Abstract
Interleukin-8 (IL-8) is elevated in the cerebrospinal fluid (CSF) of patients with meningitis and is proposed to participate in subarachnoid-space pleocytosis. However, intracisternal injection of IL-8 into rabbits failed to induce indices typical of meningitis (leukocyte, tumor necrosis factor, or protein accumulation in the CSF or histopathological changes), indicating that merely increasing the CSF level of this chemokine is insufficient to induce inflammation in this anatomical site. IL-8 treatment did not affect inflammatory responses to subsequently intracisternally administered lipopolysaccharide (LPS). IL-8 was chemotactic for rabbit neutrophils in vitro, and subcutaneous injection of IL-8 (diluted in buffer or CSF) proved the in vivo activity of this peptide and suggested the absence of an IL-8 inhibitor in normal rabbit CSF. LPS-dependent pleocytosis was only slightly diminished by intracisternally administered murine anti-rabbit IL-8 monoclonal antibody (MAb) WS-4 but was dramatically reduced by intravenously administered MAb. Therefore, elevated CSF IL-8 levels may contribute to, but cannot solely account for, neutrophil influx into the subarachnoid space during meningitis. However, inhibition of IL-8 activity of the bloodstream side of the blood-brain barrier effectively reduces pleocytosis, indicating a central role of IL-8 in neutrophil influx into CSF during bacterial meningitis. Thus, inhibition of IL-8 is a possible therapeutic target for adjunct treatment of meningitis.
In bacterial meningitis, a marked inflammatory reaction takes place in the subarachnoid space that is initiated by bacterial components (peptidoglycan, lipopolysaccharide [LPS]) that induce proinflammatory cytokines (e.g., interleukin-1 [IL-1], tumor necrosis factor alpha [TNF-α]). This inflammatory pathology has been linked to the development of neurological sequelae that follow bacteriological cure (6, 17, 55). A characteristic feature of this inflammatory response is the presence of neutrophils in the cerebrospinal fluid (CSF). IL-8, a member of the C-X-C chemokine family of peptide cytokines, is a potent mediator of inflammation. In neutrophils, the primary target cells of IL-8 and several other chemokines, IL-8 induces chemotaxis, enzyme release from storage granules, production of oxygen radicals, and upregulation of adhesion molecules (2, 63). Notably, IL-8 is regarded to play an important role in the pathology of inflammatory diseases, since (i) large quantities of this cytokine can be found in situ at inflammatory sites; (ii) many tissue cells produce IL-8 when activated by IL-1, TNF, or LPS (2, 63); and (iii) finally, anti-IL-8 antibody reduces neutrophil infiltration at the site of inflammation (35).
However, the role of chemokines in bacterial meningitis is not well understood. Experimentally, IL-8 has been detected in the CSF of rabbits with Streptococcus pneumoniae meningitis, and CSF IL-8 levels begin to rise just before commencement of pleocytosis (38); furthermore, the authors of that study report that an anti-rabbit IL-8 antibody attenuates the inflammatory response (C. Østergaard, T. L. Benfield, N. Frimodt-Møller, F. Espersen, N. Mukaida, K. Matsushima, C. G. Larsen, and J. D. Lundgren, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 2043, 1999). Macrophage inflammatory protein-1α (MIP-1α) and MIP-2 (the murine homolog of GRO, but possessing activity functionally similar to IL-8) are produced intrathecally in mice with Listeria monocytogenes infection, and antibodies to these chemokines can neutralize the chemotactic activity of CSF ex vivo (45). Haemophilus influenzae meningitis in infant rats was associated with elevated MIP-1α, MIP-2, methyl-accepting chemotaxis protein 1 (MCP-1), and regulated upon activation, normal T cell expressed and secreted chemokine (RANTES) mRNA in the subarachnoid space, and antibodies to MIP-1α and MIP-2 reduced neutrophil influx, while antibodies to MCP-1 reduced macrophage influx (15).
Many clinical studies have detected IL-8 in the CSF of meningitis patients (7, 19, 26, 29, 39, 46, 49, 51, 57, 58, 62), suggesting that this chemokine may play a role in the accumulation of neutrophils within the subarachnoid space. Clinically, there appears to be a marked difference in the duration of elevated chemokine levels between tubercular and acute bacterial meningitis, with the former displaying protracted elevated chemokine levels compared to the latter (32). Some (39, 51) but not all (7, 19, 26, 29, 49) of these clinical studies were able to correlate the CSF IL-8 concentration to neutrophil levels during bacterial meningitis, but this conclusion needs to viewed cautiously since many of these clinical samples were from single time points at indeterminate times after the induction of pleocytosis. However, there is some evidence that CSF samples obtained within 12 h of onset of clinical symptoms have higher CSF IL-8 levels than in those obtained later in the clinical course (19). Furthermore, a recent study demonstrated a correlation between CSF IL-8 levels and neutrophil levels of samples obtained within 12 h of the onset of aseptic meningitis (62). In those clinical studies considering cytokine determinations from sequential samples (26), CSF IL-8 concentrations decreased with the commencement of disease resolution. Many chemotactic peptides can be detected in the CSF of meningitis patients. C-X-C (IL-8, GROα) and C-C (MCP-1, MIP-1α, and MIP-1β) chemokines were detected in >80% of bacterial meningitis patient CSF samples; RANTES was found in only 25% of the samples (49). Furthermore, the levels of C-X-C and C-C chemokines (but not RANTES) were correlated to each other and to the leukocyte chemotactic activity of CSF, but not to neutrophil levels, and antibody to IL-8 was shown to dramatically (but incompletely) inhibit the neutrophil chemotactic activity possessed by CSF from meningitis patients (49). Although some studies (19, 57), but not all of them (65), have reported increased serum IL-8 levels in such patients, it has been proposed that IL-8 could be produced locally in the subarachnoid space during meningitis and participates in the inflammatory process (58). The possible sources of chemokines (microglia, astrocytes, and endothelia) and their roles in meningitis have been reviewed (28, 55). Although associated with damaging inflammation process, elevated IL-8 in the CNS may be beneficial since IL-8 can activate glia and induce nerve growth factor, which promote neuron survival (27, 34).
Although much evidence implicates IL-8 in the inflammatory response to bacterial meningitis, direct confirmation is lacking. The purpose of the present study was to examine the role of IL-8 in the development of LPS-induced neutrophil influx into the CSF of rabbits.
MATERIALS AND METHODS
IL-8 and related peptides.
Based upon the human amino acid sequences, IL-872 (hIL-8), neutrophil activating peptide-2 (NAP-2), and GROα were chemically synthesized (8). These chemically synthesized peptides are comparable in activity to natural or recombinant peptides (8, 56).
Purification of rbIL-8.
Rabbit IL-8 (rbIL-8) was purified from the culture medium of rabbit alveolar macrophages stimulated with LPS as described previously (56). Alveolar macrophages were collected from rabbits by bronchioalveolar lavage with pyrogen-free phosphate-buffered saline (PBS), and adherent cells were cultured for 4 days in minimal essential medium (MEM; Gibco/Life Technologies, Basel, Switzerland) containing 2.5 μg of LPS (Escherichia coli O111:B4 [Difco]; Chemie Brunschwig AG, Basel, Switzerland) per ml and 1% (vol/vol) human plasma protein (PPL; Swiss Red Cross Laboratories, Bern, Switzerland). Conditioned medium was first diluted in 20 mM KHPO4 buffer (pH 7.2) containing 20 mM NaCl and 5% (wt/vol) glycerol and then loaded onto a phosphocellulose column (P11; Whatman; Bender and Hobein, Zürich, Switzerland). Fractions were eluted with an NaCl gradient (0.02 to 2.0 M). Based upon a purification method for human IL-8 (59), fractions demonstrating the ability to release elastase from cytochalasin-treated human neutrophils were pooled. This pool was acidified (0.1% [wt/vol] trifluoroacetic acid) and loaded onto a reversed-phase high-pressure liquid chromatography C4 column (Bakerbond, 4.6 by 250 mm; Baker Research Products, Philipsburg, N.J.) and eluted with a linear gradient of 0.67% (vol/vol) acetonitrile per min. An active fraction eluting at 59 min was collected, desiccated, resuspended in 0.1% (wt/vol) trifluoroacetic acid, loaded onto a C3 column (2.1 by 100 mm; Brownlee/Applied Biosystems; Paul Bucher, Basel, Switzerland), and eluted with a linear gradient of 1% acetonitrile (vol/vol). An active fraction eluted as a single peak at 39 min and was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to yield a single band of approximately 8 kDa (silver staining; data not shown). When subjected to automated phenyl isothiocyanate degradation analysis (Applied Biosystems gas-phase sequencer model 477A; Paul Bucher), the N-terminal sequence (NH2-Ala-Val-Leu-Try-Arg-Ile-) was shown to be identical to that of rabbit IL-8 (3, 12).
In vitro chemotaxis.
Rabbit blood was collected from the lateral ear vein into 10% acid citrate dextrose, and total leukocytes were prepared from the buffy coat, washed, and resuspended in PBS containing 0.2% (wt/vol) PPL. Human neutrophils were obtained from healthy volunteers and were similarly prepared. Neutrophil migration was determined using multiwell chemotaxis chambers (Neuro Probe, Cabin John, Md.), essentially as previously described (60). Rabbit IL-8 (and the human chemokines) only induced the migration of neutrophils (not shown).
Sterile meningitis model.
The Ethical Committee of the Kantonales Veterinäramt of Basel Stadt approved the experimental protocols involving animals. Specific-pathogen-free chinchilla rabbits, 2.5 to 3 kg in weight, were obtained from Thomae, Biberach an der Riss, Germany. On the day before an experiment, rabbits were anesthetized with a combination of fentanyl and fluanisone (Hypnorm; Janssen Pharmaceutica AG, Baar, Switzerland) and were fitted with prostheses to facilitate placement within a stereotactic frame according to an established method (13). Prior to the injection of peptides, rabbits received 1.75 g of ethyl carbamate (urethane) per kg of body weight subcutaneously and then 10 mg of pentobarbital intravenously per kg to induce deep, long-term anesthesia. The animals were fixed in a stereotactic frame, and 3.5-in. spinal needles (25 g) were sited in the cisterna magna to allow repeated sampling of the CSF. Following the withdrawal of 0.4 ml of CSF, various amounts of peptide (diluted in 0.2 ml of pyrogen-free physiological saline [PFS]) were introduced into the subarachnoid space, and the needle was flushed with 0.1 ml of CSF. CSF (0.2 ml) was sampled at 2, 4, 6, and 8 h postinjection. The rate of removal of CSF did not exceed the rate of CSF formation (approximately 0.4 ml/h [50]). At 8 h, if no evidence of pleocytosis was observed, 25 ng of LPS (E. coli O111:B4; Sigma) in 0.2 ml of PFS or PFS alone was administered intracisternally, and CSF was sampled 2 and 4 h later. At 12 h after the start of the experiment, rabbits were sacrificed using an overdose of T61 (Hoescht Veterinär GmbH, Munich, Germany). At least two rabbits were injected with each dose of peptide evaluated.
Determination of indices of inflammation.
The numbers of leukocytes present in the CSF were determined by appropriately diluting CSF samples and counting the leukocytes using a Sysmex cell counter (model CC-170M; TDA Corp., Kobe, Japan). The TNF content of serum and CSF was determined by bioassay using WEHI 164 cells as indicator cells, essentially as described previously (16). Human TNF-α (Boehringer Mannheim) was used as a standard. The limit of detection was 0.05 ng/ml. CSF samples (10 μl) were assayed in triplicate, the coefficient of variation being always less than 21% and usually less than 11%. The CSF protein content was determined essentially according to the method of Lowry et al. (30). Values presented are the means ± the standard errors of the means (SEM). Statistical analyses used rank sum tests (SigmaStat; Jandel Scientific).
Histological evaluation of brain.
Rabbits were intracisternally administered 1,750 ng of hIL-8 and subsequently injected with either PFS or 25 ng of LPS at 8 h. At 12 h post-first injection, the rabbits were sacrificed and the brains were removed intact. No attempt was made to remove blood. Brains were fixed in 3.8% neutral phosphate-buffered formalin, trimmed, embedded in Paraplast (Zahner Electonic AG, Kaltbrunn, Switzerland), and sectioned nominally at 5 μm before staining with hematoxylin and eosin. Three sections, from different levels and encompassing meningeal and aqueductal surfaces, were examined histologically.
Intradermal IL-8 injection.
The day before injection of IL-8, the backs of two rabbits were closely shaved and marked to locate injection sites. hIL-8 (17,500 ng/ml) was prepared in either PFS or freshly obtained rabbit CSF, and 0.1-ml volumes were injected intradermally in separate sites; PFS and CSF alone were also injected. The animals were sacrificed 2 h postinjection, and the skin surrounding the injection site was excised, stretched over cardboard, and fixed in 3.8% neutral phosphate-buffered formalin. Tissues were subsequently trimmed, embedded in Paraplast, sectioned at 4 μm, and stained with hematoxylin and eosin. Two sections per skin sample were examined histologically.
Anti-IL-8 antibody.
The monoclonal antibody (MAb) WS-4 (murine immunoglobulin G1κ isotype), a well-characterized, specific antibody, which neutralizes the activity of rabbit IL-8 in vivo (20) and has been described previously (25), was purified from mouse ascites using protein G-Sepharose (Pharmacia Biotech, Uppsala, Sweden) according to the manufacturer's protocol. Antibody was eluted with 0.1 M Tris-glycine (pH 3.0). The pH was neutralized by the addition of 1 M Tris-HCl (pH 8.4), and the antibody was dialyzed against PBS. WS-4 was prepared in pyrogen-free solutions and tested negative for endotoxin in the Limulus assay.
Neutralization of in vivo IL-8 activity by anti-IL-8 antibody.
For neutralization of IL-8 activity in CSF, WS-4 was diluted to 2.5 mg/ml in PFS containing 125 ng of E. coli LPS per ml, and 0.2 ml of this mixture was injected intracisternally into rabbits as described above. The predicted initial concentration of WS-4 was approximately 100 to 125 ng/ml of CSF, based upon the approximate volume of CSF in rabbits of this weight being 4 to 5 ml (36). WS-4 was also administered by an intravenous infusion of a 0.9-mg/ml antibody solution in PFS that was infused into rabbits over a 20-min period via the lateral ear vein; the predicted initial concentration of this antibody was 33 to 40 μg/ml of blood (based upon an approximately blood volume of 10% of the body weight). At the end of the infusion, 25 ng of E. coli LPS was injected intracisternally as described above. To control for the neutralizing activity of WS-4, recombinant IL-8 (rIL-8; 1,750 ng) with or without WS-4 (125 μg) in PFS was injected intradermally into rabbits as described above.
RESULTS
In vitro chemotaxis.
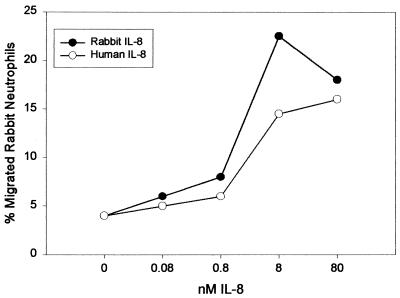
In vitro chemotaxis experiments using leukocytes purified from rabbit peripheral blood demonstrated migratory activity for both hIL-8 and rbIL-8 that was restricted to neutrophils (Fig. 1). rbIL-8 was apparently slightly more potent than hIL-8.
FIG. 1.
Chemotactic response of rabbit neutrophils to human or rabbit IL-8. Dilutions of each peptide were made in MEM containing 0.2% (wt/vol) bovine serum albumin and placed into the lower wells of a chemotaxis chamber. Freshly isolated rabbit blood buffy coat cells (800 cells/μl) in MEM plus 0.2% (wt/vol) bovine serum albumin were placed into the upper wells of a chemotaxis chamber. Cells migrating to the lower surface of the separating polycarbonate membrane were stained with DiffQuick. Migrated neutrophils were counted microscopically at ×1,000 magnification in five randomly chosen fields per well. The response was calculated as the percentage of neutrophils of the input number that migrated. The result shown is a typical example of three consistent, independent experiments.
Intracisternal injection of IL-8 does not produce pleocytosis.
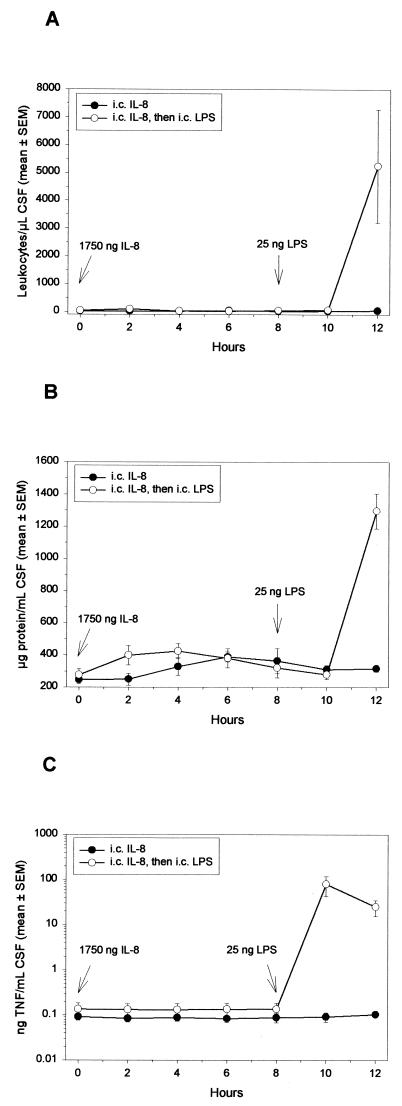
Various amounts of hIL-8 (1.75, 175, or 1,750 ng; to yield ca. 3.89 to 389 ng/ml of CSF) were injected in order to span the concentration ranges determined in clinical specimens (ca. 0.02 to 150 ng/ml of CSF) (7, 19, 26, 29, 39, 46, 49, 51, 57, 58, 62). Figure 2 demonstrates that, during an 8-h period after the intracisternal injection of 1,750 ng of hIL-8, no influx of leukocytes or accumulation of TNF or of total protein occurred in the CSF. Some rabbits were monitored for 12 h and did not demonstrate any overt subarachnoid space inflammation (Fig. 2). Similar results were obtained when smaller amounts of hIL-8 were injected and when rabbits were given 7,200 ng of hIL-8 (Table 1). hNAP-2 and hGROα (17.5, 175, or 1,750 ng), two homologous CXC chemokines with potent neutrophil-activating properties (2, 63), also failed to induce an inflammatory response (Table 1). In contrast, intracisternal injection of 25 ng of LPS into rabbits (previously injected with hIL-8) induced a vigorous inflammatory response characterized by a peak of TNF activity (<0.14 ng of TNF/ml of CSF at 8 h; 80.2 ± 38.0 ng/ml of CSF at 10 h) and marked accumulation of leukocytes (<100 leukocytes/μl of CSF at 8 h; 5,250 ± 2,037 leukocytes/μl of CSF at 12 h) and of protein (277 ± 40 μg/ml of CSF at 8 h; 1,290 ± 109 μg/ml of CSF at 12 h) within the CSF (Fig. 2). TNF was never detected in serum (<0.5 ng/ml), and there was approximately a 20% increase in circulating leukocytes. This slight leukocytosis was also observed in rabbits that received saline intracisternally (not shown). Rabbits receiving any of the chemokines did not demonstrate an attenuated inflammatory response to LPS compared to rabbits receiving PFS at 0 h and then LPS at 8 h (64 ± 12 ng of TNF/ml of CSF at 10 h; 7,000 ± 4,714 leukocytes/μl of CSF and 645 ± 64 μg of protein/ml of CSF at 12 h; n = 3; P > 0.05, rank sum test).
FIG. 2.
Effect of intracisternal injection of hIL-8 in inducing meningitis. Rabbits were administered 1,750 ng of IL-8 intracisternally at 0 h (n = 8), and some animals (n = 4) received 25 ng of LPS intracisternally at 8 h. Every 2 h, 0.2 ml of CSF was removed, and the leukocyte (A), total protein (B), and TNF (C) contents were determined. Symbols represent mean values, and the SEM either is represented by vertical bars or is encompassed by the size of the symbol.
TABLE 1.
Intracisternal administration of several CXC chemokines fails to induce pleocytosis in rabbitsa
| Treatment | No. of rabbits | Amt injected (ng) | Mean peak leukocyte count/μl of CSF ± SEM |
|---|---|---|---|
| hIL-8 | 2 | 7,200 | 120 |
| 8 | 1,750 | 70 ± 30 | |
| 2 | 175 | 70 | |
| 3 | 17.5 | 50 ± 10 | |
| hNAP-2 | 7 | 1,750 | 80 ± 20 |
| 2 | 175 | 80 | |
| 2 | 17.5 | 40 | |
| hGROα | 3 | 1,750 | 50 ± 20 |
| 2 | 175 | 120 | |
| 2 | 17.5 | 100 | |
| rbIL-8 | 4 | 1,750 | 60 ± 20 |
| 2 | 175 | 80 | |
| 2 | 17.5 | 50 | |
| Saline | 8 | 73 ± 5 | |
| LPS | 10 | 25 | 8,028 ± 1,132 |
Rabbits were injected intracisternally with saline, chemokines, or LPS in 0.2-ml volumes. CSF was sampled every 2 h for a total of 8 h, and leukocyte counts were determined by use of an automatic cell counter. Peak leukocyte levels occurred by between 6 and 8 h following the administration of chemokines or saline and at 4 h after the administration of LPS.
Although IL-8 is able to induce transmigration of neutrophils across undamaged endothelial layers in vitro (23, 47) and in vivo (11), this may not occur with the specialized endothelium of the blood-brain barrier. Prostaglandin E2 (PGE2) has been previously shown to partially disrupt the blood-brain barrier (24) and to potentiate the effects of IL-8 (10, 18). Intracisternal injection of PGE2 (5 or 0.5 μg) 2 h before or at the same time as that of hIL-8 (1,750 ng) did not result in an influx of leukocytes into the CSF over 12 h. Furthermore, the capacity of the rabbits to mount an inflammatory response to LPS was not altered following PGE2 injection (not shown). PGE2 was active in disrupting the blood-brain barrier since an approximately fourfold increase in the CSF protein content occurred following the intracisternal injection of 5 μg of PGE2 (0 h, 488 ± 41 μg of protein/ml of CSF; 12 h postinjection, 2,233 ± 222 μg of protein/ml of CSF; n = 3).
Although hIL-8 has been reported to induce strong inflammatory responses in rabbits (see, e.g., references 10, 18, and 20) and the rabbit IL-8 receptor functionally binds hIL-8 at high affinity (56), rbIL-8 was purified from natural sources and tested in this meningitis model in order to exclude the possibility of an unexpected species specificity of IL-8 within the subarachnoid space. Rabbit peptide was used in meningitis experiments analogous to those experiments using human peptides (Table 1). Intracisternal injection of 17.5, 175, or 1,750 ng of rabbit IL-8 did not result in an increase in any of the inflammatory indices measured and apparently did not affect the inflammatory response to LPS (not shown).
Histological evaluation of the effects of intracisternally administered hIL-8.
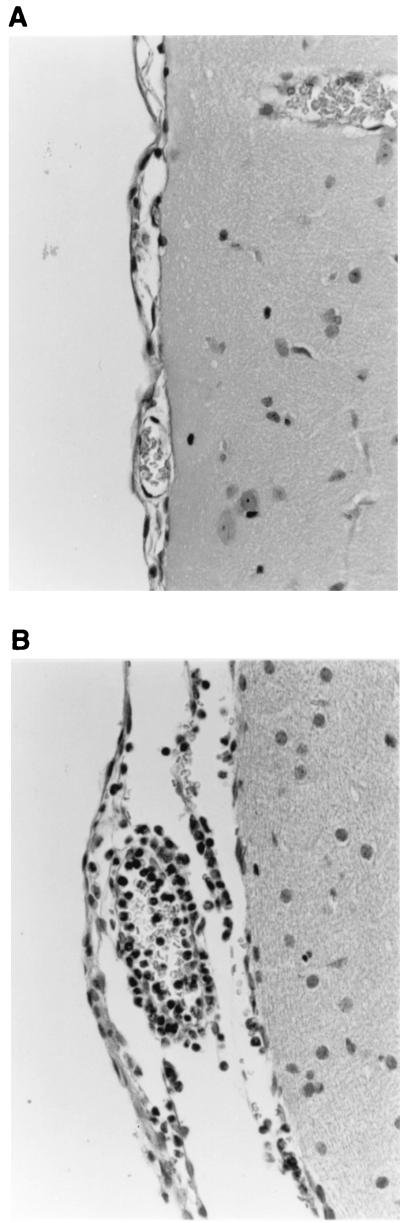
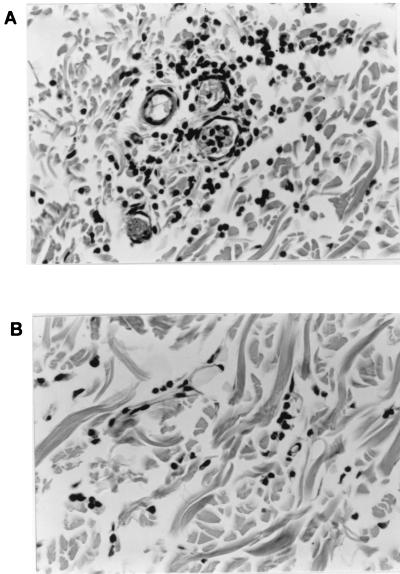
The degree of leukocytosis in the CSF may not correlate with the extent of inflammation occurring in the meninges during meningitis (37). To address this point, histological examination of the brains of rabbits that had received intracisternal hIL-8 was performed to exclude the possibility that pleocytosis was induced by hIL-8 but not associated with leukocytes appearing in the CSF. Figure 3A demonstrates that at 12 h post-intracisternal injection of 1,750 ng of hIL-8, no evidence of meningeal inflammation was apparent. Rabbits that had first received hIL-8 and then 25 ng of LPS 8 h later showed a clear accumulation of neutrophils in the meninges 4 h after the injection of LPS (Fig. 3B). Similar results were obtained with rbIL-8 (not shown).
FIG. 3.
Histological evaluation of the effects of intracisternal hIL-8. Rabbits were administered 1,750 ng of hIL-8 intracisternally and then subsequently either administered PFS or 25 ng of LPS intracisternally at 8 h. At 12 h post-first injection, rabbits were sacrificed and the brains were removed intact. Brains were fixed in buffered formalin, trimmed, embedded in Paraplast, and sectioned at 5 μm before staining with hematoxylin and eosin. Panel A shows that there are no histological alterations of the meninges of rabbits treated with hIL-8. Panel B demonstrates the formation of an acute meningitis 4 h after the injection of LPS. Inflammatory cells, predominantly neutrophils, are located in the perivascular areas and the lumen of blood vessels. Magnification, ×400.
Histological evaluation of the effects of intradermal hIL-8.
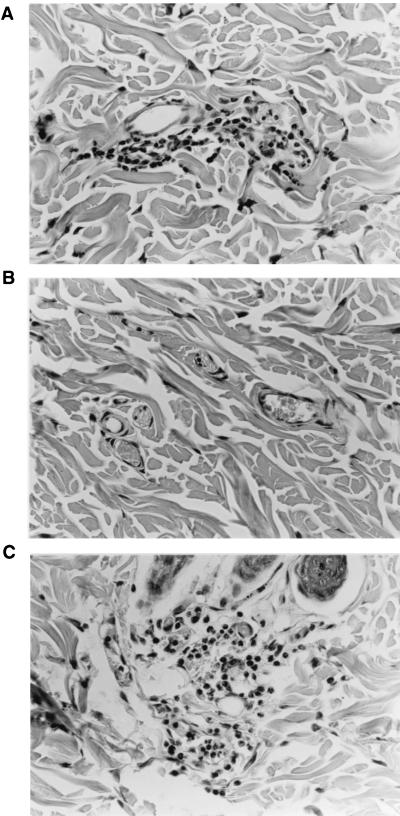
To confirm that rabbits are indeed responsive to this IL-8 preparation, 1,750 ng of hIL-8 diluted in PFS or normal rabbit CSF was injected intradermally into rabbits. Two hours later, the skin surrounding the injection site was excised and processed for histological evaluation. Figure 4 demonstrates that the hIL-8 used in this study was active in vivo, producing a distinct accumulation of neutrophils in the dermis (Fig. 4A). Similar results were obtained when rbIL-8 was used (not shown). As a control, PFS alone was inactive (Fig. 4B). Since the degree of inflammatory response was similar between hIL-8 diluted in PFS or CSF (Fig. 4C), the possibility that soluble inhibitors of IL-8 mitigate its chemotactic activity in the CSF may be excluded. Normal rabbit CSF (0.1 ml/site) was not found to be proinflammatory (not shown).
FIG. 4.
Histological evaluation of the proinflammatory effects of hIL-8 in rabbit skin. A total of 1,750 ng of hIL-8, diluted in either PFS or normal CSF, was injected into the skin of rabbits (in 0.1-ml volumes). At 2 h postinjection, skin samples were excised, fixed in buffered formalin, trimmed, embedded in Paraplast, and sectioned at 4 μm before staining with hematoxylin and eosin. hIL-8 produced a neutrophilic infiltration in perivascular areas of the dermis by 2 h postinjection (A), whereas PFS alone did not (B). Panel C shows the inflammatory response induced by hIL-8 diluted in rabbit CSF. Magnification, ×400.
Intracisternal administration of anti-IL-8 antibody reduces, but fails to prevent, LPS-induced pleocytosis.
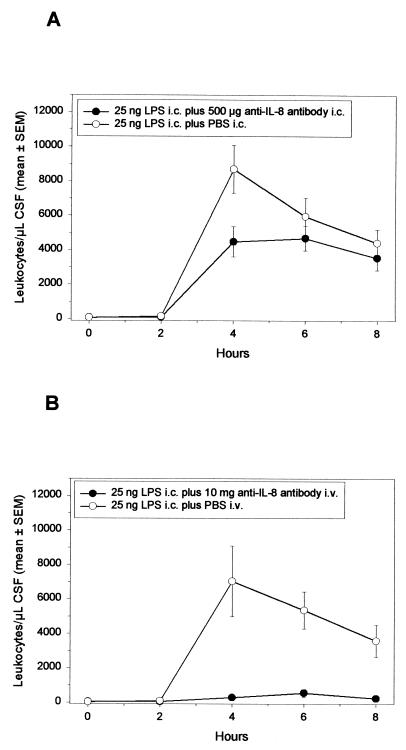
Despite being apparently inactive as a single agent, IL-8 may act in concert with other proinflammatory factors to induce neutrophil influx into the subarachnoid space. To test this possibility, LPS (25 ng) was injected either alone or together with the anti-IL-8 MAb WS-4 (500 μg) into the CSF of rabbits, and the extent of pleocytosis was monitored (Fig. 5A). Although the presence of WS-4 decreased the maximum level of leukocytes about twofold (LPS alone, 8,693 ± 1,392 leukocytes/μl of CSF; LPS plus WS-4, 4,485 ± 881 leukocytes/μl of CSF; P < 0.02, rank sum test), high intracisternal concentrations of inhibitory anti-IL-8 antibody had little effect on the overall pattern of LPS-induced neutrophil influx. The effectiveness of WS-4 in neutralizing the neutrophil chemotactic activity of rbIL-8 was confirmed by injecting rbIL-8 (1,750 ng) with or without WS-4 (125 μg) into rabbit skin (Fig. 6).
FIG. 5.
Effect of intracisternal injection of the anti-IL-8 MAb WS-4 on the pattern of LPS-induced meningitis. Rabbits were given 25 ng of LPS intracisternally at 0 h with (n = 6) or without 500 μg of WS-4 (n = 8) (A) or with 10 mg of WS-4 (n = 5) or PBS (n = 4), followed by 25 ng of LPS given intracisternally 20 min later (B). Every 2 h, 0.2 ml of CSF was removed, and subsequently the leukocyte content was determined. Symbols represent mean values, and the SEM values are represented either by vertical bars or are encompassed by the size of the symbol.
FIG. 6.
Histological evaluation of the effect of anti-IL-8 MAb WS-4 on the activity of rbIL-8 in skin. A total of 1,750 ng of rIL-8, diluted in either PFS or PFS containing 125 μg of WS-4, was injected into the skin of rabbits (in 0.1-ml volumes). At 2 h postinjection, skin samples were excised, fixed in buffered formalin, trimmed, embedded in Paraplast, and sectioned at 4 μm before staining with hematoxylin and eosin. rIL-8 produced a neutrophilic infiltration in perivascular areas of the dermis by 2 h postinjection (A) that was largely inhibited by WS-4 (B). Magnification, ×400.
Intravenous administration of anti-IL-8 antibody strongly inhibits LPS-induced pleocytosis.
Experimental data suggest that adhesion of circulating blood leukocytes to endothelium and subsequent extravagation (transmigration) are induced by chemokines immobilized or exposed on the endothelial cell surface (43, 53, 61). To test whether neutralizing antibody to IL-8 administered on the bloodstream side of the blood-brain barrier is capable of blocking LPS-dependent pleocytosis, WS-4 antibody (10 mg/rabbit) was injected intravenously before intracisternal administration of 25 ng of LPS; this WS-4 antibody dose was previously shown to block LPS-induced neutrophil influx into rabbit skin (20). The results presented in Fig. 5B indicate that pleocytosis was effectively inhibited by intravenous anti-IL-8 MAb (control, 7,030 ± 2,053 leukocytes/μl of CSF at 4 h; WS-4-treated, 544 ± 216 leukocytes/μl of CSF at 6 h; between 4 and 8 h, all the differences were statistically significant [P < 0.01, rank sum test]). Intravenous WS-4 had little effect on the pattern of TNF production within the CSF: peak concentrations may have been slightly reduced (control, 47 ± 12 ng of TNF/ml of CSF; WS-4 treated, 37 ± 10 ng/ml of TNF; P > 0.05, rank sum test), but the overall kinetics of production were unaffected by the antibody (not shown).
DISCUSSION
Given that IL-8 can induce transmigration of neutrophils across endothelia in vitro (27, 47) and in vivo (11) and that elevated CSF IL-8 concentrations occur in meningitis patients (7, 19, 26, 29, 39, 46, 49, 51, 57, 58, 62), IL-8 could perhaps be involved in the inflammatory signals that result in pleocytosis during bacterial meningitis.
However, the results obtained by the present study, in which IL-8 was administered intracisternally to rabbits, suggest that elevated CSF IL-8 levels per se are not sufficient to induce influx of neutrophils into the subarachnoid space or to damage the blood-brain barrier, as evidenced by the absence of protein leakage into the CSF. In contrast, hIL-8 and rbIL-8 preparations induced inflammatory reactions in rabbit skin, proving the activity of the peptide preparations used in the present study. Results obtained with purified rbIL-8 suggest that the observed inactivity of hIL-8 is not due to an unexpected species difference in relative potency within this anatomical site. Apparently transcytosis of CSF IL-8 does not occur or is insufficient to fully activate the specialized endothelium of the blood-brain barrier, in contrast to that occurring with other endothelia (33). Injection of PGE2 into the CSF has been previously shown to heighten the permeability of the blood-brain barrier, as indicated by increases in CSF protein levels (24), and to also potentiate the chemotactic activity of IL-8 (9, 18). However, either pretreatment or coinjection with PGE2 failed to reverse the apparent inactivity of hIL-8 present within the subarachnoid space. hIL-8 diluted in PFS or rabbit CSF resulted in a similar degree of dermal neutrophil accumulation, suggesting that there is no soluble inhibitor of IL-8 within normal CSF. Degradation of this chemokine by proteases resulting in loss of activity is unlikely, given the extreme stability of IL-8 (40).
Our results do not exclude the presence of a potent cell-associated chemokine inhibitor in neural tissue that prevents IL-8 (or GROα- or NAP-2-)-mediated responses. Since intracisternal injection of the low-molecular-weight chemotactic peptides fMLP (54) and C5a (24) and the chemokines MIP-2 and MIP-1 (44) was shown to induce neutrophilic pleocytosis, quiescent tissues within the subarachnoid space may possess an inhibitory capacity that is specific for certain chemokines. Inhibition of the activity of IL-8 can occur by a cell-bound “scavenger” chemokine receptor similar to that present on erythrocytes (14, 21); in support of this, the Duffy antigen receptor for chemokines (DARC) has been shown to be expressed in brain tissue (22). Once an inflammatory process has been initiated within the subarachnoid space, one might speculate that IL-8 is coinduced with a factor(s) (e.g., a protease) to overcome this inhibitor. However, evidence for this hypothesis requires further experimentation.
The apparent lack of IL-8 proinflammatory activity in the CSF would seem to contradict the experimental demonstration (1, 4) of a neutrophil recruitment at areas distal to the injection site following intrahippocampal injection of high amounts of IL-8 (to achieve ca. 104 to 106 ng/ml). Neutrophil accumulation in brain tissue was slow (ca. 24 h postinjection [1]), suggesting that there are important differences in the response to IL-8 depending upon the exact anatomical site in the brain where IL-8 is injected (reviewed in references 31 and 41). Although elevated CSF levels of IL-8 alone are apparently unable to invoke meningeal inflammation, this chemokine may still act in concert with other cytokines to mediate inflammation within the subarachnoid space (e.g., TNF potentiates the activity of IL-8 and NAP-2 [5, 25, 52]). However, the poor inhibition of LPS-induced pleocytosis by intracisternal anti-rabbit IL-8 MAb WS-4 would argue against a strong involvement of locally produced CSF IL-8 in the transmigration of neutrophils across the blood-brain barrier endothelium, even if acting in concert with other proinflammatory cytokines. The presence of anti-IL-8 MAb in the CSF only partially reduced the maximal leukocyte levels and little altered the kinetics of leukocyte influx. Although WS-4 is a well-characterized and specific MAb, a nonspecific effect of the antibody cannot be strictly ruled out; this suggests either that CSF IL-8 plays only a minor role or that other proinflammatory mediators, potentially including other antigenically dissimilar chemokines likely to be present in the CSF during meningitis, may render the effects of IL-8 relatively redundant. In congruence with this, although MIP-1α and MIP-2 have been implicated in experimental L. monocytogenes meningitis (45) and MIP-2 and MIP-1 administered intracisternally to rabbits induced neutrophil influx into the subarachnoid space (44), antibody neutralization of these chemokines only slightly delayed onset of inflammation during pneumococcal infection (44). Intracisternal administration of anti-rabbit IL-8 antibody did not affect the inflammatory response to pneumococcal meningitis in rabbits (Østergaard et al., Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother.). Furthermore, antibody neutralization of IL-8 only partially reduced neutrophil chemotactic activity of CSF ex vivo (49).
In contrast to the weak activity of WS-4 in the CSF, this anti-rabbit IL-8 MAb in the bloodstream was particularly effective in blocking LPS-induced neutrophil migration into the CSF, strongly implicating IL-8 as a dominant mediator in this inflammatory process. Although a nonspecific effect of this low concentration of antibody cannot be ruled out, this would suggest that the anti-IL-8 MAb in the bloodstream is neutralizing IL-8 sequestered from this receptor, possibly in the vicinity of, or associated with the surface of, activated endothelial cells of the blood-brain barrier. However, other inflammatory mediators induced by LPS may enhance the permeability of this lining barrier, allowing a chemotactic gradient of IL-8 to be established from the CSF (high IL-8 concentration) to neutrophils within meningeal or choroidal venules.
There is compelling evidence that IL-8 can be bound to the endothelial surface (42) and therefore is acting in a haptotactic manner rather than presenting a soluble molecule gradient (42, 43). In this case, the endothelium of the blood-brain barrier (or cells within the subarachnoid space), stimulated by bacterial invasion, invokes an inflammatory response producing IL-8 that crosses the endothelium by transcytosis to specific areas on the endothelium microvilli (33), binds to cell surface glycosaminoglycans (e.g., heparin sulfate [33, 53, 61] and DARC [21]), and anchors to the endothelium via the C terminus to expose the receptor-interactive N-terminal domain (21). Such surface-bound IL-8 presents a chemotactic stimulus to leukocytes but should also be accessible to anti-IL-8 antibody in the circulation. However, not all endothelial cells show the same IL-8 binding responses (33), and this needs to be determined for the specialized endothelia of the blood-brain barrier. These data would also suggest that caution should be applied in studies assessing the potential role of proinflammatory factors in meningitis when intracisternal injection is the only method of evaluation.
Chemokines have been recognized as a suitable target for therapeutic intervention against inflammatory diseases (48). Despite the presence of a large variety of chemotactic peptides in the CSF from meningitis patients (28), the present data indicate a central role for IL-8 in subarachnoid space inflammation during meningitis; a precise mechanistic depiction awaits further elucidation. Nonetheless, the data suggest IL-8 to be a well-defined target for the adjunct treatment of meningitis.
ACKNOWLEDGMENTS
We thank J. Vaxelaire, S. Kunz, and M. Hattenberger for technical assistance; Antal Rot for his review of the manuscript; Alfred Walz for his help in the purification of rIL-8; and Ian Clark-Lewis for providing synthetic hIL-8, hNAP-2, and hGROα.
REFERENCES
- 1.Andersson P-B, Perry V H, Gordon S. Intracerebral injection of proinflammatory cytokines or leukocyte chemotaxins induces minimal myelomonocytic cell recruitment to the parenchyma of the central nervous system. J Exp Med. 1992;176:255–259. doi: 10.1084/jem.176.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 3.Beaubien B C, Collins P D, Jose P J, Totty N F, Hsuan J, Waterfield M D, Williams T J. A novel neutrophil chemoattractant generated during an inflammatory reaction in the rabbit peritoneal cavity in vivo. Biochem J. 1990;271:797–801. doi: 10.1042/bj2710797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell M D, Taub D D, Perry V H. Overriding the brain's intrinsic resistance to leukocyte recruitment with intraparenchymal injections of recombinant chemokines. Neuroscience. 1996;74:283–292. doi: 10.1016/0306-4522(96)00083-8. [DOI] [PubMed] [Google Scholar]
- 5.Brandt E, Petersen F, Flad H P. Recombinant tumor necrosis factor-α potentiates neutrophil degranulation in response to host defence cytokines neutrophil-activating peptide 2 and IL-8 by modulating intracellular cyclic AMP levels. J Immunol. 1992;149:1356–1364. [PubMed] [Google Scholar]
- 6.Braun J S, Tuomanen E I. Molecular mechanisms of brain damage in bacterial meningitis. Adv Pediatr Infect Dis. 1999;14:49–71. [PubMed] [Google Scholar]
- 7.Chaka W, Heyderman R, Gangaidzo I, Robertson V, Mason P, Verhoef J, Verheul A, Hoepelman A I. Cytokine profiles in cerebrospinal fluid of human immunodeficiency virus-infected patients with cryptococcal meningitis: no leukocytosis despite high interleukin-8 levels. J Infect Dis. 1997;176:1633–1636. doi: 10.1086/517344. [DOI] [PubMed] [Google Scholar]
- 8.Clark-Lewis I, Moser B, Walz A, Baggiolini M, Scott G J, Aebersold R. Chemical synthesis, purification, and characterisation of two inflammatory proteins, neutrophil activating peptide 1 (interleukin-8) and neutrophil activating peptide 2. Biochemistry. 1991;30:3128–3135. doi: 10.1021/bi00226a021. [DOI] [PubMed] [Google Scholar]
- 9.Colditz I G. Effect of exogenous prostaglandin E2 and actinomycin D on plasma leakage induced by neutrophil-activating peptide-1/interleukin-8. Immunol Cell Biol. 1990;68:397–403. doi: 10.1038/icb.1990.53. [DOI] [PubMed] [Google Scholar]
- 10.Colditz I G, Zwahlen R D, Baggiolini M. Neutrophil accumulation and plasma leakage induced in vivo by neutrophil-activating peptide-1. J Leukoc Biol. 1990;48:129–137. doi: 10.1002/jlb.48.2.129. [DOI] [PubMed] [Google Scholar]
- 11.Colditz I G, Zwahlen R D, Dewald B, Baggiolini M. In vivo inflammatory activity of neutrophil-activating factor, a novel chemotactic peptide derived from human monocytes. Am J Pathol. 1989;134:755–760. [PMC free article] [PubMed] [Google Scholar]
- 12.Collins P D, Jose P J, Williams T J. The sequential generation of neutrophil chemoattractant proteins in acute inflammation in the rabbit in vivo. J Immunol. 1991;146:677–684. [PubMed] [Google Scholar]
- 13.Dacey R G, Sande M A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974;6:437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darbonne W C, Rice G C, Mohler M A, Apple T, Hébert C A, Valente A J, Baker J B. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Investig. 1991;88:1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diab A, Abdalla H, Li H N, Shi F D, Zhu J, Höjberg B, Lindquist L, Wretlind B, Bakhiet M, Link H. Neutralization of macrophage inflammatory protein 2 (MIP-2) and MIP-1α attenuates neutrophil recruitment in the central nervous system during experimental bacterial meningitis. Infect Immun. 1999;67:2590–2601. doi: 10.1128/iai.67.5.2590-2601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 17.Feldman W E. Relation of concentrations of bacteria and bacterial antigen in cerebrospinal fluid to prognosis in patients with bacterial meningitis. N Engl J Med. 1977;296:433–435. doi: 10.1056/NEJM197702242960806. [DOI] [PubMed] [Google Scholar]
- 18.Foster S J, Aked D M, Schröder J-M, Christophers E. Acute inflammatory effects of a monocyte-derived neutrophil-activating peptide in rabbit skin. Immunology. 1989;67:181–183. [PMC free article] [PubMed] [Google Scholar]
- 19.Halstensen A, Ceska M, Brantzaeg P, Redl H, Naess A, Waage A. Interleukin-8 in serum and cerebrospinal fluid from patients with meningococcal disease. J Infect Dis. 1993;167:471–475. doi: 10.1093/infdis/167.2.471. [DOI] [PubMed] [Google Scholar]
- 20.Harada A, Sekido N, Kuno K, Akiyama M, Kasahara T, Nakanishi I, Mukaida N, Matsushima K. Expression of recombinant rabbit IL-8 in Escherichia coli and establishment of the essential involvement of IL-8 in recruiting neutrophils into lipopolysaccharide-induced inflammatory site of rabbit skin. Int Immunol. 1993;6:681–690. doi: 10.1093/intimm/5.6.681. [DOI] [PubMed] [Google Scholar]
- 21.Horuk R, Colby T J, Darbonne W C, Schall T J, Neote K. The human erythrocyte inflammatory peptide (chemokine) receptor. Biochemical characterisation, solubilization, and development of a binding assay for the soluble receptor. Biochemistry. 1993;32:5733–5738. doi: 10.1021/bi00073a002. [DOI] [PubMed] [Google Scholar]
- 22.Horuk R, Martin A, Hesselgesser J, Hadley T, Lu Z, Wang Z, Peiper S C. The Duffy antigen receptor for chemokines: structural analysis and expression in the brain. J Leukoc Biol. 1996;59:29–38. doi: 10.1002/jlb.59.1.29. [DOI] [PubMed] [Google Scholar]
- 23.Huber A R, Kunkel S L, Todd R F, Weiss S J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- 24.Kadurugamuwa J L, Hengstler B, Bray M A, Zak O. Inhibition of complement-factor-5a-induced inflammatory reactions by prostaglandin E2 in experimental meningitis. J Infect Dis. 1989;160:715–719. doi: 10.1093/infdis/160.4.715. [DOI] [PubMed] [Google Scholar]
- 25.Ko Y-C, Mukaida N, Panyutich A, Voitenok N N, Matsushima K, Kawai T, Kasahara T. A sensitive enzyme-linked immunosorbent assay for human interleukin-8. J Immunol Methods. 1992;149:227–235. doi: 10.1016/0022-1759(92)90254-q. [DOI] [PubMed] [Google Scholar]
- 26.Kornelisse R F, Sacelkoul H F J, Mulder P H G, Suur M H, van der Straaten P J C, van der Heijden A J, Sukhai R N, Hählen K, Neijens H J, de Groot R. Interleukin-10 and soluble tumor necrosis factor receptors in cerebrospinal fluid of children with bacterial meningitis. J Infect Dis. 1996;173:1498–1502. doi: 10.1093/infdis/173.6.1498. [DOI] [PubMed] [Google Scholar]
- 27.Kossmann T, Stahel P F, Lenzlinger P M, Redl H, Dubs R W, Trentz O, Schlag G, Morganti-Kossmann M C. Interleukin-8 released into the cerebrospinal fluid after brain injury is associated with blood-brain barrier dysfunction and nerve growth factor production. J Cereb Blood Flow Metab. 1997;17:280–289. doi: 10.1097/00004647-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Lahrtz F, Piali L, Spanaus K S, Seebach J, Fontana A. Chemokines and chemotaxis of leukocytes in infectious meningitis. J Neuroimmunol. 1998;85:33–43. doi: 10.1016/s0165-5728(97)00267-1. [DOI] [PubMed] [Google Scholar]
- 29.López-Cortés L F, Cruz-Ruiz M, Gómez-Mateos J, Viciana-Fernandez P, Martinez-Marcos F J, Pachón J. Interleukin-8 in cerebrospinal fluid from patients with meningitis of different etiologies: Its possible role as a neutrophil chemotactic factor. J Infect Dis. 1995;172:581–584. doi: 10.1093/infdis/172.2.581. [DOI] [PubMed] [Google Scholar]
- 30.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Martiney J A, Cuff C, Litwalk M, Berman J, Bronsan C F. Cytokine-induced inflammation in the central nervous system revisited. Neurochem Res. 1998;23:349–359. doi: 10.1023/a:1022457500700. [DOI] [PubMed] [Google Scholar]
- 32.Mastroianni C M, Lancella L, Mengoni F, Lichtner M, Santopadre P, D'Agostino C, Ticca F, Vullo V. Chemokine profiles in the cerebrospinal fluid (CSF) during the course of pyogenic and tuberculous meningitis. Clin Exp Immunol. 1998;114:210–214. doi: 10.1046/j.1365-2249.1998.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 34.Morganti-Kossman M C, Lenzlinger P M, Hans V, Stahel P, Csuka E, Ammann E, Stocker R, Trentz O, Kossmann T. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry. 1997;2:133–136. doi: 10.1038/sj.mp.4000227. [DOI] [PubMed] [Google Scholar]
- 35.Mukaida N, Harada A, Matsushima K. Interleukin-8 (IL-8) and monocyte chemotactic and activating factor (MCAF/MCP-1), chemokines essentially involved in inflammatory and immune reactions. Cytokine Growth Factor Rev. 1998;9:9–23. doi: 10.1016/s1359-6101(97)00022-1. [DOI] [PubMed] [Google Scholar]
- 36.Mustafa M M, Ramilo O, Olsen K D, Franklin P S, Hansen E J, Beutler B, McCracken G H. Tumor necrosis factor in mediating experimental Haemophilus influenzae type b meningitis. J Clin Investig. 1989;84:1253–1259. doi: 10.1172/JCI114292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nolan C M, McAllister C K, Walters E, Beaty H N. Experimental pneumococcal meningitis IV. The effect of methyl prednisolone on meningeal inflammation. J Lab Clin Med. 1987;91:979–988. [PubMed] [Google Scholar]
- 38.Østergaard C, Benfield T L, Gesser B, Kharazmi A, Frimodt-Møller N, Espersen F, Lundgren J D. Pretreatment with granulocyte colony-stimulating factor attenuates the inflammatory response but not the bacterial load in cerebrospinal fluid during experimental pneumococal meningitis in rabbits. Infect Immun. 1999;67:3430–3436. doi: 10.1128/iai.67.7.3430-3436.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Østergaard C, Benfield T L, Sellebjerg F, Kronborg G, Lohse N, Lundgren J D. Interleukin-8 in cerebrospinal fluid from patients with septic and aseptic meningitis. Eur J Clin Microbiol Infect Dis. 1996;15:166–169. doi: 10.1007/BF01591492. [DOI] [PubMed] [Google Scholar]
- 40.Peveri P, Walz A, Dewald B, Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988;167:1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ransohoff R M, Tani M. Do chemokines mediate leukocyte recruitment in post-traumatic CNS inflammation? Trends Neurosci. 1998;21:154–159. doi: 10.1016/s0166-2236(97)01198-3. [DOI] [PubMed] [Google Scholar]
- 42.Rot A. Neutrophil attractant/activation protein-1 (interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. Eur J Immunol. 1993;23:303–306. doi: 10.1002/eji.1830230150. [DOI] [PubMed] [Google Scholar]
- 43.Rot A, Hub E, Middleton J, Pons F, Rabeck C, Thierer K, Wintle J, Wolff B, Zsak M, Dukor P. Some aspects of IL-8 pathophysiology III: chemokine interaction with endothelial cells. J Leukoc Biol. 1996;59:39–44. doi: 10.1002/jlb.59.1.39. [DOI] [PubMed] [Google Scholar]
- 44.Saukkonen K, Sande S, Cioffe C, Wolpe S, Sherry B, Cerami A, Tuomanen E. The role cytokines in the generation of inflammation and tissue damage in experimental gram-positive meningitis. J Exp Med. 1990;171:439–448. doi: 10.1084/jem.171.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seebach J, Bartholdi D, Frei K, Spanaus K-S, Ferrero E, Widmer U, Isenmann S, Strieter R M, Schwab M, Pfister H-W, Fontana A. Experimental Listeria meningoencephalitis. Macrophage inflammatory protein-1α and -2 are produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J Immunol. 1995;155:4367–4375. [PubMed] [Google Scholar]
- 46.Seki T, Joh K, Oh-Ishi T. Augmented production of interleukin-8 in cerebrospinal fluid in bacterial meningitis. Immunology. 1993;80:333–335. [PMC free article] [PubMed] [Google Scholar]
- 47.Smith W B, Gamble J R, Clark-Lewis I, Vadas M A. Interleukin-8 induces neutrophil transendothelial migration. Immunology. 1991;72:65–72. [PMC free article] [PubMed] [Google Scholar]
- 48.Sozzani S, Allavena P, Proost P, van Damme J, Mantovani A. Chemokines as targets for pharmacological intervention. Prog Drug Res. 1996;47:53–80. doi: 10.1007/978-3-0348-8998-8_2. [DOI] [PubMed] [Google Scholar]
- 49.Spanaus K-S, Nadal D, Pfister H-W, Seebach J, Widmer U, Frei K, Gloor S, Fontana A. C-X-C and C-C chemokines are expressed in the cerebrospinal fluid in bacterial meningitis and mediate chemotactic activity on peripheral blood-derived polymorphonuclear and mononuclear cells in vitro. J Immunol. 1997;158:1956–1964. [PubMed] [Google Scholar]
- 50.Spector R, Lorenzo A V. Inhibition of penicillin transport from the cerebrospinal fluid after intracisternal inoculation of bacteria. J Clin Investig. 1974;54:316–325. doi: 10.1172/JCI107767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sprenger H, Rösler A, Tonn P, Braune H J, Huffmann G, Gemsa D. Chemokines in the cerebrospinal fluid of patients with meningitis. Clin Immunol Immunopathol. 1996;80:155–161. doi: 10.1006/clin.1996.0109. [DOI] [PubMed] [Google Scholar]
- 52.Strieter R M, Kunkel S L, Showell H J, Remick D G, Phan S H, Ward P A, Marks R M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-α, LPS and IL-1β. Science. 1989;243:1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka Y, Adams D H, Shaw S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol Today. 1993;14:111–115. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- 54.Täuber M G, Borschberg U, Sande M A. Influence of granulocytes on brain edema, intracranial pressure, and cerebrospinal fluid concentrations of lactate and protein in experimental meningitis. J Infect Dis. 1988;157:456–464. doi: 10.1093/infdis/157.3.456. [DOI] [PubMed] [Google Scholar]
- 55.Täuber M G, Moser B. Cytokines and chemokines in meningeal inflammation: biology and clinical implications. Clin Infect Dis. 1999;28:1–12. doi: 10.1086/515079. [DOI] [PubMed] [Google Scholar]
- 56.Thomas K M, Taylor L, Prado G, Romero J, Moser B, Car B, Walz A, Baggiolini M, Navarro J. Functional and ligand binding specificity of the rabbit neutrophil IL-8 receptor. J Immunol. 1994;152:2496–2500. [PubMed] [Google Scholar]
- 57.van Deuren M, van der Ven-Jongekrig J, Bartelink A K M, van Dalen R, Sauerwein R W, van der Meer J W M. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J Infect Dis. 1995;172:433–439. doi: 10.1093/infdis/172.2.433. [DOI] [PubMed] [Google Scholar]
- 58.van Meir E, Ceska M, Effenberger F, Walz A, Grouzmann E, Desbaillets I, Frei K, Fontana A, de Tribolet N. Interleukin-8 is produced in neoplastic and infectious diseases of the human nervous system. Cancer Res. 1992;52:4297–4305. [PubMed] [Google Scholar]
- 59.Walz A, Peveri P, Aschauer H, Baggiolini M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem Biophys Res Commun. 1987;149:755–761. doi: 10.1016/0006-291x(87)90432-3. [DOI] [PubMed] [Google Scholar]
- 60.Walz A, Burgener R, Car B D, Baggiolini M, Kunkel S L, Strieter R M. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to IL-8. J Exp Med. 1991;174:1355–1362. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witt D P, Lander A D. Differential binding of chemokines to glycosaminoglycan subpopulations. Curr Biol. 1994;4:394–400. doi: 10.1016/s0960-9822(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 62.Yokoyama T, Oda M, Ogura S, Horiuchi T, Seno Y. Relationship of interleukin-8 and colony-stimulating factors to neutrophil migration in aseptic meningitis. Acta Paediatr. 1996;85:303–307. doi: 10.1111/j.1651-2227.1996.tb14021.x. [DOI] [PubMed] [Google Scholar]
- 63.Zlotnik A, Morales J, Hedrick J A. Recent advances in chemokines and chemokine receptors. Crit Rev Immunol. 1999;19:1–47. [PubMed] [Google Scholar]