Abstract
Group B streptococci (GBS) colonize the female genital and rectal tracts and can cause invasive infection in susceptible newborns. An optimally effective GBS vaccine should induce mucosal and systemic immunity. In this study, we investigate the local and systemic immune responses to GBS type III capsular polysaccharide (CPS) after mucosal vaccination of mice via intranasal, peroral, rectal, and vaginal routes, with GBS type III CPS conjugated with recombinant cholera toxin B subunit (GBS III CPS-rCTB). Cholera toxin (CT) was added as an adjuvant. Immunoglobulin G (IgG) and IgA antibodies to the CPS were tested in serum, lungs, and intestinal, rectal, and vaginal extracts by enzyme-linked immunosorbent assay. The conjugated CPS administered by intranasal, peroral, rectal, and vaginal routes was much more effective at inducing both mucosal and systemic antibody responses to GBS III CPS than was unconjugated CPS. The CPS-specific immune responses in various organs were dependent on the route of immunization. Generally, the highest levels of IgA and IgG were generated in the regions or sites of the conjugate exposure. Thus, intranasal vaccination elicited the highest anti-CPS IgA and IgG antibody levels in the lungs, whereas peroral administration in the intestinal site and vaginal vaccination elicited the highest antibody levels in the vagina. Rectal vaccination was superior to the other routes in inducing high antibody levels in the rectum. The four routes of mucosal vaccination also induced distant antibody responses to CPS. Rectal vaccination induced high specific IgA levels in the vagina and intestine, and oral administration induced high specific IgA levels in the lungs and rectum. All four routes of vaccination with the conjugate elicited similarly high levels of anti-CPS IgG in serum. Intranasal vaccination with different doses of the conjugate (10, 30, and 80 μg of CPS) did not have a significant influence on the anti-CPS specific antibody responses. Intranasal immunization induced better antibody responses when one dose of the conjugate was divided and given on three consecutive days compared to administration of the full dose on one occasion. In conclusion, rectal and vaginal vaccination may be the best way of stimulating anti-CPS immune responses in the rectal and vaginal tracts, while high levels of anti-CPS antibodies in the lungs can be achieved after intranasal administration. The vaccination regimen thus might influence the mucosal immune response to CPS. This conjugate may serve as an effective mucosal vaccine for preventing mucosal colonization and invasive infection caused by GBS.
Since the 1970s the group B streptococci (GBS) have been reported to be an important cause of invasive infections in newborns and infants. GBS are the prime cause of bacteremia, pneumonia, and meningitis in newborns and of endometriosis in postpartum women. An increasing incidence of GBS infections in infants more than 3 months old has been noted. In recent years, the organism has also been recognized as a pathogen in adults with severe underlying diseases (7, 8, 22, 23, 28).
The prevalence of genital colonization with GBS in women varies from 2 to 35% in different studies from many parts of the world; in The United States, GBS are found in the vaginal flora of about 25% of all women (18, 23, 27, 29). In most studies, the GBS rectal carriage rate has been similar to the genital carriage rates (23). The GBS colonization rate in the pharynx is approximately 5% (4, 9). In The United States, about 1% of children born to mothers infected with GBS will develop bacteremia and pneumonia (8, 23, 28). The bacterial colonization of the vaginal, rectal, and respiratory tracts appears to be the initial step to infection in sensitive hosts: from the mucosa the organism can enter the bloodstream and cause invasive infection. Therefore, mucosal immunity should be an important first-line defense against the pathogen in humans, while systemic immunity by serum antibodies can protect against invasive infection. Transplacentally transferred serum IgG antibodies from the mother would primarily confer immune protection for the newborn, although breast milk immunoglobulin A (IgA) antibodies may also contribute to protection. Consequently, for optimal efficacy a GBS vaccine should be designed to induce both mucosal and systemic immunity against GBS infection.
GBS capsular polysaccharides (CPS) are known to be important bacterial virulence factors and protective antigens. Seven serotypes of GBS are associated with invasive diseases. However, the most common serotype of GBS is type III (12). Similar to the vaccine development approach taken against many other encapsulated bacteria, experimental vaccines against GBS have been prepared in the form of CPS conjugated to a carrier protein such as tetanus toxoid (17, 26). The GBS CPS conjugate vaccines tested so far have been designed to induce systemic immune responses in adult females based on the notion that placental transfer of maternal serum IgG should protect neonates (17, 26). It has been reported that GBS colonization of the rectum in humans can induce specific IgA antibodies in the cervical-vaginal tract (14). Thus, natural immunity against GBS following colonization or infection may involve the mucosal immune system as well.
In accordance with this, our efforts have focused on developing a mucosal GBS conjugate vaccine capable of inducing mucosal immunity together with high serum antibody titers. In a previous study, we described the development of GBS III CPS conjugate vaccines linked to recombinant cholera toxin B subunit (rCTB) using different coupling methods, and we investigated the immunogenicity of these conjugates in a mouse model (24). The rCTB, which by itself is a safe mucosal immunogen and protective antigen in a licensed oral vaccine against cholera (21), proved to be an effective mucosal carrier protein for GBS III CPS and induced mucosal anti-CPS specific responses in the lungs and vagina when administrated by the intranasal (i.n.) route. In contrast, subcutaneous vaccination with the GBS III CPS-protein conjugates induced a strong serum antibody response but did not effectively stimulate any mucosal immune response (24).
The aims of this study were (i) to investigate the best vaccination route of the conjugate to obtain strong immune responses in the serum, lungs, vagina, and rectum and (ii) to identify the effect of different i.n. vaccination doses and schedules of the GBS III CPS-rCTB conjugate vaccine on the anti-CPS systemic and mucosal immune responses.
MATERIALS AND METHODS
CPS antigen and cholera proteins.
GBS type III CPS was purified from a culture medium of Streptococcus agalactiae M732 as described previously (24). The purified type III CPS contained <1% (wt/wt) of protein. The sialic acid content was 18 to 20% (wt/wt). The purified type III CPS did not react with group-specific antiserum, as demonstrated by double diffusion (not shown). Recombinant CTB was purified from Vibrio cholerae 358 as described previously (15). Purified cholera toxin (CT) was obtained from List Biological Laboratories, Inc. (Campbell, Calif.).
Preparation of GBS type III CPS-rCTB conjugate.
The synthesis of the type III CPS-rCTB conjugate was performed as described elsewhere (24) with some modifications. GBS type III CPS (20 mg) in 2 ml of phosphate-buffered saline (PBS; pH 7.0) was incubated in darkness at room temperature for 1.5 h with 4 mM sodium m-periodate (Sigma Chemical Co., St. Louis, Mo.). Glycerol was then added to consume any residual periodates. The mixture was passed through a Sephadex G-25 column (Pharmacia Fine Chemicals, Uppsala, Sweden) and lyophilized. The oxidized CPS (15 mg) was dissolved in 0.1 M sodium bicarbonate (pH 9.0) and mixed with rCTB (15 mg). Sodium cyanoborohydride (Aldrich Chemie, Steinheim, Germany) was added to a final concentration of 20 mg/ml, and the mixture was incubated at 37°C for 4 to 5 days. The progress of the conjugation was monitored by analyzing aliquots of the mixture at various points of time, using fast-performance liquid chromatography. A Superose 6 HR 10/30 column (Pharmacia Fine Chemicals) with PBS as eluant at a flow rate of 0.5 ml/min was used. Conjugation was indicated by a progressive increase in a broad high-molecular-weight protein peak monitored by measurement of the UV absorbancy at 280 nm. After the conjugation was completed, sodium borohydride (10 mg/ml) was added to the reaction mixture to reduce the remaining free aldehyde groups. The conjugate was purified by gel filtration on a Sephacryl S-300 HR 16/60 column (Pharmacia Fine Chemicals) and eluted with PBS to separate the conjugate from unbound rCTB. Fractions collected in the void volume were pooled, dialyzed against PBS, and concentrated. Two batches of the conjugate were pooled and used in this study.
Analyses of conjugate.
The CPS content was measured by means of a phenol sulfuric assay with purified type III CPS as a standard (6). The content of protein was estimated with a Bio-Rad protein assay in which purified rCTB was used as the standard.
The immunological reactivity of type III CPS and rCTB in the conjugate was determined by means of a GM1 ganglioside receptor-binding variant of an enzyme-linked immunosorbent assay (GM1-ELISA) as described previously (3). In brief, polystyrene microwell plates (Nunc, Roskilde, Denmark) were coated overnight with GM1 ganglioside (0.3 nmol/ml). The tested conjugates were then added in threefold serial dilutions and, after incubation, a hyperimmune rabbit serum to a GBS type III strain or a mouse monoclonal antibody to rCTB (LT39) was added. The antibodies bound to the CPS or rCTB antigen were detected by means of alkaline phosphatase goat anti-rabbit IgG and horseradish peroxidase goat anti-mouse IgG (Jackson) conjugates and corresponding enzyme substrates, respectively. The reactivates of CPS and CTB were expressed as the dilutions of the conjugates giving cutoff absorbances of 0.4 above the background level, and the amount of each component was calculated based on the comparisons with concomitantly tested CPS and rCTB standards (24).
Immunizations of mice.
C57BL/6 female mice, 8 to 10 weeks old, were obtained from B&K Universal (Stockholm, Sweden). All mice were treated subcutaneously with 10 mg of medroxyprogesteroneacetate (Depo-Provera; Upjohn Company, Kalamazoo, Mich.) 10 and 3 days prior to immunization.
The immunization schedules, administration doses and routes used in this study are shown in Table 1. Each group contained four or five mice. The CPS-rCTB conjugate and a mixture of free CPS and rCTB containing 2 mg of CPS and 2.2 mg of rCTB per ml were used for immunization.
TABLE 1.
Immunization scheme
| Antigen and group no.a | Route of immunization | CPS dose (μg) in antigen | CT dose (μg) |
|---|---|---|---|
| CPS-rCTB conjugate | |||
| 1 | i.n. | 30 | 2.5 |
| 2 | p.o. | 80 | 10 |
| 3 | Rectal | 80 | 10 |
| 4 | Vaginal | 80 | 10 |
| CPS plus rCTB mixture | |||
| 5 | i.n. | 30 | 2.5 |
| 6 | p.o. | 80 | 10 |
| 7 | Rectal | 80 | 10 |
| 8 | Vaginal | 80 | 10 |
| CPS-rCTB conjugate | |||
| 9 | i.n. | 3.5b | 0.5b |
| 10 | i.n. | 10b | 0.5b |
| 11 | i.n. | 27b | 0.5b |
Four or five mice were included in each group as described in Materials and Methods.
One dose of the conjugate indicated in the table was given on three consecutive days.
The immunization routes tested were i.n., peroral (p.o.), rectal, or vaginal. A dose corresponding to 30 μg of CPS together with 2.5 μg of CT as adjuvant was given by i.n. immunization (15 μl) and to 80 μg of CPS plus 10 μg of CT for the rectal and vaginal immunizations (50 μl). For the p.o. immunization, a dose corresponding to 80 μg of CPS together with 10 μg of CT in 0.3 ml of 3% Na2CO3 was given by gastric intubation. Each immunization dose was given on one occasion and was repeated on two or more occasions at intervals of 12 to 14 days. The mice were sacrificed 7 to 10 days after the last immunization.
In a separate experiment with i.n. immunization, groups of mice were given CPS-rCTB conjugate at dosages of 10, 30, or 80 μg per round at intervals of 12 to 14 days. Since i.n. immunization of mice is preferably performed using a volume not exceeding 15 to 20 μl per administration, the concentration of the conjugate was not enough to administer the highest dosage on a single occasion; therefore, each of these dosages were divided into three equal aliquots and administered in each round on three consecutive days in a volume of 15 μl, including 0.5 μg of CT as adjuvant in each administration. These mice were also sacrificed 7 to 10 days after the last immunization.
The mice were lightly anesthetized with methoxyflurane (Schering-Plough animal Health Corp. Union) before all immunizations. Blood samples were taken from the tail vein before each immunization round.
The perfusion extraction method was used to collect organ specimens as described elsewhere (15). A 100-μl dose of a 1% solution of heparin (Lövens Kemiske) in PBS was injected intraperitoneally in the anaesthetized mouse, and blood was drawn from the subclavian vein. The mice were killed immediately after the bleeding and extensively infused with about 20 ml of 0.1% heparin–PBS through the heart to remove as much blood from the tissues as possible. The lungs, small intestines, rectum, and vagina were collected and weighed before storage in the freezer at −20°C in a PBS solution (1 ml per g of tissue) containing 2 mM phenylmethylsulfonyl fluoride, 0.1 mg of trypsin inhibitor from soybeans (Sigma Chemical Co.) per ml, and 0.05 M EDTA. To permeabilize the cell membranes, saponin (Sigma) was added to a final concentration of 2% (wt/vol), and samples were treated at 4°C overnight. The organs were then spun down, and the supernatants were analyzed for antibody titers by ELISA.
Serologic methods.
Antibodies to GBS III CPS were estimated by ELISA using a biotinylated type III CPS as antigen as described earlier (25). In brief, plates (Greiner) were coated with 5 μg of avidin (Sigma Chemical Co.) per ml and then incubated with 2 μg of biotinylated GBS type III CPS per ml. Tested samples were added in threefold serial dilutions. Anti-mouse IgG (Jackson) and IgA (Southern Biotechnology Associates, Inc., Birmingham, Ala.) horseradish peroxidase conjugates were used, and the ELISA was finally developed with ortho-phenylenediamine (Sigma Chemical Co.) and H2O2. A pool of serum from mice immunized with the CPS-CTB conjugate was used as a positive control. The concentration of antibodies was expressed as reciprocal sample dilutions (titers), giving absorbances of 0.4 above the background level for serum IgG and IgA and 0.3 above the background level for IgG and IgA in the extracts of organs.
Statistics.
The geometric mean (GM), standard deviation (SD), and standard error of the mean (SEM) values were calculated for all of the ELISA titers. A Student's t test with a Bonferroni correction was used to compare mean values of antibody titers in different groups of mice. Statistical significance was designated as a P value of <0.05.
RESULTS
Characterization of the CPS-rCTB conjugate.
The gel filtration profiles of the two batches of conjugate prepared were practically indistinguishable and showed that a major portion of the rCTB was conjugated to CPS. The fractions containing the largest-molecular-size material (the void volume) were collected in order to avoid the unconjugated CPS. These fractions from the two batches were pooled; the ratio (weight/weight) of CPS and rCTB in the resulting final conjugate was found to be 0.90:1, and the yield of the total CPS recovered in the conjugate was 23%.
The immunologic reactivity of the conjugated CPS was analyzed by GM1-ELISA using immune serum to the homologous GBS type III strain. The conjugated CPS reacted, down to a concentration of 2.7 ng/ml, with the anti-GBS type III hyperimmune serum. GM1-ELISA was also used to study the receptor-binding capacity of the conjugated CTB. The conjugated rCTB reacted, down to a concentration of 2.0 ng/ml, with anti-CTB mouse monoclonal antibodies, which was a finding similar to that of unconjugated rCTB and indicating that the conjugated rCTB had essentially the same GM1 binding capacity as the unconjugated rCTB.
Mucosal antibody responses to different routes of immunization.
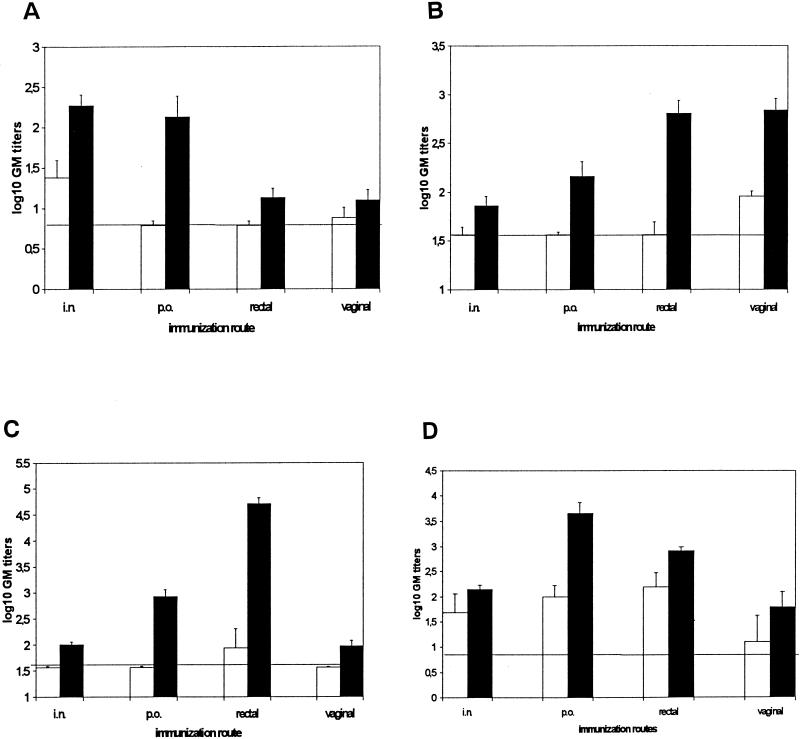
The IgA anti-CPS mucosal antibody responses in the lungs, vagina, rectum, and small intestines were examined after different routes of immunization with three doses of CPS, either conjugated to or, for comparison, simply mixed with rCTB and by either route administered together with a small amount of CT as adjuvant. The unconjugated CPS mixed with rCTB only infrequently gave rise to specific IgA titer increases in the lung, vagina, or rectum (Fig. 1A to C), whereas it could induce a detectable IgA anti-CPS response in the intestine after all four routes of immunization (Fig. 1D). Higher anti-CPS IgA antibody responses were found in all organs after immunization with the conjugated CPS than with unconjugated CPS. Thus, both i.n. and p.o. immunizations with the conjugate elicited significantly higher levels of CPS-specific IgA antibodies in the lungs (P <0.01) (Fig. 1A); p.o., rectal, and vaginal immunizations elicited significantly higher levels of CPS-specific IgA antibodies in the vagina (P < 0.01) (Fig. 1B); all four routes of immunization elicited significantly higher levels of CPS-specific IgA antibodies in the rectum (i.n., p.o., and rectal immunization, P < 0.01; vaginal immunization, P < 0.05) (Fig. 1C); and p.o. and rectal immunizations elicited significantly higher levels of CPS-specific IgA antibodies in the intestines (P < 0.01 and P < 0.05, respectively) (Fig. 1D).
FIG. 1.
GBS III CPS-specific IgA titers in the lungs (A), vagina (B), rectum (C), and intestines (D) after i.n., p.o., rectal, and vaginal immunizations with a CPS-rCTB conjugate and a mixture of free CPS and rCTB mixed with a small amount of CT. The dose of the conjugate and the mixture of free CPS and rCTB used were 30 μg of CPS plus 2.5 μg of CT per dose for i.n. immunization or 80 μg of CPS plus 10 μg of CT per dose for p.o., rectal, and vaginal immunizations. Antibody titers are given as log10 of the GM titer ± the SEM. Horizontal lines indicate maximal titers seen in preimmunization samples. White bars, titers of a mixture of CPS and rCTB; black bars, titers of the CPS-rCTB conjugate.
The levels of IgA antibodies to CPS varied substantially in different organs depending on the routes of immunization with the conjugate. Generally, the highest specific IgA levels were seen in the organs that had been directly exposed to the immunogen. Thus, i.n. immunization with the conjugate resulted in higher levels of CPS-specific IgA antibodies in the lungs than did rectal and vaginal administration (i.n. versus rectal and vaginal, P < 0.01) (Fig. 1A). Likewise, vaginal administration resulted in significantly higher levels of CPS-specific IgA antibodies in vaginal extracts than those achieved by i.n. or p.o. immunization (vaginal versus i.n., P < 0.01; vaginal versus p.o., P < 0.05) (Fig. 1B). The strongest CPS-specific IgA titer rises in rectal extracts were seen in the mice that received the conjugate by rectal immunization (rectal versus i.n., p.o., and vaginal immunization, P < 0.01) (Fig. 1C). Finally, in the intestine, p.o. immunization with the conjugate elicited higher anti-CPS IgA than immunizations via the i.n. and vaginal routes (p.o. versus i.n. and vaginal immunization, P < 0.01) (Fig. 1D).
However, at the same time, all four routes of mucosal immunization with the conjugate also elicited detectable CPS-specific IgA responses at adjacent or distant mucosal sites. Thus, i.n. vaccination induced a measurable IgA response in the rectum, vagina, and intestines (Fig. 1B to D). After p.o. administration of the conjugate, significant IgA antibody titers were found also in lungs and rectal tissue extracts (Fig. 1A and C). Particularly high “disseminated” levels of specific IgA antibodies were found in vaginal and intestinal extracts after rectal administration (Fig. 1B and D), and the antibody levels achieved in these extracts after rectal immunization were only marginally (vagina) or moderately (intestine) lower than those seen after the optimal “homologous” routes of immunization. In contrast, only relatively low levels of anti-CPS IgA responses were found at distant mucosal sites after vaginal administration (Fig. 1A, C, and D).
The anti-CPS IgG titers (GM) in the lungs, vaginas, rectums, and intestines in the unimmunized mice (control group) were low (between 1:5 and 1:36). The CPS-rCTB conjugate elicited significantly higher levels of CPS specific IgG antibody responses in the lungs, vaginas, rectums, and intestines than did the mixture of CPS and rCTB, regardless of the immunization route (P < 0.01) (not shown). The GM IgG titers after the i.n., p.o., rectal, and vaginal immunizations ranged from 1:316 to 1:870 in the lungs, 1:1,096 to 1:4,466 in the vagina, 1:954 to 1:15,848 in the rectum, and 1:257 to 1:602 in the intestines.
Serum antibody responses.
The specific serum IgG and IgA anti-CPS antibodies obtained after the different routes of immunization with the CPS-rCTB conjugate and, for comparison, with the corresponding mixtures of free CPS and rCTB, respectively, are shown in Table 2. All routes of mucosal immunization—i.n., p.o., rectal, and vaginal—with the conjugate elicited higher serum CPS-specific IgG responses than did immunization with CPS mixed with rCTB (P < 0.01). There was no statistical difference in the levels of anti-CPS IgG in serum obtained after the different routes of immunization, although there was a tendency toward stronger IgG responses in serum after the rectal and i.n. immunizations compared to the p.o. or vaginal vaccination. Significantly stronger serum IgA anti-CPS responses were found after p.o. and rectal administration than after i.n. or vaginal vaccinations (P < 0.01).
TABLE 2.
Serum anti-CPS antibody responses
| Immunization route | Antigen | Anti-CPS serum GM titer (range)
|
|
|---|---|---|---|
| IgG | IgA | ||
| i.n. | CPS − rCTB conjugate | 17,368 (12,302–24,434) | 27 (10–70) |
| CPS + rCTB mixture | 556 (381–728) | <10 | |
| p.o. | CPS − rCTB conjugate | 6,583 (2,355–18,365) | 723 (271–1,922) |
| CPS + rCTB mixture | 97 (55–168) | <10 | |
| Rectal | CPS − rCTB conjugate | 19,288 (12,644–29,376) | 285 (93–851) |
| CPS + rCTB mixture | 129 (118–140) | <10 | |
| Vaginal | CPS − rCTB conjugate | 10,816 (6,516–17,619) | <10 |
| CPS + rCTB mixture | 188 (107–325) | <10 | |
| Preimmunization sera | 145 (128–162) | <10 | |
Antibody responses to different dosage and schedules of i.n. immunization.
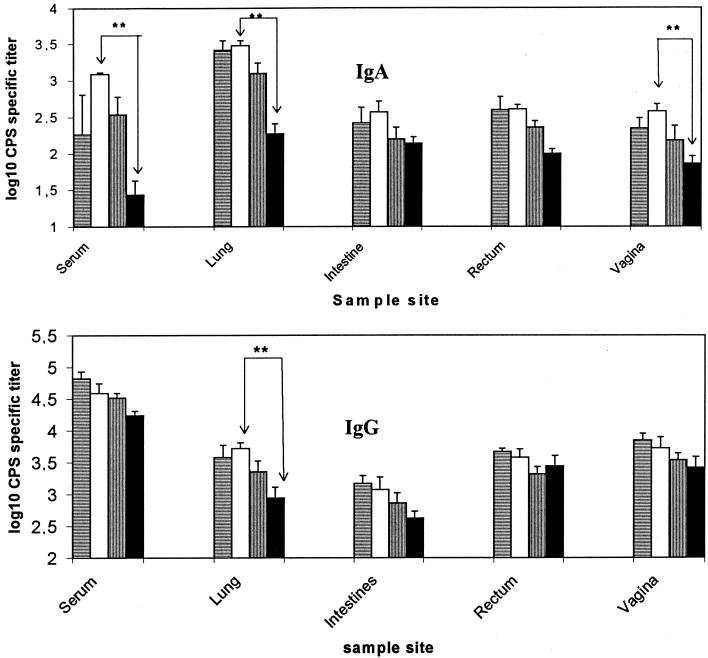
Since the results described above indicated that immunization with the conjugate by the i.n. route resulted in the strongest combined serum IgG and at least lung mucosal IgA responses and since it would be practically much easier to administer a vaccine by the i.n. route than through the vaginal or rectal immunizations, it was important to further elucidate this route of immunization to see whether further improved responses could be obtained by, for instance, increasing the dosage of conjugate administered i.n. Therefore, three groups of mice were given the CPS-rCTB conjugate in dosages corresponding to 10, 30, and 80 μg of CPS, together with 1.5 μg of CT per dose i.n., with the middle dosage corresponding to the amount given in the previous experiment. To enable the administration of the highest of these dosages without exceeding the 20-μl volume found in previous studies to be suitable for i.n. immunization, each dose was divided into three equal parts and given on three consecutive days. All three groups of mice responded with elevated levels of IgG and IgA antibodies in serum, as well as in mucosal organ extracts. Administration of 30 μg of the conjugated CPS resulted in the highest level of IgA mucosal responses, but the differences between antibody levels elicited by the different doses of conjugate was not significant (Fig. 2). However, a striking finding was that the administration of the 30-μg dose of conjugate, divided and given on three consecutive days, was significantly more effective at inducing high anti-CPS responses in serum and organs than was the same amount of conjugate given as an individual dose, even though in the latter instance a higher dose of CT (2.5 μg rather than a total of 1.5 μg) was used. Indeed, in serum, the lungs, and the vagina, 5 to 30 times higher levels of IgA titers were observed (P < 0.01).
FIG. 2.
GBS III CPS-specific IgA and IgG titers in serum and mucosa after i.n. immunization with a CPS-rCTB conjugate at different dosages and schedules. The conjugate doses used were 10 μg of CPS plus 1.5 μg of CT (horizontal striped bars), 30 μg of CPS plus 1.5 μg of CT (white bars), and 80 μg of CPS plus 1.5 μg of CT (vertical striped bars), which were divided and given on three consecutive days per round. Black bars represent the group of mice immunized with 30 μg of CPS plus 2.5 μg of CT given on one occasion per round. Stars denote significant differences between two groups (∗∗, P < 0.01).
DISCUSSION
A vaccine against GBS targeted for women in childbearing age should preferably induce a combination of mucosal and systemic immunity. In this study, we addressed the question of whether the GBS type III CPS conjugated to rCTB is an effective mucosal vaccine for stimulating both mucosal and systemic immunity, and we compared in particular the relative effectiveness of mucosal immunization by different routes and regimens. Since the adjuvant capacity of CT in p.o. and i.n. immunizations of mice has been shown previously to maximize mucosal antibody responses (2, 5, 10), we included a subclinical dose of free CT as adjuvant in all immunization protocols of this study. The CPS was conjugated to rCTB by means of reductive amination method, which due to some modifications in the procedure resulted in a higher content of CPS in the conjugates and a higher yield than previously reported, while still retaining full GM1 receptor-binding and CPS antigenic activities (24). As expected, the conjugated CPS became strongly immunogenic and, as shown in the study, proved to be superior to unconjugated CPS in stimulating systemic IgG after all routes of immunization. After i.n., p.o., rectal, and vaginal immunization with the conjugate, higher mucosal IgA anti-CPS immune responses were obtained in the lungs, intestines, rectum, and vagina, respectively.
i.n. immunization with protein antigens is clearly a more effective route than parenteral immunization in order to stimulate mucosal immune responses. Animal and human experiments have shown that i.n. vaccination can induce specific antibody responses in the salivary, nasal, pulmonary, and even distant genital tracts (1, 15, 19, 20). We have previously demonstrated that i.n. immunization of the GBS III CPS-protein conjugate containing rCTB induces higher levels of specific IgA antibodies in serum and lungs than s.c. immunization (24). The results of this study confirm that i.n. immunization with the CPS-rCTB conjugate can induce not only a strong CPS-specific systemic antibody response but also a significant immune response in the lungs. However, in contrast to several reports with protein antigens including CTB-protein conjugates which have shown substantial genital mucosal immune responses after i.n. immunization (15, 20), the anti-CPS response in the vagina after i.n. immunization with the CPS-rCTB conjugate was low and significantly lower than after immunization by the vaginal and rectal routes. This may reflect a different mechanism underlying the immune responses against the conjugated CPS and protein antigen (13).
The female genital tract may be inhabited by GBS, from which the bacteria can be transferred during parturition to the respiratory tract of the neonate and cause serious invasive infection. Effective mucosal vaccination against GBS may be needed to stimulate CPS-specific immune responses in the female genital mucosa as well. The results of the systemic and local immune responses after vaginal immunization with protein antigens are contradictory (11, 15, 16; E. L. Parr and M. B. Parr, Abstr. 10th Int. Cong. Mucosal Immunol. [EFIS], abstr. 23.1, p. 96; M. W. Russell, H. Y. Wu, G. Hajishengallis, M. H. Martin, S. M. Michalek, S. K. Hollingshead, T. D. Connell, and D. Metzger, Abstr. 10th Int. Congr. Mucosal Immunol. [EFIS], abstr. 41.1, p. 153). Most studies have shown that vaginal vaccination of women and experimental animal usually results in responses largely confined to the genital tract. However, it has also been reported that in mice vaginal administration of CT fails to induce either local or distant IgA response (11). Our study showed that vaginal immunization with the CPS-rCTB conjugate together with CT as adjuvant resulted in a markedly increased level of anti-CPS IgG in serum and of both IgA and IgG in the vaginal tract. The CPS-specific responses in mucosal tissues were largely limited to the vagina following vaginal immunization. The different effectiveness and distribution of the immune response after vaginal immunization observed in different studies may be due to the different features of the protein and CPS antigens used. GBS III CPS is a T-cell-independent antigen. The conjugated rCTB may at the same time influence the uptake of antigen by dendritic cells present in the vaginal epithelium and may also influence priming, migration, and homing directions of activated specific B cells in both afferent and efferent pathways.
Since GBS bacteria colonize the rectum of females and are then transmitted from the rectum to the vagina, the induction of local immunity against GBS in the rectum is equally important as genital immunity for protection against GBS infection. The rectal mucosa of mice and humans are known to be rich in antigen-transporting M cells and organized mucosal lymphoid tissue. Rectal immunizations of mice, as well as humans, have resulted in high levels of IgA antibodies in rectal and vaginal secretions (11, 13, 16, 30). Our finding is consistent with these reports. Rectal immunization with the CPS-rCTB conjugate elicited a high level of anti-CPS IgA and IgG antibodies not only in rectal extracts but also in adjacent vaginal and intestinal sites. Moreover, the highest level of serum-specific IgG antibodies was recorded following rectal immunization. This finding suggests that rectal immunization with the GBS III CPS-rCTB conjugate, although less convenient than p.o. or i.n. immunizations, might be preferable to other routes of immunizations in order to prevent the GBS colonization of the rectal and female genital tracts and subsequent invasive infection in the bloodstream.
Taken together, the anti-CPS responses after mucosal immunization with a GBS III CPS-rCTB conjugate have the following distinguishing features. (i) The strongest mucosal responses are generated in regions or sites exposed to antigen. The immunization strategy of rectal and vaginal vaccination may be optimal for inducing anti-GBS CPS immune responses in the rectal and female genital tracts and i.n. immunization for the respiratory tract. (ii) i.n. vaccination with different doses of the conjugate did not have a significant influence on the anti-CPS specific antibody responses within the certain range. (iii) A good effect of mucosal vaccine may be achieved by prolonging the contact with the antigens and the mucosal surface. Thus, this study indicates that the GBS type III CPS-rCTB conjugate may serve as mucosal vaccine in order to induce both effective specific systemic and mucosal responses including the vaginal, rectal, and respiratory tracts.
ACKNOWLEDGMENT
The study was supported financially by the Swedish Medical Research Council (project 16x-3383).
REFERENCES
- 1.Abraham E. Intranasal immunization with bacterial polysaccharide containing liposomes enhances antigen-specific pulmonary secretory antibody response. Vaccine. 1992;10:461–468. doi: 10.1016/0264-410x(92)90395-z. [DOI] [PubMed] [Google Scholar]
- 2.Bergquist C, Johansson E L, Lagergard T, Holmgren J, Rudin A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect Immun. 1997;65:2676–2684. doi: 10.1128/iai.65.7.2676-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergquist C, Lagergard T, Lindblad M, Holmgren J. Antibody responses in serum and lungs to intranasal immunization with Haemophilus influenzae type b polysaccharide conjugated to cholera toxin B subunit and tetanus toxiod. APMIS. 1998;106:800–806. doi: 10.1111/j.1699-0463.1998.tb00226.x. [DOI] [PubMed] [Google Scholar]
- 4.Chretien J H, Mcginniss C G, Thmopson J. Group B beta-hemolytic streptococci causing pharyngitis. J Clin Microbiol. 1979;10:263–266. doi: 10.1128/jcm.10.3.263-266.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czerkinsky C, Russell M W, Lycke N, Lindblad M, Holmgren J. Oral administration of a streptococcal antigen coupling to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect Immun. 1989;57:1072–1077. doi: 10.1128/iai.57.4.1072-1077.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 7.Farley M M, Harvey R C, Stull T. A population-based assessment of invasive disease due to group B streptococcus in non-pregnant adults. N Engl J Med. 1993;294:753–756. doi: 10.1056/NEJM199306243282503. [DOI] [PubMed] [Google Scholar]
- 8.Feldman P G. Prevention of group B streptococcal infection in neonates. J Infect Dis. 1998;11:325–329. doi: 10.1097/00001432-199806000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Ferrieri P, Blair L L. Pharyngeal colonization of GBS: detection by three methods. J Clin Microbiol. 1977;6:136–139. doi: 10.1128/jcm.6.2.136-139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajishengallis G, Russell M W, Michalek S M. Comparison of an adherence domain and a structural region of Streptococcus mutans antigen I/II in protective immunity against dental caries in rats after intranasal immunization. Infect Immun. 1998;66:1740–1743. doi: 10.1128/iai.66.4.1740-1743.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haneberg B, Kendall D, Amerongen H M, Apter F M, Kraehenbuhl J P, Neutra M R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994;62:15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrison L H, Elliott J A, Dwyer D M, Libonati J P, Ferrieri P, Billmann L, Schuchat A. Serotype distribution of invasive group B streptococcal isolates in Maryland: implications for vaccine formulation. J Infect Dis. 1998;177:998–1002. doi: 10.1086/515260. [DOI] [PubMed] [Google Scholar]
- 13.Hopkins S, Kraehenbuhl J P, Schödel F, Potts A, Peterson D, Grandi P D, Nardelli-Haefliger D. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect Immun. 1995;63:3279–3286. doi: 10.1128/iai.63.9.3279-3286.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hordnes K, Tynning T, Kvam A I, Bevanger L, Brown T A, Jonsson R, Hangeberg B. Cervical secretions in pregnant women colonized rectally with group B streptococci have high levels of antibodies to serotype III polysaccharide capsular antigen and protein R. Scand J Immunol. 1998;47:179–188. doi: 10.1046/j.1365-3083.1998.00283.x. [DOI] [PubMed] [Google Scholar]
- 15.Johansson E L, Rask C, Fredriksson M, Eriksson K, Czerkinsky C, Holmgren J. Antibodies and antibody-secreting cell in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect Immun. 1998;66:514–520. doi: 10.1128/iai.66.2.514-520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozlowski P A, Cu-uvin S, Neutra M R, Flanigan T P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretion of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagergard T, Shilach J, Robbins J B, Schneerson R. Synthesis and immunological properties of conjugates composed of group B streptococcus type III capsular polysaccharide covalently bound to tetanus toxoid. Infect Immun. 1990;58:687–694. doi: 10.1128/iai.58.3.687-694.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang S T, Lau S P, Chan S H, Fok T F, Murai T, Kaneko Y. Perinatal colonization of group B streptococcus—an epidemiological study in a Chinese population. Aust N Z Obstet Gynaecol. 1986;26:138–141. doi: 10.1111/j.1479-828x.1986.tb01550.x. [DOI] [PubMed] [Google Scholar]
- 19.Russell M W, Moldoveanu Z, White P L, Sibert G J, Mestecky J, Michalek S M. Salivary, nasal, genital and systemic antibody responses in monkey immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect Immun. 1996;64:1272–1283. doi: 10.1128/iai.64.4.1272-1283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudin A, Johansson E, Bergquist C, Holmgrem J. Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of human. Infect Immun. 1998;66:3390–3396. doi: 10.1128/iai.66.7.3390-3396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez J, Johansson S, Löwenadler B, Svennerholm A M, Holmgren J. Recombinant cholera toxin B subunit and gene fusion protein for oral vaccination. Rev Microbiol. 1990;141:971–979. doi: 10.1016/0923-2508(90)90137-f. [DOI] [PubMed] [Google Scholar]
- 22.Schuchat A. Group B streptococcus. Lancet. 1999;353:51–55. doi: 10.1016/S0140-6736(98)07128-1. [DOI] [PubMed] [Google Scholar]
- 23.Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513. doi: 10.1128/cmr.11.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen, X. Z., T. Lagergård, Y. H. Yang, M. Lindblad, M. Fredriksson, and J. Holmgren. Preparation and preclinical evaluation of experimental group B streptococcus type III polysaccharide-cholera toxin B subunit conjugate vaccine for intranasal immunization. Vaccine, in press. [DOI] [PubMed]
- 25.Sutton A, Vann W, Karpas A, Stein K E, Schneerson R. An avidin-biotin based ELISA for quantitation of antibody to bacterial polysaccharides. J Immunol Methods. 1985;82:215–224. doi: 10.1016/0022-1759(85)90353-9. [DOI] [PubMed] [Google Scholar]
- 26.Wessels M R, Paoletti L C, Kasper D L, DiFabio J L, Michon F, Holme K, Jennings H J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B streptococcus. J Clin Investig. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamane N, Yuki M, Kyono K. Isolation and characterization of group B streptococci from genitourinary tracts in Japan. Tohoku J Exp Med. 1983;141:327–335. doi: 10.1620/tjem.141.327. [DOI] [PubMed] [Google Scholar]
- 28.Zangwill K M, Schuhat A, Wenger J D. Group B streptococcal disease in the United States, 1990: report from a multistate active surveillance system. Morb Mortal Wkly Rep CDC Surveil Summ. 1992;41:25–32. [PubMed] [Google Scholar]
- 29.Zhang J H, Yuan L, Yang Y H. Perinatal colonization of group B streptococcus: a study of 600 cases in Beijing Tiantan hospital. Chin J Epidemiol. 1995;16:36–38. [PubMed] [Google Scholar]
- 30.Zhou F, Kraehenbuhl J P, Neutra M R. Mucosal IgA response to rectally administered antigen formulated in IgA-coated liposomes. Vaccine. 1995;13:637–644. doi: 10.1016/0264-410x(94)00029-m. [DOI] [PubMed] [Google Scholar]