SUMMARY
Ancient DNA has revealed multiple episodes of admixture in human prehistory during geographic expansions associated with cultural innovations. One important example is the expansion of Neolithic agricultural groups out of the Near East into Europe and their consequent admixture with Mesolithic hunter-gatherers.1–4 Ancient genomes from this period provide an opportunity to study the role of admixture in providing new genetic variation for selection to act upon, and also to identify genomic regions that resisted hunter-gatherer introgression and may thus have contributed to agricultural adaptations. We used genome-wide DNA from 677 individuals spanning Mesolithic and Neolithic Europe to infer ancestry deviations in the genomes of admixed individuals and to test for natural selection after admixture by testing for deviations from a genome-wide null distribution. We find that the region around the pigmentation-associated gene SLC24A5 shows the greatest overrepresentation of Neolithic local ancestry in the genome (|Z| = 3.46). In contrast, we find the greatest overrepresentation of Mesolithic ancestry across the major histocompatibility complex (MHC; |Z| = 4.21), a major immunity locus, which also shows allele frequency deviations indicative of selection following admixture (p = 1 × 10−56). This could reflect negative frequency-dependent selection on MHC alleles common in Neolithic populations or that Mesolithic alleles were positively selected for and facilitated adaptation in Neolithic populations to pathogens or other environmental factors. Our study extends previous results that highlight immune function and pigmentation as targets of adaptation in more recent populations to selection processes in the Stone Age.
In brief
Davy et al. study natural selection after admixture between hunter-gatherer and Neolithic farmer-associated individuals in ancient Europe. In later populations, they find an excess of Early Neolithic ancestry at a pigmentation-associated gene and of Mesolithic ancestry at the MHC locus, suggesting adaptive admixture.
RESULTS AND DISCUSSION
Despite the evidence from studies of ancient DNA that admixture among Holocene populations is ubiquitous, less is known about how admixture provided variation for natural selection to act upon during transitional periods. Given that Holocene admixture was often associated with dramatic migrations or changes in lifestyle, we might expect an important role for adaptive introgression. Perhaps the best-studied example of ancient admixture is in the Mesolithic-Neolithic transition in Europe. As Early Neolithic groups expanded across Europe from Anatolia in the period from 10,000 to 5,000 years ago,1–3,5–8 they admixed with local Mesolithic hunter-gatherers, and by 6,000 years ago derived 20%–30% of their ancestry from these local groups.1,4,5,9 The admixed Neolithic ancestry thus found itself in a new cultural and geographical landscape, with a hypothesized increase in infectious disease load due to population density and proximity to domestic animals.10
Previous studies of natural selection in the European Neolithic have either compared allele frequencies or haplotype structure with other ancient and modern populations.5,11–14 However, no study to date has specifically attempted to identify adaptive admixture, despite the fact that this mode of adaptation has been repeatedly observed in humans. A recent study identified two optimal approaches to detect adaptive admixture in data from present-day populations, based on allele frequencies and local ancestry.15 Here, we adapt these two approaches to ancient populations with a new framework to obtain p values from genome-wide null distributions, investigating adaptive admixture in 677 Mesolithic and Early and Middle Neolithic (admixed Neolithic) individuals.
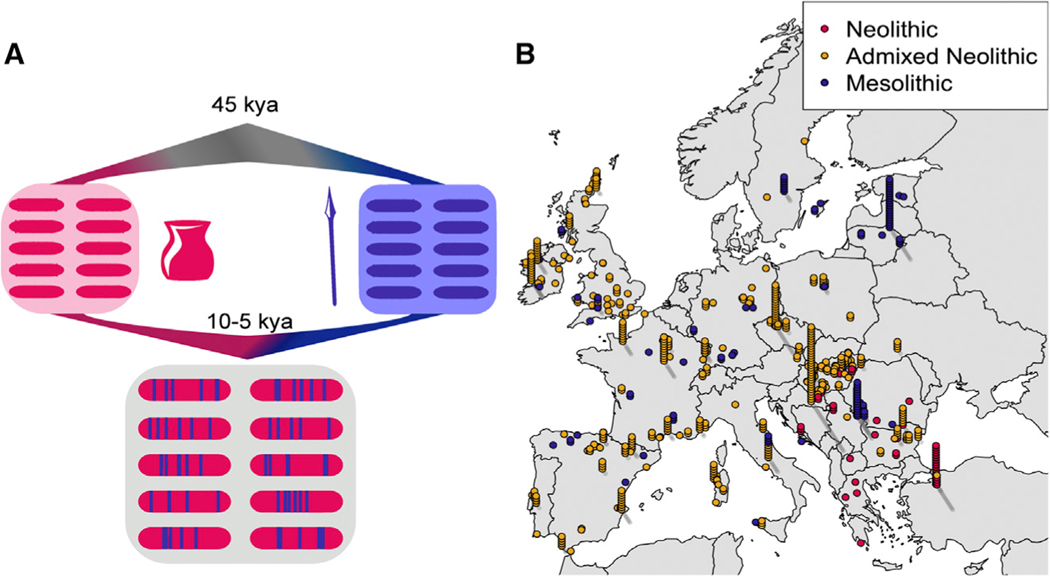
We clustered individuals with genome-wide ancient DNA data from Europe and Anatolia1,2,4,5,9,16–39 in the past ~15,000 years and assigned them to one of three groups: 125 Mesolithic and Upper Palaeolithic hunter-gatherer ancestry individuals, 55 Early Neolithic individuals from Anatolia and the Balkans without evidence for substantial hunter-gatherer admixture, and later admixed Neolithic individuals with substantial Mesolithic admixture. In total, our analysis contains 677 ancient individuals, spanning 7,500 years across West Eurasia (Figures 1A, 1B, and S1).
Figure 1. Admixture model and geographic distribution of Neolithic and Mesolithic individuals with genome-wide ancient DNA.
(A) An illustration of the genetic history of the Neolithic-Mesolithic transition in Western Eurasia.
(B) ‘‘Casino-plot’’ of individuals included for analyses, colored by the ancestry group for which those individuals were used in this paper. For sites with multiple samples, we stack those individuals above the reported coordinates. See also Figure S1.
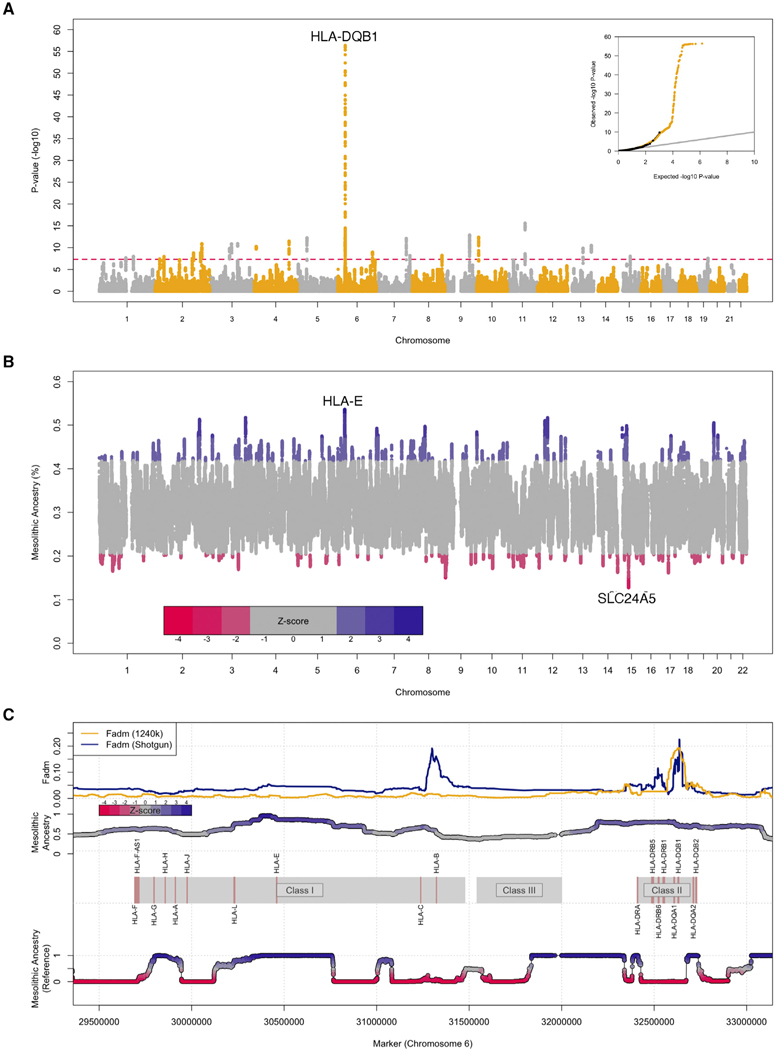
We first used an approach to find natural selection that was admixture-unaware, searching for increased differentiation as the squared allele frequency difference between populations, the f2 statistic.40 We computed the average for each SNP and 25 SNPs flanking SNPs on each side (i.e. in 51 base pair [bp] sliding windows), and obtain p values by fitting a gamma distribution to a null sample of 532 approximately independent loci, separated by at least 5 million base pairs (Mb). We observed no statistically significant outliers in the Mesolithic-Neolithic or Mesolithic-admixed Neolithic contrasts (Figure S2), which is likely due to the relatively deep divergence between these groups, i.e., variance in allele frequencies resulting from genetic drift obscuring signals of loci that are differentiated due to natural selection. However, in the Neolithic-admixed Neolithic contrast we observe a highly significant excess differentiation across the MHC region on chromosome 6, centered upon HLA-DQB1 (p = 1 × 10−21). This suggests natural selection at the MHC in the period covered by the data from Neolithic and admixed-Neolithic groups, although this analysis does not address whether that might be due to adaptive Mesolithic admixture.
To search for adaptive admixture, we applied a statistic based on previous work5 termed Fadm,15 which tests for deviations from the expected allele frequencies given the genome-wide average mixture proportions of the contributing ancestries. We again observe an excess signal across the MHC, centered upon HLA-DQB1 (p = 1 × 10−56) (Figure 2A). To confirm that our findings were not driven by ascertainment bias in the 1.2 million SNP panel,41 we analyzed the MHC region in 173 whole-genome shotgun sequences from 65 Mesolithic, 25 Neolithic, and 83 admixed Neolithic individuals4,5,8,9,17,18,25–27,30,33,39,42–45 (STAR Methods, Table S2) and observed a peak concordant to the 1240k results in both position and summary statistic stretching across the class II MHC region (Figure 2C).
Figure 2. Genome-wide significant signals of adaptive admixture.
(A) Manhattan plot of p values from the Fadm scan across the genome for deviations from expected admixed allele frequencies. Inset, quantile-quantile plot of expected and observed p values. P values were obtained by fitting a gamma distribution to a null sample of 532 approximately independent loci, separated by at least 5 million base pairs (Mb).
(B) Local ancestry deviations (LAD) in the Middle Neolithic across the genome, with top peaks of each ancestry labeled.
(C) Zoomed-in region of the MHC (chromosome 6), with statistics derived from 1240k and whole-genome shotgun data across the MHC regions I, II, and III on chromosome 6.
We next sought to quantify the direction of admixture by searching for deviations in local ancestry across the genome (local ancestry deviation [LAD]15). We inferred local ancestry in 537 admixed Middle Neolithic individuals with genome-wide SNP data using ancestryHMM,46 which estimates local ancestry in low-coverage genomic data using allele frequencies from two populations. We computed standard errors and Z scores for LAD using an approximately independent subsample of the genome-wide distribution consisting of 555 sites separated by at least 5 Mb (STAR Methods).
The greatest excess of Neolithic ancestry centered on SLC24A5 (Figures 2D and S3C), with a peak of +17.82% (|Z| = 3.46). The derived SLC24A5 allele, which is carried on the Neolithic ancestry background, is one of the two alleles which contributes most to light skin pigmentation in present-day West Eurasian-ancestry populations.47 It has previously been shown to have been at relatively high frequency in the Neolithic population and absent in the Mesolithic hunter-gatherers,5 and our results show that the selection removed hunter-gatherer ancestry at this locus in later admixed Neolithic groups.
Meanwhile, the lowest amount of Neolithic ancestry is found at the MHC region on chromosome 6. Within this locus, the region of highest Mesolithic ancestry is centered on HLA-E, with a peak excess of +23.1% (|Z| = 4.21), and a secondary peak centered upon the class II region of +17.18% (|Z| = 3.10). This region of elevated Mesolithic ancestry continues as a contiguous region which extends across the class II region of the MHC, with an average of ancestry across the entire MHC (between positions 28,477,797–33,448,354 of chromosome 6) of +9.16% (|Z| = 1.60). (Figure 2C). Bias in LAD analysis at the HLA locus has been suggested to be expected in the direction of the source ancestry with higher diversity,48 unlike what we observe here. We also run ancestryHMM on the human reference genome to test the hypothesis of biased mapping but find that the reference is inferred to have both Neolithic and Mesolithic ancestry at this locus (Figure 2C). AncestryHMM and other local ancestry approaches have not been extensively used with ancient genetic data and may be sensitive to particular properties of these data. However, the LAD results are largely concordant with the Fadm results, suggesting that the approach is robust.
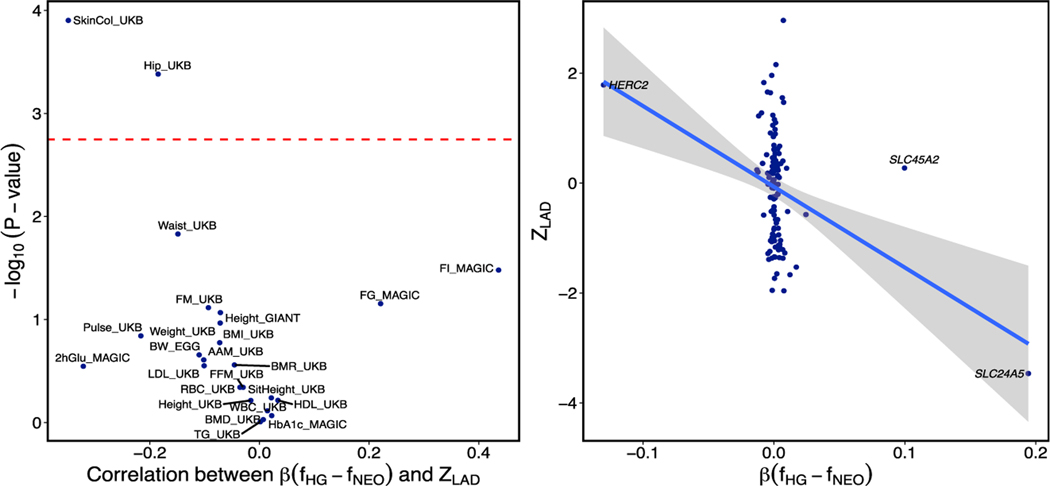
It is also possible that adaptive admixture acted on multiple variants with small effect, spread across the genome. To test for evidence of such polygenic selection, we computed the Pearson correlation between the LAD and the implied local ancestry effect size for 38 traits in the UK BioBank, using genome-wide significant SNPs thinned to be approximately independent49 (Figure 3A). We see significant evidence of correlation between trait scores and LAD in skin color (p = 1.2 × 10−4), consistent with adaptive admixture around SLC24A5. Indeed, this signal is solely driven by two loci (Figure 3B), wherein a HERC2 variant with a skew towards Mesolithic ancestry (Z = 1.8) also contributes to a lighter level of skin pigmentation alongside SLC24A5. Without these two loci, there is no significant evidence of polygenic selection (p = 0.42). We also observe a weaker but significant correlation for hip size (Figure 3A).
Figure 3. Test for polygenic adaptive admixture.
(A) Pearson correlation (p values) of polygenic traits against local ancestry.
(B) Correlation of LAD Z scores with skin color SNP effect size weighted by the signed allele frequency difference between the two source populations.
The Neolithic transition brought about drastic changes in demography, culture, and diet, as well exposure to novel pathogens and increased potential of zoonotic disease. In admixed Neolithic individuals, we found excess Neolithic farmer ancestry at the pigmentation locus SLC24A5 and excess Mesolithic ancestry at the MHC immunity locus. Previous studies also found evidence of natural selection at SLC24A5 in European populations47,50 and showed that the allele was almost fixed in Anatolian farmers and was introduced into Western Europe during the Neolithic5,12,33,51 but our study now further demonstrates that subsequent selection during the Middle Neolithic was rapid enough to result in the removal of Mesolithic ancestry across the wider locus, covering an approximately 3 Mb region. In a similar but opposite process, the MHC locus has previously been demonstrated to have undergone selection in the ancestors of present-day Europeans5,52 and specifically in Neolithic Europe.11 Here, we obtain further robust results for selection at the MHC locus corrected for multiple testing and demonstrate that this process specifically increased hunter-gatherer ancestry at the locus.
In contrast to SLC24A5, the second high-effect pigmentation variant in HERC displays an excess of Mesolithic ancestry (+10.79%, |Z| = 1.90). Together with the third high-effect pigmentation variant at SLC45A2, which arrived in Europe via later expansions from the steppe, selection on pigmentation in Europe thus targeted variants from each of the three major ancestral populations.4 This highlights the prominent role of admixture in the evolution of skin pigmentation in Western Eurasia. That this signal is not found in the allele frequency-based analysis with Fadm can likely be attributed to the small absolute change in allele frequency between our Neolithic populations, confirming recent demonstrations that local ancestry can in some cases be more powerful than allele frequency analysis for detecting selection in admixed populations.15
Evidence of selection on Mesolithic ancestry across the MHC locus highlights its role in facilitating adaptation in immunity during the Neolithic transition in Europe. One hypothesis is that this reflects the fact that Neolithic populations were expanding into environments containing pathogens to which Mesolithic populations had already adapted. This is contrary to the idea that the pathogen load in Neolithic populations was solely driven by increased population density and proximity to zoonotic vectors via animal husbandry. On the other hand, although examples of putative adaptive admixture involving the MHC have been previously described,48,53,54 no clear link between the alleles under selection within this region and a specific pathogen has been identified.
Another possibility is that this adaptation reflects negative frequency-dependent selection because pathogens adapt to the most common alleles in the population, making rare alleles beneficial.55 In particular, MHC class II genes have been suggested to experience a red-queen-like arms race between the binding ability of antigen-presenting proteins and pathogens’ ability to escape this binding and subsequent immune response.56 Under this model, HLA alleles unseen by a given pathogen will have higher fitness initially following admixture owing to rarity. Thus, selection on the minor Mesolithic ancestry could simply reflect admixture introducing rarer variants into Neolithic populations, increasing diversity at the locus. This can also be explained as selection for heterozygosity in class II genes to diversify the immunity within the population. One caveat to this interpretation is that we are pooling samples across large regions and time periods, and there is a possibility that our results reflect more localized differentiation or natural selection in regions and periods with more sampled diversity. On the other hand, we note that a similar effect has been observed in present-day populations; Cuadros-Espinoza et al.15 find adaptive admixture at the MHC, reporting, similarly, ~50% local ancestry for the minority admixing ancestry derived from west rainforest hunter-gatherers in agricultural Western Bantu speakers, echoing previous analyses.53,57
We note that the HLA appears to be a frequent target of natural selection and the processes described above are not exclusive. Extensive evidence of selection has been detected both in scans focusing on the past few millennia,5,11,14,52,58 and following introgression from archaic humans.54,59 Future studies, including whole-genome shotgun data in tandem with improved functional annotation, may shed further light on this adaptive process.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Requests for further information should be directed to and will be fulfilled by the lead contact, Pontus Skoglund (pontus.skoglund@crick.ac.uk)
Materials availability
This study did not generate new unique reagents.
Data and code availability
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request. This paper analyses existing, publicly available data. These accession numbers for the datasets are listed in Tables S1 and S2. Code from this paper used to generate all figures and analyses from allele frequency data is available at https://github.com/Tom-Davy/mesoneo_admixture.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
No experimental models were used in the study.
METHOD DETAILS
Data preparation
We first used clustering approaches on a large set of previously published1–9,16,18,24,25,27,28,30,33,37,44,45,51,71–84 individuals to identify those individuals exclusively or almost exclusively Hunter-Gatherers (Mesolithic, Western or Siberian Hunter-Gatherers [WHG, SHG]) or Neolithic ancestry (Early Neolithic, which we identify using Neolithic Anatolia as a baseline). We downloaded v50 of 10,391 individuals (3,589 ancient) from the Reich lab https://reich.hms.harvard.edu/downloadable-genotypes-present-day-and-ancient-dna-data-compiled-published-papers, accessed July 19th 2022 for the 1240K set of SNPs. We ran a first set of clustering with ADMIXTURE from K = 3 to K = 11 on all ancient individuals, removing relatives and duplicates based on assignment as such in the Reich lab compendium. Given the choice, we select the highest coverage individual or family member. We then selected individuals clustering with known examples that typify Mesolithic or Neolithic ancestry, or were clearly admixed, at K = 5, dated to >5kya, excluding siblings, parents, and duplicates. We then further filtered on age, setting a max age for Mesolithic, Neolithic and Admixed Neolithic of 12kya, 8.5kya and 8kya respectively, retaining 677 individuals, which we recluster alone with 40 individuals labeled MSL to confirm ancestry proportions and classification of ancestry (Figure S1). Using PLINK, we then make a subset of files containing only individuals fitting into either Mesolithic (EHG + WHG) or admixed Neolithic ancestry at K = 3 in this cluster, alongside a geographically and temporally conservative Neolithic cluster. We then remove sites below 99% mappability as defined on the human reference genome (hs37d5).
Confirmation of HLA signal in whole-genome shotgun data
To confirm the presence of the HLA signal, we collected a set of 99 individuals (Table S2), including some used in the original analyses, which had whole-genome shotgun genomes, and added 74 published and unpublished genomes available pre-publication under the Ft. Lauderdale principles from the AGDP (https://reich.hms.harvard.edu/ancient-genome-diversity-project).
We compile all shotgun individuals using htslib with the pileup flag into randfa format, filtering for a base quality score of 20 and a mappability quality score of 30. We thus only use this data for estimating Fadm in a restricted region comprising the MHC region. We base our defined populations on the annotation in the 1240k Reich compendium v50.0. We filter the MHC region for SNPs with a minimum allele frequency higher than 0.05, and only retain the intersect between these SNPs and the hs37d5 mappability filter for sites > 0.99, retaining 21,007 sites using BEDTools.67
Testing the hypothesis of mapping bias in the MHC region
The MHC locus can be difficult to analyze, due to the high allelic diversity, especially across the class II genes. Hence, we sought to understand if there could be any underlying bias in the reference genome that could be driving signals here (Figure 2C). We examine pairwise f2 statistics between the reference genome and our ancient populations, alongside targeting the human reference genome in a LAD analysis under the same conditions as our initial local ancestry analysis. We see no overall bias in f2 at the locus where Fadm reports selection. Furthermore, we show that the reference genome contains both Mesolithic and Neolithic ancestries across the MHC, with our top two local ancestry signals in the locus contained within different ancestries within the reference genome.
QUANTIFICATION AND STATISTICAL ANALYSIS
Detecting selective sweeps
We filtered the full set of SNPs by conditioning on observing at least 20 non-missing pseudohaploid genotypes in the Neolithic out of a maximum of 51. We apply a sliding window of 51 SNPs. We utilized the statistic, i.e. , where is the frequency of a given SNP in the admixed population and y is an expectation of allele frequency derived by the contributing ancestry proportions (AncMESOLITHIC and AncNEOLITHIC) weighted by allele frequency: ( pMESOLITHIC * AncMESOLITHIC + pNEOLITHIC * AncNEOLITHIC). AncMESOLITHIC was set to 0.3 and AncNEOLITHIC to 0.7. In all cases, the given statistic is calculated on a per-SNP basis before a sliding-window is applied across the genome with a step size of 1. We derive the f2 analyses in an identical manner. We annotate genes using the gencode v39liftover37 annotation files,60 and denote them based on the centering of the window where the center SNP is the median value of the SNP window, which is always odd-numbered.
For each analysis, we then draw a null distribution from this sliding-window whole-genome distribution, ensuring that each sliding-window datum that contributes to the null distribution is sampled with an array of SNPs that contain no positions less than 5Mb away from the previous sample. A gamma distribution is then fitted to this null distribution using the R61 package fitdistrplus v1.1–362 with the flags ““gamma”, method = “mle”, keepdata = T”’. We then derive p values for the genome-wide distribution from this null-fitted gamma distribution.
Detection of local ancestry outliers
To analyze biases in local ancestry, we bring the same panel of 677 individuals (Table S1) forward to analysis with ancestryHMM.46 We derive frequency counts for both the contributing ancestries (Mesolithic, Neolithic) and similarly obtain this for each individual admixed Neolithic sample via PLINK v1.9b.63 We then run ancestry HMM with a prior of 30% Mesolithic and 70% Neolithic ancestry, and an Ne of 10,000, which we take to be a fair assumption of Mid- to Late Neolithic effective population size,84 via the following command line: ancestry_hmm -i ../hmm.$c.input.tsv -s $hmm_input_file -a 2 0.3 0.7 -p 0 0 0.3 -p 1 35 0.7 –ne 10000. We derive Z scores first by taking a null distribution as in the analysis of LA values and calculate Z scores for the genome-wide distribution using that distribution’s mean.
Detection for biased inheritance of polygenic trait alleles
As in Mathieson and Terhorst,49 we start with 28 quantitative traits of interest, subsetting SNPs with a GWAS p value of P < 1e−8 overlapping the 1240k SNP array. We then iteratively prune for each trait, subsetting the smallest p value and removing all other associations within 250Kb. Departing from Mathieson & Terhost, we weight these pruned effect sizes by the difference in allele frequency between Mesolithic and Neolithic, sum these across the genome for each trait and test for correlation to the LAD derived in the local ancestry analysis via a Pearson correlation test.
Supplementary Material
KEY RESOURCES TABLE
Highlights.
Genomic evidence for adaptive admixture in Stone Age Europe
Excess of Neolithic farmer ancestry around the pigmentation-associated gene SLC24A5
Excess of hunter-gatherer ancestry in the major histocompatibility complex (MHC)
Post-admixture selection occurred upon variants from both admixing ancestries
ACKNOWLEDGMENTS
We thank Mateja Hajdinjak for help with analyses, and Leo Speidel and Garrett Hellenthal for helpful comments. We thank David Reich for permission to analyze the MHC region of the AGDP dataset. I.M. was funded by the NIGMS ([R35GM133708] to I.M.). P.S. was supported by the EMBO Young Investigator Programme, the Vallee Foundation, the European Research Council (grant no. 852558), the Wellcome Trust (217223/Z/19/Z), and Francis Crick Institute core funding (FC001595) from Cancer Research UK, the UK Medical Research Council, and the Wellcome Trust. This research was funded in whole, or in part, by the Wellcome Trust (FC001595 and 217223/Z/19/Z). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental information can be found online at https://doi.org/10.1016/j.cub.2023.02.049.
REFERENCES
- 1.Haak W, Lazaridis I, Patterson N, Rohland N, Mallick S, Llamas B, Brandt G, Nordenfelt S, Harney E, Stewardson K, et al. (2015). Massive migration from the steppe was a source for Indo-European languages in Europe. Nature 522, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofmanová Z, Kreutzer S, Hellenthal G, Sell C, Diekmann Y, Díez-Del-Molino D, van Dorp L, López S, Kousathanas A, Link V, et al. (2016). Early farmers from across Europe directly descended from Neolithic Aegeans. Proc. Natl. Acad. Sci. USA 113, 6886–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omrak A, Günther T, Valdiosera C, Svensson EM, Malmström H, Kiesewetter H, Aylward W, Storå J, Jakobsson M, and Götherström A. (2016). Genomic evidence establishes Anatolia as the source of the European Neolithic gene pool. Curr. Biol 26, 270–275. [DOI] [PubMed] [Google Scholar]
- 4.Lazaridis I, Patterson N, Mittnik A, Renaud G, Mallick S, Kirsanow K, Sudmant PH, Schraiber JG, Castellano S, Lipson M, et al. (2014). Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature 513, 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathieson I, Lazaridis I, Rohland N, Mallick S, Patterson N, Roodenberg SA, Harney E, Stewardson K, Fernandes D, Novak M, et al. (2015). Genome-wide patterns of selection in 230 ancient Eurasians. Nature 528, 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassidy LM, Martiniano R, Murphy EM, Teasdale MD, Mallory J, Hartwell B, and Bradley DG (2016). Neolithic and Bronze Age migration to Ireland and establishment of the insular Atlantic genome. Proc. Natl. Acad. Sci. USA 113, 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilinç GM, Koptekin D, Atakuman Ç., Sümer AP, Dönertaş HM, Yaka R, Bilgin CC, Büyükkarakaya AM, Baird D, Altinişik E, et al. (2017). Archaeogenomic analysis of the first steps of Neolithization in Anatolia and the Aegean. Proc. Biol. Sci 284, 20172064. 10.1098/rspb.2017.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kilinç GM, Omrak A, Özer F, Günther T, Büyükkarakaya AM, Biçakçi E, Baird D, Dönertaş HM, Ghalichi A, Yaka R, et al. (2016). The Demographic Development of the First Farmers in Anatolia. Curr. Biol 26, 2659–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipson M, Szécsényi-Nagy A, Mallick S, Pósa A, Stégmár B, Keerl V, Rohland N, Stewardson K, Ferry M, Michel M, et al. (2017). Parallel palaeogenomic transects reveal complex genetic history of early European farmers. Nature 551, 368–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrett R, Kuzawa CW, McDade T, and Armelagos GJ (1998). EMERGING AND RE-EMERGING INFECTIOUS DISEASES: The Third Epidemiologic Transition. Annu. Rev. Anthropol 27, 247–271. 10.1146/annurev.anthro.27.1.247. [DOI] [Google Scholar]
- 11.Childebayeva A, Rohrlach AB, Barquera R, Rivollat M, Aron F, Szolek A, Kohlbacher O, Nicklisch N, Alt KW, Gronenborn D, et al. (2022). Population Genetics and Signatures of Selection in Early Neolithic European Farmers. Mol. Biol. Evol 39, msac108. 10.1093/molbev/msac108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju D, and Mathieson I. (2021). The evolution of skin pigmentation-associated variation in West Eurasia. Proc. Natl. Acad. Sci. USA 118. e2009227118. 10.1073/pnas.2009227118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerner G, Neehus A-L, Abel L, Casanova J-L, Patin E, Laval G, and Quintana-Murci L. (2022). Genetic adaptation to pathogens and increased risk of inflammatory disorders in post-Neolithic Europe. Preprint at bioRxiv. 10.1101/2022.07.02.498543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irving-Pease EK, Refoyo-Martínez A, Ingason A, Pearson A, Fischer A, Barrie W, Sjögren K-G, Halgren AS, Macleod R, Demeter F, et al. (2022). The selection landscape and genetic legacy of Ancient Eurasians. Preprint at bioRxiv. 10.1101/2022.09.22.509027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuadros-Espinoza S, Laval G, Quintana-Murci L, and Patin E. (2022). The genomic signatures of natural selection in admixed human populations. Am. J. Hum. Genet 109, 710–726. 10.1016/j.ajhg.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allentoft ME, Sikora M, Sjögren KG, Rasmussen S, Rasmussen M, Stenderup J, Damgaard PB, Schroeder H, Ahlström T, Vinner L, et al. (2015). Population genomics of Bronze Age Eurasia. Nature 522, 167–172. [DOI] [PubMed] [Google Scholar]
- 17.Antonio ML, Gao Z, Moots HM, Lucci M, Candilio F, Sawyer S, Oberreiter V, Calderon D, Devitofranceschi K, Aikens RC, et al. (2019). Ancient Rome: a genetic crossroads of Europe and the Mediterranean. Science 366, 708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brace S, Diekmann Y, Booth TJ, van Dorp L, Faltyskova Z, Rohland N, Mallick S, Olalde I, Ferry M, Michel M, et al. (2019). Ancient genomes indicate population replacement in Early Neolithic Britain. Nat. Ecol. Evol 3, 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunel S, Bennett EA, Cardin L, Garraud D, Barrand Emam H, Beylier A, Boulestin B, Chenal F, Ciesielski E, Convertini F, et al. (2020). Ancient genomes from present-day France unveil 7,000 years of its demographic history. Proc. Natl. Acad. Sci. USA 117, 12791–12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandes DM, Mittnik A, Olalde I, Lazaridis I, Cheronet O, Rohland N, Mallick S, Bernardos R, Broomandkhoshbacht N, Carlsson J, et al. (2020). The spread of steppe and Iranian-related ancestry in the islands of the western Mediterranean. Nat. Ecol. Evol 4, 334–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandes DM, Strapagiel D, Borówka P, Marciniak B, Żądzińska E, Sirak K, Siska V, Grygiel R, Carlsson J, Manica A, et al. (2018). A genomic Neolithic time transect of hunter-farmer admixture in central Poland. Sci. Rep 8, 14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fregel R, Méndez FL, Bokbot Y, Martín-Socas D, Camalich-Massieu MD, Santana J, Morales J, Ávila-Arcos MC, Underhill PA, Shapiro B, et al. (2018). Ancient genomes from North Africa evidence prehistoric migrations to the Maghreb from both the Levant and Europe. Proc. Natl. Acad. Sci. USA 115, 6774–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Q, Posth C, Hajdinjak M, Petr M, Mallick S, Fernandes D, Furtwängler A, Haak W, Meyer M, Mittnik A, et al. (2016). The genetic history of Ice Age Europe. Nature 534, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furtwängler A, Rohrlach AB, Lamnidis TC, Papac L, Neumann GU, Siebke I, Reiter E, Steuri N, Hald J, Denaire A, et al. (2020). Ancient genomes reveal social and genetic structure of Late Neolithic Switzerland. Nat. Commun 11, 1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gamba C, Jones ER, Teasdale MD, McLaughlin RL, Gonzalez-Fortes G, Mattiangeli V, Domboróczki L, Kővári I, Pap I, Anders A, et al. (2014). Genome flux and stasis in a five millennium transect of European prehistory. Nat. Commun 5, 5257. 10.1038/ncomms6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González-Fortes G, Jones ER, Lightfoot E, Bonsall C, Lazar C, Grandal-d’Anglade A, Garralda MD, Drak L, Siska V, Simalcsik A, et al. (2017). Paleogenomic evidence for multi-generational mixing between Neolithic farmers and Mesolithic hunter-gatherers in the lower danube basin. Curr. Biol 27, 1801–1810.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Günther T, Malmström H, Svensson EM, Omrak A, Sánchez-Quinto F, Kılınç GM, Krzewińska M, Eriksson G, Fraser M, Edlund H, et al. (2018). Population genomics of Mesolithic Scandinavia: Investigating early postglacial migration routes and high-latitude adaptation. PLoS Biol. 16, e2003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Günther T, Valdiosera C, Malmström H, Ureña I, Rodriguez-Varela R, Sverrisdóttir ÓO, Daskalaki EA, Skoglund P, Naidoo T, Svensson EM, et al. (2015). Ancient genomes link early farmers from Atapuerca in Spain to modern-day Basques. Proc. Natl. Acad. Sci. USA 112, 11917–11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harney É, Cheronet O, Fernandes DM, Sirak K, Mah M, Bernardos R, Adamski N, Broomandkhoshbacht N, Callan K, Lawson AM, et al. (2021). A minimally destructive protocol for DNA extraction from ancient teeth. Genome Res. 31, 472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones ER, Zarina G, Moiseyev V, Lightfoot E, Nigst PR, Manica A, Pinhasi R, and Bradley DG (2017). The Neolithic transition in the Baltic was not driven by admixture with Early European Farmers. Curr. Biol 27, 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marcus JH, Posth C, Ringbauer H, Lai L, Skeates R, Sidore C, Beckett J, Furtwängler A, Olivieri A, Chiang CWK, et al. (2020). Genetic history from the Middle Neolithic to present on the Mediterranean island of Sardinia. Nat. Commun 11, 939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martiniano R, Cassidy LM, Ó ‘Maoldúin R, McLaughlin R, Silva NM, Manco L, Fidalgo D, Pereira T, Coelho MJ, Serra M, et al. (2017). The population genomics of archaeological transition in west Iberia: Investigation of ancient substructure using imputation and haplotype-based methods. PLoS Genet. 13, e1006852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olalde I, Allentoft ME, Sánchez-Quinto F, Santpere G, Chiang CWK, DeGiorgio M, Prado-Martinez J, Rodrıģuez JA, Rasmussen S, Quilez J, et al. (2014). Derived immune and ancestral pigmentation alleles in a 7,000-year-old Mesolithic European. Nature 507, 225–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittnik A, Wang C-C, Pfrengle S, Daubaras M, Zariņa G, Hallgren F, Allmäe R, Khartanovich V, Moiseyev V, Tõrv M, et al. (2018). The genetic prehistory of the Baltic Sea region. Nat. Commun 9, 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narasimhan VM, Patterson N, Moorjani P, Lazaridis I, Lipson M, Mallick S, Rohland N, Bernardos R, Kim AM, Nakatsuka N, et al. (2018). The Genomic Formation of South and Central Asia. Preprint at bioRxiv. 10.1101/292581. [DOI] [Google Scholar]
- 36.Novak M, Olalde I, Ringbauer H, Rohland N, Ahern J, Balen J, Janković I, Potrebica H, Pinhasi R, and Reich D. (2021). Genome-wide analysis of nearly all the victims of a 6200 year old massacre. PLoS One 16, e0247332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olalde I, Schroeder H, Sandoval-Velasco M, Vinner L, Lobón I, Ramirez O, Civit S, García Borja P, Salazar-García DC, Talamo S, et al. (2015). A Common Genetic Origin for Early Farmers from Mediterranean Cardial and Central European LBK Cultures. Mol. Biol. Evol 32, 3132–3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olalde I, Mallick S, Patterson N, Rohland N, Villalba-Mouco V, Silva M, Dulias K, Edwards CJ, Gandini F, Pala M, et al. (2019). The genomic history of the Iberian Peninsula over the past 8000 years. Science 363, 1230–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassidy LM, Maoldúin RÓ ., Kador T, Lynch A, Jones C, Woodman PC, Murphy E, Ramsey G, Dowd M, Noonan A, et al. (2020). A dynastic elite in monumental Neolithic society. Nature 582, 384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reich D, Thangaraj K, Patterson N, Price AL, and Singh L. (2009). Reconstructing Indian population history. Nature 461, 489–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lachance J, and Tishkoff SA (2013). SNP ascertainment bias in population genetic analyses: why it is important, and how to correct it. Bioessays 35, 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hofmanová Z, Kreutzer S, Hellenthal G, Sell C, Diekmann Y, Díez-Del-Molino D, and Burger J. (2016). Early farmers from across Europe directly descended from Neolithic Aegeans. Proc. Natl. Acad. Sci. USA 113, 6886–6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones ER, Gonzalez-Fortes G, Connell S, Siska V, Eriksson A, Martiniano R, McLaughlin RL, Gallego Llorente M, Cassidy LM, Gamba C, et al. (2015). Upper Palaeolithic genomes reveal deep roots of modern Eurasians. Nat. Commun 6, 8912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olalde I, Brace S, Allentoft ME, Armit I, Kristiansen K, Booth T, Rohland N, Mallick S, Szécsényi-Nagy A, Mittnik A, et al. (2018). The Beaker phenomenon and the genomic transformation of northwest Europe. Nature 555, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mathieson I, Alpaslan-Roodenberg S, Posth C, Szécsényi-Nagy A, Rohland N, Mallick S, Olalde I, Broomandkhoshbacht N, Candilio F, Cheronet O, et al. (2018). The genomic history of southeastern Europe. Nature 555, 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corbett-Detig R, and Nielsen R. (2017). A hidden Markov model approach for simultaneously estimating local ancestry and admixture time using next generation sequence data in samples of arbitrary ploidy. PLoS Genet. 13, e1006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lamason RL, Mohideen M-APK, Mest JR, Wong AC, Norton HL, Aros MC, Jurynec MJ, Mao X, Humphreville VR, Humbert JE, et al. (2005). SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science 310, 1782–1786. [DOI] [PubMed] [Google Scholar]
- 48.Mendoza-Revilla J, Chacón-Duque JC, Fuentes-Guajardo M, Ormond L, Wang K, Hurtado M, Villegas V, Granja V, Acuña-Alonzo V, Jaramillo C, et al. (2021). Disentangling signatures of selection before and after European colonization in Latin Americans. Preprint at bioRxiv. 10.1101/2021.11.15.467418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathieson I, and Terhorst J. (2022). Direct detection of natural selection in Bronze Age Britain. Preprint at bioRxiv. 10.1101/2022.03.14.484330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voight BF, Kudaravalli S, Wen X, and Pritchard JK (2006). A map of recent positive selection in the human genome. PLoS Biol. 4, e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skoglund P, Malmström H, Omrak A, Raghavan M, Valdiosera C, Günther T, Hall P, Tambets K, Parik J, Sjögren KG, et al. (2014). Genomic Diversity and Admixture Differs for Stone-Age Scandinavian Foragers and Farmers. Science 344, 747–750. [DOI] [PubMed] [Google Scholar]
- 52.Field Y, Boyle EA, Telis N, Gao Z, Gaulton KJ, Golan D, Yengo L, Rocheleau G, Froguel P, McCarthy MI, and Pritchard JK (2016). Detection of human adaptation during the past 2000 years. Science 354, 760–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patin E, Lopez M, Grollemund R, Verdu P, Harmant C, Quach H, Laval G, Perry GH, Barreiro LB, Froment A, et al. (2017). Dispersals and genetic adaptation of Bantu-speaking populations in Africa and North America. Science 356, 543–546. [DOI] [PubMed] [Google Scholar]
- 54.Abi-Rached L, Jobin MJ, Kulkarni S, McWhinnie A, Dalva K, Gragert L, Babrzadeh F, Gharizadeh B, Luo M, Plummer FA, et al. (2011). The shaping of modern human immune systems by multiregional admixture with archaic humans. Science 334, 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lenz TL (2018). Adaptive value of novel MHC immune gene variants. Proc. Natl. Acad. Sci. USA 115, 1414–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phillips KP, Cable J, Mohammed RS, Herdegen-Radwan M, Raubic J, Przesmycka KJ, van Oosterhout C, and Radwan J. (2018). Immunogenetic novelty confers a selective advantage in host-pathogen coevolution. Proc. Natl. Acad. Sci. USA 115, 1552–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lopez M, Choin J, Sikora M, Siddle K, Harmant C, Costa HA, Silvert M, Mouguiama-Daouda P, Hombert J-M, Froment A, et al. (2019). Genomic evidence for local adaptation of Hunter-gatherers to the African rainforest. Curr. Biol 29, 2926–2935.e4. [DOI] [PubMed] [Google Scholar]
- 58.Souilmi Y, Tobler R, Johar A, Williams M, Grey ST, Schmidt J, Teixeira JC, Rohrlach A, Tuke J, Johnson O, et al. (2022). Admixture has obscured signals of historical hard sweeps in humans. Nat. Ecol. Evol 6, 2003–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yasukochi Y, and Ohashi J. (2017). Elucidating the origin of HLA-B*73 allelic lineage: Did modern humans benefit by archaic introgression? Immunogenetics 69, 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frankish A, Diekhans M, Jungreis I, Lagarde J, Loveland JE, Mudge JM, Sisu C, Wright JC, Armstrong J, Barnes I, et al. (2021). GENCODE 2021. Nucleic Acids Res. 49, D916–D923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Team, R.C. (2021). R: A language and environment for statistical computing (R Foundation for Statistical Computing; ). [Google Scholar]
- 62.Delignette-Muller ML, Dutang C, and Others. (2015). fitdistrplus: An R package for fitting distributions. J. Stat. Softw 64, 1–34. [Google Scholar]
- 63.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, and Lee JJ (2015). Second generation PLINK: rising to the challenge of larger and richer datasets. GigaScience 4, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alexander DH, Novembre J, and Lange K. (2009). Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bonfield JK, Marshall J, Danecek P, Li H, Ohan V, Whitwham A, Keane T, and Davies RM (2021). HTSlib: C library for reading/writing high-throughput sequencing data. GigaScience 10, 10.1093/gigascience/giab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skoglund P, Mallick S, Bortolini MC, Chennagiri N, Hünemeier T, Petzl-Erler ML, Salzano FM, Patterson N, and Reich D. (2015). Genetic evidence for two founding populations of the Americas. Nature 525, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quinlan AR, and Hall IM (2010). BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemon J. (2006). Plotrix: a package in the red light district of R. R. News 6, 8–12. [Google Scholar]
- 69.Wickham H, and Seidel D. (2022). scales: Scale Functions for Visualization. https://scales.r-lib.org. https://github.com/r-lib/scales.
- 70.Dowle M, and Srinivasan A. (2022). data.table: Extension of ‘data.frame’ https://r-datatable.com. https://Rdatatable.gitlab.io/data.table. https://github.com/Rdatatable/data.table.
- 71.Lazaridis I, Nadel D, Rollefson G, Merrett DC, Rohland N, Mallick S, Fernandes D, Novak M, Gamarra B, Sirak K, et al. (2016). Genomic insights into the origin of farming in the ancient Near East. Nature 536, 419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Skoglund P, Malmström H, Raghavan M, Storå J, Hall P, Willerslev E, Gilbert MTP, Götherström A, and Jakobsson M. (2012). Origins and genetic legacy of Neolithic farmers and hunter-gatherers in Europe. Science 336, 466–469. [DOI] [PubMed] [Google Scholar]
- 73.Yaka R, Mapelli I, Kaptan D, Doğu A, Chyleński M, Erdal ÖD ., Koptekin D, Vural KB, Bayliss A, Mazzucato C, et al. (2021). Variable kinship patterns in Neolithic Anatolia revealed by ancient genomes. Curr. Biol 31, 2455–2468.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Coutinho A, Günther T, Munters AR, Svensson EM, Götherström A, Storå J, Malmström H, and Jakobsson M. (2020). The Neolithic Pitted Ware culture foragers were culturally but not genetically influenced by the Battle Axe culture herders. Am. J. Phys. Anthropol 172, 638–649. [DOI] [PubMed] [Google Scholar]
- 75.Sánchez-Quinto F, Malmström H, Fraser M, Girdland-Flink L, Svensson EM, Simões LG, George R, Hollfelder N, Burenhult G, Noble G, et al. (2019). Megalithic tombs in western and northern Neolithic Europe were linked to a kindred society. Proc. Natl. Acad. Sci. USA 116, 9469–9474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Immel A, Pierini F, Rinne C, Meadows J, Barquera R, Szolek A, Susat J, Böhme L, Dose J, Bonczarowska J, et al. (2021). Genome-wide study of a Neolithic Wartberg grave community reveals distinct HLA variation and hunter-gatherer ancestry. Commun. Biol 4, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rivollat M, Jeong C, Schiffels S, Küçükkalıpçi i., Pemonge M-H, Rohrlach AB, Alt KW, Binder D, Friederich S, Ghesquiè re E, et al. (2020). Ancient genome-wide DNA from France highlights the complexity of interactions between Mesolithic hunter-gatherers and Neolithic farmers. Sci. Adv 6, eaaz5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Skourtanioti E, Erdal YS, Frangipane M, Balossi Restelli F, Yener KA, Pinnock F, Matthiae P, Ö zbal R, Schoop U-D, Guliyev F, et al. (2020). Genomic History of Neolithic to Bronze Age Anatolia, Northern Levant, and Southern Caucasus. Cell 181, 1158– 1175.e28. [DOI] [PubMed] [Google Scholar]
- 79.Villalba-Mouco V, van de Loosdrecht MS, Posth C, Mora R, Martínez-Moreno J, Rojo-Guerra M, Salazar-García DC, Royo-Guillén JI, Kunst M, Rougier H, et al. (2019). Survival of Late Pleistocene Hunter-Gatherer Ancestry in the Iberian Peninsula. Curr. Biol 29, 1169–1177.e7. [DOI] [PubMed] [Google Scholar]
- 80.Mathieson I, Roodenberg SA, Posth C, Szécsényi-Nagy A, Rohland N, Mallick S, Olalde I, Broomandkhoshbacht N, Candilio F, Cheronet O, et al. (2017). The Genomic History Of Southeastern Europe. Preprint at bioRxiv. 10.1101/135616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sánchez-Quinto F, Schroeder H, Ramirez O, Ávila-Arcos MC, Pybus M, Olalde I, Velazquez AMV, Marcos MEP, Encinas JMV, Bertranpetit J, et al. (2012). Genomic Affinities of Two 7,000-Year-Old Iberian Hunter-Gatherers. Curr. Biol 22, 1494–1499. [DOI] [PubMed] [Google Scholar]
- 82.Saag L, Varul L, Scheib CL, Stenderup J, Allentoft ME, Saag L, Pagani L, Reidla M, Tambets K, Metspalu E, et al. (2017). Extensive Farming in Estonia Started through a Sex-Biased Migration from the Steppe. Curr. Biol 27, 2185–2193.e6. [DOI] [PubMed] [Google Scholar]
- 83.Broushaki F, Thomas MG, Link V, López S, van Dorp L, Kirsanow K, Hofmanová Z, Diekmann Y, Cassidy LM, Díez-Del-Molino D, et al. (2016). Early Neolithic genomes from the eastern Fertile Crescent. Science 353, 499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Marchi N, Winkelbach L, Schulz I, Brami M, Hofmanová Z, Blöcher J, Reyna-Blanco CS, Diekmann Y, Thiéry A, Kapopoulou A, et al. (2022). The genomic origins of the world’s first farmers. Cell 185, 1842–1859.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request. This paper analyses existing, publicly available data. These accession numbers for the datasets are listed in Tables S1 and S2. Code from this paper used to generate all figures and analyses from allele frequency data is available at https://github.com/Tom-Davy/mesoneo_admixture.