Abstract
Context
Multiple tumors in the same patient suggest a genetic predisposition. Here, we report a patient who presented with several unusual types of malignant and benign tumors, presumably due to a pathogenic germline PMS1 mutation.
Case
A 69-year-old woman presented with a 2-year history of abdominal pain and diarrhea. A computed tomography scan of the abdomen revealed a gastrointestinal neuroendocrine tumor (GiNET) with liver metastases and a nonfunctional benign adrenal adenoma. Bilateral large lung nodules were thought to be also metastases from the GiNET but turned out to be differentiated thyroid cancer metastases, which later progressed to anaplastic thyroid cancer (ATC) and led to the patient's demise. A right sphenoid wing meningioma causing partial hypopituitarism was diagnosed during her evaluation. A mammogram and a breast ultrasound revealed a 0.3-cm left breast nodule. Due to the multiplicity of her tumors, whole exome sequencing was performed. This revealed a previously described PMS1 deletion mutation causing a frameshift and truncation (NM_000534c.1258delC, p.His420Ilefs*22) but no other pathogenic variant in other cancer genes. DNA isolated from the ATC tumor tissue showed loss of heterozygosity of the same mutation, highly suggestive of its pathogenic role in thyroid cancer and presumably other tumors.
Conclusion
This case reports several tumors including thyroid cancer, GiNET, adrenal adenoma, meningioma, and breast nodule, likely due to the PMS1 mutation found in this patient.
Keywords: PMS1 mutation, thyroid cancer, neuroendocrine tumor, meningioma, breast tumor
Cancer is predominantly a sporadic disease. Only a handful of syndromes are associated with an increased risk of cancer [1‐3]. However, with the introduction of next-generation sequencing, it has become clear that germline variants predisposing to an increased risk of cancer are not uncommon [4‐6]. Among the common familial cancer syndromes are multiple endocrine neoplasia type 2, Von Hippel Lindau syndrome (VHL), Li Fraumani syndrome, Gardner syndrome, and many others [7]. Lynch syndrome (LS), also known as hereditary nonpolyposis colorectal cancer syndrome, is one of the most common cancer predisposition syndromes [8, 9]. It is due to mutations in any of the genes involved in DNA mismatch repair (MMR), including MLH1, MSH2, MSH6, PMS2, EPCAM [9]. The most common manifestations of this syndrome is colorectal cancer [9, 10]. Other types of cancer described in LS include endometrial, ovarian, gastric, small intestinal, hepatobiliary, pancreatic, brain, skin, and urinary tract cancers [11, 12]. The postmeiotic segregation increased 1 (PMS1) gene is considered a minor gene in the MMR group. Mutations in this gene has been described in several cases of breast and colon cancer and less frequently in other types of cancer, including lung, hepatocellular, and ovarian cancer but not in classic LS. This report describes a patient with an unusual presentation of multiple benign and malignant tumors in whom a previously reported germline PMS1 mutation was found. She presented with a gastrointestinal neuroendocrine tumor (GiNET) of the midgut and was also found to have a nonfunctioning adrenal adenoma, a breast nodule, a meningioma and metastatic thyroid cancer. Evaluation of one of these tumors (anaplastic thyroid cancer [ATC]) in which tumor tissue was available showed loss of heterozygosity, strongly suggesting that this mutation played a role in its pathogenesis. Although thyroid cancer was reported once in a patient with a PMS1 mutation, GiNET, adrenal tumors, and meningioma have not previously been reported in association with mutations in this gene. The loss of heterozygosity of this mutation in ATC tumor tissue suggests that this mutation is pathogenic in ATC and, presumably, in the other tumors that this patient had.
Patients and Methods
The patient is described in detail in the “Results”. After obtaining institutional review board approval and informed consent from the patient, we isolated DNA from peripheral leukocytes using a QIAamp DNA Blood Mini Kit (Cat. No. 51104, QIAGEN GmbH, Germany) and from the lung biopsy tissue using a QIAamp DNA FFPE Tissue Kit (Cat. No. 56404, QIAGEN GmbH, Germany).
Whole Exome Sequencing
Whole exome sequencing of the leukocyte DNA was performed using the Ion Torrent platform (AmpliSeq kit). Briefly, 100 ng of DNA was amplified using AmpliSeq HiFi mix (Life Technologies, Carlsbad, CA, USA) for 10 cycles. Polymerase chain reaction (PCR) products were pooled, followed by primer digestion using FuPa reagent (Life Technologies, Carlsbad, CA, USA). This was followed by a ligation step using Ion P1 and Ion Xpress Barcode adapters. The library was purified and quantified using quantitative PCR and the Ion Library Quantification Kit (Life Technologies, Carlsbad, CA, USA). The emulsion of the libraries was done using a Ion OneTouch System to attach the DNA fragments to the Ion Sphere particles. The final step in the library preparation was the enrichment of the Ion Sphere particles using Ion OneTouch ES (Life Technologies, Carlsbad, CA, USA). Following that, the library was loaded on to the sequencing chip, which was then inserted into the Ion Proton instrument (Life Technologies, Carlsbad, CA, USA) for sequencing.
Bioinformatics Analysis
We used the Torrent Suite (https://github.com/iontorrent/TS) analysis kit for bioinformatics analysis using the manufacturer's recommended parameters for base calling and alignment. This was followed by checking the reads for quality and trimming the low-quality parts. The reads were aligned with the reference human genome (version hg19) using the manufacturer's recommended parameters. Following alignment, we used the variant calling pipeline of the Torrent suite, both of which are based on the BWA-GATK pipeline, but they were tuned more closely to the Ion Torrent technology by including flow signal information and the library of common sequencing error motifs to improve accuracy.
The next step was running an in-house–developed annotation pipeline. This pipeline was based on the Annovar package (http://annovar.openbioinformatics.org), which includes about 40 databases. We also added more information tracks, including variant frequencies from the Saudi Human Genome Program database.
The list of annotated variants per the patient's sample was filtered to remove the small intronic sequences and synonymous variants. The remaining variants were then prioritized based on the following criteria: (1) the existence of genes that are known to be related to familial cancer or multiple tumor syndromes, (2) the effect score (whether the variant is truncating/damaging or not), and (3) the frequency in public databases and Saudi population (Saudi Genome database with more than 16000 individual sequencing data). Firstly, we compared all the variants with our internal database (Saudi Genome Database) to see whether any of the variants were already associated with a disease. Secondly, we checked whether the variant has been reported in Human Gene Mutation Database (HGMD). Thirdly, we reviewed whether the variant has already been published in ClinVar. Fourthly, we excluded variants with minor allele frequency >1% in gnomAD. Putative variants were assessed for potential pathogenicity by in silico prediction tools as recommended by American College of Medical Genetics and Genomics (ACMG) guidelines. In addition to PMS1, we specifically looked for pathogenic, likely pathogenic, or variants of unknown significance in specific genes that are associated with cancer (MLH1, MSH2, MSH6, PMS2, EPCAM RET, MEN1, DICER1, CDKN1B, VHL, NF1, BRCA1, BRCA2, TP53, TSC1, TSC2, GCGR, PI3KCA, PTEN, TP53, NF1, NF2, and SDHx). We did not find pathogenic, likely pathogenic, or variants of unknown significance in any of these genes.
Quality of Sequencing
Sequencing quality was assessed using different metrics. These showed that the target regions are well covered by the next-generation sequencing reads (99.6% total average coverage at 1× and 96.88% average coverage at 20×) with an average depth of 224 (ie, each base in the target region is covered by 224 reads on average).
PCR and Sanger Sequencing
The part of PMS1 exon 9 that contains the mutation was amplified by PCR and directly sequenced. The primers used are forward: 5′-CTGATGACGACTTGTTATGGACC and reverse: 5′-CCCTGCTCCACTCATCTGC. The PCR conditions were as follows: initial denaturation for 4 minutes at 94 °C, followed by 35 cycles of denaturation at 94 °C for 30 seconds, annealing at 56 °C for 30 seconds, and extension at 72 °C for 45 seconds followed by a final extension step at 72 °C for 4 minutes, cooling, and storing at 4 °C. The PCR product was resolved on 2% agarose gel and sequenced using the same primers in forward and reverse directions using the dideoxy chain termination method (Applied Biosystems ABI 3730XL DNA Analyzer Sequencer).
Results
Patient
A 69-year-old woman presented in 2020 with a 2-year history of recurrent right lower quadrant abdominal pain and loose bowel motions 4 to 5 times per day. A computed tomography scan (CT) of the abdomen and chest revealed a 2-cm ileal mass, an adjacent 6-cm mesenteric mass (Fig. 1A ), 3 liver lesions (Fig. 1B), and a 4-cm right adrenal mass (Fig. 2C). It also showed extensive bilateral lung nodules consistent with metastases (Fig. 1D). The patient underwent a laparoscopic exploration of the abdomen and a liver biopsy of 1 of the liver lesions. These revealed a well-differentiated metastatic neuroendocrine tumor, grade 1 with Ki67 index of 2% (Fig. 2), which was positive for chromogranin A and synaptophysin. Hormonal and radiological evaluation of the right adrenal mass showed that it was nonfunctional and likely benign. The patient was thought to have a widely metastatic GiNET to the liver and lungs. She was referred to our hospital for further management. On initial evaluation, the patient gave a history of thyroid surgery 15 years ago in a small community hospital; the details of the operation were unavailable to the patient and could not be obtained. She was not informed about the diagnosis, had no follow-up after thyroid surgery, and was not placed on thyroxine hormone replacement therapy. Her past medical history was otherwise noncontributory. Her family history was negative for cancer, NETs, or chronic illnesses. Physical examination was unremarkable except for a small healed thyroidectomy scar without neck masses or lymph nodes. Her vital signs were normal.
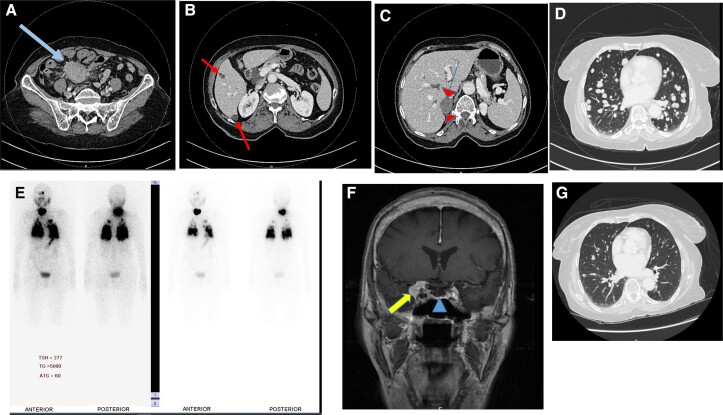
Figure 1.
A composite diagram showing axial sections of contrast-enhanced computed tomography of the pelvis and lower abdomen (A), the liver (B), and the right adrenal gland (C). These show a large mesenteric neuroendocrine tumor (arrow), liver metastases (arrows), and right adrenal mass (arrowheads). A CT scan of the chest (D) shows extensive bilateral lung metastases from thyroid cancer. This is confirmed on the anterior and posterior views of the I-123 whole body scan (E) which show extensive radioiodine uptake in the lungs and thyroid lobes. (F) A coronal section of a contrast-enhanced T1 weighted image of magnetic resonance imaging of the head showing a right sphenoid wing meningioma (arrow) and secondary partial empty sella (arrowhead). (G) Follow-up CT scan of the chest after 2 I-131 therapies showing remarkable reduction in the size and number of the lung metastases seen before I-131 therapy in D.
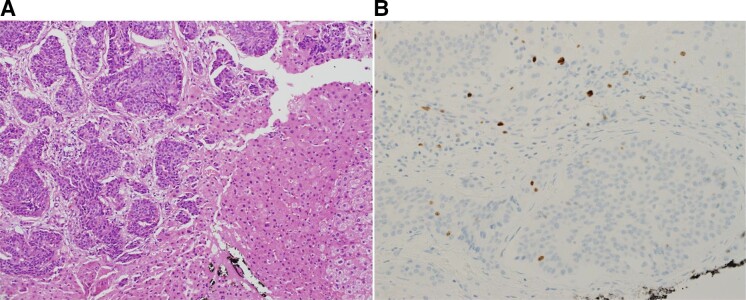
Figure 2.
(A) Hematoxylin and eosin stain of liver biopsy showing nests of well-differentiated neuroendocrine cells between normal liver cells. The tumor cells are round and uniform without mitotic activity. (B) Ki-67 stain showing several positive tumor cell nuclei in the lower half of the field (∼2%). The upper half show positive nuclei of inflammatory and stromal cells.
She was clinically mildly hypothyroid. The chest examination was clear, and the heart examination was normal without raised jugular venous pressure, added sounds, or murmurs. The abdomen was soft and lax with no palpable masses, hepatomegaly, or splenomegaly and no ascites. Bowel sounds were normal. Skin, musculoskeletal and neurological examinations were all normal. Due to the low-grade nature of the GiNET and the minimal liver metastases, we doubted that the extensive lung metastases originated from the GiNET. Considering her history of partial thyroidectomy and the classic appearance of thyroid cancer metastases on her lung CT scan, we suspected thyroid cancer as a source. Nonstimulated serum thyroglobulin came back >5000 ng/dL (normal range 1.4-78) with negative antithyroglobulin antibodies. A diagnostic I-123 whole-body scan confirmed metastatic thyroid cancer in the lungs (Fig. 1E). Thyroid ultrasonography showed evidence of left hemithyroidectomy with an intact right lobe containing 2 small nodules 3 and 2 mm in size and no suspicious lymph nodes. A fine-needle aspiration biopsy from the 3-mm nodule showed benign follicular cells. Due to the widespread lung metastases, we decided to treat her with radioactive iodine (I-131) rather than subjecting her to another thyroid surgery. It was noted that her thyrotropin (TSH) was just mildly elevated at 6.5 mU/L (normal range 0.4-4.2) despite a significant drop in her free thyroxine level of 8.8 pmol/L (normal range 12-22). In addition, luteinizing hormone (6.3 IU/L) was inappropriately low (normal range for postmenopausal women 7.7-58.5 IU/L), and follicle-stimulating hormone (18 u/L) was also inappropriately low (normal range for postmenopausal women 25.8-134.8 u/L). For these reasons, we suspected a pituitary disease and a magnetic resonance imaging of the pituitary gland was requested. This revealed a right sphenoid wing meningioma of 1.8 cm impinging on the right side of the pituitary gland with secondary partial empty sella (Fig. 1F). A short synacthen test was normal with baseline cortisol 228 pmol/L (normal range 166-507) and poststimulation cortisol level 866 pmol/L (normal response >550 pmol/L). Dehydroepiandrosterone sulfate was low, suggesting mild chronic adrenocorticotropin deficiency. Serum aldosterone and plasma renin were not measured since the patient was normotensive. Twenty-four–hour urine catecholamines were normal.
A mammogram and breast ultrasound revealed a 3-mm lesion in the left breast (BI-RADS 2).
For the GiNET, surgery was deemed difficult due to extensive mesenteric tethering by the tumor which would result in extensive bowel resection if surgery is perfomred. Therefore, surgery was not persued and the patient was started on monthly long-acting somatostatin analogue (octreotide LAR) 20 mg intramuscularly. The GiNETs remained stable on follow-up CT scans and magnetic resonance imaging done 6 and 12, and 24 months later. The meningioma was managed conservatively without intervention and continued to be stable. For thyroid cancer, the patient was treated with I-131 twice, 109 mCi and 110 mCi, in January 2020 and January 2021. Follow-up CT scan of the lungs, serum thyroglobulin, and I-123 whole body scan showed remarkable improvement in the size and activity of the lung nodules and the residual thyroid tissue (Fig. 1G). In May 2022, a repeated CT scan of the chest showed an increasing size of the lung metastases. In September 2022, an I-123 diagnostic whole body scan showed positive uptake in the bilateral lung metastases. The patient was treated with a third dose of I-131 with 78 mCi-administered activity. In December 2022, she presented with cough and increasing shortness of breath for 10 days. Chest X-ray and CT scan of the chest showed massive left pleural effusion and a significant increase in the size of the lung nodules, with new lung and large pleural nodules. A CT-guided lung biopsy revealed transformation of thyroid cancer to ATC (Fig. 3). Molecular testing revealed no druggable genetic alterations. Specifically, BRAFV600E, RET-PTC, NTRK, and ALK fusions were all negative. The patient's condition rapidly deteriorated and needed intubation and intensive care unit admission. She could not be weaned off the ventilator and passed away 11 days after her admission to the intensive care unit.
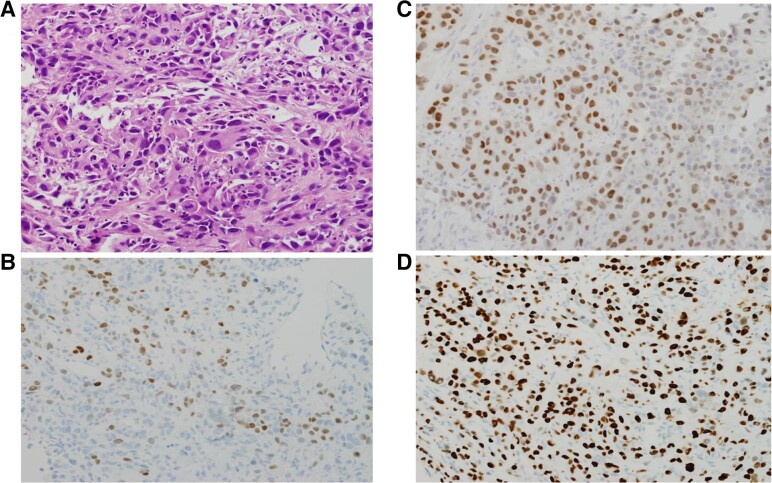
Figure 3.
(A) Hematoxylin and eosin stain of transthoracic lung biopsy showing pleomorphic tumor cells with significant mitotic activity in a disorganized pattern consistent with anaplastic thyroid cancer (ATC). Some cells show hobnail morphology, suggesting that the original tumor was papillary thyroid cancer. These cells are positive for TTF1 (B), PAX8 (C), which are features of thyroid origin, and P53 (D), a common feature of ATC.
Genetic Analysis
The bioinformatics analysis of whole exome sequencing revealed a previously described variant in PMS1 (NM_000534:c.1258delC, p.His420Ilefs*22). This was confirmed by PCR and direct Sanger sequencing (Fig. 4A and 4B). This mutation leads to frameshift and truncation 22 codons downstream of the deletion site. The lung biopsy sample confirmed the loss of heterozygosity at this mutation site (Fig. 4C and 4D).
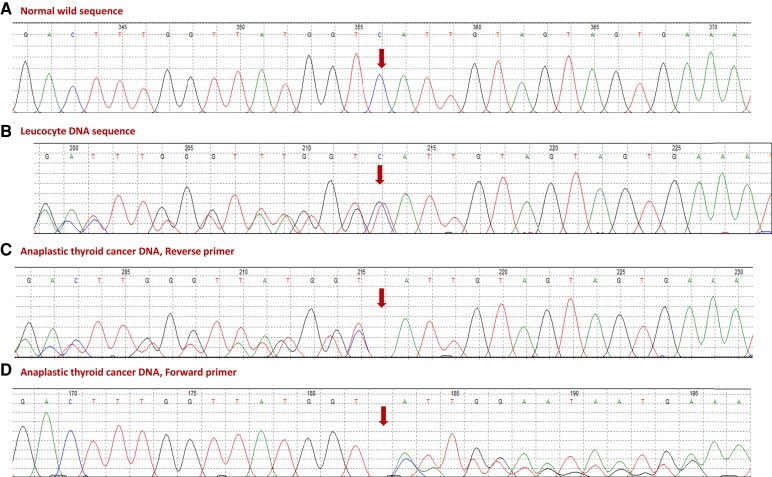
Figure 4.
A chromatogram showing (A) normal sequence of part of PMS1 exon 9 (NM_000534 and (B) the patient's germline DNA sequence showing a heterozygous deletion mutation (NM_000534:c.1258delC, p.His420Ilefs*22). The 2 lower panels show loss of heterozygosity of the same mutation in anaplastic thyroid cancer (ATC) in using reverse (C) and forward (D) primers. The double sequence following the loss of heterozygosity is due to contamination of tumor DNA with genomic DNA from the heavy lymphocytic infiltration of the ATC.
Discussion
In this report, we describe a case of multiple benign and malignant tumors with a PMS1 deletion mutation, previously described in patients with breast cancer and in a single case of thyroid cancer but not with the other tumors that this patient had [13, 14]. In a cohort of 8085 consecutive nonselected Chinese breast cancer patients screened for germline mutations using a 64-cancer gene panel, 16 cases (0.2%) were found to have PMS1 germline mutations [13]. Of these 16 mutations, 6 were the same mutation found in our patient (NM_000534:c.1258delC, p.H420fs) [13]. In another report from China, screening of 937 consecutive breast cancer patients with high-risk features for underlying genetic mutations revealed germline mutations in around 24% of them, with 1 patient having the same PMS1 mutation described in this report [14]. Our patient had a breast nodule that seemed benign based on breast ultrasonography and mammography. More unusually, she had a meningioma, thyroid cancer, GiNET, and an adrenal nodule. Except for a single case of differentiated thyroid cancer in the context of next-generation sequencing screening for cancer gene mutations in a large sample of patients with thyroid cancer [15], these tumors have never been described in association with this mutation. Although heterozygous in the peripheral blood DNA, this germline PMS1 mutation was biallelic in the ATC tumor (loss of heterozygosity, Fig. 4), implying that it is pathogenic in ATC and presumably in the other tumors of this patient. Whether this collection of tumors and the presence of the PMS1 mutation represent an atypical case of LS remains speculative only.
This specific PMS1 mutation has not been previously described in LS. LS is most commonly associated with colorectal cancer, with a lifetime risk of 20% to 70% [12]. The average age at the time of diagnosis of colorectal cancer is 44-61 years [12]. This is followed by endometrial cancer with a lifetime risk of 15% to 70% and an average age at diagnosis of 48-62 years [12]. Other common cancers in LS include gastric (lifetime risk 6-13% and average age at diagnosis of 56 years) and ovarian cancer (lifetime risk 4-12% and average age at diagnosis of 42.5 years) [12]. Other less common cancers that may occur in LS (overall life time risk is 15%) include small intestine, hepatobiliary, pancreatic, brain, skin, and urinary tract cancers [12]. On repeated extensive evaluations of this patient with several CT scans, F18-fluorodeoxyglucose and gallium-68 positron emission tomography CT scans, and upper and lower gastrointestinal endoscopies, none of these more typical tumors of LS was diagnosed. In LS, most mutations occur in MSH2 and MLH1 [16]. These genes are called the major DNA MMR genes [17]. Other MMR genes are less frequently mutated and are called minor genes [17]. MSH2 mutations are more likely associated with extracolonic cancers, including endometrial, ovarian, renal, and gastric cancers [16]. PMS2 mutations may present with a combination of colorectal cancer and brain glioblastomas [18]. PMS1 is considered a minor MMR gene. This gene has 12 exons and is located on chromosome 2q31.1. It encodes a 932-amino acid protein, PMS1 homolog 1. This protein belongs to the DNA MMR mutL/hexB family. However, its role in DNA repair is not certain, but it can form heterodimers with MLH1, a known DNA MMR protein. PMS1 mutations may cause hereditary nonpolyposis colorectal cancer, either alone or in combination with mutations in other genes involved in LS. The deletion mutation we describe in this report (NM_000534:c.1258delC, p.His420Ilefs*22) is located on exon 9, leading to frameshift and truncation 22 codons downstream. It leads to the loss of a significant part of the carboxy terminal part of the protein. It has been most frequently associated with breast cancer [13, 14, 19, 20]. Only a few cases of nonbreast cancer have been described, including 1 case of hepatocellular cancer [21], a case of colorectal cancer [22], a case of combined breast and ovarian cancer [23], a case of mesothelioma in a patient with history of asbestosis [24], and a case of papillary thyroid cancer detected in screening for germline mutations in a number of cancer genes in patients with different types of cancer [15]. The case we described in this report is unique in many aspects, including the unusual combination of tumors she had (GiNET, thyroid cancer, breast and adrenal nodules, and a meningioma) and absence of colorectal or endometrial cancer. Therefore, it is questionable whether we can consider it a case of LS or not. However, it is a case with several benign and malignant tumors and a pathogenic PMS1 mutation. The loss of heterozygosity in the ATC (Fig. 4) strongly suggests its pathogenic role in this tumor and presumably in the other tumors that this patient had based on its known role in LS.
Apart from about 20% to 25% of cases of medullary thyroid cancer that are familial as part of multiple endocrine neoplasia syndromes type 2a or 2b [25], epithelial cell–derived thyroid cancer (commonly referred to as differentiated thyroid cancer) is sporadic in more than 90% of cases [26]. However, about 5% to 9% of patients have genetic predispositions and are frequently referred to as familial nonmedullary thyroid cancer (FNMTC) [26]. These cases either develop as part of familial cancer predisposition syndromes or as an isolated type of cancer affecting more than 1 family member [26, 27]. The syndromic forms of FNMTC include Cowden syndrome caused by germline mutations in PTEN, familial adenomatosis polyposis syndrome (Gardner syndrome) due to mutations in APC, Werner syndrome due to mutations in WRO, Carney syndrome due to mutations in PPARKA1 and DICER1 syndrome due to mutations in DICER1 [26, 28]. The genetics of the nonsyndromic FNMTC are less clear [27]. Several genes and chromosomal loci have been identified in different families, but there is a lack of consistency [26, 27]. These genes include HABP2, FOXE1, SGPR1, and many others [26, 27]. Thyroid cancer has not been described in LS.
Although about 10% of pancreatic NET (PanNET) are part of underlying genetic syndromes, GiNETs are mostly sporadic [29]. PanNETs can be part of multiple endocrine neoplasia type 1 (MEN1 or CDKN1B), VHL, neurofibromatosis type 1 (NF1), tuberous sclerosis (TSC1 or TSC2), Mahvash syndrome (GCGR) and familial insulinomatosis syndrome (MAFA) [29]. PanNET and GiNET more commonly carry somatic mutations in epigenetic regulator genes rather than oncogenes and tumor suppressor genes [29]. These somatic genetic alterations are different between PanNETs and GiNETs. Both PanNETs and GiNETs have not been previously reported in LS.
Finally, meningiomas are the most common intracranial tumors and are usually benign [30]. Like thyroid cancer, they are primarily sporadic but are also associated with known cancer predisposition syndromes [31]. They are seen in cases of neurofibromatosis 2, Gorlin, Li Fraumani, Cowden, VHL, and MEN1 syndromes [31]. Somatic mutations are common in meningiomas [32, 33]. The most common abnormality that occurs in about 50% of these tumors is the 22q12 chromosome deletion that encodes for the tumor suppressor gene neurofibromin 2 (NF2) [32, 34]. Other alterations involve a number of signaling pathways (reviewed in Maggio et al [31]). To our knowledge, meningiomas have not been reported in LS.
This case strongly suggests that thyroid cancer is due to the PMS1 mutation she had, and the GiNET, meningioma, and the breast and adrenal nodules are likely also to be due to this PMS1 mutation. However, this is not definite, as those tumors are not uncommon, especially in older people.
In summary, we describe a case of multiple benign and malignant tumors, including thyroid cancer, metastatic GiNET, a meningioma, an adrenal nodule, and a breast nodule of unclear malignancy potential in an elderly lady who carries a previously described pathogenic PMS1 mutation, suggesting that this mutation is the underlying genetic alteration of these tumors. This is further supported by the loss of heterozygosity in 1 of these tumors, ATC.
Acknowledgment
We would like to thank the patient and her family for their cooperation and participation in this study.
Abbreviations
- ATC
anaplastic thyroid cancer
- CT
computed tomography
- FNMTC
familial nonmedullary thyroid cancer
- GiNET
gastrointestinal neuroendocrine tumor
- LS
Lynch syndrome
- MMR
mismatch repair
- PCR
polymerase chain reaction
- PMS1
postmeiotic segregation increased 1
- VHL
Von Hippel Lindau syndrome
Contributor Information
Balgees Alghamdi, Department of Molecular Oncology, King Faisal Specialist Hospital & Research Centre, Riyadh 11211, Saudi Arabia.
Hindi Al-Hindi, Department of Pathology and Laboratory Medicine, King Faisal Specialist Hospital & Research Centre, Riyadh 11211, Saudi Arabia.
Avaniyapuram Kannan Murugan, Department of Molecular Oncology, King Faisal Specialist Hospital & Research Centre, Riyadh 11211, Saudi Arabia.
Ali S Alzahrani, Email: aliz@kfshrc.edu.sa, Department of Molecular Oncology, King Faisal Specialist Hospital & Research Centre, Riyadh 11211, Saudi Arabia; Department of Medicine, King Faisal Specialist Hospital & Research Centre, Riyadh 11211, Saudi Arabia.
Funding
No specific funding was allocated for this work but it was supported from the general support of the Research Centre of the King Faisal Specialist Hospital & Research Centre, Riyadh, Saudi Arabia.
Disclosures
The authors have no conflict of interest related to the work described in this article.
Data Availability
Data included in this report have been deposited to the Gene Bank and awaiting processing. Meanwhile, these data are available from the corresponding author upon request.
References
- 1. Moore EC, Ioannou L, Ruseckaite R, Serpell J, Ahern S. Hereditary endocrine tumors and associated syndromes: a narrative review for endocrinologists and endocrine surgeons. Endocr Pract. 2021;27(11):1165‐1174. [DOI] [PubMed] [Google Scholar]
- 2. Schiffman JD, Geller JI, Mundt E, Means A, Means L, Means V. Update on pediatric cancer predisposition syndromes. Pediatr Blood Cancer. 2013;60(8):1247‐1252. [DOI] [PubMed] [Google Scholar]
- 3. Garber JE, Offit K. Hereditary cancer predisposition syndromes. J Clin Oncol. 2005;23(2):276‐292. [DOI] [PubMed] [Google Scholar]
- 4. Mittal A, Deo SVS, Gogia A, et al. Profile of pathogenic mutations and evaluation of germline genetic testing criteria in consecutive breast cancer patients treated at a north Indian tertiary care center. Ann Surg Oncol. 2022:29(2):1423‐1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2015;373(24):2336‐2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pritchard AL, Johansson PA, Nathan V, et al. Germline mutations in candidate predisposition genes in individuals with cutaneous melanoma and at least two independent additional primary cancers. PLoS One. 2018;13(4):e0194098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magrin L, Fanale D, Brando C, et al. POLE, POLD1, and NTHL1: the last but not the least hereditary cancer-predisposing genes. Oncogene. 2021;40(40):5893‐5901. [DOI] [PubMed] [Google Scholar]
- 8. Olave MC, Graham RP. Mismatch repair deficiency: the what, how and why it is important. Genes Chromosomes Cancer. 2022;61(6):314‐321. [DOI] [PubMed] [Google Scholar]
- 9. Maratt JK, Stoffel E. Identification of Lynch syndrome. Gastrointest Endosc Clin N Am. 2022;32(1):45‐58. [DOI] [PubMed] [Google Scholar]
- 10. Drogan C, Kupfer SS. Colorectal cancer screening recommendations and outcomes in Lynch syndrome. Gastrointest Endosc Clin N Am. 2022;32(1):59‐74. [DOI] [PubMed] [Google Scholar]
- 11. Biller LH, Creedon SA, Klehm M, Yurgelun MB. Lynch syndrome-associated cancers beyond colorectal cancer. Gastrointest Endosc Clin N Am. 2022;32(1):75‐93. [DOI] [PubMed] [Google Scholar]
- 12. Duraturo F, Liccardo R, Rosa MD, Izzo P. Genetics, diagnosis and treatment of Lynch syndrome: old lessons and current challenges. Oncol Lett. 2019;17(3):3048‐3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun J, Meng H, Yao L, et al. Germline mutations in cancer susceptibility genes in a large series of unselected breast cancer patients. Clin Cancer Res. 2017;23(20):6113‐6119. [DOI] [PubMed] [Google Scholar]
- 14. Li JY, Jing R, Wei H, et al. Germline mutations in 40 cancer susceptibility genes among Chinese patients with high hereditary risk breast cancer. Int J Cancer. 2019;144(2):281‐289. [DOI] [PubMed] [Google Scholar]
- 15. Siraj AK, Masoodi T, Bu R, et al. Expanding the spectrum of germline variants in cancer. Hum Genet. 2017;136(11-12):1431‐1444. [DOI] [PubMed] [Google Scholar]
- 16. Yurgelun MB, Kulke MH, Fuchs CS, et al. Cancer susceptibility gene mutations in individuals with colorectal cancer. J Clin Oncol. 2017;35(10):1086‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liccardo R, De Rosa M, Izzo P, Duraturo F. Novel implications in molecular diagnosis of Lynch syndrome. Gastroenterol Res Pract. 2017;2017:2595098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Agostini M, Tibiletti MG, Lucci-Cordisco E, et al. Two PMS2 mutations in a Turcot syndrome family with small bowel cancers. Am J Gastroenterol. 2005;100(8):1886‐1891. [DOI] [PubMed] [Google Scholar]
- 19. Hata C, Nakaoka H, Xiang Y, et al. Germline mutations of multiple breast cancer-related genes are differentially associated with triple-negative breast cancers and prognostic factors. J Hum Genet. 2020;65(7):577‐587. [DOI] [PubMed] [Google Scholar]
- 20. Shao D, Cheng S, Guo F, et al. Prevalence of hereditary breast and ovarian cancer (HBOC) predisposition gene mutations among 882 HBOC high-risk Chinese individuals. Cancer Sci. 2020;111(2):647‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Wu Y, Suo P, et al. Identification of a novel germline frameshift mutation p.D300fs of PMS1 in a patient with hepatocellular carcinoma: a case report and literature review. Medicine (Baltimore). 2020;99(5):e19076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schrader KA, Cheng DT, Joseph V, et al. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol. 2016;2(1):104‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castéra L, Krieger S, Rousselin A, et al. Next-generation sequencing for the diagnosis of hereditary breast and ovarian cancer using genomic capture targeting multiple candidate genes. Eur J Hum Genet. 2014;22(11):1305‐1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Betti M, Casalone E, Ferrante D, et al. Germline mutations in DNA repair genes predispose asbestos-exposed patients to malignant pleural mesothelioma. Cancer Lett. 2017;405:38‐45. [DOI] [PubMed] [Google Scholar]
- 25. Mathiesen JS, Effraimidis G, Rossing M, et al. Multiple endocrine neoplasia type 2: a review. Semin Cancer Biol. 2022;79:163‐179. [DOI] [PubMed] [Google Scholar]
- 26. Diquigiovanni C, Bonora E. Genetics of familial non-medullary thyroid carcinoma (FNMTC). Cancers (Basel). 2021;13(9):2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cirello V. Familial non-medullary thyroid carcinoma: clinico-pathological features, current knowledge and novelty regarding genetic risk factors. Minerva Endocrinol (Torino). 2021;46(1):5‐20. [DOI] [PubMed] [Google Scholar]
- 28. Peiling Yang S, Ngeow J. Familial non-medullary thyroid cancer: unraveling the genetic maze. Endocr Relat Cancer. 2016;23(12):R577‐R595. [DOI] [PubMed] [Google Scholar]
- 29. Asa SL, La Rosa S, Basturk O, Adsay V, Minnetti M, Grossman AB. Molecular pathology of well-differentiated gastro-entero-pancreatic neuroendocrine tumors. Endocr Pathol. 2021;32(1):169‐191. [DOI] [PubMed] [Google Scholar]
- 30. Maggio I, Franceschi E, Tosoni A, et al. Meningioma: not always a benign tumor. A review of advances in the treatment of meningiomas. CNS Oncol. 2021;10(2):CNS72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maggio I, Franceschi E, Di Nunno V, et al. Discovering the molecular landscape of meningioma: the struggle to find new therapeutic targets. Diagnostics (Basel). 2021;11(10):1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brastianos PK, Horowitz PM, Santagata S, et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet. 2013;45(3):285‐289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Clark VE, Erson-Omay EZ, Serin A, et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science. 2013;339(6123):1077‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wellenreuther R, Kraus JA, Lenartz D, et al. Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am J Pathol. 1995;146(4):827‐832. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in this report have been deposited to the Gene Bank and awaiting processing. Meanwhile, these data are available from the corresponding author upon request.