Abstract
Volume overload promotes pulmonary hypertension (PH) through pulmonary venous hypertension. However, PH with elevated pulmonary vascular resistance (hereafter PH-PVR) may develop in patients with diseases of volume overload, such as heart failure or chronic kidney disease (CKD). In such cases, volume management alone may be insufficient to slow PH progression. An accurate, noninvasive method to screen for PH-PVR in these diseases would facilitate early targeted therapy. We integrated invasive hemodynamic and echocardiography data collected from a single-center clinical cohort and identified patients with CKD or heart failure at the time of assessment. We applied penalized regression to derive a risk score of clinical parameters and echocardiography data associated with PH-PVR and categorized patients into low- (≤5 points), intermediate- (6–10 points), or high-risk (>10 points) groups. Using an internal validation strategy, we evaluated the ability of this risk score to predict PH-PVR and determined the association of this risk classification with 3-year all-cause mortality. Of 2422 patients, 42.4% had PH-PVR. In adjusted analyses, tricuspid regurgitant velocity, right ventricular function, BMI, heart rate, and hemoglobin most strongly associated with PH-PVR. The risk score significantly associated with PH-PVR (age-adjusted odds ratio 11.69 for the highest-risk group, 95% confidence interval [CI] 6.54–20.92). The high-risk group also associated with a significantly higher risk of 3-year all-cause mortality in adjusted analyses (hazard ratio 1.85, 95% CI 1.50–2.27). In conclusion, a noninvasive risk score derived from echocardiography and clinical parameters significantly associated with PH-PVR and all-cause mortality in a cohort of patients with CKD and heart failure.
Keywords: pulmonary hypertension, heart failure, chronic kidney disease, echocardiography
INTRODUCTION
Pulmonary hypertension (PH) is categorized into hemodynamic subtypes based on pulmonary vascular resistance (PVR) and pulmonary capillary wedge pressure (PCWP) as measured by right heart catheterization.1 While diseases of volume overload, such as CKD and heart failure, can induce PH through elevated left-sided pressures, these diseases can also adversely affect vascular biology and increase PVR leading to poor outcomes.2,3 This elevated PVR may promote PH alone or in concert with elevated PCWP. Patients with PH and elevated PVR (hereafter termed PH-PVR) may benefit from early application of pulmonary vasodilator therapy, even in select patients with volume overload.4–6 Echocardiography provides an effective screening tool for the diagnosis of PH in both CKD and HF cohorts;7,8 in other populations, echocardiography can also estimate PVR.9,10 A noninvasive method to screen for PH-PVR in diseases of volume overload would identify high-risk patients in whom definitive diagnostic procedures should proceed without delay to facilitate early targeted therapy, if indicated. We determined how select clinical parameters and echocardiography data associated with PH-PVR in patients with either CKD or heart failure referred for right heart catherization; developed and internally validated a risk score comprised of parameters which are strongly associated with PH-PVR; and determined the association of this risk classification with all-cause mortality.
METHODS
The study cohort included patients within Duke University Hospital referred for right heart catheterization between 1/1/2011 and 12/31/2014 with echocardiograms available ≤6 months before or after of catheterization. Patients with either CKD or heart failure were selected for this study. To identify patients with CKD, we calculated the estimated glomerular filtration rate (eGFR) from the median creatinine over the 6-month period prior to catheterization using the CKD-EPI equation. We defined CKD as an estimated glomerular filtration rate (eGFR) of <60 ml/min/1.73 m2; proteinuria data were not available. Heart failure diagnosis was indicated by providers at the time of catheterization and collected in the Duke Databank for Cardiovascular Disease (DDCD). Exclusion criteria included age <18 years; missing creatinine, catherization, or echocardiogram data; kidney transplantation; and right heart catheterizations performed during hospitalizations for acute coronary syndrome or for evaluation for heart or lung transplantation. We included only the first study which met the inclusion/exclusion criteria. The Duke University Health Systems Institutional Review Board reviewed and approved this study.
We collected demographic information on age, sex, and self-reported race from the DDCD at the time of catherization. We consolidated race into three categories: White, Black, and other. Diagnoses for diabetes mellitus, hypertension, chronic obstructive pulmonary disease, obstructive sleep apnea, scleroderma, and systemic lupus erythematosus were obtained from the DDCD intake form and supplemented by diagnostic codes. We also collected the body mass index (BMI), systolic blood pressure, heart rate, and hemoglobin obtained closest to the time of the right heart catherization. Patients with a heart rate ≥200 beats per minute were excluded.
We collected the following echocardiography data: tricuspid annular plane systolic excursion (TAPSE), tricuspid regurgitant velocity (TRV), left ventricular ejection fraction, left atrial anteroposterior diameter, right ventricular function (normal, mildly decreased, moderately decreased, or severely decreased), mid right ventricular diameter, and left ventricular diastolic (normal, grade 1 dysfunction, grade 2 dysfunction, or grades 3–4 dysfunction). We estimated right atrial pressure based on inferior vena cava size and respiratory variation and consolidated the estimated pressure into three values: 3 mmHg, 8 mmHg, or 15 mmHg. Echocardiograms which reported <50% of these measurements were excluded. Echocardiography measurements were reviewed for clear data outliers which resulted in the exclusion of one patient for a TAPSE >8 cm and another patient for a left atrial diameter of >11 cm.
We defined PH as a mean pulmonary artery pressure of >20 mmHg on right heart catheterization and categorized by hemodynamic subtype (Supplemental Materials).1 We grouped patients into two phenotypes: PH-PVR versus normal PVR. The PH-PVR phenotype included cases of pre-capillary and combined pre- and post-capillary PH; all other patients were grouped into the normal PVR phenotype. We excluded outliers with either a negative PVR (2 patients) or PVR >100 Wood Units (2 patients). Vital status in the DDCD was determined using a combination of longitudinal follow-up data collected for all patients in the DDCD after left-heart catheterization and National Death Index query for the remaining patients.
We stratified the study cohort by PVR phenotype (Table 1) and PH hemodynamic subtypes (Supplementary Materials) and reported baseline demographic data, clinical parameters, and echocardiography measurements. Categorical data were presented as number and percentage of patients. Continuous variables were reported as mean ± standard deviation (SD) if normally distributed or as median (interquartile range) if otherwise. To compare differences between continuous variables in the PH-PVR and normal PVR phenotypes, we used unpaired t-tests (when normally distributed) and Wilcoxon rank sum tests (when skewed). We used Pearson’s Chi square tests to compare differences in categorical variables.
Table 1.
Clinical Characteristics Stratified by PVR Phenotype
| Characteristic | Total Cohort (N = 2422) |
PH-PVR (N = 1026) |
Normal PVR (N = 1396) |
P-value |
|---|---|---|---|---|
| Age (years) | 65.2 ± 14.4 | 65.1 ± 14.1 | 65.3 ± 14.7 | 0.69 |
| Women | 1089 (45.0%) | 541 (52.7%) | 548 (39.3%) | <0.001 |
| White | 1684 (70.0) | 639 (62.8) | 1045 (75.3) | <0.001 |
| Black | 636 (26.4) | 333 (32.7) | 303 (21.8) | |
| Other | 85 (3.5) | 45 (4.4) | 40 (2.9) | |
| eGFR (ml/min/1.73m2) | 58.2 ± 24.9 | 57.2 ± 24.5 | 58.9 ± 25.1 | 0.10 |
| BMI (kg/m2) | 29.5 ± 7.2 | 29.2 ± 7.3 | 29.8 ± 7.1 | 0.05 |
| Systolic Blood Pressure (mmHg) | 132.5 ± 22.4 | 132.3 ± 22.5 | 132.7 ± 22.3 | 0.71 |
| Heart Rate (bpm) | 82.7 ± 23.4 | 84.6 ± 22.2 | 81.2 ± 24.1 | <0.001 |
| Hemoglobin (g/dl) | 12.1 ± 2.1 | 12.2 ± 2.1 | 12.0 ± 2.2 | <0.001 |
| Hypertension | 1545 (63.8%) | 648 (63.2%) | 897 (64.3%) | 0.61 |
| CKD | 1393 (57.5%) | 608 (59.3%) | 785 (56.2%) | 0.15 |
| Heart failure | 2123 (87.7%) | 912 (88.9%) | 1211 (86.8%) | 0.13 |
| Diabetes | 721 (29.8%) | 328 (32.0%) | 393 (28.2%) | 0.05 |
| COPD | 14 (0.6%) | 3 (0.3%) | 11 (0.8%) | 0.19 |
| OSA | 222 (9.2%) | 89 (8.7%) | 133 (9.5%) | 0.52 |
| SLE | 25 (1.0%) | 14 (1.4%) | 11 (0.8%) | 0.24 |
| Scleroderma | 2 (0.9%) | 14 (1.4%) | 8 (0.6%) | 0.07 |
| TAPSE (cm) | 1.7 ± 0.6 | 1.6 ± 0.5 | 1.8 ± 0.6 | <0.001 |
| TRV (m/s) | 3.1 ± 0.7 | 3.3 ± 0.7 | 2.8 ± 0.5 | <0.001 |
| LVEF (IQR) | 50.0 (30.0) | 48.0 (31.0) | 50.0 (29.0) | 0.07 |
| Right Atrial Pressure (mmHg) | <0.001 | |||
| 3 | 1142 (49.7%) | 388 (39.5%) | 754 (57.4%) | |
| 8 | 528 (23.0%) | 254 (25.8%) | 274 (20.9%) | |
| 15 | 627 (27.3%) | 341 (34.7%) | 286 (21.8%) | |
| Left atrial dimension (cm) | 4.4 ± 0.9 | 4.4 ± 0.9 | 4.4 ± 0.8 | 0.32 |
| Diastolic Dysfunction | <0.001 | |||
| Normal | 92 (6.9%) | 28 (5.0%) | 64 (8.2%) | |
| Grade 1 | 656 (49.1%) | 246 (44.2%) | 656 (49.1%) | |
| Grade 2 | 135 (10.1%) | 47 (8.5%) | 91 (11.3%) | |
| Grades 3–4 | 452 (33.9%) | 235 (42.3%) | 223 (27.7%) | |
| RV function | <0.001 | |||
| Normal | 1347 (55.8%) | 401 (39.2%) | 946 (68.0%) | |
| Mild decrease | 522 (21.6%) | 263 (25.7%) | 259 (18.6%) | |
| Moderate decrease | 392 (16.2%) | 257 (25.1%) | 135 (9.7%) | |
| Severe decrease | 155 (6.4%) | 103 (10.1%) | 52 (3.7%) | |
| RV dimension, mid (cm) | 3.2 ± 0.9 | 3.4 ± 0.9 | 3.0 ± 0.9 | <0.001 |
Abbreviations: PVR, pulmonary vascular resistance; eGFR, estimated glomerular filtration rate; BMI, body mass index; bpm, beats per minute; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnea; SLE, systemic lupus erythematosus; TAPSE, tricuspid annular systolic plane excursion; TRV, tricuspid regurgitant velocity; LVEF, left ventricular ejection fraction; RV, right ventricular.
We first determined the association of each with PH-PVR using univariate logistic regression. We then adjusted for age, race, sex, and diabetes in multivariable logistic regression models. To investigate whether eGFR or heart failure modified the association between any parameter and PH-PVR, we repeated regression analyses with the addition of interaction terms, first for eGFR and then for heart failure. Parameters with a significant interaction p-value for eGFR were further stratified by eGFR: <30 ml/min/1.73 m2, 30–59 ml/min/1.73 m2, and ≥60 ml/min/1.73 m2. Parameters for which heart failure significantly modified the association with PH-PVR were stratified by the presence or absence of heart failure diagnosis.
To construct the risk score to screen for PH-PVR, we randomly divided the study cohort into training (70%) and validation (30%) cohorts. We excluded left ventricular diastolic function and mid right ventricular diameter due to data missingness, but retained them as auxiliary variables for multiple imputation (Supplemental Material). Using multiple imputations by chained equations (MICE) R package (version 3.11.0), we generated 10 imputed datasets. To facilitate inclusion in risk score calculations, we used the cutpoint R package (version 1.0.32) to select the optimal cutpoint for continuous variables which maximizes Youden’s index (sensitivity + specificity − 1). We applied Least Absolute Shrinkage Selector Operator (LASSO) regression to select candidate variables within each of the 10 imputed training datasets. Based on prior analyses,11,12 we included candidate variables selected in >50% of the imputed training datasets in the final risk score. Each of these variables were included in a logistic regression model with PH-PVR as the outcome to generate odds ratios (OR); we assigned points to each variable by dividing each OR by the lowest OR. Since two variables included in the risk score were significantly modified by either eGFR (hemoglobin) or heart failure diagnosis (TRV), we also constructed a weighted risk score which modified the point total assigned to these variables based on the presence of CKD or heart failure diagnosis. We divided the scores into low risk (≤5 points), intermediate risk (6–10 points), and high risk (>10 points) categories.
Using the low-risk category as the referent, we used unadjusted and age-adjusted logistic regression to determine the OR for PH-PVR based on the risk score category in the validation cohort. In sensitivity analyses, we evaluated the risk score model performance after limiting the validation cohort to just patients with heart failure or CKD; we also assessed model performance in a validation cohort without imputation for data missingness by assigning zero points for any missing value. All statistical analyses were conducted using R (version 3.6.3).
To evaluate the association of the risk score classification with mortality, we compared 3-year survival curves between risk classification groups using the log rank test in the un-imputed data cohort. We then evaluated the unadjusted, age-adjusted, and fully adjusted (age, sex, race, and diabetes diagnosis) associations with mortality. Since the survival curves violated the proportional hazards assumption, we included an interaction term for time.
RESULTS
The consort diagram for the study population is depicted in Figure 1. The clinical characteristics in the total study cohort stratified by PVR phenotype is presented in Table 1. The clinical characteristics stratified by each hemodynamic PH subtype is presented in the Supplemental Materials. The median number of days between echocardiogram and invasive hemodynamic assessment was −2.0 days (interquartile range −12.0 days, 0 days). Of the 2422 patients in the volume overload cohort, 1026 (42%) of patients had PH with an elevated PVR. PH-PVR was more common in women and persons of Black race. Among the differences in clinical parameters, heart rate at the time of right heart catheterization tended to be higher and BMI was lower in persons with PH-PVR. Hemoglobin was slightly but significantly higher in the PH-PVR cohort. Key differences in echocardiography measurements included a higher prevalence of abnormal right atrial pressure and right ventricular function in persons with PH-PVR. Severe diastolic dysfunction was also more common in PH-PVR. TAPSE was significantly lower and TRV significantly higher in persons with PH-PVR.
Figure 1.
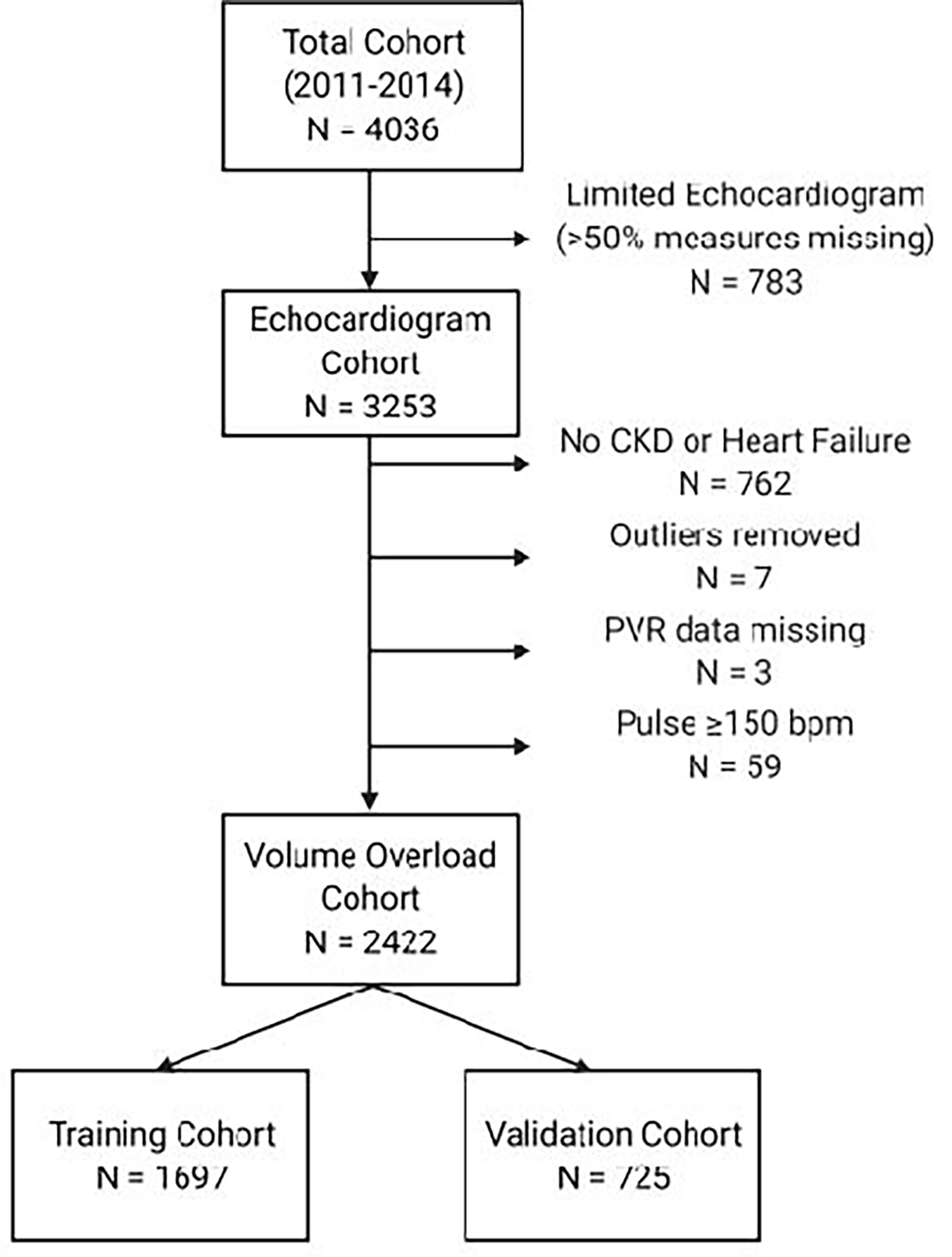
Study Consort Diagram.
Abbreviations: CKD, chronic kidney disease; PVR, pulmonary vascular resistance; bpm, beats per minute.
The unadjusted and adjusted OR for PH-PVR for each candidate risk factor is presented in Table 2. In adjusted analyses, the echocardiography measurements most strongly associated with PH-PVR were TRV and right ventricular function. Clinical parameters associated with PH-PVR included BMI, heart rate, and hemoglobin. Figure 2 depicts the factors related to PH-PVR that were significantly modified by either eGFR (Figure 2A) or heart failure (Figure 2B) diagnosis. The most notable interactions included hemoglobin (OR increased with worsening CKD) and TRV (OR decreased with heart failure diagnosis). The risk factors selected by LASSO regression with assigned point values are listed in Table 3 for both the standard risk score and the weighted risk score which accounts for the significant interactions between TRV and heart failure and between hemoglobin and CKD.
Table 2.
Association of Clinical Parameters and Echocardiography Measurements with PH-PVR Phenotype
| Characteristic | OR (95% CI) | P-value | aORa (95% CI) | P-value |
|---|---|---|---|---|
| Age (per year) | 1.00 (0.99–1.00) | 0.69 | N/A | N/A |
| Women | 1.73 (1.47–2.03) | <0.001 | N/A | N/A |
| Race | ||||
| White | 1 (ref) | N/A | N/A | N/A |
| Black | 1.80 (1.50–2.16) | <0.001 | N/A | N/A |
| Other | 1.84 (1.19–2.86) | 0.006 | N/A | N/A |
| BMI (per kg/m2) | 0.99 (0.98–1.00) | 0.04 | 0.98 (0.97–0.99) | 0.001 |
| Systolic Blood Pressure (per 10 mmHg) |
0.99 (0.96–1.03) | 0.71 | 0.96 (0.92–1.00) | 0.07 |
| Heart Rate (per 10 bpm) | 1.06 (1.03–1.10) | 0.001 | 1.06 (1.02–1.10) | 0.002 |
| Hemoglobin (per 1 g/L increase) | 1.07 (1.03–1.11) | <0.001 | 1.12 (1.08–1.17) | <0.001 |
| TAPSE (per 1 cm increase) | 0.48 (0.40–0.57) | <0.001 | 0.43 (0.35–0.51) | <0.001 |
| TRV (per 1 m/s increase) | 5.53 (4.51–6.85) | <0.001 | 5.42 (4.39–6.74) | <0.001 |
| LVEF (per 10% increase) | 0.94 (0.90–0.99) | 0.03 | 0.89 (0.84–0.94) | <0.001 |
| Right Atrial Pressure (mmHg) | ||||
| 3 | 1 (ref) | N/A | 1 (ref) | N/A |
| 8 | 1.80 (1.46–2.22) | <0.001 | 1.93 (1.56–2.40) | <0.001 |
| 15 | 2.32 (1.90–2.83) | <0.001 | 2.63 (2.14–3.25) | <0.001 |
| Left atrial dimension (per 1 cm increase) |
0.95 (0.86–1.04) | 0.32 | 1.07 (0.96–1.19) | 0.20 |
| Diastolic Dysfunction | ||||
| Normal | 1 (ref) | N/A | 1 (ref) | N/A |
| Grade 1 | 1.37 (0.86–2.23) | 0.19 | 1.36 (0.84–2.26) | 0.22 |
| Grade 2 | 1.22 (0.69–2.17) | 0.49 | 1.32 (0.74–2.40) | 0.35 |
| Grades 3–4 | 2.68 (1.54–4.06) | <0.001 | 2.94 (1.80–4.91) | <0.001 |
| RV function | ||||
| Normal | 1 (ref) | N/A | 1 (ref) | N/A |
| Mild decrease | 2.40 (1.95–2.95) | <0.001 | 2.92 (2.34–3.65) | <0.001 |
| Moderate decrease | 4.49 (3.54–5.71) | <0.001 | 5.60 (4.34–7.27) | <0.001 |
| Severe decrease | 4.67 (3.44–6.69) | <0.001 | 5.97 (4.13–8.71) | <0.001 |
| RV dimension, mid (cm) | 1.59 (1.40–1.81) | <0.001 | 1.73 (1.51–1.98) | <0.001 |
Adjusted for demographics (age, race, sex), diabetes
Abbreviations: BMI, body mass index; TAPSE, tricuspid annular systolic plane excursion; TRV, tricuspid regurgitant velocity; LVEF, left ventricular ejection fraction; RV, right ventricular.
Figure 2.
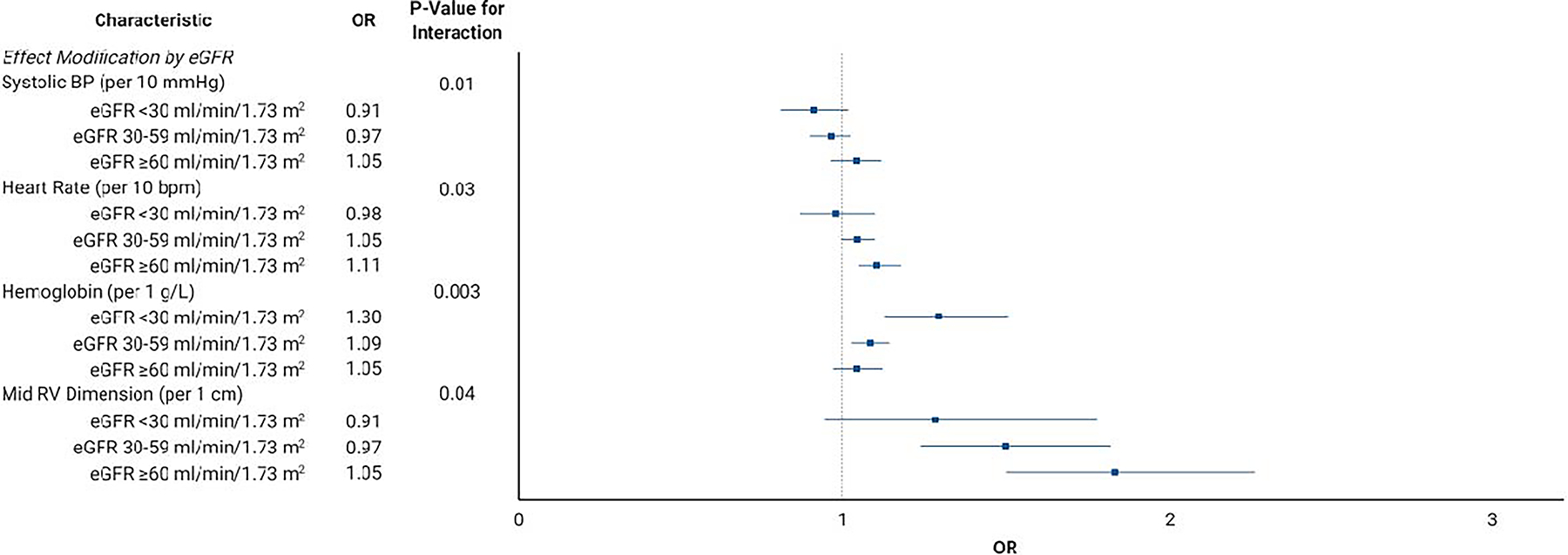
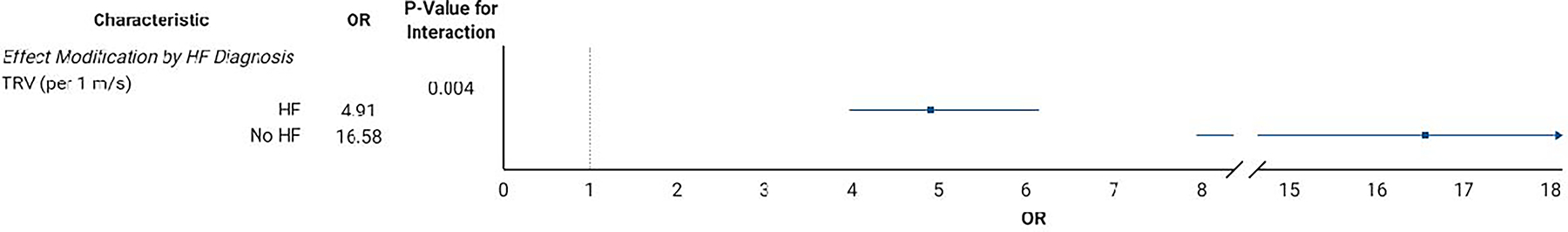
Effect Modification by (A) Estimated Glomerular Filtration Rate and (B) Heart Failure Diagnosis.
Abbreviations: eGFR, estimated glomerular filtration rate; bpm, beats per minute; RV, right ventricular; HF, heart failure; TRV, tricuspid regurgitant velocity; OR, odds ratio.
Table 3.
Standard and Weighted Risk Scores
| Risk Factor | Standard Score | Weighted Score | ||
|---|---|---|---|---|
| Strata | Points | Strata | Points | |
| Sex | Female | 2 | Female | 2 |
| Male | 0 | Male | 0 | |
| Race | Black Race | 1.5 | Black Race | 1.5 |
| Non-Black, Non-White Race | 1 | Non-Black, Non-White Race | 1 | |
| White Race | 0 | White Race | 0 | |
| TRV | ≥3.2 m/s | 4 | ≥3.2 m/s + no HF history | 8 |
| ≥3.2 m/s + HF history | 4 | |||
| <3.2 m/s | 0 | <3.2 m/s | 0 | |
| TAPSE | ≤1.65 cm | 1.5 | ≤1.65 cm | 1.5 |
| >1.65 cm | 0 | >1.65 cm | 0 | |
| RV Function | Moderate-Severely Reduced | 2.5 | Moderate-Severely Reduced | 2.5 |
| Mildly Reduced | 1.5 | Mildly Reduced | 1.5 | |
| Normal | 0 | Normal | 0 | |
| Right Atrial Pressure | 15 mmHg | 1.5 | 15 mmHg | 1.5 |
| 8 mmHg | 1 | 8 mmHg | 1 | |
| 3 mmHg | 0 | 3 mmHg | 0 | |
| LVEF | >55% | 1 | >55% | 1 |
| ≤55% | 0 | ≤55% | 0 | |
| Heart Rate | ≥60 bpm | 1.5 | ≥60 bpm | 1.5 |
| <60 bpm | 0 | <60 bpm | 0 | |
| BMI | <30 kg/m2 | 1 | <30 kg/m2 | 1 |
| ≥30 kg/m2 | 0 | ≥30 kg/m2 | 0 | |
| Hemoglobin | ≥11.2 g/L | 1.5 | ≥11.2 g/L + CKD | 2 |
| ≥11.2 g/L + no CKD | 1.5 | |||
| <11.2 g/L | 0 | <11.2 g/L | 0 | |
| Maximum Score | 18 | 22.5 | ||
| Low Risk ≤5 points, Intermediate Risk 6–10 points, High Risk >10 points | ||||
Abbreviations: BMI, body mass index; TAPSE, tricuspid annular systolic plane excursion; TRV, tricuspid regurgitant velocity; LVEF, left ventricular ejection fraction; RV, right ventricular.
Table 4 presents the unadjusted and age-adjusted OR for PH-PVR stratified by the standard and weighted risk scores in the validation cohort. The age-adjusted OR was nominally higher for the weighted risk score. In sensitivity analyses, the risk score performed similarly in both the heart failure and CKD cohorts (Supplementary Materials). The association diminished when we assigned a score of zero for missing risk factors (Supplementary Materials).
Table 4.
Association of Standard and Weighted Risk Scores with PH-PVR Phenotype
| Risk Score | %Patients with Score | Unadjusted OR (95% CI) | P Value | Age-Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|---|
| Standard Risk Score | |||||
| ≤5 (Low) |
164 (23%) | 1 (ref) | N/A | 1 (ref) | N/A |
| 6–10 (Intermediate) | 353 (49%) | 3.89 (2.28–6.64) | <0.001 | 3.94 (2.31–6.72) | <0.001 |
| >10 (High) |
208 (29%) | 11.52 (6.43–20.63) | <0.001 | 11.69 (6.54–20.92) | <0.001 |
| Weighted Risk Score | |||||
| ≤5 (Low) |
142 (20%) | 1 (ref) | N/A | 1 (ref) | N/A |
| 6–10 (Intermediate) | 364 (50%) | 4.68 (2.49–8.78) | <0.001 | 4.71 (2.51–8.85) | <0.001 |
| >10 (High) |
219 (30%) | 13.68 (6.87–27.23) | <0.001 | 13.80 (6.92–27.48) | <0.001 |
Abbreviations: OR, odds ratio; CI, confidence interval.
The survival curve stratified by risk score for the study cohort without data imputation is presented in Figure 3. In both unadjusted and adjusted analyses, the intermediate- and high-risk scores associated with a significantly higher risk of 3-year mortality (Table 5).
Figure 3.
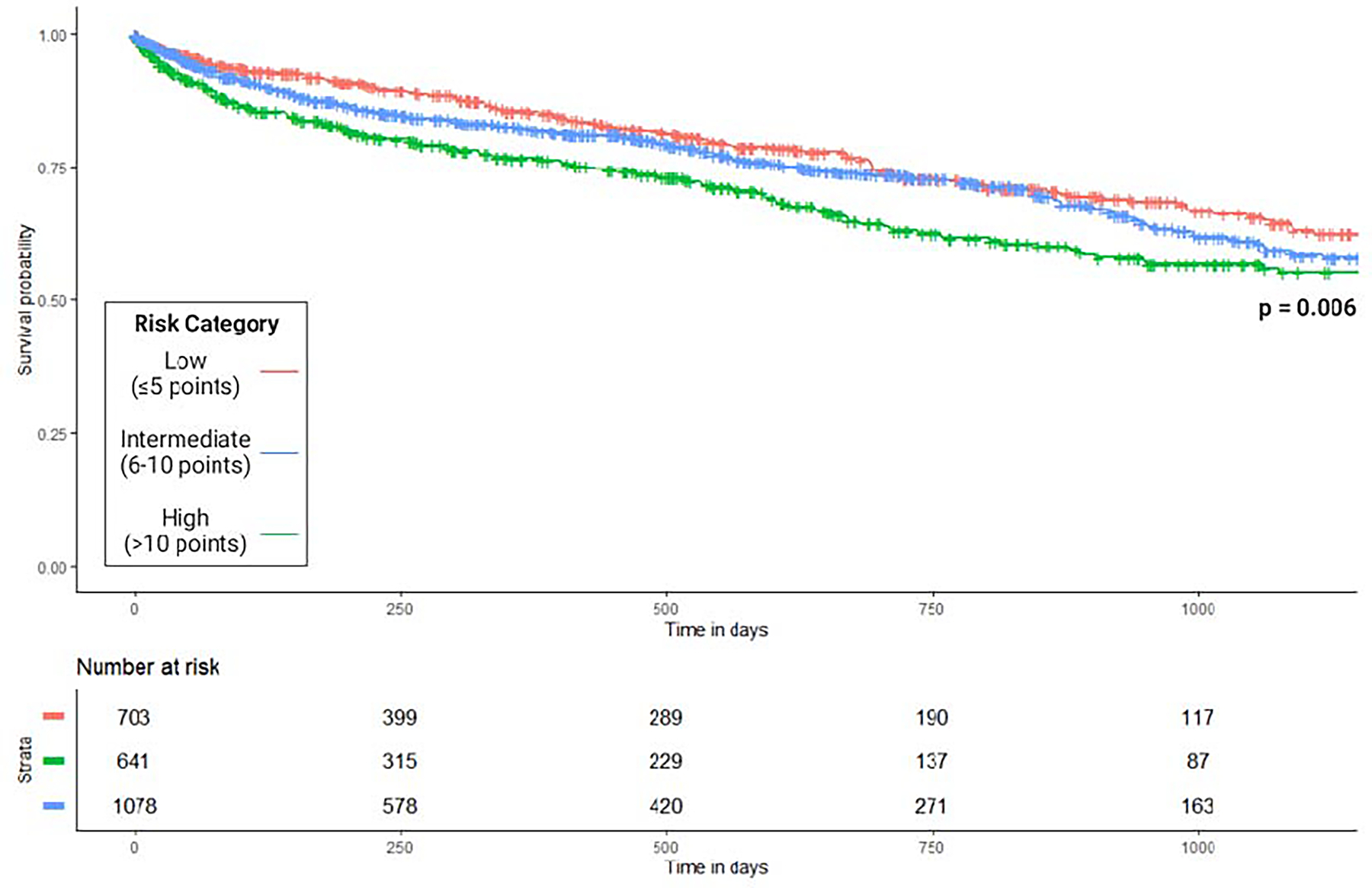
Kaplan-Meier Survival Curve Stratified by Standard Risk Score Classification.
Table 5.
Association of the Standard Risk Score with 3-Year All-Cause Mortality without Data Imputation
| Risk Category | Unadjusted OR (95% CI) | P Value | Age-Adjusted OR (95% CI) | P Value | Adjusted OR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| ≤5 (Low) |
1 (ref) | N/A | 1 (ref) | N/A | 1 (ref) | N/A |
| 6–10 (Intermediate) |
1.31 (1.09–1.58) | 0.004 | 1.37 (1.13–1.65) | 0.001 | 1.39 (1.15–1.67) | 0.001 |
| >10 (High) |
1.68 (1.37–2.06) | <0.001 | 1.79 (1.46–2.20) | <0.001 | 1.85 (1.50–2.27) | <0.001 |
Abbreviations: HR, hazard ratio; CI, confidence interval. Adjusted for age, sex, race, and diabetes mellitus diagnosis.
DISCUSSION
In a cohort of 2422 patients with either CKD or heart failure, a risk score derived from clinical parameters and echocardiography measurements identified patients with PH-PVR with moderate accuracy and was associated with all-cause mortality. A weighted risk score accounting for select interactions between eGFR or heart failure and factors included in the risk score nominally increased the association with PH-PVR above the standard risk score. This study is the first to evaluate the performance of noninvasive risk factors as a method to screen for PH-PVR in a population restricted to CKD or heart failure. Due to the high prevalence of PH due to volume overload in this population and the potential for alternative therapeutic approaches for patients with elevated PVR, such a noninvasive screening tool would allow clinicians to identify patients who may not improve with volume management alone to facilitate earlier definitive diagnosis by right heart catheterization and application of targeted therapies.
Echocardiography has been used to screen for elevated PVR in other populations, including measurements not available in our study such as right ventricular outflow tract velocity time integral or indices of pulmonary pressure reflection.9,10,13,14 In this study, TRV, right ventricular function, and TAPSE strongly associated with PH-PVR. These markers of right ventricular systolic function each variably associate with outcomes in PH.10,15–19 In contrast, an abnormal left ventricular ejection fraction associated with lower odds of PH-PVR; this finding likely reflects the substantial prevalence of isolated post-capillary PH in patients with heart failure with a reduced ejection fraction.20 This combination of left- and right-sided echocardiography measurements improved the accuracy of screening for PH-PVR.
Select clinical parameters significantly associated with PH-PVR in this study. Consistent with prior studies,21 we report an increased odds of PH-PVR for women. While idiopathic PAH predominantly affects persons of White race, persons of Black race are more likely to present with PAH associated with other diseases.22 This important distinction likely underlies why Black race and other non-White races associated with an increased odds of PH-PVR in our study cohort. Heart rate less than 60 beats-per-minute associated with a lower risk of PH-PVR in this study. While not previously applied to discern hemodynamic PH subtypes, a lower resting heart rate associates with a reduced risk of a composite of death or lung transplant in persons with PAH.23,24 While BMI exhibits a complicated and at times paradoxical relationship with PH,23–25 we report an increased risk of PH-PVR at BMI <30 kg/m2. While anemia serves as a risk factor for mortality in persons with PH,26,27 the strong link with heart failure may explain the inverse association between lower hemoglobin less than 11.2 g/L and PH-PVR uncovered in our study.28 Compared to other populations, these clinical parameters may play a larger role in discerning PVR elevation in persons at risk for volume overload.
We acknowledge several limitations to the study design. First, all data were all collected within the context of clinical care. This limitation enriches both the overall prevalence of PH and the proportion of patients with elevated PVR in the study population. Additionally, we cannot assess interobserver variability for study measurements. Echocardiogram and right heart catherization may be separated by a meaningful intervention which influences the relationship between these two diagnostic modalities. The echocardiograms in this study did not report measurements known to associate with PVR, including right ventricular outflow tract acceleration velocity-time integral or the presence of notching in the pulmonary artery outflow signal associates with pre-capillary PH.10,29 While we eliminated limited echocardiograms, select measurements were still absent from a proportion of echocardiograms. The absence of these measurements does not imply the absence of pathology.30 Another limitation introduced by the use of clinical data is the definition of CKD based on a median of 6 months of creatinine data; rapid progression of CKD or acute kidney injury prior to right heart catheterization may lead to misclassification. Lastly, despite our robust sample size, external validation better ensures generalizability. The ideal cohort for such validation is an asymptomatic population with prospective data collection; however, the invasive nature of right heart catheterization limits the feasibility of large studies in such a cohort.
This study also has strengths. The large population with granular data collection of clinical parameters, echocardiography, and right heart catherization data provides a robust study cohort. To our knowledge, this study is the first to noninvasively screen for elevated PVR in a population with CKD. A risk score to screen for elevated PVR has numerous clinical and research applications, including earlier suspicion for PH-PVR which facilitates timely diagnosis and application of targeted therapies; improved pre-transplant assessment of kidney transplant candidates; and selection of enriched populations for clinical studies of PH in CKD and/or heart failure. In summary, we derived a risk score from clinical parameters and echocardiography measurements to identify patients at high risk for PH due to a PVR phenotype with moderate accuracy in a population with a high prevalence of volume overload.
Supplementary Material
Footnotes
DISCLOSURES
The authors report no relevant financial disclosures or conflicts of interest related to this study. This study was funded by the Duke University Division of Nephrology. Dr. Edmonston is supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health under award number K23HL155734.
References
- 1.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Group ESCSD. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 2.Assad TR, Hemnes AR, Larkin EK, Glazer AM, Xu M, Wells QS, Farber-Eger EH, Sheng Q, Shyr Y, Harrell FE, Newman JH, Brittain EL. Clinical and Biological Insights Into Combined Post- and Pre-Capillary Pulmonary Hypertension. J Am Coll Cardiol 2016;68:2525–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edmonston DL, Parikh KS, Rajagopal S, Shaw LK, Abraham D, Grabner A, Sparks MA, Wolf M. Pulmonary Hypertension Subtypes and Mortality in CKD. Am J Kidney Dis 2020;75(5):713–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghio S, Crimi G, Temporelli PL, Traversi E, La Rovere MT, Cannito A, Vizza D, Scelsi L, Raineri C, Guazzi M, Oltrona Visconti L. Haemodynamic effects of an acute vasodilator challenge in heart failure patients with reduced ejection fraction and different forms of post-capillary pulmonary hypertension. Eur J Heart Fail 2018;20:725–734. [DOI] [PubMed] [Google Scholar]
- 5.Packer M, McMurray J, Massie BM, Caspi A, Charlon V, Cohen-Solal A, Kiowski W, Kostuk W, Krum H, Levine B, Rizzon P, Soler J, Swedberg K, Anderson S, Demets DL. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J Card Fail 2005;11:12–20. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Villa F, Farrero M, Sionis A, Castel A, Roig E. Therapy with sildenafil or bosentan decreases pulmonary vascular resistance in patients ineligible for heart transplantation because of severe pulmonary hypertension. J Heart Lung Transplant 2010;29:817–818. [DOI] [PubMed] [Google Scholar]
- 7.Edmonston DL, Rajagopal S, Wolf M. Echocardiography to Screen for Pulmonary Hypertension in CKD. Kidney Int Rep 2020;5:2275–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanzarini L, Fontana A, Campana C, Klersy C. Two simple echo-Doppler measurements can accurately identify pulmonary hypertension in the large majority of patients with chronic heart failure. J Heart Lung Transplant 2005;24:745–754. [DOI] [PubMed] [Google Scholar]
- 9.Abbas AE, Franey LM, Marwick T, Maeder MT, Kaye DM, Vlahos AP, Serra W, Al-Azizi K, Schiller NB, Lester SJ. Noninvasive assessment of pulmonary vascular resistance by Doppler echocardiography. J Am Soc Echocardiogr 2013;26:1170–1177. [DOI] [PubMed] [Google Scholar]
- 10.Opotowsky AR, Ojeda J, Rogers F, Prasanna V, Clair M, Moko L, Vaidya A, Afilalo J, Forfia PR. A simple echocardiographic prediction rule for hemodynamics in pulmonary hypertension. Circ Cardiovasc Imaging 2012;5:765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh AYY, Budd M, Deng D, Gadawska I, Cote HCF. A Monochrome Multiplex Real-Time Quantitative PCR Assay for the Measurement of Mitochondrial DNA Content. J Mol Diagn 2018;20:612–620. [DOI] [PubMed] [Google Scholar]
- 12.Austin PC, Lee DS, Ko DT, White IR. Effect of Variable Selection Strategy on the Performance of Prognostic Models When Using Multiple Imputation. Circ Cardiovasc Qual Outcomes 2019;12:e005927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bech-Hanssen O, Lindgren F, Selimovic N, Rundqvist B. Echocardiography can identify patients with increased pulmonary vascular resistance by assessing pressure reflection in the pulmonary circulation. Circ Cardiovasc Imaging 2010;3:424–432. [DOI] [PubMed] [Google Scholar]
- 14.Abbas AE, Fortuin FD, Schiller NB, Appleton CP, Moreno CA, Lester SJ. A simple method for noninvasive estimation of pulmonary vascular resistance. J Am Coll Cardiol 2003;41(6):1021–7 [DOI] [PubMed] [Google Scholar]
- 15.Mazurek JA, Vaidya A, Mathai SC, Roberts JD, Forfia PR. Follow-up tricuspid annular plane systolic excursion predicts survival in pulmonary arterial hypertension. Pulm Circ 2017;7:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006;174:1034–1041. [DOI] [PubMed] [Google Scholar]
- 17.Gopal DM, Doldt B, Finch K, Simms RW, Farber HW, Gokce N. Relation of novel echocardiographic measures to invasive hemodynamic assessment in scleroderma-associated pulmonary arterial hypertension. Arthritis Care Res (Hoboken) 2014;66:1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Austin C, Alassas K, Burger C, Safford R, Pagan R, Duello K, Kumar P, Zeiger T, Shapiro B. Echocardiographic assessment of estimated right atrial pressure and size predicts mortality in pulmonary arterial hypertension. Chest 2015;147:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin C, Burger C, Kane G, Safford R, Blackshear J, Ung R, Ray J, Alsaad A, Alassas K, Shapiro B. High-risk echocardiographic features predict mortality in pulmonary arterial hypertension. Am Heart J 2017;189:167–176. [DOI] [PubMed] [Google Scholar]
- 20.Gerges M, Gerges C, Pistritto AM, Lang MB, Trip P, Jakowitsch J, Binder T, Lang IM. Pulmonary Hypertension in Heart Failure. Epidemiology, Right Ventricular Function, and Survival. Am J Respir Crit Care Med 2015;192:1234–1246. [DOI] [PubMed] [Google Scholar]
- 21.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010;137:376–387. [DOI] [PubMed] [Google Scholar]
- 22.Al-Naamani N, Paulus JK, Roberts KE, Pauciulo MW, Lutz K, Nichols WC, Kawut SM. Racial and ethnic differences in pulmonary arterial hypertension. Pulm Circ 2017;7:793–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hildenbrand FF, Fauchere I, Huber LC, Keusch S, Speich R, Ulrich S. A low resting heart rate at diagnosis predicts favourable long-term outcome in pulmonary arterial and chronic thromboembolic pulmonary hypertension. A prospective observational study. Respir Res 2012;13:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouchery-Bardet H, Creveuil C, Bauer F, Beygui F, Champ-Rigot L, Saloux E, Labombarda F, Roule V, Sabatier R, Legallois D, Zalcman G, Bergot E, Milliez P. Heart rate and risk of death among patients with Pulmonary Hypertension: A 12-lead ECG analysis. Respir Med 2017;132:42–49. [DOI] [PubMed] [Google Scholar]
- 25.Frank RC, Min J, Abdelghany M, Paniagua S, Bhattacharya R, Bhambhani V, Pomerantsev E, Ho JE. Obesity Is Associated With Pulmonary Hypertension and Modifies Outcomes. J Am Heart Assoc 2020;9:e014195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura A, Kasamatsu N, Hashizume I, Shirai T, Hanzawa S, Momiki S, Sasaki K, Kinoshita M, Okada O, Tatsumi K, Kuriyama T. Effects of hemoglobin on pulmonary arterial pressure and pulmonary vascular resistance in patients with chronic emphysema. Respiration 2000;67:502–506. [DOI] [PubMed] [Google Scholar]
- 27.Krasuski RA, Hart SA, Smith B, Wang A, Harrison JK, Bashore TM. Association of anemia and long-term survival in patients with pulmonary hypertension. Int J Cardiol 2011;150:291–295. [DOI] [PubMed] [Google Scholar]
- 28.Anand IS, Gupta P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018;138:80–98. [DOI] [PubMed] [Google Scholar]
- 29.Opotowsky AR, Clair M, Afilalo J, Landzberg MJ, Waxman AB, Moko L, Maron BA, Vaidya A, Forfia PR. A simple echocardiographic method to estimate pulmonary vascular resistance. Am J Cardiol 2013;112:873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Leary JM, Assad TR, Xu M, Farber-Eger E, Wells QS, Hemnes AR, Brittain EL. Lack of a Tricuspid Regurgitation Doppler Signal and Pulmonary Hypertension by Invasive Measurement. J Am Heart Assoc 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


