Abstract
Infection of the J774 murine macrophage-derived cell line with Listeria monocytogenes results in several elevations of intracellular calcium during the first 15 min of infection. These appear to result from the actions of secreted bacterial proteins, including phosphatidylinositol-specific phospholipase C (PI-PLC), a broad-range phospholipase C, and listeriolysin O (LLO) (S. J. Wadsworth and H. Goldfine, Infect. Immun. 67:1770–1778, 1999). We have measured hydrolysis of host PI and the activation of host polyphosphoinositide-specific PLC and host phospholipase D (PLD) during infection with wild-type and mutant L. monocytogenes. Elevated hydrolysis of host PI occurred within the first 10 min of infection and was dependent on both bacterial PI-PLC and LLO, both of which were required for the earliest elevations of intracellular calcium in the host cell. A more rapid hydrolysis of host PI was observed at 30 min after infection, at the time when wild-type bacteria have been internalized. Activation of host PLC, also occurred in the first 10 min of infection but was not dependent on the presence of bacterial PI-PLC. Similar observations were made in murine bone marrow-derived macrophages. In J774 cells, activation of host PLD was observed after 20 min of infection and was dependent on bacterial LLO. Mutants in the bacterial phospholipases produced levels of PLD activation similar to those produced by the wild type. Phorbol myristate acetate (PMA) also activated host PLD, while long-term treatment with PMA resulted in loss of the ability of L. monocytogenes to activate host PLD, suggesting an involvement of protein kinase C (PKC) in the activation of PLD. Rottlerin, an inhibitor of PKC δ in J774 cells, also inhibited the activation of PLD, but hispidin, an inhibitor of PKC βI and βII, did not. Pretreatment of J774 cells with the PLD inhibitor, 2,3-diphosphoglycerate partially inhibited escape of the bacteria from the primary phagocytic vacuole.
The earliest events in the interaction of Listeria monocytogenes with mammalian cells appear to involve the activities of bacterial secreted proteins before internalization of these bacteria. On infection of the J774 murine macrophage cell line, these activities delay uptake of wild-type bacteria into the phagosome (36). Subsequent growth in the cytoplasm and cell-to-cell spread are completely dependent on the ability of the bacterium to mediate escape from a vacuole (12, 26, 35). Two genes, hly and plcA, in a cluster of six genes on the bacterial chromosome have been implicated in escape from the primary vacuole of a macrophage. They encode listeriolysin O (LLO) and a phosphatidylinositol-specific phospholipase C (PI-PLC), respectively. A third gene, prfA, which is adjacent to plcA, encodes a positive regulatory protein, PrfA, which is required for the induction of all genes in this virulence cluster (25, 29). LLO has been shown to be absolutely required for escape from the primary vacuole of a macrophage (12, 26) and for mouse virulence (9, 13, 18, 26, 29). Assays for escape from the primary vacuole show that mutants in PI-PLC are between 30 and 65% less likely to be found in the cytoplasm of a bone marrow-derived macrophage than a wild-type strain at 1.5 h postinfection (7, 33). These mutants show reduced growth compared to the wild type in mouse liver; however, the mouse 50% lethal dose is only slightly increased upon intravenous infection (7).
PI-PLC of L. monocytogenes is a member of a family of homologous enzymes secreted by gram-positive bacteria. Like other bacterial PI-PLCs, the enzyme from L. monocytogenes has high specificity for PI with no detectable activity on PI-4-P or PI-4,5-P2, eukaryotic lipids involved in intracellular signaling. It has relatively low activity on glycosyl-PI-anchored eukaryotic membrane proteins, which are actively cleaved by other bacterial PI-PLCs (14, 16).
The ability of L. monocytogenes to escape from a phagosome, grow in the cytoplasm, and spread from cell to cell is essential for the pathogenesis of this food-borne, human and animal pathogen. In humans, infections with L. monocytogenes tend to occur in immunocompromised adults, pregnant women, and the elderly. They can produce septic abortions of the fetus and meningoencephalitis and are often fatal (11, 28).
Since bacterial LLO and PI-PLC activities appear to be important for elevation of intracellular Ca2+ in host cells (36), it seemed possible that there is a connection between escape from the vacuole and activation of certain host cell functions that are dependent on elevated intracellular Ca2+. Among these is the activation of host PLC isoforms, which hydrolyze PI-4-P and PI-4,5-P2 (27, 31). The hydrolysis of host phosphoinositides by bacterial and host PLCs also results in the formation of diacylglycerol (DAG), which is an activator of eukaryotic protein kinase C (PKC) isoforms (24). Activation of the classical isoforms of PKC also requires elevated intracellular Ca2+. Since PKC has been implicated in activation of phospholipase D (PLD) (31) and PLD influences the internalization of another facultative intracellular pathogen, Mycobacterium tuberculosis (20), we have also examined the activation of this host function in infected J774 cells. Our studies show that there is an LLO- and a PI-PLC-dependent hydrolysis of host PI in J774 cells. Activation of J774 cell polyphosphoinositide PLC and PLD was also observed, and these activities were completely dependent on the expression of bacterial LLO.
MATERIALS AND METHODS
Bacterial strains and mammalian cells.
The wild-type L. monocytogenes strain used in this study was 10403S, belonging to serotype 1 (4). The mutant strains derived from strain 10403S were strain DP-L2161 (Δhly) (17), strain DP-L1552 (ΔplcA) (7), strain DP-L1935 (ΔplcB), and strain DP-L1936 (ΔplcA ΔplcB) (33). These strains and the J774 murine macrophage cell line were obtained from Daniel A. Portnoy (University of California, Berkeley) and grown as described previously (36). Murine bone marrow macrophages and J774 cells were propagated as described by Portnoy et al. (26), except that bone marrow macrophages were routinely grown in 60-mm petri dishes (Lab-Tek; American Scientific Products, McGaw Park, Ill.).
Infection of cells prelabeled with [2-3H]inositol.
Bone marrow-derived macrophages and J774 cells were plated in six-well dishes at 1.3 × 106 or 1.0 × 106 cells/well, respectively. After overnight growth in 1.5 ml of Dulbecco modified Eagle medium (DMEM) minus inositol containing 10% fetal bovine serum (FBS) and 10 μCi of myo-[2-3H]inositol (Amersham) per well, the medium was removed, and 2 ml of prewarmed DMEM plus 10% FBS was added. After 10 min at 37°C, 20 μl of 1 M LiCl was added to each well. A suspension of L. monocytogenes was prepared by inoculation of 0.5 ml of an overnight culture grown in brain heart infusion (BHI) broth into 3.5 ml of fresh BHI broth followed by growth on a rotator at 37°C for 2 h. After centrifugation of 1 ml of this logarithmic-phase culture in a microcentrifuge for 1 min and washing with 1 ml of phosphate-buffered saline (PBS), the bacteria were suspended in PBS to provide a density, i.e., A620, of 1.2. After a further 10 min at 37°C in the CO2 incubator, the monolayer of cells was infected with 60 μl of the bacterial suspension. Control cultures received an equal volume of PBS. For incubations longer than 30 min, the medium was completely removed at 30 min and replaced with fresh medium containing 10 mM LiCl, and gentamicin was added to a final concentration of 50 μg/ml. To harvest the cells at the times indicated, the dishes were placed on ice; after removal of the medium, 0.5 ml of ice-cold 4% perchloric acid was added, and the cells were scraped and transferred to screw-cap Eppendorf microcentrifuge tubes. The wells were scraped twice more with 0.2 ml of cold 4% perchloric acid, and the washes were combined with the first extract. The cell suspensions were centrifuged at 2,000 rpm at 4°C. The supernatants from two parallel cultures were combined, diluted to 5 ml with deionized water, and neutralized to pH 7.5 to 8.0 with KOH solutions. The precipitate was centrifuged for 5 min at 2,000 rpm. The supernatants were transferred to fresh tubes and diluted to 10 ml. Carrier inositol-1-P (IP), inositol-1,4-P2 (IP2), and inositol-1,4,5-P3 (IP3), 10 μg of each, were added.
Separation of IP and polyphosphoinositide hydrolysis products.
IP, IP2, and IP3 were separated on 1-ml columns of Dowex AG1X8, 200-400 mesh-formate form as described by Berridge et al. (3) and as modified by Watson (38). Then, 2-ml fractions were collected, and tritium was measured by liquid scintillation spectrometry. Chromatography of radioactive standards of IP2 and IP3 was used to verify the expected elution and separation of these compounds.
Assay of PLD.
J774 cells were grown in Iscove modified Dulbecco medium (IMDM; BioWhittaker) supplemented with 2 mM glutamine and 100 μg of nonessential amino acids (Gibco-BRL) per ml (1). The cells were grown overnight in six-well plates at 106 cells/well in 2 ml of supplemented IMDM plus 10% FBS, and the lipids were labeled with 5 μCi of [9,10-3H]palmitic acid (Amersham) per well. One hour before infection, the medium was removed, the cells were washed with prewarmed PBS, and 2 ml of supplemented IMDM plus 1 mg of bovine serum albumin BSA (fatty acid-free; Sigma) per ml was added. At 5 min before infection, ethanol at 20 μl per well was added. A 60-μl suspension of the appropriate L. monocytogenes strain, prepared as described above, was added, and the cells were returned to the incubator.
The infection was stopped by removing the medium, chilling the plates on ice, and washing the cells twice with PBS at 0°C. Then, 1% methanolic HCl at 0°C (0.75 ml/well) was added, and the cells were scraped off and transferred to 13-by-100-mm glass tubes; any remaining cells were transferred twice with 0.3 ml of 1% methanolic HCl. Carriers, 10 μg each of phosphatidic acid (PA) and phosphatidylethanol (PEt), were added, and the cells were extracted by the method of Bligh and Dyer (5). The lipid solutions were evaporated to dryness and dried in a vacuum desiccator.
The lipids were chromatographed on Whatman LKD 60 thin-layer plates in the upper phase of the solvent ethyl acetate–iso-octane–acetic acid–water (13:2:3:10 [vol/vol/vol/vol]) (1) and visualized by spraying them with 0.001% Primulin (Sigma) in acetone–H2O (4:1). The PA and PEt bands were scraped into liquid scintillation vials for radioassay. The amount of PEt formed is expressed as the percent counts per minute (cpm) relative to total lipid counts per minute.
Measurement of escape from the primary vacuole.
Escape of L. monocytogenes from the primary phagocytic vacuole was measured by labeling bacteria with fluorescien isothiocyanate (FITC) and rhodamine-phalloidin as previously described (21, 36). Briefly, cells which had been labeled with FITC prior to infection were labeled with rhodamine-phalloidin after infection. Phalloidin binds to polymerized actin, which is only associated with bacteria in the cytosol.
RESULTS
Formation of inositol-P upon infection of J774 cells with L. monocytogenes.
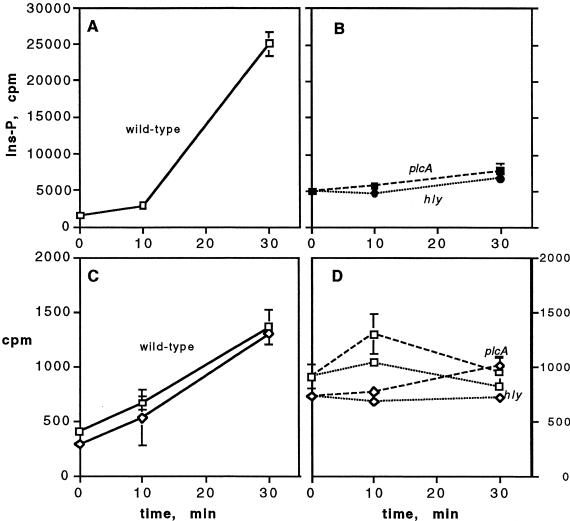
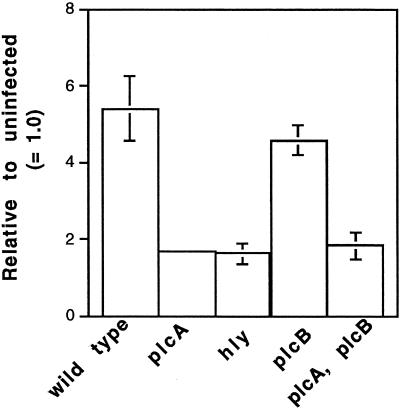
When J774 cells were infected with washed L. monocytogenes, there was a significant increase in [3H]IP within 10 min of infection (P < 0.0001, n = 3). However, a much more rapid release was observed at 30 min after infection (Fig. 1A and data not shown). The release of [3H]IP was greatly diminished upon infection with mutants with deletions in hly or plcA, the genes for LLO and PI-PLC, respectively (Fig. 1B and 2). A mutant with a deletion in plcB, the gene for the broad-range PLC (BR-PLC), produced the same amount of [3H]IP as infection with the wild type, and a mutant with deletions in both plcA and plcB gave the same low level of [3H]IP as the mutant in plcA alone (Fig. 2). Thus, the observed hydrolysis of phosphoinositide was dependent on the ability of L. monocytogenes to produce LLO and PI-PLC during these early stages of infection. These requirements are consistent with a need for both LLO and PI-PLC for elevation of intracellular calcium in infected J774 cells (36) and for the activation of PKC δ and βII isoforms (S. J. Wadsworth and H. Goldfine, unpublished data).
FIG. 1.
Formation of [3H]IP, IP2, and IP3 in J774 cells prelabeled with [3H]inositol after infection with L. monocytogenes. Radioactive products of PI (inositol-P), PIP (IP2), and PIP2 (IP3) hydrolysis were extracted from infected cells at the times indicated and separated by ion-exchange chromatography as described in the text. (A and B) [3H]IP after infection with the strains indicated. (C and D) [3H]IP2 (□) and [3H]IP3 (◊) after infection with the ΔplcA strain (dashed lines) and Δhly strain (dotted lines). Data for the wild type represent the mean ± the standard deviation (SD) (n = 3) from a representative experiment, and data for the mutant strains represent the mean ± the standard error of the mean (SEM) (n = 2) from a representative experiment.
FIG. 2.
Formation of [3H]IP in J774 cells prelabeled with [3H]inositol after infection with L. monocytogenes strains. IP was extracted from infected cells at 30 min after infection and separated by ion-exchange chromatography as described in the text. The data represent the means ± the SEM for wild-type (n = 12), ΔplcA (n = 4), Δhly (n = 3), ΔplcB (n = 5), and ΔplcA ΔplcB (n = 4) strains. Differences between wild-type and the Δhly, ΔplcA, and ΔplcA ΔplcB strains were all significant (P < 0.01, Mann-Whitney).
Activation of host polyphosphoinositide-PLC during infection of J774 cells with L. monocytogenes.
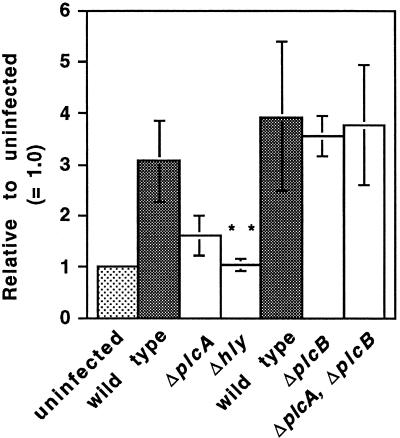
Mammalian isoforms of PLC preferentially hydrolyze PI-4,5-P2 and PI-4-P to give rise to IP3 and IP2, respectively (31). Elevated formation of [3H]IP3 and [3H]IP2 was readily observed at 30 min and was variably observed at 10 min after infection with the wild type (Fig. 1C). A similar increase in these tritiated products was not observed upon infection with a mutant in LLO and was lower with the mutant in bacterial PI-PLC, but the latter did not meet a test of statistical significance (Fig. 1D and 3). A BR-PLC deletion mutant activated host PLC to the same extent as the wild-type as did, surprisingly, the double mutant, ΔplcA ΔplcB (Fig. 3). Thus, the broad-range phospholipase of L. monocytogenes is not required for activation of host PLC nor, apparently, is activity of L. monocytogenes PI-PLC needed in the absence of the BR-PLC.
FIG. 3.
Formation of [3H]IP3 in J774 cells prelabeled with [3H]inositol after infection with L. monocytogenes strains. [3H]IP3 was extracted from infected cells at 30 min after infection and separated by ion-exchange chromatography as described in the text. The data represent the means ± the SEM for wild-type (n = 6), ΔplcA (n = 5), and Δhly (n = 5) strains and, in a separate set of experiments, for wild-type (n = 3), ΔplcB (n = 3), and ΔplcA ΔplcB (n = 3) strains. The difference between wild-type and Δhly strains was significant (P < 0.01, Mann-Whitney).
Activation of host PLC also occurs in murine bone marrow-derived macrophages infected with L. monocytogenes.
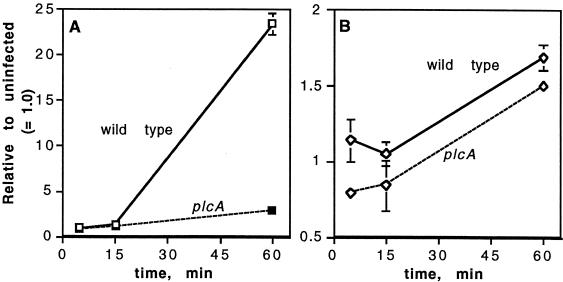
The formation of [3H]IP was also observed in murine bone marrow-derived macrophages infected with the wild-type strain with a time course similar to that observed with J774 cells. As in J774 cells, the formation of [3H]IP was much lower with the PI-PLC mutant (Fig. 4A). The activation of host phospholipases by the wild type was also evidenced by the formation of IP3. Infection with the PI-PLC mutant resulted in as much IP3 formation as did infection with the wild type (Fig. 4B). Earlier experiments in which bone marrow-derived macrophages were infected with L. monocytogenes grown to the stationary phase showed that a mutant in LLO produced similarly reduced levels of [3H]IP as the PI-PLC mutant, both of which were much lower than was observed with the wild type (data not shown).
FIG. 4.
Formation of [3H]IP and [3H]IP3 in murine bone marrow-derived macrophages prelabeled with [3H]inositol after infection with L. monocytogenes. Radioactive products of PI hydrolysis (IP) (A) and PIP2 hydrolysis (IP3) (B) were extracted from infected cells at the times indicated and separated by ion-exchange chromatography as described in the text. The data represent the means ± the SEM of two separate experiments.
Activation of host phospholipase D in J774 cells infected with wild-type and mutant L. monocytogenes.
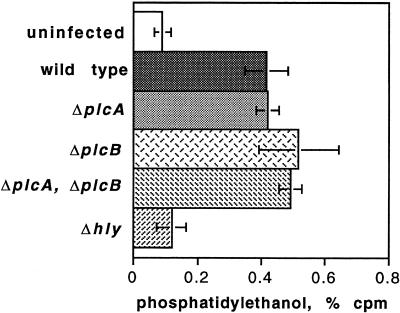
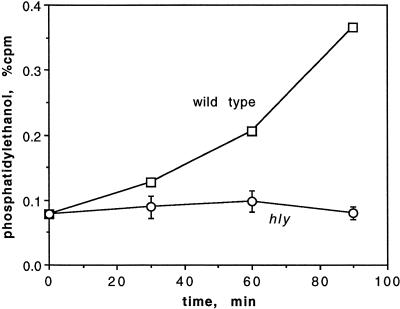
Many of the agonists that stimulate eukaryotic polyphosphoinositide-specific PLC also result in activation of PLD, and it appears that in some systems activation of PLD occurs downstream of PLC activation. Furthermore, PLD has been implicated in vesicular membrane traffic (31). Consequently, we examined the activation of PLD in J774 cells infected with L. monocytogenes. Eukaryotic PLD catalyzes transphosphatidylation to ethanol from various membrane lipids with phosphatidylcholine as the preferred donor for the membrane-bound forms, which do not require Ca2+ (31). Thus, in cells prelabeled with [3H]palmitic acid, the formation of [3H]PEt in the presence of ethanol serves as a measure of PLD activity. Infection with wild-type L. monocytogenes and all mutants studied except for a mutant in LLO activated PLD to a similar extent in J774 cells (Fig. 5). These results were obtained at 90 min after infection. The kinetics of activation of PLD upon wild-type infection of J774 cells and upon infection with all mutants except the LLO deletion strain were similar, and the results of a typical experiment are shown in Fig. 6. Several experiments showed that there was no detectable increase in PEt during the first 15 min of infection (data not shown); however, PLD activity at 30 min was readily seen.
FIG. 5.
Formation of [3H]PEt in J774 cells prelabeled with [9,10-3H]palmitate at 90 min after infection with L. monocytogenes strains. [3H]PEt was extracted from the cells and separated by thin-layer chromatography as described in the text. The data represent the means ± the SD of two separate experiments carried out in duplicate, in which each mutant strain was compared with the wild type in the same experiment. For wild type, n = 22; for all other strains, n = 4. The difference between the wild-type and the Δhly infections was highly significant (P < 0.0001, unpaired t test). The difference in [3H]PEt between uninfected control cells and the cells infected with the Δhly strain was not significant.
FIG. 6.
Formation of [3H]PEt in J774 cells infected with wild-type L. monocytogenes and with the Δhly strain. The data are from one of two experiments with the Δhly strain, each done in duplicate.
Evidence for the participation of PKC in the activation of PLD has been obtained in several experimental systems; however, the results have been mixed (10, 31). As in many cell systems, short-term activation of PKC by phorbol 12-myristate 13-acetate (PMA) resulted in a time-dependent activation of PLD (Table 1). In agreement with this, overnight pretreatment with PMA decreased the activation of PLD upon wild-type infection to a nearly basal level (Table 2). Also in agreement, the treatment of cells with rottlerin (Calbiochem), an inhibitor of PKC δ, resulted in strong inhibition of the PLD activation caused by L. monocytogenes infection. However, hispidin (Calbiochem), which inhibits both PKC βI and βII, but not PKC δ, had no effect on PLD activation (Table 3). Both PKC inhibitors partially inhibited escape from the primary phagocytic vacuole (Wadsworth and Goldfine, unpublished).
TABLE 1.
Short-term treatment with PMA activates PLD in J774 cells
| Treatment | Mean PLD activitya |
|---|---|
| None | 0.09, 0.11 |
| PMA, 100 nM, 15 min | 0.15 |
| PMA, 100 nM, 30 min | 0.31, 0.32 |
| PMA, 100 nM, 60 min | 0.40 |
| L. monocytogenes infection, 90 min | 0.42 |
cpm in PEt. Values are percentages of total lipid cpm. Each value represents the mean of duplicate wells.
TABLE 2.
Overnight (18 h) pretreatment with PMA inhibits activation of PLD with wild-type L. monocytogenes in J774 cells
| Pretreatment | Mean PLD activation (%)a ± SEM |
|---|---|
| None | 100 |
| PMA, 200 nM | 24 ± 10 |
| PMA, 500 nM | 19 ± 7 |
Percent PLD activation observed during a 90-min infection with wild-type L. monocytogenes. The data are the results of two separate experiments done in duplicate (±SEM).
TABLE 3.
Effects of PKC inhibitors on PLD activation by infection of J774 cells with L. monocytogenes
| Pretreatment | Mean PLD activation (%)a ± SEM |
|---|---|
| None | 100 |
| Rottlerin | |
| 10 μMb | 38.7 ± 15.4 |
| 25 μM | 2.4 ± 1.0 |
| Hispidin, 5 μMb | 102 ± 5 |
Percent PLD activation observed during a 90-min infection with wild-type L. monocytogenes. The data represent the results of two separate experiments done in duplicate (±SEM).
Diluted in IMDM and added 30 min before infection.
Inhibition of PLD by the competitive inhibitor 2,3-DPG results in decreased escape from the primary vacuole.
We studied the effects of the competitive PLD inhibitor 2,3-diphosphoglycerate (2,3-DPG) (20) on PLD activation in J774 cells upon infection with wild-type L. monocytogenes. Treatment of uninfected J774 cells with 2,3-DPG surprisingly resulted in variable stimulation of basal PLD activity (5 mM, 151% ± 59% of the no-drug control; 10 mM, 212% ± 183% of the control). After infection, PLD activation was inhibited 91% ± 19% by 5 mM 2,3-DPG and 86% ± 12% by 10 mM 2,3-DPG (three separate experiments at each concentration, carried out in duplicate). Measurement of the effects of 2,3-DPG treatment on the escape from the primary vacuole of wild-type bacteria showed significant inhibition at both concentrations. The PLD inhibitor 2,3-DPG was found to partially inhibit escape from the primary vacuole as follows: with no pretreatment, there was 45% escape in 1.5 h; with 2,3-DPG at 5 and 10 mM, the percent escape values were 29 and 23%, respectively. (The percent escape values refer to the percentage of internalized bacteria associated with polymerized actin at 1.5 h. The data were pooled from two separate experiments.)
DISCUSSION
The observations that infection of murine macrophage-derived J774 cells with wild-type L. monocytogenes resulted in two brief elevations of intracellular Ca2+ within the first 5 min of infection, followed by a third prolonged elevation lasting for more than 30 min, and that the same pattern of calcium fluxes was not observed upon infection with mutants in LLO, PI-PLC, or BR-PLC led to the questions concerning the origins of these fluxes and their effects on other host cellular activities. The importance of these observations was heightened by the finding that pharmacological inhibition of the induced calcium signals resulted in changes in the kinetics of bacterial uptake into these cells and the efficiency of escape from the primary phagocytic vacuole (36). In parallel with studies on calcium signaling in J774 cells, we have carried out studies on the hydrolysis of host phosphoinositides and the activation of PLD induced by wild-type and mutant L. monocytogenes.
The observed formation of [3H]IP in cells prelabeled overnight with [3H]inositol beginning during the first 10 min of infection was, like the first and second elevations of intracellular calcium ([Ca2+]i), dependent on the expression of both LLO and PI-PLC. Early formation of [3H]IP did not require the expression of BR-PLC, which is not required for the first, brief elevation of [Ca2+]i (36). Therefore, the observed increase in [3H]IP release is likely to result from the activity of bacterial PI-PLC. It does not appear to result from activation of host PLCs, which preferentially hydrolyze PI-4-P and PI-4,5-P2 to produce IP2 and IP3, and subsequent hydrolysis of these by phosphatases to yield IP. This conclusion is based on our finding that infection with the double ΔplcA ΔplcB mutant lacking both PI-PLC and BR-PLC caused accumulation of IP2 and IP3 equal to that of wild-type infection in J774 cells but did not result in increased IP formation (Fig. 2 and 3).
We had previously hypothesized that the first brief [Ca2+]i elevation resulted from activation of the calcium-independent PKC δ found in J774 cells (32) upon release of DAG formed by the action of PI-PLC. Presumably, either pores or membrane perturbations induced by LLO permit access of PI-PLC to host PI (30, 36). Our measurements of PI hydrolysis show release of [3H]IP during the first 10 min of infection, but the background level in J774 cells is too high to measure early release of either IP or DAG (33). However, studies on PKC δ show that it is activated within the first min of infection of J774 cells with wild-type L. monocytogenes and that its activation required expression of both LLO and PI-PLC (Wadsworth and Goldfine, unpublished). Thus, there is excellent agreement between rapid PKC δ activation, the earliest inward Ca2+ flux, and the hydrolysis of host PI, with the ability of L. monocytogenes to express LLO and PI-PLC.
The more rapid release of [3H]IP beginning between 20 and 30 min after infection mirrors the kinetics of internalization of the wild type, which is inhibited during the first 5 min and approaches a plateau at 20 min after infection. It also corresponds temporally with the third elevation of [Ca2+]i (36). Enhanced activity of bacterial PI-PLC after entry into the phagosome is presumably the result of the increased expression of LLO and PI-PLC observed after bacterial internalization (6, 19, 22). This increased activity was presumably not dependent on elevated [Ca2+]i since it was observed with the BR-PLC deletion mutant, which produces only a brief spike in [Ca2+]i at the first minute after infection. This is consistent with the lack of a Ca2+ requirement for bacterial PI-PLCs in general and for that of L. monocytogenes (16, 23).
Activation of host PLC.
The release of IP2 and IP3, indicating activation of host phosphoinositide PLC, was also dependent on expression of LLO. In most experiments with J774 cells and the PI-PLC deletion mutant, the release of IP2 and IP3 was lower than wild-type, but there was considerable scatter in these data. Indeed, PI-PLC expression was not required for host PLC activation in the absence of BR-PLC (Fig. 3), which suggests that a product of BR-PLC is inhibitory for host PLC activation. These could include DAG derived from lipids other than PI (37), ceramide, or metabolic products derived from these lipid intermediates. Since the mutant lacking both PI-PLC and BR-PLC did not produce any [Ca2+]i elevation in J774 cells (36), elevated [Ca2+]i is not a requirement for activation of host PLC in this system. Eukaryotic PLCs require Ca2+ for catalytic activity and are activated by elevated [Ca2+]i, but activation by the G protein-dependent pathway occurs in the resting physiological range of ∼100 nM (2, 31), which is the level we have observed in uninfected J774 cells (36). It is also important to note that the presence of bacterial PI-PLC, which causes hydrolysis of PI and could thus affect the pool of cellular PI-4,5-P2, does not interfere with the stimulation of IP3 release.
Surprisingly, there does not appear to be a connection between the activation of host PLC, which gives rise to IP3, a mediator of release of Ca2+ from intracellular stores, and the elevations of [Ca2+]i observed in J774 cells after infection. Infection with the double phospholipase mutant of L. monocytogenes resulted in the same level of host PLC activation as with the wild type, yet it produced no elevation of [Ca2+]i (36). Release of calcium from intracellular stores in these cells may result from signaling molecules other than IP3. One candidate is sphingosine-1-P (15, 34), which can be produced as a result of the formation of ceramide by the action of L. monocytogenes BR-PLC (33), followed by hydrolysis of the N-acyl group and phosphorylation of the resulting sphingosine. Some evidence for this hypothesis is the finding that an L. monocytogenes construct in which the gene for the PC-PLC of B. cereus, which has essentially no sphingomyelinase activity, replaced plcB (39) produced much lower [Ca2+]i levels than did the wild type after 10 min of infection of J774 cells (Wadsworth and Goldfine, unpublished).
Activation of host PLD.
Of the mutants studied, only the strain deficient in LLO was unable to activate host PLD. Thus, bacterial PI-PLC and BR-PLC activities and elevated host [Ca2+]i are not required for PLD activation, since the double phospholipase mutant activated PLD but did not increase [Ca2+]i (36). Short-term treatment with PMA stimulated PLD to the same level observed on infection with L. monocytogenes and overnight pretreatment with PMA inhibited PLD activation (Table 1), suggesting an involvement of PKCs in the activation of PLD. The finding that rottlerin, an inhibitor of PKC δ in J774 cells, strongly inhibited PLD activation is also consistent with a role for PKC in PLD activation; however, hispidin, an inhibitor of both PKC βI and βII, but not PKC δ, did not block PLD activation in infected J774 cells (Table 3). These findings are consistent with many observations suggesting a connection between PKCs and mammalian PLC and PLD activation; however, there is no established mechanism for activation of PLD by PKC, and the results with inhibitors have been variable (31). The finding that PLD activation occurred after 20 min of infection compared to rapid changes in [Ca2+]i and rapid activation of PKC isoforms (Wadsworth and Goldfine, unpublished) suggests that PLD activation is downstream of these signals and may involve more complicated and possibly parallel pathways (2, 8).
The observation that PLD activation, like escape from the primary vacuole, is dependent on LLO expression and occurs during the time when the phagosome is beginning to undergo lysis suggests a connection between these phenomena. This notion is bolstered by the finding that the PLD inhibitor 2,3-DPG resulted in decreased escape from the primary vacuole. However, PI-PLC loss results in decreased efficiency of escape from the primary vacuole (7, 33, 36) but does not affect PLD activation. Also, as noted above, hispidin partially inhibits escape from the primary vacuole but had no effect on PLD activation. Kusner et al. have observed a tight coupling of phagocytosis of M. tuberculosis and opsonized zymosan and activation of PLD in human macrophages (20). At this time it is not possible to state that similar tight coupling exists in the fate of L. monocytogenes during infections. Clearly, further studies will be required to dissect these complex and long-range interactions.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (GM52797) and the University Research Foundation of the University of Pennsylvania.
REFERENCES
- 1.Balsinde J, Balboa M A, Insel P A, Dennis E A. Differential regulation of phospholipase D and phospholipase A2 by protein kinase C in P388D1 macrophages. Biochem J. 1997;321:805–809. doi: 10.1042/bj3210805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandyopadhyay R, Basu M. Involvement of PL-D in the alternate signal transduction pathway of macrophages induced by an exernal stimulus. Mol Cell Biochem. 2000;203:127–133. doi: 10.1023/a:1007055804978. [DOI] [PubMed] [Google Scholar]
- 3.Berridge M J, Downes C P, Hanley M R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982;206:587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop D K, Hinrichs D J. Adoptive transfer of immunity to Listeria monocytogenes: the influence of in vitro stimulation on lymphocyte subset requirements. J Immunol. 1987;139:2005–2009. [PubMed] [Google Scholar]
- 5.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 6.Bubert A, Sokolovic Z, Chun S K, Papatheodorou L, Simm A, Goebel W. Differential expression of Listeria monocytogenes virulence genes in mammalian host cells. Mol Gen Genet. 1999;261:323–336. doi: 10.1007/pl00008633. [DOI] [PubMed] [Google Scholar]
- 7.Camilli A, Tilney L G, Portnoy D A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993;8:143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockcroft S. G-protein-regulated phospholipases C, D and A2-mediated signalling in neutrophils. Biochim Biophys Acta. 1992;1113:135–160. [PubMed] [Google Scholar]
- 9.Cossart P, Vicente M F, Mengaud J, Baquero F, Perez-Diaz J C, Berche P. Listeriolysin O is essential for virulence of Listeria monocytogenes: direct evidence obtained by gene complementation. Infect Immun. 1989;57:3629–3636. doi: 10.1128/iai.57.11.3629-3636.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Exton J H. Regulation of phospholipase D. Biochim Biophys Acta. 1999;1439:121–133. doi: 10.1016/s1388-1981(99)00089-x. [DOI] [PubMed] [Google Scholar]
- 11.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaillard J L, Berche P, Mounier J, Richard S, Sansonetti P. In vitro model of penetration and intracellular growth of Listeria monocytogenes in the human enterocyte-like cell line Caco-2. Infect Immun. 1987;55:2822–2829. doi: 10.1128/iai.55.11.2822-2829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaillard J L, Berche P, Sansonetti P. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect Immun. 1986;52:50–55. doi: 10.1128/iai.52.1.50-55.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gandhi A J, Perussia B, Goldfine H. Listeria monocytogenes phosphatidylinositol (PI)-specific phospholipase C has low activity on glycosyl-PI anchored proteins. J Bacteriol. 1993;175:8014–8017. doi: 10.1128/jb.175.24.8014-8017.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gijsbers S, Mannaerts G P, Himpens B, van Veldhoven P P. N-acetyl-sphingenine-1-phosphate is a potent calcium mobilizing agent. FEBS Lett. 1999;453:269–272. doi: 10.1016/s0014-5793(99)00735-8. [DOI] [PubMed] [Google Scholar]
- 16.Goldfine H, Knob C. Purification and characterization of Listeria monocytogenes phosphatidylinositol-specific phospholipase C. Infect Immun. 1992;60:4059–4067. doi: 10.1128/iai.60.10.4059-4067.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones S, Portnoy D A. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect Immun. 1994;62:5608–5613. doi: 10.1128/iai.62.12.5608-5613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kathariou S, Metz P, Hof H, Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987;169:1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klarsfeld A D, Goossens P L, Cossart P. Five Listeria monocytogenes genes preferentially expressed in infected mammalian cells: plcA, purH, purD, pyrE and an arginine ABC transporter gene, arpJ. Mol Microbiol. 1994;13:585–597. doi: 10.1111/j.1365-2958.1994.tb00453.x. [DOI] [PubMed] [Google Scholar]
- 20.Kusner D J, Hall C F, Schlesinger L S. Activation of phospholipase D is tightly coupled to the phagocytosis of Mycobacterium tuberculosis or opsonized zymosan by human macrophages. J Exp Med. 1996;184:585–595. doi: 10.1084/jem.184.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marquis H, Doshi V, Portnoy D A. Broad-range phospholipase C and metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect Immun. 1995;63:4531–4534. doi: 10.1128/iai.63.11.4531-4534.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moors M A, Levitt B, Youngman P, Portnoy D A. Expression of listeriolysin O and ActA by intracellular and extracellular Listeria monocytogenes. Infect Immun. 1999;67:131–139. doi: 10.1128/iai.67.1.131-139.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moser J, Gerstel B, Meyer J E W, Chakraborty T, Wehland J, Heinz D W. Crystal structure of the phosphatidylinositol-specific phospholipase C from the human pathogen Listeria monocytogenes. J Mol Biol. 1997;273:269–282. doi: 10.1006/jmbi.1997.1290. [DOI] [PubMed] [Google Scholar]
- 24.Newton A C. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 25.Portnoy D A, Chakraborty T, Goebel W, Cossart P. Molecular determinants of Listeria monocytogenes pathogenesis. Infect Immun. 1992;60:1263–1267. doi: 10.1128/iai.60.4.1263-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Portnoy D A, Jacks P S, Hinrichs D J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988;167:1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhee S G, Bae Y S. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 28.Schuchat A, Robinson K, Wenger J D, Harrison L H, Farley M, Reingold A L, Lefkowitz L, Perkins B A. Bacterial meningitis in the United States in 1995. N Engl J Med. 1997;337:970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 29.Sheehan B, Kocks C, Dramsi S, Gouin E, Klarsfeld A D, Cossart P. Molecular and genetic determinants of the Listeria monocytogenes infectious process. Curr Top Microbiol Immunol. 1994;192:187–216. doi: 10.1007/978-3-642-78624-2_9. [DOI] [PubMed] [Google Scholar]
- 30.Sibelius U, Chakraborty T, Krögel B, Wolf J, Rose F, Schmidt R, Wehland J, Seeger W, Grimminger F. The listerial exotoxins listeriolysin and phosphatidylinositol-specific phospholipase C synergize to elicit endothelial cell phosphoinositide metabolism. J Immunol. 1996;157:4055–4060. [PubMed] [Google Scholar]
- 31.Singer W D, Brown H A, Sternweis P C. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu Rev Biochem. 1997;66:475–509. doi: 10.1146/annurev.biochem.66.1.475. [DOI] [PubMed] [Google Scholar]
- 32.Smith E R, Jones P L, Boss J M, Merrill A H., Jr Changing J774A.1 cells to new medium perturbs multiple signaling pathways, including the modulation of protein kinase C by endogenous sphingoid bases. J Biol Chem. 1997;272:5640–5646. doi: 10.1074/jbc.272.9.5640. [DOI] [PubMed] [Google Scholar]
- 33.Smith G A, Marquis H, Jones S, Johnston N C, Portnoy D A, Goldfine H. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect Immun. 1995;63:4231–4237. doi: 10.1128/iai.63.11.4231-4237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spiegel S, Merrill A H., Jr Sphingolipid metabolism and cell growth regulation. FASEB J. 1996;10:1388–1397. doi: 10.1096/fasebj.10.12.8903509. [DOI] [PubMed] [Google Scholar]
- 35.Tilney L G, Portnoy D A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989;109:1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wadsworth S J, Goldfine H. Listeria monocytogenes phospholipase C-dependent calcium signaling modulates bacterial entry into J774 macrophage-like cells. Infect Immun. 1999;67:1770–1778. doi: 10.1128/iai.67.4.1770-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakelam M J O. Diacylglycerol—when is it an intracellular messenger? Biochim Biophys Acta. 1998;1436:117–126. doi: 10.1016/s0005-2760(98)00123-4. [DOI] [PubMed] [Google Scholar]
- 38.Watson F. Phospholipases involved in activation of the neutrophil NADPH-oxidase. Methods. 1996;9:578–590. doi: 10.1006/meth.1996.0065. [DOI] [PubMed] [Google Scholar]
- 39.Zückert W R, Marquis H, Goldfine H. Modulation of enzymatic activity and biological function of Listeria monocytogenes broad-range phospholipase C by amino acid substitutions and by replacement with the Bacillus cereus ortholog. Infect Immun. 1998;66:4823–4831. doi: 10.1128/iai.66.10.4823-4831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]