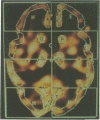
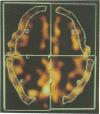
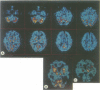
Abstract
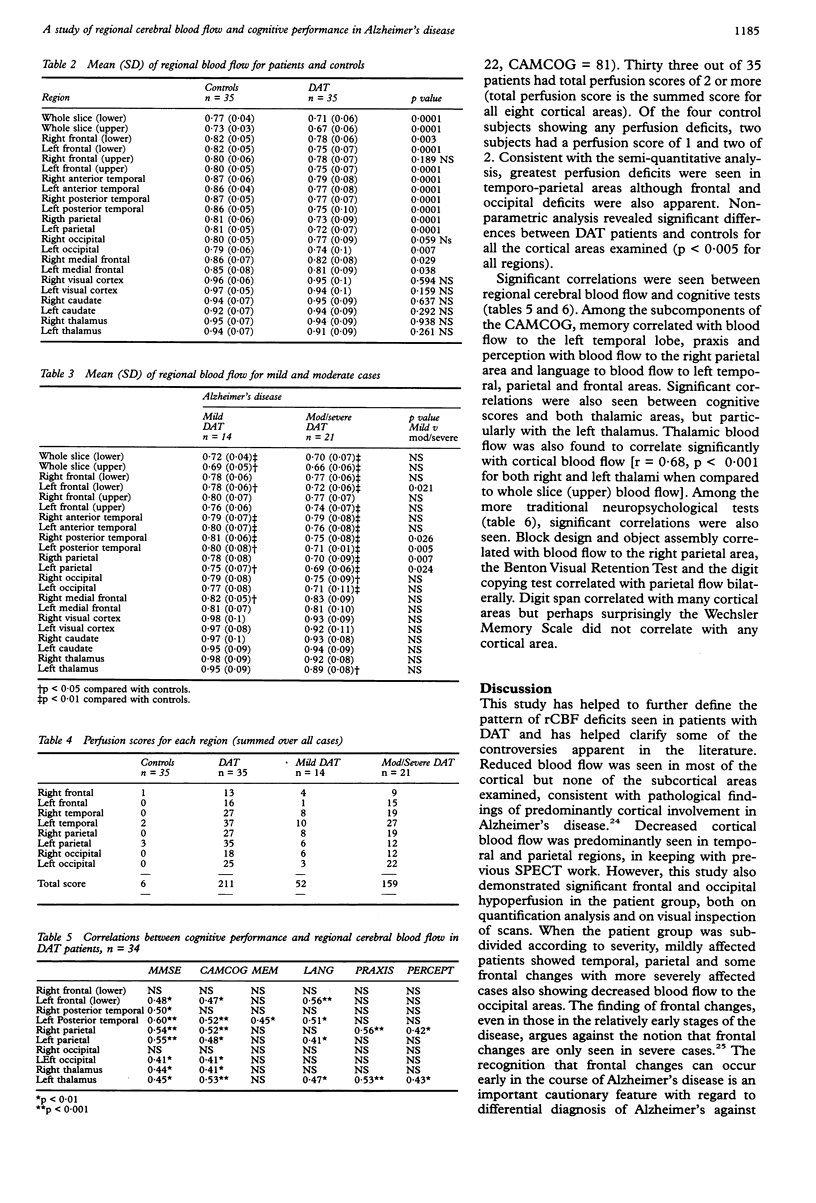
Thirty five patients with dementia of the Alzheimer type (DAT) and 35 controls matched for age, sex and handedness were investigated using single photon emission computer tomography (SPECT) with 99m technetium HMPAO. Regional cerebral blood flow (rCBF) was assessed semi-quantitatively in 18 cortical and 4 subcortical areas by normalising mean information density in each region to cerebellar mean information density. Analysis revealed significantly reduced rCBF to temporal, parietal, frontal and left occipital cortex in the patients whilst blood flow to subcortical areas showed no differences between the 2 groups. In addition, significant left-sided cortical hypoperfusion was seen in the DAT group but not in controls. When patients were sub-divided on the basis of disease severity, those with mild disease showed temporal, parietal and left frontal changes with more severely affected patients also showing right frontal and left occipital involvement. rCBF patterns did not distinguish between presenile and senile onset cases once duration and severity of illness were controlled. Eight cortical areas were also rated visually for perfusion deficits on a simple 4 point scale. Perfusion deficits were detected in 34 of 35 patients but in only 4 of 35 controls. In the DAT group significant correlations were found between many of the neuropsychological tests used and rCBF. Memory correlated with left temporal activity, praxis, perception, object assembly and block design with right parietal activity and language with activity throughout the left hemisphere. Significant correlations were also seen between subcortical and cortical blood flow, possibly explaining the correlations observed between many of the neuropsychological tests and thalamic blood flow.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns A., Philpot M. P., Costa D. C., Ell P. J., Levy R. The investigation of Alzheimer's disease with single photon emission tomography. J Neurol Neurosurg Psychiatry. 1989 Feb;52(2):248–253. doi: 10.1136/jnnp.52.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H., Eslinger P., Damasio A. R., Rizzo M., Huang H. K., Demeter S. Quantitative computed tomographic analysis in the diagnosis of dementia. Arch Neurol. 1983 Nov;40(12):715–719. doi: 10.1001/archneur.1983.04050110033004. [DOI] [PubMed] [Google Scholar]
- Deutsch G., Tweedy J. R. Cerebral blood flow in severity-matched Alzheimer and multi-infarct patients. Neurology. 1987 Mar;37(3):431–438. doi: 10.1212/wnl.37.3.431. [DOI] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frackowiak R. S., Pozzilli C., Legg N. J., Du Boulay G. H., Marshall J., Lenzi G. L., Jones T. Regional cerebral oxygen supply and utilization in dementia. A clinical and physiological study with oxygen-15 and positron tomography. Brain. 1981 Dec;104(Pt 4):753–778. doi: 10.1093/brain/104.4.753. [DOI] [PubMed] [Google Scholar]
- Frlich L., Eilles C., Ihl R., Maurer K., Lanczik M. Stage-dependent reductions of regional cerebral blood flow measured by HMPAO-SPECT in dementia of Alzheimer type. Psychiatry Res. 1989 Sep;29(3):347–350. doi: 10.1016/0165-1781(89)90085-1. [DOI] [PubMed] [Google Scholar]
- Geaney D. P., Soper N., Shepstone B. J., Cowen P. J. Effect of central cholinergic stimulation on regional cerebral blood flow in Alzheimer disease. Lancet. 1990 Jun 23;335(8704):1484–1487. doi: 10.1016/0140-6736(90)93028-n. [DOI] [PubMed] [Google Scholar]
- Gemmell H. G., Sharp P. F., Besson J. A., Crawford J. R., Ebmeier K. P., Davidson J., Smith F. W. Differential diagnosis in dementia using the cerebral blood flow agent 99mTc HM-PAO: a SPECT study. J Comput Assist Tomogr. 1987 May-Jun;11(3):398–402. doi: 10.1097/00004728-198705000-00005. [DOI] [PubMed] [Google Scholar]
- Gustafson L., Edvinsson L., Dahlgren N., Hagberg B., Risberg J., Rosén I., Fernö H. Intravenous physostigmine treatment of Alzheimer's disease evaluated by psychometric testing, regional cerebral blood flow (rCBF) measurement, and EEG. Psychopharmacology (Berl) 1987;93(1):31–35. doi: 10.1007/BF02439583. [DOI] [PubMed] [Google Scholar]
- Habert M. O., Spampinato U., Mas J. L., Piketty M. L., Bourdel M. C., de Recondo J., Rondot P., Askienazy S. A comparative technetium 99m hexamethylpropylene amine oxime SPET study in different types of dementia. Eur J Nucl Med. 1991;18(1):3–11. doi: 10.1007/BF00177677. [DOI] [PubMed] [Google Scholar]
- Hunter R., McLuskie R., Wyper D., Patterson J., Christie J. E., Brooks D. N., McCulloch J., Fink G., Goodwin G. M. The pattern of function-related regional cerebral blood flow investigated by single photon emission tomography with 99mTc-HMPAO in patients with presenile Alzheimer's disease and Korsakoff's psychosis. Psychol Med. 1989 Nov;19(4):847–855. doi: 10.1017/s0033291700005560. [DOI] [PubMed] [Google Scholar]
- Hunter R., Wyper D. J., Patterson J., Hansen M. T., Goodwin G. M. Cerebral pharmacodynamics of physostigmine in Alzheimer's disease investigated using single-photon computerised tomography. Br J Psychiatry. 1991 Mar;158:351–357. doi: 10.1192/bjp.158.3.351. [DOI] [PubMed] [Google Scholar]
- Jagust W. J., Reed B. R., Seab J. P., Budinger T. F. Alzheimer's disease. Age at onset and single-photon emission computed tomographic patterns of regional cerebral blood flow. Arch Neurol. 1990 Jun;47(6):628–633. doi: 10.1001/archneur.1990.00530060036013. [DOI] [PubMed] [Google Scholar]
- Johnson K. A., Mueller S. T., Walshe T. M., English R. J., Holman B. L. Cerebral perfusion imaging in Alzheimer's disease. Use of single photon emission computed tomography and iofetamine hydrochloride I 123. Arch Neurol. 1987 Feb;44(2):165–168. doi: 10.1001/archneur.1987.00520140035014. [DOI] [PubMed] [Google Scholar]
- Koss E., Friedland R. P., Ober B. A., Jagust W. J. Differences in lateral hemispheric asymmetries of glucose utilization between early- and late-onset Alzheimer-type dementia. Am J Psychiatry. 1985 May;142(5):638–640. doi: 10.1176/ajp.142.5.638. [DOI] [PubMed] [Google Scholar]
- Mayeux R., Stern Y., Spanton S. Heterogeneity in dementia of the Alzheimer type: evidence of subgroups. Neurology. 1985 Apr;35(4):453–461. doi: 10.1212/wnl.35.4.453. [DOI] [PubMed] [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E. M. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Montaldi D., Brooks D. N., McColl J. H., Wyper D., Patterson J., Barron E., McCulloch J. Measurements of regional cerebral blood flow and cognitive performance in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1990 Jan;53(1):33–38. doi: 10.1136/jnnp.53.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moossy J., Zubenko G. S., Martinez A. J., Rao G. R., Kopp U., Hanin I. Lateralization of brain morphologic and cholinergic abnormalities in Alzheimer's disease. Arch Neurol. 1989 Jun;46(6):639–642. doi: 10.1001/archneur.1989.00520420059023. [DOI] [PubMed] [Google Scholar]
- Najlerahim A., Bowen D. M. Biochemical measurements in Alzheimer's disease reveal a necessity for improved neuroimaging techniques to study metabolism. Biochem J. 1988 Apr 1;251(1):305–308. doi: 10.1042/bj2510305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D., Di Piero V., Vallar G., Cappa S., Messa C., Bottini G., Berti A., Passafiume D., Scarlato G., Gerundini P. Technetium-99m HM-PAO-SPECT study of regional cerebral perfusion in early Alzheimer's disease. J Nucl Med. 1988 Sep;29(9):1507–1514. [PubMed] [Google Scholar]
- Rossor M. N., Iversen L. L., Reynolds G. P., Mountjoy C. Q., Roth M. Neurochemical characteristics of early and late onset types of Alzheimer's disease. Br Med J (Clin Res Ed) 1984 Mar 31;288(6422):961–964. doi: 10.1136/bmj.288.6422.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M., Tym E., Mountjoy C. Q., Huppert F. A., Hendrie H., Verma S., Goddard R. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry. 1986 Dec;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- Seltzer B., Sherwin I. A comparison of clinical features in early- and late-onset primary degenerative dementia. One entity or two? Arch Neurol. 1983 Mar;40(3):143–146. doi: 10.1001/archneur.1983.04050030037006. [DOI] [PubMed] [Google Scholar]
- Ware B. R., Klein J. W. Assembly of actin filaments studied by laser light scattering and fluorescence photobleaching recovery. Biophys J. 1986 Jan;49(1):147–149. doi: 10.1016/S0006-3495(86)83629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]