Abstract
Background:
A precision medicine approach in type 2 diabetes requires identification of clinical and biological features that are reproducibly associated with differences in clinical outcomes with specific anti-hyperglycaemic therapies. Robust evidence of such treatment effect heterogeneity could support more individualized clinical decisions on optimal type 2 diabetes therapy.
Methods:
We performed a pre-registered systematic review of meta-analysis studies, randomized control trials, and observational studies evaluating clinical and biological features associated with heterogenous treatment effects for SGLT2-inhibitor and GLP1-receptor agonist therapies, considering glycaemic, cardiovascular, and renal outcomes.
Results:
After screening 5,686 studies, we included 101 studies of SGLT2-inhibitors and 75 studies of GLP1-receptor agonists in the final systematic review. The majority of papers had methodological limitations precluding robust assessment of treatment effect heterogeneity. For glycaemic outcomes, most cohorts were observational, with multiple analyses identifying lower renal function as a predictor of lesser glycaemic response with SGLT2-inhibitors and markers of reduced insulin secretion as predictors of lesser response with GLP1-receptor agonists. For cardiovascular and renal outcomes, the majority of included studies were post-hoc analyses of randomized control trials (including meta-analysis studies) which identified limited clinically relevant treatment effect heterogeneity.
Conclusions:
Current evidence on treatment effect heterogeneity for SGLT2-inhibitor and GLP1-receptor agonist therapies is limited, likely reflecting the methodological limitations of published studies. Robust and appropriately powered studies are required to understand type 2 diabetes treatment effect heterogeneity and evaluate the potential for precision medicine to inform future clinical care.
Plain language summary:
This review identifies research that helps understand which clinical and biological factors that are associated with different outcomes for specific type 2 diabetes treatments. This information could help clinical providers and patients make better informed personalized decisions about type 2 diabetes treatments. We focused on two common type 2 diabetes treatments: SGLT2-inhibitors and GLP1-receptor agonists, and three outcomes: blood glucose control, heart disease, and kidney disease. We identified some potential factors that are likely to lessen blood glucose control including lower kidney function for SGLT2-inhibitors and lower insulin secretion for GLP1-receptor agonists. We did not identify clear factors that alter heart and renal disease outcomes for either treatment. Most of the studies had limitations, meaning more research is needed to fully understand the factors that influence treatment outcomes in type 2 diabetes.
INTRODUCTION
Two of the most recently introduced anti-hyperglycaemic drug classes, SGLT2-inhibitors (SGLT2i) and GLP1-receptor agonists (GLP1-RA), have been shown in randomized clinical trials not only to reduce glycaemia1 but also to lower the risk of renal and cardiovascular disease (CVD) outcomes among high-risk individuals with type 2 diabetes (T2D)2–5. Based on average treatment effects reported in placebo-controlled trials, current T2D clinical consensus guidelines recommend a stratified approach to treatment selection, preferentially recommending these drug classes independent of their glucose lowering effect for individuals with cardiovascular or renal comorbidity. Specifically, people with heart failure and/or chronic kidney disease are recommended to initiate SGLT2i and people with prior CVD or high risk for CVD are recommended to initiate either an SGLT2i or a GLP1-RA. In addition, these drugs are recommended as second-line glucose lowering medications to be added after metformin6.
A limitation of the current stratified approach to SGLT2i and GLP1-RA treatment in clinical guidelines is that is informed by trial recruitment strategies, and consequential accumulation of evidence of treatment benefits only for specific subgroups with or at high risk of cardiorenal disease, rather than from an understanding of how the benefits and risks of each drug class vary across the whole spectrum of T2D. A more comprehensive approach to treatment selection would require recognition of the extreme heterogeneity in the demographic, clinical, and biological features of people with T2D, and the impact of this heterogeneity on drug-specific clinical outcomes. Identification of robust and reproducible patterns of heterogenous treatment effects is plausible as, at the individual patient level, responses to the same drug treatment appears to vary greatly7. A greater understanding of population-wide heterogenous treatment effects and enhanced capacity to predict individual treatment responses is needed to advance towards the central goal of precision type 2 diabetes medicine—where demographic, clinical, biological, or other patient-level features may be used to match individuals to their optimal anti-hyperglycaemic regimen as part of routine T2D diabetes care.
To assess the evidence base for treatment effect heterogeneity for SGLT2i and GLP1-RA, we undertook a systematic literature review to summarize key findings from studies that specifically examined interactions between individual-level biomarkers and the effects of these drug classes on clinical outcomes. Although biomarkers may connote laboratory-based measurements in traditional contexts, herein we broadly conceptualized biomarkers as individual-level demographic, clinical, and biological features including both laboratory measures as well as genetic and genomic markers. We focused on three categories of outcomes relevant to T2D care: (1) glycaemic response (as measured by hemoglobin A1c; HbA1c); (2) CVD outcomes; and (3) renal outcomes. Our review was guided by the following research question: In a population with T2D, treated with SGLT2i or GLP1-RA, what are the biomarkers associated with heterogenous treatment effects in glycaemic, CVD, and renal outcomes? Each of the three outcomes were evaluated separately for each of the two drug classes for a total of 6 sub-studies. Given the heterogeneity of the T2D population, we anticipated that we would find one or more biomarkers modifying the effects of SGLT2i and GLP1-RA.
The Precision Medicine in Diabetes Initiative (PMDI) was established in 2018 by the American Diabetes Association (ADA) in partnership with the European Association for the Study of Diabetes (EASD). The ADA/EASD PMDI includes global thought leaders in precision diabetes medicine who are working to address the burgeoning need for higher quality, individualized diabetes prevention and care through precision medicine8. This Systematic Review is written on behalf of the ADA/EASD PMDI as part of a comprehensive evidence evaluation in support of the 2nd International Consensus Report on Precision Diabetes Medicine.
METHODS
We conducted a systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines9. See Appendix 1 for a checklist of each component. We first developed and iterated a protocol for the review (CRD42022303236). As above, our review was guided by the following research question: In a population with T2D, treated with SGLT2i and GLP1-RA, what are the biomarkers associated with heterogenous treatment effects in glycaemic, CVD, and renal outcomes?
Search Strategy
The search strategy for this review was developed for each drug class (SGLT2i and GLP1-RA) and outcome (glycaemic, cardiovascular, and renal) to capture studies specifically evaluating treatment effect heterogeneity associated with demographic, clinical, and biological features in people with type 2 diabetes. Potential effect modifiers of interest comprised age, sex, ethnicity, clinical features, routine blood tests, metabolic markers, and genetics; all search terms were based on medical subject subheadings (MeSH) terms and are reported in Supplementary Material section 1. SGLT2i and GLP1-RA were evaluated at drug class level and we did not aim to identify within-class heterogeneity in treatment effects. Electronic searches were performed in PubMed and Embase by two independent academic librarians in February 2022. Forwards and backwards citation searching was conducted but grey literature and white papers were not searched.
Inclusion criteria
To be included, studies were required to meet the following criteria:
Full-text English-language publications of RCTs, meta-analyses, post-hoc analyses of RCTs, pooled cohort analyses, prospective and retrospective observational analyses published in peer-reviewed journals.
Adult populations with type 2 diabetes taking at least one of either SGLT2i or GLP1-RA with sample size >100 for the active drug of interest.
At least a 4 month potential follow up period after initiation of the drug class of interest.
Randomized control trials (RCTs) required a comparison against placebo or an active comparator antihyperglycaemic drug. Observational studies did not require a comparator group.
Pre-specified aim of the study to examine heterogeneity in treatment outcome, such as biomarker-treatment interactions, stratified analyses, or heterogeneity-focused machine learning approaches.
Reported differential effects of the drug class on an outcome of interest with respect to a biomarker.
All individual trial or observational cohorts included in a meta-analysis or pooled cohort analysis must have met the inclusion criteria stated above.
We further excluded studies based on the following criteria: studied type 1 or other forms of non-type 2 diabetes; included children/minors; inpatient studies; conference proceeding abstracts, editorials, opinions papers, book chapters, clinical trial registries, case reports, commentaries, narrative reviews, or non-peer reviewed studies; did not adequately adjust for confounders (individual RCTs and observational studies only, this criteria was not applied for meta-analyses and pooled cohort analyses); did not address the question of treatment response heterogeneity for biomarkers of interest.
Titles and abstracts were independently screened by pairs of research team members to identify potentially eligible studies; these were then independently evaluated for inclusion in the full-text review. Any discrepancies were discussed with a third author until reaching consensus. Discrepancies were discussed as part of larger group meetings to ensure consistency in decisions across reviewer pairs.
Data Extraction and Quality Assessment
Pairs of authors independently reviewed the main reports and supplementary materials and extracted the following data for each of the included papers:
Publication (PMID, journal, publication year, first author, title, study type)
Study (setting and region, study time period, follow up period)
Population (overall characteristics, ethnicity)
Intervention (Drug class, specific therapies, treatment/comparator arm sizes)
Statistical analysis (outcome, outcome measurement, subgroups/predictors analysed with respect to biomarkers, statistical model, covariate set)
Results (relevant figures and tables, main findings, methodology, quality)
After data were extracted, information was synthesized by drug class and outcome and further examined by biomarkers or subgroups analyzed within each study. Results were extracted within these subsections and summarized for each paper, where general trends in results for each subsection were outlined.
Risk of bias evaluations using the Joanna Briggs Institute (JBI) critical appraisal tool for cohort studies10 was also conducted alongside the data extraction by each pair of authors. This was used to determine the extent of bias within the study’s design, execution, and analysis, specifically within the population, outcome measurements, and statistical modelling. Further detail on the risk of bias can be seen in Supplementary Figure 2. Additionally, the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework11 was applied at the outcome level for each drug class to determine the quality of evidence and certainty of effects for these subsections; an overall GRADE evaluation for all evidence was also provided.
Outcomes
Three outcome categories were assessed in the included studies: (1) changes in HbA1c associated with treatment; (2) CVD outcomes limited to cardiovascular (CV)-related death, non-fatal myocardial infarction, non-fatal stroke, hospitalization for angina, coronary artery bypass graft, percutaneous coronary intervention, hospitalization for heart failure, carotid endarterectomy, and peripheral vascular disease; and (3) renal outcomes including development of chronic kidney disease, and longitudinal changes in markers of renal function including eGFR and albuminuria. Specific measurements and their procedures for each category of outcome varied across the included studies. Summaries of the included papers assessing each outcome for each drug class are reported in Supplementary Tables 1a–f.
RESULTS
Literature search and screening results
Supplementary Figure 1 depicts the outcomes of the study screening processes for SGLT2i (1a) and GLP1-RA (1b).
For SGLT2i, a total of 3415 unique citations underwent title and abstract screening. 3076 were determined to not meet the pre-defined eligibility criteria. The remaining 339 full-text articles were screened, through which process 238 articles were excluded. The most common reasons for exclusion included studies which did not report on heterogeneity of treatment response (126 studies), studies reporting only univariate or unadjusted associations (41 studies), and studies that did not meet inclusion criteria (64 studies). In total, 101 studies were identified for inclusion based on the systematic search.
For GLP1-RA, a total of 2270 unique citations underwent title and abstract screening. 2109 were determined to not meet the pre-defined eligibility criteria. The remaining 161 full-text articles were screened, through which process 86 articles were excluded. The most common reasons for exclusion included studies that did not meet inclusion criteria (39 studies), and studies reporting only univariate or unadjusted associations (26 studies), and studies which did not report on heterogeneity of treatment response (17 studies). In total, 75 studies were identified for inclusion.
Description of included studies
Included studies for CVD and renal outcomes were predominantly secondary analyses of industry-funded placebo-controlled trials (RCT), or meta-analyses of these trials, with a smaller number of observational studies. For glycaemic outcomes, most studies were observational. Supplementary Table 1 (a–f) shows all included studies – for each of GLP1-RA and SGLT2i, split by glycaemic, CVD, and renal outcomes, and including information on study population size, examined biomarkers, and significant findings. Summaries of the individual RCTs informing included meta-analyses are detailed in Supplementary Table 2.
SGLT2i, GLP1-RA AND GLYCAEMIC OUTCOMES
Study quality for assessment of heterologous treatment effects for both SGLT2i and GLP1-RA was variable with strong methodological limitations for the study of predictors of glycaemia treatment response common. A core weakness with many studies was a lack of head-to-head comparisons between therapies, which is required to separate broader prognostic factors (that predict response to any glucose-lowering therapy) from drug-specific factors that are associated with differential treatment response. Put otherwise, even when data suggested that a biomarker was associated with glycaemic response, it was not clear if this factor was helpful for choosing between therapies due to the lack of an active comparator. Other common methodological weaknesses included the use of arbitrary subgroups (rather than assessment of continuous predictors), small numbers in comparator subgroups which limited statistical power, dichotomized outcomes (responder analysis), multiple testing, and lack of adjustment for key potential confounders.
SGLT2i
Of 27 studies that met our inclusion/exclusion criteria, 9 observational studies (usually retrospective analysis of healthcare records), 5 post-hoc analysis of individual randomized control trials (RCTs), 10 pooled analyses of individual data from multiple RCTs, and 3 RCT meta-analyses were included (Supplementary Table 1e). All included studies assessed routine clinical characteristics and routinely measured clinical biomarkers (Table 1). No pharmacogenetic, or, with the exception of one study of HOMA-B,12 non-routine biomarker studies were identified.
Table 1:
Summary of evidence for treatment effect heterogeneity for SGLT2-inhibitor and GLP1-receptor agonist therapies for glycaemic, cardiovascular (including heart failure), and renal outcomes
| GLP1-RA | SGLT2i | Plain language summary | |||||
|---|---|---|---|---|---|---|---|
| Glycaemic outcomes | GRADE EVIDENCE C | GRADE EVIDENCE B | |||||
| Biomarker | N (observational) | N (RCT) | N (Meta-analysis and pooled RCT) | N (observational) | N (RCT) | N (Meta-analysis and pooled RCT) | |
| Glycaemia Biomarkers | 1834–38,42,44,45,51,52,58,59,134–139 | 1129,41,43,47,53,56,60,140–143 | 150 | 421,144–146 | 224,29 | 312,22,23 | Greater glycaemic response for both SGLT2i and GLP1-RA seen in individuals with higher baseline HbA1c, No studies available to comparing the relative efficacy of SGLT2i to GLP1-RA at different baseline HbA1c levels. |
| Renal Function | 636,37,42,51,59,136 | 429,41,56,60 | 161 | 2145,146 | 313,15,29 | 416,17,19,23 | GLP1-RA: no evidence that renal function alters glycaemic response. SGLT2i: Lesser glycaemic response for renally impaired patients and those with lower baseline kidney function (eGFR). |
| BMI | 1534–38,42,45,51,54,55,58,59,134,136,137 | 1129,41,43,53,56,57,60,141,142,147,148 | 0 | 418,21,144,145 | 224,29 | 219,23 | No consistent evidence of significant modifying effects of BMI on glycaemic response for either SGLT2i or GLP1-RA. |
| Age | 1334–38,42,44,51,58,59,134,136,137 | 729,41,43,56,60,142,149 | 0 | 414,21,144,145 | 129 | 619,23,30–32,150 | GLP1-RA: No evidence that age alters glycaemic response with GLP1-RA. SGLT2i: some studies suggest older age may be associated with reduced glycaemia response, however analyses usually did not adjust for eGFR which may confound this association as eGFR declines with age. |
| Diabetes duration | 1534–39,42,44,45,51,58,59,134,136,138 | 529,43,47,56,60 | 146 | 3144–146 | 129 | 123 | SGLT2i: No consistent effect of diabetes duration on glycaemic response. GLP1-RA: Longer diabetes duration (or proxies such as insulin treatment) associated with lesser glycaemic response. |
| Sex | 1334–38,42,45,51,52,59,134,136,137 | 629,43,47,53,56,60 | 0 | 421,27,144,145 | 129 | 319,23,150 | No consistent evidence of significant modifying effects of sex on glycaemic response for either SGLT2i or GLP1-RA. |
| Ethnicity | 237,64 | 529,56,60,62,65 | 163 | 127 | 129 | 412,23,25,26 | No consistent evidence of differences in glycaemic response across ethnic groups for either SGLT2i or GLP1-RA. |
| Genetics | 240,134 | 166 | 0 | 0 | 0 | 0 | SGLT2i: no studies examined genetic factors. GLP1-RA: Two small studies suggest variants rs163184 and rs10305420 may be associated with lesser response in individuals of Chinese ethnicity. |
| Non-routine biomarkers | 1234,35,37,38,42,45,51,54,59,136,137,151 | 341,60,152 | 0 | 0 | 1153 | 112 | SGLT2i: No evidence of heterogeneity in treatment response for measures of insulin secretion and insulin resistance, or for patients with obstructive sleep apnea. GLP1-RA: Observational studies suggest markers of lower insulin secretion including lower fasting C-peptide, lower urine C-peptide-to-creatinine ratio, and positive GAD or IA2 islet autoantibodies are associated with lesser glycaemic response. In contrast, post-hoc RCT analyses found insulin secretion does not modify glycaemic outcome. This may reflect trial inclusion criteria as participants had relatively higher beta-cell function compared with the observational cohorts. |
| Cardiovascular disease | GRADE EVIDENCE B | GRADE EVIDENCE B | |||||
| Race/ethnicity | 0 | 0 | 373,80,87 | 0 | 2154,155 | 473,75,80,107 | No heterogeneity by race/ethnicity for SGLT2is; Potential increased cardiovascular benefit in Asians associated with GLP1-RA use, but results inconsistent. |
| History of CVD | 3102–104 | 8122,156–161 | 780,87–92,95,96 | 4101–103,106 | 383,162,163 | 52,77,80,92,109 | No consistent impact on SGLT2i or GLP1-RA outcomes. |
| Age | 2104,105 | 3118,159,164 | 380,89,91 | 2101,106 | 1165 | 475–77,80 | No consistent heterogeneity by age for SGLT2is; No evidence of age effect for GLP1-RAs. |
| Sex | 399,104,105 | 1159 | 580,87,89,91,93,96 | 499,101,106,107 | 3165–167 | 476,80,168,169 | No consistent heterogeneity by sex for SGLT2is or GLP1-RAs. |
| Renal function | 1105 | 2124,170 | 580,81,87,89,91 | 2101,106 | 613,114–116,165,171 | 52,79–81,92 | No consistent heterogeneity by renal function on cardiovascular outcomes for SGLT2is; No evidence of heterogeneity by renal function on cardiovascular outcomes for GLP1-RAs. |
| BMI | 0 | 1123 | 580,87,89,91,94 | 1107 | 284,165 | 376,80,94 | No consistent heterogeneity by BMI for SGLT2is; Some inconsistent evidence suggesting higher baseline BMI may improve cardiovascular efficacy of GLP1-RAs. |
| Genetics | 0 | 0 | 0 | 0 | 0 | 0 | |
| Non-routine biomarkers | 0 | 586,172–175 | 0 | Greater benefit of SGLT2i in those with high levels of 3 biomarkers: hs Cardiac Troponin T, soluble suppression of tumorigenesis-2 (sST2), and insulin-like growth factor binding protein 7 (IGFBP7) levels | |||
| Heart Failure | GRADE EVIDENCE B | GRADE EVIDENCE B | |||||
| Ethnicity | 0 | 1176 | 173 | 0 | 4154,155,177,178 | 473–76 | Possibly greater relative benefit of SGLT2i in Asian and Black compared to white ethnicity; Potential increased efficacy of GLP1-RAs in Asian ethnicity. |
| Age | 0 | 2159,164 | 191 | 1101 | 1177 | 376 Bhatia 2021; 75 | No heterogeneity by age for SGLT2is or GLP1-RAs. |
| Sex | 0 | 2176 | 191 | 299,101 | 3166,167,177 | 376,78,169 | No heterogeneity by sex for SGLT2is or GLP1-RAs. |
| BMI | 0 | 0 | 191 | 0 | 384,177,178 | 276,78 | No consistent heterogeneity by BMI for SGLT2is or GLP1-RAs. |
| History of CVD | 2102,103 | 3159,160,176 | 390–92 | 498,101–103 | 483,162,163,179 | 42,74,78,82 | No consistent heterogeneity by CVD history for SGLT2is or GLP1-RAs. |
| History of HF | 0 | 0 | 0 | 1103 | 185 | 42,74,78,82 | No consistent heterogeneity by HF history for SGLT2is; No analysis on heterogeneity by HF history performed for GLP1-RAs. |
| HF severity/score | 0 | 0 | 0 | 1100 | 3157,180,181 | 174 | Greater relative benefit of SGLT2i in those with NYHA class II vs class III/IV in one meta-analysis; No analysis of heterogeneity by HF severity/score performed for GLP1-RAs. |
| Renal function | 0 | 0 | 191 | 2100,101 | 613,114–116,171,177 | 52,74,78,79,82 | No consistent heterogeneity by renal function. Single meta-analysis showed greater SGLT-2 benefit with lower eGFR and higher ACR; No evidence for heterogeneity by renal function for GLP1-RAs. |
| Genetics | 0 | 0 | 0 | 0 | 0 | 0 | |
| Non-routine biomarkers | 0 | 0 | 0 | 0 | 686,153,172–174,182 | 0 | No heterogeneity across a variety of non-routine biomarkers. No analysis on heterogeneity by non-routine biomarkers performed for GLP1-RAs. |
| Renal (eGFR changes / CKD progression / composite outcomes of these with or without ACR changes) | GRADE EVIDENCE B | GRADE EVIDENCE B | |||||
| Baseline HBA1c | 0 | 0 | 0 | 0 | 0 | 0 | |
| Renal Function | 0 | 3119,124,125 | 15 | 2126,127 | 6111,112,114–116,124 | 52,79,108–110 | Generally no relationship between either eGFR or ACR and GLP1-RA benefit. Greater relative benefit of SGLT2i in those with higher eGFR (although inconsistent results with some studies showing no impact and 1 observational study finding the opposite relationship [Koh 2021]). Generally no relationship between ACR/proteinuria and SGLT2i benefit. |
| BMI | 0 | 2}119,123 | 0 | 1126 | 284,178 | 176 | Greater GLP1-RA benefit with lower BMI but not seen consistently. Generally no effect on SGLT2i benefit |
| Age | 0 | 1118 | 0 | 2126,127 | 0 | 176 | No effect on GLP1-RA or SGLT2i benefit |
| Diabetes duration | 0 | 1121 | 0 | 0 | 0 | 176 | No effect on GLP1-RA or SGLT2i benefit |
| Sex | 0 | 0 | 0 | 1126 | 1167 | 176 | No effect on SGLT2i benefit |
| Ethnicity | 0 | 0 | 0 | 0 | 2178,183 | 176 | No effect on SGLT2i benefit |
| Genetics | 0 | 0 | 0 | 0 | 0 | 0 | |
| Non-routine biomarkers | 0 | 0 | 0 | 0 | 586,117,172–174 | 0 | No effect on SGLT2i benefit |
| Blood pressure/hypertension | 0 | 2119,120 | 0 | 1126 | 0 | 176 | No effect on GLP1-RA or SGLT2i benefit |
| History of CVD/HF | 0 | 2119,122 | 0 | 1126 | 3162,163,184 | 32,77,109 | No effect on GLP1-RA or SGLT2i benefit |
| Renal (albuminuria changes) | GRADE EVIDENCE B | GRADE EVIDENCE B | |||||
| Baseline HBA1c | 0 | 0 | 0 | 0 | 0 | 0 | |
| Renal Function | 0 | 2119,125 | 15 | 0 | 0 | 1110 | Greater GLP1-RA benefit with higher ACR although not seen consistently. No relationship between eGFR and GLP1-RA. No effect on SGLT2i benefit |
| BMI | 0 | 1119 | 0 | 0 | 0 | 0 | No effect on GLP1-RA benefit |
| Age | 0 | 0 | 0 | 0 | 0 | 0 | |
| Diabetes duration | 0 | 0 | 0 | 0 | 0 | 0 | |
| Sex | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ethnicity | 0 | 0 | 0 | 0 | 1183 | 0 | No effect on SGLT2i benefit |
| Genetics | 0 | 0 | 0 | 0 | 0 | 0 | |
| Non-routine biomarkers | 0 | 0 | 0 | 0 | 1117 | 0 | Single trial found greater SGLT2i benefit at higher IGFBP7 |
| Blood pressure/hypertension | 0 | 1119 | 0 | 0 | 0 | 0 | No effect on GLP1-RA benefit |
| History of CVD/HF | 0 | 1119 | 0 | 0 | 0 | 0 | No effect on GLP1-RA benefit |
A key finding across multiple studies including appropriately adjusted analysis of RCT and observational data was that HbA1c reduction with SGLT2i is substantially reduced with lower eGFR13–19. In pooled RCT data for canagliflozin 300mg, 6-month HbA1c reduction was estimated to be 11.0 mmol/mol for participants with eGFR ≥90 mL/min/1.73 m2, compared to 6.7 mmol/mol for those with eGFR 45-6019. With empagliflozin 25mg, 6-month HbA1c reduction was 9.6 mmol/mol at eGFR ≥90, and 4.3 mmol/mol at eGFR 30-6016.
A further finding confirmed by multiple robust studies is that in keeping with other glucose-lowering agents higher baseline HbA1c is associated with greater HbA1c lowering with SGLT2 inhibitors, including verses placebo12,18,20–24. Active comparator studies suggested that higher baseline HbA1c may predict greater relative HbA1c response to SGLT2i therapy in comparison to DPP4i and sulfonylurea therapy12,22,23. Notably, an individual participant data meta-analysis of two RCTs showed greater improvement with empagliflozin (6-month HbA1c decline per unit higher baseline HbA1c [HbA1c slope] −0.49%] compared to sitagliptin (6-month HbA1c slope −0.29% [95%CI −0.42, −0.15]) and glimepiride (12-month HbA1c slope: empagliflozin −0.52% [95%CI −0.59, −0.44]; glimepiride −0.32% [95%CI −0.39, −0.25])22.
A number of studies assessing differences in glycaemic response to SGLT2i by ethnicity suggest that initial glycaemic response to this medication class does not vary by ethnicity25–29. Similarly, many studies also showed that response did not vary meaningfully vary by sex. Some studies suggested older age may be associated with reduced glycaemia response; however, analyses usually did not adjust for eGFR which may confound this association as eGFR declines with age14,20,29–32.
GLP1-RA
Of 49 studies that met our inclusion/exclusion criteria, 24 observational studies, 6 post-hoc analysis of individual randomized control trials (RCTs), and 19 meta-analyses were included (Supplementary Table 1f). The majority of included studies assessed routine clinical characteristics and routinely measured clinical biomarkers, although 3 studies evaluated genetic variants, and 15 studies evaluated non-routine biomarkers (Table 1).
Studies consistently identified baseline HbA1c as a predictor of greater HbA1c response. For other clinical features, the strongest evidence was that in many observational studies markers of lower insulin secretion (including longer diabetes duration [or proxies such as insulin treatment], lower fasting C-peptide, lower urine C-peptide-to-creatinine ratio, and positive GAD or IA2 islet autoantibodies) were associated with lesser glycaemic response to GLP1-RA33–46. One large prospective study (n=620) observed clinically relevant reductions in HbA1c response with GLP1-RA in individuals with GAD or IA2 autoantibodies (mean HbA1c reduction −5.2 vs. −15.2 mmol/mol without autoantibodies) or C-peptide <0.25 nmol/L (mean HbA1c reduction −2.1 vs. −15.3 mmol/mol with C-peptide >0.25 nmol/L). In contrast, post-hoc RCT analyses has found T2D duration47 and beta-cell function48,49 do not modify glycaemic outcome. This may reflect trial inclusion criteria as included participants had relatively higher beta-cell function, and were less-commonly insulin-treated, compared with the observational cohorts48.
Few studies contrasted HbA1c outcome for GLP1-RA versus a comparator drug. One meta-analysis showed a greater HbA1c reduction with the GLP1-RA liraglutide compared to other antidiabetic drugs (sitagliptin, glimepiride, rosiglitazone, exenatide, and insulin glargine) across all baseline HbA1c categories (n=1,804)50, a finding supported for the GLP1-RA dulaglutide compared to glimepiride and insulin glargine51.
Overall, there was no consistent evidence for effect modification by body mass index (BMI), sex, age or kidney function, with studies reporting contrasting, or null, associations for these clinical features36,37,41–43,47,51–61. In comparative analysis, one large observational study found that markers of insulin resistance (including higher HOMA-IR, BMI, fasting triglycerides, and HDL) do not alter GLP1-RA response, but are associated with lesser DPP4-inhibitor response54.
There was limited evidence for differences by ethnicity. One large pooled RCT analysis (N=2,355) suggested greater HbA1c response in Asian participants compared to those of other ethnicities, but other studies have not identified differences in response across ethnic groups62–65. Similarly, limited studies evaluated pharmacogenetics, although two small studies suggest variants rs163184 and rs10305420, but not rs3765467, may be associated with lesser response in Chinese patients40,66.
SGLT2i, GLP1-RA AND CARDIOVASCULAR OUTCOMES
SGLT2i: Evidence from clinical trials
Of 65 studies, 58 were post-hoc meta-analysis of RCTs or meta-analysis of multiple RCTs. Heart failure (HF) was common as a secondary outcome. The majority of studies were derived from EMPA-REG67 and the CANVAS program68, although more recent meta-analyses included up to 12 cardiovascular outcome trials (CVOTs) with different inclusion criteria, treatments, primary outcomes, and follow-up duration (Supplementary Table 2a). Most studies included only participants with established CVD or elevated cardiovascular risk, although some studies were restricted to patients with pre-existing heart failure or chronic kidney disease. While most CVOTs and meta-analyses included only patients with type 2 diabetes, some meta-analyses also included data from patients without diabetes in the EMPEROR-P69, EMPEROR-R70, DAPA-HF71 and DAPA-CKD72 RCTs. Studies primarily focused on relative rather than absolute treatment effects and one of two primary outcomes: 3-point MACE which was a composite of cardiovascular death, non-fatal MI, and non-fatal stroke; or composite heart failure outcomes including hospitalized heart failure and cardiovascular death. The longest duration of follow-up was in the CANVAS CVOT with a median follow-up of 5.7 years, although most other included CVOTs had durations of 1 to 4 years.
On average, in relative terms SGLT2i reduce the risk of cardiovascular disease (MACE) by 10% (HR 0.90 [95%CI 0.85, 0.95]), and heart failure hospitalization by 32% (HR 0.68 [95%CI 0.61, 0.76]) in individuals at with or at high-risk of CVD2. The majority of meta-analyses of CVOTs found no significant interactions for MACE or heart failure outcomes across a variety of biomarkers (Supplementary Table 1a). Several meta-analyses found no interactions by age, sex, and adiposity for MACE or heart failure outcomes. Four meta-analyses examined interactions by race for MACE outcomes and found no interactions. Three meta-analyses have consistently identified a greater relative heart failure benefit of SGLT2i in people of Black and Asian ethnicity73–75 (HR SGLT2i versus placebo 0.60 [95% CI 0.47, 0.74]) compared to White individuals (HR 0.82 [95% CI 0.73, 0.92])73, however one meta-analysis reported no difference between Caucasian and non-Caucasian individuals76.
Contemporary meta-analysis incorporating the CREDENCE and VERTIS-CV trials alongside EMPA-REG, CANVAS, and DECLARE suggests history of cardiovascular disease does not modify the efficacy of SGLT2i for MACE2,77. One meta-analysis suggests heart failure severity modifies the efficacy of SGLT2i’s for heart failure outcome (composite outcome of cardiovascular death or hospitalization for heart failure) with greater efficacy in patients with NYHA heart failure class II (HR SGLT2i versus placebo 0.66 [95%CI 0.59, 0.74]) than class III or IV (HR 0.86 [95%CI 0.75, 0.99])74. Other meta-analyses that examined treatment effect heterogeneity using heart failure history as a binary predictor did not find significant interactions2,78.
A recent meta-analysis79 that included 6 CVOTs of patients with diabetes and 4 CVOTs of patients with and without diabetes found that eGFR did not alter the relative benefit of SGLT2 inhibitors for MACE and heart failure outcomes2,74,78,80–82; however, a greater relative benefit was reported for MACE in those with higher baseline albuminuria (ACR>300mg/g HR 0.74 95%CI 0.66, 0.84; ACR 30-300mg/g HR 0.95 [95%CI 0.82, 1.10]) ACR<30mg/g HR 0.87 [95%CI 0.77, 0.98]).
We identified many secondary analyses of single CVOTs, which largely found no interactions by biomarkers (Supplementary Table 1a). Single studies identified potential effect modification for MACE by history of CVD83, and obesity84, and history of heart failure for heart failure outcome85, but these associations were not replicated across the other studies or in multi-RCT meta-analysis. In a secondary analysis of CANVAS, participants with higher levels of biomarkers of cardiovascular stress (high-sensitivity cardiac troponin T (hs-cTnT), soluble suppression of tumorigenesis-2 (sST2), and insulin-like growth factor binding protein 7 (IGFBP7)) level had greater relative benefit for MACE; for a multimarker score summing high levels of these 3 biomarkers the relative benefit of SGLT2i for no abnormal biomarkers was HR: 0.99; 95% CI: 0.66-1.49, 1 abnormal biomarker (HR: 1.34; 95% CI: 0.94-1.89), 2 abnormal biomarkers (HR: 0.61; 95% CI: 0.45-0.82), and 3 abnormal biomarkers (HR: 0.46; 95% CI:0.18-1.17; Pinteraction trend =0.005)86. Unlike meta-analyses, studies based on single RCTs typically performed multivariable adjustment for potential confounders.
GLP1-RA: Evidence from clinical trials
Of the 35 studies that investigated heterogeneity in effect of GLP1-RAs on cardiovascular health and met our inclusion criteria, 15 were meta-analyses of RCTs or pooled analyses of multiple RCTs, 15 were post-hoc analyses of RCTs, and 5 were observational studies (Supplementary Table 1b). Most studies used data collected from the LEADER, SUSTAIN 6, and EXSCEL trials, however in total the data from 7 CVOTs were used (Supplementary Table 2b). The majority of these CVOTs investigated the effect of previous cardiovascular disease on the cardiovascular efficacy of GLP1-RAs using 3-point MACE as a primary outcome, and with heart failure being a common secondary outcome, focusing on relative rather than absolute benefit. The population of 6 of the 7 CVOTs had established cardiovascular disease or high cardiovascular disease risk. The CVOT with the longest median follow up was REWIND with a median follow up of 5.4 years, and the median follow up of the other CVOTs ranged from 1 to 4 years.
Contemporary meta-analysis data suggests GLP1-RA reduce the relative risk of cardiovascular disease (MACE) by 14% (HR 0.86 [95%CI 0.80-0.93]), and heart failure hospitalization by 11% (HR 0.89 [95%CI 0.82, 0.98]) compared to placebo3. Several large meta-analyses examining heterogenous treatment effects in placebo-controlled CVOTs have been conducted for GLP1-RA73,80,81,87–94, with the majority of studies focusing on whether prior established CVD modifies the relative effect of GLP1-RA on MACE and/or heart failure. Two meta-analyses reported the relative MACE benefit of GLP-RA may be restricted to those with established CVD80,87, the largest of which included 7 RCTs and reported a 14% relative risk reduction with GLP1-RA specific to individuals with established CVD (with CVD: HR 0.86 [95%CI 0.80, 0.93]; at high-risk of CVD: HR 0.94 [95% CI 0.82, 1.07])80. However, this risk difference is not conclusive and has not been replicated in other meta-analyses and pooled RCT analyses88–90,95,96, including an individual participant level re-analysis of the SUSTAIN and PIONEER RCTs which evaluated baseline CVD risk as a continuous rather than subgroup-level variable97.
Differential relative effects of GLP1-RAs on MACE have been reported by ethnicity in two out of three meta-analyses73,80,87: one showed a benefit of GLP1-RA treatment compared to placebo in Asian (HR 0.76 [95%CI 0.61, 0.96]) and Black (HR 0.77 [95%CI 0.59, 0.99]) individuals, but not in white individuals (HR 0.95 [95%CI 0.88, 1.02])87; the second another showed a significantly greater benefit of GLP1-RA for MACE in Asian compared to White individuals (HR Asian 0.68 [95%CI 0.53, 0.84]; White 0.87 [95% 0.81, 0.94])73. For other clinical features including sex, BMI/obesity, baseline kidney disease, duration of diabetes, baseline HbA1c, background glucose lowering medications, and prior history of microvascular disease, the overall body of evidence from meta-analyses does not provide robust evidence to support differential effects of GLP1-RA on CVD outcomes (Table 1).
SGLT2i and GLP1-RA: Evidence from observational studies
10 observational studies met our inclusion criteria, with studies primarily reporting relative rather than absolute risk differences98–107. These studies comparing SGLT2i and GLP1-RA individually with other oral therapies (predominantly DPP4i) generally reported average relative benefits for CVD and heart failure outcomes in-line with placebo-controlled trials, with no consistent pattern of subgroup level differences across studies (Supplementary Table 1a and 1b).
A few observational studies compared SGLT2i and GLP1-RA CVD outcomes. In a US claims-based study with follow-up to two years (n=47,343), Htoo et al.103 reported a higher relative risk of MACE with SGLT2i compared to GLP1-RA specific to individuals without CVD and heart failure (Relative risk [RR] 1.31 [95% CI 1.09, 1.56]), and a higher risk of stroke with SGLT2i versus GLP1-RA specific to individuals without CVD (No CVD without heart failure: RR 1.62 [95%CI 1.10, 2.38]; No CVD with heart failure: RR 3.30 [95%CI 1.22, 8.97]). In contrast, over a median follow-up of 7 months Patorno et al.102 reported a lower relative risk of myocardial infarction with SGLT2i compared to GLP1-RA in US claims data specific to individuals with a history of CVD (n=156,825; HR 0.82 [95%CI 0.71, 0.95]), with no differences in stroke outcomes irrespective of CVD status. Both studies reported a consistent benefit of SGLT2i over GLP1-RA for heart failure. Raparelli et al.99 identified potential differences by sex in the Truven Health MarketScan database (n=167,341), with, compared to sulfonylureas and over a median follow-up of 4.5 years, a greater relative reduction with GLP1-RA for females (HR 0.57 [95%CI 0.48, 0.68]) compared to males (HR 0.82 [95%CI 0.71, 0.95]), but a similar benefit for both sexes with SGLT2i (females HR 0.58 [95%CI 0.57, 0.83]; males HR 0.69 [95%CI 0.57, 0.83]).
SGLT2i, GLP1-RA AND RENAL OUTCOMES
SGLT2i: Evidence from clinical trials
29 studies met our inclusion criteria. These included 2 analyses of observational data, 20 post-hoc analyses of individual RCTs, and 7 trial meta-analyses (Supplementary Table 1c). All of the post-hoc RCT analyses and all but 1 of the meta-analyses used only data from the 12 SGLT2i cardiovascular/renal RCTs shown in Supplementary Table 2a, which therefore provided most of the evidence in our review. These trials included people with type 2 diabetes with and without pre-existing cardiovascular disease, and had composite renal endpoints incorporating two or more of the following: changes in eGFR/serum creatinine, end-stage renal disease, changes in urine albumin:creatinine ratio (ACR), and/or death from renal causes; which differed between trials. Most studies assessed routine clinical characteristics, especially renal function as measured by eGFR or urine ACR or a combination of both. In addition, 4 post-hoc RCT analyses examined non-routine plasma biomarkers. We found no genetic studies.
On average, SGLT2i have a relative benefit for a number of renal outcomes including kidney disease progression (HR 0.63, 95%CI 0.58,0.69) and acute kidney injury (HR 0.77, 95%CI 0.70, 0.84)4. Placebo-controlled trial meta-analyses of subgroups found no evidence for heterogeneity of SGLT2i treatment effects on relative renal outcomes by age76, use of other glucose-lowering drugs76, use of blood pressure/cardiovascular medications76,108, blood pressure76, BMI76, diabetes duration76, Caucasian ethnicity76, history of cardiovascular disease or heart failure2,77 or sex76.
For baseline eGFR, an early meta-analysis that included EMPA-REG, CANVAS and DECLARE reported greater effect of SGLT2i on renal outcomes in those with higher eGFR109 but both a later meta-analysis that added CREDENCE108 and a recent meta-analysis that added two further studies (SCORED and DAPA-CKD, including some participants without diabetes)79 showed no effect of baseline eGFR on renal outcomes with SGLT2i. For urine ACR, meta-analyses of subgroups found no evidence for greater SGLT2i effect with higher UACR2,79,108,110. Single RCTs found no heterogeneity of treatment effect by eGFR and UACR, or subgroups defined by the combination of these two 111–115, with the except of Neuen et al.116 which showed a greater SGLT2i effect in preventing eGFR decline relative to placebo for those with higher UACR, and heterogeneity in a composite renal outcome by UACR. Overall there was limited or no evidence to support modifying effects of baseline eGFR or UACR on the effect of SGTL2i on renal function outcomes.
A few post-hoc analyses of the CANVAS RCT considered non-routine biomarkers, with most showing no interaction with SGLT2i treatment and renal outcomes. Two RCTs studied the effect of SGLT2i on renal outcomes at differing plasma IGFBP7 levels. One study reported an interaction of IGFBP7 with SGLT2i treatment for progression of albuminuria (>96.5ng/ml FIR 0.64; <=96.5ng/ml HR 0.95, Pinteraction = 0.003)117 but no effect was seen for the composite renal endpoint in two studies86,117. The biomarker panel (sST2, IGFBP7, hs-cTnT) that showed a strong interaction with SGLT2i for MACE outcomes (see above) did not show any interaction for renal outcomes86.
GLP1-RA: Evidence from clinical trials
7 studies met our inclusion criteria: all post-hoc RCT analyses, 6 of individual trials (or multiple trials analysed separately) and 1 pooled analysis of two RCTS (Supplementary Table 1d). These studies used data from 5 of the 7 GLP1-RA cardiovascular outcome trials shown in Supplementary Table 2b, with renal outcomes only a secondary endpoint. Most of these trials had composite renal endpoints as per the SGLT2i cardiovascular/renal trials, whilst some examined changes in either eGFR or urine ACR only. All studies assessed routine clinical characteristics, especially renal function as measured by eGFR or urine ACR. No studies of genetics or non-routine biomarkers were identified. The overall sample sizes were small and subgroup analyses underpowered to show a subgroup by treatment interaction for renal outcomes.
Overall, GLP1-RA reduce the relative risk of albuminuria over 2 years by 24% versus placebo (HR 0.76 [95% CI 0.73-0.80; P<0.001), and similarly reduce the relative risk of a 40% reduction in eGFR (HR, 0.86 [95% CI 0.75-0.99]; P=0.039)5. Studies found no heterogeneity of GLP1-RA relative treatment effect by age118, blood pressure119,120, diabetes duration121, history of cardiovascular disease/heart failure119,122 or use of RAS inhibitors119. For BMI, a post-hoc analysis of EXSCEL (Exenatide) found a greater GLP1-RA effect on reducing rate of eGFR decline in those with lower BMI (BMI≤30kg/m2 treatment difference 0.26 mL/min/1.73m2/year [95% CI 0.04, 0.48] vs BMI>30 kg/m2 −0.12 [−0.26, 0.03], Pinteraction=0.005)119. However, Verma et al.123 found no significant interaction by BMI subgroup with GLP1-RA treatment for a composite renal outcome in LEADER (Liraglutide) or SUSTAIN 6 (Semaglutide).
For baseline eGFR, a pooled analysis of LEADER and SUSTAIN-6 reported a significant interaction, with lower eGFR associated with greater GLP1-RA effect in reducing eGFR decline: Semaglutide 1.0mg vs placebo, eGFR<60 difference in decline 1.62 ml/min/1.73m2/year vs eGFR>=60 difference in decline 0.64ml/min/1.73m2/year, Pinteraction=0.057; Liraglutide 1.8mg vs placebo, eGFR<60 difference in decline 0.67 ml/min/1.73m2/year vs 0.15ml/min/1.73m2/year, Pinteraction=0.008)5. However, a study of Exenatide LAR found no treatment heterogeneity for this same outcome by eGFR category119 and in a further analysis of LEADER the renal composite endpoint was used with no interaction reported by baseline eGFR category124. The overall evidence does not support an effect of baseline eGFR on the relative renal benefit for GLP1-RA as an overall drug class.
For baseline UACR, a pooled analysis of LEADER and SUSTAIN-65 and EXSCEL119 showed a greater benefit of GLP1-RA on eGFR reduction or eGFR slope with higher UACR however there was either no significant interaction5 or no formal interaction test was reported119. For ELIXA, Muskiet et al.125 did not find a significant interaction of UACR category on eGFR decline. A further study found no association between UACR and GLP1-RA effect on reducing a composite renal outcome124.
Two studies found that GLP1-RAs more effectively reduced UACR in those with higher UACR. In a pooled analysis of LEADER and SUSTAIN-6, those with normalbuminuria had a 20% (95%CI 15%, 25%) reduction in UACR compared to placebo; those with microalbuminuria had a 31% (95%CI 25-37%) reduction; those with macroalbuminuria had a 19% (95%CI 7-30%) Pinteraction=0.0215. In ELIXA, least-squares mean percentage change in UACR was −1·69% (SE 5·10; 95% CI −11·69 to 8·30; p=0·7398) in participants with normoalbuminuria, −21·10% (10·79; −42·25 to 0·4; p=0·0502) in participants with microalbuminuria, and −39·18% (14·97; −68·53 to −9·84; p=0·0070) in participants with macroalbuminuria in favour of lixisenatide; a formal test for interaction was not reported125. A third study found no treatment heterogeneity for this same outcome119.
In summary, the included studies showed conflicting results for renal outcomes of GLP1-RA, though the majority were underpowered to detect heterogenous treatment effects. The most consistent finding was that a higher UACR is associated with greater GLP1-RA reduction in UACR relative to placebo, but this does not translate to benefits in eGFR defined measures of renal function. There were no other biomarkers that robustly predicted benefit from GLP1-RA for the renal outcomes examined.
SGLT2i and GLP1-RA: Evidence from observational studies
There were no observational studies for GLP1-RA and renal outcomes included, and no comparison studies between people treated with GLP1-RA and SGLT2i. Observational studies comparing SGLT2i to other glucose-lowering drugs confirmed the lack of treatment effect heterogeneity associated with age126,127, use of blood pressure/cardiovascular medications127, blood pressure (Koh 2021), history of cardiovascular disease126 and sex126, but one study in a Korean population found greater SGLT2i benefit on progression to end stage renal impairment with higher BMI (BMI<25kg/m2, HR 0.80 (95%CI 0.51, 1.25); BMI≤25kg/m2 HR 0.27 (0.16, 0.44), Pinteraction=0.002) and with abdominal obesity compared to without126. This is not consistent with results from meta-analysis of RCTs.
SUMMARY OF QUALITY ASSESSMENT
To evaluate risk of bias, we used the JBI critical appraisal tool for cohort studies as the best flexible tool for the range of studies included. Due to our screening criteria no manuscripts that passed full text screening were excluded due to risk of bias. The checklist results for the 11 points in the appraisal checklist are shown as a heatmap in Supplementary Figure 2a (SGLT2i) and 2b (GLP1-RA).
Additionally, the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework was applied at the outcome level for each drug class to determine the quality of evidence and certainty of effects for these subsections. For SGLT2i Cardiovascular (GRADE B), SGLT2i renal (GRADE B), GLP1-RA Cardiovascular (GRADE B) and GLP1-RA Renal (GRADE B), the majority of our evidence base comes from industry-funded, CVOTs (RCT designs), including post-hoc analyses of individual trials as well as meta-analyses. There were limited observational studies.
For the two glycaemia outcomes, we scored the SGLT2i glycaemia outcomes as GRADE B but the GLP1-RA as GRADE C. This reflects that a larger proportion of the studies included for evaluation of GLP1-RA outcomes were observational (24/49). By contrast, for SGLT2i glycaemia outcomes there were (18) RCT/meta-analyses and (9) observational studies.
DISCUSSION
This systematic review provides a comprehensive review of observational and RCT-based studies of people with type 2 diabetes specifically examining heterologous treatment effects for SGLT2i and GLP1-RA therapies on glycaemic, cardiovascular, and renal outcomes. We assessed evidence for treatment effect modification for a wide range of demographic, clinical and biological features, including pharmacogenetic markers. Each of the three clinical outcomes were evaluated separately for each drug class for a total of 6 sub-studies. Overall, our review identified limited evidence for treatment effect heterogeneity for glycaemia, cardiovascular, and renal outcomes for the two drug classes. We summarize the key findings below.
For glycaemic response, there was strong evidence that reduced renal function is associated with lower efficacy of SGLT2i. For GLP1-RA, markers of reduced insulin secretion, either directly measured (e.g. c-peptide or HOMA-B) or proxy measures such as diabetes duration, were associated with reduced glycaemic response to GLP1-RA in the majority of observational studies. As with other glucose-lowering drug classes, a greater glycaemic response with both SGLT2i and GLP1-RA was seen at higher baseline HbA1c. We did not identify any studies examining whether the relative efficacy of SGLT2i compared to GLP1-RA is altered by baseline HbA1c levels.
For both CVD and heart failure outcomes, RCT meta-analyses do not support differences in the relative efficacy of either GLP1-RA or SGLT2i based on an individuals’ prior CVD status. However, this finding should be interpreted cautiously as all RCTs to-date have predominantly included participants with, or at high-risk of, CVD, thereby excluding the majority of the wider T2D population at lower risk. However, meta-analyses suggest the relative effects of both drug classes may be greater in people of non-white ethnicity. In particular, those of Asian and African ethnicity (compared to White Europeans) have been shown to have a greater relative benefit for hospitalization for heart failure/CV death (but not MACE) with SGLT2i, and MACE for GLP1-RA.
When evaluating renal outcomes, there was no consistent evidence of treatment heterogeneity for SGLT2i, but for GLP1-RA there was greater reduction in proteinuria in those with higher baseline proteinuria.
This limited evidence could reflect a true lack of heterogenous treatment effects, but is more likely to reflect an absence of clinical studies that were well designed or sufficiently powered to robustly identify and characterise treatment effect heterogeneity. Although five of the six sub-studies we evaluated were evaluated at GRADE B, there were methodological concerns with many of the included studies. As individual RCTs are by design powered only for the main effect of treatment128, our primary focus when reporting were meta-analyses of post-hoc subgroup analyses of RCTs, however we found the subgroup analyses in these studies primarily focused on stratification by baseline risk for the outcome in question e.g. baseline HbA1c on glycaemic response, CKD stage or albuminuria on renal outcomes, and CVD risk or established CVD for CVD outcomes. Other common subgroups included those defined by BMI, age, sex or other routinely collected clinical characteristics, with very few studies evaluating non-routine biomarkers or pharmacogenetic markers (as highlighted in Table 1). A major limitation was that studies predominantly focused on conventional “one-at-a-time” approaches to subgroup analysis, with very few studies assessing continuous features (such as BMI) on a continuous scale which is required to maximize power to detect treatment effect heterogeneity128,129.
It is also important to recognize that almost all the studies evaluating cardiovascular and renal endpoints included in our systematic review focused on the relative effect of a biomarker/stratifier on the outcome, as most studies reported a hazard ratio compared with a placebo arm for the outcome of interest (e.g. MACE, incident renal disease). This does not recognize that baseline absolute risk of these endpoints is likely to differ significantly across these strata; so although, for example, there was no difference in relative benefit of an SGLT2i by age, this means that on the absolute scale benefit will increase with age (as underlying absolute risk increases) and it is this absolute benefit that should be considered when deciding on whether to initiate SGLT2i treatment.
A significant finding of our review is the lack of robust comparative effectiveness studies directly examining treatment effect heterogeneity for these two major drug classes, either head-to-head or compared with other major anti-hyperglycaemic therapies. Insight into effect modification for a single drug class is not sufficient to support clinical translation of a precision medicine approach. The lack of direct comparisons between therapies obscures the interpretation of biomarkers with regards to whether they function as broad prognostic factors, which may be relevant to any (or at least multiple) drug class, or as markers of heterogenous treatment effects specific to a particular drug class. An evidence base that includes more high-quality studies on heterogeneity in the comparative effectiveness of SGLT2i, GLP1-RA, and other drug classes is needed to advance the field towards clinically useful precision diabetes medicine. For cardiovascular and renal outcomes, these studies need to incorporate both absolute outcome risk and relative estimates of treatment effects in order to usefully inform clinical decision making. Only when this evidence is available can precision medicine support more individualised treatment decisions, allowing providers to select an optimal therapy from a set of multiple options informed by each medication’s risk/benefit profile specific to the characteristics of an individual patient.
We identified the following additional, high-level gaps in our review:
Limited head-to-head comparative effectiveness studies examining treatment effect heterogeneity.
A lack of robust studies integrating multiple clinical features and biomarkers. The majority of studies only tested single biomarkers in “one-at-a-time” subgroup analysis.
Few studies focused on pharmacogenetics or non-routine biomarkers.
Few studies conducted in low-middle income countries, required for an equitable global approach to precision type 2 diabetes medicine.
Few RCT meta-analyses based on individual-level participant data, precluding robust evaluation of between-trial heterogeneity and individual-level confounders.
An absence of confirmatory studies. We identified no prospective studies testing a priori hypotheses of potential treatment effect modifiers, or studies conducting independent validation of previously described heterogenous treatment effects.
A lack of population-based data representing individuals treated in routine care. As cardiovascular and renal trials have focused on high-risk participants, the benefits of SGLT2i and GLP1-RA for primary prevention is a major unanswered question.
Few cardiovascular and renal outcome studies considering treatment effect modification on the absolute as well as relative risk scale.
A focus on short-term glycaemic outcomes, with limited studies investigating durability of glycaemic response or time to glycaemic failure.
Of note, several studies published since our data extraction was completed in February 2022 are worth highlighting as they fill some of these evidence gaps: the TriMaster study – an ‘n-of-1’ precision medicine RCT of SGLT2i, DPP4i and TZD that established that individuals with higher renal function (eGFR >90 ml/min/1.73 m2) have a greater HbA1c response with SGLT2i vs DPP4i relative to those with eGFR 60–90 ml/min/1.73 m2 130; a similarly designed two-way crossover trial in New Zealand which identified a greater relative benefit of TZD therapy compared to DPP4i in people with obesity and/or hypertriglyceridemia131; a study using large-scale observational data and post-hoc analysis of individual participant-level data from 14 RCTs that specifically investigated differential treatment effects with SGLT2i and DPP4i, and developed a treatment selection model to predict HbA1c response on the two therapies based on an individuals’ routine clinical characteristics132; and a robust study across observational and multiple RCTs identifying pharmacogenetic markers of differential glycaemic response to GLP1-RA133.
This review highlights the need for several research priorities to advance our limited understanding of heterogenous treatment effects among individuals with type 2 diabetes. We outline priorities for research to advance the field towards a translational model of evidence-based, empirical precision diabetes medicine (Figure 1), and highlight the recent Predictive Approaches to Treatment effect Heterogeneity (PATH) Statement to guide this research129. In the future, with a greater understanding of heterogenous treatment effects and enhanced capacity to predict individual treatment responses, precision treatment in type 2 diabetes may be able to integrate demographic, clinical, biological or other patient-level features to match individuals to their optimal anti-hyperglycaemic regimen.
Figure 1.
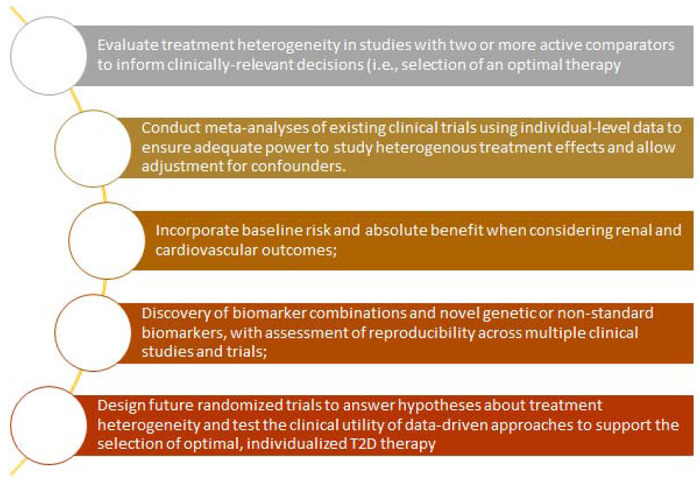
Priorities for future research to advance the field towards a translational model of evidence-based, empirical precision diabetes medicine
Conclusion
There is limited evidence of treatment effect heterogeneity with SGLT2i and GLP1-RA for glycaemic, cardiovascular and renal outcomes in people with type 2 diabetes. This lack of evidence likely reflects the methodological limitations of the current evidence base. Robust future studies to fill the research gaps identified in this review are required for precision medicine in type 2 diabetes to translate to clinical care.
Supplementary Material
Acknowledgements:
The ADA/EASD Precision Diabetes Medicine Initiative, within which this work was conducted, has received the following support: The Covidance license was funded by Lund University (Sweden) for which technical support was provided by Maria Bjorklund and Krister Aronsson (Faculty of Medicine Library, Lund University, Sweden). Administrative support was provided by Lund University (Malmo, Sweden), University of Chicago (IL, USA), and the American Diabetes Association (Washington D.C., USA). The Novo Nordisk Foundation (Hellerup, Denmark) provided grant support for in-person writing group meetings (PI: L Phillipson, University of Chicago, IL). This study was supported by the National Institute for Health and Care Research Exeter Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Funding:
KGY and JMD are supported by Research England’s Expanding Excellence in England (E3) fund. ARK is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR002490. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. SR is funded by an US Department of Veterans Affairs Award IK2-CX001907, and a Webb-Waring Biomedical Research Award from the Boettcher Foundation. JMD is funded by the EFSD Rising Star Fellowship Programme, the UK Medical Research Council (MR/N00633X/1), and a BHF-Turing Cardiovascular Data Science Award (SP/19/6/34809). The funders had no role in the study design, data extraction or interpretation, writing of the article, or the decision to submit for publication.
Conflict of interest:
APM declares previous research funding from Eli Lilly and Company, Pfizer, and AstraZeneca. ERP has received honoraria for speaking from Lilly, Novo Nordisk and Illumina. All other authors declare no conflict of interest.
Appendix 1: PRISMA CHECKLIST
| Section and Topic | Item # | Checklist item | Location where item is reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Page 1 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Page 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Page 3 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Page 3 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Page 4 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Page 4 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | Supplementary Material Pages 1 - 6 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | Page 4 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | Page 5 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g. for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Page 5 |
| 10b | List and define all other variables for which data were sought (e.g. participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Page 5 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Page 5 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g. risk ratio, mean difference) used in the synthesis or presentation of results. | Page 5 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g. tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Page 5 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | NA | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Page 5 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | Page 5 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g. subgroup analysis, meta-regression). | NA | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | NA | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | NA |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | Pages 4 - 5 |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Page 6; Supplementary Figure 1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Not Reported | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Supplementary Table 1 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Supplementary Figure 2 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g. confidence/credible interval), ideally using structured tables or plots. | Results (text), pages 6 - 13; Table 1 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | Results (text), pages 6 - 13; Table 1 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g. confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | Results (text), pages 6 - 13 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | Results (text), pages 6 - 13 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | Results (text), pages 6 - 13 | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | Not Reported |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Results (text), pages 6 - 13 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Page 14 |
| 23b | Discuss any limitations of the evidence included in the review. | Page 14 | |
| 23c | Discuss any limitations of the review processes used. | Not Reported | |
| 23d | Discuss implications of the results for practice, policy, and future research. | Page 15 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Page 4 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | Page 4 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | Page 4 | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | Page 17 |
| Competing interests | 26 | Declare any competing interests of review authors. | Page 17 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | Page 17 |
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71
For more information, visit: http://www.prisma-statement.org/
Data Availability:
Template data collection forms and the data extracted from included studies are available upon request.
References
- 1.Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative Effectiveness of Glucose-Lowering Drugs for Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Annals of internal medicine 2020; 173(4). [DOI] [PubMed] [Google Scholar]
- 2.McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: A Meta-analysis. JAMA Cardiol 2021; 6(2): 148–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sattar N, Lee M, Kristensen S, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. The lancet Diabetes & endocrinology 2021; 9(10). [DOI] [PubMed] [Google Scholar]
- 4.Group NDoPHRS, Consortium SiM-AC-RT. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet (London, England) 2022; 400(10365). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaman AM, Bain SC, Bakris GL, et al. Effect of the Glucagon-Like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients With Type 2 Diabetes: Pooled Analysis of SUSTAIN 6 and LEADER. Circulation 2022; 145(8): 575–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies MJ, Aroda VR, Collins BS, et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022; 45(11): 2753–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dennis JM. Precision Medicine in Type 2 Diabetes: Using Individualized Prediction Models to Optimize Selection of Treatment. Diabetes 2020; 69(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nolan JJ, Kahkoska AR, Semnani-Azad Z, et al. ADA/EASD Precision Medicine in Diabetes Initiative: An International Perspective and Future Vision for Precision Medicine in Diabetes. Diabetes Care 2022; 45(2): 261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joanna Briggs Institute. Critical Appraisal Checklist for Cohort Studies. 2017. https://jbi.global/sites/default/files/2021-10/Checklist_for_Cohort_Studies.docx.
- 11.Guyatt G, Oxman A, Akl E, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of clinical epidemiology 2011; 64(4). [DOI] [PubMed] [Google Scholar]
- 12.Matthews DR, Zinman B, Tong C, Meininger G, Polidori D. Glycaemic efficacy of canagliflozin is largely independent of baseline β-cell function or insulin sensitivity. Diabetic Medicine 2016; 33(12): 1744–7. [DOI] [PubMed] [Google Scholar]
- 13.Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and Clinical Outcomes in Patients With Type 2 Diabetes Mellitus, Established Cardiovascular Disease, and Chronic Kidney Disease. Circulation 2018; 137(2): 119–29. [DOI] [PubMed] [Google Scholar]
- 14.Scheerer MF, Rist R, Proske O, Meng A, Kostev K. Changes in HbA1c, body weight, and systolic blood pressure in type 2 diabetes patients initiating dapagliflozin therapy: a primary care database study. Diabetes, Metabolic Syndrome and Obesity 2016; 9: 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherney DZI, Cosentino F, Pratley RE, et al. The differential effects of ertugliflozin on glucosuria and natriuresis biomarkers: Prespecified analyses from VERTIS CV. Diabetes, Obesity & Metabolism 2022; 24(6): 1114–22. [DOI] [PubMed] [Google Scholar]
- 16.Cherney DZI, Cooper ME, Tikkanen I, et al. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney International 2018; 93(1): 231–44. [DOI] [PubMed] [Google Scholar]
- 17.Yamout H, Perkovic V, Davies M, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes and stage 3 nephropathy. American Journal of Nephrology 2014; 40(1): 64–74. [DOI] [PubMed] [Google Scholar]
- 18.Lee JY, Cho Y, Lee M, et al. Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors. Diabetes & Metabolism Journal 2019; 43(2): 158–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert RE, Weir MR, Fioretto P, et al. Impact of Age and Estimated Glomerular Filtration Rate on the Glycemic Efficacy and Safety of Canagliflozin: A Pooled Analysis of Clinical Studies. Can J Diabetes 2016; 40(3): 247–57. [DOI] [PubMed] [Google Scholar]
- 20.Wilding J, Bailey C, Rigney U, Blak B, Kok M, Emmas C. Dapagliflozin therapy for type 2 diabetes in primary care: Changes in HbA1c, weight and blood pressure over 2 years follow-up. Primary Care Diabetes 2017; 11(5): 437–44. [DOI] [PubMed] [Google Scholar]
- 21.Chen JF, Peng YS, Chen CS, et al. Use and effectiveness of dapagliflozin in patients with type 2 diabetes mellitus: a multicenter retrospective study in Taiwan. PeerJ 2020; 8: e9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeFronzo RA, Ferrannini E, Schernthaner G, et al. Slope of change in HbA1c from baseline with empagliflozin compared with sitagliptin or glimepiride in patients with type 2 diabetes. Endocrinology, Diabetes and Metabolism 2018; 1(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Parigi A, Tang W, Liu D, Lee C, Pratley R. Machine Learning to Identify Predictors of Glycemic Control in Type 2 Diabetes: An Analysis of Target HbA1c Reduction Using Empagliflozin/Linagliptin Data. Pharmaceutical Medicine 2019; 33(3): 209–17. [DOI] [PubMed] [Google Scholar]
- 24.Inzucchi SE, Davies MJ, Khunti K, et al. Empagliflozin treatment effects across categories of baseline HbA1c, body weight and blood pressure as an add-on to metformin in patients with type 2 diabetes. Diabetes, Obesity & Metabolism 2021; 23(2): 425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scheen AJ. SGLT2 Inhibitors as Add-On Therapy to Metformin for People with Type 2 Diabetes: A Review of Placebo-Controlled Trials in Asian versus Non-Asian Patients. Diabetes, Metabolic Syndrome and Obesity 2020; 13: 2765–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai X, Gao X, Yang W, et al. No disparity of the efficacy and all-cause mortality between Asian and non-Asian type 2 diabetes patients with sodium-glucose cotransporter 2 inhibitors treatment: A meta-analysis. J Diabetes Investig 2018; 9(4): 850–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montvida O, Verma S, Shaw JE, Paul SK. Cardiometabolic risk factor control in black and white people in the United States initiating sodium-glucose co-transporter-2 inhibitors: A real-world study. Diabetes, Obesity & Metabolism 2020; 22(12): 2384–97. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Tarasenko L, Pong A, et al. Efficacy and safety of ertugliflozin across racial groups in patients with type 2 diabetes mellitus. Current Medical Research and Opinion 2020; 36(8): 1277–84. [DOI] [PubMed] [Google Scholar]
- 29.Frías JP, Hardy E, Ahmed A, et al. Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly alone, or dapagliflozin alone added to metformin monotherapy in subgroups of patients with type 2 diabetes in the DURATION-8 randomized controlled trial. Diabetes, Obesity & Metabolism 2018; 20(6): 1520–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Shao X, Liu Z. Efficacy and safety of sodium-glucose co-transporter 2 inhibitors in the elderly versus non-elderly patients with type 2 diabetes mellitus: a meta-analysis. Endocrine Journal 2022. [DOI] [PubMed] [Google Scholar]
- 31.Pratley R, Dagogo-Jack S, Charbonnel B, et al. Efficacy and safety of ertugliflozin in older patients with type 2 diabetes: A pooled analysis of phase III studies. Diabetes, Obesity & Metabolism 2020; 22(12): 2276–86. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair A, Bode B, Harris S, et al. Efficacy and safety of canagliflozin compared with placebo in older patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. BMC Endocrine Disorders 2014; 14: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones AG, McDonald TJ, Shields BM, et al. Markers of β-Cell Failure Predict Poor Glycemic Response to GLP-1 Receptor Agonist Therapy in Type 2 Diabetes. Diabetes Care 2016; 39(2): 250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapolla A, Frison V, Bettio M, et al. Correlation between baseline characteristics and clinical outcomes in a large population of diabetes patients treated with liraglutide in a real-world setting in Italy. Clinical Therapeutics 2015; 37(3): 574–84. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Peralta F, Lecube A, Fernández-Mariño A, et al. Interindividual differences in the clinical effectiveness of liraglutide in Type 2 diabetes: a real-world retrospective study conducted in Spain. Diabetic Medicine 2018; 35(11): 1605–12. [DOI] [PubMed] [Google Scholar]
- 36.Simioni N, Berra C, Boemi M, et al. Predictors of treatment response to liraglutide in type 2 diabetes in a real-world setting. Acta Diabetologica 2018; 55(6): 557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thong KY, McDonald TJ, Hattersley AT, et al. The association between postprandial urinary C-peptide creatinine ratio and the treatment response to liraglutide: a multi-centre observational study. Diabetic Medicine 2014; 31(4): 403–11. [DOI] [PubMed] [Google Scholar]
- 38.Wang T, Zhang F, Wang X, et al. Predictive factors associated with glycaemic response to exenatide in Chinese patients with type 2 diabetes mellitus. Journal of Clinical Pharmacy and Therapeutics 2020; 45(5): 1050–7. [DOI] [PubMed] [Google Scholar]
- 39.Thong KY, McGowan BM, Htay T, et al. Insulin treatment and longer diabetes duration both predict poorer glycaemic response to liraglutide treatment in type 2 diabetes: The Association of British Clinical Diabetologists Nationwide Liraglutide Audit. British Journal of Diabetes and Vascular Disease 2015; 15(4): 169–72. [Google Scholar]
- 40.Yu M, Wang K, Liu H, Cao R. GLP1R variant is associated with response to exenatide in overweight Chinese Type 2 diabetes patients. Pharmacogenomics 2019; 20(4): 273–7. [DOI] [PubMed] [Google Scholar]
- 41.Feng P, Yu DM, Chen LM, et al. Liraglutide reduces the body weight and waist circumference in Chinese overweight and obese type 2 diabetic patients. Acta Pharmacologica Sinica 2015; 36(2): 200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Dalmazi G, Coluzzi S, Baldassarre MPA, et al. Exenatide Once Weekly: Effectiveness, Tolerability, and Discontinuation Predictors in a Real-world Setting. Clinical Therapeutics 2020; 42(9): 1738–49.el. [DOI] [PubMed] [Google Scholar]
- 43.Blonde L, Chava P, Dex T, Lin J, Nikonova EV, Goldenberg RM. Predictors of outcomes in patients with type 2 diabetes in the lixisenatide GetGoal clinical trials. Diabetes, Obesity & Metabolism 2017; 19(2): 275–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berra CC, Resi V, Mirani M, et al. Clinical efficacy and predictors of response to dulaglutide in type-2 diabetes. Pharmacological Research 2020; 159:104996. [DOI] [PubMed] [Google Scholar]
- 45.Anichini R, Cosimi S, Di Carlo A, et al. Gender difference in response predictors after 1-year exenatide therapy twice daily in type 2 diabetic patients: a real world experience. Diabetes, Metabolic Syndrome and Obesity 2013; 6:123–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seufert J, Bailey T, Barkholt Christensen S, Nauck MA. Impact of diabetes duration on achieved reductions in glycated haemoglobin, fasting plasma glucose and body weight with liraglutide treatment for up to 28-weeks: a meta-analysis of seven phase III trials. Diabetes, Obesity & Metabolism 2016; 18(7): 721–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallwitz B, Dagogo-Jack S, Thieu V, et al. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes, Obesity & Metabolism 2018; 20(2): 409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathieu C, Del Prato S, Botros FT, et al. Effect of once weekly dulaglutide by baseline beta-cell function in people with type 2 diabetes in the AWARD programme. Diabetes, Obesity & Metabolism 2018; 20(8): 2023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bonadonna RC, Blonde L, Antsiferov M, et al. Lixisenatide as add-on treatment among patients with different β-cell function levels as assessed by HOMA-β index. Diabetes/Metabolism Research and Reviews 2017; 33(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henry RR, Buse JB, Sesti G, et al. Efficacy of antihyperglycemic therapies and the influence of baseline hemoglobin A(1C): a meta-analysis of the liraglutide development program. Endocrine Practice 2011; 17(6): 906–13. [DOI] [PubMed] [Google Scholar]
- 51.Yoo JH, Cho YK, Lee J, et al. Clinical Efficacy and Parameters Affecting the Response to Dulaglutide Treatment in Patients with Type 2 Diabetes: A Retrospective, Real-World Data Study. Diabetes Ther 2019; 10(4): 1453–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berkovic MC, Bilic-Curcic I, Herman Mahecic D, Gradiser M, Grgurevic M, Bozek T. Long-Term Effectiveness of Liraglutide in Association with Patients’ Baseline Characteristics in Real-Life Setting in Croatia: An Observational, Retrospective, Multicenter Study. Diabetes Ther 2017; 8(6): 1297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petri KCC, Ingwersen SH, Flint A, Zacho J, Overgaard RV. Exposure-response analysis for evaluation of semaglutide dose levels in type 2 diabetes. Diabetes, Obesity & Metabolism 2018; 20(9): 2238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dennis JM, Shields BM, Hill AV, et al. Precision Medicine in Type 2 Diabetes: Clinical Markers of Insulin Resistance Are Associated With Altered Short- and Long-term Glycemic Response to DPP-4 Inhibitor Therapy. Diabetes Care 2018; 41(4): 705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chitnis AS, Ganz ML, Benjamin N, Langer J, Hammer M. Clinical Effectiveness of Liraglutide Across Body Mass Index in Patients with Type 2 Diabetes in the United States: A Retrospective Cohort Study. Advances in Therapy 2014; 31(9): 986–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaw JE, Gallwitz B, Han J, Hardy E, Schernthaner G. Variability in and predictors of glycaemic responses after 24 weeks of treatment with exenatide twice daily and exenatide once weekly. Diabetes, Obesity & Metabolism 2017; 19(12): 1793–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolffenbuttel BH, Van Gaal L, Durán-Garcia S, Han J. Relationship of body mass index with efficacy of exenatide twice daily added to insulin glargine in patients with type 2 diabetes. Diabetes, Obesity & Metabolism 2016; 18(8): 829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yale JF, Bodholdt U, Catarig AM, et al. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: pooled analysis of data from four SURE studies by baseline characteristic subgroups. BMJ Open Diabetes Res Care 2022; 10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee J, Cho YK, Kim HS, Jung CH, Park JY, Lee WJ. Dulaglutide as an Add-on to Insulin in Type 2 Diabetes; Clinical Efficacy and Parameters Affecting the Response in Real-World Practice. Diabetes, Metabolic Syndrome and Obesity 2019; 12: 2745–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wysham C, Guerci B, D’Alessio D, Jia N, Botros FT. Baseline factors associated with glycaemic response to treatment with once-weekly dulaglutide in patients with type 2 diabetes. Diabetes, Obesity & Metabolism 2016; 18(11): 1138–42. [DOI] [PubMed] [Google Scholar]
- 61.Davidson JA, Brett J, Falahati A, Scott D. Mild renal impairment and the efficacy and safety of liraglutide. Endocrine Practice 2011; 17(3): 345–55. [DOI] [PubMed] [Google Scholar]
- 62.DeSouza C, Cariou B, Garg S, Lausvig N, Navarria A, Fonseca V. Efficacy and Safety of Semaglutide for Type 2 Diabetes by Race and Ethnicity: A Post Hoc Analysis of the SUSTAIN Trials. J Clin Endocrinol Metab 2020; 105(2). [DOI] [PubMed] [Google Scholar]
- 63.Shomali ME, Ørsted DD, Cannon AJ. Efficacy and safety of liraglutide, a once-daily human glucagon-like peptide-1 receptor agonist, in African-American people with Type 2 diabetes: a meta-analysis of sub-population data from seven phase III trials. Diabetic Medicine 2017; 34(2): 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nunes AP, Loughlin AM, Qiao Q, et al. Tolerability and Effectiveness of Exenatide Once Weekly Relative to Basal Insulin Among Type 2 Diabetes Patients of Different Races in Routine Care. Diabetes Therapy 2017; 8(6): 1349–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davidson JA, Ørsted DD, Campos C. Efficacy and safety of liraglutide, a once-daily human glucagon-like peptide-1 analogue, in Latino/Hispanic patients with type 2 diabetes: post hoc analysis of data from four phase III trials. Diabetes, Obesity & Metabolism 2016; 18(7): 725–8. [DOI] [PubMed] [Google Scholar]
- 66.Geng Z, Li Q, Huang R, et al. KCNQ1 variant rs163184 is a potential biomarker of glycemic response to exenatide. Pharmacogenomics 2022; 23(6): 355–61. [DOI] [PubMed] [Google Scholar]
- 67.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. 10.1056/NEJMoa1504720 2015. [DOI] [PubMed]
- 68.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. 10.1056/NEJMoa1611925 2017. [DOI] [PubMed]
- 69.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. 10.1056/NEJMoa2107038 2021. [DOI]
- 70.Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. 10.1056/NEJMoa2022190 2020. [DOI] [PubMed]
- 71.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. 10.1056/NEJMoa1911303 2019. [DOI] [PubMed]
- 72.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. 10.1056/NEJMoa2024816 2020. [DOI]
- 73.Lee MMY, Ghouri N, McGuire DK, Rutter MK, Sattar N. Meta-analyses of Results From Randomized Outcome Trials Comparing Cardiovascular Effects of SGLT2is and GLP-1RAs in Asian Versus White Patients With and Without Type 2 Diabetes. Digbetes Care 2021; 44(5): 1236–41. [DOI] [PubMed] [Google Scholar]
- 74.Qiu M, Ding LL, Zhou HR. Factors affecting the efficacy of SGLT2is on heart failure events: A meta-Analysis based on cardiovascular outcome trials. Cardiovascular Diagnosis and Therapy 2021; 11(3): 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhattarai M, Salih M, Regmi M, et al. Association of Sodium-Glucose Cotransporter 2 Inhibitors With Cardiovascular Outcomes in Patients With Type 2 Diabetes and Other Risk Factors for Cardiovascular Disease: A Meta-analysis. JAMA Netw Open 2022; 5(1): e2142078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang R, Liu SY, Zhao LM. Impact of demographic characteristics and antihyperglycemic and cardiovascular drugs on the cardiorenal benefits of SGLT2 inhibitors in patients with type 2 diabetes mellitus: A protocol for systematic review and meta-analysis. Medicine (Baltimore) 2021; 100(47): e27802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giugliano D, Longo M, Caruso P, Maiorino MI, Bellastella G, Esposito K. Sodium-glucose co-transporter-2 inhibitors for the prevention of cardiorenal outcomes in type 2 diabetes: An updated meta-analysis. Diabetes, Obesity & Metabolism 2021; 23(7): 1672–6. [DOI] [PubMed] [Google Scholar]
- 78.Bhatia K, Jain V, Gupta K, et al. Prevention of heart failure events with sodium-glucose co-transporter 2 inhibitors across a spectrum of cardio-renal-metabolic risk. European Journal of Heart Failure 2021; 23(6): 1002–8. [DOI] [PubMed] [Google Scholar]
- 79.Chun KJ, Jung HH. SGLT2 Inhibitors and Kidney and Cardiac Outcomes According to Estimated GFR and Albuminuria Levels: A Meta-analysis of Randomized Controlled Trials. Kidney Med 2021; 3(5): 732–44.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.D’Andrea E, Kesselheim AS, Franklin JM, Jung EH, Hey SP, Patorno E. Heterogeneity of antidiabetic treatment effect on the risk of major adverse cardiovascular events in type 2 diabetes: a systematic review and meta-analysis. Cardiovascular Diabetology 2020; 19(1): 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawai Y, Uneda K, Yamada T, et al. Comparison of effects of SGLT-2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in type 2 diabetes mellitus patients with/without albuminuria: A systematic review and network meta-analysis. Diabetes Research and Clinical Practice 2022; 183:109146. [DOI] [PubMed] [Google Scholar]
- 82.Li N, Zhou G, Zheng Y, et al. Effects of SGLT2 inhibitors on cardiovascular outcomes in patients with stage 3/4 CKD: A meta-analysis. PloS One 2022; 17(1): e0261986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Furtado RHM, Bonaca MP, Raz I, et al. Dapagliflozin and Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus and Previous Myocardial Infarction. Circulation 2019; 139(22): 2516–27. [DOI] [PubMed] [Google Scholar]
- 84.Oyama K, Raz I, Cahn A, et al. Obesity and effects of dapagliflozin on cardiovascular and renal outcomes in patients with type 2 diabetes mellitus in the DECLARE-TIMI 58 trial. European Heart Journal 2021. [DOI] [PubMed] [Google Scholar]
- 85.Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus: Results From the CANVAS Program. Circulation 2018; 138(5): 458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vaduganathan M, Sattar N, Xu J, et al. Stress Cardiac Biomarkers, Cardiovascular and Renal Outcomes, and Response to Canagliflozin. Journal of the American College of Cardiology 2022; 79(5): 432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Q, Liu L, Gao L, Li Q. Cardiovascular safety of GLP-1 receptor agonists for diabetes patients with high cardiovascular risk: A meta-analysis of cardiovascular outcomes trials. Diabetes Research and Clinical Practice 2018; 143: 34–42. [DOI] [PubMed] [Google Scholar]
- 88.Marsico F, Paolillo S, Gargiulo P, et al. Effects of glucagon-like peptide-1 receptor agonists on major cardiovascular events in patients with Type 2 diabetes mellitus with or without established cardiovascular disease: a meta-analysis of randomized controlled trials. European Heart Journal 2020; 41(35): 3346–58. [DOI] [PubMed] [Google Scholar]
- 89.He L, Yang N, Xu L, et al. Subpopulation Differences in the Cardiovascular Efficacy of Long-Acting Glucagon-Like Peptide 1 Receptor Agonists in Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Diabetes Therapy 2020; 11(9): 2121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giugliano D, Maiorino MI, Bellastella G, Longo M, Chiodini P, Esposito K. GLP-1 receptor agonists for prevention of cardiorenal outcomes in type 2 diabetes: An updated meta-analysis including the REWIND and PIONEER 6 trials. Diabetes, Obesity & Metabolism 2019; 21(11): 2576–80. [DOI] [PubMed] [Google Scholar]
- 91.Mannucci E, Dicembrini I, Nreu B, Monami M. Glucagon-like peptide-1 receptor agonists and cardiovascular outcomes in patients with and without prior cardiovascular events: An updated meta-analysis and subgroup analysis of randomized controlled trials. Diabetes, Obesity & Metabolism 2020; 22(2): 203–11. [DOI] [PubMed] [Google Scholar]
- 92.Zelniker TA, Wiviott SD, Raz I, et al. Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation 2019; 139(17): 2022–31. [DOI] [PubMed] [Google Scholar]
- 93.Singh AK, Singh R. Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: A systematic review and meta-analysis of cardio-vascular outcome trials. Diabetes & Metabolic Syndrome 2020; 14(3): 181–7. [DOI] [PubMed] [Google Scholar]
- 94.Uneda K, Kawai Y, Yamada T, et al. Systematic review and meta-analysis for prevention of cardiovascular complications using GLP-1 receptor agonists and SGLT-2 inhibitors in obese diabetic patients. Scientific Reports 2021; 11(1): 10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Husain M, Bain SC, Jeppesen OK, et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes, Obesity & Metabolism 2020; 22(3): 442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qiu M, Ding LL, Zhang M, Lin JH, Huang H, Li KK. The effects of the baseline characteristics on the efficacy of glp-1 ras in reducing cardiovascular events in type 2 diabetes: A meta-analysis. International Journal of Clinical and Experimental Medicine 2020; 13(10): 7437–45. [Google Scholar]
- 97.Husain M, Bain SC, Holst AG, Mark T, Rasmussen S, Lingvay I. Effects of semaglutide on risk of cardiovascular events across a continuum of cardiovascular risk: combined post hoc analysis of the SUSTAIN and PIONEER trials. Cardiovascular Diabetology 2020; 19(1): 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim YG, Han SJ, Kim DJ, Lee KW, Kim HJ. Association between sodium glucose co-transporter 2 inhibitors and a reduced risk of heart failure in patients with type 2 diabetes mellitus: a real-world nationwide population-based cohort study. Cardiovascular Diabetology 2018; 17(1): 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raparelli V, Elharram M, Moura CS, et al. Sex Differences in Cardiovascular Effectiveness of Newer Glucose-Lowering Drugs Added to Metformin in Type 2 Diabetes Mellitus. J Am Heart Assoc 2020; 9(1): e012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Becher PM, Schrage B, Ferrannini G, et al. Use of sodium-glucose co-transporter 2 inhibitors in patients with heart failure and type 2 diabetes mellitus: data from the Swedish Heart Failure Registry. European Journal of Heart Failure 2021; 23(6): 1012–22. [DOI] [PubMed] [Google Scholar]
- 101.Idris I, Zhang R, Mamza JB, et al. Lower risk of hospitalization for heart failure, kidney disease and death with sodium-glucose co-transporter-2 inhibitors compared with dipeptidyl peptidase-4 inhibitors in type 2 diabetes regardless of prior cardiovascular or kidney disease: A retrospective cohort study in UK primary care. Diabetes, Obesity & Metabolism 2021; 23(10): 2207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patorno E, Htoo PT, Glynn RJ, et al. Sodium-Glucose Cotransporter-2 Inhibitors Versus Glucagon-like Peptide-1 Receptor Agonists and the Risk for Cardiovascular Outcomes in Routine Care Patients With Diabetes Across Categories of Cardiovascular Disease. Annals of Internal Medicine 2021; 174(11): 1528–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Htoo PT, Buse J, Cavender M, et al. Cardiovascular Effectiveness of Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists in Older Patients in Routine Clinical Care With or Without History of Atherosclerotic Cardiovascular Diseases or Heart Failure. J Am Heart Assoc 2022; 11(4): e022376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang CT, Yang CY, Ou HT, Kuo S. Comparative cardiovascular safety of GLP-1 receptor agonists versus other glucose-lowering agents in real-world patients with type 2 diabetes: a nationwide population-based cohort study. Cardiovascular Diabetology 2020; 19(1): 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen JJ, Wu CY, Jenq CC, et al. Association of Glucagon-Like Peptide-1 Receptor Agonist vs Dipeptidyl Peptidase-4 Inhibitor Use With Mortality Among Patients With Type 2 Diabetes and Advanced Chronic Kidney Disease. JAMA Netw Open 2022; 5(3): e221169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Birkeland KI, Jørgensen ME, Carstensen B, et al. Cardiovascular mortality and morbidity in patients with type 2 diabetes following initiation of sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL Nordic): a multinational observational analysis. Lancet Diabetes Endocrinol 2017; 5(9): 709–17. [DOI] [PubMed] [Google Scholar]
- 107.Chan YH, Chen SW, Chao TF, Kao YW, Huang CY, Chu PH. Impact of the initial decline in estimated glomerular filtration rate on the risk of new-onset atrial fibrillation and adverse cardiovascular and renal events in patients with type 2 diabetes treated with sodium-glucose co-transporter-2 inhibitors. Diabetes, Obesity & Metabolism 2021; 23(9): 2077–89. [DOI] [PubMed] [Google Scholar]
- 108.Neuen BL, Young T, Heerspink HJL, et al. SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2019; 7(11): 845–54. [DOI] [PubMed] [Google Scholar]
- 109.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019; 393(10166): 31–9. [DOI] [PubMed] [Google Scholar]
- 110.Bae JH, Park EG, Kim S, Kim SG, Hahn S, Kim NH. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Scientific Reports 2019; 9(1): 13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bakris G, Oshima M, Mahaffey KW, et al. Effects of Canagliflozin in Patients with Baseline eGFR <30 ml/min per 1.73 m(2): Subgroup Analysis of the Randomized CREDENCE Trial. Clinical Journal of the American Society of Nephrology 2020; 15(12): 1705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jardine M, Zhou Z, Lambers Heerspink HJ, et al. Kidney, Cardiovascular, and Safety Outcomes of Canagliflozin according to Baseline Albuminuria: A CREDENCE Secondary Analysis. Clinical Journal of the American Society of Nephrology 2021; 16(3): 384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mosenzon O, Wiviott SD, Heerspink HJL, et al. The Effect of Dapagliflozin on Albuminuria in DECLARE-TIMI 58. Diabetes Care 2021; 44(8): 1805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neuen BL, Ohkuma T, Neal B, et al. Relative and Absolute Risk Reductions in Cardiovascular and Kidney Outcomes With Canagliflozin Across KDIGO Risk Categories: Findings From the CANVAS Program. American Journal of Kidney Diseases 2021; 77(1): 23–34.e1. [DOI] [PubMed] [Google Scholar]
- 115.Wanner C, Inzucchi SE, Zinman B, et al. Consistent effects of empagliflozin on cardiovascular and kidney outcomes irrespective of diabetic kidney disease categories: Insights from the EMPA-REG OUTCOME trial. Diabetes, Obesity & Metabolism 2020; 22(12): 2335–47. [DOI] [PubMed] [Google Scholar]
- 116.Neuen BL, Ohkuma T, Neal B, et al. Effect of Canagliflozin on Renal and Cardiovascular Outcomes across Different Levels of Albuminuria: Data from the CANVAS Program. Journal of the American Society of Nephrology 2019; 30(11): 2229–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Januzzi JL Jr., Butler J, Sattar N, et al. Insulin-Like Growth Factor Binding Protein 7 Predicts Renal and Cardiovascular Outcomes in the Canagliflozin Cardiovascular Assessment Study. Diabetes Care 2021; 44(1): 210–6. [DOI] [PubMed] [Google Scholar]
- 118.Riddle M, Gerstein H, Xavier D, et al. Efficacy and Safety of Dulaglutide in Older Patients: A post hoc Analysis of the REWIND trial. The Journal of clinical endocrinology and metabolism 2021; 106(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.van der Aart-van der Beek AB, Clegg LE, Penland RC, et al. Effect of once-weekly exenatide on estimated glomerular filtration rate slope depends on baseline renal risk: A post hoc analysis of the EXSCEL trial. Diabetes, Obesity & Metabolism 2020; 22(12): 2493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leiter LA, Bain SC, Bhatt DL, et al. The effect of glucagon-like peptide-1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across baseline blood pressure categories: Analysis of the LEADER and SUSTAIN 6 trials. Diabetes, Obesity & Metabolism 2020; 22(9): 1690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Verma S, Bain SC, Monk Fries T, et al. Duration of diabetes and cardiorenal efficacy of liraglutide and semaglutide: A post hoc analysis of the LEADER and SUSTAIN 6 clinical trials. Diabetes, Obesity & Metabolism 2019; 21(7): 1745–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marso S, Baeres F, Bain S, et al. Effects of Liraglutide on Cardiovascular Outcomes in Patients With Diabetes With or Without Heart Failure. Journal of the American College of Cardiology 2020; 75(10). [DOI] [PubMed] [Google Scholar]
- 123.Verma S, McGuire DK, Bain SC, et al. Effects of glucagon-like peptide-1 receptor agonists liraglutide and semaglutide on cardiovascular and renal outcomes across body mass index categories in type 2 diabetes: Results of the LEADER and SUSTAIN 6 trials. Diabetes, Obesity & Metabolism 2020; 22(12): 2487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mosenzon O, Bain SC, Heerspink HJL, et al. Cardiovascular and renal outcomes by baseline albuminuria status and renal function: Results from the LEADER randomized trial. Diabetes, Obesity & Metabolism 2020; 22(11): 2077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muskiet MHA, Tonneijck L, Huang Y, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2018; 6(11): 859–69. [DOI] [PubMed] [Google Scholar]
- 126.Koh ES, Han K, Nam YS, et al. Renal outcomes and all-cause death associated with sodium-glucose co-transporter-2 inhibitors versus other glucose-lowering drugs (CVD-REAL 3 Korea). Diabetes, Obesity & Metabolism 2021; 23(2): 455–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nagasu H, Yano Y, Kanegae H, et al. Kidney Outcomes Associated With SGLT2 Inhibitors Versus Other Glucose-Lowering Drugs in Real-world Clinical Practice: The Japan Chronic Kidney Disease Database. Diabetes Care 2021; 44(11): 2542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kent DM, Steyerberg E, van Klaveren D. Personalized evidence based medicine: predictive approaches to heterogeneous treatment effects. BMJ 2018; 363: k4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kent D, Paulus J, van Klaveren D, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) Statement. Annals of internal medicine 2020; 172(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shields BM, Dennis JM, Angwin CD, et al. Patient stratification for determining optimal second-line and third-line therapy for type 2 diabetes: the TriMaster study. Nature Medicine 2022; 29(2): 376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brandon R, Jiang Y, Yeu R, et al. Stratified glucose-lowering response to vildagliptin and pioglitazone by obesity and hypertriglyceridemia in a randomized crossover trial. Frontiers in endocrinology 2023; 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dennis JM, Young KG, McGovern AP, et al. Derivation and validation of a type 2 diabetes treatment selection algorithm for SGLT2-inhibitor and DPP4-inhibitor therapies based on glucose-lowering efficacy: cohort study using trial and routine clinical data. medRxiv 2021: 2021.11.11.21265959. [Google Scholar]
- 133.Dawed AY, Mari A, Brown A, et al. Pharmacogenomics of GLP-1 receptor agonists: a genome-wide analysis of observational data and large randomised controlled trials. The Lancet Diabetes & Endocrinology 2023; 11(1): 33–41. [DOI] [PubMed] [Google Scholar]
- 134.Kyriakidou A, Kyriazou AV, Koufakis T, et al. Clinical and Genetic Predictors of Glycemic Control and Weight Loss Response to Liraglutide in Patients with Type 2 Diabetes. J Pers Med 2022; 12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McAdam-Marx C, Nguyen H, Schauerhamer MB, et al. Glycemic Control and Weight Outcomes for Exenatide Once Weekly Versus Liraglutide in Patients with Type 2 Diabetes: A 1-Year Retrospective Cohort Analysis. Clinical Therapeutics 2016; 38(12): 2642–51. [DOI] [PubMed] [Google Scholar]
- 136.Gorgojo-Martínez JJ, Gargallo-Fernández MA, Brito-Sanfiel M, Lisbona-Catalán A. Real-world clinical outcomes and predictors of glycaemic and weight response to exenatide once weekly in patients with type 2 diabetes: The CIBELES project. International Journal of Clinical Practice 2018; 72(3): e13055. [DOI] [PubMed] [Google Scholar]
- 137.Brekke L, Buysman E, Grabner M, et al. The Use of Decomposition Methods in Real-World Treatment Benefits Evaluation for Patients with Type 2 Diabetes Initiating Different Injectable Therapies: Findings from the INITIATOR Study. Value in Health 2017; 20(10): 1252–9. [DOI] [PubMed] [Google Scholar]
- 138.Carrington MJ, Chan YK, Stewart S, Sjouke B, Brazilek R, Cohen N. Long-term tolerance and efficacy of adjunctive exenatide therapy on glycaemic control and bodyweight in type 2 diabetes: a retrospective study from a specialist diabetes outpatient clinic. Internal Medicine Journal 2014; 44(4): 345–53. [DOI] [PubMed] [Google Scholar]
- 139.Mirabelli M, Chiefari E, Tocci V, et al. Clinical effectiveness and safety of once-weekly glp-1 receptor agonist dulaglutide as add-on to metformin or metformin plus insulin secretagogues in obesity and type 2 diabetes. Journal of Clinical Medicine 2021; 10(5): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yu M, Yuan GY, Zhang B, Wu HY, Lv XF. Efficacy and Safety of Dulaglutide by Baseline HbA1c in Chinese Patients with Type 2 Diabetes: A Post Hoc Analysis. Diabetes Therapy 2020; 11(5): 1147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yabe D, Deenadayalan S, Horio H, et al. Efficacy and safety of oral semaglutide in Japanese patients with type-2 diabetes: A subgroup analysis by baseline variables in the PIONEER-9 and PIONEER-10 trials. J Diabetes Investig 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Terauchi Y, Yabe D, Kaneto H, et al. Benefits of the fixed-ratio combination of insulin glargine 100-units/mL and lixisenatide (iGlarLixi) in Japanese people with type 2 diabetes: A subgroup and time-to-control analysis of the LixiLan JP phase 3 trials. Diabetes, Obesity & Metabolism 2020; 22 Suppl 4: 35–47. [DOI] [PubMed] [Google Scholar]
- 143.Gentilella R, Romera I, Nicolay C, Buzzetti R, Vázquez LA, Sesti G. Change in HbA(1c) Across the Baseline HbA(1c) Range in Type 2 Diabetes Patients Receiving Once-Weekly Dulaglutide Versus Other Incretin Agents. Diabetes Therapy 2019; 10(3): 1113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cho YK, Lee J, Kang YM, et al. Clinical parameters affecting the therapeutic efficacy of empagliflozin in patients with type 2 diabetes. PloS One 2019; 14(8): e0220667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brown RE, Gupta N, Aronson R. Effect of Dapagliflozin on Glycemic Control, Weight, and Blood Pressure in Patients with Type 2 Diabetes Attending a Specialist Endocrinology Practice in Canada: A Retrospective Cohort Analysis. Diabetes Technology & Therapeutics 2017; 19(11): 685–91. [DOI] [PubMed] [Google Scholar]
- 146.Zhou FL, Watada H, Tajima Y, et al. Identification of subgroups of patients with type 2 diabetes with differences in renal function preservation, comparing patients receiving sodium-glucose co-transporter-2 inhibitors with those receiving dipeptidyl peptidase-4 inhibitors, using a supervised machine-learning algorithm (PROFILE study): A retrospective analysis of a Japanese commercial medical database. Diabetes, Obesity & Metabolism 2019; 21(8): 1925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Montanya E, Fonseca V, Colagiuri S, Blonde L, Donsmark M, Nauck MA. Improvement in glycated haemoglobin evaluated by baseline body mass index: a meta-analysis of the liraglutide phase III clinical trial programme. Diabetes, Obesity & Metabolism 2016; 18(7): 707–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Eto K, Naito Y, Seino Y. Evaluation of the efficacy and safety of lixisenatide add-on treatment to basal insulin therapy among T2DM patients with different body mass indices from GetGoal trials. Diabetology and Metabolic Syndrome 2015; 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boustani MA, Pittman It, Yu M, Thieu VT, Varnado OJ, Juneja R. Similar efficacy and safety of once-weekly dulaglutide in patients with type 2 diabetes aged ≥65 and <65 years. Diabetes, Obesity & Metabolism 2016; 18(8): 820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blonde L, Woo V, Mathieu C, et al. Achievement of treatment goals with canagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of randomized controlled trials. Current Medical Research and Opinion 2015; 31(11): 1993–2000. [DOI] [PubMed] [Google Scholar]
- 151.Gimeno-Orna JA, Verdes-Sanz G, Borau-Maorad L, Campos-Fernández J, Lardiés-Sánchez B, Monreal-Villanueva M. Baseline ALT levels as a marker of glycemic response to treatment with GLP-1 receptor agonists. Endocrinol Nutr 2016; 63(4): 164–70. [DOI] [PubMed] [Google Scholar]
- 152.Yang K, Wang H, Wei R, et al. High baseline FGF21 levels are associated with poor glucose-lowering efficacy of exenatide in patients with type 2 diabetes. Acta Diabetologica 2021; 58(5): 595–602. [DOI] [PubMed] [Google Scholar]
- 153.Neeland IJ, Eliasson B, Kasai T, et al. The Impact of Empagliflozin on Obstructive Sleep Apnea and Cardiovascular and Renal Outcomes: An Exploratory Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2020; 43(12): 3007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ. Empagliflozin and Cardiovascular Outcomes in Asian Patients With Type 2 Diabetes and Established Cardiovascular Disease - Results From EMPA-REG OUTCOM(®). Circulation Journal 2017; 81(2): 227–34. [DOI] [PubMed] [Google Scholar]
- 155.Kaku K, Wanner C, Anker SD, et al. The effect of empagliflozin on the total burden of cardiovascular and hospitalization events in the Asian and non-Asian populations of the EMPA-REG OUTCOME trial of patients with type 2 diabetes and cardiovascular disease. Diabetes, Obesity & Metabolism 2022; 24(4): 662–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Verma S, Poulter NR, Bhatt DL, et al. Effects of Liraglutide on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus With or Without History of Myocardial Infarction or Stroke. Circulation 2018; 138(25): 2884–94. [DOI] [PubMed] [Google Scholar]
- 157.Verma S, Sharma A, Zinman B, et al. Empagliflozin reduces the risk of mortality and hospitalization for heart failure across Thrombolysis In Myocardial Infarction Risk Score for Heart Failure in Diabetes categories: Post hoc analysis of the EMPA-REG OUTCOME trial. Diabetes, Obesity & Metabolism 2020; 22(7): 1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mentz RJ, Bethel MA, Merrill P, et al. Effect of Once-Weekly Exenatide on Clinical Outcomes According to Baseline Risk in Patients With Type 2 Diabetes Mellitus: Insights From the EXSCEL Trial. J Am Heart Assoc 2018; 7(19): e009304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Leiter LA, Bain SC, Hramiak I, et al. Cardiovascular risk reduction with once-weekly semaglutide in subjects with type 2 diabetes: a post hoc analysis of gender, age, and baseline CV risk profile in the SUSTAIN 6 trial. Cardiovascular Diabetology 2019; 18(1): 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fudim M, White J, Pagidipati NJ, et al. Effect of Once-Weekly Exenatide in Patients With Type 2 Diabetes Mellitus With and Without Heart Failure and Heart Failure-Related Outcomes: Insights From the EXSCEL Trial. Circulation 2019; 140(20): 1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Badjatiya A, Merrill P, Buse JB, et al. Clinical Outcomes in Patients With Type 2 Diabetes Mellitus and Peripheral Artery Disease: Results From the EXSCEL Trial. Circulation: Cardiovascular Interventions 2019; 12(12): e008018. [DOI] [PubMed] [Google Scholar]
- 162.Verma S, Mazer CD, Fitchett D, et al. Empagliflozin reduces cardiovascular events, mortality and renal events in participants with type 2 diabetes after coronary artery bypass graft surgery: subanalysis of the EMPA-REG OUTCOME® randomised trial. Diabetologia 2018; 61(8): 1712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bonaca MP, Wiviott SD, Zelniker TA, et al. Dapagliflozin and Cardiac, Kidney, and Limb Outcomes in Patients With and Without Peripheral Artery Disease in DECLARE-TIMI 58. Circulation 2020; 142(8): 734–47. [DOI] [PubMed] [Google Scholar]
- 164.Gilbert MP, Bain SC, Franek E, et al. Effect of Liraglutide on Cardiovascular Outcomes in Elderly Patients: A Post Hoc Analysis of a Randomized Controlled Trial. Annals of Internal Medicine 2019; 170(6): 423–6. [DOI] [PubMed] [Google Scholar]
- 165.Inzucchi SE, Khunti K, Fitchett DH, et al. Cardiovascular Benefit of Empagliflozin Across the Spectrum of Cardiovascular Risk Factor Control in the EMPA-REG OUTCOME Trial. J Clin Endocrinol Metab 2020; 105(9): 3025–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zinman B, Inzucchi SE, Wanner C, et al. Empagliflozin in women with type 2 diabetes and cardiovascular disease - an analysis of EMPA-REG OUTCOME®. Diabetologia 2018; 61(7): 1522–7. [DOI] [PubMed] [Google Scholar]
- 167.O’Donoghue ML, Kato ET, Mosenzon O, et al. The efficacy and safety of dapagliflozin in women and men with type 2 diabetes mellitus. Diabetologia 2021; 64(6): 1226–34. [DOI] [PubMed] [Google Scholar]
- 168.Mahmoud AN, Elgendy IY, Saad M, et al. Does Gender Influence the Cardiovascular Benefits Observed with Sodium Glucose Co-Transporter-2 (SGLT-2) Inhibitors? A Meta-Regression Analysis. Cardiol Ther 2017; 6(1): 129–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rådholm K, Zhou Z, Clemens K, Neal B, Woodward M. Effects of sodium-glucose co-transporter-2 inhibitors in type 2 diabetes in women versus men. Diabetes, Obesity & Metabolism 2020; 22(2): 263–6. [DOI] [PubMed] [Google Scholar]
- 170.Mann JFE, Fonseca V, Mosenzon O, et al. Effects of Liraglutide Versus Placebo on Cardiovascular Events in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease. Circulation 2018; 138(25): 2908–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zelniker TA, Raz I, Mosenzon O, et al. Effect of Dapagliflozin on Cardiovascular Outcomes According to Baseline Kidney Function and Albuminuria Status in Patients With Type 2 Diabetes: A Prespecified Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiol 2021; 6(7): 801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Verma S, Ji Q, Bhatt DL, et al. Association between uric acid levels and cardio-renal outcomes and death in patients with type 2 diabetes: A subanalysis of EMPA-REG OUTCOME. Diabetes, Obesity & Metabolism 2020; 22(7): 1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sen T, Li J, Neuen BL, et al. Association Between Circulating GDF-15 and Cardio-Renal Outcomes and Effect of Canagliflozin: Results From the CANVAS Trial. J Am Heart Assoc 2021; 10(23): e021661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sen T, Li J, Neuen BL, et al. Effects of the SGLT2 inhibitor canagliflozin on plasma biomarkers TNFR-1, TNFR-2 and KIM-1 in the CANVAS trial. Diabetologia 2021; 64(10): 2147–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Oikonomou EK, Suchard MA, McGuire DK, Khera R. Phenomapping-Derived Tool to Individualize the Effect of Canagliflozin on Cardiovascular Risk in Type 2 Diabetes. Diabetes Care 2022; 45(4): 965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Barbery C, Giczewska A, White J, et al. Effect of once-weekly exenatide on hospitalization for acute coronary syndrome or coronary revascularization in patients with type 2 diabetes mellitus. American heart journal 2021; 239. [DOI] [PubMed] [Google Scholar]
- 177.Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME® trial. European Heart Journal 2016; 37(19): 1526–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ji Q, Ji L, Mu Y, et al. Effect of empagliflozin on cardiorenal outcomes and mortality according to body mass index: A subgroup analysis of the EMPA-REG OUTCOME trial with a focus on Asia. Diabetes, Obesity & Metabolism 2021; 23(8): 1886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fitchett D, Inzucchi SE, Cannon CP, et al. Empagliflozin Reduced Mortality and Hospitalization for Heart Failure Across the Spectrum of Cardiovascular Risk in the EMPA-REG OUTCOME Trial. Circulation 2019; 139(11): 1384–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Berg DD, Wiviott SD, Scirica BM, et al. Heart Failure Risk Stratification and Efficacy of Sodium-Glucose Cotransporter-2 Inhibitors in Patients With Type 2 Diabetes Mellitus. Circulation 2019; 140(19): 1569–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Savarese G, Uijl A, Lund LH, et al. Empagliflozin in Heart Failure With Predicted Preserved Versus Reduced Ejection Fraction: Data From the EMPA-REG OUTCOME Trial. Journal of Cardiac Failure 2021; 27(8): 888–95. [DOI] [PubMed] [Google Scholar]
- 182.Sharma A, Ofstad AP, Ahmad T, et al. Patient Phenotypes and SGLT-2 Inhibition in Type 2 Diabetes: Insights From the EMPA-REG OUTCOME Trial. JACC Heart Fail 2021; 9(8): 568–77. [DOI] [PubMed] [Google Scholar]
- 183.Kadowaki T, Nangaku M, Hantel S, et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: Results from the EMPA-REG OUTCOME(®) trial. J Diabetes Investig 2019; 10(3): 760–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Böhm M, Slawik J, Brueckmann M, et al. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: data from the EMPA-REG OUTCOME trial. European Journal of Heart Failure 2020; 22(1): 126–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Template data collection forms and the data extracted from included studies are available upon request.


