Abstract
Aims
We investigated the association of fasting triglycerides with cardiovascular disease (CVD) mortality.
Methods and results
This cohort study included US adults from the National Health and Nutrition Examination Surveys from 1988 to 2014. CVD mortality outcomes were ascertained by linkage to the National Death Index records. Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of triglycerides for CVD mortality. The cohort included 26 570 adult participants, among which 3978 had diabetes. People with higher triglycerides had a higher prevalence of diabetes at baseline. The cohort was followed up for a mean of 12.0 years with 1492 CVD deaths recorded. A 1-natural-log-unit higher triglyceride was associated with a 30% higher multivariate-adjusted risk of CVD mortality in participants with diabetes (HR, 1.30; 95% CI, 1.08–1.56) but not in those without diabetes (HR, 0.95; 95% CI, 0.83–1.07). In participants with diabetes, people with high triglycerides (200–499 mg/dL) had a 44% (HR, 1.44; 95% CI, 1.12–1.85) higher multivariate-adjusted risk of CVD mortality compared with those with normal triglycerides (<150 mg/dL). The findings remained significant when diabetes was defined by fasting glucose levels alone, or after further adjustment for the use of lipid-lowering medications, or after the exclusion of those who took lipid-lowering medications.
Conclusion
This study demonstrates that fasting triglycerides of ≥200 mg/dL are associated with an increased risk of CVD mortality in patients with diabetes but not in those without diabetes. Future clinical trials of new treatments to lower triglycerides should focus on patients with diabetes.
Keywords: Hypertriglyceridaemia, Mortality, CVD, Diabetes, Risk factor
Time of primary review: 41 days
Translational perspective.
There has been much interest in investigating whether lowering triglyceride levels protects against cardiovascular disease (CVD). However, 12 of 13 randomized controlled trials since 2010 have not demonstrated any effect of lowering triglycerides on CVD events and mortality. Our study found that elevated triglycerides were associated with enhanced CVD mortality in those with diabetes, but not in those without diabetes. The results of our study may offer some guidance for future clinical trials investigating the effect of lowering triglycerides on CVD: both diabetes and hypertriglyceridaemia may need to be used as inclusion criteria.
1. Introduction
Cardiovascular disease (CVD) is the leading global cause of mortality and a major contributor to disability.1 CVD prevalence nearly doubled from 271 million in 1990 to 523 million in 2019, and the number of CVD deaths increased from 12.1 million in 1990 to 18.6 million in 2019.1 Therefore, it is of high importance to identify modifiable risk factors for CVD and to decrease CVD mortality.
There has been much interest in investigating whether lowering triglyceride levels protects against CVD. However, 12 of 13 randomized controlled trials since 20102–14 (see Supplementary material online, Table S1) have not demonstrated any effect of lowering triglycerides (via omega-3 fatty acid, niacin, or fibrate) on CVD events and mortality, challenging the belief that lowering triglycerides lowers CVD risk.
Recent reports suggest that triglycerides may be important for the pathogenesis of diabetes, a disease that can increase CVD risk. For example, higher baseline triglycerides were associated with higher risk of new-onset of diabetes15–17 and diabetes-caused mortality18 in cohort studies.
The bezafibrate infarction prevention (BIP) trial showed that in patients with established coronary heart disease, high baseline triglycerides predicted high all-cause mortality after adjustment for baseline diabetes diagnosis.19 However, whether there is an interaction between diabetes and triglycerides in predicting CVD mortality is unknown.
This cohort study aimed to investigate the association of fasting triglycerides with CVD mortality in US adult participants with or without diabetes who attended the National Health and Nutrition Examination Surveys (NHANES) from 1988 to 2014.
2. Methods
2.1. Study participants
This cohort study included participants from NHANES III (1988–1994) and the subsequent eight cycles of NHANES from 1999 to 2014.20,21 The inclusion criteria included age of ≥20 years and presence of fasting triglyceride data, resulting in a cohort of 27 184 people. The following were excluded: those who were pregnant (n = 582), those without a follow-up time or with a follow-up time of 0 month (n = 30), and those without diabetes status (n = 2). Therefore, 26 570 participants were included in the final analysis.
The National Centre for Health Statistics Research Ethics Review Board approved all study protocols.18,20,22 All procedures were performed following the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all participants.
2.2. Diabetes definition
Diabetes was defined as presence of any of the following: self-reported physician diagnosis of diabetes, use of insulin or oral diabetes medications, haemoglobin A1c (HbA1c) ≥6.5%, or fasting glucose ≥140 mg/dL (≥7.8 mmol/L) in NHANES III (1988–1994) or ≥126 mg/dL (≥7.0 mmol/L) in NHANES 1999–2014.23 NHANES was not conducted between 1995 and 1998. The use of different fasting plasma glucose levels for diabetes diagnosis was due to the change in diagnostic criteria over time, and the fasting glucose level for diabetes was ≥140 mg/dL before the American Diabetes Association criteria in 1997.24
2.3. Fasting triglycerides classification
The baseline concentration of fasting (fasting time ≥8 h23,25) triglycerides in the serum was directly retrieved from the NHANES website.18 Triglyceride levels were classified into four groups according to the recommendation by the National Cholesterol Education Program (NCEP) Expert Panel,26 i.e. normal (<150 mg/dL), borderline high (150–199 mg/dL), high (200–499 mg/dL), and very high (≥500 mg/dL).
2.4. CVD mortality
Data on mortality were directly retrieved from NHANES-linked mortality files.18,20,22 To evaluate mortality status, the National Centre for Health Statistics conducted probabilistic matching to link the NHANES data with death certificate records from the National Death Index (NDI) records. CVD mortality was defined as mortality from heart diseases or cerebrovascular diseases, as previously reported.20 Follow-up time was defined as the time (in months) from when the blood was drawn at the Mobile Examination Centre until death, or until the end of follow-up (i.e. 31 December 2015), whichever occurred first.18,20,22
2.5. Covariates
Confounding covariates were similar to previous reports.18,20,22,27 They included age (continuous), sex (male or female), ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, or other),28 obesity (underweight, normal, overweight, obese, or unknown), education (<high school, high school, >high school, or unknown), poverty–income ratio (<130%, 130–349%, ≥ 350%, or unknown), and survey periods (1988–1991, 1991–1994, 1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, or 2013–2014). Lifestyle confounders included physical activity (inactive, insufficiently active, or active), alcohol consumption (never, <1 drink per week, 1–6 drinks per week, ≥7 drinks per week, or unknown), and smoking status (past smoker, current smoker, non-smoker, or unknown). Clinical confounders included self-reported physician diagnosis of hypertension (yes, no, or unknown), self-reported physician diagnosis of hypercholesterolaemia (yes, no, or unknown), diabetes (yes or no), family history of diabetes (yes, no, or unknown), duration of diabetes (≥10 years, <10 years, or unknown), and diabetes medications (insulin only, oral medications only, both insulin and oral medications, or unknown).
2.6. Statistical analyses
Statistical analysis methods were similar to previous reports.18,20,22 Data were presented as mean and standard deviation for normally distributed continuous variables or median and interquartile range for non-normally continuous distributed variables or percentages for categorical variables. Difference in age was analysed using Student’s t-test between those with or without diabetes or one-way analysis of variance (ANOVA) among four triglyceride groups. Differences in non-normally distributed continuous variables (triglyceride, glucose, and HbA1c) were analysed using the Mann–Whitney U test between those with or without diabetes or using Kruskal–Wallis one-way ANOVA among four triglyceride groups. Differences among categorical variables were analysed using Pearson’s χ2 test. Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of triglycerides for CVD mortality, with adjustment for age, sex, ethnicity, obesity, poverty–income ratio, education, physical activity, alcohol consumption, smoking status, survey period, hypercholesterolaemia, hypertension, diabetes, family history of diabetes, duration of diabetes, and diabetes medications. Triglyceride was treated as a continuous variable (natural log-transformed) or a categorical variable (normal, borderline high, high, and very high).26 Subgroup analyses were conducted in those with or without pre-existing CVD which was defined as prior diagnosis of myocardial infarction or stroke, or in those with various levels of low-density lipoprotein cholesterol (≤55, 55.1–70, 70.1–100, and >100 mg/dL).29,30
Sensitivity analyses were conducted by defining diabetes according to fasting plasma glucose alone, or by further adjustment for the use of lipid-lowering medications, or by exclusion of those who took lipid-lowering medications. Sensitivity analyses were also conducted by adjusting for total cholesterol (continuous), high-density lipoprotein (HDL) cholesterol (continuous), or non-HDL cholesterol (continuous) instead of hypercholesterolaemia,31 or by adjusting for systolic blood pressure (continuous) instead of hypertension status.
The restricted cubic spline model (with five knots at 5th, 27.5th, 50th 72.5th, and 95th percentiles)32 was used to examine the shape of the association between triglycerides and CVD mortality in participants with or without diabetes, with adjustment for age, sex, ethnicity, obesity, poverty–income ratio, education, physical activity, alcohol consumption, smoking status, survey period, hypercholesterolaemia, hypertension, family history of diabetes, duration of diabetes, and diabetes medications.
The null hypothesis was rejected with a two-tailed P-value of <0.05. Restricted cubic spline analyses were performed using SAS® OnDemand for Academics (SAS Institute Inc, Cary, NC, USA) and all other analyses were performed using SPSS version 27.0 (IBM SPSS Statistics for Windows; IBM Corporation, Armonk, NY, USA).
3. Results
3.1. General characteristics
This study included 26 570 adult participants, among whom 3978 had diabetes. The baseline characteristics of the participants are displayed in Tables 1 and 2. People with higher triglycerides had a higher prevalence of diabetes, and people with diabetes had higher triglycerides compared with those without diabetes. People with diabetes (compared with those without diabetes), as well as those with higher triglycerides, were more likely to be males, had less income and education, and had a higher prevalence of obesity, hypercholesterolaemia, and hypertension (Tables 1 and 2).
Table 1.
Baseline characteristics of 26 570 US adults stratified by diabetes status
| Participants without diabetes | Participants with diabetes | All participants | P-value | |
|---|---|---|---|---|
| Sample size | 22 592 | 3978 | 26 570 | NA |
| Age, years, mean (SD) | 47 (18) | 61 (14) | 49 (19) | <0.001 |
| Sex (female), % | 51.3 | 49.6 | 51.1 | 0.047 |
| Ethnicity, % | <0.001 | |||
| ȃHispanic | 27.1 | 30.8 | 27.7 | |
| ȃNon-Hispanic white | 46.1 | 39.6 | 45.1 | |
| ȃNon-Hispanic black | 21.7 | 24.0 | 22.1 | |
| ȃOther | 5.1 | 5.5 | 5.1 | |
| Triglyceride, mg/dL, median (IQR) | 105 (74–153) | 142 (98–209) | 109 (77–161) | <0.001 |
| FPG, mg/dL, median (IQR) | 96 (89–102) | 133 (114–169) | 97 (90–107) | <0.001 |
| HbA1c, %, median (IQR) | 5.3 (5.1–5.6) | 6.7 (6.0–7.8) | 5.4 (5.1–5.8) | <0.001 |
| Obesity, % | ||||
| ȃUnderweight | 1.8 | 0.7 | 1.7 | <0.001 |
| ȃNormal | 34.8 | 15.1 | 31.9 | |
| ȃOverweight | 34.6 | 31.9 | 34.2 | |
| ȃObese | 27.8 | 50.2 | 31.2 | |
| ȃUnknown | 0.9 | 2.1 | 1.1 | |
| Poverty–income ratio, % | ||||
| ȃ<130% | 27.8 | 33.3 | 28.6 | <0.001 |
| ȃ130–349% | 36.9 | 37.9 | 37.0 | |
| ȃ ≥ 350% | 27.1 | 19.3 | 26.0 | |
| ȃUnknown | 8.2 | 9.4 | 8.4 | |
| Education, % | ||||
| ȃ<High school | 30.5 | 43.0 | 32.4 | <0.001 |
| ȃHigh school | 26.0 | 24.1 | 25.7 | |
| ȃ>High school | 43.2 | 32.7 | 41.7 | |
| ȃUnknown | 0.3 | 0.2 | 0.3 | |
| Physical activity, % | ||||
| ȃInactive | 28.0 | 18.5 | 26.6 | <0.001 |
| ȃInsufficiently active | 38.2 | 29.3 | 36.9 | |
| ȃActive | 33.7 | 52.2 | 36.5 | |
| Alcohol consumption, % | ||||
| ȃ0 drink/week | 16.0 | 27.3 | 17.7 | <0.001 |
| ȃ<1 drink/week | 22.5 | 20.8 | 22.2 | |
| ȃ1–6 drinks/week | 21.8 | 11.6 | 20.3 | |
| ȃ ≥ 7 drinks/week | 13.7 | 9.6 | 13.1 | |
| ȃUnknown | 26.0 | 30.7 | 26.7 | |
| Smoking status, % | ||||
| ȃPast smoker | 23.8 | 17.4 | 22.9 | <0.001 |
| ȃCurrent smoker | 23.5 | 34.2 | 25.1 | |
| ȃNon-smoker | 52.6 | 48.3 | 51.9 | |
| Hypercholesterolaemia, % | 22.8 | 45.2 | 26.2 | <0.001 |
| Hypertension, % | 26.9 | 60.2 | 31.9 | <0.001 |
| Diabetes, % | 0 | 100 | 15.0 | NA |
| Family history of diabetes, % | 40.5 | 60.1 | 43.4 | <0.001 |
| Diabetes duration ≥10 years, % | 0.0 | 22.2 | 3.3 | NA |
| Use of diabetes medications, % | ||||
| ȃInsulin only | 0.0 | 5.9 | 0.9 | NA |
| ȃOral medications only | 0.0 | 38.8 | 5.8 | |
| ȃBoth | 0.0 | 5.6 | 0.8 |
HbA1c, haemoglobin A1c; FPG, fasting plasma glucose; IQR, interquartile range; NA, not applicable; SD, standard deviation.
Table 2.
Baseline characteristics of 26 570 US adults stratified by triglyceride categories
| Triglyceride (mg/dL) | P-value | ||||
|---|---|---|---|---|---|
| <150 | 150–199 | 200–499 | ≥500 | ||
| Sample size | 18 802 | 3714 | 3726 | 328 | NA |
| Age, years, mean (SD) | 47 (19) | 53 (18) | 53 (17) | 49 (15) | <0.001 |
| Sex (female), % | 53.3 | 48.7 | 44.3 | 28.7 | <0.001 |
| Ethnicity, % | |||||
| ȃHispanic | 43.1 | 49.3 | 50.8 | 47.9 | <0.001 |
| ȃNon-Hispanic white | 26.3 | 13.5 | 10.5 | 8.2 | |
| ȃNon-Hispanic black | 25.2 | 32.5 | 34.4 | 39.6 | |
| ȃOther | 5.4 | 4.8 | 4.3 | 4.3 | |
| Triglyceride, mg/dL, median (IQR) | 89 (68–114) | 170 (159–183) | 251 (220–305) | 642 (551–834) | <0.001 |
| FPG, mg/dL, median (IQR) | 96 (89–104) | 101 (94–111) | 103 (95–118) | 110 (98–173) | <0.001 |
| HbA1c, %, median (IQR) | 5.4 (5.1–5.7) | 5.5 (5.2–5.9) | 5.6 (5.2–6.1) | 5.6 (5.2–7.4) | <0.001 |
| Obesity, % | |||||
| ȃUnderweight | 2.1 | 0.6 | 0.5 | 0.3 | |
| ȃNormal | 37.6 | 20.7 | 15.9 | 11.0 | <0.001 |
| ȃOverweight | 32.4 | 37.9 | 38.5 | 45.1 | |
| ȃObese | 26.8 | 39.7 | 43.9 | 43.0 | |
| ȃUnknown | 1.0 | 1.2 | 1.2 | 0.6 | |
| Poverty–income ratio, % | |||||
| ȃ<130% | 27.9 | 28.9 | 31.1 | 33.8 | <0.001 |
| ȃ130–349% | 37.2 | 36.8 | 36.3 | 39.9 | |
| ȃ≥350% | 26.7 | 24.6 | 24.1 | 19.2 | |
| ȃUnknown | 8.2 | 9.7 | 8.5 | 7.0 | |
| Education, % | |||||
| ȃ<High school | 29.9 | 37.9 | 38.4 | 39.6 | <0.001 |
| ȃHigh school | 25.6 | 25.6 | 26.3 | 25.9 | |
| ȃ>High school | 44.2 | 36.1 | 35.1 | 34.5 | |
| ȃUnknown | 0.3 | 0.3 | 0.2 | 0.0 | |
| Physical activity, % | |||||
| ȃInactive | 27.8 | 24.1 | 22.9 | 27.1 | <0.001 |
| ȃInsufficiently active | 37.1 | 35.5 | 37.4 | 34.5 | |
| ȃActive | 35.0 | 40.4 | 39.7 | 38.4 | |
| Alcohol consumption, % | |||||
| ȃ0 drink/week | 17.0 | 19.2 | 19.8 | 18.9 | <0.001 |
| ȃ<1 drink/week | 22.6 | 22.5 | 20.6 | 18.0 | |
| ȃ1–6 drinks/week | 21.3 | 17.7 | 18.2 | 19.8 | |
| ȃ≥7 drinks/week | 12.9 | 13.2 | 13.5 | 18.9 | |
| ȃUnknown | 26.3 | 27.4 | 27.9 | 24.4 | |
| Smoking status, % | |||||
| ȃPast smoker | 22.1 | 24.1 | 24.9 | 28.4 | <0.001 |
| ȃCurrent smoker | 23.2 | 29.4 | 30.3 | 29.0 | |
| ȃNon-smoker | 54.6 | 46.4 | 44.7 | 42.7 | |
| Hypercholesterolaemia, % | 21.8 | 33.6 | 39.0 | 48.2 | <0.001 |
| Hypertension, % | 28.7 | 37.6 | 41.1 | 41.8 | <0.001 |
| Diabetes, % | 11.4 | 19.8 | 25.8 | 40.9 | <0.001 |
| Family history of diabetes, % | 41.8 | 45.5 | 48.7 | 53.7 | <0.001 |
| Diabetes duration ≥10 years, % | 2.7 | 4.0 | 5.2 | 7.9 | <0.001 |
| Use of diabetes medications, % | |||||
| ȃInsulin only | 0.8 | 0.9 | 1.5 | 1.5 | <0.001 |
| ȃOral medications only | 4.4 | 7.6 | 10.3 | 15.9 | |
| ȃBoth | 0.7 | 0.9 | 1.4 | 2.7 | |
HbA1c, haemoglobin A1c; FPG, fasting plasma glucose; IQR, interquartile range; NA, not applicable; SD, standard deviation.
3.2. Association of fasting plasma triglycerides with CVD mortality
This cohort was followed up for a mean of 12.0 years with a total of 318 346 person-years of follow-up. During the follow-up, 1492 CVD deaths were recorded.
A 1-natural-log-unit increase in triglycerides was not associated with CVD mortality in the whole cohort nor the non-diabetic subcohort (Table 3). However, it was associated with a 30% higher multivariate-adjusted risk of CVD mortality in participants with diabetes (HR, 1.30; 95% CI, 1.08–1.56; Table 3). Restricted cubic spline analyses showed that the association between triglycerides and CVD mortality risks in participants with diabetes was not linear (P = 0.011, Supplementary material online, Figure S1). When triglycerides were treated as a categorical variable, similar results were obtained, and people with high triglycerides (200–499 mg/dL) had a 44% higher multivariate-adjusted risk of CVD mortality compared with those with normal triglycerides (<150 mg/dL) in the subcohort of participants with diabetes (Table 4). Interaction analyses confirmed that diabetes status interacted with triglycerides for CVD mortality risks (P = 0.015, Supplementary material online, Table S2).
Table 3.
Triglyceride (natural log-transformed) and risk for CVD mortality among 26 570 adults
| Models | HR | 95% CI | P-value |
|---|---|---|---|
| Overall (n = 26 570) | |||
| ȃModel 1 | 1.24 | 1.12–1.36 | <0.001 |
| ȃModel 2 | 1.16 | 1.05–1.28 | 0.005 |
| ȃModel 3 | 1.12 | 1.01–1.24 | 0.033 |
| ȃModel 4 | 1.06 | 0.95–1.17 | 0.315 |
| Participants without diabetes (n = 22 592) | |||
| ȃModel 1 | 1.05 | 0.93–1.18 | 0.463 |
| ȃModel 2 | 0.97 | 0.86–1.10 | 0.641 |
| ȃModel 3 | 0.94 | 0.83–1.07 | 0.359 |
| ȃModel 4 | 0.95 | 0.83–1.07 | 0.382 |
| Participants with diabetes (n = 3978) | |||
| ȃModel 1 | 1.41 | 1.18–1.68 | <0.001 |
| ȃModel 2 | 1.31 | 1.09–1.57 | 0.004 |
| ȃModel 3 | 1.28 | 1.06–1.53 | 0.009 |
| ȃModel 4 | 1.30 | 1.08–1.56 | 0.006 |
CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Model 1: adjusted for age, sex, and ethnicity. Model 2: adjusted for age, sex, ethnicity, obesity, poverty–income ratio, education, physical activity, alcohol consumption, smoking status, and survey period. Model 3: adjusted for all the factors in Model 2 plus hypercholesterolaemia and hypertension. Model 4: adjusted for all the factors in Model 3 plus diabetes, family history of diabetes, duration of diabetes, and diabetes medications.
Table 4.
Triglyceride categories and risk for CVD mortality among 26 570 adults
| Triglyceride (mg/dL) | n | HRa | 95% CI | P-valueb | P for trend |
|---|---|---|---|---|---|
| All participants (n = 26 570) | |||||
| ȃ<150 (normal) | 18 802 | 1 | Reference | NA | 0.668 |
| ȃ150–199 (borderline high) | 3714 | 1.02 | 0.88–1.17 | 0.830 | |
| ȃ200–499 (high) | 3726 | 1.04 | 0.91–1.20 | 0.557 | |
| ȃ≥500 (very high) | 328 | 1.25 | 0.86–1.83 | 0.243 | |
| Participants without diabetes (n = 22 592) | |||||
| ȃ<150 (normal) | 16 656 | 1 | Reference | NA | 0.575 |
| ȃ150–199 (borderline high) | 2978 | 0.97 | 0.83–1.14 | 0.718 | |
| ȃ200–499 (high) | 2764 | 0.88 | 0.74–1.05 | 0.160 | |
| ȃ≥500 (very high) | 194 | 0.93 | 0.48–1.81 | 0.840 | |
| Participants with diabetes (n = 3978) | |||||
| ȃ<150 (normal) | 2146 | 1 | Reference | NA | 0.015 |
| ȃ150–199 (borderline high) | 736 | 1.10 | 0.83–1.46 | 0.496 | |
| ȃ200–499 (high) | 962 | 1.44 | 1.12–1.85 | 0.004 | |
| ȃ≥500 (very high) | 134 | 1.66 | 1.01–2.70 | 0.044 | |
CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Adjusted for age, sex, ethnicity, obesity, poverty–income ratio, education, physical activity, alcohol consumption, smoking status, survey period, hypercholesterolaemia, hypertension, diabetes, family history of diabetes, duration of diabetes, and diabetes medications.
Compared with those with normal triglycerides.
Subanalyses showed that triglycerides were positively associated with CVD mortality in participants with diabetes, regardless of pre-existing CVD status (Table 5). In addition, the positive association between triglycerides and CVD mortality in people with diabetes was only presented in those with low-density lipoprotein cholesterol concentrations ranging from 70.1 to 100 mg/dL (see Supplementary material online, Table S3).
Table 5.
Triglyceride (natural log-transformed) and risk for CVD mortality among 26 413a adults, stratified by diabetes and pre-existing CVDb
| Groups | n | HRc | 95% CI | P-value |
|---|---|---|---|---|
| Participants without diabetes | ||||
| ȃWithout pre-existing CVD | 21 283 | 0.88 | 0.76–1.02 | 0.088 |
| ȃWith pre-existing CVD | 1179 | 1.01 | 0.77–1.31 | 0.967 |
| Participants with diabetes | ||||
| ȃWithout pre-existing CVD | 3309 | 1.29 | 1.02–1.63 | 0.032 |
| ȃWith pre-existing CVD | 642 | 1.54 | 1.09–2.18 | 0.015 |
CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
A total of 157 participants were excluded due to unknown status of pre-existing CVD. Therefore, the remaining 26 413 participants were included in the analysis.
Pre-existing CVD was defined as prior diagnosis of myocardial infarction or stroke.
Adjusted for age, sex, ethnicity, obesity, poverty–income ratio, education, physical activity, alcohol consumption, smoking status, survey period, hypercholesterolaemia, hypertension, family history of diabetes, duration of diabetes, and diabetes medications.
3.3. Sensitivity analyses
Sensitivity analyses showed that the association of triglycerides with CVD mortality did not materially change after diabetes was re-defined according to fasting glucose alone (see Supplementary material online, Tables S4 and S5), or after further adjustment for the use of lipid-lowering medications (see Supplementary material online, Table S6), or after exclusion of those who took lipid-lowering medications (see Supplementary material online, Table S7), or after adjusting for systolic blood pressure instead of hypertension status (see Supplementary material online, Table S8). In addition, adjustment for total cholesterol, HDL cholesterol, or non-HDL cholesterol instead of hypercholesterolaemia did not abolish the association between triglycerides and CVD mortality in people with diabetes (see Supplementary material online, Tables S9–S11).
4. Discussion
This study found that elevated triglycerides were associated with enhanced CVD mortality in those with diabetes, but not in those without diabetes, in a large cohort of US adults. The positive association between triglycerides and CVD mortality in people with diabetes was independent of prior diagnosis of CVD.
In epidemiological studies, diabetes has often been defined by self-reported physician diagnosis and use of diabetes medications. However, using self-reported diagnosis to identify diabetes could be inaccurate,33 and in a similar manner diabetes medications and HbA1c.34 Therefore, sensitivity analyses were conducted by defining diabetes using era-specific fasting plasma glucose alone or using the single fasting plasma glucose level of ≥126 mg/dL. As lipid-lowering medications could affect triglyceride levels,35 sensitivity analyses were also conducted by further adjustment for the use of those medications or by exclusion of those who took those medications. These sensitivity analyses did not materially affect the results. In addition, family history of diabetes, diabetes duration, and diabetes medications were adjusted for in all the analyses. Therefore, this study supports the conclusion that elevated fasting triglycerides were associated with increased possibility of CVD mortality in people with diabetes.
The current study was observational in nature and therefore could not establish whether elevated triglycerides are merely a marker of risk or a causative factor. Mendelian randomization studies showed that genetically higher triglycerides were associated with increased CVD risk,36 suggesting that elevated triglycerides are pathogenic and thus a potential therapeutic target.
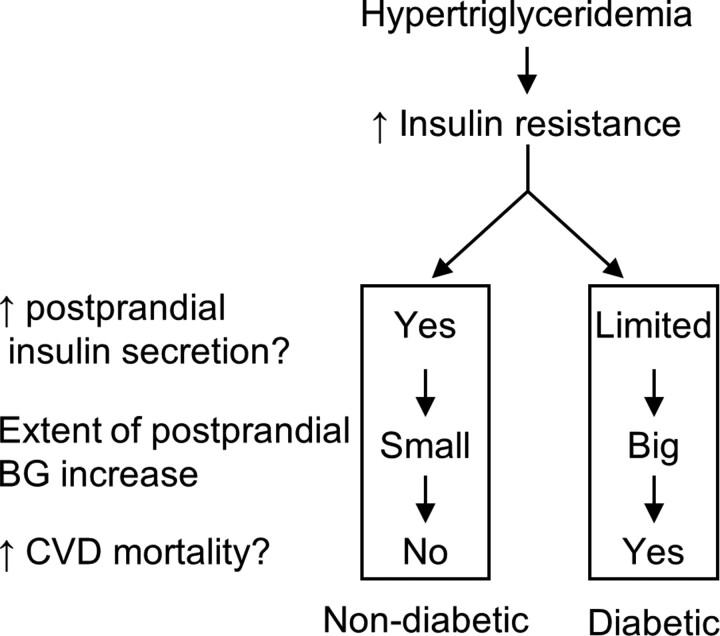
This study indicates that hypertriglyceridaemia was associated with CVD mortality preferentially in people with diabetes as opposed to those without diabetes. The reason for this is not clear. The authors propose the following hypothesis for diabetes-induced sensitization to hypertriglyceridaemia (Figure 1).
Figure 1.
The proposed hypothesis of diabetes-induced sensitization to hypertriglyceridaemia-associated CVD mortality. High triglycerides induce insulin resistance. In people without diabetes, increased insulin resistance could be compensated by higher insulin secretion to maintain postprandial glucose homeostasis. However, in people with diabetes, the compensation capacity is limited, leading to a greater increase in postprandial BG, and ultimately, enhanced CVD mortality. ↑, increase; BG, blood glucose; CVD, cardiovascular disease.
Triglyceride-induced insulin resistance and impaired insulin secretion in diabetic patients might explain the proposed hypothesis. Infusion of triglycerides into rats induced insulin resistance as assessed by the euglycaemic-hyperinsulinaemic clamp study.37 Consistently, triglycerides in humans were positively associated with insulin resistance, the latter being assessed by homeostatic model assessment for insulin resistance.18 In people without diabetes, increased insulin resistance associated with high triglycerides could be compensated by higher insulin secretion to maintain postprandial glucose homeostasis. However, the insulin secretion in people with diabetes is impaired,38 and therefore, increased insulin resistance associated with high triglycerides could not be sufficiently compensated by an increase in insulin secretion. Consequently, high triglycerides could lead to a much higher plasma glucose increase after a meal in people with diabetes than in those without the disease. This may be supported by the following observation: triglycerides were linearly associated with plasma glucose in both non-diabetic and diabetic adults after adjustment for multiple confounders; however, the standardized coefficient (β) was 0.074 in non-diabetic adults, whereas it was 0.292 in diabetic adults.18 Plasma glucose is positively associated with CVD mortality25; consequently, the detrimental effect of triglycerides could be sensitized by diabetes.
The proposed diabetes-induced sensitization to hypertriglyceridaemia hypothesis is consistent with previous reports that higher baseline triglycerides were associated with new-onset of diabetes in various populations including Americans,15 Japanese,16 and Chinese,17 as well as with diabetes-caused mortality in people without diabetes at baseline.18 About 55% of patients with Type 2 diabetes have a triglyceride level higher than normal (i.e. >150 mg/dL),39 and this might, at least in part, explain why people with diabetes have a higher CVD risk (about two-fold on average) compared with those without the disease.40,41
The proposed diabetes-induced sensitization to hypertriglyceridaemia hypothesis is supported by the ACCORD-Lipid study.14 That study showed that although lowering triglycerides by fenofibrate did not decrease CVD risk in the overall cohort of patients with Type 2 diabetes,14 the therapy showed a 31% lower CVD event rate in the subcohort of diabetic patients with a combination of hypertriglyceridaemia and low HDL cholesterol.14 Our hypothesis is also supported by a study that showed that higher triglycerides are associated with CVD mortality in patients with diabetes,42 although, unlike the current study, that study42 had a small sample size (562 patients) and only 15 CVD deaths recorded.
Findings from the Baltimore Coronary Observational Long-Term Study (COLTS) appear to not support our proposed hypothesis, as higher triglycerides remained a significant risk factor for new CVD events after exclusion of Type 2 diabetes after 18 years of follow-up.43 However, the COLTS study finding may not reject the hypothesis, as during the 18-year follow-up, higher triglycerides may have led to diabetes formation in some participants, as higher triglycerides were associated with new-onset of diabetes15–17 and diabetes-caused mortaltiy18 in people without diabetes.
In addition, the 22-year mortality data from the BIP trial showed that, in patients with established coronary heart disease, high baseline triglycerides were associated with high all-cause mortality independent of baseline diabetes diagnosis,19 which may argue against such a diabetes-sensitization hypothesis. However, whether there was an interaction between diabetes and triglycerides in the association between triglycerides and all-cause mortality in that cohort19 was not investigated.
The findings of the current study suggest that people who may benefit most from triglyceride-lowering therapies are those with both diabetes and hypertriglyceridaemia. This study might provide a new explanation for why the majority of recently completed randomized controlled trials failed to demonstrate that lowering triglycerides protects against CVD, i.e. none of these trials used both diabetes and hypertriglyceridaemia as inclusion criteria (see Supplementary material online, Table S12). Among these 13 trials, only three used hypertriglyceridaemia as an inclusion criterion (see Supplementary material online, Table S12): Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial (REDUCE-IT),9 Long-Term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridaemia (STRENGTH),10 and Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides, Impact on Global Health Outcomes (AIM-HIGH) trials.12 The AIM-High trial had a low percentage of participants with diabetes (33.9%),12 whereas the REDUCE-IT9 and STRENGTH10 trials had a higher prevalence of diabetes (58.5 and 70%, respectively; see Supplementary material online, Table S12). Hypertriglyceridaemia together with a high prevalence of diabetes might explain why REDUCE-IT9 showed that lowering triglycerides reduced CVD risk. However, why the STRENGTH10 trial did not achieve its primary endpoint is not clear. It could be due to low treatment adherence44 and other reasons including chance.
Our study might provide some guidance for current and future clinical trials investigating the effect of lowering triglycerides on CVD. Presently, at least 29 current and future trials registered on the ClinicalTrials.gov website are designed to investigate the protective effect against CVD by triglyceride-lowering therapies via omega-3 fatty acid (see Supplementary material online, Table S13), niacin (see Supplementary material online, Table S14), or fibrate (see Supplementary material online, Table S15). Among these 29 trials, 9 have a status of active but not recruiting, 15 recruiting, and 5 not yet recruiting (see Supplementary material online, Tables S13–S15). However, only one of the 29 trials uses high triglycerides as an inclusion criterion (NCT04562467, Supplementary material online, Table S13) and none of them use both diabetes and high triglycerides as inclusion criteria.
The current study seemed inconsistent with the REDUCE-IT, as the latter showed that the triglyceride-lowering drug icosapent ethyl reduced CVD events in both diabetic and non-diabetic patients.9 However, the exact mechanisms underlying the CVD-lowering effect of icosapent ethyl are unclear, and it is possible that lowering triglycerides might not be the main mechanism. This speculation seemed to be supported by the following observation: (i) baseline triglyceride levels (≥150 vs. <150 mg/dL or ≥200 vs. <200 mg/dL) had no influence on the CVD-lowering effect of icosapent ethyl; and (ii) the attainment of triglyceride levels of ≥150 vs. <150 mg/dL at 1 year after randomization had no influence on the efficacy of icosapent ethyl.9 The REDUCE-IT investigators9 suggested that other mechanisms may contribute to the observed beneficial effect of icosapent ethyl and the proposed mechanisms included antiplatelet effect, stabilization or regression of coronary plaque, and anti-inflammatory effect associated with icosapent ethyl.
4.1. Strengths and limitations
This study has similar strengths to those previously reported.18,20,22 In brief, strengths include a large sample size (n = 26 570), a prospective study design, the use of a nationally representative sample of US adults, and adjustment for a large number of confounding factors. This study also has a number of limitations as previously reported18,20,22: (i) triglycerides were only measured at one time point, which may result in misclassification. Nevertheless, such misclassification would tend to result in an underestimate rather than an overestimate of risk due to the effect of regression dilution bias; (ii) mortality outcomes were ascertained by linkage to the NDI records with a probabilistic match, which may lead to misclassification. However, a prior validation study showed that the matching method had high accuracy (98.5%).45 In addition, this study does not represent the whole US population. The Hispanic subcohort counted for 27.7% of the whole cohort, which is higher than the percentage of the Hispanic subpopulation in the USA (18.7% in 2020). This difference was explained by the NHANES design: the NHANES cycles from 1988 to 2014 were designed to oversample the Hispanic subpopulation. This oversampling aimed to obtain sufficient numbers of Hispanic persons and to increase the reliability and precision of estimates of health status indicators for this subpopulation. Therefore, the results of the current study may not be extrapolated to the entire US population.
5. Conclusion
This study demonstrated that higher fasting triglycerides were associated with a higher risk of CVD mortality in people with diabetes but not in those without diabetes. Triglycerides may be a therapeutic target for lowering CVD mortality in people with both diabetes and hypertriglyceridaemia. A diabetes-induced sensitization to hypertriglyceridaemia hypothesis was proposed to describe the association between hypertriglyceridaemia and CVD mortality (Figure 1).
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Authors’ contributions
Conceptualization: Y.W., Y.F.; data analysis: Y.W.; writing—original draft preparation, Y.W., Y.F.; writing—review and editing: Y.W., Y.F., D.J.M., F.J.C., C.G.S., G.R.D., J.G.; funding acquisition, Y.W.
Supplementary Material
Contributor Information
Yutang Wang, Discipline of Life Science, School of Science, Psychology and Sport, Federation University Australia, University Drive, Mt Helen, Ballarat, VIC, 3350, Australia.
Yan Fang, Discipline of Life Science, School of Science, Psychology and Sport, Federation University Australia, University Drive, Mt Helen, Ballarat, VIC, 3350, Australia.
Dianna J Magliano, Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia.
Fadi J Charchar, Discipline of Life Science, School of Science, Psychology and Sport, Federation University Australia, University Drive, Mt Helen, Ballarat, VIC, 3350, Australia.
Christopher G Sobey, Centre for Cardiovascular Biology and Disease Research and Department of Microbiology, Anatomy, Physiology and Pharmacology, School of Agriculture, Biomedicine and Environment, La Trobe University, Melbourne, VIC, Australia.
Grant R Drummond, Centre for Cardiovascular Biology and Disease Research and Department of Microbiology, Anatomy, Physiology and Pharmacology, School of Agriculture, Biomedicine and Environment, La Trobe University, Melbourne, VIC, Australia.
Jonathan Golledge, Queensland Research Centre for Peripheral Vascular Disease, College of Medicine and Dentistry, James Cook University, Townsville, QLD, Australia; Department of Vascular and Endovascular Surgery, The Townsville University Hospital, Townsville, QLD, Australia.
Funding
This work was supported by a grant from the National Health and Medical Research Council of Australia (grant number 1062671).
Data availability
All data in the current analysis are publicly available on the NHANES website.
References
- 1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, de Oliveira G M, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group . Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 2010;122:2152–2159. [DOI] [PubMed] [Google Scholar]
- 3. Kromhout D, Giltay EJ, Geleijnse JM. N-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 2010;363:2015–2026. [DOI] [PubMed] [Google Scholar]
- 4. Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The ORIGIN Trial Investigators, Bosch J, Gerstein HC, Dagenais GR, Díaz R, Dyal L, Jung H, Maggiono AP, Probstfield J, Ramachandran A, Riddle MC, Rydén LE, Yusuf S. N-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med 2012;367:309–318. [DOI] [PubMed] [Google Scholar]
- 6. Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, Marzona I, Milani V, Silletta MG, Tognoni G, Marchioli R. N-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med 2013;368:1800–1808. [DOI] [PubMed] [Google Scholar]
- 7. The ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med 2018;379:1540–1550. [DOI] [PubMed] [Google Scholar]
- 8. Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D'Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RTJR, Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 10. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, Ridker PM, Ray KK, Katona BG, Himmelmann A, Loss LE, Rensfeldt M, Lundström T, Agrawal R, Menon V, Wolski K, Nissen SE. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 2020;324:2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, Nilsen DWT, Tveit A, Fagerland MW, Solheim S, Seljeflot I, Arnesen H. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction. Circulation 2021;143:528–539. [DOI] [PubMed] [Google Scholar]
- 12. The AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 13. The HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–212. [DOI] [PubMed] [Google Scholar]
- 14. Elam M, Lovato L, Ginsberg H. The ACCORD-lipid study: implications for treatment of dyslipidemia in type 2 diabetes mellitus. Clin Lipidol 2011;6:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klimentidis YC, Chougule A, Arora A, Frazier-Wood AC, Hsu CH. Triglyceride-Increasing alleles associated with protection against type-2 diabetes. PLoS Genet 2015;11:e1005204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fujihara K, Sugawara A, Heianza Y, Sairenchi T, Irie F, Iso H, Doi M, Shimano H, Watanabe H, Sone H, Ota H. Utility of the triglyceride level for predicting incident diabetes mellitus according to the fasting status and body mass index category: the Ibaraki prefectural health study. J Atheroscler Thromb 2014;21:1152–1169. [DOI] [PubMed] [Google Scholar]
- 17. Zhao J, Zhang Y, Wei F, Song J, Cao Z, Chen C, Zhang K, Feng S, Wang Y, Li WD. Triglyceride is an independent predictor of type 2 diabetes among middle-aged and older adults: a prospective study with 8-year follow-ups in two cohorts. J Transl Med 2019;17:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y. Higher fasting triglyceride predicts higher risks of diabetes mortality in US adults. Lipids Health Dis 2021;20:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klempfner R, Erez A, Sagit BZ, Goldenberg I, Fisman E, Kopel E, Shlomo N, Israel A, Tenenbaum A. Elevated triglyceride level is independently associated with increased all-cause mortality in patients with established coronary heart disease. Circ Cardiovasc Qual Outcomes 2016;9:100–108. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y. Stage 1 hypertension and risk of cardiovascular disease mortality in United States adults with or without diabetes. J Hypertens 2022;40:794–803. [DOI] [PubMed] [Google Scholar]
- 21. Fang Y, Wang Y. Fasting status modifies the association between triglyceride and all-cause mortality: a cohort study. Health Sci Rep 2022;5:e642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Fang Y. Postabsorptive homeostasis model assessment for insulin resistance is a reliable biomarker for cardiovascular disease mortality and all-cause mortality. Diabetes Epidemiol Manag 2021;6:100045. [Google Scholar]
- 23. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care 2021;44:S15–S33. [DOI] [PubMed] [Google Scholar]
- 24. Wan Z, Guo J, Pan A, Chen C, Liu L, Liu G. Association of Serum 25-hydroxyvitamin D concentrations with all-cause and cause-specific mortality among individuals with diabetes. Diabetes Care 2021;44:350–357. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Fang Y. Late non-fasting plasma glucose predicts cardiovascular mortality independent of hemoglobin A1c. Sci Rep 2022;12:7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Charchar FJ. Establishment of sex difference in circulating uric acid is associated with higher testosterone and lower sex hormone-binding globulin in adolescent boys. Sci Rep 2021;11:17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y. Definition, prevalence, and risk factors of low sex hormone-binding globulin in US adults. J Clin Endocrinol Metab 2021;106:e3946–e3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Ferrari GM, Stevens SR, Ambrosio G, Leonardi S, Armstrong PW, Green JB, Wamil M, Holman RR, Peterson ED. Low-density lipoprotein cholesterol treatment and outcomes in patients with type 2 diabetes and established cardiovascular disease: insights from TECOS. Am Heart J 2020;220:82–88. [DOI] [PubMed] [Google Scholar]
- 30. Sharma A, Zheng Y, Ezekowitz JA, Westerhout CM, Udell JA, Goodman SG, Armstrong PW, Buse JB, Green JB, Josse RG, Kaufman KD, McGuire DK, Ambrosio G, Chuang LM, Lopes RD, Peterson ED, Holman RR. Cluster analysis of cardiovascular phenotypes in patients with type 2 diabetes and established atherosclerotic cardiovascular disease: a potential approach to precision medicine. Diabetes Care 2022;45:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. The Emerging Risk Factors Collaboration, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis. New York: Springer; 2015. [Google Scholar]
- 33. Schneider ALC, Pankow JS, Heiss G, Selvin E. Validity and reliability of self-reported diabetes in the atherosclerosis risk in communities study. Am J Epidemiol 2012;176:738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Comino EJ, Tran DT, Haas M, Flack J, Jalaludin B, Jorm L, Harris MF. Validating self-report of diabetes use by participants in the 45 and up study: a record linkage study. BMC Health Serv Res 2013;13:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Branchi A, Fiorenza AM, Rovellini A, Torri A, Muzio F, Macor S, Sommariva D. Lowering effects of four different statins on serum triglyceride level. Eur J Clin Pharmacol 1999;55:499–502. [DOI] [PubMed] [Google Scholar]
- 36. Virani SS, Morris PB, Agarwala A, Ballantyne CM, Birtcher KK, Kris-Etherton PM, Ladden-Stirling AB, Miller M, Orringer CE, Stone NJ. 2021 ACC expert consensus decision pathway on the management of ASCVD risk reduction in patients with persistent hypertriglyceridemia. J Am Coll Cardiol 2021;78:960–993. [DOI] [PubMed] [Google Scholar]
- 37. Dong ZH, Lin HY, Chen FL, Che XQ, Bi WK, Shi SL, Wang J, Gao L, He Z, Zhao JJ. Berberine improves intralipid-induced insulin resistance in murine. Acta Pharmacol Sin 2021;42:735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vaag A, Henriksen JE, Madsbad S, Holm N, Beck-Nielsen H. Insulin secretion, insulin action, and hepatic glucose production in identical twins discordant for non-insulin-dependent diabetes mellitus. J Clin Invest 1995;95:690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bruckert E, Baccara-Dinet M, Eschwege E. Low HDL-cholesterol is common in European type 2 diabetic patients receiving treatment for dyslipidaemia: data from a pan-European survey. Diabet Med 2007;24:388–391. [DOI] [PubMed] [Google Scholar]
- 40. Sattar N. Revisiting the links between glycaemia, diabetes and cardiovascular disease. Diabetologia 2013;56:686–695. [DOI] [PubMed] [Google Scholar]
- 41. Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010;375:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chan WB, Tong PC, Chow CC, So WY, Ng MC, Ma RC, Osaki R, Cockram CS, Chan JC. Triglyceride predicts cardiovascular mortality and its relationship with glycaemia and obesity in Chinese type 2 diabetic patients. Diabetes Metab Res Rev 2005;21:183–188. [DOI] [PubMed] [Google Scholar]
- 43. Miller M, Seidler A, Moalemi A, Pearson TA. Normal triglyceride levels and coronary artery disease events: the Baltimore coronary observational long-term study. J Am Coll Cardiol 1998;31:1252–1257. [DOI] [PubMed] [Google Scholar]
- 44. Wang Y. Omega-3 fatty acids effect on major cardiovascular events in patients at high cardiovascular risk. JAMA 2021;325:1333–1333. [DOI] [PubMed] [Google Scholar]
- 45. Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation 2006;114:1388–1394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in the current analysis are publicly available on the NHANES website.