Abstract
We have evaluated the ability of two carbohydrate biopolymers, chitosan and gellan, to enhance antibody responses to subunit influenza virus vaccines delivered to the respiratory tracts of mice. Groups of mice were vaccinated three times intranasally (i.n.) with 10 μg of purified influenza B/Panama virus surface antigens (PSAs), which consist of hemagglutinin (HA) and neuraminidase (NA), either alone or admixed with chitosan or gellan solutions. Separate groups were vaccinated subcutaneously (s.c.) with PSAs adsorbed to Alhydrogel or chitosan or gellan alone i.n. Serum antibody responses were determined by enzyme-linked immunosorbent assay (ELISA) for influenza virus-specific immunoglobulin G (IgG) and by HA inhibition (HAI) and NA inhibition (NAI) assays. The local respiratory immune response was measured by assaying for influenza virus-specific IgA antibody in nasal secretions and by enumerating nasal and pulmonary lymphocytes secreting IgA, IgG, and IgM anti-influenza virus-specific antibodies by enzyme-linked immunospotting (ELISPOT). When administered alone i.n., B/Panama PSA was poorly immunogenic. Parenteral immunization with B/Panama PSA with Alhydrogel elicited high titers of anti-B/Panama antibodies in serum but a very poor respiratory anti-B/Panama IgA response. In contrast, i.n. immunization with PSA plus chitosan stimulated very strong local and systemic anti-B/Panama responses. Gellan also enhanced the local and serum antibody responses to i.n. PSA but not to the same extent as chitosan. The ability of chitosan to augment the immunogenicity of influenza vaccines given i.n. was confirmed using PSA prepared from an influenza A virus (A/Texas H1N1).
Outbreaks of influenza are responsible for devastating global morbidity and high mortality in high-risk groups, such as the elderly and those with underlying pulmonary or cardiac disease. Although influenza is usually fatal only in certain populations, it also accounts for significant absenteeism in the work force. The influenza viruses of humans and other mammals are largely spread by aerosols, and the virus is distributed throughout the respiratory tract (1). Infection with influenza virus results in both a serum and a local secretory antibody response in the respiratory tract. The protective antibody response to influenza virus is directed at the surface glycoproteins hemagglutinin (HA) and neuraminidase (NA) (1).
Although the correlation between serum HA inhibition (HAI) titers and protection from influenza is well established in humans, protection has also been correlated with anti-HA antibody in nasal washings (13, 15). Secretory immunoglobulin A (IgA) forms the major part of this mucosal antibody response to influenza virus infection, and several groups have demonstrated that HA-specific IgA confers protection against influenza virus in mice (1, 33, 42, 43, 52).
Secretory IgA neutralizes virus infectivity efficiently, and polymeric IgA is superior to IgG at neutralizing influenza virus in vitro (2, 40). Mazanec et al. (35) proposed a three-tiered view of the role of IgA in mucosal defense. As well as inhibiting viral attachment and penetration, IgA may have a role in mediating recovery from infection by neutralizing intracellular virus directly within epithelial cells and by binding virus in the mucosal lamina propria and excreting it through the adjacent epithelium (35).
Natural infection induces better cross-reactive protection against subtype variants than conventional parenteral vaccines. This has been attributed to the induction of cross-protecting IgA antibodies in the respiratory tract (33, 52). Parenteral immunization with current influenza vaccines is generally efficient at eliciting serum antibody but not secretory IgA responses (14, 36, 56). An ideal influenza vaccine would induce both local respiratory and systemic immune responses. Generally, vaccines need to be applied topically to mucosal surfaces to elicit a good mucosal immune response. However, conventional inactivated or subunit vaccines are often poorly immunogenic when given mucosally (17, 23).
A number of approaches have been investigated in order to improve antibody responses to antigens delivered mucosally. These include encapsulation of the antigens or their coadministration with mucosal adjuvants, such as cholera toxin, Escherichia coli heat-labile toxin, or derivatives thereof (7, 8, 12, 18, 29, 49, 51, 53, 54).
In this study, we have investigated the ability of two nontoxic carbohydrate biopolymers, gellan and chitosan, to increase the immunogenicity of intranasally (i.n.) administered influenza virus vaccines. Both substances can improve the delivery of drugs across mucous membranes (5, 12, 25, 30, 47). Gellan is an extracellular, anionic polysaccharide produced by Pseudomonas elodea. Its monosaccharide units are β-d glucose, β-d glucuronic acid, and α-l-rhamnose. Chitosan is a cationic polysaccharide (consisting of repeating units of N-acetyl-d-glucosamine and d-glucosamine), derived by the partial deacetylation of chitin (obtained from the shells of crustaceans). Chitosan has a number of uses, including sustained or targeted drug delivery (12, 20, 22, 25). Chitosan is susceptible to lysozyme digestion, making it relatively biodegradable, and it has low toxicity compared with other natural polysaccharides (20, 22, 55).
MATERIALS AND METHODS
Preparation of influenza virus vaccines.
Influenza virus antigen preparations were supplied by Evans Medical (Liverpool, United Kingdom). Surface glycoproteins HA and NA were prepared from B/Panama/45/90 and A/Texas H1N1 influenza viruses as described previously (9). This preparation, called purified surface antigens (PSAs), adopts a characteristic rosette structure as a result of the removal of the majority of viral lipid during the preparation (44). The vast majority of protein in PSA preparations is HA. PSA was dialyzed against phosphate-buffered saline (PBS, pH 7.2), to remove preservative and was concentrated or diluted to vaccine strength prior to use. The protein concentrations were determined by the Markwell adaptation of the Lowry procedure (34), using bovine serum albumin (BSA) as a standard.
Vaccine formulations for i.n. delivery were prepared by mixing antigen 1:1 (vol/vol) with either 1% [wt/vol] chitosan solution (chitosan glutamate [Protasan UP G210; Pronova, Oslo, Norway] in saline at pH 6) or 0.4% [wt/vol] gellan solution (gellan [Kelco Division, Merck & Co., Inc., Rahway, N.J.] in water). Both gellan and chitosan were supplied as endotoxin free. For subcutaneous (s.c.) vaccination, PSA was adsorbed to Alhydrogel (0.5%) overnight.
Immunization and sampling of mice.
Adult female (6- to 8-week-old) BALB/c mice (Harlan Olac, Bicester United Kingdom) were used (12 per group). For i.n. immunizations, mice were lightly anesthetized with halothane, and 20 μl of sample (10 μl per nostril) was applied to the external nares with a micropipette (Gilson). Mice were immunized s.c. by injection of 200 μl of sample into the skin folds in the back of the neck. For all routes, mice received 10 μg of PSA per dose. Control mice receiving chitosan or gellan alone i.n. were included. In the case of B/Panama PSA, 12 mice per group were vaccinated on day 1 and boosted on days 30 and 57. Serum and nasal-wash samples were obtained 21 days after the first dose, 14 days after the second dose, and 14 to 16 days after the third dose (four mice were sacrificed after each dose). Lymphocytes were isolated from the lung and nasal mucosa of sacrificed animals after the third dose for analysis by enzyme-linked immunospotting (ELISPOT) assay. For A/Texas PSA, five mice/group were immunized i.n. with 10 μg of PSA alone or in combination with chitosan. Mice were vaccinated on day 1 and boosted on days 28 and 57. Serum and nasal-wash samples were obtained at 15 days post-final dose, and lymphocytes were isolated from the lung and nasal mucosa of sacrificed animals for analysis by ELISPOT assay.
Measurement of the serum response. (i) Serum anti-influenza virus IgG ELISA.
Individual animal samples were analyzed for anti-influenza virus antibodies by enzyme-linked immunosorbent assay (ELISA). Ninety-six-well microtiter plates (Costar, High Wycombe, United Kingdom) were coated at 4°C overnight with 50 μl of B/Panama or A/Texas whole inactivated virus (WIV), per well, at 20 μg/ml in PBS. Plates were washed three times with PBS-Tween (0.05% [vol/vol]) and then blocked with PBS containing 1% BSA for 1 h at 37°C. After washing, serially diluted samples in PBS-Tween containing 0.1% BSA were added to the wells, and the plates were incubated for 2 h at 37°C. Following incubation and a washing stage, antibody binding was revealed by the sequential addition of anti-mouse IgG biotin conjugate (Sigma) and streptavidin-horseradish peroxidase conjugate (DAKO) and finally ς-phenylenediamine substrate. Optical density was read on a Ceres 900 ELISA reader (BioTek) at 490 nm. Optical density dilution curves were plotted (post-background correction), and titer values were determined. Endpoint titers were defined as the highest dilutions giving optical densities of 0.3 (after correction for background).
(ii) HAI.
Nonspecific inhibitors of hemagglutination were removed from the sera using kaolin (ICN-FLOW). Treated sera were tested by the standard procedure (21) using chick erythrocytes. Endpoint was determined as the dilution of sera at which 100% settling of chick erythrocytes occurred and was estimated by interpolation when the endpoint occurred between two dilutions in adjacent wells. Titers were given as the reciprocal of serum dilutions giving 100% inhibition of HA.
(iii) Neuraminidase antigen inhibition (NAI).
Sera were assayed according to an adaptation of the procedure of Lambré et al. (31). Briefly, the method involves coating the wells of a 96-well microtiter plate (ICN-FLOW) with fetuin (100 μl per well of a 1.05-mg/ml fetuin in 0.1 M bicarbonate buffer [pH 9.6]), a natural substrate of the NA enzyme. Following a blocking stage, a 1 in 75 dilution of test or control (normal mouse serum) serum was added to the well in combination with a defined amount of purified challenge virus and incubated for 5 or 17 h at 25°C. Active NA catalyzes the removal of terminal α-ketosidically linked sialic acid from fetuin resulting in the unmasking of α-d-galactose-N-acetyl-galactosamine (α-d-gal-NAc-gal). This exposure of α-d-gal-NAc-gal was revealed by the addition of peanut agglutinin horseradish-peroxidase conjugate (100 μl per well of a 3.3-μg/ml conjugate in 0.01 M PBS [pH 7.2]), followed by ς-phenylenediamine substrate and measurement of color development spectrophotometrically. The intensity of chromophore development is proportional to the level of NA activity. As a consequence, the lower the intensity of chromophore, the higher the anti-NA antibody component. Absolute NA activity was quantified relative to a standard curve, and thereby the percentage inhibition titer compared to that for the challenge virus control was determined. Of the NA activity of the challenge virus, 10% was inhibited nonspecifically by the normal mouse serum. Therefore, only samples having NAI activity of 20% and greater were recorded as positive.
Measurement of local anti-influenza virus antibody response in mice.
Local responses were determined by ELISA assaying influenza virus-specific IgA response in nasal-lavage samples and by measuring influenza virus-specific antibody-secreting cells (ASCs) to B/Panama or A/Texas influenza virus with an ELISPOT assay.
(i) Nasal-wash anti-influenza virus IgA ELISA.
Nasal washes were obtained from sacrificed animals as described previously (46). Neat and serial diluted samples of nasal-wash fluid were assayed for IgA using the ELISA method as described above with an anti-mouse IgA biotin conjugate (Sigma) instead of an anti-mouse IgG biotin conjugate. The endpoint titer was determined as the highest dilution of nasal-wash sample that gave an optical density of 0.1 (after background correction). To correct for variations in nasal-lavage efficiency, the total IgA in each sample was determined by ELISA, as previously described (16). Finally, the nasal anti-influenza virus IgA responses were expressed as a ratio of specific response (endpoint titer) in the lavage divided by the total amount of IgA (in micrograms) measured in the same sample.
(ii) Anti-influenza virus ELISPOT assay.
Lymphocytes from the nasal mucosa and pulmonary tissue were assayed for anti-influenza virus specificity by ELISPOT. Pulmonary lymphocytes were isolated as previously described (45). Nasal lymphocytes were isolated as follows: after sacrifice of animals, the nasal mucosa and turbinate bones were dissected from the nasal cavity using a dissecting microscope. Isolated tissues were placed in cold sterile PBS and then macerated between the frosted ends of two sterile glass microscope slides. Large fragments were removed by brief centrifugation (up to 460 × g, then immediately brake). The supernatant was retained and centrifuged at 460 × g for 7 min to pellet cells. To remove erythrocytes, the pellet was suspended in lysing buffer (0.15 M NH4Cl–0.01 M KHCO3–0.1 mM Na2EDTA) and incubated for 10 min at room temperature. Cells were then pelleted (200 × g for 10 min at 4°C) and washed twice in PBS. Cells were resuspended in RPMI 1640 complete medium (10% fetal calf serum–2 mM glutamine–100 U of penicillin per ml–100 μg of streptomycin per ml) plus 5 × 10−5 M β-mercaptoethanol. Cells were counted by trypan blue exclusion before being plated into an ELISPOT assay. The ELISPOT assay was performed in 24-well plates (Costar) as described previously (45) using B/Panama or A/Texas WIV as antigen (0.5 ml of 40-μg/ml WIV per well); responses were recorded as ASCs per 106 mononuclear cells.
Statistical analysis.
Where indicated, the data were analyzed for statistical significance by single-factor analysis of variance or t test as appropriate.
RESULTS
Serum antibody responses.
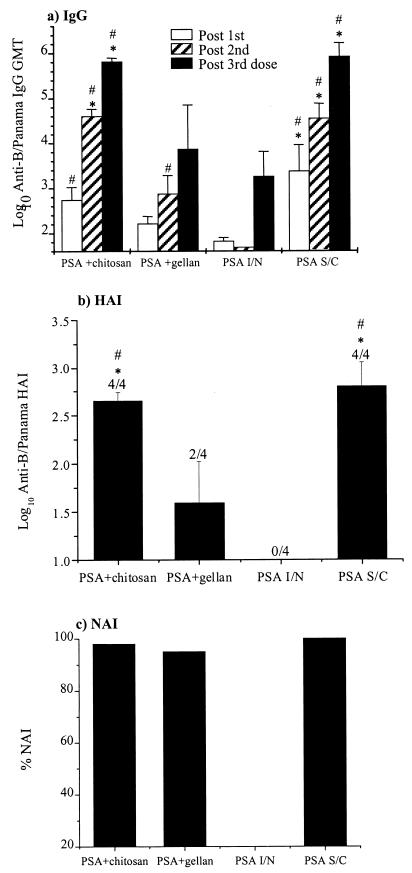
The serum antibody responses to the B/Panama PSA influenza virus vaccines in mice are shown in Fig. 1. Serum antibody responses were measured by ELISA for total anti-influenza virus IgG and by HAI and NAI assays. The anti-influenza virus serum antibody response in mice given PSA alone i.n. was very weak in all of the assays, even in serum samples taken after the third dose of vaccine. None of the mice in this group generated a detectable serum HAI response. In contrast, chitosan and gellan both enhanced the serum antibody response to the i.n. administered PSA, with chitosan (at the concentrations used) having the greatest effect. At all time points the mean serum IgG titers and the HAI titers of the PSA-plus-chitosan-immunized mice were significantly greater (P < 0.05) than those of the PSA i.n. immunized mice. This was the case for the PSA-plus-gellan group only for the post-second-dose IgG titers. Also the serum IgG (post-second and -third doses) and HAI titers for the PSA-plus-chitosan-immunized mice were significantly greater (P < 0.05) than those of the PSA-plus-gellan-immunized mice. Anti-influenza virus antibody responses were not measurable in any of the samples taken from the control mice administered chitosan or gellan alone i.n.
FIG. 1.
Serum antibody response to B/Panama PSA. Mice were immunized three times with B/Panama, and serum samples were obtained after each immunization as detailed in the text. (a) Anti-B/Panama IgG ELISA. The columns represent the GMT, and the error bars represent the standard errors of the mean (SEM). (b) Anti-B/Panama HAI. Columns represent the mean log10 HAI, and the error bars show the SEM; numbers above error bars show the number of sera giving a positive response per number of sera tested. (c) NAI. NAI was assayed on pooled post-third-dose sera diluted 1/75. The means of the different groups at the equivalent time points were compared to determine if they were significantly different (P < 0.05). #, mean significantly greater than that of the PSA i.n. group; ∗, mean significantly greater than that of the PSA + gellan group. Abbreviations: I/N, intranasally administered; S/C, subcutaneously administered.
For the serum anti-B/Panama virus IgG, the kinetics and magnitude of the response of mice in the chitosan-PSA group and the PSA-plus-Aldhydrogel s.c. group were very similar and were not significantly different (Fig. 1a). The mean HAI titer was higher in the sera from mice immunized s.c. with PSA than in the sera from the PSA-plus-chitosan i.n. mice (Fig. 1b). However, the difference was not statistically significant, and the variation in the HAI response was also greater in the former group. The post-third-dose serum from one of the mice in the PSA-plus-Aldhydrogel s.c. group had very high IgG and HAI titers, and if this was omitted, the HAI geometric mean titer (GMT) is higher in the group receiving chitosan-PSA than in the s.c. immunized group, although not significantly so.
The results of the NAI assay indicate that pooled post-third-dose sera from mice in the gellan-plus-PSA and chitosan-plus-PSA groups were equally effective in inhibiting NA (Fig. 1c). In this assay a single dilution of sera was assayed. To investigate why the activities of sera from mice in the PSA-plus-chitosan and PSA-plus-gellan groups were so similar, sera were tested individually for NAI activity. All of the sera from the PSA-plus-chitosan mice inhibited 98% of the NA activity, whereas the sera from the PSA-plus-gellan mice inhibited 37%, 56%, 69%, and 98% of the NAI activity (data not shown).
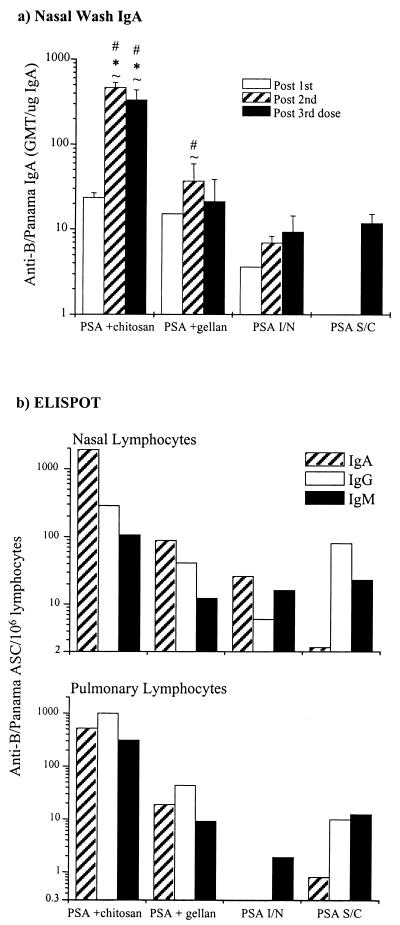
Local respiratory antibody responses to B/Panama.
Nasal secretory IgA responses to B/Panama PSA are shown in Fig. 2a. All mice in the i.n. immunized groups, but not the s.c. immunized group, had detectable anti-influenza virus IgA antibodies in their nasal lavage fluid. However, PSA alone i.n. was a poor mucosal immunogen compared to PSA admixed with gellan or, in particular, chitosan. After 2 doses of vaccine, all mice in the chitosan-PSA group had detectable anti-B/Panama IgA in their nasal secretions, whereas in none of the other groups did all mice in the group show evidence of an IgA response even after three immunizations. The post-second and -third-dose-specific IgA titers in the PSA-plus-chitosan group were significantly higher (P < 0.05) than those of all other groups. Gellan also boosted the nasal secretory IgA response to influenza virus antigens but to a lesser extent than chitosan. An improvement in response, over PSA alone i.n., was evident after two doses, but there was no significant difference between the two groups after three doses. This was due, in part, to large differences in responses to PSA plus gellan between individual mice. Administration of PSA s.c. with Alhydrogel proved to be a poor stimulant of secretory IgA, even after three doses.
FIG. 2.
Local respiratory antibody response to B/Panama PSA. (a) Nasal-wash anti-B/Panama IgA ELISA. The columns represent the specific anti-B/Panama IgA GMTs corrected for total nasal-wash IgA, and the error bars show the SEMs. The means of the different groups at the equivalent time points were compared to determine if they were significantly different (P < 0.05). #, mean significantly greater than that of the PSA i.n. (“I/N”) group; ∗, mean significantly greater than that of the PSA + gellan group; ∼, mean significantly greater than that of the PSA s.c. (“S/C”) group. (b) Respiratory anti-B/Panama ASC response assayed by ELISPOT. Anti-B/Panama ASC responses on lymphocytes isolated from the nasal mucosa and lungs were assayed by ELISPOT on tissues recovered from mice after the third dose of vaccine. Columns represent the response of lymphocytes pooled from four mice per group.
To obtain information on if and how the quality and quantity of the local antibody response differed in the upper and lower respiratory tract in response to vaccination with the different B/Panama PSA formulations, an ELISPOT assay was used (Fig. 1b). Lymphocytes were isolated from the nasal mucosa and lung parenchyma after mice had received three doses of vaccine and were assayed for cells secreting IgA, IgG, and IgM antibody specific for B/Panama WIV.
In all groups except the s.c. immunized mice, B cells secreting IgA specific for B/Panama influenza virus predominated in the nasal cavity, and B cells secreting virus-specific IgG or IgM predominated in the lung (Fig. 1b). The ELISPOT results confirmed those obtained by the IgA ELISA on the nasal lavage fluid. There were a far greater number of influenza virus-specific IgG, IgA, and IgM ASCs in the nasal and lung tissues of mice in the PSA-plus-chitosan group than in any of the other groups. In fact, the PSA-plus-chitosan formulation generated approximately 50-fold more specific ASCs in the nose and 1,000-fold more specific ASCs in the lungs than PSA alone i.n. Gellan slightly enhanced the nasal IgA and lung IgG response to B/Panama influenza virus compared to the response seen in mice receiving PSA alone i.n.
Responses to A/Texas PSA vaccines.
The above results indicated that chitosan was able to greatly improve the immunogenicity of PSA prepared from a B influenza virus. To ensure that the results we obtained were not specific for antigens prepared from B viruses, we performed a study using PSA prepared from A/Texas virus. Mice were immunized three times i.n. with A/Texas PSA alone or admixed with chitosan, and the local and systemic anti-A/Texas antibody responses were determined (Fig. 3 and 4).
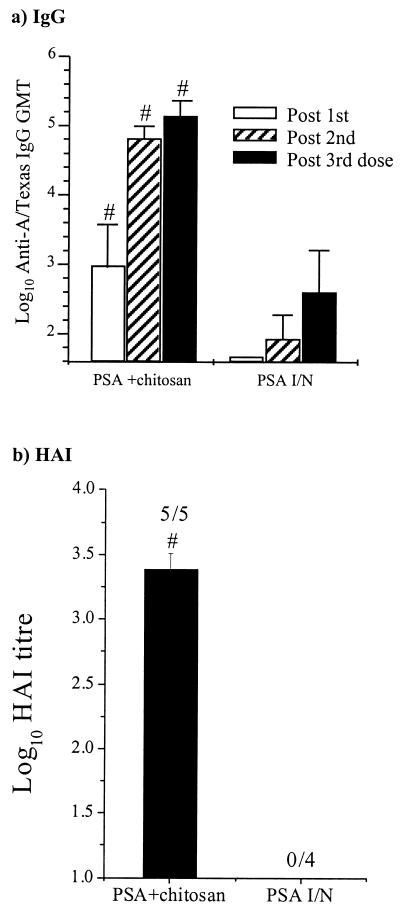
FIG. 3.
Serum antibody response to A/Texas PSA. Mice were immunized, and serum samples were obtained after each immunization as detailed above. (a) Anti-A/Texas IgG ELISA. The columns represent the mean GMTs, and the error bars represent the SEMs. (b) Anti-A/Texas HAI. Columns represent the mean log10 HAIs, and the numbers above the columns show the number of sera giving a positive response per number tested. The means of the different groups were compared to determine if they were significantly different (P < 0.05). #, mean significantly greater than that of the PSA i.n. (I/N) group.
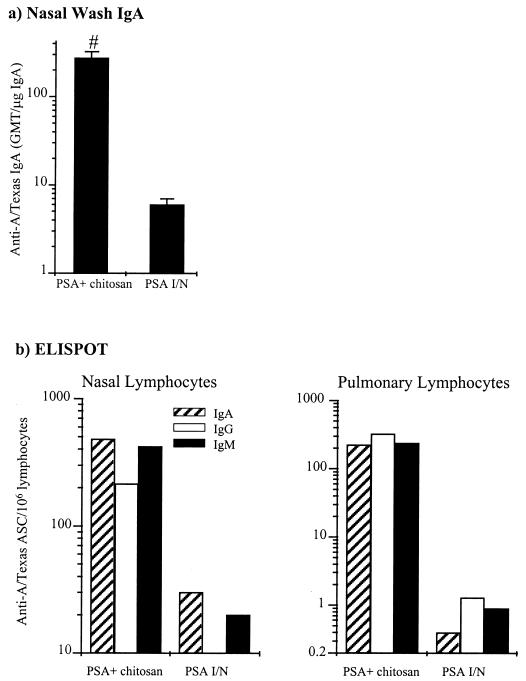
FIG. 4.
Local respiratory antibody response to A/Texas PSA. (a) Nasal-wash anti-anti-A/Texas IgA ELISA. The columns represent the anti-A/Texas IgA GMTs corrected for total nasal-wash IgA, and the error bars represent the SEMs. The means of the different groups were compared to determine if they were significantly different (P < 0.05). #, mean significantly greater than that of the PSA i.n. (“I/N”) group. (b) Respiratory anti-A/Texas ASC response. Anti-A/Texas ASC responses on lymphocytes isolated from the nasal mucosa and lungs were assayed after the third dose by ELISPOT. Columns represent the response of lymphocytes pooled from five mice per group.
Serum antibody responses to A/Texas PSA.
The serum IgG and HAI anti-A/Texas antibody responses are shown in Fig. 3. The serum response to PSA alone i.n. was poor, with all mice failing to generate a detectable serum HAI response even after the third dose. In contrast all mice immunized with A/Texas PSA plus chitosan gave an HAI response. The enhanced serum response in mice immunized with A/Texas along with chitosan was also reflected in the results from the anti-A/Texas IgG ELISA. The IgG anti-A/Texas GMT of the PSA-plus-chitosan mice following the primary immunization was higher than that of mice that had been immunized three times i.n. with PSA alone.
Local respiratory tract antibody responses to A/Texas PSA.
Nasal-wash IgA and nasal and respiratory ELISPOT responses to A/Texas PSA are shown in Fig. 4. Chitosan significantly (P < 0.05) enhanced the nasal-secretory-IgA response to A/Texas PSA administered i.n. There were far greater numbers of lymphocytes secreting specific antibody to A/Texas in the lung and nasal mucosa of mice immunized with A/Texas PSA plus chitosan than in mice immunized with A/Texas PSA alone. In fact, the A/Texas PSA-plus-chitosan formulation generated approximately 25-fold more specific ASCs in the nose and 400-fold more specific ASCs in the lungs than A/Texas PSA alone.
These results demonstrate that chitosan is capable of augmenting the local and systemic antibody responses to immunogens prepared from both A-type and B-type influenza viruses administered to the respiratory tract of mice.
DISCUSSION
B/Panama PSA was highly immunogenic when given parenterally with Alhydrogel, as evidenced by the high titers of serum anti-B/Panama antibodies produced by immunized mice. However, parenteral immunization stimulated only a very weak secretory antibody response. B/Panama PSA also proved to be a poor mucosal immunogen when administered alone i.n. Both chitosan and gellan enhanced both the local and systemic antibody responses to B/Panama PSA administered i.n., but, at the concentrations of carbohydrates used, chitosan was much more effective in this regard than gellan, in terms of the increases in influenza virus-specific serum IgG, HAI and NAI, nasal secretory IgA, and respiratory tract anti-influenza virus ASCs. Also in terms of the number of animals that responded and the consistency of the responses in individual mice, chitosan was superior to gellan. The serum antibody responses induced by i.n. immunization with PSA plus chitosan were similar to those induced by s.c. immunization of PSA with Alhydrogel adjuvant. However, the local antibody responses to B/Panama in the mice immunized i.n. with PSA plus chitosan were far superior to those in the s.c. immunized mice. The enhancement of the immunogenicity of i.n. administered influenza virus PSA by chitosan was not restricted to vaccine prepared from B viruses; the immunogenicity of A/Texas PSA administered i.n. was also significantly enhanced by chitosan.
The HAI assay is less sensitive than the anti-influenza virus IgG ELISA. However, the HAI assay measures functional activity of the antibody and is a recognized reliable measure of protective antibody against influenza virus. The HAI and anti-influenza virus IgG responses in this study showed good correlation. Also, mice that demonstrated good HAI responses generally gave good NAI responses, showing that both antigens were presented to the immune system and providing a degree of validation for the NAI test method used. Anti-NA antibodies can contribution to protection against influenza virus by inhibiting the release of virus from infected cells, possibly by cross-linking budding virus particles (1, 48).
The numbers of influenza virus-specific ASCs in both the upper and lower respiratory tracts of mice were greater in animals immunized i.n. with chitosan plus PSA than in mice in the other groups. Except for the parenterally immunized mice, IgA ASCs predominated in the nasal mucosa, and, in general, IgG ASCs were the predominant isotype in the lungs. As mentioned, the serum IgG anti-B/Panama response was similar in mice immunized s.c. with B/Panama PSA plus Alhydrogel and mice immunized with PSA plus chitosan i.n. However, there were many more IgG anti-influenza virus ASCs in the respiratory tracts (in particular the lower respiratory tracts) of the PSA-plus-chitosan mice than in the respiratory tracts of PSA-plus-Alhydrogel mice. This indicates that the majority of the IgG ASCs in the respiratory tracts of i.n. immunized mice are probably of local origin and are not the result of seeding from systemic lymphoid organs.
The mode of action of chitosan in enhancing serum and secretory antibody responses to i.n. applied influenza vaccine will require further investigation. It may be that chitosan facilitates greater uptake of antigens across the nasal mucosa by slowing down mucociliary clearance, thus maintaining the contact of antigen with the mucosa for a greater length of time (4, 6, 32). Gellan forms gels in the presence of mono- or divalent cations and so should also hinder mucociliary clearance by forming a gel on contact with the nasal mucosa. However, gellan solution was not as successful as chitosan solution at enhancing antibody responses to influenza virus antigens. It has also been suggested that chitosan can transiently affect the permeability of epithelia by modulating the gating properties of tight junctions (3, 19, 25). Chitosan transiently enhanced the nasal absorption of insulin in rats (25). However, the size of the immunogen used in this study is much greater than that of insulin, HA trimers have a molecular mass of greater than 200 kDa, and in PSA preparations they form rosettes in the absence of lipid, due to their amphipathic nature (44). It may be, therefore, that chitosan augments the immunogenicity of chitosan by a means other than merely increasing uptake across mucosal epithelia. Recently, chitosan was shown to enhance the local and systemic immune responses to several i.n. administered Bordetella pertussis proteins (26). However, in contrast to the present study, the B. pertussis antigens used are good mucosal immunogens in their own right (26, 45, 46, 49).
Some studies have shown that chitosan can activate components of the nonspecific immune system such as macrophages and can induce nonspecific immunity to bacteria, fungi, and tumors (38, 41, 50). Chitin and chitosan are both components of fungal cell walls (10, 11). Therefore, chitosan may provide a “danger signal” and be acting as an adjuvant, as do other conserved cellular components of microorganisms (27, 28). In contrast, extracellular polysaccharides, such as gellan, can vary greatly from one bacteria to another (even within the same bacterial species), are often poorly immunogenic, and are not known to act as adjuvants.
During natural infection, influenza virus is deposited throughout the respiratory tract (1). Secretory anti-influenza virus IgA response in the upper and lower respiratory tract combined with a local pulmonary and systemic anti-influenza virus IgG response should provide high-level protection against influenza virus infection. An i.n. administered influenza virus vaccine would have a number of advantages over conventional parenteral vaccines, including the acceptability of the vaccine among “needlephobics” as well as the induction of protective antibody in the respiratory tract (37).
Although Oh et al. (39) demonstrated that i.n. immunization of humans with high-concentration influenza vaccine (three times the current parenteral dose) elicited local and serum antibody responses, it is generally considered that such a high-dose vaccine would not be commercially viable. A high concentration of influenza antigens may have been required because of the difficulty of transporting large molecules across epithelial membranes and/or enzyme degradation and removal of the vaccine from the mucosal surface by the mucociliary clearance mechanism (24).
Several methods have previously been described for enhancing the immune response to influenza vaccines administered to the respiratory tract. These include the use of wild-type and nontoxic derivatives of cholera toxin and E. coli heat-labile toxin (7, 8, 18, 29, 51, 53, 54). The safety of these adjuvants in humans has not yet been demonstrated. In contrast, chitosans have been administered to humans by a variety of routes without toxic effects (20, 22, 25). Also, after cellulose, chitin (from which chitosan is derived) is the most plentiful biopolymer in the world. Influenza virus PSA plus chitosan is therefore an excellent candidate for an inactivated mucosal influenza vaccine.
REFERENCES
- 1.Ada G L, Jones P D. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:2–53. doi: 10.1007/978-3-642-71272-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong S J, Dimmock N J. Neutralization of influenza virus by low concentrations of hemagglutinin-specific polymeric immunoglobulin A inhibits viral fusion activity, but activation of the ribonucleoprotein is also inhibited. J Virol. 1992;66:3823–3832. doi: 10.1128/jvi.66.6.3823-3832.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artursson P, Lindmark S, Davis S, Illum L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2) Pharm Res. 1994;11:1358–1361. doi: 10.1023/a:1018967116988. [DOI] [PubMed] [Google Scholar]
- 4.Aspden T J, Adler J, Davis S S, Skaugrud O, Illum L. Chitosan as a nasal delivery system: evaluation of the effect of chitosan on mucociliary clearance rate in the palate model. Int J Pharm. 1995;122:69–78. [Google Scholar]
- 5.Aspden T J, Illum L, Skaugrud O. Chitosan as a nasal delivery system: evaluation of insulin absorption enhancement and effect on nasal membrane integrity using rat models. Eur J Pharm Sci. 1996;4:23–31. [Google Scholar]
- 6.Aspden T J, Mason J D, Jones N, Lowe J, Skaugrud O, Illum L. Chitosan as a nasal delivery system: the effect of chitosan on in vitro and in vivo mucociliary transport rates. J Pharm Sci. 1997;86:509–513. doi: 10.1021/js960182o. [DOI] [PubMed] [Google Scholar]
- 7.Barackman J D, Ott G, O'Hagan D T. Intranasal immunization of mice with influenza vaccine in combination with the adjuvant LT-R72 induces potent mucosal and serum immunity which is stronger than that with traditional intramuscular immunization. Infect Immun. 1999;67:4276–4279. doi: 10.1128/iai.67.8.4276-4279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barchfeld G L, Hessler A L, Chen M, Pizza M, Rappuoli R, Van Nest G A. The adjuvants MF59 and LT-K63 enhance the mucosal and systemic immunogenicity of subunit influenza vaccine administered intranasally in mice. Vaccine. 1999;17:695–704. doi: 10.1016/s0264-410x(98)00252-7. [DOI] [PubMed] [Google Scholar]
- 9.Brady M I, Furminger I G. A surface antigen influenza vaccine. 1. Purification of haemagglutinin and neuraminidase proteins. J Hyg. 1976;77:161–172. doi: 10.1017/s002217240002458x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briza P, Ellinger A, Winkler G, Breitenbach M. Chemical composition of the yeast ascospore wall. The second outer layer consists of chitosan. J Biol Chem. 1988;263:11569–11574. [PubMed] [Google Scholar]
- 11.Bulawa C E. Genetics and molecular biology of chitin synthesis in fungi. Annu Rev Microbiol. 1993;47:505–534. doi: 10.1146/annurev.mi.47.100193.002445. [DOI] [PubMed] [Google Scholar]
- 12.Cahill E S, O'Hagan D T, Illum L, Barnard A, Mills K H, Redhead K. Immune responses and protection against Bordetella pertussis infection after intranasal immunization of mice with filamentous haemagglutinin in solution or incorporated in biodegradable microspheres. Vaccine. 1995;13:455–462. doi: 10.1016/0264-410x(94)00008-b. [DOI] [PubMed] [Google Scholar]
- 13.Clements M L, Murphy B R. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986;23:66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements M L, O'Donnell S, Levine M M, Chanock R M, Murphy B R. Dose response of A/Alaska/6/77 (H3N2) cold-adapted reassortant vaccine virus in adult volunteers: role of local antibody in resistance to infection with vaccine virus. Infect Immun. 1983;40:1044–1051. doi: 10.1128/iai.40.3.1044-1051.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Couch R B, Kasel J A, Six H R, Cate T R. The basis for immunity to influenza in man. In: Nayak D P, editor. Genetic variation among influenza viruses. New York, N.Y: Academic Press; 1981. pp. 535–546. [Google Scholar]
- 16.Cropley I, Douce G, Roberts M, Chatfield S, Pizza M, Marsili I, Rappuoli R, Dougan G. Mucosal and systemic immunogenicity of a recombinant, non-ADP-ribosylating pertussis toxin: effects of formaldehyde treatment. Vaccine. 1995;13:1643–1648. doi: 10.1016/0264-410x(95)00134-m. [DOI] [PubMed] [Google Scholar]
- 17.Czerkinsky C, Anjuere F, McGhee J R, George-Chandy A, Holmgren J, Kieny M P, Fujiyashi K, Mestecky J F, Pierrefite-Carle V, Rask C, Sun J B. Mucosal immunity and tolerance: relevance to vaccine development. Immunol Rev. 1999;170:197–222. doi: 10.1111/j.1600-065X.1999.tb01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Haan L, Verweij W R, Feil I K, Holtrop M, Hol W G, Agsteribbe E, Wilschut J. Role of GM1 binding in the mucosal immunogenicity and adjuvant activity of the Escherichia coli heat-labile enterotoxin and its B subunit. Immunology. 1998;94:424–430. doi: 10.1046/j.1365-2567.1998.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dodane V, Khan M A, Merwin J R. Effect of chitosan on epithelial permeability and structure. Int J Pharm. 1999;182:21–32. doi: 10.1016/s0378-5173(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 20.Felt O, Buri P, Gurny R. Chitosan: a unique polysaccharide for drug delivery. Drug Dev Ind Pharm. 1998;24:979–993. doi: 10.3109/03639049809089942. [DOI] [PubMed] [Google Scholar]
- 21.Hierholzer J C, Suggs M T. Standardized viral hemagglutination and hemagglutination-inhibition tests. I. Standardization of erythrocyte suspensions. Appl Microbiol. 1969;18:816–823. doi: 10.1128/am.18.5.816-823.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirano S. Chitin biotechnology applications. Annu Rev Biotechnol. 1996;2:237–258. doi: 10.1016/s1387-2656(08)70012-7. [DOI] [PubMed] [Google Scholar]
- 23.Holmgren J, Czerkinsky C, Lycke N, Svennerholm A M. Mucosal immunity: implications for vaccine development. Immunobiology. 1992;184:157–179. doi: 10.1016/S0171-2985(11)80473-0. [DOI] [PubMed] [Google Scholar]
- 24.Illum L. The nasal delivery of peptides and proteins. Trends Biotechnol. 1991;9:284–289. doi: 10.1016/0167-7799(91)90091-u. [DOI] [PubMed] [Google Scholar]
- 25.Illum L, Farraj N F, Davis S S. Chitosan as a novel delivery system for peptide drugs. Pharmaceutical Res. 1994;11:1186–1189. doi: 10.1023/a:1018901302450. [DOI] [PubMed] [Google Scholar]
- 26.Jabbal-Gill I, Fisher A N, Rappuoli R, Davis S S, Illum L. Stimulation of mucosal and systemic antibody responses against Bordetella pertussis filamentous haemagglutinin and recombinant pertussis toxin after nasal administration with chitosan in mice. Vaccine. 1998;16:2039–2046. doi: 10.1016/s0264-410x(98)00077-2. [DOI] [PubMed] [Google Scholar]
- 27.Johnson A G. Molecular adjuvants and immunomodulators: new approaches to immunization. Clin Microbiol Rev. 1994;7:277–289. doi: 10.1128/cmr.7.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klinman D M, Barnhart K M, Conover J. CpG motifs as immune adjuvants. Vaccine. 1999;17:19–25. doi: 10.1016/s0264-410x(98)00151-0. [DOI] [PubMed] [Google Scholar]
- 29.Komase K, Tamura S, Matsuo K, Watanabe K, Hattori N, Odaka A, Suzuki Y, Kurata T, Aizawa C. Mutants of Escherichia coli heat-labile enterotoxin as an adjuvant for nasal influenza vaccine. Vaccine. 1998;16:248–254. doi: 10.1016/s0264-410x(97)00176-x. [DOI] [PubMed] [Google Scholar]
- 30.Kublik H, Muller B W. Rheological properties of polymer solutions as carriers for nasal drug delivery systems. Eur J Pharm Biopharm. 1993;39:192–196. [Google Scholar]
- 31.Lambre C R, Terzidis H, Greffard A, Webster R G. Measurement of anti-influenza neuraminidase antibody using a peroxidase-linked lectin and microtitre plates coated with natural substrates. J Immunol Methods. 1990;135:49–57. doi: 10.1016/0022-1759(90)90255-t. [DOI] [PubMed] [Google Scholar]
- 32.Lehr C M, Bouwstra J A, Schacht E H, Junginger H E. In vitro evaluation of mucoadhesive properties of chitosan and some other natural polymers. Int J Pharm. 1992;78:43–48. [Google Scholar]
- 33.Liew F Y, Russell S M, Appleyard G, Brand C M, Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984;14:350. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- 34.Markwell M A, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 35.Mazanec M B, Coudret C L, Fletcher D R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meitin C A, Bender B S, Small J P. Influenza immunisation: intranasal live vaccinia recombinant contrasted with parenteral inactivated vaccine. Vaccine. 1991;9:751–756. doi: 10.1016/0264-410x(91)90292-e. [DOI] [PubMed] [Google Scholar]
- 37.Nicholson K G. Influenza. Curr Opin Infect Dis. 1994;7:168–172. [Google Scholar]
- 38.Nishimura K, Nishimura S, Nishi N, Saiki I, Tokura S, Azuma I. Immunological activity of chitin and its derivatives. Vaccine. 1984;2:93–99. doi: 10.1016/s0264-410x(98)90039-1. [DOI] [PubMed] [Google Scholar]
- 39.Oh Y, Ohta K, Kuno-Sakai H, Kim R, Kimura M. Local and systemic influenza haemagglutinin-specific antibody responses following aerosol and subcutaneous administration of inactivated split influenza vaccine. Vaccine. 1992;10:506–511. doi: 10.1016/0264-410x(92)90348-n. [DOI] [PubMed] [Google Scholar]
- 40.Outlaw M C. Insights into neutralisation of animal viruses gained from study of influenza virus. Epidemiol Infect. 1991;106:205–220. doi: 10.1017/s0950268800048354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peluso G, Petillo O, Ranieri M, Santin M, Ambrosio L, Calabro D, Avallone B, Balsamo G. Chitosan-mediated stimulation of macrophage function. Biomaterials. 1994;15:1215–1220. doi: 10.1016/0142-9612(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 42.Renegar K B, Small P A. Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Renegar K B, Small P A. Passive transfer of local immunity to influenza virus infection by IgA antibody. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 44.Renfrey S, Watts A. Morphological and biochemical characterisation of influenza vaccines commercially available in the United Kingdom. Vaccine. 1994;12:747–752. doi: 10.1016/0264-410x(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 45.Roberts M, Bacon A, Rappuoli R, Pizza M, Cropley I, Douce G, Dougan G, Marinaro M, McGhee J, Chatfield S. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect Immun. 1995;63:2100–2108. doi: 10.1128/iai.63.6.2100-2108.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts M, Cropley I, Chatfield S, Dougan G. Protection of mice against respiratory Bordetella pertussis infection by intranasal immunisation with P.69 and FHA. Vaccine. 1993;11:866–872. doi: 10.1016/0264-410x(93)90363-3. [DOI] [PubMed] [Google Scholar]
- 47.Rozier A, Grove M J, Plazonnet B. Gelrite: a novel, ion-activated, in-situ gelling polymer for ophthalmic vehicles. Effect on bioavailability of timolol. Int J Pharm. 1989;57:163–168. [Google Scholar]
- 48.Schulman J L. Immunology of influenza. In: Kilbourne E, editor. Influenza viruses and influenza. New York, N.Y: Academic Press; 1975. pp. 373–393. [Google Scholar]
- 49.Shahin R, Leef M, Eldridge J, Hudson M F, Gilley R. Adjuvanticity and protective immunity elicited by Bordetella pertussis antigens encapsulated in poly(dl-lactide-co-glycolide) microspheres. Infect Immun. 1995;63:1195–1200. doi: 10.1128/iai.63.4.1195-1200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki K, Okawa Y, Hashimoto K, Suzuki S, Suzuki M. Protecting effect of chitin and chitosan on experimentally induced murine candidiasis. Microbiol Immunol. 1984;28:903–912. doi: 10.1111/j.1348-0421.1984.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 51.Tamura S, Asanuma H, Tomita T, Komase K, Kawahara K, Danbara H, Hattori N, Watanabe K, Suzuki Y, Nagamine T, Aizawa C, Oya A, Kurata T. Escherichia coli heat-labile enterotoxin B subunits supplemented with a trace amount of the holotoxin as an adjuvant for nasal influenza vaccine. Vaccine. 1994;12:1083–1089. doi: 10.1016/0264-410x(94)90177-5. [DOI] [PubMed] [Google Scholar]
- 52.Tamura S, Funato H, Hirabayashi Y, Kikuta K, Suzuki Y, Nagamine T, Aizawa C, Nakagawa M, Kurata T. Functional role of respiratory tract haemagglutinin-specific IgA antibodies in protection against influenza. Vaccine. 1990;8:479–485. doi: 10.1016/0264-410x(90)90250-p. [DOI] [PubMed] [Google Scholar]
- 53.Tamura S I, Samegai Y, Kurata H, Nagamine T, Aizawa C, Kurata T. Protection against influenza virus infection by vaccine inoculated intranasally with cholera toxin B subunit. Vaccine. 1988;6:409–413. doi: 10.1016/0264-410x(88)90140-5. [DOI] [PubMed] [Google Scholar]
- 54.Thanoo B C, Sunny M C, Jayakrishnan A. Cross-linked chitosan microspheres: preparation and evaluation as a matrix for the controlled release of pharmaceuticals. J Pharm Pharmacol. 1992;44:283–286. doi: 10.1111/j.2042-7158.1992.tb03607.x. [DOI] [PubMed] [Google Scholar]
- 55.Varum K M, Myhr M M, Hjerde R J, Smidsrod O. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydr Res. 1997;299:99–101. doi: 10.1016/s0008-6215(96)00332-1. [DOI] [PubMed] [Google Scholar]
- 56.Walker R I. New strategies for using mucosal vaccination to achieve more effective immunisation. Vaccine. 1994;12:387–400. doi: 10.1016/0264-410x(94)90112-0. [DOI] [PubMed] [Google Scholar]