Abstract
The four evolutionary stages have brought us to Industry 4.0. Industry 4.0 is nothing but the 4th Industrial Revolution which will change the production processes. The implementation of Industry 4.0 in the pharmaceutical sector will make the manufacturing of complex drugs easier. The arrival of Industry 4.0 and its advanced technologies such as artificial intelligence (AI), robotics, and the Internet of Things (IoT) makes the processes flexible. Industry 4.0 was introduced to reduce the human workforce and make the complicated processes unchallenging. It is used in all aspects of pharmaceutical sector like analysis, diagnosis, manufacturing, and packaging. The main aim of this paper is to comprehensively elucidate how Industry 4.0 has played a significant role in sustainable development (SD). Industry 4.0 in sustainability decreases the research efforts and examines the research sector’s opportunities. This paper also discusses the impact of Industry 4.0 on sustainable development. Industry 4.0 constructs a bridge between industry and sustainability leading to sustainable development. Sustainability can be achieved by adopting innovative techniques of Industry 4.0 in manufacturing. Moreover, Industry 4.0 provides potential benefits for enhancing pharmaceutical production concerning flexibility, expenses, standards, and safety. It is noticed that Industry 4.0 has a beneficial impact on sustainable development by implementing advanced technologies leading to flexible manufacturing processes.
Keywords: Industry 4.0, Pharmaceutical sector, Sustainable development
Introduction
The pharmaceutical sector is a complicated and determined business model of study, advancement, production, and promotion of recent chemical entities (NCEs) and bioproducts (proteins, peptides, monoclonal antibodies, vaccines, etc.) created to accentuate human well-being (Northrup 2005). “Advanced analytics” which are developed from Industry 4.0 are employed all across the value chain of pharmaceutical companies, including research and development, safety, production, and regulation. The pharmaceutical industry is a crucial segment of the healthcare system, which deals with the assembly and promotion of pharmaceuticals, biological products, and therapeutic devices used to diagnose and treat diseases and conducts research to develop new products for human welfare. So, it would be crucial to keep up the standard of the final products to stop health hazards as many pharmaceutical products are lifesaving (Ugvekar et al. 2021). The pharmaceutical manufacturing unit is a simple precondition for realizing the international organization’s sustainable advancement goals (Lakner et al. 2019). Manufacturing new, effective, and less expensive medications requires to control the population’s well-being, encouragement, abilities, contributing to reducing poverty, a primary sustainability development goal (Lakner et al. 2019). New technologies are essential for water and energy preservation (Argiyantari et al. 2020). Efforts to boost the bargain-basement of medicaments reduce unfairness (Lakner et al. 2019). In the pharmaceutical industry, machine learning has been used for automated quality evaluation of emulsions. The boosting importance of the “green chemistry” approach within the pharmaceutical sector directly contributes to the declining carbon footprint, responsible manufacturing and consumption, and mitigation of temperature change. In summary, it can be concluded that expanding the pharmaceutical sector is a critical element in achieving the United Nation’s sustainable advancement objective (Lakner et al. 2019).
The pharmaceutical sector provides therapeutic agents to treat diseases. It enhances the health of the population. The pharmaceutical industry manufactures new drugs that improve patients’ quality of life worldwide by researching and developing. The pharmaceutical sector is a crucial advantage to worldwide wealth. The pharmaceutical sector frequently attempts to create new therapies that help people live longer and healthier lives. The pharmaceutical sector contributes directly to the world gross domestic product and supports many workers by producing medical products. The pharmaceuticals are used for diagnosing and curing the disease, but sometimes it has an undesirable effect. However, contaminants such as pharmaceuticals in potable water systems have been reported as one of humanity’s fundamental problems (Peake et al. 2016). Recent surveys stated that many pharmaceuticals are partially eradicated at sewage treatment plants, rivaling the levels of some pesticides (Jones et al. 2003). Pharmaceuticals are potentially omnipresent pollutants because they can be found in any environment inhabited by human beings (Jones et al. 2003). Recent investigations stated that drugs are sometimes detected on surface water, groundwater, and drinking water. It can cause a threat to aquatic lives. During the COVID pandemic, the pharmaceutical sector was positively impacted by researching and manufacturing the medications used to treat the COVID-infected patients. Industry 4.0 in the production of vaccine will enhance quality, improve efficacy, and improve compliance with the data-regulated regulatory requirements.
Conventional technology is a three-step conversion process of energies which was used before the era of Industry 4.0. Traditional manufacturing of pharmaceutical processes does not appropriately address the necessity of military and civilian patient populations and healthcare providers because traditional manufacturing is not enough capable of monitoring and controlling automated and complicated manufacturing process to produce personalized products expertly and beneficially. Conventional technologies have reached their limits for material development, including medical applications. The limitations of conventional drug delivery systems are low therapeutic indices and poor water solubility. The advancement of new medications is overpriced, and regular production operations typically occupying batch processing are often ineffective. Pharma 4.0 could benefit the pharmaceutical sector through automatization. Major obstacles faced by the previous technologies or revolutions include overpriced production of new medications, non-transparent processes, and ineffective regular production, leading to batch failures (Steinwandter et al. 2019). The United Kingdom experienced dreadful air pollution from burning coal during the First Industrial Revolution. Even other Industrial Revolutions have led to extreme ecological impacts such as material utilization, waste, and carbon emissions (Chiarini 2021). Conventional pharmaceutical manufacturing, designed in rigid patterns and for limited products, can no longer be adapted to the new manufacturing trend of small batch and multi-variety (Argiyantari et al. 2020). Conventional technology has contributed to the levels of pathogenicity and increased global warming (Dai 2006). However, conventional manufacturing lacks the capability of monitoring and managing automated and complicated production to produce personalized and small-lot products effectively and beneficially (Shi et al. 2020). The limitations of conventional technology can be bypassed through the execution of Industry 4.0 in the pharmaceutical industry. Thus, conventional production cannot cope with the challenges of speedily developing technologies.
Industry 4.0 is the phrase used to express the execution of “smart” devices that can interface autonomously along the value chain (Santos & Lima 2018). In the recent years, the idea of Industry 4.0 portrays the Fourth Industrial Revolution, which is explained as a new extent of association and control over the product lifecycle value chain, with importance on client needs that become more personalized (da Silveira et al. 2019). The companies, the supply chains, the departments, and the resources became more cohesive by implementing Industry 4.0 (Carina Acioli et al. 2021). The insight of Industry 4.0 layouts a new archetype in the manufacturing sector, which is signalized by a new level of sociotechnical interactivity (Cimini et al. 2019). Industry 4.0 contributes to sustainable development by achieving paradigms for the circular economy and enabling new sustainable business models. In health, the idea of Industry 4.0 comprises the digitalization of clinical, therapeutic, and research lab data implementing the computerization of various hand-operated processes used in hospitals and general health conditions (da Silveira et al. 2019). The effective use of Industry 4.0 tools and technology should provide chances to manage huge amounts of data. In contrast, the ongoing Fourth Industrial Revolution requires industries to act self-sustaining without an apparent demand for increased productivity from the market side (Prajwal et al. 2020a). It is broadly accepted that techniques related to Industry 4.0 will notably influence current manufacturing and establishment of future industries (Santos & Lima 2018). The term “Industry 4.0” is frequently used to refer to the digital manufacturing idea. The beginning of Industry 4.0 has received the spectacular attention of the business and study community. Experts assess that Industry 4.0 and correlated programs alongside this line will significantly influence social life.
Advanced technologies such as artificial intelligence (AI) and the Internet of Things (IoT) are used in the pharmaceutical sector. Nowadays, the use of AI in the pharmaceutical industry has increased and is expected to grow. AI can save time and money while providing better outcomes to the formulations. AI duplicates human intelligence in machines. Over the last few years, the use of AI in the pharmaceutical industry has redefined how researchers develop new drugs (Thuemmler and Bai 2017). AI can be applied mainly in researching and developing crucial, life-changing medications. AI has been speculated to play a significant role in radiology and radiotherapy. AI can influence almost every aspect of clinical trials, including patient satisfaction, observing drug adherence, medical record sharing, analysis, and design (Henstock 2020). IoT is an intelligent network that decreases a person’s labor and uses a human-computerization system. IoT comprises hardware and software architecture. IoT plays a revolutionary role in the field of the pharmaceutical sector. The blueprint of the pharmaceutical industry with IoT is crucial in effortless conduction (Thuemmler and Bai 2017). IoT makes it unchallenging to connect with distinct people and supply chain processes into a single network with increased ability and decreased costs with protection and security. IoT provides healthcare services in the field of the pharmaceutical sector. In diagnostic-based therapies, the production of drugs for small-scale manufacturing uses advanced technology (e.g., AI and IoT).
Role of Industry 4.0 in the pharmaceutical sector
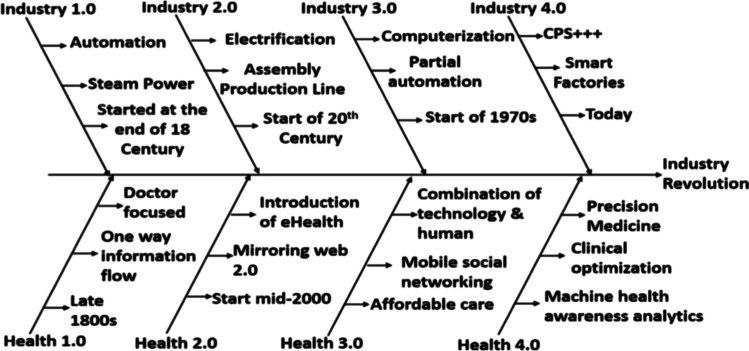
The four transformative stages of manufacturing have brought us to Pharma 4.0. Pharma 4.0 is the upgradation of the pharmaceutical industry to incorporate advanced technologies and digital strategies. Adoption of ideas of Industry 4.0 in the pharmaceutical sector could resolve hurdles faced by the pharmaceutical sector. The role of Industry 4.0 in the pharmaceutical sector is to design and manufacture innovational and customized products as per the varying customer taste and demands within no time, economically, and efficiently. The sum of the International Council of Harmonisation (ICH) and Industry 4.0 equals the Pharma 4.0 operating model. Industry 4.0 in the pharmaceutical sector has let the pharmacy industries relish competitive advantages (Ding 2018; Lakner et al. 2019; Scherer 2000). Industry 4.0 holds the potential to generate positive sustainability influence along the whole value chain through a rise in efficiency by facilitating circular economy solutions, enabling transparency, and the ability to trace through the customization of products. Implementation of Industry 4.0 plays a major role in the modernization of the pharmaceutical and biopharmaceutical sectors. Industry 4.0 can decrease the ecological effect of a product, a process, or a process based on footprint information accessibility and traceable study (Rezeoa 2021). Industry 4.0 is the application of flexible automation, cyber-physical systems, IoT, cognitive robotics, extensive data computer modeling, and 3D printing. The four evolutionary manufacturing stages have brought us to Pharma 4.0 (Fig. 1).
Fig. 1.
Industrial revolution from Industry 1.0 to Industry 4.0 along with health revolution from Health 1.0 to Health 4.0
The four generations of the industrial revolutions are mechanization, automation, computerization, and informatization depicted in Fig. 1 (Sisodia & Jindal 2021). The First Industrial Revolution started with steam power on mechanization. The evolution of the Second Industrial Revolution is mass production. The Third Industrial Revolution, i.e., Industry 3.0, is completely focused on automation. The Fourth Industrial Revolution, i.e., Industry 4.0, in the pharmaceutical sector is known as Pharma 4.0 (Prajwal et al. 2020b).
As Industry 4.0 came into existence, the market demands were much more intensive and effortful (Wan et al. 2019). An essential element of Pharma 4.0, i.e., digitalization, will connect everything, building new stages of accuracy and adjustability for a digitalized plant floor. Industry 4.0 was proposed to improve production to realize short product life cycles and maximum mass customization cost-effectively. Health 4.0 includes the objectives of Industry 4.0 for the digitization of labs and to apply automation in several techniques used in general health care and hospitals (da Silveira et al. 2019). Industry 4.0 in the pharma sector provides better instruments for product protection and supply chain security. Pharma 4.0 may support increased production, customized medications, additive manufacturing, localized 3D printing of therapies, and even a future where human beings are no longer confidentially engaged with manufacturing. Pharma 4.0 represents a modification of the pharmaceutical industry, which involves collecting and studying data through “machines,” enabling more adaptable and effective processes (Ding 2018). Industry 4.0 offers potential benefits for improving pharmaceutical production concerning flexibility, expenses, standards, and safety (Barenji et al. 2019; Mostofi & Jain 2021; Reinhardt et al. 2021).
Industry 4.0 and digital transformation promise to enhance capability and upgrade medication production processes. In the recent past, the technologies of Industry 4.0 have been used in the pharmaceutical sector and have been rigidly controlled by numerous shareholders to ensure safety and secure the well-being of the whole society (Arden et al. 2021; Steinwandter et al. 2019) Pharma 4.0 is powered by processing massive information, interconnectivity, cooperative robotics, AI, and distributed cloud service-based architectures. Industry 4.0 in the pharma sector helps leverage greater efficiency of functions, e.g., a decrease in resource utilization. Health 4.0 is used to improve the capability of medical practitioners by intensifying their speed for analyzing patient data and enabling them to optimize the resources so that patients’ health can be improved. Industry 4.0 enhances the production flows and optimizes the whole value chain or all life cycle stages of products from product concept to its advancement, manufacturing, utilization, maintenance, and recycling. Industry 4.0 represents a smart production networking where instruments and products link without the involvement of humans. Moreover, higher data density decreases productivity and inspires the pharmaceutical sector to produce more modified and customized medicinal products suitable for individualized medication treatment instead of the current “one-size-fits-all” approach (Barenji et al. 2019; Chiacchio et al. 2019; Ding 2018). Execution of the technologies and objectives of Industry 4.0 in the biopharmaceutical sector offers opportunities for important gains in biomedicines’ advancement, manufacturing, and availability. Industry 4.0 is a strategy aiming to build a connection between manufacturing apparatus and products based on hyper-connected technology and combining the overall manufacturing processes (Shi et al. 2020).
Transforming the current pharmaceutical sector to Pharma 4.0 requires a new production and process data capture approach. The turning up technologies of Industry 4.0 promote sustainable value creation and lead to a more energetic, smart, and customized pharma industry, thereby enabling pharmaceutical industries to gain competitive advantages (Ding 2018). Integrating Industry 4.0 elements will also improve the pharmaceutical manufacturing plant into a “reconfigurable factory,” which may provide mass customization of personalized medications for distinct demands. The innovations of Industry 4.0 will help pharma industries fulfill more complicated standards and necessities for products (Reinhardt et al. 2021). The hypothesis elements of Industry 4.0 are expected to enable a series of technological revolutions, incremental advancements, and practical solutions for direct implementation in the management of biomedicines’ manufacturing and the production techniques themselves. Adaptive and creative technologies will help the pharmaceutical sector exhibit more robust and agile production processes characterized by lesser obstructions, lesser defects, and better levels of quality control (Arden et al. 2021). The pharmaceutical and biopharmaceutical industry has seen major changes in its operating model and footprint over the recent few years because of Industry 4.0 (Gautam & Pan 2016). The vertical integration of Industry 4.0 will enhance the pharma manufacturing plant to a “reconfigurable factory” in which a highly adjustable, agile, and smart manufacturing line can support mass customization of personalized medications for distinct demands (Barenji et al. 2019). Industry 4.0 is a method of achieving high levels of productivity. Industry 4.0 in the pharmaceutical sector gives tailored drug treatments, advanced drug delivery tools, and medication development. Together, Industry 4.0 and cloud manufacturing can unleash the full potential of the pharmaceutical manufacturing sector (Reinhardt et al. 2021). Pharma 4.0 conceptualizes highly systematic automated techniques, which could be batch continuous, or a hybrid of these, operated by an integrated manufacturing control strategy.
The health sector faces much more difficulties regarding services, such as managing various datasets developed in recent years (da Silveira et al. 2019). Thus, it was necessary to bring revolutionary change in the industrial field. Hence, Industry 4.0 arrived to face the challenges. Advanced technologies are used to bring a positive impact. The technologies involved in Industry 4.0 are big data analysis, cloud computing, IoT, AI, cyber security, etc. Extending borders of modernization with the cooperation of the IoT, Internet of Services, and other advanced technologies can improve the health sector (da Silveira et al. 2019). With the help of AI, adaptive robotics is used to produce innovative products for the health sector. Cloud computing technology enhances the ability of the service of manufacturing systems to create information-driven decisions. Big data analysis is used to build a successful extensive data architecture with Industry 4.0. Cyber security is used to overcome threats like data loss, monetary theft, attack on the framework, and pharmaceutical instruments. Each technology has a different function and objective in Industry 4.0 (Fig. 2).
Fig. 2.
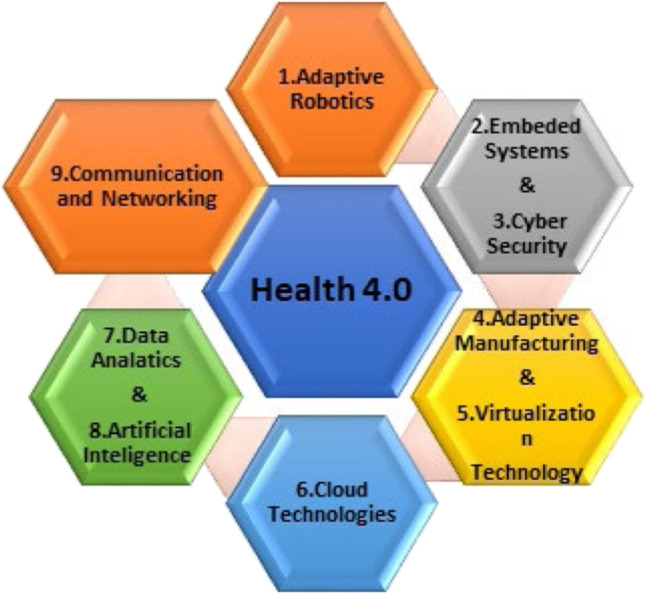
Application of Industry 4.0 in pharma and health sector
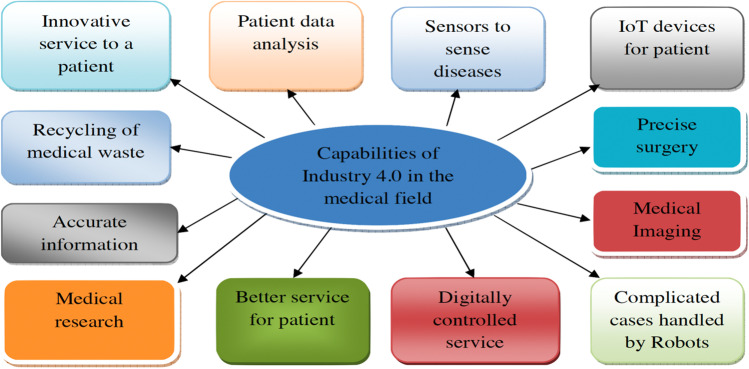
Industry 4.0 provides different potentials and advanced opportunities for health care. With the help of innovative technologies, Industry 4.0 has a beneficial impact on the hospital management system. Figure 3 shows the potential of Industry 4.0 in the pharmaceutical field. Data analytics is used to maintain the patient’s data and provide valuable information, such as the patient’s symptoms. Smart sensors provide details on temperature, blood pressure, and other patient condition. The magnificent ability of Industry 4.0 is waste recycle. It helps prevent the medical field from deteriorating the environment (Fig. 3). Advanced technologies positively impact medical research. Industry 4.0 imparts flexibility in pattern, producing by which the manufacturing system is digitally controlled (Singh et al. 2019).
Fig. 3.
Classification of Industry 4.0 in the medical field
Shi et al. (2020) have introduced a smart factory in Industry 4.0. Smart factories combine physical and cyber technologies to add complexity and precision to related technologies to improve manufacturing process performance, quality, controllability, and transparency. The features of smart factories are sensors, interoperability, virtual reality techniques, and robotics and AI. The advancement of information technologies and other advanced technologies has brought opportunities to smart factories. Industries can lead to sustainable development by using smart factories. The smart factory aims to achieve self-adaptable and flexible production systems. The objective of a smart factory is to enhance the transparency of complicated structures and processes used in the production process. Everything in the smart factory is interconnected, switches data, and naturally combines the actual world with the digital world. Smart factories can make the manufacturing process adaptive and reconfigurable and meet customers’ needs (Arden et al. 2021). Smart factories build strong relationships with suppliers and customers. The smart factory does not mean a factory without the involvement of human beings. Instead, it aims to satisfy the market’s requirement at an acceptable cost.
Acioli et al. (2021) have evaluated the co-relation between Industry 4.0 and sustainability. The main aim was to express how Industry 4.0 can support objectives of sustainability. Industry 4.0 prevents the deterioration of the environment by reducing production of scrap wastes. Advanced technologies of Industry 4.0 can meet the demands of the present. With the help of advanced technologies, the ideas of computer integrated manufacturing and flexible manufacturing system could be further advanced and executed at a lower cost. The aim of Industry 4.0 in sustainable development is to decrease the efforts put into the research, recognize the objectives of the investigation, and examine the opportunities of the research sector in the future.
Djunaedi (2019) aims to build social sustainability in the pharmaceutical sector by implementing Industry 4.0. The objective is to study the effect of information-intensive services and the effect of supply chain integration on social sustainability performance. Industry 4.0 involves using information communication technology (ICT) and cyber-physical system (CPS) in the production processes and enterprise architecture. There is an increase in the focus of the research sector in the medical field because of Industry 4.0 and its increasing interest and unapproachable scientific research. The Fourth Industrial Revolution introduced the sustainable pharmaceutical supply chain, and this supply chain improved the management of products and the communication between different sectors improved.
Grzybowska and Lupicka (2017) evaluated that the development of information technology, internationalization, and competition between sectors leads to the advancement of Industry 4.0. Manufacturing processes involving Industry 4.0 are self-adaptive and flexible. Industry 4.0 provides the latest changes to the manufacturing industry. Some competencies like creativity, decision-making, conflict solving, problem solving, analytical skills, efficiency orientation, and research skills are used for analysis. Competencies are important for the effective conduction of research and understanding the process and result of the research. Automation, robotization, and computerization have positively impacted the pharmaceutical industry worldwide. The quality of life and the welfare of the whole society is improved by the development of advanced technologies and Industry 4.0.
Barenji et al. (2019) evaluated that the 4th Industrial Revolution could improve the manufacturing system’s intelligence. Manufacturing systems can be converted into smart factories by enabling the connection between instruments and decreasing the need for human interventions. Cyber-physical system is significant in preventing threats in the manufacturing process. Cyber-physical system and cloud services in various parts of the organization enable optimization using complicated algorithms and influential computing resources. Cyber security is used to avoid the disruption of extensive data in the manufacturing industries. AI in smart factories provides decentralization, real-time capability, virtualization, and modularity. The current pharmaceutical manufacturing industries are facing many challenges in product quality and product cost. Thus, the development of smart factories will overcome these challenges in the pharmaceutical manufacturing industry.
Aksu and Yeğen (2021) evaluated that the aim of the Fourth Industrial Revolution in this new world is to create a society where the problems of sector life which is also known as industry life and social life can be solved. With the arrival of Industry 4.0, the workforce has been vanished, and the manufacturing industries adopted advanced technologies like cyber systems, IoT, and AI. Industry 4.0 will make the production process more manageable and decrease efforts. Quality by design and process analytical techniques initiative will bring development in safe and effective treatment. Quality by design is used to increase the quality of the products manufactured.
Duvoisin et al. (2018) stated that additive manufacturing plays a key role in industrial economic competitiveness. The use of additive manufacturing is expanding in Industry 4.0 because of the new development. Productivity is expanding with the rapid growth in manufacturing technology. Processes like fused deposition modeling, selective laser sintering, stereolithography, and photopolymerization are available for 3D printing. A broad range of materials used in aerospace, automotive, dental, jewelry, oil, orthopedics, etc. are covered by 3D printing technology. 3D printing technology is used in Industry 4.0 for the efficiency of the process and to reduce complexity. Undoubtedly, 3D printing technology will lead to a significant industrial revolution. To reduce complexity and save time and cost, additive manufacturing plays a major role in Industry 4.0.
Prajwal et al. (2020b) studied the impact of IoT in health care. It is evaluated that the IoT is prospering in the world of the health sector. IoT plays a significant role in home automation. Home automation is the automatic and electronic control on features used in household activity and appliances. IoT controls light and temperature, surveillance cameras, traffic signals, health care, and many more. IoT in health care is used in blood pressure monitoring, glucose monitoring, and ECG monitoring. In hospital management, IoT is used to detect any fault in the management and decrease the medical instruments’ downtime. IoT in the health sector enhances life services throughout the world. IoT not only improves performance but also provides new benefits like cost reduction, reduction in human labor, better use of assets, and better business opportunities.
Chiacchio et al. (2019) evaluated that the Fourth Industrial Revolution boosted the industrial sector. The pharmaceutical sector and the supply chain ensure the quality and safety of the products. For modernizing the factory by enabling the technologies of Industry 4.0, improvements are made by the company. Using Industry 4.0, falsified medications can be detected quickly and withdrawn easily from the process. Traceability is an important factor in the production of safe medicines. Advanced technology is used to automate the generation of serial numbers, labeling, and packaging in the pharmaceutical sector. Serialization has positively impacted the pharmaceutical industry. Serialization in pharmaceutical industry means assigning a unique code or unique serial number to each pharmaceutical package. It has positively impacted the pharmaceutical industry by reduction in product loss, efficiency in expiry date management, and improvement in sales forecasting accuracy.
Lassila (2020) evaluated that Industry 4.0 in the pharmaceutical sector can solve the obstacles like risk reduction, difficulties in manufacturing, and analysis faced by the pharmaceutical industry. Automatization and fewer human interventions with Industry 4.0 made the industry more efficient. Pharma 4.0 has the potential to overcome the problems like the expensive development of pharmaceuticals and batch failures. CPS integrates the physical world with the digital world. AI is used in researching new and effective medications, which is beneficial for the pharmaceutical industry. Advanced analytics in the pharmaceutical industry involves research and development, safety, manufacturing, and supply chain. Machine learning is applied in many factors like automatic quality evolution of emulsion, phase classification, and forecasting of the particle size distribution.
Acioli et al. (2021) investigated the effects of Industry 4.0 innovations on the real and virtual worlds. The technologies like autonomous robotics, the IoT, AI, data analytics, and cyber security advance Industry 4.0. IoT connects various platforms with technologies. IoT works as a bridge between the digital and physical world. The supply chain evolves with an increase in the changes in the market in all aspects like social, economic, financial, and technological. 3D printing can reduce the shortage of medical supplies. Thus, 3D printing is used in COVID-19 to overcome the shortage of medical instruments used to treat the Coronavirus. The technologies of Industry 4.0, IoT, data analytics, cyber security, and cloud computing, have been functional in the marketing and management of the supply chain.
Sarfraz et al. (2022) evaluated the importance of Industry 4.0 in producing and dispensing the COVID-19 vaccine. The technologies of Industry 4.0 are used in manufacturing vaccines to improve quality and enhance efficacy. Technologies like digitization and big data analytics are used for better production of vaccines on a large scale. Vaccine 4.0 has a significant role in manufacturing vaccines and ensuring their quality. Vaccine 4.0 analyzes the development of a process, quality assessment, performance of a process, product stability, optimized maintenance, and compliance. Vaccine tracing through smartphone technology is used in the follow-ups for booster shots of the coronavirus vaccine. Industry 4.0 is used in the production of a vaccine to decrease inequalities.
Giltinan (1996) evaluated that the manufacturing processes use the technologies of Industry 4.0 to improve the process. The main principles of Pharma 4.0 are decision-making, digitalization, traceability, and Workforce 4.0. AI can learn human language and provides meaningful conclusions. Cloud technologies depend on infrastructure, platforms, and software services. Traditional manufacturing industries could not manage this massive amount of data, so Industry 4.0 developed big data analytics to collect the data. Big data analytics, AI, and cloud computing are used to understand the complicated nature of the production process. Industry 4.0 in the pharmaceutical sector has defined the manufacturing sector differently.
Thuemmler and Bai (2017) evaluated that smart pharmaceuticals provide electronic connectivity that compiles, analyzes, and stores the data. Industry 4.0 has mainly focused on research and development in the pharmaceutical sector. Big data analytics and mobile health are used in diagnostics in a significant way. Adoption of Industry 4.0 advances the development of technologies. Information technology plays an essential role in the management of manufacturing operations. AI is used to connect product development and manufacturing operations. The advancement of analytical quality-by-design is used to understand the purpose, quality, accuracy, specificity, etc. of research. Industry 4.0 in the pharma sector can fill the gaps in the manufacturing industry.
The previous Industrial Revolution used mechanization, mass production, and automation. The Fourth Industrial Revolution uses cyber security, the IoT, AI, and other technologies that positively impact manufacturing industries. Advanced technologies like robotics and AI decrease the greenhouse effect, carbon emission, and pollution. Industry 4.0 is used to produce more complex drugs and reduces their side effects. Industry 4.0 builds a connection between industry and sustainability, which leads to sustainable development. Industry 4.0 is used to achieve a high-performance manufacturing system and effective production of medications. Industry 4.0 connects the real and virtual worlds, providing a new aspect to the pharmaceutical sector in research, manufacturing, packaging, and distribution. Sustainable development mainly depends on the advancing technologies of Industry 4.0 by strengthening the manufacturing process and decreases the efforts in analysis. It is used in the development that meets the present’s needs and does not compromise the future. Industry 4.0 has a beneficial effect on sustainable development (Table 1).
Table 1.
Comprehensive review on Industry 4.0 in the pharma sector
| Technology | Application | Country | Reference |
|---|---|---|---|
| •Internet of Things (IoT) | • Used in the management of the positions of inventory in hospital pharmacy racks | New Zealand | Mostofi & Jain (2021) |
| •Adaptive robotics | •Used in price deduction, decreasing the interval in operations and manufacturing time | India | Sisodia & Jindal (2021) |
| •Cloud technologies | •Enhances the ability of the service of manufacturing systems for information-driven conclusions | ||
| •3D printing | •Introduce more complicated medication profiles to decrease side effects and improves drug compliance | United Kingdom | Ding (2018) |
| •Artificial intelligence (AI) | •Used to replace human visual inquiry of packaging caps and vials by using machine vision technology | United States | Arden et al. (2021) |
| •Digitization | •Involves knowledge about variability of raw materials and worldwide tracking of materials across facilities | ||
| •Cloud-based big data analysis | •Drug consumers’ orders might be studied for fine-grain research of pharmaceutical sector trend | China | Wan et al. (2019) |
| •Big data analysis | •It assembles and stores of massive amount of data at high speed | Portugal | Silva et al. (2020) |
| •Robotics | •Robotics reduces the exposure of operators to repetitive processes and biological risk | ||
| •Artificial intelligence (AI) | •AI provides complicated models for predictions and conclusion-making based on developed algorithm study | ||
| •Digitization | •Statistical process is used in measuring and evaluation of stability of process and process ability | Rezeoa (2021) | |
| •Augmented and virtual reality | •In manufacturing industries to solve hurdles related to changing needs from customers and suppliers | Cork | Reinhardt et al. (2020) |
| •3D printing | •Used in fabricating solid oral dosage form, customized products, and advanced objects with developed characteristics and complicated, lightweight design | ||
| •Digital twin and simulation | •Real-time simulation with digital twin is used for examining and procedure optimization before the task is being commanded, thereby driving down machine setup time and enhancing quality | ||
| •Metrology and sensing technology | •This technology motivated the advancement of dedicated solutions and the emergence of profile monitoring | Portugal | Reis & Gins (2017) |
| •Internet of Things (IoT) | •IoT is used to connect more instruments and semifinished products, to link with each other through standard technologies to take benefits of integrated information processing | Turkey | Bianchi et al. (2019) |
| •Big data analysis | •It is used for decreasing price, improving conclusion-making, enhancing products and services | ||
| •Autonomous robot | •Used to analyze, pick, pack, sort, make, examine, or transfer materials of different sizes and weights quickly and more efficiently than ever | ||
| •Cyber security | •Used to prevent disruption of large data network caused by intelligent manufacturing systems, data sharing, and virus | ||
| •Artificial intelligence (AI) | •Used to simulate human intelligence and cognition to study and interpret comprehensive complicated healthcare and medical data | Turkey | Sisodia & Jindal (2021) |
| •Smart sensors | •Used to recognize materials and measure processes, which may be embedded or installed in machines, products, tools, collecting stations, logistic vehicles, etc | Italy | Chiarini (2021) |
| •Collaborative robot (Cobots) | •Cobots allow closer work between humans and robots and are naturally secured | ||
| •Additive manufacturing | •It permits the layer-upon-layer production of a 3D object from a digital file |
Challenges
The use of advanced technologies is not always straightforward. Even Industry 4.0 has faced some challenges. Finding a skilled staff to run the industry is challenging because the technologies are very advanced. Industry 4.0 connects various technologies, and the main concern is security (Rezeoa 2021). There is no going back once developments like these have shown their worth. Either you have to accept it or cannot do anything about it because you will be left behind. The staff working in the industries does not understand the implementation of smart factories (Shi et al. 2020). A high bandwidth network is required for the execution of smart factories. Using big data analytics for improving the performance of smart factories is challenging (Shi et al. 2020). Sometimes the advancing technologies of Industry 4.0 can negatively impact sustainable development (Wagner). Another challenge is the lack of infrastructure or environment in which the technologies of Industry 4.0 integrate. High investment is required in adopting the advanced technologies of Industry 4.0 (Silva et al. 2020). Human labor and their opportunities to work will decrease, leading to unemployment. Insufficient expertise and training will prevent sustainable development in the pharmaceutical supply chain (Reinhardt et al. 2021). Unproductive cooperation, teamwork in supply chain, and poor customer awareness will also inhibit sustainability. The main drawback of Industry 4.0 is that the workforce is replaced by automation (Acioli et al. 2021). With the implementation of Industry 4.0, financial problems and ecological issues can be considered carefully and figured out (Acioli et al. 2021). The lack of law-making measures for the growth of cloud computing, AI, and cyber security in developing countries (Sarfraz et al. 2022). The challenges also include the unsolved issues of protection of data, data privacy, and transparency of data. Using advancing technologies of Industry 4.0 poses a threat to information or data security. In the setting of Industry 4.0, industries need to decrease network vulnerabilities. There is no support from the government to the industries using technologies of Industry 4.0. Communication with the information’s purpose is a crucial challenge for Industry 4.0. Because of the infrastructure hindrance and underlying instrument’s vulnerabilities, a tremendous amount of privacy and secured information is vulnerable to attacks (Wan et al. 2019). Once these advancements have shown their fortune, there is no going back.
Economic sustainability
The technologies of Industry 4.0 could address its high cost and challenges in estimating its entire financial benefits and economic efficacy. The role of Industry 4.0 in economic sustainability leads to smart growth and reduction in cost of living and other processes.
Social sustainability
Industry 4.0 makes more comfortable and safe environment for working. It improves health of the employees working in the industry. It reduces poverty and increases levels of education. Social sustainability can be assured by supplying accessible medicaments and affordable therapy to developing countries with needful and underfinanced wellness program.
Environmental sustainability
Industry 4.0 increases efficiency of energy and there is decrease in scrap waste which is produced during manufacturing process. Industry 4.0 is employed in creating products that are easy to recycle which decreases the pollution.
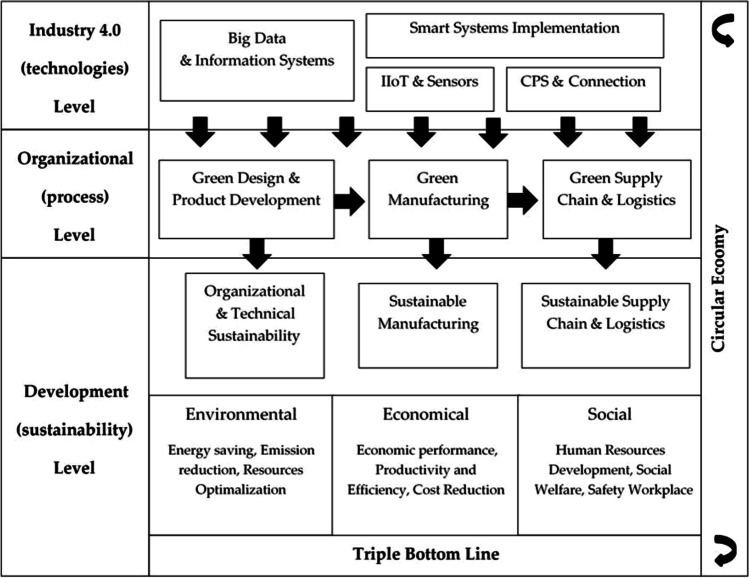
The framework of the Sustainability Green Industry shows the relations between sustainability, Industry 4.0, and green processes. This framework has three levels technological, process, and development (Industry, n.d.). This framework explains a significant part of Industry 4.0 and the outcomes of sustainability. The future scope of Industry 4.0 is related to security, where information technology threats affect manufacturing industries. The technologies of Industry 4.0 will strengthen the manufacturing process more in the future. Industry 4.0 in the pharmaceutical sector will help industries to obtain SD (Ding 2018). Information technology provides accurate information, allowing industries to decrease decision-making delays (Ding 2018). In the future, the information collected by smart pharmaceuticals will lower hospitalization and other costs. The technologies of Industry 4.0, like cyber security, are improved to decrease the threat to information. Industry 4.0 will help to create innovative environments in the research and development sector. With the help of technologies of Industry 4.0, the pharmaceutical industry will make its presence more potent in the growing market. Industry 4.0 in the upcoming years will solve data transparency and protection issues. Industry 4.0 in vaccine production will ensure worldwide dispensation and production of a vaccine to eliminate upcoming outbreaks. The advancing technologies of Industry 4.0 will overcome all the challenges mentioned above and smooth the working of the pharmaceutical sector. The technologies of Industry 4.0 will develop more and face challenges in the future and will positively impact sustainable development. The developing technologies will meet the demands of the market. Industry 4.0 will solve the hurdles in the future in every aspect (Fig. 4).
Fig. 4.
Sustainability framework
Conclusions
This paper represents a comprehensive study of the impact of the Fourth Industrial Revolution, known as Industry 4.0 in pharmaceutical manufacturing for sustainable development. The technologies of Industry 4.0 are used to overcome the challenges faced by the industry. The implementation of Industry 4.0 in the pharmaceutical industry has made the manufacturing process unchallenging. And even in the health sector, diagnosis and treatment have become easier. By adopting Industry 4.0, the industries have become autonomous and flexible. Industry 4.0 in the pharmaceutical industry efficiently designs and devises innovative and personalized products according to customers’ tastes. Industry 4.0 in the pharmaceutical industry has been employed to ensure safety and the protection of the health of the whole society. Advanced technologies of Industry 4.0 like AI, IoT, robotics, cloud computing, and big data analytics positively impacted the pharmaceutical industry. These technologies are used in various aspects of the pharmaceutical and health sectors. Every technology has a different function in Industry 4.0. The technologies of Industry 4.0 are used in manufacturing vaccines to improve quality and enhance the efficacy of vaccines. Industry 4.0 decreases pollution, carbon emission, greenhouse effect, and other things which deteriorate the environment. Advanced technologies led to the introduction of smart factories. The objective of smart factories is to achieve self-adaptable manufacturing process. Sustainable development can be achieved by implementing Industry 4.0. Industry 4.0 can connect the real and virtual world, providing the pharmaceutical sector with a new aspect. Industry 4.0 in sustainable development decreases the efforts put into the research, recognizes the investigation’s objectives, and examines the opportunities of the research sector in the future. With the arrival of the Fourth Industrial Revolution, a sustainable pharmaceutical supply chain was introduced, and this chain improved the management of products. Sustainability depends on the advancing technologies of Industry 4.0. Thus, Industry 4.0 has a beneficial impact on sustainable development.
Acknowledgements
The authors are grateful to the Department of Chemical Engineering, School of Energy Technology, Pandit Deendayal Energy University for the permission to publish this review.
Author contribution
All the authors make substantial contribution in writing the manuscript. DS, PP, and MS participated in drafting the manuscript. DS, PP, and MS wrote the main manuscript; all the authors discussed the review and implication on the manuscript at all stages.
Data availability
All relevant data and material are presented in the main paper.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Devansh Sharma, Email: Devansh.sharma.drx@gmail.com.
Manan Shah, Email: manan.shah@spt.pdpu.ac.in.
References
- Acioli C, Scavarda A, Reis A. Applying Industry 4.0 technologies in the COVID–19 sustainable chains. Int J Product Perform Manag. 2021;70(5):988–1016. doi: 10.1108/IJPPM-03-2020-0137. [DOI] [Google Scholar]
- Aksu B, Yeğen G. Mini-review Industry 4.0 elements for pharmaceutical development and manufacture. J Health Sci A J Health Sci. 2021;3(1):45–50. [Google Scholar]
- Arden NS, Fisher AC, Tyner K, Yu LX, Lee SL, Kopcha M (2021) Industry 4.0 for pharmaceutical manufacturing: preparing for the smart factories of the future. In International Journal of Pharmaceutics (Vol. 602). 10.1016/j.ijpharm.2021.120554 [DOI] [PubMed]
- Argiyantari B, Simatupang TM, Basri MH. Pharmaceutical supply chain transformation through application of the lean principle: a literature review. J Ind Eng Manag. 2020;13(3):475–494. doi: 10.3926/jiem.3100. [DOI] [Google Scholar]
- Barenji RV, Akdag Y, Yet B, Oner L. Cyber-physical-based PAT (CPbPAT) framework for Pharma 4.0. Int J Pharm. 2019;567(June):0–45. doi: 10.1016/j.ijpharm.2019.06.036. [DOI] [PubMed] [Google Scholar]
- Bianchi P, Durán C, Labory S (2019) Impact of Industry 4.0 on manufacturing. Transform Ind Policy Digit Age 11–11. 10.4337/9781788976152.00006
- Chiacchio F, D’Urso D, Compagno L, Chiarenza M, Velardita L. Towards a blockchain based traceability process: a case study from pharma industry. IFIP Adv Inf Commun Technol. 2019;566:451–457. doi: 10.1007/978-3-030-30000-5_56. [DOI] [Google Scholar]
- Chiarini A. Industry 4.0 technologies in the manufacturing sector: are we sure they are all relevant for environmental performance? Bus Strateg Environ. 2021;30(7):3194–3207. doi: 10.1002/bse.2797. [DOI] [Google Scholar]
- Cimini C, Pezzotta G, Pinto R, Cavalieri S. Industry 4.0 technologies impacts in the manufacturing and supply chain landscape: an overview. Stud Comput Intell. 2019;803:109–120. doi: 10.1007/978-3-030-03003-2_8. [DOI] [Google Scholar]
- da Silveira F, Neto IR, Machado FM, da Silva MP, Amaral FG. Analysis of Industry 4.0 technologies applied to the health sector: systematic literature review. Stud Syst Decis Control. 2019;202:701–709. doi: 10.1007/978-3-030-14730-3_73. [DOI] [Google Scholar]
- Dai L (2006) From conventional technology to carbon nanotechnology. The fourth industrial revolution and the discoveries of C60, carbon nanotube and nanodiamond. Carbon Nanotechnol 3–11. 10.1016/B978-044451855-2/50004-8
- Ding B. Pharma Industry 4.0: literature review and research opportunities in sustainable pharmaceutical supply chains. Process Saf Environ Prot. 2018;119:115–130. doi: 10.1016/j.psep.2018.06.031. [DOI] [Google Scholar]
- Djunaedi Building social sustainability of pharmaceutical industry through Industry 4.0 implementation. Pol J Manag Stud. 2019;20(1):149–158. doi: 10.17512/pjms.2019.20.1.13. [DOI] [Google Scholar]
- Duvoisin C, Horst D, Sousa M (2018) Additive manufacturing at Industry 4.0: a review Cite this paper St at e-of-t he-Art Survey of AdditiveManufacturing Technologies, Met hods, and Mat erials. Int J Eng Tech Res 8:3. www.erpublication.org
- Gautam A, Pan X. The changing model of big pharma: Impact of key trends. Drug Discov Today. 2016;21(3):379–384. doi: 10.1016/j.drudis.2015.10.002. [DOI] [PubMed] [Google Scholar]
- Giltinan D. Getting ready for independence. Adopt Foster. 1996;20(1):59–60. doi: 10.1177/030857599602000113. [DOI] [Google Scholar]
- Grzybowska K, Lupicka A. Economics & management innovations ( ICEMI ): key competencies for Industry 4.0. Volkson Press. 2017;1(1):250–253. [Google Scholar]
- Henstock P. Artificial intelligence in pharma: positive trends but more investment needed to drive a transformation. Arch Pharmacol Ther. 2020;2(2):24–28. doi: 10.33696/pharmacol.2.017. [DOI] [Google Scholar]
- Industry, SG (n.d.) Sustainability Green Industry 4.0 | Encyclopedia MDPI. https://encyclopedia.pub/entry/1556
- Jones OAH, Voulvoulis N, Lester JN. Potential impact of pharmaceuticals on environmental health. Bull World Health Organ. 2003;81(10):768–769. [PMC free article] [PubMed] [Google Scholar]
- Lakner Z, Kiss A, Popp J, Zéman Z, Máté D, Oláh J. From basic research to competitiveness: an econometric analysis of the global pharmaceutical sector. Sustain (switzerland) 2019;11(11):1–17. doi: 10.3390/su11113125. [DOI] [Google Scholar]
- Lassila M (2020) Case study in the context of Pharma 4.0: binary pass / fail classification of pharmaceutical product batches based on raw material batch properties.
- Mostofi A, Jain V. Inventory management and control of deteriorating pharmaceutical products sing Industry 4.0. 2021 IEEE 8th Int Conf Ind Eng Appl ICIEA. 2021;2021:394–400. doi: 10.1109/ICIEA52957.2021.9436744. [DOI] [Google Scholar]
- Northrup J. The pharmaceutical sector. Bus Healthc Innov. 2005 doi: 10.1017/CBO9780511488672.003. [DOI] [Google Scholar]
- Peake BM, Braund R, Tong AYC, Tremblay LA (2016) Impact of pharmaceuticals on the environment. In The Life-Cycle of Pharmaceuticals in the Environment (Issue 1). Elsevier Ltd. 10.1016/b978-1-907568-25-1.00005-0
- Prajwal AT, Muddukrishna BS, Vasantharaju SG. Pharma 4.0–impact of the internet of things on health care. Int J Appl Pharm. 2020;12(5):64–69. doi: 10.22159/ijap.2020v12i5.38633. [DOI] [Google Scholar]
- Prajwal AT, Muddukrishna BS, Vasantharaju SG. Pharma 4.0–impact of the internet of things on health care. Int J Appl Pharm. 2020;12(5):64–69. doi: 10.22159/ijap.2020v12i5.38633. [DOI] [Google Scholar]
- Reinhardt IC, Oliveira DJC, Ring DDT. Current perspectives on the development of Industry 4.0 in the pharmaceutical sector. J Ind Inf Integr. 2020;18(January):100131. doi: 10.1016/j.jii.2020.100131. [DOI] [Google Scholar]
- Reinhardt IC, Oliveira JC, Ring DT (2021) Industry 4.0 and the future of the pharmaceutical industry. Pharm Eng 1–11.
- Reis MS, Gins G (2017) Industrial process monitoring in the big data/industry 4.0 era: from detection, to diagnosis, to prognosis. Processes 5(3). 10.3390/pr5030035
- Rezeoa S (2021) Strategy and planning and implementation and impact for the pharmaceutical industry with and by Industry 4.0 by Stefan Rezepa :: SSRN. Lang Competence Part Lifelong Learn Book Sci Artic 4:1–14. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3955697
- Santos BP, Lima TM (2018) Industry 4.0: an overview. II.
- Sarfraz A, Sarfraz Z, Sarfraz M, Abdul Razzack A, Bano S, Singh Makkar S, Thevuthasan S, Paul T, Khawar Sana M, Azeem N, Felix M, Cherrez-Ojeda I (2022) Industry 4.0 technologies for the manufacturing and distribution of COVID-19 vaccines. J Prim Care Commun Health 13. 10.1177/21501319211068638 [DOI] [PMC free article] [PubMed]
- Scherer FM. Chapter 25 The pharmaceutical industry. Handb Health Econ. 2000;1(PART B):1297–1336. doi: 10.1016/S1574-0064(00)80038-4. [DOI] [Google Scholar]
- Shi Z, Xie Y, Xue W, Chen Y, Fu L, Xu X. Smart factory in Industry 4.0. Syst Res Behav Sci. 2020;37(4):607–617. doi: 10.1002/sres.2704. [DOI] [Google Scholar]
- Silva F, Resende D, Amorim M, Borges M. A field study on the impacts of implementing concepts and elements of industry 4.0 in the biopharmaceutical sector. J Open Innov Technol Market Complex. 2020;6(4):1–19. doi: 10.3390/joitmc6040175. [DOI] [Google Scholar]
- Singh M, Sachan S, Singh A, Singh KK. Internet of Things in pharma industry: possibilities and challenges. In Emergence of Pharmaceutical Industry Growth with Industrial IoT Approach: Elsevier Inc.; 2019. [Google Scholar]
- Sisodia A, Jindal R. A meta-analysis of industry 4.0 design principles applied in the health sector. Eng Appl Artif Intell. 2021;104(June 2020):104377. doi: 10.1016/j.engappai.2021.104377. [DOI] [Google Scholar]
- Steinwandter V, Borchert D, Herwig C. Data science tools and applications on the way to Pharma 4.0. Drug Discov Today. 2019;24(9):1795–1805. doi: 10.1016/j.drudis.2019.06.005. [DOI] [PubMed] [Google Scholar]
- Thuemmler C, Bai C (2017) Health 4.0: how virtualization and big data are revolutionizing healthcare. Health 4.0 How Virtualization Big Data Are Revolutionizing Healthc 1–254. 10.1007/978-3-319-47617-9
- Ugvekar N, Kamath KK, Subramanyam EVS, Shabaraya AR. A review on change management system in pharmaceutical industry. Nishi et al Int J Drug Regul Aff. 2021;9(3):37–41. doi: 10.22270/ijdra.v9i3.482. [DOI] [Google Scholar]
- Wan J, Tang S, Li D, Imran M, Zhang C, Liu C, Pang Z. Reconfigurable smart factory for drug packing in healthcare industry 4.0. IEEE Trans Ind Inf. 2019;15(1):507–516. doi: 10.1109/TII.2018.2843811. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data and material are presented in the main paper.