 (2.18–4.33) | |
| Kestenbaum et al, 2003178 | 124141 | Case-control study | Preeclampsia | Cerebrovascular disease | 2.53 (1.70–3.77) | |
| Lykke et al, 2009179 | 782287 | Retrospective cohort | Gestational hypertension, mild preeclampsia, severe preeclampsia | Stroke | 12.9–14.6 | Gestational hypertension 1.58 (1.32–1.89); mild preeclampsia 1.50 (1.36–1.66); severe preeclampsia 1.66 (1.29–2.14) |
CI indicates confidence interval; HR, hazard ratio; OR, odds ratio; SGA, small for gestational age; and TIA, transient ischemic attack.
*Defined as preeclampsia between 16 and 36 weeks.
The basis of the association between preeclampsia and future stroke is not entirely known but is hypothesized to be possibly related to genetic factors; shared risk factors (hypertension, dyslipidemia, endothelial dysfunction) between preeclampsia/eclampsia or other pregnancy complications and stroke; unmasking of underlying metabolic or vascular disease; or the induction during pregnancy of cardiovascular or cerebrovascular abnormalities that persist long-term.188 To assess the contribution by preeclampsia/eclampsia to future risk for CVD and stroke and the possible impact that lifestyle interventions may have on this risk, Berks et al189 performed a series of literature-based calculations on risk estimates. First, using a meta-analysis cumulative OR for stroke as the starting point, they found that preeclampsia increased the odds of stroke by 1.55-fold after correction for cardiovascular risk factors (interquartile range 1.76–1.98). This result suggests that CVD risk factors antecedent to pregnancy did not fully explain the risk for CVD after preeclampsia. They hypothesized that preeclampsia/eclampsia is a risk factor rather than a marker for stroke and CVD. The authors then calculated the effect of literature-based cumulative benefits of lifestyle interventions (dietary habits, exercise, and smoking cessation) on this risk for stroke with preeclampsia. They found the OR for the effect of lifestyle interventions on the risk for CVD after a preeclamptic pregnancy to be 0.91 (interquartile range, 0.87–0.96), which suggests that these interventions could reduce the risk of stroke in this population. Although one limitation of this research was the extrapolation of lifestyle interventions performed in older populations to a younger population of women 1 to 30 years after preeclampsia, prospective studies are warranted on the basis of the implication that lifestyle interventions in these women might be effective.189
Preeclampsia and Pregnancy Outcomes: Summary and Gaps
Hypertensive disorders of pregnancy and other complications (preterm birth, small size for gestational age, and first-trimester bleeding) are associated with increased risk of stroke during pregnancy, immediately after delivery, and years after delivery. This risk has been quantified in large retrospective studies, mostly in northern European populations. Prospective studies on the pathophysiology underlying the association between hypertensive disorders of pregnancy and stroke, especially in diverse populations, are needed, because it is not known whether prepregnancy risk factors or pregnancy-associated factors predispose these women to subsequent risk of stroke. Research also suggests that clinicians are not aware of the association between adverse pregnancy outcomes and CVD and stroke, which suggests a need for better clinician and patient education.190 Although a limited number of studies have examined cardiovascular and stroke risk factors and documented increased risk for events long-term in women with these disorders, there are no prospective randomized controlled trials assessing interventions to reduce stroke risk in this population with clear risk factors (preeclampsia, gestational diabetes). There is a need for high-quality studies of women with a history of adverse pregnancy outcomes to define their trajectory for the development of cerebrovascular disease and then to develop screening, risk stratification, and preventive strategies. Insufficient evidence exists to inform any recommendation for screening, prevention, or treatment in women with a history of pregnancy complications or adverse pregnancy outcomes.
Preeclampsia and Pregnancy Outcomes: Recommendations
Prevention of Preeclampsia
Women with chronic primary or secondary hypertension or previous pregnancy-related hypertension should take low-dose aspirin from the 12th week of gestation until delivery (Class I; Level of Evidence A).
Calcium supplementation (of ≥1 g/d, orally) should be considered for women with low dietary intake of calcium (<600 mg/d) to prevent preeclampsia (Class I; Level of Evidence A).
Treatment of Hypertension in Pregnancy and Post Partum
Severe hypertension in pregnancy should be treated with safe and effective antihypertensive medications, such as methyldopa, labetalol, and nifedipine, with consideration of maternal and fetal side effects (Class I; Level of Evidence A).
Consideration may be given to treatment of moderate hypertension in pregnancy with safe and effective antihypertensive medications, given the evidence for possibly Increased stroke risk at currently defined systolic and diastolic BP cutoffs, as well as evidence for decreased risk for the development of severe hypertension with treatment (although maternal-fetal risk-benefit ratios have not been established) (Class IIa; Level of Evidence B).
Atenolol, angiotensin receptor blockers, and direct renin inhibitors are contraindicated in pregnancy and should not be used (Class III; Level of Evidence C).
After giving birth, women with chronic hypertension should be continued on their antihypertensive regimen, with dosage adjustments to reflect the decrease in volume of distribution and glomerular filtration rate that occurs after delivery. They should also be monitored carefully for the development of postpartum preeclampsia (Class IIa; Level of Evidence C).
Prevention of Stroke in Women With a History of Preeclampsia
Because of the increased risk of future hypertension and stroke 1 to 30 years after delivery in women with a history of preeclampsia (Level of Evidence B), it is reasonable to (1) consider evaluating all women starting 6 months to 1 year post partum, as well as those who are past childbearing age, for a history of preeclampsia/eclampsia and document their history of preeclampsia/eclampsia as a risk factor, and (2) evaluate and treat for cardiovascular risk factors including hypertension, obesity, smoking, and dyslipidemia (Class IIa; Level of Evidence C).
Cerebral Venous Thrombosis
Cerebral venous thrombosis (CVT) is a stroke type that is caused by thrombus formation in ≥1 of the venous sinuses and manifests primarily as headache. CVT makes up 0.5% to 1% of all strokes but is the stroke type that shows the most prominent differential sex prevalence.191,192 In adulthood, the majority of affected individuals are women, who represent >70% of cases in most studies193–200 (Table 7). The overall adult incidence of CVT is 1.32 per 100 000 person-years (95% CI, 1.06–1.61) and is higher in women (1.86 per 100 000; 95% CI, 1.44–2.36) than men (0.75 per 100 000; 95% CI, 0.49–1.09).198 This sex difference is even more notable in women aged 31 to 50 years, in whom the incidence may be as high as 2.78 per 100 000 person-years (95% CI, 1.98–3.82). Women tend to be younger (median age 34 years) than men (median age 42 years) at the time of diagnosis.193,198 Guidelines for the evaluation and treatment of CVT were published recently.200 Therefore, only interim studies with an emphasis on sex-specific factors are presented in this guideline.
Table 7.
CVT and Recurrence Rates in Published Studies
| Recurrence Rate, % | |||||
|---|---|---|---|---|---|
| Study | Subjects Enrolled, n | % Female | CVT | Other Thrombosis | Length of Follow-up |
| ISCVT94 | 624 | 74.5 | 2.2 | 4.3 | 16 mo |
| VENOPORT96 | 142 | 71 | 2.0 | 8.0 | 16 y |
| Martinelli et al197 | 145 | 73 | 3.0 | 7.0 | 6y |
| Coutinho et al193 | 94 | 72 | NA | NA | NA |
| Dentali etal199 | 706 | 73.7 | 4.4 | 6.5 | 40 mo |
CVT indicates cerebral venous thrombosis; ISCVT, International Study on Cerebral Vein and Dural Sinus Thrombosis; NA, not available; and VENOPORT, Cerebral Venous Thrombosis Portuguese Collaborative Study Group.
Risk Factors
The female predominance of CVT has been attributed to hormonal factors (primarily oral contraceptive [OC] use and pregnancy), because the incidence is sex-independent in children and in the elderly.201,202 A link between thrombophilia and CVT has been relatively well established for several inherited conditions, including antithrombin III, protein C, and protein S deficiency and factor V Leiden.200 Many exogenous provoking factors for venous thrombosis have been described, such as cancer, infection, and hematologic and autoimmune conditions.191,192 However, 2 major risk factors are female specific: OC use and pregnancy. The use of OCs is associated with an increased risk of CVT,200 a risk that is increased significantly in women with an underlying hereditary prothrombotic factor, such as factor V Leiden or prothrombin gene mutation.203 Pregnancy and OC use are considered transient risk factors and do not necessarily indicate a higher risk for recurrence. Most pregnancy-related CVT occurs in the third trimester or puerperium.200,204
Treatment and Recurrence
The standard therapy for acute CVT is anticoagulation with intravenous unfractionated heparin or subcutaneous low-molecular-weight heparin (LMWH) followed by oral anticoagulation.200 There are no large studies of the use of newer anticoagulants that are currently only approved for use in patients with nonvalvular AF or deep venous thrombosis205; therefore, warfarin is usually recommended. Management and imaging recommendations are provided in detail in prior guidelines200 and are summarized below. There are no secondary prevention trials of duration of anticoagulation in adults with CVT; therefore, guidelines are based solely on observational data.
Recurrence rates range from 2% to 5% in most studies, although many of these studies did not provide long-term follow-up of patients, and the level of anticoagulation at the time of recurrence was often not reported. In the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT), recurrence of CVT was seen in 2.2% of patients, and other recurrent thrombotic events were seen in 4.3%, with a mean follow-up of 16 months194,196 (Table 7). A recent large, retrospective, multinational study performed follow-up of 706 patients for a median of 40 months and tracked prespecified risk factors and conditions such as infections, trauma, OC use, pregnancy, puerperium, HT, recent neurosurgical procedures, and the presence of myeloproliferative neoplasms.199 Significantly more women than men had at least 1 risk factor (61.0% versus 45.7%; P<0.05). Recurrence rates were again low (4.4% for recurrent CVT and 6.5% with a recurrent venous thromboembolism [VTE] in a different site), which led to an overall incidence of recurrence of 23.6 events per 1000 patient-years (95% CI, 17.8–28.7). Most events occurred after anticoagulation discontinuation. Somewhat surprisingly, the recurrence rate was similar in patients with unprovoked CVT and in patients with CVT secondary to known risk factors (22.8 events/1000 patient-years [95% CI, 15.9–32.6] versus 27.0 events/1000 patient-years [95% CI, 20.4–36.0]). A previous VTE was the only significant predictor of recurrence with multivariate analysis (hazard ratio [HR], 2.70; 95% CI, 1.25–5.83; P<0.011).199 Many of the recurrent VTEs occurred in women when the first CVT occurred during pregnancy/puerperium or was secondary to OC or HT use; however, neither female sex (HR, 1.37; 95% CI, 0.83–2.25), pregnancy/puerperium (HR, 1.05; 95% CI, 0.48–2.28), or use of OC/HT (HR, 0.72; 95% CI, 0.45–1.14) was an independent risk factor for VTE recurrence.199 This was in contrast to the results from a study by Martinelli et al,197 which found that male sex was a risk factor for recurrence (HR, 9.66; 95% CI, 2.86–32.7). The higher risk in men could potentially be attributable to more correctable or transient risk factors in women (use of OCs, pregnancy, etc) or may simply reflect the fact that this study enrolled fewer patients (n=145) and may have been under-powered for sex-specific analysis.197
Recurrence tends to occur within the first year of the index CVT. Patients with severe thrombophilia (antithrombin, protein C, or protein S deficiency; antiphospholipid antibodies; or combined abnormalities) have an increased risk of VTE (adjusted HR, 4.71; 95% CI, 1.34–16.5).200,203,206 The recurrent event is more often a VTE than a recurrent CVT, and providers should have a high index of suspicion for other thrombotic complications (pulmonary embolism, deep venous thrombosis) in patients with a prior CVT.
Sex Differences in Outcome
Overall, patients with CVT have lower mortality and better functional outcomes than most stroke subtypes.191,192 Predictors for poor outcome include age, malignancy, central nervous system infection, and intracranial hemorrhage.191 The mortality rate was only 2.8% in the most recent large study, and in general, patients had good functional outcomes (89.1% of patients had a complete recovery, with a modified Rankin score of 0–1).199 A post hoc analysis of patients followed up in the ISCVT found that male sex was associated with poorer outcomes at follow-up (HR, 1.59; 95% CI, 1.01–2.52) and that significantly more women recovered completely after 6 months (81% versus 71%, P=0.01).193 This was driven in large part by improved outcomes in a subset of women who had an identified “gender-specific risk factor” (OC use, pregnancy, puerperium, and hormone replacement therapy), present in 65% of women.193 Women with other underlying risk factors for CVT unrelated to these sex-specific factors had similar outcomes as males. Logistic regression analysis confirmed that the absence of sex-specific risk factors was a strong and independent predictor of poor outcome in women with CVT (OR, 3.7; 95% CI, 1.9–7.4). Although there was a trend toward higher mortality in males, this was not significant.193 No association between sex and mortality rates was seen in the recent Nationwide Inpatient Sample of 3488 patients; however, the mortality was higher in that cohort (4.39%), which contained a surprisingly large number of patients with pyogenic CVT.207,208 In a larger sample of 11 400 records from the Nationwide Inpatient Sample data set, the most common condition associated with CVT was pregnancy/puerperium (seen in 24.6% of patients). These women had a low mortality rate (0.4%), but despite this, male sex was associated with decreased mortality (2.1%) on multivariate analysis (OR, 0.62; 95% CI, 0.43–0.87, P=0.006).209 The use of the Nationwide Inpatient Sample data is limited, because only inpatient data are recorded, results may be prone to coding errors, initial stroke severity is not recorded, and information on the presence of sex-specific risk factors is undoubtedly incomplete. Currently, data on sex specific functional outcomes are lacking.
Pregnancy-Associated CVT
Pregnancy and the puerperium period are times of increased risk for venous thrombosis for women, including CVT. The incidence of CVT during pregnancy and the puerperium is estimated at 1 in 2500 deliveries to 1 in 10 000 deliveries in Western countries, with increased odds ranging from 30% to 13-fold higher (ORs, 1.3–13).210–212 The greatest risk periods for CVT include the third trimester and the first 4 postpartum weeks.211 Up to 73% of CVTs in women occur during the puerperium.212 Cesarean delivery appears to be associated with a higher risk of CVT after adjustment for age, vascular risk factors, presence of infections, hospital type, and location (OR, 3.10; 95% CI, 2.26–4.24).
Future Pregnancies and Recurrence
Prior guidelines have summarized the studies examining the outcome and complication rates of pregnancy in women who had CVT.200 These studies found that the risk of complications during future pregnancies was low. There was a high proportion of spontaneous abortion, consistent with emerging observational trials.213 On the basis of the available evidence, CVT is not a contraindication for future pregnancies; however, many of the patients followed up for recurrences were maintained on preventive antithrombotic medication. Considering the additional risk that pregnancy confers to women with a history of CVT, prophylaxis with LMWH during future pregnancies and the postpartum period may be beneficial.200
CVT: Summary and Gaps
There is a striking sex difference in CVT incidence that is related to hormonal factors and pregnancy. Long-term oral anticoagulation is recommended for patients at high risk of recurrence because of thrombophilia, but overall recurrence rates are low, even with subsequent pregnancy. Long-term data on sex differences in recurrence and on functional outcomes are lacking.
CVT: Recommendations
In patients with suspected CVT, routine blood studies consisting of a complete blood count, chemistry panel, prothrombin time, and activated partial thromboplastin time should be performed (Class I; Level of Evidence C).
Screening for potential prothrombotic conditions that may predispose a person to CVT (eg, use of contraceptives, underlying inflammatory disease, infectious process) is recommended in the initial clinical assessment (Class I; Level of Evidence C).
Testing for prothrombotic conditions, including protein C, protein S, or antithrombin deficiency; antiphospholipid syndrome; prothrombin G20210A mutation; and factor V Leiden can be beneficial for the management of patients with CVT. Testing for protein C, protein S, and antithrombin deficiency is generally indicated 2 to 4 weeks after completion of anticoagulation. There is a very limited value of testing in the acute setting or in patients taking warfarin (Class IIa; Level of Evidence B).
In patients with provoked CVT (associated with a transient risk factor), vitamin K antagonists may be continued for 3 to 6 months, with a target international normalized ratio of 2.0 to 3.0 (Class IIb; Level of Evidence C).
In patients with unprovoked CVT, vitamin K antagonists may be continued for 6 to 12 months, with a target international normalized ratio of 2.0 to 3.0 (Class IIb; Level of Evidence C).
For patients with recurrent CVT, VTE after CVT, or first CVT with severe thrombophilia (ie, homozygous prothrombin G20210A; homozygous factor V Leiden; deficiencies of protein C, protein S, or antithrombin; combined thrombophilia defects; or antiphospholipid syndrome), indefinite anticoagulation may be considered, with a target international normalized ratio of 2.0 to 3.0 (Class IIb; Level of Evidence C).
For women with CVT during pregnancy, LMWH in full anticoagulant doses should be continued throughout pregnancy, and LMWH or vitamin K antagonist with a target international normalized ratio of 2.0 to 3.0 should be continued for ≥6 weeks post partum (for a total minimum duration of therapy of 6 months) (Class I; Level of Evidence C).
It is reasonable to advise women with a history of CVT that future pregnancy is not contraindicated. Further investigations regarding the underlying cause and a formal consultation with a hematologist or maternal fetal medicine specialist are reasonable (Class IIa; Level of Evidence B).
It is reasonable to treat acute CVT during pregnancy with full-dose LMWH rather than unfractionated heparin (Class IIa; Level of Evidence C).
For women with a history of CVT, prophylaxis with LMWH during future pregnancies and the postpartum period is reasonable (Class IIa; Level of Evidence C).
Oral Contraceptives
On the basis of a US Department of Health and Human Services survey conducted from 2006 to 2008, 10.7 million women aged 15 to 44 years in the United States used the pill form of contraception.214 As alternative forms of hormonal contraception such as the transdermal patch, vaginal ring, and intrauterine devices are increasingly used, the risk of stroke with these formulations also needs to be evaluated. The risk of stroke is very low in the age group of women who use contraception, but the incidence rises steeply from 3.4 per 100 000 at ages 15 to 19 years to 64.4 per 100 000 in women aged 45 to 49 years.144
IS Risk
The cumulative risk of stroke in women using OC pills has been summarized in 4 different meta-analyses, with many of the same individual cohort or case-control studies included in each. A meta-analysis of 16 case-control and cohort studies between 1960 and 1999 estimated a 2.75-fold increased odds (95% CI, 2.24–3.38) of stroke associated with any OC use.215 A later meta-analysis of 20 studies published between 1970 and 2000 that separated the studies by design (case-control versus cohort) found no increased risk of stroke in the cohort studies but an increased risk with OC use in case-control studies (OR, 2.13; 95% CI, 1.59–2.86).216 Importantly, only 2 of the 4 cohort studies reported strokes by subtype, and risk was increased for IS but not hemorrhagic strokes.216 An additional meta-analysis of studies from 1980 to 2002 limited only to low-dose combined OCs (second and third generation only) also showed a comparable increased risk with OC use (OR, 2.12; 95% CI, 1.56–2.86).217 Lastly, a systematic review of progestogen-only OCs revealed no significant increased risk of stroke with this form of contraceptive.218
Two additional large cohort studies have been published since these meta-analyses. The first is the Women’s Lifestyle and Health Cohort Study. This cohort comprised 49259 Swedish women who were followed up from 1991 to 1992 until 2004.219 In the 285 cases of incident stroke that included ischemic, hemorrhagic, and unknown types, there was no significant association between OC use, duration, or type of OC. Reproductive factors, such as age at first birth, duration of breastfeeding, age at menarche, mean menstrual cycle days at age 30 years, and parity, were not associated with stroke after adjustment for cigarette smoking, hypertension, diabetes mellitus, alcohol, body mass index (BMI), education, and physical activity.219
The second study estimated rates of IS only (excluding hemorrhagic stroke and transient ischemic attacks [TIAs]) in women aged 15 to 49 years and the RRs associated with use of various doses and formulations of hormonal contraception in Denmark.144 In this population-based cohort of ≈1.6 million women, the crude incidence of IS in contraceptive users was 21.4 per 100 000 person-years. The adjusted RR for ethinyl estradiol doses from 30 to 40 μg ranged from 1.40 (95% CI, 0.97–2.03) to 2.20 (1.79–2.69), whereas the RR for the 20-μg dose ranged from 0.88 (0.22–3.53) to 1.53 (1.26–1.87). Progestin-only formulations were not associated with IS. The transdermal patch was associated with a nonsignificant increased risk in a small number of cases (RR, 3.15; 95% CI, 0.79–12.60), whereas the vaginal ring was associated with a 2.49-fold increased risk (95% CI, 1.41–4.41). In addition, duration of use did not change the risk estimates.144 Although this study followed a very large number of women, it is limited because risk factors and stroke cases were based on administrative data. The authors concluded that the RR of IS with intermediate-dose ethinyl estradiol and different progestin types was lower than that reported in other studies and that the transdermal and vaginal ring routes of contraception conferred a similar risk as pills.144
Hemorrhagic Stroke Risk
Data regarding risk with OC use have been less consistent for hemorrhagic stroke. The World Health Organization reported an overall slightly increased risk of hemorrhagic stroke (both intracerebral and subarachnoid) with OC use; however, this risk was present in developing countries but not in Europe.220 Also, European women >35 years of age were at increased risk of SAH, whereas women in developing nations were at increased risk of both ICH and SAH. Women with hypertension and who smoked cigarettes were also at increased risk.221 In the Swedish Women’s Lifestyle and Health Cohort, there was a significant decrease in hemorrhagic stroke among women who were parous (versus nulliparous; HR, 0.5; 95% CI, 0.2–0.8) and a nonsignificant increase in women who started OC use after 30 years of age (HR, 2.3; 95% CI, 0.8–6.8) and stopped using OCs based on doctor recommendation for medical reasons (adjusted HR, 2.1; 95% CI, 0.9–5.0).219
Hemorrhagic stroke in young women is relevant in Asia, where the risk of this type of stroke is disproportionately higher than in Europe and North America. A recent case-control study of Chinese women evaluated the association between the single-nucleotide polymorphisms rs10958409 GA/AA (located near SOX17, a transcription factor that modulates cardiovascular development and endothelial cell biology) and rs1333040 CT/TT (located near CDKN2A, CDKN2B, and ANRIL, which regulate p53 activity) and risk of ischemic and hemorrhagic stroke in OC users and nonusers.222 Women with the rs10958409 GA/AA or rs1333040 CT/TT genotypes (associated with susceptibility of intracranial aneurysm) had an increased overall risk of stroke, which increased to an OR of 6.06 (95% CI, 1.69–21.81) and 14.48 (95% CI, 1.56–134.43), respectively, in OC users <50 years of age. The rs1333040 single-nucleotide polymorphism was a significant risk with OC use only for hemorrhagic stroke, not IS.222 This study is important because it demonstrates not only the gene-drug interaction but also some potential mechanisms for how OCs might lead to hemorrhage in specific at-risk populations.222
Additional Risk Factors for Stroke in Women Using OCs
Besides the well-established risk associated with older age, cigarette smoking, hypertension, and migraine headaches,223 the Risk of Arterial Thrombosis in Relation to Oral Contraceptives (RATIO) study from the Netherlands showed that women who were obese (OR, 4.6; 95% CI, 2.4–8.9) and had a history of hypercholesterolemia (OR, 10.8; 95% CI, 2.3–49.9) were also at an increased risk from OC use compared with women with these risk factors who did not use OCs.224
The RATIO investigators have performed multiple analyses to identify prothrombotic mutations in women with stroke who were and were not OC users (Table 8). They found that women using OCs who were heterozygous for factor V Leiden (OR, 11.2; 95% CI, 4.2–29.0) and methyl tetrahydrofolate reductase or MTHFR 677TT mutation (OR, 5.4; 95% CI, 2.4–12.0) were at increased risk of IS. There may have been some synergism between OCs and these mutations, because the increased risk was not evident in nonusers with these mutations.225 In addition, this study also showed an association with a genetic variation of factor XIII.226 In the assessment of acquired antiphospholipid antibodies, the presence of β2 glycoprotein-1 antibodies was associated with 2.3-fold increased odds of stroke (95% CI, 1.4–3.7), but there was no association with anticardiolipin or antiprothrombin antibodies. The prevalence of lupus anticoagulant was 17% in women with IS, and the OR was very high at 43.1 (95% CI, 12.2–152.0).227 The OR increased to 201 (95% CI, 22.1–1828.0) in women who were also using OCs, although this was based on a very small number of outcomes. This is another example of the amplification of IS risk in a condition that is already associated with arterial thromboembolism and VTE.227
Table 8.
Odds of Ischemic Stroke With the Presence of Genetic or Acquired Prothrombotic Factors With and Without OC Use in the RATIO Cohort
| Adjusted OR (95% CI) | ||||
|---|---|---|---|---|
| Study | Case/Control, n | Biomarker (Genetic or Acquired) | Non-OC Users | OC Users |
| Slooter et al225 | 193/767 | FVL | 0.4 (0.1–1.9)* | 11.2(4.3–29.0)* |
| MTHFR 677TT | 1.1 (0.5–2.4)* | 5.4(2.4–12.0)* | ||
| Pruissen et al226 | 190/767 | FXIII Tyr204Phe | 8.8 (4.3–18)† | 20 (9–46)‡ |
| Urbanus et al227 | 175/628 | Lupus anticoagulant (Ratios/c ≥1.15) | 33.6 (6.8–167)* | 201.0(22.1–1828.0)* |
| Andersson et al228 | 175/638 | vWF >90th percentile | 1.6 (0.8–3.5)‡ | 11.4(5.2–25.3)‡ |
| ADAMTS13 ≤10th percentile | 1.8 (0.8–4.3) ‡ | 5.1 (2.4–11.2)‡: | ||
ADAMTS13 indicates a disintegrin and metalloproteinase with the thrombospondin type I repeat 13; CI, confidence interval; FVL, factor V Leiden mutation; FXIII, factor XIII; MTHFR, methylenetetrahydrofolate reductase; OC, oral contraceptive; OR, odds ratio; RATIO, Risk of Arterial Thrombosis in Relation to Oral Contraceptives; Ratios/c, normalized ratios for lupus anticoagulant screen and lupus anticoagulant–confirm coagulation times; and vWF, von Willebrand factor.
Adjusted for age, residence area, and index year.
Adjusted for age at index date, index year, area of residence, hypercholesterolemia, hypertension, diabetes mellitus, and smoking.
Adjusted for age, year of event/index year, area of residence, hypercholesterolemia, hypertension, diabetes mellitus, and smoking.
The RATIO investigators also assessed the association between OC use and endothelial dysfunction. They reported that an increase in von Willebrand factor levels and low ADAMTS13 levels were associated with increased odds of IS and MI in young women in the RATIO cohort, with a further increase in the OR with OC use.228 The largest effect of OC use was in women with von Willebrand factor levels >90th percentile, for whom the OR for stroke was 1.6 (95% CI, 0.8–3.5) in nonusers and increased to 11.4 (95% CI, 5.2–25.3) in OC users. The results of this study demonstrate that OC use appears to further increase the risk of stroke in the setting of endothelial dysfunction. Additional research should be focused on the validation of von Willebrand factor and ADAMTS13 as risk factors for stroke with OC use in other racial/ethnic and geographic populations, as well as exploration of the value of measuring these biomarkers in women before initiation of OCs.
Should women be screened for thrombophilia before hormonal contraception is prescribed for them? This question has been addressed in a large systematic review and meta-analysis of the risk of VTE in the high-risk settings of OC use and pregnancy.229 Although there are 15-fold odds of VTE in women with the factor V Leiden mutation who are using OCs (95% CI, 8.66–28.15), the absolute risk is low because of the low prevalence of this and other thrombophilias and VTE. For other hereditary thrombophilias, including prothrombin gene mutation, as well as protein C and antithrombin deficiencies, the odds of VTE increased in combination with OC use, but the odds of VTE stayed the same with protein S deficiency.229 IS and CVT are much less common than VTE,144 so the yield of routine screening would be even lower for these conditions. Selective screening based on prior personal or family history of VTE is proposed to be more cost-effective than universal screening in women who initiate OCs or desire to become pregnant.229 The cost-effectiveness analysis in this meta-analysis was designed for prevention of VTE, but adaptation to stroke screening in young women should also include obesity, diabetes mellitus, hyperlipidemia, hypertension, and cigarette smoking.
Another very important risk factor for stroke in young women is migraine aura, which has some evidence supporting a further increase in risk for women who also use OCs. An analysis of the Stroke Prevention in Young Women study, a population-based, case-control study of 386 women aged 15 to 49 years with incident stroke and 614 age- and ethnicity-matched control subjects, showed that women with probable migraine with visual aura were at 1.5-fold increased odds (95% CI, 1.1–2.0) of stroke compared with control subjects.230 Women with this migraine type who also smoked cigarettes and used OCs had 7.0-fold higher odds (95% CI, 1.3–22.8) of stroke than women with probable migraine with visual aura who did not smoke or use OCs; however, women with probable migraine with visual aura who were OC users but nonsmokers did not have a significantly increased odds of stroke, which suggests the risk with both OC use and smoking in women with probable migraine with visual aura is additive.230 This was a biethnic cohort of black (representing a higher proportion of cases) and white women, whereas many of the large cohorts were limited to a northern European population. A consensus statement from both headache and stroke experts suggests screening for and treatment of all traditional stroke risk factors in women with migraine but does not state that low-dose OC use is contraindicated.231
Hormonal Contraception and BP
The impact of OC use on BP, an important stroke risk factor, and other hemodynamic parameters is somewhat controversial. A study of BP and hemodynamic measurements in young women (mean age 20 years) in the United Kingdom (ENIGMA Study) showed that women using OCs had a marginal but significantly higher systolic BP (mean 112±12 versus 110±11 mm Hg in nonusers; P=0.04) and an increased arterial pulse wave velocity, a measure of aortic stiffness232; however, in the multivariate model, mean arterial pressure, age, and heart rate were associated with arterial pulse wave velocity but not OC use.232
Several systematic reviews cover the topic of OC use and hypertension in women. Summarizing the data through 2005, one review estimated the odds of IS for women with hypertension using OCs were 1.73 (95% CI, 0.83–3.60) and concluded that there was no synergistic increase in risk because the odds of stroke in normotensive women using OCs were similar.233 A systematic review of studies that examined BP after initiation of OCs demonstrated mixed results from studies of follow-up BPs. Generally, the mean BPs were most often well below 140/90 mm Hg. Importantly, only a very small percentage (≈2%) of women developed hypertension.234 A systematic review of studies that collected outcomes based on measurement of BP before initiation of OCs235 found 2 case-control studies that met criteria for inclusion.220,236 Both studies demonstrated a higher OR of IS in women without versus with BP measurement before initiation of OCs, although the CIs overlapped.220,236 A separate case-control study showed no difference in hemorrhagic stroke based on preinitiation BP measurement.221 Taken together, these limited data suggest that OCs appear to marginally increase BP, albeit infrequently leading to hypertension, and that measurement of BP before OC initiation may be an important preventive measure to detect women at risk of stroke.
OCs: Summary and Gaps
The relative increase in stroke risk with low-dose OCs is small, approximately 1.4 to 2.0 times that of non-OC users.144 On the basis of the longitudinal data from the Danish population-based study, among 10 000 women who use the 20-μg dose of desogestrel with ethinyl estradiol for 1 year, 2 women will have arterial thrombosis and 6.8 will have venous thrombosis.144 The risk of stroke with OC use also appears to be lower than the risk associated with pregnancy (≈3 per 10000 deliveries).143
Despite the overall low risk of stroke from hormonal contraception, certain subgroups of women, particularly those who are older, smoke cigarettes, or have hypertension, diabetes mellitus, obesity, hypercholesterolemia, or prothrombotic mutations, may be at higher risk for stroke. Estimates are based primarily on case-control studies and a smaller number of cohort studies primarily from northern European countries, which limits generalizability to other populations. Further research is needed to better understand the subgroups of women who may be at risk for hemorrhagic stroke associated with OCs based on age, race/ethnicity, genetic makeup, and parity. In addition, research assessing the value of specific biomarkers of endothelial function, such as von Willebrand factor and ADAMTS13, before and during OC use, as well as after an arterial thrombotic event, is warranted.
OCs: Recommendations
OCs may be harmful in women with additional risk factors (eg, cigarette smoking, prior thromboembolic events) (Class III; Level of Evidence B). 224,225
Among OC users, aggressive therapy of stroke risk factors may be reasonable (Class IIb; Level of Evidence
Routine screening for prothrombotic mutations before initiation of hormonal contraception is not useful (Class III; Level of Evidence A).229
Measurement of BP before initiation of hormonal contraception is recommended (Class I; Level of Evidence B).220,235,236
Menopause and Postmenopausal HT
Menopause Onset
Exposure to endogenous estrogen has been hypothesized to be protective for stroke in premenopausal women; however, given logistical difficulties in collecting longitudinal data on endogenous hormones and the large sample sizes that would be required to study stroke in younger women, no study has investigated the relationship between endogenous hormones and stroke as women transition through menopause. The association between onset of menopause and stroke risk has been the subject of 2 recent reviews, one focusing on the association of age at menopause and stroke risk and the other focusing on the association of premature or early menopause and stroke risk.237,238 In the first review by Lisabeth and Bushnell,237 the authors concluded that the few studies that considered the association between age at menopause and incident stroke had inconsistent findings. The findings of these studies were summarized briefly. Hu et al239 found that age at natural menopause was not associated with risk of total stroke, IS, or hemorrhagic stroke among 35 616 women in the Nurse’s Health Study who reported no use of HT. In a cohort study of 5731 postmenopausal Korean women who did not use HT, no association was found between age at natural menopause and risk of total stroke, IS or hemorrhagic stroke.240 Lisabeth et al,241 using data from the Framingham Heart Study (n=1430), found that women with natural menopause before age 42 years had twice the IS risk (RR, 2.03; 95% CI, 1.16–3.56) as women who had natural menopause at ≥42 years of age after adjustment for age, risk factors, and postmenopausal estrogen use. Results from a Japanese cohort also suggested that women who underwent menopause before 40 years of age were more likely to have an IS than those with menopause between 50 and 54 years of age after adjustment for age and risk factors (RR, 2.57; 95% CI, 1.20–5.49); however, findings appeared to be largely driven by women with surgical menopause.242 A case-control study conducted in Spain found no association between menopause at <53 years of age and the odds of noncardioembolic stroke after accounting for age and risk factors.243
In the review by Rocca et al,238 7 observational cohort studies were summarized to determine whether early or premature menopause is associated with stroke. The finding from 3 of the studies not yet discussed previously are briefly summarized.244 Using data from the Nurse’s Health Study, Parker et al245 found that after multivariable adjustment, women with hysterectomy with bilateral oophorectomy had a slightly elevated risk of total stroke compared with women with hysterectomy with ovarian conservation (HR, 1.14; 95% CI, 0.98–1.33), and this association did not reach significance. In further analysis of these data limited to women with hysterectomy who had never used estrogen therapy, the authors found a larger statistically significant association between oophorectomy and total stroke (HR, 1.85; 95% CI, 1.09–3.16) in all women and in women with hysterectomy before age 50 years (HR, 2.19; 95% CI, 1.16–4.14). A more recent analysis of a Swedish cohort found that women who underwent an oophorectomy before age 50 years had an increased risk of total stroke (HR, 1.47; 95% CI, 1.16–1.87) compared with women with no hysterectomy and no oophorectomy.246 Finally, in a recent analysis of data from the WHI focused on women with a history of hysterectomy (without capture of age before natural menopause), no association was found for oophorectomy versus ovarian conservation and risk of total stroke in all women (HR, 1.04; 95% CI, 0.87–1.24) or those women without a history of hormone use (HR, 1.31; 95% CI, 0.92–1.87) after multivariable adjustment.247
Menopause Onset: Summary and Gaps
Results of existing studies of the association between age at menopause or premature or early menopause, whether natural or surgical, and stroke risk appear to suggest increased risk of stroke with earlier onset of menopause, although the evidence is not entirely consistent. Few data on the association of other surrogate markers for endogenous hormone exposures, such as lifetime estrogen exposure, duration of ovarian activity, or time since menopause, and stroke risk exist. Additional studies are needed to determine the influence of the onset of menopause on stroke risk. Studies should aim to determine whether the association between menopause onset and stroke is limited to ischemic events and whether and how the type of menopause (natural or surgical) may impact this association.
Postmenopausal HT
Early observational evidence suggested a potential benefit of HT on cerebrovascular disease248; however, even as early as 2002, evidence was emerging that HT may have detrimental effects. A review of 29 observational studies found no clear evidence that HT use benefited stroke risk in postmenopausal women.249 Subsequent randomized clinical trials for both the primary and secondary prevention of stroke in women randomized to HT have been universally negative (Table 9). Two large clinical trials examined women with established vascular disease: the Heart and Estrogen/Progestin Replacement Study (HERS) and Women’s Estrogen for Stroke Trial (WEST).250,251 Stroke events (including any stroke and IS) were similar for women allocated to an estrogen or to placebo.
Table 9.
Association Between HT Use and Stroke Risk in Randomized Controlled Trials of Perimenopausal and Postmenopausal Women
| Trial | Total No. | Average Age, y | HT Regimen | Vascular Disease | Follow-up | Any Stroke, HR (95% CI) |
|---|---|---|---|---|---|---|
| HERS (2001)250 | 2763 | 66.7 | 0.625 mg of CEE plus 2.5 mg of MPA (n=1380) vs placebo (n=1383) | Yes (CAD) | 4.1 y | 1.1 (0.9–1.7) |
| WEST251 | 664 | 71 | Estrogen (1 mg of estradiol) vs placebo | Yes (CVD); nondisabling ischemic stroke or TIA within preceding 90 d (CVD) | 1.1 (0.8–1.6) | |
| HERS II252 | 2321 | 66.7 | 0.625 mg of CEE plus 2.5 mg of MPA (n=1380) vs placebo (n=1383) | Yes (CAD) | 6.8 y (2.7–y unblinded follow-up to HERS) | 1.09(0.75–1.6) |
| WHI253–256 | 16608 | 63 | CEE+MPA | No* | 1.3 (1.0–1.7) | |
| WHI253–256 | 10739 | 63 | CEE | No* | 1.4 (1.1–1.7) | |
| Estonian trial257 | Open HT, 494; open control, 494; blind HT, 404; blind placebo, 373 | Open HT, 58.6; open control, 58.9; blind HT, 58.5; blind placebo, 59 | CEE 0.625 mg/d plus 2.5 mg/d MPA or CEE 0.625 mg/d plus 5 mg/d MPA, If <3 y had passed since menopause at recruitment | No* | 2.0–5.0 y | 1.24(0.85–1.82) |
| DOPS258 | Randomly allocated (open label, n=1006) to HT (n=502) or no treatment (n=504) | 49.7 | Intact uterus: 2 mg of synthetic 17β-estradiol for 12 d, 2 mg of 17β-estradiol plus 1 mg of norethisterone acetate for 10 d, and 1 mg of 17β-estradiol for 6 d. Prior hysterectomy: 17β-estradiol 2 mg/d. Enrolled within 24 mo of last menses. | No* | 11 y (randomized treatment phase) and continued observational follow-up (16 y) | 11 -y follow-up, 0.77(0.35–1.70); 16-y follow-up, 0.89(0.48–1.65; P=0.71) |
CAD indicates coronary artery disease; CEE, conjugated equine estrogen; CI, confidence interval; CVD, cardiovascular disease; DOPS, Danish Osteoporosis Prevention Study; HERS, Heart and Estrogen/Progestin Replacement Study; HR, hazard ratio; HT, hormone therapy; MPA, medroxyprogesterone acetate; WEST, Women’s Estrogen for Stroke Trial; and WHI, Women’s Health Initiative trial for women with a uterus (CEE+MPA) or without a uterus (CEE).
Self-reported as no history of acute myocardial infarction, stroke, or transient ischemic attack in the previous 6 months.259
Findings from the WHI HT trials were reported soon after those from HERS and WEST. The multicenter WHI randomized women into groups according to use of conjugated equine estrogen (CEE) and medroxyprogesterone or CEE alone, based on hysterectomy status.253–256 These women, unlike those in previous randomized trials, did not have documented vascular disease (no self-reported history of acute MI, stroke, or TIA in the previous 6 months); however, they were considerably older than women in previous observational trials, with a mean age of 63 years. Additional analyses of the WHI focused on specific subgroups of women to determine those at particularly high risk; the subgroups were outlined in previous AHA guidelines.19 The risk of stroke with CEE was limited to IS (HR, 1.55; 95% CI, 1.19–2.01) and not hemorrhagic stroke (HR, 0.64; 95% CI, 0.35–1.18). There was no difference based on stroke pathogenic subtype, severity, or mortality. Women with no prior history of CVD were at higher risk (HR, 1.73; 95% CI, 1.28–2.33) than women with a prior history (HR, 1.01; 95% CI, 0.58–1.75). Women using HT who were 50 to 59 years of age had a lower risk (HR, 1.09; 95% CI, 0.54–2.21) than those 60 to 69 years of age (HR, 1.72; 95% CI, 1.17–2.54) or those 70 to 79 years of age (HR, 1.52; 95% CI, 1.02–2.29) compared with nonusers. These risk estimates did not vary by race or other baseline risk factors, including aspirin or statin use or BP.19
There is also little compelling evidence that HT is effective at preventing deterioration of cognitive function in postmenopausal women. The Women’s Health Initiative Memory Study (WHIMS), a subgroup of women enrolled in the WHI, found that for women ≥65 years of age, HT did not reduce the incidence of either dementia or mild cognitive impairment.260,261 HT had an adverse effect on global cognitive function,262,263 which was greater among women with lower cognitive function at initiation of treatment. Subsequent magnetic resonance imaging studies in a subset of these women found greater brain atrophy264 but not a significantly higher volume of subclinical cerebrovascular lesions in treated women.265 The adverse effects were most evident in women experiencing cognitive deficits before initiation of HT.
Similar results have been reported in randomized clinical trials for selective estrogen receptor modulators and other hormonally active compounds (including raloxifene266,267 and tibolone,268 a commonly used therapy in Europe with both estrogenic and androgenic properties). Raloxifene (60 mg versus placebo) had no effect on the risk of nonfatal stroke (HR, 1.10; 95% CI, 0.92–1.32) but increased the risk of fatal strokes (HR, 1.49; 95% CI, 1.00–1.24; P=0.05).
There has been an increasing recognition that the timing of HT initiation may play a critical role in the overall effect of HT.269,270 An analysis of the WHI subjects was performed to test this hypothesis, and interestingly, women <10 years from menopause had no increased risk of coronary heart disease events with any CEE (alone or CEE plus medroxyprogesterone; HR, 0.76; 95% CI, 0.50–1.16), whereas women ≥20 years post menopause had an elevated risk (HR, 1.28; 95% CI, 1.03–1.58; P for trend=0.02). There was, however, no trend for increased stroke based on years since menopause.267 The Estonian trial of HT, a study of women 50 to 64 years of age, also confirmed the findings of the WHI, with a trend toward an increase in cerebrovascular events in women taking HT257 (Table 9). The Kronos Early Estrogen Prevention Study (KEEPS) is an ongoing trial of women 42 to 58 years of age who are within 36 months of their final menstrual period and who were randomized to estrogen replacement in low doses (0.45 mg of CEE), transdermal formulation (50 μg/wk), or combined with cyclic oral, micronized progesterone 200 mg for 12 days each month.271 The primary outcomes are progression of subclinical atherosclerosis as measured by carotid intima-media thickness and coronary calcium scores, rather than stroke; however, this study will directly assess HT initiated soon after menopause. This study has completed enrollment, and initial results were presented at the meeting of the North American Menopause Society on October 3, 2012 (http://www.keepstudy.org); however, until the full data are available, no statements can be made regarding the safety of HT in this subset of women. The effective duration of use for benefit is currently unknown.
Only 1 randomized trial, the open-label Danish Osteoporosis Prevention Study (DOPS),258 specifically examined healthy women aged 45 to 58 years who were recently postmenopausal or had perimenopausal symptoms in combination with recorded postmenopausal serum follicle-stimulating hormone values (n=1006). Women had a mean time since menopause of 0.6 years, with last menstrual bleeding 3 to 24 months before study entry. Stroke was a designated (secondary) end point. A total of 502 women were randomly allocated to receive HT and 504 to receive no treatment (control; Table 9). Importantly, this study had an open-label design, and the patients in the control arm did not receive placebo, which could have influenced compliance in the HT arm. After 11 years, the trial was stopped secondary to concerns of potentially harmful effects of HT that had been seen in other trials, such as the WHL Women were followed up as an observational cohort for an additional 5 years. There was no increase in stroke, VTE, or breast cancer in treated women. HT initiated in these recently postmenopausal, younger women significantly reduced the risk of the combined end point of mortality, MI, or heart failure. Stroke rates did not differ between groups.258
Transdermal estradiol may represent a safer alternative than oral estrogens, because treatment does not appear to increase the risk of VTE and stroke and may reduce the risk of MI compared with nonusers. In a nested case-control study examining patients in general practices in the United Kingdom that included 15 710 cases of stroke and almost 60 000 randomly selected, matched control subjects from women aged 50 to79 years, transdermal estradiol was not associated with an increased risk of stroke, whereas oral estrogens significantly increased stroke risk.272 Importantly, dose effects, even from transdermal use, were seen in this population, because low-dose products that contained ≤50 μg of estrogen did not increase stroke risk (rate ratio, 0.81; 95% CI, 0.62–1.05) compared with no use, but high-dose patches that contained >50 μg did increase risk (rate ratio, 1.89; 95% CI, 1.15–3.11). Until randomized blinded studies are performed that demonstrate the safety of transdermal therapy, this treatment is not recommended for stroke prevention on the basis of available evidence, although this treatment is approved for relief of menopausal symptoms.
Postmenopausal HT: Summary and Gaps
An increased risk of stroke is associated with the tested forms of HT, which include CEE/medroxyprogesterone in standard formulations. A recent analysis and review of 9 randomized controlled trials,273 a review of HT trials by Henderson and Lobo,274 and a Cochrane review that included 23 studies275 all reached the conclusion that HT, in the formulations prescribed in prior studies, does not reduce stroke risk and may increase the risk of stroke. There are insufficient data to assess the risk of long-term HT use initiated in perimenopausal women or postmenopausal women <50 years of age; however, the data on this subject are often conflicting, and information regarding risk with newer HT regimens continues to emerge. There is no benefit of raloxifene or tamoxifen for stroke prevention, and raloxifene may increase the risk of fatal stroke. Tibolone is also associated with an increased risk of stroke. Prospective randomized trials of alternative forms of HT are ongoing, although the primary outcomes are an intermediate measurement of subclinical atherosclerosis and not stroke. The use of HT for other indications needs to be informed by the risk estimate for vascular outcomes provided by the clinical trials that have been reviewed. HT is associated with a small to moderate improvement in sexual function, particularly in pain, when used in women with menopausal symptoms or in early postmenopause (within 5 years of amenorrhea), but this treatment cannot be recommended currently for stroke prevention in unselected post-menopausal women.276 Limitations of prior trials included low adherence, high attrition, inadequate power to detect risks for low-incidence outcomes such as stroke, and evaluation of few regimens. Further research is needed to better understand the subgroups of women who may be at risk for stroke associated with HT and to optimize the timing and route of administration, as well as the dose and type of hormone used. Much less is known about the use of HT and the risk of either ICH or SAH.
Postmenopausal HT: Recommendations
HT (CEE with or without medroxyprogesterone) should not be used for primary or secondary prevention of stroke in postmenopausal women (Class III; Level of Evidence A).
Selective estrogen receptor modulators, such as raloxifene, tamoxifen, or tibolone, should not be used for primary prevention of stroke (Class III; Level of Evidence A).
Risk Factors More Common in Women Than Men
Migraine With Aura
The prevalence of migraine in the population is ≈18.5%, and for migraine with aura, it is 4.4%.277 Women are 4 times more likely to have migraines than men.277 Very rarely are migraines associated with stroke, however. Migraine with aura is defined as a typical migraine headache plus the presence of homonymous visual disturbance, unilateral paresthesias or numbness, unilateral weakness, or aphasia or unclassifiable speech difficulty that might typically precede the migraine headache.278 This type of migraine is associated with double the risk for IS based on meta-analyses of diverse cohorts of patients. The most recently published meta-analysis reported a 2.5-fold increase in IS in patients with migraine with aura (OR, 2.51; 95% CI, 1.52–4.14),279 similar to a previous analysis that showed an OR of 2.16 (95% CI, 1.53–3.03).280 The association between migraine aura and IS is higher in women than men. In women with migraine with aura, the risk increases even more in those using oral contraceptives (OR, 7.02; 95% CI, 1.51–32.68) and in cigarette smokers (OR, 9.03; 95% CI, 4.22–19.34).280
The absolute risk of migraine-associated stroke is relatively low. On the basis of data from the Women’s Health Study (WHS), migraine with aura accounted for 4 additional IS cases per 10 000 women per year when migraine with aura was the assumed underlying cause of stroke.281 The risk is higher with increasing frequency of migraine282 and if the aura does not include nausea and vomiting.283 Data from the WHS suggest that migraine with aura is associated with increased risk of TIA (RR, 1.56; 95% CI, 1.03–2.36) and nondisabling stroke (RR, 2.33; 95% CI, 1.37–3.97) compared with women with no history of migraine284 and that the presence of migraine with aura does not modify the beneficial effects of aspirin.285 Therefore, migraine aura appears to be associated with a better prognosis because of the link to milder strokes and TIAs than with non-migraine-associated strokes in the WHS.
Migraines with aura have also been associated with a risk of hemorrhagic stroke in the WHS, but this association was stronger in the subset of women with fatal hemorrhagic stroke and in women <55 years of age.286 In pregnant women with an International Classification of Diseases, 9th Revision, hospital code for migraine, there was a large association with hemorrhagic stroke (OR, 9.1; 95% CI, 3.0–27.8); however, in the pregnant population, the risk of vascular diseases was closely associated with a concomitant diagnosis of preeclampsia/eclampsia.287
Interestingly, there is an emerging literature on the association between migraines and preeclampsia.288–290 The most recent analysis of the United Kingdom Obstetric Surveillance System found 30 cases of antenatal stroke, for an estimated incidence of 1.5 cases per 100 000 women who delivered babies (95% CI, 1.0–2.1). Factors associated with increased risk of antenatal stroke were history of migraine (adjusted OR, 8.5; 95% CI, 1.5–62.1), gestational diabetes (adjusted OR, 26.8; 95% CI, 3.2–∞), and preeclampsia or eclampsia (adjusted OR, 7.7; 95% CI, 1.3–55.7).146
Migraine With Aura: Summary and Gaps
Migraine with aura (but not without aura) is associated with risk for IS and hemorrhagic stroke in women, especially those <55 years of age, although the absolute risk is low, and these women appear to have a good poststroke prognosis. Not only do the majority of studies with both men and women support this as a predominantly female issue, but the largest cohorts that have been studied are limited to women. There are not sufficient data to recommend specific approaches to treat migraine with the intention of lowering risk of stroke. Migraine treatment with triptans is contraindicated in patients with a history of cerebrovascular disease or coronary heart disease,291 as explicitly stated in guidelines from the American Academy of Neurology. As for the risk of treatment with triptans in women with migraine with aura, there are no data to guide this decision other than the observational data related to higher risk of stroke among those who smoke cigarettes or use OCs (see “Oral Contraceptives”). Given the number of studies that consistently show a higher risk of stroke in younger women with migraine with aura, it may be reasonable to include this in a woman-specific risk profile. The general recommendations for men and women with migraine with aura and stroke are as stated in the primary prevention guideline.19
Migraine With Aura: Recommendations
Because there is an association between higher migraine frequency and stroke risk, treatments to reduce migraine frequency might be reasonable, although evidence is lacking that this treatment reduces the risk of first stroke (Class IIb; Level of Evidence C).
Because of the increased stroke risk seen in women with migraine headaches with aura and smoking, it is reasonable to strongly recommend smoking cessation in women with migraine headaches with aura (Class IIa; Level of Evidence B).
Obesity, Metabolic Syndrome, and Lifestyle Factors
By the year 2030, an estimated 86% of Americans will be overweight or obese.292 Obesity affects a disproportionate number of women in the United States; in 2007 to 2008, the age-adjusted prevalence of obesity in the United States was 35.2% in women compared with 32.0% in men.293 Non-Hispanic black women have the highest prevalence of obesity (49.6% in 2007–2008).294
The distribution of obesity has important cardiovascular ramifications. In 1947, Vague295 coined the term android obesity to describe the high-risk form of obesity, at that time more frequently found in men, in which the body fat is concentrated in the abdominal area; he introduced the term gynoid obesity to describe the low-risk lower-body adiposity, more frequently found in premenopausal women. Abdominal obesity (defined as waist circumference >88 cm in women and >102 cm in men), however, is now far more prevalent in women than men, and android obesity is a misnomer. In fact, data from NHANES in 2007 to 2008 revealed that among adults ≥20 years old, age-adjusted prevalence of abdominal obesity was 61.8% in women compared with 43.7% in men.293 In addition, premenopausal women are increasingly likely to have abdominal obesity; a recent study of women aged 35 to 54 years in the United States revealed that from 1988–1994 until 1999–2004, the prevalence of abdominal obesity increased from 47.4% to 58.9%.296 The escalating obesity epidemic may counter the tremendous advances that have been made in smoking cessation and hypertension and dyslipidemia awareness and control in the United States. Understanding the effects of obesity and abdominal adiposity on stroke risk, and the potentially differential impact of these conditions on women compared with men, may elucidate avenues for reducing the incidence and morbidity of stroke.
Association Between Obesity and Abdominal Adiposity and Stroke Risk and Outcomes
Obesity is an independent risk factor for stroke. Studies have revealed a graded association between BMI and stroke risk; the risk of total stroke or IS rises linearly with increasing BMI and in a stepwise fashion for higher BMI categories.297–299 Obesity affects stroke risk in both men and women, even after adjustment for factors such as age, physical activity, smoking, alcohol consumption, and comorbid conditions such as hypertension and diabetes mellitus297–307 (Table 10). There is no clear evidence that obesity has a stronger impact on stroke risk in women than in men (Table 10).
Table 10.
Relationship Between Elevated BMI and Stroke
| HR (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| First Author | Study Site | n | Follow-up, y | Study Type | End Point | BMI, kg/m2 | Women | Men |
| Bazzano297 | China | 154736 | Mean: 8.3 | Prospective | Clinically defined | 25–29.9 | 1.35 (1.24–1.47) | 1.49(1.39–1.61) |
| cohort | total stroke* | vs 18.5–24.9 | ||||||
| ≥30 | 1.73 (1.51–1.98) | 1.68(1.43–1.97) | ||||||
| Hu300 | Finland | 49996 | Mean: 19.5 | Prospective | Clinically defined | 25–29.9 | 1.02(0.90–1.16) | 1.13(1.01–1.27) |
| cohort | total stroke† | vs 18.5–24.9 | ||||||
| ≥30 | 1.12(0.97–1.29) | 1.32 (1.14–1.53) | ||||||
| Winter307 | Germany | 1137 | ≈1 | Case-control | Clinically defined | 25–29.9 vs <25.0 | 1.17(0.60–2.28) | 1.36(0.82–2.25) |
| total stroke, TIA‡ | 30–34.9 vs <25.0 | 1.63(0.78–3.37) | 0.99(0.55–1.80) | |||||
| Saito298 | Japan | 71 722 | Median: 7.9 | Prospective | Clinically defined | 27–29.9 | 1.29 (1.01–1.65) | 1.09(0.88–1.36) |
| cohort | total stroke§ | vs 23.0–24.9 | ||||||
| ≥30 vs 23.0–24.9 | 2.16(1.60–2.93) | 1.25(0.86–1.84) | ||||||
| Kurth299 | USA | 39053 | Mean: 10 | Prospective | Clinically defined | 30–34.9 vs <20.0 | 1.37(0.83–2.28) | NA |
| cohort | total stroke‖· | ≥35 vs <20.0 | 2.05 (1.18–3.55) | |||||
| Kurth308 | USA | 21 414 | 12.5 | Prospective | Clinically defined | 27–29.9 vs <23 | N/A | 1.51 (1.19–1.92) |
| cohort | total stroke¶ | |||||||
| ≥30 vs <23 | N/A | 2.00 (1.48–2.71) | ||||||
| Yatsuya301 | USA | 13549 | Median: 16.9 | Prospective | Clinically defined | 28.6–32.0 | Black: 1.15(0.62–2.13) | Black: 1.33 (0.70–2.55) |
| cohort | ischemic stroke# | White: 1.49(0.89–2.50) | White: 1.85 (1.17–2.94) | |||||
| ≥32 | Black: 1.43(0.81–2.53) | Black: 2.12 (1.13–4.00) | ||||||
| White: 1.78 (1.08–2.93) | White: 1.85 (1.08–3.17) | |||||||
| Zhang303 | China | 67083 | Mean: 7.3 | Prospective | Clinically defined | 24.4–26.5 | 1.51 (1.30–1.74) | NA |
| cohort | total stroke** | vs <21.1 | ||||||
| ≥26.6 vs <21.1 | 1.71 (1.49–1.97) | |||||||
| Lu304 | Sweden | 33578 | Mean: 11 | Prospective | Clinically defined | 25–29.9 | 1.2 (0.9–1.7) | NA |
| cohort | total stroke†† | vs 20.0–24.9 | ||||||
| ≥30 vs 20.0–24.9 | 1.4 (0.8–2.4) | |||||||
| Rexrode305 | USA | 116759 | 16 | Prospective | Clinically defined | 29–31.9 vs <21.0 | 1.90(1.28–2.82) | NA |
| cohort | total stroke‡‡ | ≥32 vs <21.0 | 2.37 (1.60–3.50) | |||||
| Wang306 | China | 26607 | 11 | Prospective | Clinically defined | 25–29.9 | 1.42(1.16–1.73) | 1.63(1.35–1.96) |
| cohort | total stroke§§ | vs 18.5–24.9 ≥30 vs 18.5–24.9 | 1.57 (1.06–2.31) | 2.20 (1.47–3.30) | ||||
BMI indicates body mass index; CI, confidence interval; HR, hazard ratio; NA, not available; and T1A, transient ischemic attack.
Adjusted for age, smoking, alcohol consumption, physical inactivity, education, residence in northern China, and residence in urban area.
Adjusted for age, study year, smoking, physical activity, educational level, family history of stroke, alcohol consumption, systolic blood pressure, total cholesterol level, and history of diabetes mellitus.
Matched for age and sex and adjusted for physical inactivity, smoking, history of hypertension, and history of diabetes mellitus.
Adjusted for age, smoking, alcohol consumption, sports and physical exercise, medications or past history of hypertension or diabetes mellitus, and Japan Public Health Center community.
Adjusted for age, smoking status, exercise, alcohol consumption, and postmenopausal hormone use.
Adjusted for age, smoking, alcohol consumption, exercise, history of angina, parental history of myocardial infarction at age < 60 y, and randomized treatment assignment.
Adjusted for age, education, smoking status, cigarette-years, alcohol consumption, and physical activity.
Adjusted for age; education; occupation; family income; menopausal status; use of oral contraceptives, hormone therapy (HT), and aspirin; amount of exercise; cigarette smoking; alcohol consumption; and intakes of saturated fat, vegetables, fruits, and sodium comparing the highest versus lowest quintiles of BMI.
Adjusted for age, smoking, alcohol intake, age at first childbirth, years of education, and oral contraceptive use.
Adjusted for age, smoking, oral contraceptive use, menopausal status, HT, and time period.
Adjusted for age, educational level, smoking status, and alcohol consumption.
Numerous epidemiological and metabolic studies have shown that abdominal obesity has a stronger correlation with insulin resistance, atherogenic dyslipidemia, diabetes mellitus, and CVD than other distributions of body fat.309 Abdominal obesity can be measured by use of waist circumference, waist-to-hip ratio, and waist-to-stature ratio. As with BMI, there is a graded association between abdominal obesity and stroke risk. A study of 67 000 women found a 2% relative increase in total stroke risk with each 1-unit increase in waist circumference.303 Other studies have shown similar associations between abdominal obesity and stroke risk.304,307,310,311 Although many studies have shown that abdominal obesity is associated with stroke in women, even after adjustment for age, lifestyle habits, and medical comorbidities,301,303,307,310–312 some studies have shown that the association is no longer significant in multivariable models300,313,314 (Table 11). Studies of sex differences in the effect of abdominal obesity on stroke risk have had conflicting results (Table 11).
Table 11.
Abdominal Obesity and Stroke in Women and Men
| First Author | Study Site | No. of Subjects | Follow-up, y | Study Type | End Point | Stroke Subtypes | Measure of Abdominal Obesity | HR or OR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | ||||||||
| Hu300 | Finland | 49996 | Mean: 19.5 | Prospective cohort | Clinically defined stroke* | Ischemic, hemorrhagic | WC 4th vs 1st quartile WHR 4th vs 1st quartile |
HR: 1.25 (0.78–2.01) HR: 1.07 (0.70–1.63) |
HR: 1.57 (1.10–2.25) HR: 1.55 (1.06–2.26) |
| Winter307 | Germany | 1137 | Undefined | Case-control | Clinically defined stroke, TIA† | Ischemic, hemorrhagic | F: WC ≥88 cm vs <80 cm M: WC>102 cm vs <94 cm F: WHR ≥0.85 vs <0.85 M: WHR ≥ 1.0 vs <1.0 |
OR: 4.49 (2.13–9.46) OR: 7.77 (3.87–15.61) |
OR: 3.71 (2.18–6.32) OR: 4.13 (2.70–6.30) |
| Suk312 | USA | 1718 | ≈4 | Case-control | Clinically defined stroke‡ | Ischemic | F: WHR ≥0.86 vs <0.86 M: WHR ≥0.93 vs <0.93 | OR: 2.6 (1.7–4.2) | OR: 3.2 (1.9–5.5) |
| Yatsuya301 | USA | 13549 | Median: 16.9 | Prospective cohort | Clinically defined stroke§ | Ischemic | WC 5th vs 1st quintile WHR 5th vs 1st quintile |
Black HR: 1.65(1.03–2.65) White HR: 1.97(1.23–3.15) Black HR: 2.45(1.55–3.87) White HR: 1.76 (1.08–2.88) |
Black HR: 3.19(1.53–6.67) White HR: 2.15(1.14–4.03) Black HR: 1.69(0.91–3.15) White HR: 2.55 (1.42–4.57) |
| Zhang303 | China | 67083 | Mean: 7.3 | Prospective cohort | Clinically defined stroke‖ | Ischemic, hemorrhagic | WC 5th vs 1st quintile WHR 5th vs 1st quintile WSR 5th vs 1st quintile |
HR: 1.77 (1.53–2.05) HR: 1.59 (1.37–1.85) HR: 1.91 (1.61–2.27) |
NA NA NA |
| Lu304 | Sweden | 33578 | Mean: 11 | Prospective cohort |
Clinically defined stroke¶ | Ischemic, hemorrhagic | WC 4th vs 1st quartile WHR ≥0.88 5th vs 1st quintile WSR 5th vs 1st quintile |
HR: 2.3 (1.2–4.3) HR: 2.4 (1.3–4.2) HR: 2.5 (1.5–4.3) |
NA NA NA |
| Furukawa313 | Japan | 5474 | Mean: 11.7 | Prospective cohort | Clinically defined stroke# | Not stated | WC 4th vs 1st quartile | HR: 2.64 (1.16–6.03) | HR: 1.40(0.82–2.41) |
CI indicates confidence interval; HR, hazard ratio; F, female; M, male; NA, not applicable; OR, odds ratio; TIA, transient ischemic attack; WC, waist circumference; WHR, waist-hip ratio; and WSR, waist-to-stature ratio.
Adjusted for age; study year; smoking; physical activity; education level; family history of stroke; alcohol, vegetable, fruit, sausage, and bread consumption; systolic blood pressure; total cholesterol level; and history of diabetes mellitus.
Matehed for age and sex and adjusted for physical inactivity, smoking, history of hypertension, and history of diabetes mellitus.
Matched by age, sex, and race/ethnicity, and adjusted for hypertension, diabetes mellitus, any cardiac disease, current smoking status, no physical activity, moderate alcohol drinking, level of LDL cholesterol, level of HDL cholesterol, and education.
Adjusted for age, education, smoking status, cigarette-years, usual alcohol consumption, and physical activity.
Adjusted for age; education; occupation; family income; menopausal status; use of oral contraceptives; hormone therapy; aspirin; amount of exercise; cigarette smoking; alcohol consumption; and intakes of saturated fat, vegetables, fruits, and sodium comparing the highest versus lowest quintiles.
Adjusted for age, smoking, alcohol intake, age at first birth, years of education, and ever use of oral contraceptives by the time of cohort enrollment.
Adjusted for age, smoking, and drinking status.
The impact of obesity on poststroke outcomes remains unclear. One retrospective analysis of cross-sectional and prospective data from a nationally representative survey of the US adult population followed up from survey participation in 1988 to 1994 through mortality assessment in 2000 revealed that the overall risk for all-cause mortality among stroke survivors increased per 1 kg/m2 of higher BMI (P=0.030), but an interaction between age and BMI (P=0.009) revealed that the association of higher BMI with mortality risk was strongest in younger individuals and declined linearly with increasing age.315 On the other hand, a post hoc analysis of the Telemedical Project for Integrative Stroke Care (TEMPiS) revealed that mortality risk was lower in overweight patients (HR, 0.69; 95% CI, 0.56–0.86) and lowest in obese (HR, 0.50; 95% CI, 0.35–0.71) and very obese (HR, 0.36; 95% CI, 0.20–0.66) patients compared with those with normal BMI.316 It is unclear whether obesity has a differential impact on stroke outcomes in women compared with men.317
Metabolic Syndrome
Metabolic syndrome, a combination of cardiometabolic risk factors that tend to cluster together (insulin resistance, abdominal adiposity, dyslipidemia, and hypertension) affects approximately one third of the US adult population.318 Analysis of data from NHANES 2003 to 2006 revealed that 36.1% of men and 32.4% of women in the United States had metabolic syndrome (P=0.063).318 Numerous studies have shown an association between metabolic syndrome and stroke in both men and women303,310,311,314,319–326 (Table 12). The exact mechanism whereby metabolic syndrome affects cardiovascular risk is unknown; it is thought that components of the syndrome synergistically increase vascular risk through mechanisms that include insulin resistance, hypercoagulability, endothelial dysfunction, and inflammation. Studies suggest that metabolic syndrome confers a higher stroke risk on women than men,303,310,320,321 and metabolic syndrome accounts for a larger percentage of stroke events in women than in men (30% versus 4%, respectively).310 The mechanisms for this difference are not completely understood.
Table 12.
Metabolic Syndrome and Stroke
| First Author | Study Site | n | Follow-up, y | Type of Study | End Point | Stroke Subtypes | MetSD Definition | HR, OR, or RR (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Women | Men | ||||||||
| Boden-Albala310 | USA | 3298 | Median: 6.4 | Prospective cohort | Clinically defined stroke* | Ischemic | NCEP-ATP III | HR: 2.0 (1.3–3.1) | HR: 1.1 (0.6–1.9) |
| Chen319 | Taiwan | 3453 | Mean: 10.4 | Prospective cohort | Clinically defined stroke† | Ischemic | NCEP-ATP III | HR: 2.5 (0.7–8.4) | HR: 5.8 (2.0–16.5) |
| Ninomiya320 | USA | 10357 | NA | Cross-sectional | Self-reported stroke‡ | Undefined | NCEP-ATP III | OR: 2.20 (1.56–3.11) | OR: 1.93 (1.34–2.78) |
| Najarian321 | USA | 2097 | 14 | Prospective cohort | Clinically defined stroke§ | Ischemic, hemorrhagic | NCEP-ATP III | RR: 2.81 (1.48–5.33) | RR: 1.57 (0.88–2.79) |
| Takahashi324 | Japan | 1493 | Mean: 6.4 | Prospective cohort | Clinically defined stroke‖ | Ischemic | Other | RR: 23.1 (2.7–196) | NA |
| Li326 | USA Europe, Asia | 92732 | Variable | Review of prospective cohort | Clinically defined stroke | Ischemic, hemorrhagic | NCEP-ATP III, WHO | RR: 1.54 (1.28–1.82) | RR: 1.46 (1.28–1.65) |
ATP III indicates Adult Treatment Panel III; CI, confidence interval; EGIR, European Group for the Study of Insulin Resistance; HR, hazard ratio; IDF, International Diabetes Federation; MetSD, metabolic syndrome; NA, not available; NCEP, National Cholesterol Education Program; OR, odds ratio; RR, relative risk; and WHO, World Health Organization.
Adjusted for MetSD, age, education, insurance status, any physical activity, smoking, moderate alcohol use, and cardiac disease.
Adjusted for age, age squared, residential township, smoking, alcohol intake, physical activity level, parental history of stroke, and education level.
Adjusted for age, sex, race, and cigarette smoking.
Adjusted for age; systolic blood pressure; treatment for hypertension; history of cardiovascular disease, atrial fibrillation, and/or left ventricular hypertrophy; and smoking status.
Adjusted for age and smoking.
Pathophysiologic Mechanisms of Obesity, Abdominal Adiposity, and Metabolic Syndrome That Affect Stroke Risk
The pathophysiological mechanisms by which general obesity increases stroke risk remain unclear. One proposed mechanism is that obesity is associated with a prothrombotic and proinflammatory state.328–332 BMI is directly associated with fibrinogen, factor VII, plasminogen activator inhibitor, and tissue-type plasminogen activator antigen levels in both men and women.328 Similar associations are present between abdominal obesity and hemostatic factors. These associations persist after controlling for age, smoking, total and high-density lipoprotein cholesterol, triglycerides, glucose level, BP, and use of antihypertensive medications.328 In addition, higher levels of acute phase reactants such as C-reactive protein may decrease endothelial cell production of nitric oxide, which may in turn instigate a cascade of events leading to vasoconstriction, leukocyte adherence, platelet activation, oxidation, and thrombosis.333,334 Attenuation of the protective effect of high-density lipoprotein cholesterol314 attributable to general obesity may also play a role. The biological pathways by which abdominal adiposity increases stroke risk are also not yet understood, but platelet activation, inflammation, endothelial dysfunction, or an overactive endocannabinoid system335 may all serve key roles in the process. In addition, increased very low-density lipoprotein production caused by the high lipolytic activity of abdominal adipose tissue304,312 may increase stroke risk.
Lifestyle
Lifestyle factors such as a healthy diet,336–338 physical activity,339–343 abstinence from smoking,344–346 moderate alcohol intake,347,348 and maintenance of a healthy BMI299,308 reduce the risk of CVD and mortality. Adherence to a combination of healthy lifestyle practices has been shown to decrease stroke incidence in women327 and improve outcomes after stroke in both men and women.349 All-cause mortality after stroke decreases with higher numbers of healthy behaviors (1–3 factors versus none: HR, 0.12 [95% CI, 0.03–0.47]; 4–5 factors versus none: HR, 0.04 [95% CI, 0.01–0.20]; 4–5 factors versus 1–3 factors: HR, 0.38 [95% CI, 0.22–0.66]; trend P=0.04). Similar effects are observed for cardiovascular mortality after stroke (4–5 factors versus none: HR, 0.08 [95% CI, 0.01–0.66]; 1–3 factors versus none: HR, 0.15 [95% CI, 0.02–1.15]; 4–5 factors versus 1–3 factors: HR, 0.53 [95% CI, 0.28–0.98]; trend P=0.18).349
A multitude of randomized controlled trials of lifestyle interventions targeting individuals at high risk for diabetes mellitus and CVD have been conducted.350–362 Although many of the studies have proved effective in improving lifestyle habits and vascular risk factors in the short term, it has proved more challenging to maintain such changes and reduce cardiovascular events. For example, in the WHI Randomized Controlled Dietary Modification Trial, over a mean of 8.1 years, the dietary intervention reduced total fat intake and increased intakes of vegetables, fruits, and grains but did not significantly reduce the risk of coronary heart disease, stroke, or cardiovascular death in postmenopausal women and achieved only modest effects on cardiovascular risk factors.358 In addition, a 2-year behaviorally based physical activity and diet program implemented to reduce obesity in a primary care setting showed a significant reduction in waist circumference at 6 and 12 months, but the reduction in waist circumference was sustained in men but not women at 24 months.361 On the other hand, a recent primary prevention trial of an energy-unrestricted Mediterranean diet supplemented with either extra-virgin olive oil or nuts in high-risk people without CVD revealed that the groups randomly assigned to a Mediterranean diet with extra-virgin olive oil and to a Mediterranean diet with nuts had lower odds of MI, stroke, or cardiovascular death (HR, 0.70 [95% CI, 0.54–0.92] and HR 0.72 [95% CI, 0.54–0.96], respectively) compared with usual care.362 Regarding components of the primary end point, only the comparisons of stroke risk reached statistical significance (HR, 0.61; 95% CI, 0.44–0.86).362 Prespecified subgroup analyses revealed that men derived a significant benefit, whereas women did not. Further studies are needed to determine whether these results can be replicated, particularly because they are based on subgroup analyses. In addition, the pathophysiology underlying these potential sex differences is poorly understood and deserves additional exploration.
Although lifestyle habits have an important impact on post-stroke outcomes, there are no published trials of lifestyle interventions for secondary stroke prevention. The Healthy Eating and Lifestyle After Stroke (HEALS) trial, a randomized controlled trial of an occupational therapist–led series of 6 group clinics aimed at changing lifestyle habits among stroke survivors, is attempting to address this knowledge gap.363 Finally, little is known regarding how and whether lifestyle interventions should be tailored in women.
Obesity, Abdominal Adiposity, and Metabolic Syndrome: Summary and Gaps
In the United States, ≈1 in 3 individuals is obese. The prevalence of obesity is higher in women than men and is expected to increase over time in both sexes. Prospective studies have shown that obesity, abdominal adiposity, and metabolic syndrome are independent risk factors for stroke in both men and women. Further research is needed to determine whether sex modifies the impact of these conditions on stroke risk and outcomes. Healthy lifestyle practices, including maintaining a normal BMI, eating a diet rich in fruits and vegetables, moderate alcohol use, abstaining from smoking, and regular exercise, are associated with lower stroke incidence and better outcomes after stroke; however, little is known about sex differences in the effect of healthy lifestyle on stroke incidence and outcomes. Further research is needed to determine effective lifestyle interventions for preventing stroke occurrence and recurrence in women. Research is needed to develop lifestyle interventions that are effective for both primary and secondary stroke prevention and for tailoring such interventions in women.
Obesity, Metabolic Syndrome, and Lifestyle Factors: Recommendations
A healthy lifestyle consisting of regular physical activity, moderate alcohol consumption (<1 drink/d for nonpregnant women), abstention from cigarette smoking, and a diet rich in fruits, vegetables, grains, nuts, olive oil, and low in saturated fat (such as the DASH [Dietary Approaches to Stop Hypertension] diet) is recommended for primary stroke prevention in women with cardiovascular risk factors (Class I; Level of Evidence B).
Lifestyle interventions focusing on diet and exercise are recommended for primary stroke prevention among individuals at high risk for stroke (Class I; Level of Evidence B).
Atrial Fibrillation
AF is the most common arrhythmia and a major modifiable risk factor for stroke. AF increases 4- to 5-fold the risk of IS and is associated with higher death and disability.19 The attributable risk of stroke from AF increases with age, from 1.5% for those aged 50 to 59 years to nearly 25% for those aged ≥80 years.19,364 Whites carry the highest prevalence of AF compared with blacks, Hispanics, Asians, and other ethnic groups.365–367 The overall number of men and women with AF is similar, but ≈60% of AF patients aged >75 years are women.368,369
Given that AF increases with age and that women have greater life expectancy, there will be an increasing number of elderly women with AF as the population ages.19 For example, in Get With The Guidelines–Stroke, one third of hospital admissions for stroke were for stroke patients ≥80 years of age, and AF was identified in 15.6% of men and 20.4% of women (P<0.0001).370 Moreover, women with AF are slightly less likely to receive anticoagulation therapy than men (88% versus 89.7%; adjusted OR, 0.93; 95% CI, 0.88–0.98).371 Similar findings were observed in other studies.71,372–376
Risk Stratification for Women With AF
The occurrence of stroke is one of the most feared complications in patients with AF. Risk stratification tools, such as the CHADS2 and CHA2DS2-VASc scores, are useful in guiding the decision making for anticoagulation therapy.377,378 The CHADS2 score uses a point system that includes congestive heart failure (1 point), hypertension (1 point), age ≥75 years (1 point), diabetes mellitus (1 point), and 2 points for prior stroke/TIA.378 This scheme has been tested in several independent cohorts of patients with AF, with a score of 0 points indicating low risk (0.5% to 1.7%); 1 point, moderate risk (1.2% to 2.2% per year); and ≥2 points, high risk (1.9% to 7.6% per year).364
Female sex is an independent predictor of stroke in patients with AF.379–383 This has been incorporated into other risk stratification tools used in the decision making for anticoagulation prophylaxis.380
The CHA2DS2-VASc score can be considered an extension of the CHADS2 with extra points added for female sex (1 point), previous MI, peripheral arterial disease or aortic plaque (1 point), and age 65 to 74 years (1 point) or ≥75 years (2 points). The American College of Cardiology/AHA/European Society of Cardiology guidelines included similar risk stratification strategies as CHADS2, with the inclusion of left ventricular ejection fraction <35% in the high-risk category. The CHA2DS2-VASc score has been recommended recently by the European Society of Cardiology for risk classification.368,384–387
Two large cohort studies have both confirmed an age-sex interaction in patients with AF, which suggests a higher risk of stroke in women ≥75 years with AF compared with men.372,382 For example, in a large study that included 100 802 patients with nonvalvular AF in Sweden, the incident risk of IS was greater in women than in men (6.2% versus 4.2% per year, P<0.0001).382
Another large population-based study comprising 39 398 men (47.2%) and 44 115 women (52.8%) aged ≥65 years with AF from Canada showed a higher crude stroke incidence in women (2.02 per 100 person-years; 95% CI, 1.95–2.10) than men (1.61 per 100 person-years; 95% CI, 1.54–1.69; P<0.001). The observed difference was mainly driven by women aged ≥75 years. The stroke incidence per 100 person-years among participants aged ≥75 years was 2.38 (95% CI, 2.28–2.49) in women and 1.95 (95% CI, 1.84–2.07) in men (P<0.001). The stroke risk was significantly higher for women both for those taking or not taking warfarin. The multivariable analysis also revealed a similar increased risk of stroke in women (adjusted HR, 1.14 [95% CI, 1.07–1.22]; P<0.001) after adjustment for baseline comorbid conditions, individual components of the CHADS2 score, and warfarin treatment.372 There was a significant (P=0.02) interaction of age with sex, with the increased stroke risk confined to women aged ≥75 years. The age-sex interaction on stroke risk in patients with AF was again confirmed recently in a large Swedish nationwide drug registry study.372,382 Interestingly, warfarin use was associated with greater stroke reduction in women (60%; RR, 0.4; 95% CI, 0.3–0.5) than in men (40%; RR, 0.6; 95% CI, 0.5–0.8).380
Appropriateness of Anticoagulation by Sex
The appropriateness of anticoagulation in women with AF <65 years of age with no other major or clinically relevant risk factors is controversial. The European Society of Cardiology recommends anticoagulation for patients with AF and a CH2DS2-VASc score ≥1.387 The Canadian Cardiovascular Society recommends anticoagulation for AF patients with a CHADS2 score ≥1.388 According to these guidelines, anticoagulation would be recommended for all women with AF alone (and no other risk factors).
Two large observational studies provide some guidance. The Swedish study (n=100 802) found that the risk of stroke among patients with AF aged ≤65 years without risk factors was comparably low in both women (0.7%) and men (0.5%).382 Another nationwide study from Denmark that included 73 538 patients with AF looked at the short- and long-term risk of thromboembolic events by combining the risk factors included in CHA2DS2-VASc.389 The authors found that female sex alone was the weaker risk factor, showing a nonsignificant increase in the risk of thromboembolic events at 1- (HR, 1.24; 95% CI, 0.89–1.73), 5- (HR, 0.86; 95% CI, 0.70–1.06) and 10-year (0.82; 95% CI, 0.68–1.00) follow-up. The increased risk of thromboembolic events rose in women aged 65 to 74 years to 2.82 (95% CI, 2.21–3.60) at 1 year, 2.10 (95% CI, 1.81–2.45) at 5 years, and 2.06 (95% CI, 1.80–2.36) at 10 years.389
The AHA provides recommendations based on CHADS2 The great majority of patients being assessed at stroke prevention clinics have a CHADS2 score ≥2, the clinical situation with strong evidence in favor of anticoagulation for high-risk patients (Class I; Level of Evidence A).377 These data suggest that women, especially those aged ≥75 years, have a higher stroke risk, and most benefit from anticoagulation therapy. Younger women (<65 years old) with AF alone (no other risk factors) have a lower risk of stroke. No specific sex benefits were observed when rate versus rhythm control strategies were compared.390
Evidence From Randomized Clinical Trials of New Oral Anticoagulants
Most recent randomized clinical trials of new oral anticoagulants showed better stroke risk reduction and lower risk of intracranial bleeding. The benefit in women was comparable to that in men391–393 (Table 13). The RELY (Randomized Evaluation of Long-term Anticoagulant Therapy) study randomly assigned 18113 patients (36.4% women) with AF and a risk of stroke (mean CHADS2 score 2.2) to receive fixed doses of dabigatran (110 or 150 mg twice daily) or adjusted-dose warfarin. The median follow-up period was 2.0 years. The primary outcome was stroke or systemic embolism.
Table 13.
Primary and Secondary Outcomes Among Participants in the ARISTOTLE, RELY, and ROCKET AF Trials Stratified by Sex
| ARISTOTLE | RELY | ROCKET AF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | No. of Events (%/y) | n | No. of Events (%/y) | n | No. of Events (%/y) | |||||
| Apixaban | Warfarin | Dabigatran 110 mg | Dabigatran 150 mg | Warfarin | Rivaroxaban | Warfarin | ||||
| Primary outcome | ||||||||||
| Men | 11785 | 132 (1.2) | 160 (1.5) | 11514 | 52(1.35) | 42(1.10) | 57 (1.49) | 8553 | 103 (1.52) | 136 (1.95) |
| Women | 6416 | 80 (1.4) | 105 (1.8) | 6598 | 40(1.86) | 25(1.14) | 45 (2.03) | 5590 | 86 (1.97) | 107(2.47) |
| Major bleeding | ||||||||||
| Men | 11747 | 225 (2.3) | 294 (3.0) | 11514 | 113(2.92) | 129(3.37) | 138(3.63) | 8591 | 260 (3.92) | 253 (3.68) |
| Women | 6393 | 102 (1.9) | 168(3.3) | 6598 | 60 (2.79) | 72 (3.23) | 77 (3.46) | 5645 | 135(3.11) | 133(3.10) |
ARISTOTLE indicates Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation; RELY, Randomized Evaluation of Long-term Anticoagulant Therapy; and ROCKET AF, Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation.
ARISTOTLE (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation) was a randomized, double-blind trial comparing apixaban (5 mg twice daily) versus warfarin (target international normalized ratio, 2.0–3.0) in 18201 patients with AF and at least 1 additional risk factor for stroke (mean CHADS2 score 2.1). Women constituted one third (35.3%) of participants. The primary outcome was ischemic or hemorrhagic stroke or systemic embolism.391,391a
ROCKET AF (Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation) was a double-blind trial that randomly assigned 14264 patients with nonvalvular AF who were at increased risk for stroke to receive either rivaroxaban (20 mg daily) or dose-adjusted warfarin. Women represented 39.7% of the participants. The primary end point was stroke or systemic embolism.393 Of note, the risk of stroke was greater among participants in ROCKET AF than in RELY or ARISTOTLE, because the mean CHADS2 score was 3.47, with no patients in the lowest categories. Although all 3 trials using new oral anticoagulants consistently showed a greater number of events per year among women, none demonstrated a differential benefit by sex for the primary or secondary outcomes (P value for the interaction was not significant).391–393 These results should be interpreted with caution, because none of these studies were powered to determine a sex difference in the efficacy of new oral anticoagulants over warfarin.
In addition, women with AF had on average 30% higher concentrations of dabigatran than their male counterparts for the same given dose. The effect is most likely attributable to the average 30% lower creatinine clearance in women. No dose adjustment is required according to the product monograph or approval by regulatory agencies.394–396 Limited information is available among patients who participated in RELY with a body weight <50 kg.392 There were no differences in serum concentrations by sex for apixaban and rivaroxaban.391,393 Finally, some questions remain regarding the incomplete or scarce information on sex differences in the dosing of new oral anticoagulants.
AF: Summary and Gaps
AF is a major modifiable stroke risk factor, independently associated with a 4- to 5-fold increased risk of IS. AF is responsible for 1.5% to 25% of all IS depending on the age group. Anticoagulation is the most effective therapeutic strategy to decrease the risk of stroke. Risk stratification tools, such as CH2DS2-VASc, are useful to stratify the risk of stroke and assist clinicians in the decision to initiate anticoagulation therapy. New oral anticoagulants are a useful alternative to warfarin for the prevention of stroke and systemic thromboembolism; however, caution about overdosing must be used considering the additive effect of age, sex, renal function, and concomitant medications (acetylsalicylic acid, clopidogrel, nonsteroidal anti-inflammatory drugs, P-glycoprotein inhibitors) in increasing the concentrations of new oral anticoagulants.
Future research is needed to determine the appropriate dose of new oral anticoagulants in older women with a lower weight (eg, <50 kg) who are also exposed to other comorbidities (eg, renal impairment) that influence the pharmacokinetics of these agents. In addition, none of the trials of new anticoagulants were powered to determine a sex difference in efficacy versus warfarin.
AF: Recommendations
Risk stratification tools in AF that account for age- and sex-specific differences in the incidence of stroke are recommended (Class I; Level of Evidence A).
Considering the increased prevalence of AF with age and the higher risk of stroke in elderly women with AF, active screening (in particular of women >75 years of age) in primary care settings using pulse taking followed by an ECG as appropriate is recommended (Class I; Level of Evidence B).
Oral anticoagulation in women aged ≤65 years with AF alone (no other risk factors; women with CHADS2=0 or CHA2DS2-VASc=1) is not recommended (Class III; Level of Evidence B). Antiplatelet therapy is a reasonable therapeutic option for selected low-risk women (Class IIa; Level of Evidence B).
New oral anticoagulants are a useful alternative to warfarin for the prevention of stroke and systemic thromboembolism in women with paroxysmal or permanent AF and prespecified risk factors (according to CHA2DS2-VASc) who do not have a prosthetic heart valve or hemodynamically significant valve disease, severe renal failure (creatinine clearance <15 mL/min), lower weight (<50 kg), or advanced liver disease (impaired baseline clotting function) (Class I; Level of Evidence A).
Depression and Psychosocial Stress
In addition to being a well-recognized sequela of stroke,397 depression is associated with increased risk of stroke incidence among both women and men. In the INTERSTROKE case-control study of stroke participants from 22 countries,92 investigators found that self-reported depression (defined as feeling sad, blue, or depressed for ≥2 consecutive weeks during the past 12 months) was associated with a 35% increased odds of stroke (95% CI, 1.10–1.66) after adjustment for age, sex, and region. In addition, psychosocial stress (defined with a combined measure of general stress at home and in the workplace and categorized by permanent or several periods of stress versus no or some periods of stress in the past year) was associated with a 30% increased odds of stroke (95% CI, 1.06–1.60) in the adjusted model. Although the multivariable models were adjusted for sex, there were no sex-specific analyses performed in this cohort. In the Nurses’ Health Study, women with a history of depression had a 29% increased risk of incident total stroke (multivariate RR, 1.29; 95% CI, 1.13–1.48).398 In a meta-analysis of prospective studies of depression and stroke, the pooled adjusted HRs were 1.45 (95% CI, 1.29–1.63) for total stroke and 1.25 (95% CI, 1.11–1.40) for IS. The cumulative ORs for studies that included only men or only women revealed no difference in the strength of association between depression and stroke compared with the cumulative OR for studies that included both sexes.399
Depression and Psychosocial Stress: Summary and Gaps
Depression is associated with increased risk of stroke. More research is needed to understand the mechanisms underlying the association between depression and stroke, as well as to determine which women with depression may be at risk, such as those who are treated versus untreated, and whether self-reported measures such as those used in the INTERSTROKE study are the most accurate to determine stroke risk. There is also a lack of sex-specific analyses in many of the cohorts that have assessed multiple risk factors. Because depression and psychosocial stressors are more common in women, it may be reasonable to test these risk factors in a woman-specific stroke risk score.
Strategies for Prevention of Stroke: Are They Different in Women?
Representation of Women in Stroke Clinical Trials
Women account for less than half of all subjects enrolled in National Institutes of Health–funded stroke prevention clinical drug trials of the past decade. It has also been recognized that women have been underrepresented in clinical trials in surgery, overall CVD, and cancer.400–403 Sex disparities in cerebrovascular disease clinical trial participation have been less well examined.404 The low proportion of women in clinical stroke prevention trials limits the generalization of results across the sexes. Conventional subgroup analyses in women are commonly flawed by type II error. As a result, it remains unclear whether current evidence-based practices apply to women, who represent half of all stroke victims.
In response to recognition that overall, women were under-represented in National Institutes of Health–sponsored trials, the National Institutes of Health Revitalization Act (public law 103–43), which required inclusion of women in clinical trials of diseases affecting women, was enacted in 1993.402,405 Unfortunately, to date, the legislation appears to have had little impact. A review of articles published in The New England Journal of Medicine after enactment of the legislation reported that there was no change in the overall rate of enrollment of females, which remained at roughly 25%.406 Neurological clinical trials were among the most successful in enrolling women, with a participation rate of 45%. A subsequent review of National Institutes of Health-funded studies found that only 24% of enrolled drug study populations were women.400 This lack of recruitment continues in recent trials. For example, only 20% of women were recruited in the WARCEF trial (Warfarin Versus Aspirin Use in Patients With Impaired LV Function) as recently as 2012.407 Factors that may play a role in the apparent underrepresentation of women in clinical trials may be sex differences in disease prevalence and age at onset in women. A systematic review of enrollment of women in trials of coronary artery disease, heart failure, arrhythmia, and primary prevention reported that after accounting for age- and sex-specific differences in disease prevalence, the enrollment gaps for women narrowed, ranging from 3% for primary prevention to 13% for heart failure. Stroke was not included in this analysis, however.408 The percentage of women enrolled in recent stroke prevention trials of carotid disease and antiplatelet agents ranges from 25% to 53%, with an average of 34%, which is generally below the stroke prevalence rates by sex (Tables 14 and 15).
Table 14.
Representation of Women in Carotid Intervention Trials
| Trial | Total Patients (% Women) |
|---|---|
| NASCET409 | 663 (32) |
| NASCET moderate410 | 2303 (29) |
| ECST411 | 3035 (28) |
| ACAS412 | 1662 (34) |
| ACST413 | 3120 (35) |
| EVA-3S414 | 520 (25) |
| SPACE415 | 1207 (28) |
| CREST416 | 2522 (35) |
ACAS indicates Asymptomatic Carotid Atherosclerosis Study; ACST, Asymptomatic Carotid Surgery Trial; CREST, Carotid Revascularization Endarterectomy Versus Stenting Trial; ECST, European Carotid Surgery Trial; EVA-3S, Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis; NASCET, North American Symptomatic Carotid Endarterectomy Trial; NASCET moderate, North American Symptomatic Carotid Endarterectomy Trial (patients with moderate stenosis only); and SPACE, Stent-Protected Angioplasty Versus Carotid Endarterectomy.
Table 15.
Representation of Women in Antiplatelet Trials
| Trial | Total Patients Enrolled (% Women) |
|---|---|
| ACE417 | 2806 (30) |
| ESPS-2418 | 6604 (42) |
| CAPRI E419 | 15 480(30) |
| MATCH420 | 7624 (37) |
| AAASPS421 | 1824(53) |
| ESPRIT422 | 2714(35) |
| PROFESS423 | 20 438 (37) |
| SPS3424 | 3021 (37) |
AAASPS indicates African American Antiplatelet Stroke Prevention Study; ACE, ASA [acetylsalicylic acid] and Carotid Endarterectomy Trial; CAPRIE, Clopidogrel Versus Aspirin in Patients at Risk of Ischaemic Events; ESPS-2, European Stroke Prevention Study 2; ESPRIT, European/Australasian Stroke Prevention in Reversible Ischaemia Trial; MATCH, Management of Atherosclerosis With Clopidogrel in High-Risk Patients; PRoFESS, Prevention Regimen for Effectively Avoiding Second Strokes; and SPS3, Secondary Prevention of Small Subcortical Strokes.
CEA Versus Medical Management for Symptomatic or Asymptomatic Carotid Stenosis
Differences in the anatomy of the internal carotid arteries and differences in the composition of plaque between women and men have fueled speculation that there may be differential risk or benefit to intervention. Compared with men, women have smaller-caliber internal carotid arteries and shorter stenotic segments.425,426 CEA is also performed less often in women, likely because of the lower incidence of high-grade symptomatic stenosis.427,428 In a retrospective cohort study at Kaiser Permanente Medical Care Plan, for the period 2003 to 2004, 299 patients were identified on the basis of a diagnosis of TIA and carotid stenosis (>70%), that is, symptomatic stenosis.427 Approximately half (47%) were women. Women were less likely to undergo CEA (36.4% versus 53.8% in men; P=0.004). Being female remained an independent predictor for not receiving CEA after adjustment for age, number of TIAs, specificity of TIA symptoms, and degree of stenosis. In patients who underwent CEA, the time to surgery was longer in women (mean of 35 days) than men (mean of 18 days; P=0.03),427 whereas the current recommendations are to perform CEA within 2 weeks of symptoms of TIA or mild stroke.20 In the surgical subgroup, women were older and less likely to have coronary artery disease. Outcomes were similar in men and women in both the CEA and medical management groups.427
In subgroup analyses of some trials comparing medical management to CEA in patients with symptomatic or asymptomatic carotid stenosis, women appeared to derive less benefit from surgery than men, potentially because of an increased risk of perioperative events411–413; however, the data were inconclusive because of small sample sizes within sex strata and the post hoc nature of some of the analyses. Only 1 trial, the Asymptomatic Carotid Surgery Trial, conducted a prespecified secondary analysis by sex group.413 Although for all the subgroup analyses, the results were similar to that first found in the Asymptomatic Carotid Atherosclerosis Study,412 they were statistically significant only for the European Carotid Surgery Trial, but this was a post hoc analysis.411
CEA Versus Carotid Artery Stenting
Carotid artery stenting (CAS) has emerged as an alternative strategy for the management of carotid stenosis.414–416,429,430 In a meta-analysis of 3 European trials comparing CEA to CAS in symptomatic patients, there was no interaction between sex and 120-day outcome.431 The North American Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST) assigned 2502 patients with symptomatic and asymptomatic carotid stenosis to either CEA or CAS. Roughly 35% (n=872) of the subjects were women. Rates of stroke, MI, or death of any cause during the periprocedural period or any ipsilateral stroke within 4 years after randomization for CAS compared with CEA were 6.2% versus 6.8% in men (HR, 0.99; 95% CI, 0.66–1.46) and 8.9% versus 6.7% in women (HR 1.35; 95% CI, 0.82–2.23).416 Periprocedural events (from randomization to 30 days after the procedure) occurred in 4.3% of 807 men versus 6.8% of 455 women assigned to CAS and 4.9% of 823 men versus 3.8% of 417 women assigned to CEA, with significant interaction (P=0.064).432 The CAS-to-CEA treatment difference was also affected by age (P=0.02), with older patients faring better with CEA and younger patients faring better with CAS.433 There was no evidence (P=0.45) that this effect by age was different for women than men. Although data from CREST suggest that women with CAS (relative to CEA) may be at higher risk for the composite outcome of stroke, death, or MI during the periprocedural period, this finding should be interpreted with caution pending confirmation from other trials.
At present, there may not be sufficient data to conclude that high-grade symptomatic or asymptomatic carotid stenosis should be managed differently in men and women with regard to the type of procedure, or whether it should be managed medically. Without definitive data, the recommendations in the guidelines for prevention of first or recurrent stroke should be applied to women.19,20,434
Aspirin for Prevention of Stroke in Women
One of the seminal trials of primary prevention of CVD, including stroke, in women is the WHS, a randomized trial of 100 mg of aspirin on alternate days versus placebo in 39876 initially asymptomatic women 45 years of age, followed up for 10 years for a first major vascular event (nonfatal MI, nonfatal stroke, or cardiovascular death).435 Although there was a nonsignificant 9% reduction (RR, 0.91; 95% CI, 0.80–1.03; P=0.13) in the combined primary end point among women, the study found a 17% reduction in the risk of stroke (RR, 0.83; 95% CI, 0.69–0.99; P=0.04). This was based on a 24% reduction in the risk of IS (RR, 0.76; 95% CI, 0.63–0.93; P=0.009) and a nonsignificant increase in the risk of hemorrhagic stroke (RR, 1.24; 95% CI, 0.82–1.87; P=0.31). The overall average stroke rates were 0.11% per year in aspirin-treated women and 0.13% per year in placebo-treated women (RR, 0.02% per year; number needed to treat, 5000). An important adverse outcome, gastrointestinal hemorrhage requiring transfusion, was more frequent in the aspirin group (RR, 1.40; 95% CI, 1.07–1.83; P=0.02 and absolute risk increase, 0.01% per year; number needed to harm, 10000). The most consistent benefit for aspirin was in women ≥65 years of age at study entry, among whom the risk of major cardiovascular events was reduced by 26% (RR, 0.74; 95% CI, 0.59–0.92; P=0.008), including a 30% reduction in the risk of IS (RR, 0.70; 95% CI, 0.49–1.00; P=0.05); however, the benefit was reduced when the combination of IS and hemorrhagic stroke was considered (RR, 0.78; 95% CI, 0.57–1.08; P=0.13). On the basis of the WHS results, aspirin is recommended for primary prevention for women after consideration of the 10-year risk of CVD and whether this and age outweigh the risk of hemorrhage.21
A large, sex-specific meta-analysis of aspirin for primary prevention of IS (separate from other cardiovascular events) showed that women appeared to benefit from aspirin (OR, 0.76; 95% CI, 0.63–0.93), whereas men showed no benefit (OR, 1.00; 95% CI, 0.72–1.41).436 For heart disease, however, men benefited from aspirin for prevention against coronary heart disease, whereas women did not. As in men, aspirin allocation in primary prevention trials increased the risk of hemorrhagic stroke and major gastrointestinal and extracranial bleeds among women and resulted in uncertain net value.
There is no evidence of a differential effect of antiplatelet agents for secondary stroke in women compared with men.418,419,422,423,437 The Antithrombotic Trialists meta-analysis of aspirin for the primary and secondary prevention of vascular events (MI, stroke, vascular death) also included individual patient data from each of the trials.437 They found similar proportional reductions in risk for men and women for the combined outcome; however, there was a trend toward benefit for primary prevention in women versus men, although this was no longer the case when the P value was adjusted for multiple comparisons.437 In secondary prevention (among patients who already have occlusive vascular disease), aspirin appears to result in a greater absolute benefit for stroke prevention, with similar magnitude of effect for women and men, and a 19% reduction in stroke risk.437
Strategies for Prevention of Stroke in Women: Summary and Gaps
In all clinical trials of primary and secondary stroke prevention, women need to be included in sufficient numbers for preplanned subgroup analysis, with reasonable statistical power to provide for valid analysis to test for sex interaction.405 Questions about the benefits and risks of carotid procedures in women with asymptomatic high-grade carotid stenosis and symptomatic treatment for moderate (50%–69%) carotid stenosis remain unanswered. Until further studies are performed, the recommendations for prevention of stroke in women with carotid disease (symptomatic or asymptomatic) remain the same as for men, as published in the primary and secondary prevention guidelines.19,20 For aspirin, the recommendations below for women are as published in the AHA’s “Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women.”21
Strategies for Prevention of Stroke in Women: Recommendations
Women with asymptomatic carotid stenosis should be screened for other treatable risk factors for stroke, and appropriate lifestyle changes and medical therapies should be instituted (Class I; Level of Evidence C). 19
In women who are to undergo CEA, aspirin is recommended unless contraindicated, because aspirin was used in every major trial that demonstrated efficacy of CEA (Class I; Level of Evidence C).
Prophylactic CEA performed with <3% morbidity/mortality can be useful in highly selected patients with an asymptomatic carotid stenosis (minimum 60% by angiography, 70% by validated Doppler ultrasound) (Class IIa; Level of Evidence A).
For women with recent TIA or IS within the past 6 months and ipsilateral severe (70%–99%) carotid artery stenosis, CEA is recommended if the perioperative morbidity and mortality risk is estimated to be <6% (Class I; Level of Evidence A).20
For women with recent TIA or IS and ipsilateral moderate (50%–69%) carotid stenosis, CEA is recommended depending on patient-specific factors, such as age and comorbidities, if the perioperative morbidity and mortality risk is estimated to be <6% (Class I; Level of Evidence B).20
When CEA is indicated for women with TIA or stroke, surgery within 2 weeks is reasonable rather than delaying surgery, if there are no contraindications to early revascularization (Class IIa; Level of Evidence B).20
Aspirin therapy (75–325 mg/d) is reasonable in women with diabetes mellitus unless contraindicated (Class IIa; Level of Evidence B).21
If a high-risk (ie, 10-year predicted CVD risk ≥10%) woman has an indication for aspirin but is intolerant of aspirin therapy, clopidogrel should be substituted (Class I; Level of Evidence B).21
Aspirin therapy can be useful in women ≥65 years of age (81 mg/d or 100 mg every other day) if BP is controlled and the benefit for IS and MI prevention is likely to outweigh the risk of gastrointestinal bleeding and hemorrhagic stroke (Class IIa; Level of Evidence B) and may be reasonable for women <65 years of age for IS prevention (Class IIb; Level of Evidence B).21
Data Shaping Development and Validation of Risk Scores
Use of Sex-Specific Stroke Risk Prediction Scores
Risk scores have been advocated to clinically classify individuals according to their overall risk to guide prevention and treatment recommendations. The goal of such scores is to accurately classify individuals as high or low risk so that high-risk individuals receive appropriate interventions to reduce risk.
Although there are several general cardiovascular risk scores,438, 439 there are fewer stroke-specific scores. Risk prediction models that are specific to women have not been developed. Furthermore, whether risk factors differ by age and racial/ethnic group has not been established. The Framingham stroke risk calculator does take sex into account.440 The number of points assigned to particular conditions differs by sex, with diabetes mellitus, systolic BP, and AF garnering a higher number of points in women than men, whereas women with existing CVD receive fewer points than do men (Table 16). Moreover, the 10-year probability of stroke from the Framingham Stroke Risk Score (FSRS) is lower for women than for men with the same score (Table 17); however, few other widely validated stroke prediction scores for the general population are available. Data in minorities and women are limited.
Table 16.
Risk Factors and Points Included in the Framingham Stroke Risk Score for 10-Year Stroke Risk Prediction in Women*
| Predictors | 0 | +1 | +2 | +3 | +4 | +5 | +6 | +7 | +8 | +9 | +10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 54–56 | 57–59 | 60–62 | 63–64 | 65–67 | 68–70 | 71–73 | 74–76 | 77–78 | 79–81 | 82–84 |
| SBP (untreated), mm Hg | 95–106 | 107–118 | 119–130 | 131–143 | 144–155 | 156–167 | 168–180 | 181–192 | 193–204 | 205–216 | |
| SBP (treated), mm Hg | 95–106 | 107–113 | 14–119 | 120–125 | 126–131 | 132–139 | 140–148 | 149–160 | 161–204 | 204–216 | |
| Diabetes mellitus | No | Yes | |||||||||
| Cigarette smoking | No | Yes | |||||||||
| Prior CVD† | No | Yes | |||||||||
| AF | No | Yes | |||||||||
| LVH on ECG | No | Yes | Yes |
AF indicates atrial fibrillation; CVD, cardiovascular disease; ECG, electrocardiogram; LVH, left ventricular hypertrophy; and SBP, systolic blood pressure.
CVD is defined as any of the following: history of myocardial infarction, angina pectoris, coronary insufficiency, intermittent claudication, or congestive heart failure.
Table 17.
Ten-Year Stroke Probability in Women According to Framingham Stroke Risk Score
| Points | 10-y Probability, % | Points | 10-y Probability, % | Points | 10-y Probability, % |
|---|---|---|---|---|---|
| 1 | 1 | 11 | 8 | 21 | 43 |
| 2 | 1 | 12 | 9 | 22 | 50 |
| 3 | 2 | 13 | 11 | 23 | 57 |
| 4 | 2 | 14 | 13 | 24 | 64 |
| 5 | 2 | 15 | 16 | 25 | 71 |
| 6 | 3 | 16 | 19 | 26 | 78 |
| 7 | 4 | 17 | 23 | 27 | 84 |
| 8 | 4 | 18 | 27 | ||
| 9 | 5 | 19 | 32 | ||
| 10 | 6 | 20 | 37 |
The FSRS was derived in a predominantly white, middle- to older-aged population in a single community. The FSRS was applied to the Cardiovascular Health Study (CHS) population of men and women >65 years of age, and it was found that the FSRS model predicted stroke less effectively in elderly women than men. A CHS stroke risk model and applet were created that improved discrimination in women (area under the receiver operating characteristic curve of 0.73 for FSRS in women compared with 0.77 for the CHS model in women; P=0.044).441 Additional variables included in the CHS score included time to walk 15 feet (functional status) and creatinine. When the FSRS was used to evaluate risk in a group of elderly French men and women (aged 65–84 years at baseline), it overestimated the risk substantially (by a factor of 4.4 in women and 3.7 in men), which led to a recalibrated stroke score for that population.442 Therefore, comparative data are needed on how well risk scores perform in women versus men and in different racial and ethnic populations.
Several stroke risk scores were developed exclusively for use in men,443,444 but woman-specific models are needed to more accurately reflect risk across the lifespan. Specific risk factors are unique to or more common in women that have not been included in traditional risk assessment (Table 3). Prospective research is needed for these woman-specific risk factors, including pregnancy-related risk factors and hormonal exposure (OCs and postmenopausal HT), as well as changes in hormone status across the lifespan (menarche, menopause, and oophorectomy). For example, preeclampsia clearly increases risk of stroke in the peripartum period and has been listed as a risk factor for CVD in women in the 2011 AHA “Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women”21; however, relatively limited data on long-term risk of stroke among women with a history of preeclampsia are available.180 In addition, several less traditional risk factors, including psychosocial stress and depression, were important in the INTERSTROKE study92 and other observational studies398,399 but are not included in traditional risk scores.440,445 Women have higher rates of depression, and thus, these factors may be of greater impact for women.446 Additionally, there are potential differences in the risk of stroke among older individuals with AF, with higher rates in women despite warfarin therapy, as discussed above.372 Whether biomarkers, in addition to lipid measures, additionally contribute to risk stratification in women is unclear. C-reactive protein, an inflammatory marker that is also included in the Reynolds Cardiovascular Risk Score,439,447 was found to improve prediction of risk of IS, particularly cardioembolic stroke, in older women in the WHI.448 Lipoprotein-associated phospholipase A2 was associated with improvement in the prediction of large-artery strokes.448 Additional evaluation of other biomarkers for risk prediction will be of continued interest.
Woman-Specific Risk Score: Summary and Gaps
Consideration of the risk factors that are unique to women and are more prevalent or differentially increase risk, compared with men, may improve the accuracy of stroke risk assessment compared with current risk scores. This is especially true for younger women of reproductive age. Prospective data on the long-term stroke risk of women with a history of preeclampsia and other pregnancy-related complications are also needed.449 Further development of stroke risk prediction models in women could be achieved by use of data from large, diverse longitudinal studies including the WHI, WHS, Nurses’ Health Study, ARIC study, Multi-Ethnic Study of Atherosclerosis (MESA), and Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Sex-specific and race- and ethnic-specific analyses need to be conducted explicitly to determine whether current and future risk scores perform equally well in white, black, and Latina women.
Conclusions and Summary
In this guideline, we have summarized the current evidence and provided summaries and gaps for prevention focused on the risk factors that are either unique to or more common in women than men. Some of the recommendations in this guideline were formerly associated with other prevention guidelines but have been assimilated because of the focus on women. In addition, we have summarized the data that support the development of woman-specific stroke risk profiles, which might more accurately reflect a woman’s future risk of stroke than some of the currently available stroke risk profiles.
Prevention efforts for women would be enhanced if future epidemiological studies provided more detail on stroke subtype, especially hemorrhagic stroke, in addition to accounting for age and sex. Similarly, it is important to improve stroke awareness and provide more rigorous education to women at younger ages, including childbearing ages, because of women’s increased risk of stroke with age; the risks of stroke associated with pregnancy, gestational hypertension, and hormonal contraception; and the onset of stroke risk factors such as obesity, hypertension, and diabetes mellitus, which occur at younger ages. Future research focused on risk profile development is urgently needed to appropriately tailor prevention strategies for women. There is a need for recognition of women’s unique sex-specific stroke risk factors, and a risk score that includes these factors would thereby identify women at risk. Until sex-specific risk is better understood, prevention and management of stroke and cardiovascular risk factors remains essentially the same for men and women.
Footnotes
Disclosures
| Writing Group Member | Employment | Research Grant | Other Research Support | Speakers' Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Cheryl Bushnell | Wake Forest School of Medicine | NIH/NINDS† | World Federation of Neurology† | None | None | None | None | None |
| Louise D. McCullough | University of Connecticut | NIH/NINDS† | None | Genentech* | None | None | None | None |
| Issam A. Awad | University of Chicago | NIH/NINDS† | None | None | None | None | None | None |
| Monique V. Chireau | Duke University Medical Center | None | None | None | None | None | None | None |
| Wende N. Fedder | Alexian Brothers Health System | None | None | None | None | None | None | None |
| Karen L. Furie | The Warren Alpert Medical School of Brown University/Lifespan | AHA†; NINDS† | None | None | None | None | UpToDate* | JNNP Deputy Editor†; Stroke Vice Editor† |
| Virginia J. Howard | University of Alabama at Birmingham | NIH† | None | None | None | None | Spouse has relationship with Abbott Vascular*; spouse has relationship with Bayer* | None |
| Judith H. Lichtman | Yale University | AHA†; NINDS† | None | None | None | None | None | None |
| Lynda D. Lisabeth | University of Michigan | NIH† | None | None | None | None | None | None |
| IIeana L. Piña | Montefiore Medical Center | None | None | None | None | None | None | None |
| Mathew J. Reeves | Michigan State University | None | None | None | None | None | None | None |
| Kathryn M. Rexrode | Brigham and Women’s Hospital | NIH/NHLBI† | None | None | None | None | None | None |
| Gustavo Saposnik | University of Toronto | None | None | None | None | None | Bayer* | None |
| Vineeta Singh | University of California, San Francisco | None | None | None | None | None | None | None |
| Amytis Towfighi | University of Southern California; Rancho Los Amigos National Rehabilitation Center | AHA†; NIH/NINDS† | None | Boehringer Ingelheim* | None | None | None | None |
| Viola Vaccarino | Emory University | None | None | None | None | None | None | None |
| Matthew R. Walters | University of Glasgow | AstraZeneca*; GlaxoSmithKline*; Lundbeck*; Menarini*; Microtransponder, Inc*; Roche* | None | None | None | None | Bayer*; Lundbeck*; UCB Pharma* | None |
| Reviewer | Employment | Research Grant | Other Research Support | Speakers’ Bureau/Honoraria | Expert Witness | Ownership Interest | Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Jo-Ann Eastwood | University of California Los Angeles | AHA† | None | Preventive Cardiovascular Nurses: Expert exchange* | None | None | None | None |
| Michael D. Hill | University of Calgary | Covidien† (money goes to institution); Hoffmann-La Roche Canada† (money goes to institution) | Heart & Stroke Foundation Alberta† (money goes to institution); Alberta Innovates Health Solutions† (money goes to institution) | None | None | None | Merck Ltd.* | None |
| Marian Limacher | University of Florida | NIH† | None | None | None | None | None | None |
| Virginia Miller | Mayo Clinic | None | None | None | None | None | None | None |
| Latha Stead | University of Florida | None | None | None | None | None | None | None |
| Greg Zipfel | Washington University | NINDS†; AHA†; Brain Aneurysm Foundation*; Hope Center for Neurological Disorders*; Pfizer† | None | None | None | None | Pfizer* | None |
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpem SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Morelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2013 update: a report from the American Heart Association [published corrections appear in Circulation. 2013;127:e841 and Circulation. 2013;127]. Circulation. 2013;127:e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. Health, United States, 2011. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; 2012. http://www.cdc.gov/nchs/hus/contents2011.htm#031. Accessed December 31, 2012.
- 3.US Department of Health and Human Services, Administration on Aging. A profile of older Americans: 2011. http://www.aoa.gov/AoAroot/Aging_Statistics/Profile/2011/docs/2011profile.pdf. Accessed March 6, 2013.
- 4.Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly-Hayes M, Beiser A, Kase CS, Scaramucci A, D’Agostino RB, Wolf PA. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12:119–126. [DOI] [PubMed] [Google Scholar]
- 6.Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006;37:345–350. [DOI] [PubMed] [Google Scholar]
- 7.Gall SL, Tran PL, Martin K, Blizzard L, Srikanth V. Sex differences in long-term outcomes after stroke: functional outcomes, handicap, and quality of life. Stroke. 2012;43:1982–1987. [DOI] [PubMed] [Google Scholar]
- 8.Gargano JW, Reeves MJ; Paul Coverdell National Acute Stroke Registry Michigan Prototype Investigators. Sex differences in stroke recovery and stroke-specific quality of life: results from a statewide stroke registry. Stroke. 2007;38:2541–2548. [DOI] [PubMed] [Google Scholar]
- 9.Holroyd-Leduc JM, Kapral MK, Austin PC, Tu JV. Sex differences and similarities in the management and outcome of stroke patients. Stroke. 2000;31:1833–1837. [DOI] [PubMed] [Google Scholar]
- 10.Kim JS, Lee KB, Roh H, Ahn MY, Hwang HW. Gender differences in the functional recovery after acute stroke. J Clin Neurol. 2010;6:183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paolucci S, Bragoni M, Coiro P, De Angelis D, Fusco FR, Morelli D, Venturiero V, Pratesi L. Is sex a prognostic factor in stroke rehabilitation? A matched comparison. Stroke. 2006;37:2989–2994. [DOI] [PubMed] [Google Scholar]
- 12.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham Heart Study. Stroke. 2009;40:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34:1581–1585. [DOI] [PubMed] [Google Scholar]
- 14.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun. 2012;38:J282–J291. [DOI] [PubMed] [Google Scholar]
- 16.Rauch U Gender differences in anticoagulation and antithrombotic therapy. Handb Exp Pharmacol. 2012:523–542. [DOI] [PubMed] [Google Scholar]
- 17.Zuem CS, Lindemann S, Gawaz M. Platelet function and response to aspirin: gender-specific features and implications for female thrombotic risk and management. Semin Thromb Hemost. 2009;35:295–306. [DOI] [PubMed] [Google Scholar]
- 18.Haast RA, Gustafson DR, Kiliaan AJ. Sex differences in stroke. J Cereb Blood Flow Metab. 2012;32:2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, Creager MA, Culebras A, Eckel RH, Hart RG, Hinchey JA, Howard VJ, Jauch EC, Levine SR, Meschia JF, Moore WS, Nixon JV, Pearson TA; on behalf of the American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Epidemiology and Prevention, Council for High Blood Pressure Research, Council on Peripheral Vascular Disease, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke. 2011;42:e26]. Stroke. 2011;42:517–584. [DOI] [PubMed] [Google Scholar]
- 20.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D; on behalf of the American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. [DOI] [PubMed] [Google Scholar]
- 21.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Piña IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC Jr, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the American Heart Association [published corrections appear in Circulation. 2011;124:e427 and Circulation. 2011;123:e624]. Circulation. 2011;123:1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006;296:2939–2946. [DOI] [PubMed] [Google Scholar]
- 23.Heuschmann PU, Grieve AP, Toschke AM, Rudd AG, Wolfe CD. Ethnic group disparities in 10-year trends in stroke incidence and vascular risk factors: the South London Stroke Register (SLSR). Stroke. 2008;39:2204–2210. [DOI] [PubMed] [Google Scholar]
- 24.Wieberdink RG, Ikram MA, Hofman A, Koudstaal PJ, Breteler MM. Trends in stroke incidence rates and stroke risk factors in Rotterdam, the Netherlands from 1990 to 2008. Eur J Epidemiol. 2012;27:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sidney S, Rosamond WD, Howard VJ, Luepker RV; National Forum for Heart Disease and Stroke Prevention. The “Heart Disease and Stroke Statistics-2013 Update” and the need for a national cardiovascular surveillance system. Circulation. 2013;127:21–23. [DOI] [PubMed] [Google Scholar]
- 26.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–1090. [DOI] [PubMed] [Google Scholar]
- 27.Thrift AG, Dewey HM, Sturm JW, Srikanth VK, Gilligan AK, Gall SL, Macdonell RA, McNeil JJ, Donnan GA. Incidence of stroke subtypes in the North East Melbourne Stroke Incidence Study (NEMESIS): differences between men and women. Neuroepidemiology. 2009;32:11–18. [DOI] [PubMed] [Google Scholar]
- 28.Sealy-Jefferson S, Wing JJ, Sánchez BN, Brown DL, Meurer WJ, Smith MA, Morgenstern LB, Lisabeth LD. Age- and ethnic-specific sex differences in stroke risk. Gend Med. 2012;9:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howard VJ, Judd SE, Letter AJ, Kleindorfer DO, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman ES, Kissela BM, Howard G. Sex differences in stroke incidence: the REGARDS Study. Circulation 2012;125:AP287. Abstract. [Google Scholar]
- 30.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 31.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268. [DOI] [PubMed] [Google Scholar]
- 32.Shea AM, Reed SD, Curtis LH, Alexander MJ, Villani JJ, Schulman KA. Characteristics of nontraumatic subarachnoid hemorrhage in the United States in 2003. Neurosurgery. 2007;61:1131–1137. [DOI] [PubMed] [Google Scholar]
- 33.Ingall TJ, Whisnant JP, Wiebers DO, O’Fallon WM. Has there been a decline in subarachnoid hemorrhage mortality? Stroke. 1989;20:718–724. [DOI] [PubMed] [Google Scholar]
- 34.Kozák N, Hayashi M. Trends in the incidence of subarachnoid hemorrhage in Akita Prefecture, Japan. J Neurosurg. 2007;106:234–238. [DOI] [PubMed] [Google Scholar]
- 35.Sacco S, Totaro R, Toni D, Marini C, Cerone D, Carolei A. Incidence, case-fatalities and 10-year survival of subarachnoid hemorrhage in a population-based registry. Eur Neurol. 2009;62:155–160. [DOI] [PubMed] [Google Scholar]
- 36.van Munster CE, von und zu Fraunberg M, Rinkel GJ, Rinne J, Koivisto T, Ronkainen A. Differences in aneurysm and patient characteristics between cohorts of Finnish and Dutch patients with subarachnoid hemorrhage: time trends between 1986 and 2005. Stroke. 2008;39:3166–3171. [DOI] [PubMed] [Google Scholar]
- 37.Koffijberg H, Buskens E, Granath F, Adami J, Ekbom A, Rinkel GJ, Blomqvist P. Subarachnoid haemorrhage in Sweden 1987–2002: regional incidence and case fatality rates. J Neurol Neurosurg Psychiatry. 2008;79:294–299. [DOI] [PubMed] [Google Scholar]
- 38.Ostbye T, Levy AR, Mayo NE. Hospitalization and case-fatality rates for subarachnoid hemorrhage in Canada from 1982 through 1991: the Canadian Collaborative Study Group of Stroke Hospitalizations. Stroke. 1997;28:793–798. [DOI] [PubMed] [Google Scholar]
- 39.Haberman S, Capildeo R, Rose FC. Sex differences in the incidence of cerebrovascular disease. J Epidemiol Community Health. 1981;35:45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nilsson OG, Lindgren A, Ståhl N, Brandt L, Saveländ H. Incidence of intracerebral and subarachnoid haemorrhage in southern Sweden. J Neurol Neumsurg Psychiatry. 2000;69:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs BS, Boden-Albala B, Lin IF, Sacco RL. Stroke in the young in the Northern Manhattan Stroke Study. Stroke. 2002;33:2789–2793. [DOI] [PubMed] [Google Scholar]
- 42.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, Redgrave JN, Bull LM, Welch SJ, Cuthbertson FC, Binney LE, Gutnikov SA, Anslow P, Banning AP, Mant D, Mehta Z. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet. 2005;366:1773–1783. [DOI] [PubMed] [Google Scholar]
- 43.Linn FH, Rinkel GJ, Algra A, van Gijn J. Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography: a meta-analysis. Stroke. 1996;27:625–629. [DOI] [PubMed] [Google Scholar]
- 44.de Rooij NK, Linn FH, van der Plas JA, Algra A, Rinkel GJ. Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry. 2007;78:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eden SV, Meurer WJ, Sánchez BN, Lisabeth LD, Smith MA, Brown DL, Morgenstern LB. Gender and ethnic differences in subarachnoid hemorrhage. Neurology. 2008;71:731–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ingall T, Asplund K, Mähönen M, Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke. 2000;31:1054–1061. [DOI] [PubMed] [Google Scholar]
- 47.Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–2065. [DOI] [PubMed] [Google Scholar]
- 48.Kleindorfer D, Broderick J, Khoury J, Flaherty M, Woo D, Alwell K, Moomaw CJ, Schneider A, Miller R, Shukla R, Kissela B. The unchanging incidence and case-fatality of stroke in the 1990s: a population-based study. Stroke. 2006;37:2473–2478. [DOI] [PubMed] [Google Scholar]
- 49.Wardlaw JM, White PM. The detection and management of unruptured intracranial aneurysms. Brain. 2000;123(pt 2):205–221. [DOI] [PubMed] [Google Scholar]
- 50.Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251–256. [DOI] [PubMed] [Google Scholar]
- 51.Lin N, Cahill KS, Frerichs KU, Friedlander RM, Claus EB. Treatment of ruptured and unruptured cerebral aneurysms in the USA: a paradigm shift. J Neurointerv Surg. 2012;4:182–189. [DOI] [PubMed] [Google Scholar]
- 52.Ghods AJ, Lopes D, Chen M. Gender differences in cerebral aneurysm location. Front Neurol. 2012;3:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vlak MH, Rinkel GJ, Greebe P, van der Bom JG, Algra A. Trigger factors for rupture of intracranial aneurysms in relation to patient and aneurysm characteristics. J Neurol. 2012;259:1298–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim YW, Neal D, Hoh BL. Cerebral aneurysms in pregnancy and delivery: pregnancy and delivery do not increase the risk of aneurysm rupture. Neurosurgery. 2013;72:143–149. [DOI] [PubMed] [Google Scholar]
- 55.Schievink WI. Intracranial aneurysms [published correction appears in N Engl J Med. 1997;336:1267]. N E ngl J Med. 1997;336:28–40. [DOI] [PubMed] [Google Scholar]
- 56.Mhurchu CN, Anderson C, Jamrozik K, Hankey G, Dunbabin D; Australasian Cooperative Research on Subarachnoid Hemorrhage Study (ACROSS) Group. Hormonal factors and risk of aneurysmal subarachnoid hemorrhage: an international population-based, case-control study. Stroke. 2001;32:606–612. [DOI] [PubMed] [Google Scholar]
- 57.Schiller JS, Lucas JW, Ward BW, Peregoy JA. Summary health statistics for U.S. adults: National Health Interview Survey, 2010. Vital Health Stat 10. 2012;(252):l–207. [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention (CDC). Prevalence of Stroke-United States, 2006–2010. MMWR Morb Mortal Wkly Rep. 2012;61:379–382. [PubMed] [Google Scholar]
- 59.Murphy SL, Xu JQ, Kochanek KD. Deaths: final data for 2010. National Vital Statistics Report. Vol 61, No 4. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention (CDC). Ten great public health achievements: United States, 1900–1999. MMWR Morb Mortal Wkly Rep. 1999;48:241–243. [PubMed] [Google Scholar]
- 61.Towfighi A, Ovbiagele B, Saver JL. Therapeutic milestone: stroke declines from the second to the third leading organ- and disease-specific cause of death in the United States. Stroke. 2010;41:499–503. [DOI] [PubMed] [Google Scholar]
- 62.Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009;8:345–354. [DOI] [PubMed] [Google Scholar]
- 63.Lewsey JD, Gillies M, Jhund PS, Chalmers JW, Redpath A, Briggs A, Walters M, Langhorne P, Capewell S, McMurray JJ, Macintyre K. Sex differences in incidence, mortality, and survival in individuals with stroke in Scotland, 1986 to 2005. Stroke. 2009;40:1038–1043. [DOI] [PubMed] [Google Scholar]
- 64.Goldacre MJ, Duncan M, Griffith M, Rothwell PM. Mortality rates for stroke in England from 1979 to 2004: trends, diagnostic precision, and artifacts. Stroke. 2008;39:2197–2203. [DOI] [PubMed] [Google Scholar]
- 65.Sarti C, Rastenyte D, Cepaitis Z, Tuomilehto J. International trends in mortality from stroke, 1968 to 1994. Stroke. 2000;31:1588–1601. [DOI] [PubMed] [Google Scholar]
- 66.Vaartjes I, O’Flaherty M, Capewell S, Kappelle J, Bots M. Remarkable decline in ischemic stroke mortality is not matched by changes in incidence. Stroke. 2013;44:591–597. [DOI] [PubMed] [Google Scholar]
- 67.Ayala C, Croft JB, Greenlund KJ, Keenan NL, Donehoo RS, Malarcher AM, Mensah GA. Sex differences in US mortality rates for stroke and stroke subtypes by race/ethnicity and age, 1995–1998. Stroke. 2002;33:1197–1201. [DOI] [PubMed] [Google Scholar]
- 68.Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50:1413–1418. [DOI] [PubMed] [Google Scholar]
- 69.Sarti C, Stegmayr B, Tolonen H, Mahonen M, Tuomilehto J, Asplund K; WHO MONICA Project. Are changes in mortality from stroke caused by changes in stroke event rates or case fatality? Results from the WHO MONICA Project. Stroke. 2003;34:1833–1840. [DOI] [PubMed] [Google Scholar]
- 70.Zhang Y, Galloway JM, Welty TK, Wiebers DO, Whisnant JP, Devereux RB, Kizer JR, Howard BV, Cowan LD,Yeh J, Howard WJ, Wang W, Best L, Lee ET. Incidence and risk factors for stroke in American Indians: the Strong Heart Study. Circulation. 2008;118:1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, Cheung AM; for the Investigators of the Registry of the Canadian Stroke Network. Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke. 2005;36:809–814. [DOI] [PubMed] [Google Scholar]
- 72.Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the International Stroke Trial. Neuroepidemiology. 2005;24:123–128. [DOI] [PubMed] [Google Scholar]
- 73.Olsen TS, Dehlendorff C, Andersen KK. Sex-related time-dependent variations in post-stroke survival: evidence of a female stroke survival advantage. Neuroepidemiology. 2007;29:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pajunen P, Pääkkönen R, Hämäläinen H, Keskimäki I, Laatikainen T, Niemi M, Rintanen H, Salomaa V. Trends in fatal and nonfatal strokes among persons aged 35 to ≥85 years during 1991–2002 in Finland. Stroke. 2005;36:244–248. [DOI] [PubMed] [Google Scholar]
- 75.Moser DK, Kimble LP, Alberts MJ, Alonzo A, Croft JB, Dracup K, Evenson KR, Go AS, Hand MM, Kothari RU, Mensah GA, Morris DL, Pancioli AM, Riegel B, Zerwic JJ. Reducing delay in seeking treatment by patients with acute coronary syndrome and stroke: a scientific statement from the American Heart Association Council on Cardiovascular Nursing and Stroke Council. Circulation. 2006;114:168–182. [DOI] [PubMed] [Google Scholar]
- 76.Menon SC, Pandey DK, Morgenstern LB. Critical factors determining access to acute stroke care. Neurology. 1998;51:427–432. [DOI] [PubMed] [Google Scholar]
- 77.Cheung RT. Hong Kong patients’ knowledge of stroke does not influence time-to-hospital presentation. J Clin Neurosci. 2001;8:311–314. [DOI] [PubMed] [Google Scholar]
- 78.Barr J, McKinley S, O’Brien E, Herkes G. Patient recognition of and response to symptoms of TIA or stroke. Neuroepidemiology. 2006;26:168–175. [DOI] [PubMed] [Google Scholar]
- 79.Mandelzweig L, Goldbourt U, Boyko V, Tanne D. Perceptual, social, and behavioral factors associated with delays in seeking medical care in patients with symptoms of acute stroke. Stroke. 2006;37:1248–1253. [DOI] [PubMed] [Google Scholar]
- 80.Foerch C, Misselwitz B, Humpich M, Steinmetz H, Neumann-Haefelin T, Sitzer M; for the Arbeitsgruppe Schlaganfall Hessen. Sex disparity in the access of elderly patients to acute stroke care. Stroke. 2007;38:2123–2126. [DOI] [PubMed] [Google Scholar]
- 81.Pancioli AM, Broderick J, Kothari R, Brott T, Tuchfarber A, Miller R, Khoury J, Jauch E. Public perception of stroke warning signs and knowledge of potential risk factors. JAMA. 1998;279:1288–1292. [DOI] [PubMed] [Google Scholar]
- 82.Schneider AT, Pancioli AM, Khoury JC, Rademacher E, Tuchfarber A, Miller R, Woo D, Kissela B, Broderick JP. Trends in community knowledge of the warning signs and risk factors for stroke. JAMA. 2003;289:343–346. [DOI] [PubMed] [Google Scholar]
- 83.Reeves MJ, Rafferty AP, Aranha AA, Theisen V. Changes in knowledge of stroke risk factors and warning signs among Michigan adults. Cerebrovasc Dis. 2008;25:385–391. [DOI] [PubMed] [Google Scholar]
- 84.Anderson BE, Rafferty AP, Lyon-Callo S, Fussman C, Reeves MJ. Knowledge of tissue plasminogen activator for acute stroke among Michigan adults. Stroke. 2009;40:2564–2567. [DOI] [PubMed] [Google Scholar]
- 85.Christian AH, Rosamond W, White AR, Mosca L. Nine-year trends and racial and ethnic disparities in women’s awareness of heart disease and stroke: an American Heart Association national study. J Womens Health (Larchmt). 2007;16:68–81. [DOI] [PubMed] [Google Scholar]
- 86.Ferris A, Robertson RM, Fabunmi R, Mosca L. American Heart Association and American Stroke Association national survey of stroke risk awareness among women. Circulation. 2005;111:1321–1326. [DOI] [PubMed] [Google Scholar]
- 87.Deleted in proof.
- 88.Smith DB, Murphy P, Santos P, Phillips M, Wilde M. Gender differences in the Colorado Stroke Registry. Stroke. 2009;40:1078–1081. [DOI] [PubMed] [Google Scholar]
- 89.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D; European BIOMED Study of Stroke Care Group. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34:1114–1119. [DOI] [PubMed] [Google Scholar]
- 90.Förster A, Gass A, Kern R, Wolf ME, Ottomeyer C, Zohsel K, Hennerici M, Szabo K. Gender differences in acute ischemic stroke: etiology, stroke patterns and response to thrombolysis. Stroke. 2009;40:2428–2432. [DOI] [PubMed] [Google Scholar]
- 91.Gray LJ, Sprigg N, Bath PM, Boysen G, De Deyn PP, Leys D, O’Neill D, Ringelstein EB; TAIST Investigators. Sex differences in quality of life in stroke survivors: data from the Tinzaparin in Acute Ischaemic Stroke Trial (TAIST). Stroke. 2007;38:2960–2964. [DOI] [PubMed] [Google Scholar]
- 92.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, Mondo C, Damasceno A, Lopez-Jaramillo P, Hankey GJ, Dans AL, Yusoff K, Truelsen T, Diener HC, Sacco RL, Ryglewicz D, Czlonkowska A, Weimar C, Wang X, Yusuf S; INTERSTROKE Investigators. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. [DOI] [PubMed] [Google Scholar]
- 93.Hsia J, Margolis KL, Eaton CB, Wenger NK, Allison M, Wu L, LaCroix AZ, Black HR; Women’s Health Initiative Investigators. Prehypertension and cardiovascular disease risk in the Women’s Health Initiative. Circulation. 2007; 115:855–860. [DOI] [PubMed] [Google Scholar]
- 94.Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ; ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 95.Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, Mclnnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J; ASCOT Investigators. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. [DOI] [PubMed] [Google Scholar]
- 96.Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C; ONTARGET Investigators. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–1559. [DOI] [PubMed] [Google Scholar]
- 97.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) [published corrections appear in JAMA. 2004;291:2196 and JAMA. 2003;289:178]. JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 98.Dahlöf B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H; LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. [DOI] [PubMed] [Google Scholar]
- 99.Staessen JA, Fagard R, Thijs L, Celis H, Arabidze GG, Birkenhäger WH, Bulpitt CJ, de Leeuw PW, Dollery CT, Fletcher AE, Forette F, Leonetti G, Nachev C, O’Brien ET, Rosenfeld J, Rodicio JL, Tuomilehto J, Zanchetti A. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension: the Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet. 1997;350:757–764. [DOI] [PubMed] [Google Scholar]
- 100.Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, Stoyanovsky V, Antikainen RL, Nikitin Y, Anderson C, Belhani A, Forette F, Rajkumar C, Thijs L, Banya W, Bulpitt CJ; HYVET Study Group. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–1898. [DOI] [PubMed] [Google Scholar]
- 101.Julius S, Kjeldsen SE, Brunner H, Hansson L, Platt F, Ekman S, Laragh JH, Mclnnes G, Schork AM, Smith B, Weber M, Zanchetti A. VALUE trial: long-term blood pressure trends in 13,449 patients with hypertension and high cardiovascular risk. Am. J Hypertens. 2003;16:544–548. [DOI] [PubMed] [Google Scholar]
- 102.Wright JT Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG; African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial [published correction appears in JAMA. 2006;295:2726]. JAMA. 2002;288:2421–2431. [DOI] [PubMed] [Google Scholar]
- 103.Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. [DOI] [PubMed] [Google Scholar]
- 104.Wang JG, Staessen JA, Gong L, Liu L; Systolic Hypertension in China (Syst-China) Collaborative Group. Chinese trial on isolated systolic hypertension in the elderly. Arch Intern Med. 2000;160:211–220. [DOI] [PubMed] [Google Scholar]
- 105.MRC Working Party. Medical Research Council trial of treatment of hypertension in older adults: principal results. BMJ. 1992;304:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534–2544. [DOI] [PubMed] [Google Scholar]
- 107.Neal B, MacMahon S, Chapman N; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of ACE inhibitors, calcium antagonists, and other blood-pressure-lowering drugs: results of prospectively designed overviews of randomised trials. Lancet. 2000;356:1955–1964. [DOI] [PubMed] [Google Scholar]
- 108.Turnbull F, Woodward M, Neal B, Barzi F, Ninomiya T, Chalmers J, Perkovic V, Li N, MacMahon S; Blood Pressure Lowering Treatment Trialists’ Collaboration. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J. 2008;29:2669–2680. [DOI] [PubMed] [Google Scholar]
- 109.Quan A, Kerlikowske K, Gueyffier F, Boissel JP; INDANA Investigators. Pharmacotherapy for hypertension in women of different races. Cochrane Database Syst Rev. 2000;(3):CD002146. [DOI] [PubMed] [Google Scholar]
- 110.Ong KL, Tso AW, Lam KS, Cheung BM. Gender difference in blood pressure control and cardiovascular risk factors in Americans with diagnosed hypertension. Hypertension. 2008;51:1142–1148. [DOI] [PubMed] [Google Scholar]
- 111.Aronow WS, Fleg JL, Pepine CJ, Artinian NT, Bakris G, Brown AS, Ferdinand KC, Forciea MA, Frishman WH, Jaigobin C, Kostis JB, Mancia G, Oparil S, Ortiz E, Reisin E, Rich MW, Schocken DD, Weber MA, Wesley DJ, Harrington RA. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents [published corrections appear in Circulation. 2011;123:e616 and Circulation. 2011;124:el75]. Circulation. 2011;123:2434–2506. [DOI] [PubMed] [Google Scholar]
- 112.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. [DOI] [PubMed] [Google Scholar]
- 113.Gu Q, Burt VL, Paulose-Ram R, Dillon CF. Gender differences in hypertension treatment, drug utilization patterns, and blood pressure control among US adults with hypertension: data from the National Health and Nutrition Examination Survey 1999–2004. Am. J Hypertens. 2008;21:789–798. [DOI] [PubMed] [Google Scholar]
- 114.Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2012;(11):CD002003. [DOI] [PubMed] [Google Scholar]
- 115.Mounier-Vehier C, Simon T, Guedj-Meynier D, Ferrini M, Ghannad E, Hubermann JP, Jullien G, Poncelet P, Achouba A, Quéré S, Guenoun M. Gender-related differences in the management of hypertension by cardiologists: the PARITE study. Arch Cardiovasc Dis. 2012;105:271–280. [DOI] [PubMed] [Google Scholar]
- 116.Wenner MM, Stachenfeld NS. Blood pressure and water regulation: understanding sex hormone effects within and between men and women. J Physiol. 2012;590:5949–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2013;(4):CD004937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rodenburg EM, Strieker BH, Visser LE. Sex-related differences in hospital admissions attributed to adverse drug reactions in the Netherlands. Br J Clin Pharmacol. 2011;71:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharabi Y, Illan R, Kamari Y, Cohen H, Nadler M, Messerli FH, Grossman E. Diuretic induced hyponatraemia in elderly hypertensive women. J Hum Hypertens. 2002;16:631–635. [DOI] [PubMed] [Google Scholar]
- 120.Lewis CE, Grandits A, Flack J, McDonald R, Elmer PJ. Efficacy and tolerance of antihypertensive treatment in men and women with stage 1 diastolic hypertension: results of the Treatment of Mild Hypertension Study. Arch Intern Med. 1996;156:377–385. [PubMed] [Google Scholar]; 120a. Umans JG, Abalos EJ, Lindheimer MD. Antihypertensive Treatment. In: Lindheimer MD, Roberts JM, Cunningham FG, eds. Chesly’s Hypertensive Disorders in Pregnancy. Amsterdam: Academic Press, Elsevier; 2009:369–38. [Google Scholar]
- 121.American College of Obstetricians and Gynecologists. ACOG practice bulletin No. 125: chronic hypertension in pregnancy. Obstet Gynecol. 2012;119:396–407. [DOI] [PubMed] [Google Scholar]
- 122.Lindheimer MD, Taler SJ, Cunningham FG; American Society of Hypertension. ASH position paper: hypertension in pregnancy. J Clin Hypertens (Greenwich). 2009;11:214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lindheimer MD, Taler SJ, Cunningham FG. Hypertension in pregnancy. J Am Soc Hypertens. 2010;4:68–78. [DOI] [PubMed] [Google Scholar]
- 124.Collins R, Chalmers I, Peto R. Antihypertensive treatment in pregnancy. Br Med J (Clin Res Ed). 1985;291:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Duley L, Henderson-Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. 2006;(3):CD001449. [DOI] [PubMed] [Google Scholar]
- 126.Magee LA, Duley L. Oral beta-blockers for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2003;(3):CD002863. [DOI] [PubMed] [Google Scholar]
- 127.Agency for Healthcare Research and Quality (AHRQ). Management of Chronic Hypertension During Pregnancy. Evidence Report/Technology Assessment Number 14. http://archive.ahrq.gov/clinic/epcsums/preg-sum.htm. Accessed June 14, 2013.
- 128.Cockbum J, Moar VA, Ounsted M, Redman CW. Final report of study on hypertension during pregnancy: the effects of specific treatment on the growth and development of the children. Lancet. 1982;1:647–649. [DOI] [PubMed] [Google Scholar]
- 129.Lydakis C, Lip GY, Beevers M, Beevers DG. Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens. 1999;12:541–547. [DOI] [PubMed] [Google Scholar]
- 130.Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol. 1999;93:725–733. [DOI] [PubMed] [Google Scholar]
- 131.Sibai BM. Chronic hypertension in pregnancy. Obstet Gynecol. 2002;100:369–377. [DOI] [PubMed] [Google Scholar]
- 132.Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007:CD002252. [DOI] [PubMed] [Google Scholar]
- 133.Gruppo di Studio Ipertensione in Gravidanza. Nifedipine versus expectant management in mild to moderate hypertension in pregnancy. Br J Obstet Gynaecol. 1998;105:718–722. [DOI] [PubMed] [Google Scholar]
- 134.Sibai BM, Grossman RA, Grossman HG. Effects of diuretics on plasma volume in pregnancies with long-term hypertension. Am J Obstet Gynecol. 1984;150:831–835. [DOI] [PubMed] [Google Scholar]
- 135.Churchill D, Beevers GD, Meher S, Rhodes C. Diuretics for preventing pre-eclampsia. Cochrane Database Syst Rev. 2007;(1):CD004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barr M Jr, Cohen MM Jr. ACE inhibitor fetopathy and hypocalvaria: the kidney-skull connection. Teratology. 1991;44:485–495. [DOI] [PubMed] [Google Scholar]
- 137.Hanssens M, Keirse MJ, Vankelecom F, Van Assche FA. Fetal and neonatal effects of treatment with angiotensin-converting enzyme inhibitors in pregnancy. Obstet Gynecol. 1991;78:128–135. [PubMed] [Google Scholar]
- 138.Buttar HS. An overview of the influence of ACE inhibitors on fetal-placental circulation and perinatal development. Mol Cell Biochem. 1997;176:61–71. [PubMed] [Google Scholar]
- 139.Pryde PG, Sedman AB, Nugent CE, Barr M Jr. Angiotensin-converting enzyme inhibitor fetopathy. J Am Soc Nephrol. 1993;3:1575–1582. [DOI] [PubMed] [Google Scholar]
- 140.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 141.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Simes PA, Sleight P, Viigimaa M, Waeber B, Zannad F. 2013 ESH/ESC guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 142.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report [published correction appears in JAMA. 2003;290:197]. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 143.James AH, Bushnell CD, Jamison MG, Myers ER. Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol. 2005;106:509–516. [DOI] [PubMed] [Google Scholar]
- 144.Lidegaard Ø, Løkkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. N Engl J Med. 2012;366:2257–2266. [DOI] [PubMed] [Google Scholar]
- 145.Bateman BT, Schumacher HC, Bushnell CD, Pile-Spellman J, Simpson LL, Sacco RL, Berman MF. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome [published correction appears in Neurology. 2007;36:1165]. Neurology. 2006;67:424–429. [DOI] [PubMed] [Google Scholar]
- 146.Scott CA, Bewley S, Rudd A, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. Incidence, risk factors, management, and outcomes of stroke in pregnancy. Obstet Gynecol. 2012;120:318–324. [DOI] [PubMed] [Google Scholar]
- 147.Tang CH, Wu CS, Lee TH, Hung ST, Yang CY, Lee CH, Chu PH. Preeclampsia-eclampsia and the risk of stroke among peripartum in Taiwan. Stroke. 2009;40:1162–1168. [DOI] [PubMed] [Google Scholar]
- 148.Sibai B, Dekker G, Kupfermine M. Pre-eclampsia. Lancet. 2005;365:785–799. [DOI] [PubMed] [Google Scholar]
- 149.Garovic VD, August P. Preeclampsia and the future risk of hypertension: the pregnant evidence. Curr Hypertens Rep. 2013;15:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 151.Moser M, Brown CM, Rose CH, Garovic VD. Hypertension in pregnancy: is it time for a new approach to treatment? J Hypertens. 2012;30:1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sidorov EV, Feng W, Caplan LR. Stroke in pregnant and postpartum women. Expert Rev Cardiovasc Ther. 2011;9:1235–1247. [DOI] [PubMed] [Google Scholar]
- 153.Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206:470–475. [DOI] [PubMed] [Google Scholar]
- 154.Kittner SJ, Stern BJ, Feeser BR, Hebel R, Nagey DA, Buchholz DW, Earley CJ, Johnson CJ, Macko RF, Sloan MA, Wityk RJ, Wozniak MA. Pregnancy and the risk of stroke. N Engl J Med. 1996;335:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kuklina EV, Tong X, Bansil P, George MG, Callaghan WM. Trends in pregnancy hospitalizations that included a stroke in the United States from 1994 to 2007: reasons for concern? Stroke. 2011;42:2564–2570. [DOI] [PubMed] [Google Scholar]
- 156.Hofmeyr GJ, Lawrie TA, Atallah AN, Duley L. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst Rev. 2010;(8):CD001059. [DOI] [PubMed] [Google Scholar]
- 157.Duley L, Henderson-Smart DJ, Meher S, King JF. Antiplatelet agents for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659. [DOI] [PubMed] [Google Scholar]
- 158.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. J Clin Endocrinol Metab. 2007;92:3517–3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martin JN Jr, Thigpen BD, Moore RC, Rose CH, Cushman J, May W. Stroke and severe preeclampsia and eclampsia: a paradigm shift focusing on systolic blood pressure. Obstet Gynecol. 2005;105:246–254. [DOI] [PubMed] [Google Scholar]
- 160.von Dadelszen P, Magee LA. Fall in mean arterial pressure and fetal growth restriction in pregnancy hypertension: an updated metaregression analysis. J Obstet Gynaecol Can. 2002;24:941–945. [DOI] [PubMed] [Google Scholar]
- 161.Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S. Management of hypertensive disorders during pregnancy: summary of NICE guidance. BMJ. 2010;341:c2207. [DOI] [PubMed] [Google Scholar]
- 162.Duley L, Gulmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;(11):CD000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Berends AL, de Groot CJ, Sijbrands EJ, Sie MP, Benneheij SH, Pal R, Heydanus R, Oostra BA, van Duijn CM, Steegers EA. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. 2008;51:1034–1041. [DOI] [PubMed] [Google Scholar]
- 164.Sibai BM, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am. J Obstet Gynecol. 1986;155:1011–1016. [DOI] [PubMed] [Google Scholar]
- 165.Nisell H, Lintu H, Lunell NO, Möllerström G, Pettersson E. Blood pressure and renal function seven years after pregnancy complicated by hypertension. Br J Obstet Gynaecol. 1995;102:876–81. [DOI] [PubMed] [Google Scholar]
- 166.North RA, Simmons D, Bamfather D, Upjohn M. What happens to women with preeclampsia? Microalbuminuria and hypertension following preeclampsia. Aust N Z J Obstet Gynaecol. 1996;36:233–238. [DOI] [PubMed] [Google Scholar]
- 167.Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxaemia of pregnancy. Heart. 1997;77:154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marin R, Gorostidi M, Portal CG, Sánchez M, Sánchez E, Alvarez J. Long-term prognosis of hypertension in pregnancy. Hypertens Pregnancy. 2000;19:199–209. [DOI] [PubMed] [Google Scholar]
- 169.Hubel CA, Snaedal S, Ness RB, Weissfeld LA, Geirsson RT, Roberts JM, Arngrimsson R. Dyslipoproteinaemia in postmenopausal women with a history of eclampsia. BJOG. 2000;107:776–784. [DOI] [PubMed] [Google Scholar]
- 170.Wilson BJ, Watson MS, Prescott GJ, Sunderland S, Campbell DM, Hannaford P, Smith WC. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sattar N, Ramsay J, Crawford L, Cheyne H, Greer IA. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension. 2003;42:39–42. [DOI] [PubMed] [Google Scholar]
- 172.Diehl CL, Brost BC, Hogan MC, Elesber AA, Offord KP, Turner ST, Garovic VD. Preeclampsia as a risk factor for cardiovascular disease later in life: validation of a preeclampsia questionnaire. Am J Obstet Gynecol. 2008; 198:e11–e13. [DOI] [PubMed] [Google Scholar]
- 173.Mannistö T, Mendola P, Vääräsmäki M, Järvelin MR, Hartikainen AL, Pouta A, Suvanto E. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127:681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bonamy AK, Parikh NI, Cnattingius S, Ludvigsson JF, Ingelsson E. Birth characteristics and subsequent risks of maternal cardiovascular disease: effects of gestational age and fetal growth. Circulation. 2011;124:2839–2846. [DOI] [PubMed] [Google Scholar]
- 175.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ. 2001;323:1213–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797–1803. [DOI] [PubMed] [Google Scholar]
- 177.Funai EF, Friedlander Y, Paltiel O, Tiram E, Xue X, Deutsch L, Harlap S. Long-term mortality after preeclampsia. Epidemiology. 2005;16:206–215. [DOI] [PubMed] [Google Scholar]
- 178.Kestenbaum B, Seliger SL, Easterling TR, Gillen DL, Critchlow CW, Stehman-Breen CO, Schwartz SM. Cardiovascular and thromboembolic events following hypertensive pregnancy. Am J Kidney Dis. 2003;42:982–989. [DOI] [PubMed] [Google Scholar]
- 179.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53:944–951. [DOI] [PubMed] [Google Scholar]
- 180.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156:918–930. [DOI] [PubMed] [Google Scholar]
- 182.Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ. 2009;181:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Smith GN, Pudwell J, Walker M, Wen SW. Ten-year, thirty-year, and lifetime cardiovascular disease risk estimates following a pregnancy complicated by preeclampsia. J Obstet Gynaecol Can. 2012;34:830–835. [DOI] [PubMed] [Google Scholar]
- 184.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1–19. [DOI] [PubMed] [Google Scholar]
- 185.Ben-Ami S, Oron G, Ben-Haroush A, Blickstein D, Hod M, Bar J. Primary atherothrombotic occlusive vascular events in premenopausal women with history of adverse pregnancy outcome. Thromb Res. 2010;125:124–127. [DOI] [PubMed] [Google Scholar]
- 186.Schausberger CE, Jacobs VR, Bogner G, Wolfrum-Ristau P, Fischer T. Hypertensive disorders of pregnancy: a life-long risk? Geburtsh Frauenheilk. 2013;73:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Aukes AM, De Groot JC, Wiegman MJ, Aamoudse JG, Sanwikarja GS, Zeeman GG. Long-term cerebral imaging after pre-eclampsia. BJOG. 2012;119:1117–1122. [DOI] [PubMed] [Google Scholar]
- 188.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122:579–584. [DOI] [PubMed] [Google Scholar]
- 189.Berks D, Hoedjes M, Raat H, Duvekot J, Steegers E, Habbema J. Risk of cardiovascular disease after pre-eclampsia and the effect of lifestyle interventions: a literature-based study. BJOG. 2013;120:924–931. [DOI] [PubMed] [Google Scholar]
- 190.Young B, Hacker MR, Rana S. Physicians’ knowledge of future vascular disease in women with preeclampsia. Hypertens Pregnancy. 2012:31:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol 2007;6:162–170. [DOI] [PubMed] [Google Scholar]
- 192.Star M, Plaster M. Advances and controversies in the management of cerebral venous thrombosis. Neurol Clin. 2013;31:765–783. [DOI] [PubMed] [Google Scholar]
- 193.Coutinho JM, Ferro JM, Canhao P, Barmagarrementeria F, Cantú C, Bousser MG, Stam J. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40:2356–2361. [DOI] [PubMed] [Google Scholar]
- 194.Ferro JM, Canhão P, Stam J, Bousser MG, Barinagarrementeria F; ISCVT Investigators. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35:664–670. [DOI] [PubMed] [Google Scholar]
- 195.Ferro JM, Correia M, Pontes C, Baptista MV, Pita F; Cerebral Venous Thrombosis Portuguese Collaborative Study Group (Venoport). Cerebral vein and dural sinus thrombosis in Portugal: 1980–1998. Cerebrovasc Dis. 2001;11:177–182. [DOI] [PubMed] [Google Scholar]
- 196.Ferro JM, Lopes MG, Rosas MJ, Ferro MA, Fontes J; Cerebral Venous Thrombosis Portuguese Collaborative Study Group (Venoport). Long-term prognosis of cerebral vein and dural sinus thrombosis, results of the VENOPORT study. Cerebrovasc Dis. 2002;13:272–278. [DOI] [PubMed] [Google Scholar]
- 197.Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121:2740–2746. [DOI] [PubMed] [Google Scholar]
- 198.Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross-sectional study. Stroke. 2012;43:3375–3377. [DOI] [PubMed] [Google Scholar]
- 199.Dentali F, Poli D, Scoditti U, Di Minno MN, De Stefano V, Siragusa S, Kostal M, Palareti G, Sartori MT, Grandone E, Vedovati MC, Ageno W; for the CEVETIS (CErebral VEin Thrombosis International Study) Investigators. Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study [published correction appears in J Thromb Haemost. 2013;11:399]. J Thromb Haemost. 2012;10:1297–1302. [DOI] [PubMed] [Google Scholar]
- 200.Saposnik G, Barinagarrementeria F, Brown RD Jr, Bushnell CD, Cucchiara B, Cushman M, deVeber G, Ferro JM, Tsai FY; on behalf of the American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–1192. [DOI] [PubMed] [Google Scholar]
- 201.deVeber G, Andrew M, Adams C, Bjornson B, Booth F, Buckley DJ, Camfield CS, David M, Humphreys P, Langevin P, MacDonald EA, Gillett J, Meaney B, Shevell M, Sinclair DB, Yager J; Canadian Pediatric Ischemic Stroke Study Group. Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345:417–423. [DOI] [PubMed] [Google Scholar]
- 202.Ferro JM, Canhão P, Bousser MG, Stam J, Barinagarrementeria F; ISCVT Investigators. Cerebral vein and dural sinus thrombosis in elderly patients. Stroke. 2005;36:1927–1932. [DOI] [PubMed] [Google Scholar]
- 203.Martinelli I, Sacchi E, Landi G, Taioli E, Duca F, Mannucci PM. High risk of cerebral-vein thrombosis in carriers of a prothrombin-gene mutation and in users of oral contraceptives. N Engl J Med. 1998;338:1793–1797. [DOI] [PubMed] [Google Scholar]
- 204.Bousser MG, Crassard I. Cerebral venous thrombosis, pregnancy and oral contraceptives. Thromb Res. 2012;130(suppl 1):S19–S22. [DOI] [PubMed] [Google Scholar]
- 205.Ahrens I, Peter K, Lip GY, Bode C. Development and clinical applications of novel oral anticoagulants, part I: clinically approved drugs. Discov Med. 2012;13:433–443. [PubMed] [Google Scholar]
- 206.Lijfering WM, Brouwer JL, Veeger NJ, Bank I, Coppens M, Middeldorp S, Hamulyák K, Prins MH, Buller HR, van der Meer J. Selective testing for thrombophilia in patients with first venous thrombosis: results from a retrospective family cohort study on absolute thrombotic risk for currently known thrombophilic defects in 2479 relatives. Blood. 2009;113:5314–5322. [DOI] [PubMed] [Google Scholar]
- 207.Alshekhlee A, Borhani Haghighi A, Cruz-Flores S. Response to letter by Coutinho et al regarding article, “Mortality of cerebral venous-sinus thrombosis in a large national sample.” Stroke. 2012;43:e23. [DOI] [PubMed] [Google Scholar]
- 208.Borhani Haghighi A, Edgell RC, Cruz-Flores S, Feen E, Piriyawat P, Vora N, Callison RC, Alshekhlee A. Mortality of cerebral venous-sinus thrombosis in a large national sample. Stroke. 2012;43:262–264. [DOI] [PubMed] [Google Scholar]
- 209.Nasr DM, Brinjikji W, Cloft HJ, Saposnik G, Rabinstein AA. Mortality in cerebral venous thrombosis: results from the National Inpatient Sample database. Cerebrovasc Dis. 2013;35:40–44. [DOI] [PubMed] [Google Scholar]
- 210.Lanska DJ, Kryscio RJ. Peripartum stroke and intracranial venous thrombosis in the National Hospital Discharge Survey. Obstet Gynecol. 1997;89:413–418. [DOI] [PubMed] [Google Scholar]
- 211.Wilterdink JL, Easton JD. Cerebral ischemia in pregnancy. Adv Neurol 2002;90:51–62. [PubMed] [Google Scholar]
- 212.Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J; American College of Chest Physicians. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:844S–886S. [DOI] [PubMed] [Google Scholar]
- 213.Ciron J, Godenèche G, Vandamme X, Rosier MP, Sharov I, Mathis S, Larrieu D, Neau JP. Obstetrical outcome of young women with a past history of cerebral venous thrombosis. Cerebrovasc Dis. 2013;36:55–61. [DOI] [PubMed] [Google Scholar]
- 214.Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. Vital Health Stat 23. 2010;(29):l–44. [PubMed] [Google Scholar]
- 215.Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: a meta-analysis. JAMA. 2000;284:72–78. [DOI] [PubMed] [Google Scholar]
- 216.Chan W-S, Ray J, Wai EK, Ginsburg S, Hannah ME, Corey PN, Ginsberg JS. Risk of stroke in women exposed to low-dose oral contraceptives: a critical evaluation of the evidence [published correction appears in Arch Intern Med. 2005;165:2040]. Arch Intern Med. 2004; 164:741–747. [DOI] [PubMed] [Google Scholar]
- 217.Baillargeon JP, McClish DK, Essah PA, Nestler JE. Association between the current use of low-dose oral contraceptives and cardiovascular arterial disease: a meta-analysis. J Clin Endocrinol Metab. 2005;90:3863–3870. [DOI] [PubMed] [Google Scholar]
- 218.Chakhtoura Z, Canonico M, Gompel A, Thalabard JC, Scarabin PY, Plu-Bureau G. Progestogen-only contraceptives and the risk of stroke: a meta-analysis. Stroke. 2009;40:1059–1062. [DOI] [PubMed] [Google Scholar]
- 219.Yang L, Kuper H, Sandin S, Margolis KL, Chen Z, Adami HO, Weiderpass E. Reproductive history, oral contraceptive use, and the risk of ischemic and hemorrhagic stroke in a cohort study of middle-aged Swedish women. Stroke. 2009;40:1050–1058. [DOI] [PubMed] [Google Scholar]
- 220.WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Ischaemic stroke and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348:498–505. [PubMed] [Google Scholar]
- 221.WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Haemorrhagic stroke, overall stroke risk, and combined oral contraceptives: results of an international, multicentre, case-control study. Lancet. 1996;348:505–510. [PubMed] [Google Scholar]
- 222.Wang C, Li Y, Li H, Sun T, Jin G, Sun Z, Zhou J, Ba L, Huang Z, Bai J. Increased risk of stroke in oral contraceptive users carried replicated genetic variants: a population-based case-control study in China. Hum Genet. 2012;131:1337–1344. [DOI] [PubMed] [Google Scholar]
- 223.Chang CL, Donaghy M, Poulter N; World Health Organisation Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Migraine and stroke in young women: case-control study.. BMJ. 1999;318:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kemmeren JM, Tanis BC, van den Bosch MA, Bollen EL, Helmerhorst FM, van der Graaf Y, Rosendaal FR, Algra A. Risk of Arterial Thrombosis in Relation to Oral Contraceptives (RATIO) study: oral contraceptives and the risk of ischemic stroke. Stroke. 2002;33:1202–1208. [DOI] [PubMed] [Google Scholar]
- 225.Slooter AJ, Rosendaal FR, Tanis BC, Kemmeren JM, van der Graaf Y, Algra A. Prothrombotic conditions, oral contraceptives, and the risk of ischemic stroke. J Thromb Haemost. 2005;3:1213–1217. [DOI] [PubMed] [Google Scholar]
- 226.Pruissen DM, Slooter AJ, Rosendaal FR, van der Graaf Y, Algra A. Coagulation factor XIII gene variation, oral contraceptives, and risk of ischemic stroke. Blood. 2008; 111:1282–1286. [DOI] [PubMed] [Google Scholar]
- 227.Urbanus RT, Siegerink B, Roest M, Rosendaal FR, de Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardial infarction and ischaemic stroke in young women in the RATIO study: a case-control study. Lancet Neurol. 2009;8:998–1005. [DOI] [PubMed] [Google Scholar]
- 228.Andersson HM, Siegerink B, Luken BM, Crawley JT, Algra A, Lane DA, Rosendaal FR. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood. 2012;119:1555–1560. [DOI] [PubMed] [Google Scholar]
- 229.Wu O, Robertson L, Twaddle S, Lowe GD, Clark P, Greaves M, Walker ID, Langhorne P, Brenkel I, Regan L, Greer I. Screening for thrombophilia in high-risk situations: systematic review and cost-effectiveness analysis: the Thrombosis: Risk and Economic Assessment of Thrombophilia Screening (TREATS) study. Health Technol Assess. 2006;10:1–110. [DOI] [PubMed] [Google Scholar]
- 230.MacClellan LR, Giles W, Cole J, Wozniak M, Stem B, Mitchell BD, Kittner SJ. Probable migraine with visual aura and risk of ischemic stroke: the Stroke Prevention in Young Women Study. Stroke. 2007;38:2438–2445. [DOI] [PubMed] [Google Scholar]
- 231.Bousser MG, Conard J, Kittner S, de Lignieres B, MacGregor EA, Massiou H, Silberstein SD, Tzourio C; International Headache Society Task Force on Combined Oral Contraceptives & Hormone Replacement Therapy. Recommendations on the risk of ischaemic stroke associated with use of combined oral contraceptives and hormone replacement therapy in women with migraine. Cephalalgia. 2000;20:155–156. [DOI] [PubMed] [Google Scholar]
- 232.Hickson SS, Miles KL, McDonnell BJ, Yasmin, Cockcroft JR, Wilkinson IB, McEniery CM; ENIGMA Study Investigators. Use of the oral contraceptive pill is associated with increased large artery stiffness in young women: the ENIGMA study. J Hypertens. 2011;29:1155–1159. [DOI] [PubMed] [Google Scholar]
- 233.Curtis KM, Mohllajee AP, Martins SL, Peterson HB. Combined oral contraceptive use among women with hypertension: a systematic review. Contraception. 2006;73:179–188. [DOI] [PubMed] [Google Scholar]
- 234.Steenland MW, Zapata LB, Brahmi D, Marchbanks PA, Curtis KM. Appropriate follow up to detect potential adverse events after initiation of select contraceptive methods: a systematic review. Contraception. 2013;87:611–624. [DOI] [PubMed] [Google Scholar]
- 235.Tepper NK, Curtis KM, Steenland MW, Marchbanks PA. Blood pressure measurement prior to initiating hormonal contraception: a systematic review. Contraception. 2013;87:631–638. [DOI] [PubMed] [Google Scholar]
- 236.Heinemann LA, Lewis MA, Spitzer WO, Thorogood M, Guggenmoos-Holzmann I, Bruppacher R; Transnational Research Group on Oral Contraceptives and the Health of Young Women. Thromboembolic stroke in young women: a European case-control study on oral contraceptives. Contraception. 1998;57:29–37. [DOI] [PubMed] [Google Scholar]
- 237.Lisabeth L, Bushnell C. Stroke risk in women: the role of menopause and hormone therapy [published correction appears in Lancet Neurol. 2012;11:125]. Lancet Neurol. 2012;11:82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rocca WA, Grossardt BR, Miller VM, Shuster LT, Brown RD Jr. Premature menopause or early menopause and risk of ischemic stroke. Menopause. 2012;19:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, Rosner B, Stampfer MJ. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159:1061–1066. [DOI] [PubMed] [Google Scholar]
- 240.Choi SH, Lee SM, Kim Y, Choi NK, Cho YJ, Park BJ. Natural menopause and risk of stroke in elderly women. J Korean Med Sci. 2005;20:1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009;40:1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Baba Y, Ishikawa S, Amagi Y, Kayaba K, Gotoh T, Kajii E. Premature menopause is associated with increased risk of cerebral infarction in Japanese women. Menopause. 2010; 17:506–510. [DOI] [PubMed] [Google Scholar]
- 243.de Leciñana MA, Egido JA, Femández C, Martínez-Vila E, Santos S, Morales A, Martínez E, Pareja A, Alvarez-Sabín J, Casado I; PIVE Study Investigators of the Stroke Project of the Spanish Cerebrovascular Diseases Study Group. Risk of ischemic stroke and lifetime estrogen exposure. Neurology. 2007;68:33–38. [DOI] [PubMed] [Google Scholar]
- 244.Rivera CM, Grossardt BR, Rhodes DJ, Brown RD Jr, Roger VL, Melton LJ 3rd, Rocca WA. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, Shoupe D, Berek JS, Hankinson S, Manson JE. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol. 2009;113:1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32:745–750. [DOI] [PubMed] [Google Scholar]
- 247.Jacoby VL, Grady D, Wactawski-Wende J, Manson JE, Allison MA, Kuppermann M, Sarto GE, Robbins J, Phillips L, Martin LW, O’Sullivan MJ, Jackson R, Rodabough RJ, Stefanick ML. Oophorectomy vs ovarian conservation with hysterectomy: cardiovascular disease, hip fracture, and cancer in the Women’s Health Initiative Observational Study. Arch Intern Med. 2011;171:760–768. [DOI] [PubMed] [Google Scholar]
- 248.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, Ernster VL, Cummings SR. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016–1037. [DOI] [PubMed] [Google Scholar]
- 249.Paganini-Hill A Hormone replacement therapy and stroke: risk, protection or no effect? Maturitas. 2001;38:243–261. [DOI] [PubMed] [Google Scholar]
- 250.Simon JA, Hsia J, Cauley JA, Richards C, Harris F, Fong J, Barrett-Connor E, Hulley SB. Postmenopausal hormone therapy and risk of stroke: the Heart and Estrogen-progestin Replacement Study (HERS). Circulation. 2001;103:638–642. [DOI] [PubMed] [Google Scholar]
- 251.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med. 2001;345:1243–1249. [DOI] [PubMed] [Google Scholar]
- 252.Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N; HERS Research Group. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) [published correction appears in JAMA. 2002:288:10641]. JAMA. 2002:288:49–57. [DOI] [PubMed] [Google Scholar]
- 253.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Bizyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S; Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 254.Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Tomer J; WHI Investigators. Effects of conjugated equine estrogen on stroke in the Women’s Health Initiative. Circulation. 2006;113:2425–2434. [DOI] [PubMed] [Google Scholar]
- 255.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 256.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ; WHI Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. [DOI] [PubMed] [Google Scholar]
- 257.Veerus P, Hovi SL, Fischer K, Rahu M, Hokama M, Hemminki E. Results from the Estonian postmenopausal hormone therapy trial [ISRCTN35338757]. Maturitas. 2006;55:162–173. [DOI] [PubMed] [Google Scholar]
- 258.Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, Køber L, Jensen JE. Effect of hormone replacement therapy on cardiovascular events in recently postmenopausal women: randomised trial. BMJ. 2012;345:e6409. [DOI] [PubMed] [Google Scholar]
- 259.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 260.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN 3rd, Assaf AR, J ackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J; WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. [DOI] [PubMed] [Google Scholar]
- 261.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH; Women’s Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in post-menopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. [DOI] [PubMed] [Google Scholar]
- 262.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J; for the Women’s Health Initiative Memory Study. Conjugated equine estrogens and global cognitive function in post-menopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. [DOI] [PubMed] [Google Scholar]
- 263.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, Lane DS, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D; WHIMS Investigators. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–2672. [DOI] [PubMed] [Google Scholar]
- 264.Resnick SM, Espeland MA, Jaramillo SA, Hirsch C, Stefanick ML, Murray AM, Ockene J, Davatzikos C. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Coker LH, Hogan PE, Bryan NR, Kuller LH, Margolis KL, Bettermann K, Wallace RB, Lao Z, Freeman R, Stefanick ML, Shumaker SA. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI Study. Neurology. 2009;72:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Barrett-Connor E, Grady D, Sashegyi A, Anderson PW, Cox DA, Hoszowski K, Rautahaiju P, Harper KD; MORE Investigators (Multiple Outcomes of Raloxifene Evaluation). Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA. 2002:287:847–857. [DOI] [PubMed] [Google Scholar]
- 267.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Komitzer M, McNabb MA, Wenger NK; Raloxifene Use for The Heart (RUTH) Trial Investigators. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. [DOI] [PubMed] [Google Scholar]
- 268.Formoso G, Perrone E, Maltoni S, Balduzzi S, D’Amico R, Bassi C, Basevi V, Marata AM, Magrini N, Maestri E. Short and long term effects of tibolone in postmenopausal women. Cochrane Database Syst Rev. 2012;2:CD008536. [DOI] [PubMed] [Google Scholar]
- 269.Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Bamabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause [published correction appears in JAMA. 2008;299:1426]. JAMA. 2007;297:1465–1477. [DOI] [PubMed] [Google Scholar]
- 270.Grodstein F, Manson JE, Stampfer MJ, Rexrode K. Postmenopausal hormone therapy and stroke: role of time since menopause and age at initiation of hormone therapy. Arch Intern Med. 2008;168:861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: the Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:3–12. [DOI] [PubMed] [Google Scholar]
- 272.Renoux C, Dell’aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. [DOI] [PubMed] [Google Scholar]
- 273.Nelson HD, Walker M, Zakher B, Mitchell J. Menopausal hormone therapy for the primary prevention of chronic conditions: a systematic review to update the U.S. Preventive Services Task Force recommendations. Ann Intern Med. 2012;157:104–113. [DOI] [PubMed] [Google Scholar]
- 274.Henderson VW, Lobo RA. Hormone therapy and the risk of stroke: perspectives 10 years after the Women’s Health Initiative trials. Climacteric. 2012;15:229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2012;7:CD004143. [DOI] [PubMed] [Google Scholar]
- 276.Nastri CO, Lara LA, Ferriani RA, Rosa ESAC, Figueiredo JB, Martins WP. Hormone therapy for sexual function in perimenopausal and post-menopausal women. Cochrane Database Syst Rev. 2013;6:CD009672. [DOI] [PubMed] [Google Scholar]
- 277.Merikangas KR. Contributions of epidemiology to our understanding of migraine. Headache. 2013;53:230–246. [DOI] [PubMed] [Google Scholar]
- 278.Wolff’s Headache and Other Head Pain. 7th ed. Silberstein SD, Lipton RB, Dalessio DJ, eds. New York, NY: Oxford University Press; 2001. [Google Scholar]
- 279.Spector JT, Kahn SR, Jones MR, Jayakumar M, Dalai D, Nazarian S. Migraine headache and ischemic stroke risk: an updated meta-analysis. Am J Med. 2010;123:612–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kurth T, Diener HC. Migraine and stroke: perspectives for stroke physicians. Stroke. 2012;43:3421–3426. [DOI] [PubMed] [Google Scholar]
- 282.Kurth T, Schürks M, Logroscino G, Buring JE. Migraine frequency and risk of cardiovascular disease in women. Neurology. 2009;73:581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Schürks M, Buring JE, Kurth T. Migraine, migraine features, and cardiovascular disease. Headache. 2010;50:1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Rist PM, Buring JE, Kase CS, Schürks M, Kurth T. Migraine and functional outcome from ischemic cerebral events in women. Circulation. 2010;122:2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kurth T, Diener HC, Buring JE. Migraine and cardiovascular disease in women and the role of aspirin: subgroup analyses in the Women’s Health Study. Cephalalgia. 2011;31:1106–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kurth T, Kase CS, Schurks M, Tzourio C, Buring JE. Migraine and risk of haemorrhagic stroke in women: prospective cohort study. BMJ. 2010;341:c3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Bushnell CD, Jamison M, James AH. Migraines during pregnancy linked to stroke and vascular diseases: US population based case-control study. BMJ. 2009;338:b664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Adeney KL, Williams MA, Miller RS, Frederick IO, Sorensen TK, Luthy DA. Risk of preeclampsia in relation to maternal history of migraine headaches. J Matern Fetal Neonatal Med. 2005;18:167–172. [DOI] [PubMed] [Google Scholar]
- 289.Facchinetti F, Allais G, Nappi RE, D’Amico R, Marozio L, Bertozzi L, Ornati A, Benedetto C. Migraine is a risk factor for hypertensive disorders in pregnancy: a prospective cohort study. Cephalalgia. 2009;29:286–292. [DOI] [PubMed] [Google Scholar]
- 290.Sanchez SE, Qiu C, Williams MA, Lam N, Sorensen TK. Headaches and migraines are associated with an increased risk of preeclampsia in Peruvian women. Am. J Hypertens. 2008;21:360–364. [DOI] [PubMed] [Google Scholar]
- 291.Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology [published correction appears in Neurology. 2000;56:142]. Neurology. 2000;55:754–762. [DOI] [PubMed] [Google Scholar]
- 292.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK .Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring). 2008;16:2323–2330. [DOI] [PubMed] [Google Scholar]
- 293.Ford ES, Li C, Zhao G, Tsai J. Trends in obesity and abdominal obesity among adults in the United States from 1999–2008. Int J Obes (Lond). 2011;35:736–743. [DOI] [PubMed] [Google Scholar]
- 294.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. [DOI] [PubMed] [Google Scholar]
- 295.Vague J La differenciation sexuelle, feateur determinant des formes de l’obesite [in French]. Presse Med. 1947;55:339–340. [PubMed] [Google Scholar]
- 296.Towfighi A, Zheng L, Ovbiagele B. Weight of the obesity epidemic: rising stroke rates among middle-aged women in the United States. Stroke. 2010;41:1371–1375. [DOI] [PubMed] [Google Scholar]
- 297.Bazzano LA, Gu D, Whelton MR, Wu X, Chen CS, Duan X, Chen J, Chen JC, He J. Body mass index and risk of stroke among Chinese men and women. Ann Neurol. 2010;67:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Saito I, Iso H, Kokubo Y, Inoue M, Tsugane S. Body mass index, weight change and risk of stroke and stroke subtypes: the Japan Public Health Center-based prospective (JPHC) study. Int J Obes (Lond). 2011;35:283–291. [DOI] [PubMed] [Google Scholar]
- 299.Kurth T, Gaziano JM, Rexrode KM, Kase CS, Cook NR, Manson JE, Buring JE. Prospective study of body mass index and risk of stroke in apparently healthy women. Circulation. 2005;111:1992–1998. [DOI] [PubMed] [Google Scholar]
- 300.Hu G, Tuomilehto J, Silventoinen K, Sarti C, Männistö S, Jousilahti P. Body mass index, waist circumference, and waist-hip ratio on the risk of total and type-specific stroke. Arch Intern Med. 2007;167:1420–1427. [DOI] [PubMed] [Google Scholar]
- 301.Yatsuya H, Folsom AR, Yamagishi K, North KE, Brancati FL, Stevens J; Atherosclerosis Risk in Communities Study Investigators. Race- and sex-specific associations of obesity measures with ischemic stroke incidence in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Stxazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke. 2010;41:e418–e426. [DOI] [PubMed] [Google Scholar]
- 303.Zhang X, Shu XO, Gao YT, Yang G, Li H, Zheng W. General and abdominal adiposity and risk of stroke in Chinese women. Stroke. 2009;40:1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Lu M, Ye W, Adami HO, Weiderpass E. Prospective study of body size and risk for stroke amongst women below age 60. J Intern Med. 2006;260:442–450. [DOI] [PubMed] [Google Scholar]
- 305.Rexrode KM, Hennekens CH, Willett WC, Colditz GA, Stampfer MJ, Rich-Edwards JW, Speizer FE, Manson JE. A prospective study of body mass index, weight change, and risk of stroke in women. JAMA. 1997;277:1539–1545. [DOI] [PubMed] [Google Scholar]
- 306.Wang C, Liu Y,Yang Q, Dai X, Wu S, Wang W, Ji X, Li L, Fang X. Body mass index and risk of total and type-specific stroke in Chinese adults: results from a longitudinal study in China. Int J Stroke. 2013;8:245–250. [DOI] [PubMed] [Google Scholar]
- 307.Winter Y, Rohrmann S, Linseisen J, Lanczik O, Ringleb PA, Hebebrand J, Back T. Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke. 2008;39:3145–3151. [DOI] [PubMed] [Google Scholar]
- 308.Kurth T, Gaziano JM, Berger K, Kase CS, Rexrode KM, Cook NR, Buring JE, Manson JE. Body mass index and the risk of stroke in men. Arch Intern Med. 2002;162:2557–2562. [DOI] [PubMed] [Google Scholar]
- 309.Cornier MA, Després JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, Poirier P; on behalf of the American Heart Association Obesity Committee of the Council on Nutrition; Physical Activity and Metabolism; Council on Arteriosclerosis; Thrombosis and Vascular Biology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing, Council on Epidemiology and Prevention; Council on the Kidney in Cardiovascular Disease, and Stroke Council. Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011;124:1996–2019. [DOI] [PubMed] [Google Scholar]
- 310.Boden-Albala B, Sacco RL, Lee HS, Grahame-Clarke C, Rundek T, Elkind MV, Wright C, Giardina EG, DiTullio MR, Homma S, Paik MC. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke. 2008;39:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wang J, Ruotsalainen S, Moilanen L, Lepistö P, Laakso M, Kuusisto J. The metabolic syndrome predicts incident stroke: a 14-year follow-up study in elderly people in Finland. Stroke. 2008;39:1078–1083. [DOI] [PubMed] [Google Scholar]
- 312.Suk SH, Sacco RL, Boden-Albala B, Cheun JF, Pittman JG, Elkind MS, Paik MC; Northern Manhattan Stroke Study. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study. Stroke. 2003;34:1586–1592. [DOI] [PubMed] [Google Scholar]
- 313.Furukawa Y, Kokubo Y, Okamura T, Watanabe M, Higashiyama A, Ono Y’ Kawanishi K, Okayama A, Date C. The relationship between waist circumference and the risk of stroke and myocardial infarction in a Japanese urban cohort: the Suita study. Stroke. 2010;41:550–553. [DOI] [PubMed] [Google Scholar]
- 314.Milionis HJ, Filippatos TD, Derdemezis CS, Kalantzi KJ, Goudevenos J, Seferiadis K, Mikhailidis DP, Elisaf MS. Excess body weight and risk of first-ever acute ischaemic non-embolic stroke in elderly subjects. Eur J Neurol. 2007;14:762–769. [DOI] [PubMed] [Google Scholar]
- 315.Towfighi A, Ovbiagele B. The impact of body mass index on mortality after stroke. Stroke. 2009;40:2704–2708. [DOI] [PubMed] [Google Scholar]
- 316.Doehner W, Schenkel J, Anker SD, Springer J, Audebert HJ. Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: observations from the TEMPiS trial. Eur Heart J. 2013;34:268–277. [DOI] [PubMed] [Google Scholar]
- 317.Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Rnnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, Satler LF, Lindsay J Jr. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578–584. [DOI] [PubMed] [Google Scholar]
- 318.Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diabetes. 2010;2:180–193. [DOI] [PubMed] [Google Scholar]
- 319.Chen HJ, Bai CH, Yeh WT, Chiu HC, Pan WH. Influence of metabolic syndrome and general obesity on the risk of ischemic stroke. Stroke. 2006;37:1060–1064. [DOI] [PubMed] [Google Scholar]
- 320.Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A, Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. [DOI] [PubMed] [Google Scholar]
- 321.Najarian RM, Sullivan LM, Kannel WB, Wilson PW, D’Agostino RB, Wolf PA. Metabolic syndrome compared with type 2 diabetes mellitus as a risk factor for stroke: the Framingham Offspring Study. Arch Intern Med. 2006;166:106–111. [DOI] [PubMed] [Google Scholar]
- 322.Towfighi A, Ovbiagele B. Metabolic syndrome and stroke. Curr Diab Rep. 2008;8:37–41. [DOI] [PubMed] [Google Scholar]
- 323.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. [DOI] [PubMed] [Google Scholar]
- 324.Takahashi K, Bokura H, Kobayashi S, Iijima K, Nagai A, Yamaguchi S. Metabolic syndrome increases the risk of ischemic stroke in women. Intern Med. 2007;46:643–648. [DOI] [PubMed] [Google Scholar]
- 325.Milionis HJ, Rizos E, Goudevenos J, Seferiadis K, Mikhailidis DP, Elisaf MS. Components of the metabolic syndrome and risk for first-ever acute ischemic nonembolic stroke in elderly subjects. Stroke. 2005;36:1372–1376. [DOI] [PubMed] [Google Scholar]
- 326.Li W, Ma D, Liu M, Liu H, Feng S, Hao Z, Wu B, Zhang S. Association between metabolic syndrome and risk of stroke: a meta-analysis of cohort studies. Cerebrovasc Dis. 2008;25:539–547. [DOI] [PubMed] [Google Scholar]
- 327.Kurth T, Moore SC, Gaziano JM, Kase CS, Stampfer MJ, Berger K, Buring JE. Healthy lifestyle and the risk of stroke in women. Arch Intern Med. 2006;166:1403–1409. [DOI] [PubMed] [Google Scholar]
- 328.Rosito GA, D’Agostino RB, Massaro J, Lipinska I, Mittleman MA, Sutherland P, Wilson PW, Levy D, Muller JE, Tofler GH. Association between obesity and a prothrombotic state: the Framingham Offspring Study. Thromb Haemost. 2004;91:683–689. [DOI] [PubMed] [Google Scholar]
- 329.Wozniak SE, Gee LL, Wachtel MS, Frezza EE. Adipose tissue: the new endocrine organ? A review article. Dig Dis Sci. 2009;54:1847–1856. [DOI] [PubMed] [Google Scholar]
- 330.Bakhai A Adipokines: targeting a root cause of cardiometabolic risk. QJM. 2008;101:767–776. [DOI] [PubMed] [Google Scholar]
- 331.Kraja AT, Province MA, Arnett D, Wagenknecht L, Tang W, Hopkins PN, Djoussé L, Borecki IB. Do inflammation and procoagulation biomarkers contribute to the metabolic syndrome cluster? Nutr Metab (bond). 2007;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.McLaughlin T, Deng A, Gonzales O, Aillaud M, Yee G, Lamendola C, Abbasi F, Connolly AJ, Sherman A, Cushman SW, Reaven G, Tsao PS. Insulin resistance is associated with a modest increase in inflammation in subcutaneous adipose tissue of moderately obese women. Diabetologia. 2008;51:2303–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA, Stewart DJ. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. [DOI] [PubMed] [Google Scholar]
- 334.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002:106:1439–1441. [DOI] [PubMed] [Google Scholar]
- 335.JP Després. Targeting abdominal obesity and the metabolic syndrome to manage cardiovascular disease risk. Heart. 2009;95:1118–1124. [DOI] [PubMed] [Google Scholar]
- 336.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348:2599–2608. [DOI] [PubMed] [Google Scholar]
- 337.Liu S, Manson JE, Lee IM, Cole SR, Hennekens CH, Willett WC, Buring JE. Fruit and vegetable intake and risk of cardiovascular disease: the Women’s Health Study. Am J Clin Nutr. 2000;72:922–928. [DOI] [PubMed] [Google Scholar]
- 338.Huijbregts P, Feskens E, Räsänen L, Fidanza F, Nissinen A, Menotti A, Kromhout D. Dietary pattern and 20 year mortality in elderly men in Finland, Italy, and The Netherlands: longitudinal cohort study. BMJ. 1997;315:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Mensink GB, Deketh M, Mul MD, Schuit AJ, Hoffmeister H. Physical activity and its association with cardiovascular risk factors and mortality. Epidemiology. 1996;7:391–397. [DOI] [PubMed] [Google Scholar]
- 340.Lee IM, Paffenbarger RS Jr. Physical activity and stroke incidence: the Harvard Alumni Health Study. Stroke. 1998;29:2049–2054. [DOI] [PubMed] [Google Scholar]
- 341.Tanasescu M, Leitzmann MF, Rimm EB, Willett WC, Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart disease in men. JAMA. 2002;288:1994–2000. [DOI] [PubMed] [Google Scholar]
- 342.Berlin JA, Colditz GA. A meta-analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol. 1990;132:612–628. [DOI] [PubMed] [Google Scholar]
- 343.Leon AS, Connett J. Physical activity and 10.5 year mortality in the Multiple Risk Factor Intervention Trial (MRFIT). Int J Epidemiol. 1991;20:690–697. [DOI] [PubMed] [Google Scholar]
- 344.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, Hunter DJ, Hennekens CH, Speizer FE. Smoking cessation in relation to total mortality rates in women: a prospective cohort study. Ann Intern Med. 1993;119:992–1000. [DOI] [PubMed] [Google Scholar]
- 345.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Smoking cessation and decreased risk of stroke in women. JAMA. 1993;269:232–236. [PubMed] [Google Scholar]
- 346.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Smoking cessation and time course of decreased risks of coronary heart disease in middle-aged women. Arch Intern Med. 1994;154:169–175. [PubMed] [Google Scholar]
- 347.White IR, Altmann DR, Nanchahal K. Alcohol consumption and mortality: modelling risks for men and women at different ages. BMJ. 2002;325:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Liao Y, McGee DL, Cao G, Cooper RS. Alcohol intake and mortality: findings from the National Health Interview Surveys (1988 and 1990). Am J Epidemiol. 2000;151:651–659. [DOI] [PubMed] [Google Scholar]
- 349.Towfighi A, Markovic D, Ovbiagele B. Impact of a healthy lifestyle on all-cause and cardiovascular mortality after stroke in the USA. J Neurol Neurosurg Psychiatry. 2012;83:146–151. [DOI] [PubMed] [Google Scholar]
- 350.Kontogianni MD, Liatis S, Grammatikou S, Perrea D, Katsilambros N, Makrilakis K. Changes in dietary habits and their association with metabolic markers after a non-intensive, community-based lifestyle intervention to prevent type 2 diabetes, in Greece: the DEPLAN study. Diabetes Res Clin Pract. 2012;95:207–214. [DOI] [PubMed] [Google Scholar]
- 351.Khare MM, Carpenter RA, Huber R, Bates NJ, Cursio JF, Balmer PW, Nolen KN, Hudson H, Shippee SJ, Loo RK. Lifestyle intervention and cardiovascular risk reduction in the Illinois WISEWOMAN Program. J Womens Health (Larchmt). 2012;21:294–301. [DOI] [PubMed] [Google Scholar]
- 352.Costa B, Barrio F, Cabré JJ, Piñol JL, Cos X, Solé C, Bolíbar B, Basora J, Castell C, Solà-Morales O, Salas-Salvadó J, Lindström J, Tuomilehto J. Delaying progression to type 2 diabetes among high-risk Spanish individuals is feasible in real-life primary healthcare settings using intensive lifestyle intervention. Diabetologia. 2012;55:1319–1328. [DOI] [PubMed] [Google Scholar]
- 353.Parekh S, Vandelanotte C, King D, Boyle FM. Design and baseline characteristics of the 10 Small Steps Study: a randomised controlled trial of an intervention to promote healthy behaviour using a lifestyle score and personalised feedback. BMC Public Health. 2012; 12:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Vermunt PW, Milder IE, Wielaard F, de Vries JH, Baan CA, van Oers JA, Westert GP. A lifestyle intervention to reduce type 2 diabetes risk in Dutch primary care: 2.5-year results of a randomized controlled trial. Diabet Med. 2012;29:e223–231. [DOI] [PubMed] [Google Scholar]
- 355.Kanaya AM, Santoyo-Olsson J, Gregorich S, Grossman M, Moore T, Stewart AL. The Live Well, Be Well study: a community-based, translational lifestyle program to lower diabetes risk factors in ethnic minority and lower-socioeconomic status adults. Am J Public Health. 2012;102:1551–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Ratner R, Goldberg R, Haffner S, Marcovina S, Orchard T, Fowler S, Temprosa M; Diabetes Prevention Program Research Group. Impact of intensive lifestyle and metformin therapy on cardiovascular disease risk factors in the Diabetes Prevention Program. Diabetes Care. 2005;28:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Gregg EW, Chen H, Wagenknecht LE, Clark JM, Delahanty LM, Bantle J, Pownall HJ, Johnson KC, Safford MM, Kitabchi AE, Pi-Sunyer FX, Wing RR, Bertoni AG; Look AHEAD Research Group. Association of an intensive lifestyle intervention with remission of type 2 diabetes. JAMA. 2012;308:2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, Lewis CE, Limacher MC, Margolis KL, Mysiw WJ, Ockene JK, Parker LM, Perri MG, Phillips L, Prentice RL, Robbins J, Rossouw JE, Sarto GE, Schatz IJ, Snetselaar LG, Stevens VJ, Tinker LF, Trevisan M, Vitolins MZ, Anderson GL, Assaf AR, Bassford T, Beresford SA, Black HR, Brunner RL, Brzyski RG, Caan B, Chlebowski RT, Gass M, Granek I, Greenland P, Hays J, Heber D, Heiss G, Hendrix SL, Hubbell FA, Johnson KC, Kotchen JM. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–666. [DOI] [PubMed] [Google Scholar]
- 359.Parra-Medina D, Wilcox S, Salinas J, Addy C, Fore E, Poston M, Wilson DK. Results of the Heart Healthy and Ethnically Relevant Lifestyle trial: a cardiovascular risk reduction intervention for African American women attending community health centers. Am J Public Health. 2011;101:1914–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Rankin P, Morton DP, Diehl H, Gobble J, Morey P, Chang E. Effectiveness of a volunteer-delivered lifestyle modification program for reducing cardiovascular disease risk factors. Am J Cardiol. 2012;109:82–86. [DOI] [PubMed] [Google Scholar]
- 361.Ross R, Lam M, Blair SN, Church TS, Godwin M, Hotz SB, Johnson A, Katzmarzyk PT, Lévesque L, MacDonald S. Trial of prevention and reduction of obesity through active living in clinical settings: a randomized controlled trial. Arch Intern Med. 2012;172:414–424. [DOI] [PubMed] [Google Scholar]
- 362.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martinez JA, Martínez-González MA; PREDEMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 363.Towfighi A, Cheng E, Valle N, Vickrey B. HEALS (Healthy Eating And Lifestyle After Stroke): a pilot trial of a multidisciplinary lifestyle intervention program. Clinicaltrials.gov identifier: NCT01550822. http://clinicaltrials.gov/show/NCT01550822. Accessed February 21, 2013.
- 364.Stroke Risk in Atrial Fibrillation Working Group. Comparison of 12 risk stratification schemes to predict stroke in patients with nonvalvular atrial fibrillation. Stroke. 2008;39:1901–1910. [DOI] [PubMed] [Google Scholar]
- 365.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009; 158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Lip GY, Brechin CM, Lane DA. The global burden of atrial fibrillation and stroke: a systematic review of the epidemiology of atrial fibrillation in regions outside North America and Europe. Chest. 2012;142:1489–1498. [DOI] [PubMed] [Google Scholar]
- 367.Shen AY, Contreras R, Sobnosky S, Shah AI, Ichiuji AM, Jorgensen MB, Brar SS, Chen W. Racial/ethnic differences in the prevalence of atrial fibrillation among older adults: a cross-sectional study. J Natl Med Assoc. 2010;102:906–913. [DOI] [PubMed] [Google Scholar]
- 368.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS, Smith SC Jr, Priori SG, Estes NA 3rd, Ezekowitz MD, Jackman WM, January CT, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM, Jacobs AK, Anderson JL, Albert N, Buller CE, Creager MA, Ettinger SM, Guyton RA, Hochman JS, Kushner FG, Ohman EM, Tarkington LG, Yancy CW. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011; 123:e269–e367. [DOI] [PubMed] [Google Scholar]
- 369.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 370.Fonarow GC, Reeves MJ, Zhao X, Olson DM, Smith EE, Saver JL, Schwamm LH; Get With The Guidelines-Stroke Steering Committee and Investigators. Age-related differences in characteristics, performance measures, treatment trends, and outcomes in patients with ischemic stroke. Circulation. 2010;121:879–891. [DOI] [PubMed] [Google Scholar]
- 371.Reeves MJ, Fonarow GC, Zhao X, Smith EE, Schwamm LH; Get With The Guidleines-Stroke Steering Committee and Investigators. Quality of care in women with ischemic stroke in the GWTG program. Stroke. 2009;40:1127–1133. [DOI] [PubMed] [Google Scholar]
- 372.Avgil Tsadok M, Jackevicius CA, Rahme E, Humphries KH, Behlouli H, Pilote L. Sex differences in stroke risk among older patients with recently diagnosed atrial fibrillation. JAMA. 2012;307:1952–1958. [DOI] [PubMed] [Google Scholar]
- 373.Humphries KH, Kerr CR, Connolly SJ, Klein G, Boone JA, Green M, Sheldon R, Talajic M, Dorian P, Newman D. New-onset atrial fibrillation: sex differences in presentation, treatment, and outcome. Circulation. 2001;103:2365–2370. [DOI] [PubMed] [Google Scholar]
- 374.Saposnik G, Cote R, Phillips S, Gubitz G, Bayer N, Minuk J, Black S; Stroke Outcome Research Canada (SORCan) Working Group. Stroke outcome in those over 80: a multicenter cohort study across Canada. Stroke. 2008;39:2310–2317. [DOI] [PubMed] [Google Scholar]
- 375.Singer DE, Chang Y, Fang MC, Borowsky LH, Pomemacki NK, Udaltsova N, Go AS. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Thrift AG, Dewey HM, Macdonell RA, McNeil JJ, Donnan GA. Stroke incidence on the east coast of Australia: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke. 2000;31:2087–2092. [DOI] [PubMed] [Google Scholar]
- 377.Furie KL, Goldstein LB, Albers GW, Khatri P, Neyens R, Turakhia MP, Turan TN, Wood KA; on behalf of the American Heart Association Stroke Council; Council on Quality of Care and Outcomes Research; Council on Cardiovascular Nursing; Council on Clinical Cardiology; Council on Peripheral Vascular Disease. Oral antithrombotic agents for the prevention of stroke in nonvalvular atrial fibrillation: a science advisory for healthcare professionals from the American Heart Association/American Stroke Association [published corrections appear in Stroke. 2013;44:e20 and Stroke. 2012;43:e181]. Stroke. 2012;43:3442–3453. [DOI] [PubMed] [Google Scholar]
- 378.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. [DOI] [PubMed] [Google Scholar]
- 379.Coppens M, Eikelboom JW, Hart RG, Yusuf S, Lip GY, Dorian P, Shestakovska O , Connolly SJ. The CHA2DS2-VASc score identifies those patients with atrial fibrillation and a CHADS2 score of 1 who are unlikely to benefit from oral anticoagulant therapy. Eur Heart J. 2013;34:170–176. [DOI] [PubMed] [Google Scholar]
- 380.Fang MC, Singer DE, Chang Y, Hylek EM, Henault LE, Jensvold NG, Go AS. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. Circulation. 2005;112:1687–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Friberg J, Scharling H, Gadsbøll N, Truelsen T, Jensen GB; Copenhagen City Heart Study. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (The Copenhagen City Heart Study). Am J Cardiol. 2004;94:889–894. [DOI] [PubMed] [Google Scholar]
- 382.Friberg L, Benson L, Rosenqvist M, Lip GY. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Wang TJ, Massaro JM, Levy D, Vasan RS, Wolf PA, D’Agostino RB, Larson MG, Kannel WB, Benjamin EJ. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–1056. [DOI] [PubMed] [Google Scholar]
- 384.Olesen JB, Trop-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0–1: a nationwide cohort study. Thromb Haemost. 2012;107:1172–1179. [DOI] [PubMed] [Google Scholar]
- 385.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 386.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients Atrial Fibrillation) [published correction appears in Circulation. 2007;116:e138]. Circulation. 2006; 114:e257–354. [DOI] [PubMed] [Google Scholar]
- 387.Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC); Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH;. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC): [published correction appears in Europace. 2011; 13:1058]. Europace. 2010;12:1360–1420. [DOI] [PubMed] [Google Scholar]
- 388.Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, McMurtry MS, Mitchell LB, Verma A, Nattel S; Canadian Cardiovascular Society Atrial Fibrillation Guidelines Committee. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control [published correction appears in Can J Cardiol. 2012;28:396]. Can J Cardiol. 2012;28:125–136. [DOI] [PubMed] [Google Scholar]
- 389.Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, Selmer C, Ahlehoff O, Olsen AM, Gislason GH, Torp-Pedersen C. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Sullivan RM, Zhang J, Zamba G, Lip GY, Olshansky B. Relation of gender-specific risk of ischemic stroke in patients with atrial fibrillation to differences in warfarin anticoagulation control (from AFFIRM). Am J Cardiol. 2012;110:1799–1802. [DOI] [PubMed] [Google Scholar]
- 391.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O’Donnell M, Lawrence J, Lewis G, Afzal R, Yusuf S; AVERROES Steering Committee and Investigators. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011. ;364:806–817. [DOI] [PubMed] [Google Scholar]; 391a. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. [DOI] [PubMed] [Google Scholar]
- 392.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation [published correction appears in N Engl J Med. 2010;363:1877]. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 393.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. [DOI] [PubMed] [Google Scholar]
- 394.Stangier J, Stahle H, Rathgen K, Fuhr R. Pharmacokinetics and pharmacodynamics of the direct oral thrombin inhibitor dabigatran in healthy elderly subjects. Clin Pharmacokinet. 2008;47:47–59. [DOI] [PubMed] [Google Scholar]
- 395.Duffull SB, Wright DF, Al-Sallami HS, Zufferey PJ, Faed JM. Dabigatran: rational dose individualisation and monitoring guidance is needed. N Z Med J. 2012; 125:148–154. [PubMed] [Google Scholar]
- 396.PRADAXA [data sheet]. Manukau City, Auckland, New Zealand: Boehringer Ingelheim (N.Z.) Ltd; 2011. [Google Scholar]
- 397.Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:1330–1340. [DOI] [PubMed] [Google Scholar]
- 398.Pan A, Okereke OI, Sun Q, Logroscino G, Manson JE, Willett WC, Ascherio A, Hu FB, Rexrode KM. Depression and incident stroke in women. Stroke. 2011;42:2770–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review [published correction appears in JAMA. 2011;306;2565]. JAMA. 2011;306:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Geller SE, Adams MG, Carnes M. Adherence to federal guidelines for reporting of sex and race/ethnicity in clinical trials. J Womens Health (Larchmt). 2006;15:1123–1131. [DOI] [PubMed] [Google Scholar]
- 401.Goode PS, Fitzgerald MP, Richter HE, Whitehead WE, Nygaard I, Wren PA, Zyczynski HM, Cundiff G, Menefee S, Senka JM, Gao X, Weber AM; Pelvic Floor Disorders Network. Enhancing participation of older women in surgical trials. J Am Coll Surg. 2008;207:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med. 2000;343:475–480. [DOI] [PubMed] [Google Scholar]
- 403.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. [DOI] [PubMed] [Google Scholar]
- 404.Burke JF, Brown DL, Lisabeth LD, Sanchez BN, Morgenstern LB. Enrollment of women and minorities in NINDS trials. Neurology. 2011;76:354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.NIH Policy and Guidelines on the Inclusion of Women and Minorities as Subjects in Clinical Research: Amended, October 2001. National Institutes of Health Web site. http://grants1.nih.gov/grants/funding/women_min/guidelmes_amended_10_2001.htm. Accessed June 14, 2013.
- 406.Ramasubbu K, Gurm H, Litaker D. Gender bias in clinical trials: do double standards still apply? J Womens Health Gend Based Med. 2001;10:757–764. [DOI] [PubMed] [Google Scholar]
- 407.Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, Di Tullio MR, Sanford AR, Mejia V, Gabriel AP, del Valle ML, Buchsbaum R; WARCEF Investigators. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Tsang W, Alter DA, Wijeysundera HC, Zhang T, Ko DT. The impact of cardiovascular disease prevalence on women’s enrollment in landmark randomized cardiovascular trials: a systematic review. J Gen Intern Med. 2012;27:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. [DOI] [PubMed] [Google Scholar]
- 410.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD; North American Symptomatic Carotid Endarterectomy Trial Collaborators. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med. 1998;339:1415–1425. [DOI] [PubMed] [Google Scholar]
- 411.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 412.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- 413.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D; MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial [published correction appears in Lancet. 2004;364:416]. Lancet. 2004;363:1491–1502. [DOI] [PubMed] [Google Scholar]
- 414.Mas JL, Trinquart L, Leys D, Albucher JF, Rousseau H, Viguier A, Bossavy JP, Denis B, Piquet P, Gamier P, Viader F, Touzé E, Julia P, Giroud M, Krause D, Hosseini H, Becquemin JP, Hinzelin G, Houdart E, Hénon H, Neau JP, Bracard S, Onnient Y, Padovani R, Chatellier G; EVA-3S Investigators. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008;7:885–892. [DOI] [PubMed] [Google Scholar]
- 415.Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, Hennerici M, Stingele R, Fiehler J, Zeumer H, Jansen O. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial [published correction appears in Lancet Neurol. 2009;8:135]. Lancet Neurol. 2008;7:893–902. [DOI] [PubMed] [Google Scholar]
- 416.Brott TG, Hobson RW 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, Howard VJ, Moore WS, Voeks JH, Hopkins LN, Cutlip DE, Cohen DJ, Popma JJ, Ferguson RD, Cohen SN, Blackshear JL, Silver FL, Mohr JP, Lai BK, Meschia JF; CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis [published corrections appear in N Engl J Med. 2010;363:198 and N Engl J Med. 2010;363:498]. N Engl J Med. 2010;363:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Taylor DW, Barnett HJ, Haynes RB, Ferguson GG, Sackett DL, Thorpe KE, Simard D, Silver FL, Hachinski V, Clagett GP, Barnes R, Spence JD; for the ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. Lancet. 1999;353:2179–2184. [DOI] [PubMed] [Google Scholar]
- 418.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study 2: dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. [DOI] [PubMed] [Google Scholar]
- 419.CAPRIE Steering Committee. A randomised, blinded, trial of Clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet. 1996:348:1329–1339. [DOI] [PubMed] [Google Scholar]
- 420.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ; MATCH Investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. [DOI] [PubMed] [Google Scholar]
- 421.Gorelick PB, Richardson D, Kelly M, Ruland S, Hung E, Harris Y, Kittner S, Leurgans S; African American Antiplatelet Stroke Prevention Study Investigators. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial. JAMA. 2003;289:2947–2957. [DOI] [PubMed] [Google Scholar]
- 422.Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A; ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial [published correction appears in Lancet. 2007;369:274]. Lancet. 2006;367:1665–1673. [DOI] [PubMed] [Google Scholar]
- 423.Sacco RL, Diener HC, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlöf B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, Vandermaelen C, Voigt T, Weber M, Yoon BW; PRoFESS Study Group. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359:1238–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA; SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Rothwell PM, Slattery J, Warlow CP. Clinical and angiographic predictors of stroke and death from carotid endarterectomy: systematic review. BMJ. 1997;315:1571–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Kandiyil N, Altaf N, Hosseini AA, MacSweeney ST, Auer DP. Lower prevalence of carotid plaque hemorrhage in women, and its mediator effect on sex differences in recurrent cerebrovascular events. PLoS One. 2012;7:e47319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Poisson SN, Johnston SC, Sidney S, Klingman JG, Nguyen-Huynh MN. Gender differences in treatment of severe carotid stenosis after transient ischemic attack. Stroke. 2010;41:1891–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Marquardt L, Fairhead JF, Rothwell PM. Lower rates of intervention for symptomatic carotid stenosis in women than in men reflect differences in disease incidence: a population-based study. Stroke. 2010;41:16–20. [DOI] [PubMed] [Google Scholar]
- 429.International Carotid Stenting Study Investigators; Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, Lo TH, Gaines P, Dorman PJ, Macdonald S, Lyrer PA, Hendriks JM, McCollum C, Nederkoorn PJ, Brown MM. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial [published correction appears in Lancet. 2010;376:90]. Lancet. 2010;375:985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Bonati LH, Fraedrich G; Carotid Stenting Trialists’ Collaboration. Age modifies the relative risk of stenting versus endarterectomy for symptomatic carotid stenosis: a pooled analysis of EVA-3S, SPACE and ICSS. Eur J Vase Endovasc Surg. 2011;41:153–158. [DOI] [PubMed] [Google Scholar]
- 431.Carotid Stenting Trialists’ Collaboration;Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, Fraedrich G, Mali WP, Zeumer H, Brown MM, Mas JL, Ringleb PA. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet. 2010;376:1062–1073. [DOI] [PubMed] [Google Scholar]
- 432.Howard VJ, Lutsep HL, Mackey A, Demaerschalk BM, Sam AD 2nd, Gonzales NR, Sheffet AJ, Voeks JH, Meschia JF, Brott TG; CREST Investigators. Influence of sex on outcomes of stenting versus endarterectomy: a subgroup analysis of the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Lancet Neurol. 2011;10:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Voeks JH, Howard G, Roubin GS, Malas MB, Cohen DJ, Stembergh WC 3rd, Aronow HD, Eskandari MK, Sheffet AJ, Lal BK, Meschia JF, Brott TG; CREST Investigators. Age and outcomes after carotid stenting and endarterectomy: the Carotid Revascularization Endarterectomy Versus Stenting Trial. Stroke. 2011. ;42:3484–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, Hertzberg VS, McIff EB, Moore WS, Panagos PD, Riles TS, Rosenwasser RH, Taylor AJ. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extra-cranial carotid and vertebral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery [published corrections appear in Stroke. 2012;43:e80 and Stroke. 2011;42:e542]. Stroke. 2011;42:e464–e540.21282493 [Google Scholar]
- 435.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. [DOI] [PubMed] [Google Scholar]
- 436.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials [published correction appears in JAMA. 2006;295:2002], JAMA. 2006;295:306–313. [DOI] [PubMed] [Google Scholar]
- 437.Antitbrombotic Trialists’ (ATT) Collaboration; Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.D’Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008; 117:743–753. [DOI] [PubMed] [Google Scholar]
- 439.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score [published correction appears in JAMA. 2007;297:1433]. JAMA. 2007;297:611–619. [DOI] [PubMed] [Google Scholar]
- 440.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication: the Framingham Study. Stroke. 1994;25:40–43. [DOI] [PubMed] [Google Scholar]
- 441.Lumley T, Kronmal RA, Cushman M, Manolio TA, Goldstein S. A stroke prediction score in the elderly: validation and Web-based application. J Clin Epidemiol. 2002;55:129–136. [DOI] [PubMed] [Google Scholar]
- 442.Bineau S, Dufouil C, Helmer C, Ritchie K, Empana JP, Ducimetière P, Alpérovitch A, Bousser MG, Tzourio C. Framingham stroke risk function in a large population-based cohort of elderly people: the 3C study. Stroke. 2009;40:1564–1570. [DOI] [PubMed] [Google Scholar]
- 443.Moons KG, Bots ML, Salonen JT, Elwood PC, Freire de Concalves A, Nikitin Y, Sivenius J, Inzitari D, Benetou V, Tuomilehto J, Koudstaal PJ, Grobbee DE. Prediction of stroke in the general population in Europe (EUROSTROKE): is there a role for fibrinogen and electrocardiography? J Epidemiol Community Health. 2002;56(suppl 1):i30–i36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Tanne D, Yaari S, Goldbourt U. Risk profile and prediction of long-term ischemic stroke mortality: a 21-year follow-up in the Israeli Ischemic Heart Disease (IIHD) Project. Circulation. 1998;98:1365–1371. [DOI] [PubMed] [Google Scholar]
- 445.Chambless LE, Heiss G, Shahar E, Earp MJ, Toole J. Prediction of ischemic stroke risk in the Atherosclerosis Risk in Communities Study [published correction appears in Am J Epidemiol. 2004;160:927]. Am J Epidemiol. 2004; 160:259–269. [DOI] [PubMed] [Google Scholar]
- 446.Seedat S, Scott KM, Angermeyer MC, Berglund P, Bromet EJ, Brugha TS, Demyttenaere K, de Girolamo G, Haro JM, Jin R, Karam EG, Kovess-Masfety V, Levinson D, Medina Mora ME, Ono Y, Ormel J, Pennell BE, Posada-Villa J, Sampson NA, Wiliams D, Kessler RC. Cross-national associations between gender and mental disorders in the World Health Organization World Mental Health Surveys. Arch Gen Psychiatry. 2009;66:785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Cook NR, Paynter NP, Eaton CB, Manson JE, Martin LW, Robinson JG, Rossouw JE, Wassertheil-Smoller S, Ridker PM. Comparison of the Framingham and Reynolds risk scores for global cardiovascular risk prediction in the multiethnic Women’s Health Initiative. Circulation. 2012;125:1748–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Wassertheil-Smoller S, McGinn A, Allison M, Ca T, Curb D, Eaton C, Hendrix S, Kaplan R, Ko M, Martin LW, Xue X. Improvement in stroke risk prediction: role of C-reactive protein and lipoprotein-associated phospholipase A2 in the Women’s Health Initiative. Int J Stroke. October 23, 2012. doi: 10.1111/j.1747-949.2012.00860.x. http://onlinelibrary.wiley.com/doi/10.1111/j.1747-4949.2012.00860.x/abstract. Accessed January 22, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Hermes W, Franx A, van Pampus MG, Bloemenkamp KW, van der Post JA, Porath M, Ponjee G, Tamsma JT, Mol BW, de Groot CJ. 10-Year cardiovascular event risks for women who experienced hypertensive disorders in late pregnancy: the HyRAS study. BMC Pregnancy Childbirth. 2010:10:28. [DOI] [PMC free article] [PubMed] [Google Scholar]


