Abstract
Spinal muscular atrophy is a neurodegenerative disease resulting from irreversible loss of anterior horn cells owing to biallelic deletions/mutations in the survival motor neuron (SMN) 1 gene. Gene replacement therapy using an adeno-associated virus vector containing the SMN gene was approved by the US Food and Drug Administration in May 2019. We report 2 cases of transient, drug-induced liver failure after this therapy.
Spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disease characterized by progressive muscle weakness and atrophy. SMA results from irreversible loss of anterior horn cells in the spinal cord secondary to dysfunction or loss of the survival motor neuron 1 (SMN1) gene and a critical deficiency of SMN protein needed for motor neuron development. The disease has an incidence of 1 in 10 000 live births and is the most common fatal genetic disease of infancy.1–4 SMA displays a range of phenotypic expression patterns that depend on copy numbers of SMN2, a paralogous gene to SMN1 that also encodes for SMN protein, but produces functional protein at much lower levels. Children with SMA type 1 (SMA1), the most severe form of the disease, have hypotonia and severe weakness beginning in early infancy and do not achieve major developmental motor milestones.5,6 The majority of children with SMA1 require feeding and ventilatory support by 14 months of age and have a life expectancy of less than 2 years without intervention.5–9
Initial efforts to increase levels of SMN protein focused on improving the effectiveness of SMN2. In 2016, the US Food and Drug Administration approved nusinersen, an antisense oligonucleotide designed to bind the splicing silencer region on SMN2 pre-mRNA, to increase SMN2 transcription and, in turn, translation of functional SMN protein. Delivery of this therapy requires repeated intrathecal injections.10 Subsequently, onasemnogene abeparvovec-xioi (also known as AVXS-101 in clinical trials) was developed as a 1-time intravenous gene transfer therapy. It delivers a nonintegrating DNA-encoding, fully functional copy of SMN1 into motor neuron cells through the use of self-complementary adeno-associated viral vector (AAV) serotype 9 (AAV9) with a cytomegalovirus (CMV) enhancer/chicken-beta-actin hybrid promoter.11 In 15 children enrolled on the phase I trial of onasemnogene, there was marked improvement in overall survival, motor function, and achievement of motor milestones, compared with natural history controls.12 Elevated serum aminotransferase levels were described in 4 patients, but were reported to be clinically asymptomatic and reversed with oral steroids.12
Since its approval by the US Food and Drug Administration in May 2019, more than 335 children have been treated with onasemnogene commercially or via a managed access program.13 Herein we report 2 cases of subacute pediatric acute liver failure (defined by international normalized ratio [INR] of ≥2.0 or INR of ≥1.5 with encephalopathy) within 8 weeks of receiving AVXS-101.14,15 These cases raise a potential safety concern with this gene therapy and highlight important questions clinicians must anticipate and address about this gene therapy: Can it cause drug-induced liver failure? What is the mechanism of liver injury? How should children be screened before treatment to stratify their risk for developing liver injury? What medications might prevent or minimize liver injury before and after gene therapy?
Case 1
A 6-month-old ex-term male prenatally diagnosed with SMA1 (0 copies of SMN1, 2 copies of SMN2) received 4 doses of nusinersen between the ages of 12 days and 3 months. At 4 months of age after receiving AVXS-101 (33 mL of 1.1 × 1014 vector genomes/kg; 6.25 × 1014 Vg), he had an adverse event characterized by fever and vomiting, requiring hospitalization overnight. He presented 7 weeks after his AVXS-101 treatment with irritability, scleral icterus, and jaundice. He had been off steroids for approximately 1 week at this presentation, having received 1 mg/kg/day of prednisolone 1 day before dosing and for 30 days after the onasemnogene infusion, followed by a 10-day gradual taper off steroids. Of note, his serum aminotransferase levels were elevated before receiving onasemnogene: aspartate aminotransferase (AST) 216 U/L, alanine aminotransferase (ALT) 255 U/L, and gamma glutamyl transferase (GGT) 45 U/L. His total bilirubin before treatment was within normal limits. His creatinine kinase was 334 U/L before the infusion. An anti-AAV9 antibody titer before treatment was <1:25 (negative).
On examination, he was awake but irritable and displayed scleral icterus, jaundice, hypotonia, and head lag. He did not exhibit hepatosplenomegaly, ascites, or bruising. He was noted to be in acute liver failure with an INR of 5.3 (despite vitamin K administration) and elevated ALT of 2014 U/L, AST of 4447 U/L, total bilirubin of 7.6 mg/dL, direct bilirubin of 3.9 mg/dL, alkaline phosphatase of 1074 U/L, lactate dehydrogenase of 3226 U/L, and GGT of 273 U/L. The glucose, albumin, and ammonia levels were normal at 71 mg/dL, 3.7 g/dL, and 44 mmol/L, respectively. Plasma factor levels were consistent with acute liver failure, with low factor V (47%) and VII (7%) levels, and a high factor VIII level (390%). Alpha fetoprotein was markedly elevated at >155 000 ng/mL. The creatinine kinase was only mildly elevated at 222 U/L. Evaluation for infection revealed norovirus in the stool; however, other testing for infectious diseases was negative, including blood and urine cultures, hepatitis A, B, and C serologies, and polymerase chain reaction-based testing for Epstein-Barr virus (EBV), CMV, herpes simplex virus (HSV), and adenovirus. His toxicology screen was negative. A thorough metabolic workup revealed an elevated lactate level of 4 mmol/L. Other testing that was normal included serum acylcarnitine, urine succinylacetone, alpha-1-antitrypsin level, serum amino acids, urine organic acids, oxysterols, congenital disorder of glycosylation testing, and phosphorylase kinase testing. Autoimmune testing was negative, including antinuclear, anti-liver-kidney microsome, and antiactin antibodies. Serum ferritin was elevated (1520 ng/mL) and triglycerides were normal (164 mg/dL). Imaging studies including an abdominal ultrasound examination with Doppler, brain magnetic resonance imaging, and a transthoracic echocardiogram, were normal. An electroencephalogram was normal.
The patient underwent laparoscopic liver biopsy the day after presentation. Pathologic analysis showed massive ballooning degeneration with drop-out of hepatocytes in zone 3, extensive inflammation in the periportal areas (composed mainly of CD8+ T cells, with some neutrophils, eosinophils, and plasma cells), a marked bile ductular reaction with neutrophilic periductular inflammation, and moderate periportal and marked central vein fibrosis (Figure 1). Electron microscopy did not reveal abnormal mitochondria or storage products. Respiratory chain enzyme analysis of the liver tissue showed a low activity of complex II; however, whether this was a primary deficiency or secondary to poor liver function could not be determined. Adenovirus, CMV, EBV, and HSV stains of hepatic tissue were negative. Whole genome sequencing was notable for an incidental finding in the sodium voltage-gated channel alpha subunit 5 (SCN5A) gene, but no pathogenic genetic variants associated with the patient phenotype were identified.
Figure 1.
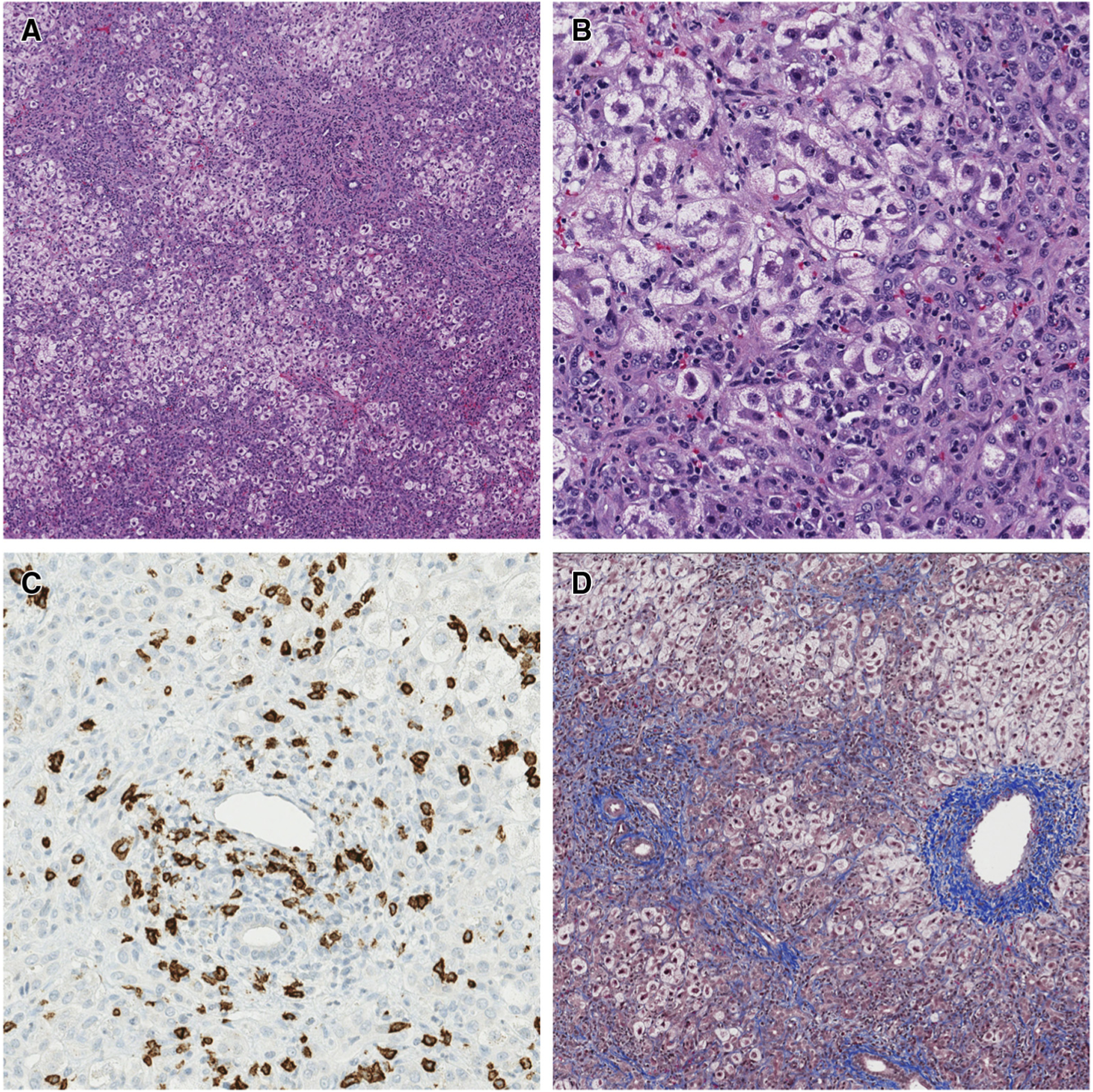
Case 1 liver histology at diagnosis of liver failure. A, Extensive ballooning degeneration of hepatocytes, most prominent in zone 3 (around central veins) (stain: hematoxylin and eosin; original magnification × 40). B, Mixed portal and parenchymal inflammation including neutrophils, eosinophils, lymphocytes, and rare plasma cells. A prominent ductular reaction is also seen (stain: hematoxylin and eosin; original magnification × 200). C, Abundant CD8+ lymphocytes present within the portal tracts (CD8 immunohistochemistry-brown stain; original magnification × 200). D, Moderate amounts of portal, perisinusoidal, and central fibrosis with occasional portal-central bridging (stain: trichrome; original magnification × 40).
Given the active inflammation on liver biopsy and the acute liver failure presentation, intravenous methylprednisone was initiated at 20 mg/kg/day for 3 days followed by a 10-5-4-3-2-1 mg/kg/day taper on hospital days 4–9. The INR and aminotransferases improved and he was discharged home on hospital day 13 on 1 mg/kg/day of oral prednisolone. He was weaned slowly off prednisolone over the course of the year, in concert with normalization of his liver tests. He is now 20 months old and continues to make motor gains. A repeat liver biopsy performed 2 months after his episode of acute liver failure showed resolution of inflammation with persistence of the fibrosis to a similar degree as previously reported.
Case 2
A 20-month old ex term female, diagnosed with SMA1 (0 copies of SMN1, 2 copies of SMN2) at 1 month of age, who had received 8 doses of nusinersen from 6 weeks to 19 months of age, presented with irritability and scleral icterus 8 weeks after receiving an AVXS-101 infusion (57.8 mL of 1.1 × 1014 vector genomes per kilogram of body weight; 11.55 × 1014 Vg). Before receiving AVXS-101, her ALT, AST, GGT, and total bilirubin levels were within normal limits and the anti-AAV9 antibody titer was negative. She had received prednisolone 2 mg/kg/day 1 day before AVXS-101, on the day of infusion, and for 2 days after the infusion, followed by 1 mg/kg/day until day 14 when her prednisolone was increased to 2 mg/kg/day owing to elevated transaminases (ALT 295 U/L, AST 207 U/L, and GGT 62 U/L). Despite the increased dose of prednisolone, her liver enzymes continued to rise. When she presented 8 weeks after infusion with irritability and icterus, her examination revealed mild hepatomegaly. She was noted to be in acute liver failure with an INR of 1.5 (despite vitamin K administration), stage 1–2 encephalopathy, ALT 1634 U/L, AST 1950 U/l, GGT 572 U/L, total bilirubin 3.5 mg/dL, direct bilirubin 2.5 mg/mL, alkaline phosphatase 437 U/L, and ammonia 103 mmol/L. The glucose and albumin were within normal limits at 118 mg/dL and 3.8 g/dL, respectively. A workup for infection was negative, including blood and urine cultures; hepatitis A, B, and C serologies; and polymerase chain reaction-based testing for EBV, CMV, HSV, and adenovirus. Metabolic work-up including acylcarnitine profile, serum amino acids, essential fatty acids, free and total carnitine, and alpha-1-antitrypsin (phenotype and level) were all within normal limits. Autoimmune testing including antinuclear, anti-liver-kidney microsome, antiactin, antineutrophil cytoplasmic, soluble liver, antihistone, and antitissue transglutaminase antibodies were all negative or normal. A transthoracic echocardiogram was normal.
The patient underwent an ultrasound-guided percutaneous liver biopsy the day after admission. Pathologic analysis revealed expanded portal triads and mild interface hepatitis composed mainly of neutrophils and CD8+ T cells, with occasional CD4+ T cells, CD20+ B cells, and eosinophils. Bile ducts had reactive changes with proliferation, confirmed by CK7 staining, with no acute cholangitis. The hepatic lobule had a lymphocytic infiltrate composed primarily of CD8+ T cells. Masson trichrome stain demonstrated mild portal fibrosis with occasional thin fibrous septae extending into the lobule with mild lobular pericellular fibrosis (Figure 2; available at www.jpeds.com). There was diffuse hepatocellular swelling involving the entire lobule with rare ballooning. EBV, CMV, HSV, adenovirus, and human herpes virus-6 staining was negative.
Figure 2.
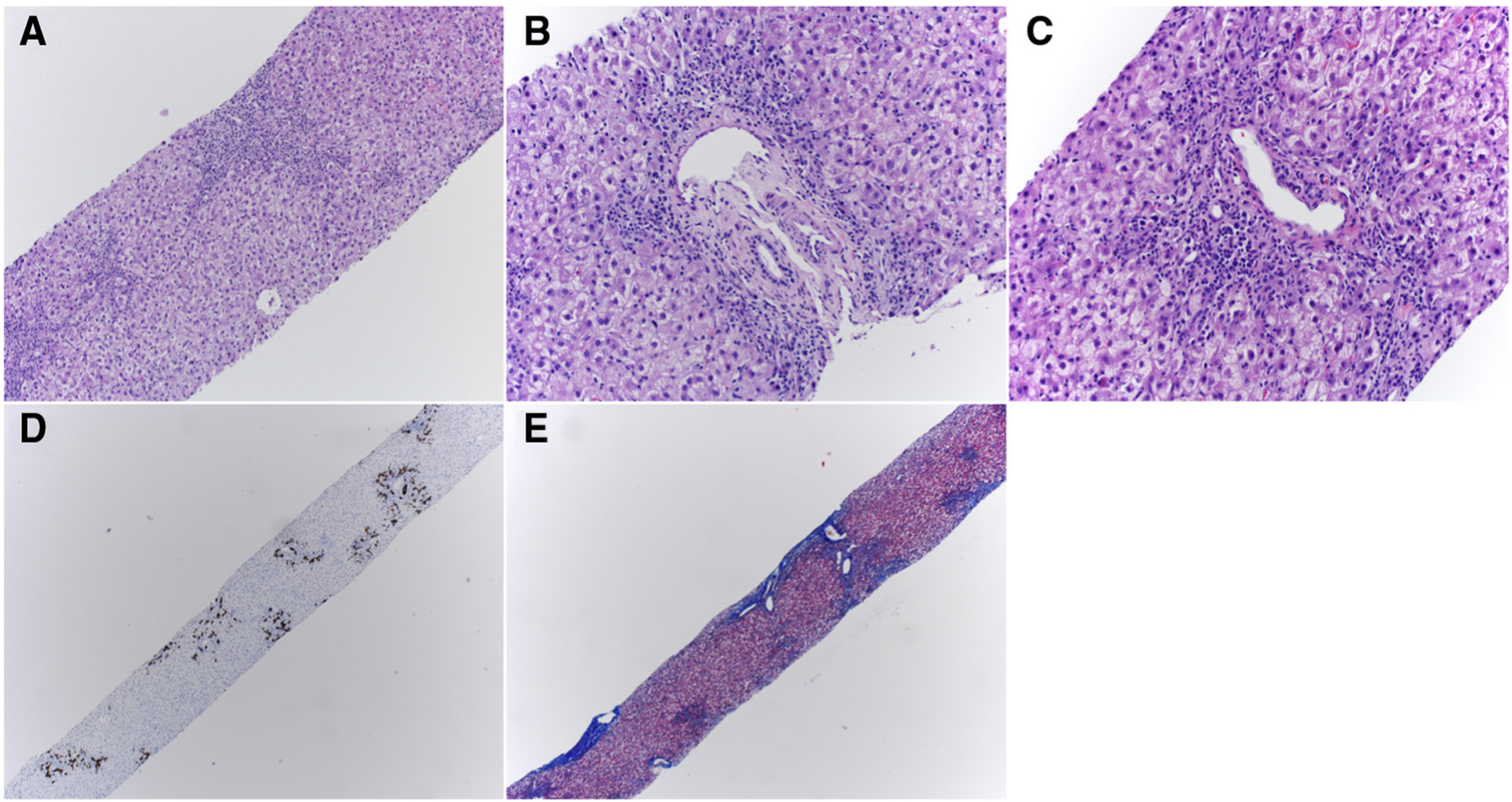
Case 2 liver histology at diagnosis of liver failure. A, Low-power view of extensive ballooning degeneration of hepatocytes and portal tract infiltrates (stain: hematoxylin and eosin; original magnification × 100). B, Mixed portal, and C, paren chymal inflammation including neutrophils, lymphocytes, and macrophages (stain: hematoxylin and eosin; original magnification × 200). D, Cytokeratin 7 immunohistochemistry (brown stain; original magnification × 100) highlighting bile ductular reaction. E, Moderate amounts of portal and central fibrosis with occasional portal-central bridging (stain: trichrome; original magnification × 100).
Given the acute inflammation on liver biopsy and liver failure, intravenous methylprednisolone was initiated at 20 mg/kg/day for 2 days, followed by 10 mg/kg/day for 3 days, and then a 3-day taper to 2 mg/kg/day, during which daily reductions in liver enzyme and INR levels were noted. She was discharged home on hospital day 8 on 2 mg/kg/day of oral prednisolone, which was subsequently weaned off over the next 3.5 months with sustained normalization of her AST, ALT, and GGT. She is now 29 months old and continues to make motor gains.
Discussion
Preclinical studies of SMN1 gene replacement therapy in primate models of SMA initially showed that the therapy was both safe and effective.16,17 However, a recent study of nonhuman primates and piglets who received an AAV vector expressing human SMN showed significant hepatotoxicity.18 All the nonhuman primates, but none of the piglets, developed aminotransferase elevations, and 1 nonhuman primate died from liver failure. The investigators theorized that the hepatotoxicity was secondary to a nonspecific systemic hyperinflammatory reaction. In an open-label trial of 15 children with SMA, 4 of 15 patients displayed elevated serum aminotransferase levels without other liver enzyme abnormalities or clinical symptoms of liver dysfunction.12
We report here 2 cases of children who experienced liver failure within 2 months of onasemnogene treatment. Both children fulfilled common terminology criteria for adverse events grade 3 hepatic failure with mild encephalopathy (inconsolable crying, sleep reversal and/or inattention to task such as poor feeding) and elevations in ALT, AST, GGT, and INR.19,20 Both also fulfilled criteria for drug-induced liver injury using Hy’s law, whereby the drug causes (1) hepatocellular injury (ALT or AST ≥3-fold above the upper limit of normal), (2) elevated serum total bilirubin >2-fold the upper limit of normal, and (3) no known alternative cause of liver damage (such as viral hepatitis, ischemia or another drug capable of causing the observed injury).21,22 It is particularly concerning that both children had an INR of ≥1.5 despite vitamin K administration, suggesting synthetic liver dysfunction. Impaired liver synthetic function signifies severe drug-induced liver injury, which carries a 10%−50% risk of eventual transplantation and/or death.21,23
These 2 cases of liver failure after gene therapy for SMA raise concerns about how this gene therapy might induce hepatocyte damage. A seemingly implausible explanation would be hepatitis from AAV infection alone, because wild-type AAV infection in humans, although common, is not associated with any known illness.24 Furthermore, the onasemnogene AAV9 vector is inactive, and no pathogenicity in humans has been previously described with other various AAV vectors.25 That said, biodistribution analyses performed on postmortem samples obtained from patients that had previously received onasemnogene therapy confirmed the known strong AAV9 tropism for the liver, with the highest concentrations of vector DNA found in the liver specimens of the deceased.26 This liver tropism, accompanied by a compensatory immune response to the viral vector capsid or the transgene housed within, is a feasible mechanism by which liver injury may be preferentially triggered with systemically administered, AAV9-facilitated gene therapy. Regarding inducible immune responses to AAV-facilitated gene therapy, prior exposure to wild-type AAV prompts antigen-specific responses that can effectively neutralize the viral vector, and thus inhibit long-term transgene expression.27 As a surrogate measure of past serotype-specific AAV exposure, individuals who are to receive onasemnogene therapy undergo testing for the presence of circulating anti-AAV9 antibodies, which was reported as negative (<1:25) for the 2 children discussed in this report.
Wild-type AAV and recombinant AAV vectors (as employed by onasemnogene) can activate pattern recognition receptors of the innate immune system, in which the best characterized pattern recognition receptor-AAV interaction has been with the engagement of Toll-like receptor 9 (TLR9).28 Activation of TLR9 results in downstream nuclear factor-κB and interferon regulatory factor signaling that ultimately promotes production of proinflammatory cytokines, such as Interleukin-6 and tumor necrosis factor-α, as well as type I interferons. The initial result of this signaling cascade is a mixed inflammatory infiltrate of neutrophils, macrophages, and natural killer cells that can later stimulate CD8+ T-cell recognition of the viral capsid that was used to introduce the transgene.29 Importantly, TLR9 signaling can be further enhanced by tissue injury, in which DNA extrava-sated from the nucleus of a host cell acts as a damageassociated molecular pattern. AAV vector-based gene therapies for other diseases have shown that AAV vectors can induce cytotoxic CD8+ T cell response to the viral capsid (eg, hemophilia) or can activate the innate immune response via the double-stranded RNA transduction pathway.11,30–39 The time course and hepatic inflammatory infiltrate noted in both cases presented here would favor an AAV vector-induced immune response as the etiology of their liver injury.
Alternatively, or in parallel, the introduction of a transgene that then produces a protein to which the host immune system is na€ıve may result in immunogenicity to the transgene protein as if it were a foreign antigen. This phenomenon is referred to as cross-reactive immunologic material-negative status.40 Although cross-reactive immunologic material-negative status is an important feature for various disease states with null mutations, patients with SMA are exposed to at least some degree of SMN protein through their copy/copies of SMN2.37,41,42 Therefore, a complete cross-reactive immunologic material-negative status is unlikely to be present in the majority of patients with SMA.
Because elevated aminotransferase levels were seen in preclinical trials and in view of these 2 cases of acute liver failure after gene therapy, all future candidate children considering therapy should undergo pretreatment screening for liver disease, in addition to the AAV9 antibody testing currently recommended for previous exposure to wild-type adenovirus. Initial liver test screening should include ALT, AST, GGT, total and direct bilirubin, and INR. Assessment of GGT is important to help differentiate whether the elevated aminotransferase levels are liver (elevated GGT) or muscle (normal GGT) in origin. INR testing is necessary to rule out liver failure. If the liver tests are elevated, then a workup for preexisting chronic liver disease should be performed by a hepatologist before the initiation of gene therapy. This treatment would include alpha-1-antitrypsin level and phenotype, autoantibodies for children >6 months of age (antinuclear, antiactin, anti-liver-kidney microsomal antibody), hepatitis B surface antigen, hepatitis C antibody, and a liver ultrasound examination to screen for anatomic abnormalities and fatty liver disease (Figure 3, A). Both patients with SMA as well as mouse models of SMA have shown an increased susceptibility to dyslipidemia, fatty acid metabolism defects, and associated fatty liver disease.43–46 These could predispose the patient to liver injury after gene therapy. Review of the literature did not reveal any cases of liver failure caused by nusinersen; however, it is possible that the combination of therapies resulted in liver injury and further research is needed to better understand how each therapy alone, and in combination, affects the liver.
Figure 3.
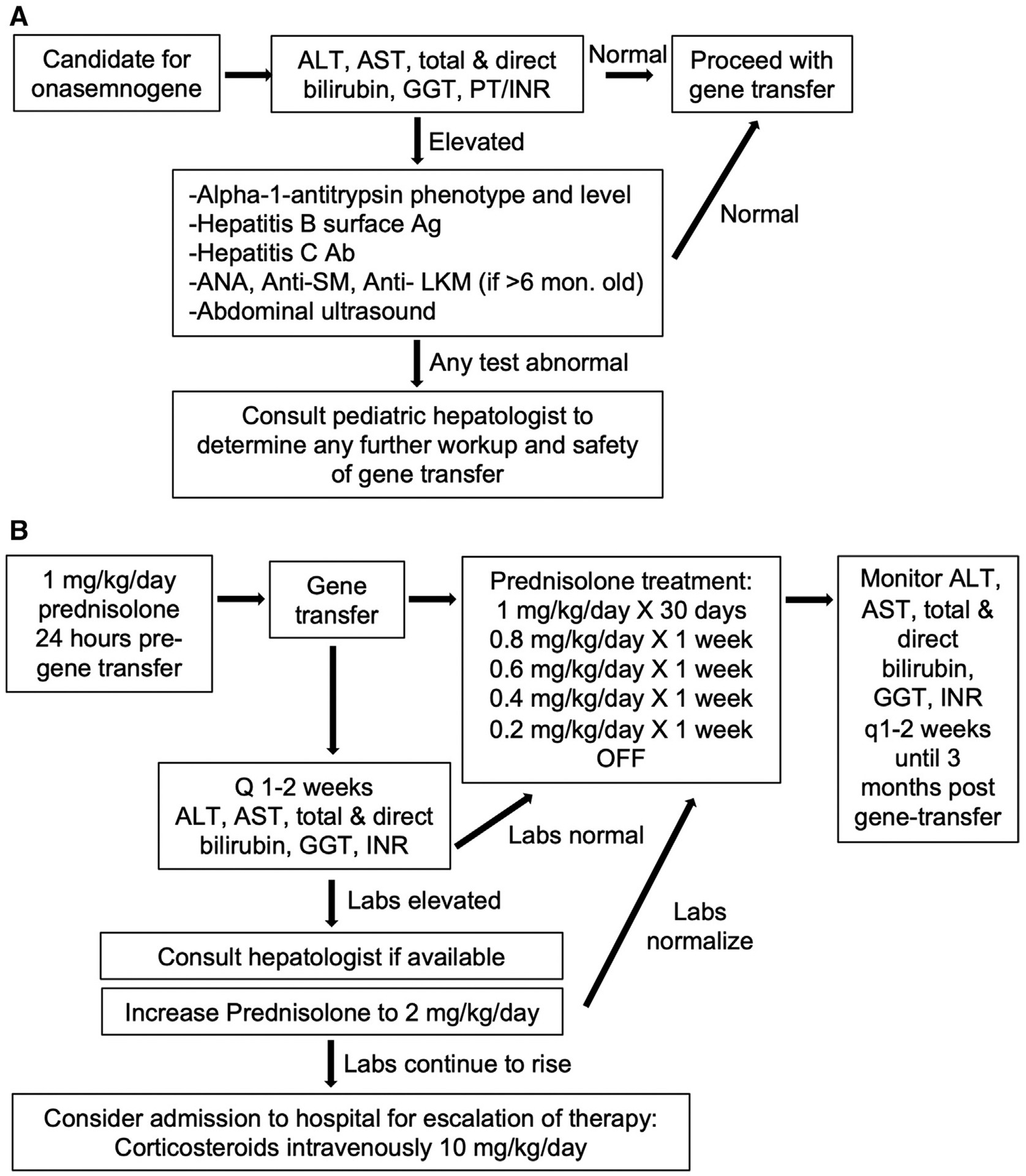
Proposed algorithm for evaluation and treatment of liver disease in association with onasemnogene. A, Pregene therapy evaluation for existing chronic liver disease or predisposition to liver disease. B, Post gene therapy monitoring for liver damage and treatment recommendations if liver damage occurs. Ab, antibody; PT, prothrombin time.
In preclinical trials of onasemnogene, elevated aminotrans-ferases improved with steroid therapy.12 As a result, the package insert for onasemnogene recommends that prednisolone (1 mg/kg/day) should be given in the 24 hours before infusion and should be continued for 30 days after infusion. If liver tests are normal at that time, then steroids can be tapered over the next 28 days, for a total of 2 months of steroid therapy26 (Figure 3, B). However, in both cases described in this report, the children developed liver failure between 3 and 8 weeks after onasemnogene therapy, despite receiving steroids before and after infusion. Both children were treated on a managed access program (before current steroid recommendations were in place), and the first child had a steroid taper that was faster than currently recommended. Therefore, it is necessary to monitor liver tests frequently in the first 2 months after onasemnogene therapy (ie, every 1–2 weeks) and escalate therapy as needed for severe hepatitis or acute liver failure. Management may include increasing the dosing of corticosteroids (ie, initial intravenous doses of 10–20 mg/kg/day) for a few days. If there is no response to high-dose corticosteroids, then consideration should be given to an immunosuppressant that is T-cell targeted (eg, tacrolimus, sirolimus, everolimus, or antithymocyte globulin), based on the observation that the liver histology shows a predominant CD8+ T-cell-mediated pathology. Alternatively, given that proinflammatory cytokines and type I interferon production induced by TLR9 engagement may be implicated in the pathogenesis of liver injury in AAV9-facilitated gene therapy, treatment with anti-IL-6, antitumor necrosis factor-α, or janus kinase inhibition may prove beneficial; however, such warrants further investigation. Conversely, pre-onasemnogene treatment with a TLR9 inhibitor and/or B-cell depletion could be appropriate mechanisms by which deleterious innate and humoral immune responses might be prevented.
Gene therapy using an AAV vector is not unique to SMA. This vector has been used for hemophilia, inherited blindness, and other neurologic diseases, and in the near future will be used for several muscular dystrophies.30,47–53 Although these treatments are exciting and life altering, we must continue to focus on safety and analyze all reports of adverse events, even after drugs come to market. Further work is needed to understand better the immune response in the setting of intravenous AAV vector gene therapy and to learn how to optimally prevent liver injury.
Acknowledgments
Funded by AHRQ K08 HS026510-01A1 (to A.F.). A.V. received personal compensation for serving on advisory boards for AveXis, Biogen, and PTC therapeutics and as speaker for AveXis; served as investigator for AVXS-101 Managed access program; and serves as an Associate Editor for Medlink Neurology. J.P. serves as an investigator/advisor for AveXis, Biogen, Scholar Rock, Sarepta, PTC Therapeutics, and Genentech. The other authors declare no conflicts of interest.
Glossary
- AAV
Adeno-associated viral vector
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- CMV
Cytomegalovirus
- EBV
Epstein-Barr virus
- GGT
Gamma glutamyl transferase
- HSV
Herpes simplex virus
- INR
International normalized ratio
- SMA
Spinal muscular atrophy
- SMA1
SMA type 1
- SMN
Survival motor neuron
- TLR9
Toll-like receptor 9
Footnotes
Portions of this study were presented at the American Academy of Neurology annual meeting, April 14, 2020, and at the Muscular Dystrophy Association clinical and scientific conference, March, 2020.
References
- 1.Sugarman EA, Nagan N, Zhu H, Akmaev VR, Zhou Z, Rohlfs EM, et al. Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72,400 specimens. Eur J Hum Genet 2012;20:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, et al. Diagnosis and management of spinal muscular atrophy: part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord 2018;28:103–15. [DOI] [PubMed] [Google Scholar]
- 3.Verhaart IEC, Robertson A, Wilson IJ, Aartsma-Rus A, Cameron S, Jones CC, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J Rare Dis 2017;12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swoboda KJ, Prior TW, Scott CB, McNaught TP, Wride MC, Reyna SP, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol 2005;57:704–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkel RS, McDermott MP, Kaufmann P, Darras BT, Chung WK, Sproule DM, et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology 2014;83:810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb SJ, Coffey CS, Yankey JW, Krosschell K, Arnold WD, Rutkove SB, et al. Baseline results of the NeuroNEXT spinal muscular atrophy infant biomarker study. Ann Clin Transl Neurol 2016;3:132–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kolb SJ, Coffey CS, Yankey JW, Krosschell K, Arnold WD, Rutkove SB, et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol 2017;82:883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iannaccone ST. Modern management of spinal muscular atrophy. J Child Neurol 2007;22:974–8. [DOI] [PubMed] [Google Scholar]
- 9.Chung BH, Wong VC, Ip P. Spinal muscular atrophy: survival pattern and functional status. Pediatrics 2004;114:e548–53. [DOI] [PubMed] [Google Scholar]
- 10.Chiriboga CA. Nusinersen for the treatment of spinal muscular atrophy. Expert Rev Neurother 2017;17:955–62. [DOI] [PubMed] [Google Scholar]
- 11.Al-Zaidy SA, Mendell JR. From clinical trials to clinical practice: practical considerations for gene replacement therapy in SMA type 1. Pediatr Neurol 2019;100:3–11. [DOI] [PubMed] [Google Scholar]
- 12.Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med 2017;377:1713–22. [DOI] [PubMed] [Google Scholar]
- 13.Zolgensma ® data shows rapid, significant, clinically meaningful benefit in SMA including prolonged event-free survival, motor milestone achievement and durability now up to 5 years post-dosing: Novartis. 2020. www.novartis.com/news/media-releases/zolgensma-data-shows-rapid-significant-clinically-meaningful-benefit-sma-including-prolonged-event-free-survival-motor-milestone-achievement-and-durability-now. Accessed March 24, 2020.
- 14.Squires RH Jr, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, et al. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr 2006;148:652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Grady JG, Schalm SW, Williams R. Acute liver failure: redefining the syndromes. Lancet 1993;342:273–5. [DOI] [PubMed] [Google Scholar]
- 16.Foust KD, Wang X, McGovern VL, Braun L, Bevan AK, Haidet AM, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol 2010;28:271–4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Meyer K, Ferraiuolo L, Schmelzer L, Braun L, McGovern V, Likhite S, et al. Improving single injection CSF delivery of AAV9-mediated gene therapy for SMA: a dose-response study in mice and nonhuman primates. Mol Ther 2015;23:477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinderer C, Katz N, Buza EL, Dyer C, Goode T, Bell P, et al. Severe toxicity in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther 2018;29:285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng VL, Li R, Loomes KM, Leonis MA, Rudnick DA, Belle SH, et al. Outcomes of children with and without hepatic encephalopathy from the Pediatric Acute Liver Failure Study Group. J Pediatr Gastroenterol Nutr 2016;63:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdf. Accessed April 21, 2020.
- 21.Guidance for industry drug-induced liver injury: premarketing clinical evaluation U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER) 2009. www.fda.gov/media/116737/download. Accessed April 21, 2020.
- 22.Reuben A Hy’s law. Hepatology 2004;39:574–8. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman H Drug-Induced Liver Disease. In: Wilkins LW, editor. Hepatotoxicity, the adverse effects of drugs and other chemicals on the liver, 2nd ed. Philadelphia: Lippincott Williams & Wilkins. p. 428–433. [Google Scholar]
- 24.Calcedo R, Morizono H, Wang L, McCarter R, He J, Jones D, et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol 2011;18:1586–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther 2010;21:704–12. [DOI] [PubMed] [Google Scholar]
- 26.Zolgensma Prescribing Information 2019. www.avexis.com/content/pdf/prescribing_information.pdf. Accessed April 20, 2020.
- 27.Li C, Narkbunnam N, Samulski RJ, Asokan A, Hu G, Jacobson LJ, et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther 2012;19:288–94. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Huang X, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest 2009;119:2388–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martino AT, Suzuki M, Markusic DM, Zolotukhin I, Ryals RC, Moghimi B, et al. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood 2011;117:6459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–7. [DOI] [PubMed] [Google Scholar]
- 31.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 2014;371:1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mingozzi F, High KA. Overcoming the host immune response to adeno-associated virus gene delivery vectors: the race between clearance, tolerance, neutralization, and escape. Annu Rev Virol 2017;4:511–34. [DOI] [PubMed] [Google Scholar]
- 34.Finn JD, Hui D, Downey HD, Dunn D, Pien GC, Mingozzi F, et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther 2010;18:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pien GC, Basner-Tschakarjan E, Hui DJ, Mentlik AN, Finn JD, Hasbrouck NC, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest 2009;119:1688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monahan PE, Sun J, Gui T, Hu G, Hannah WB, Wichlan DG, et al. Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial. Hum Gene Ther 2015;26:69–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colella P, Ronzitti G, Mingozzi F. Emerging issues in AAV-mediated in vivo gene therapy. Mol Ther Methods Clin Dev 2018;8:87–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet 2020;21:255–72. [DOI] [PubMed] [Google Scholar]
- 39.Shao W, Earley LF, Chai Z, Chen X, Sun J, He T, et al. Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berrier KL, Kazi ZB, Prater SN, Bali DS, Goldstein J, Stefanescu MC, et al. CRIM-negative infantile Pompe disease: characterization of immune responses in patients treated with ERT monotherapy. Genet Med 2015;17: 912–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson-Jones BJ, Ducore J, et al. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med 2017;377:2215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miesbach W, Meijer K, Coppens M, Kampmann P, Klamroth R, Schutgens R, et al. Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood 2018;131: 1022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deguise MO, Kothary R. New insights into SMA pathogenesis: immune dysfunction and neuroinflammation. Ann Clin Transl Neurol 2017;4: 522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deguise MO, Baranello G, Mastella C, Beauvais A, Michaud J, Leone A, et al. Abnormal fatty acid metabolism is a core component of spinal muscular atrophy. Ann Clin Transl Neurol 2019;6:1519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deguise MO, De Repentigny Y, McFall E, Auclair N, Sad S, Kothary R. Immune dysregulation may contribute to disease pathogenesis in spinal muscular atrophy mice. Hum Mol Genet 2017;26:801–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deguise MO, Chehade L, Tierney A, Beauvais A, Kothary R. Low fat diets increase survival of a mouse model of spinal muscular atrophy. Ann Clin Transl Neurol 2019;6:2340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, et al. AAV5-factor VIII gene transfer in severe hemophilia A. N Engl J Med 2017;377:2519–30. [DOI] [PubMed] [Google Scholar]
- 48.Moore NA, Morral N, Ciulla TA, Bracha P. Gene therapy for inherited retinal and optic nerve degenerations. Expert Opin Biol Ther 2018;18: 37–49. [DOI] [PubMed] [Google Scholar]
- 49.Deverman BE, Ravina BM, Bankiewicz KS, Paul SM, Sah DWY. Gene therapy for neurological disorders: progress and prospects. Nat Rev Drug Discov 2018;17:641–59. [DOI] [PubMed] [Google Scholar]
- 50.Chien YH, Lee NC, Tseng SH, Tai CH, Muramatsu SI, Byrne BJ, et al. Efficacy and safety of AAV2 gene therapy in children with aromatic L-amino acid decarboxylase deficiency: an open-label, phase 1/2 trial. Lancet Child Adolesc Health 2017;1:265–73. [DOI] [PubMed] [Google Scholar]
- 51.Leone P, Shera D, McPhee SW, Francis JS, Kolodny EH, Bilaniuk LT, et al. Long-term follow-up after gene therapy for canavan disease. Sci Transl Med 2012;4:165ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duan D Micro-dystrophin gene therapy goes systemic in Duchenne muscular dystrophy patients. Hum Gene Ther 2018;29:733–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aguti S, Malerba A, Zhou H. The progress of AAV-mediated gene therapy in neuromuscular disorders. Expert Opin Biol Ther 2018;18:681–93. [DOI] [PubMed] [Google Scholar]


