Abstract
Biologics, a class of medicines grown in and purified from genetically engineered cell cultures, have transformed the management of many cancers and rare diseases, such as paroxysmal nocturnal hemoglobinuria. As prescription drug spending has increased and exclusivity periods have expired, manufacturers have developed biosimilars–biologics that may be more affordable and highly similar to a licensed biological therapeutic, with no clinically meaningful differences in terms of safety or efficacy. With biosimilars gaining regulatory approval around the globe and broadening patient access to biologics, this review aims to help rare disease healthcare providers familiarize themselves with biosimilars, understand their development and regulatory approval process, and address practical considerations that may facilitate their use.
Introduction
Biologics, which include hormones, blood products, cytokines, growth factors, vaccines, and monoclonal antibodies, have emerged as indispensable options in the treatment of cancer and other serious health conditions; however, their use has significantly increased healthcare spending.1 As exclusivity periods for many biologics have expired, manufacturers have developed products called “biosimilars,” which are biological medicines that are highly similar to an approved reference product (RP), with no clinically meaningful differences in terms of safety or efficacy. The European Medicines Agency (EMA) was the first regulatory authority to establish a biosimilar approval framework based on safety, efficacy, and quality.2 Biosimilar recombinant human growth hormone (Omnitrope®, Sandoz GmbH, Kundl, Austria) was the first medicine to be approved through the EMA biosimilar regulatory pathway in 2006.3 Since then, dozens of biosimilar medicines have been approved and used in clinical practice with no evidence to date that they perform any differently from the RP on a population level.4 In the US, the Biologics Price Competition and Innovation Act (BPCIA) of 2009 authorized the US Food and Drug Administration (FDA) to oversee a biosimilar approval pathway.5 Modeled with the same intention of the law that allows the development and the approval of generic alternatives to small-molecule drugs, BPCIA was designed to encourage competition and innovation.6 A biosimilar of the granulocyte colony-stimulating factor filgrastim7 (Zarxio®, Sandoz Inc., Princeton, NJ, USA) was the first biosimilar approved in the US, in March 2015.4,7
Despite the endorsement of biosimilars by regulatory authorities around the world, they remain underused.8 This review provides an overview of the expanding knowledge base regarding biosimilars. We seek to help rare disease healthcare providers (HCP) familiarize themselves with biosimilars and understand how they are developed, as well as address practical considerations to facilitate their use.
What are biosimilars?
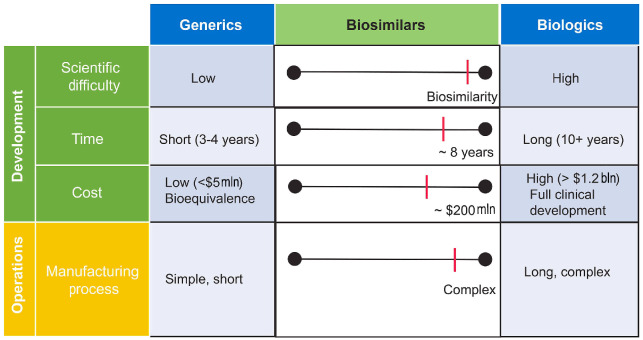
A biosimilar may be defined, in part, as a biologic agent that is highly similar to a licensed RP (Online Supplementary Table S1), the off-patent product to which they offer an alternative.2,5,9,10 Biosimilars have no clinically meaningful differences from originator biologics in function, purity, potency, pharmacokinetics (PK), pharmacodynamics (PD), clinical efficacy, safety, and immunogenicity. To better explain what biosimilars are, it helps to understand what they are not. Biosimilars are fundamentally different from generic drugs (Figure 1). A generic drug is a small molecule with a well-defined structure that is identical to its RP. In addition, generics are usually produced by chemical synthesis, a wholly reproducible process that is generally faster and lower in cost than the development of biologics. They are also indistinguishable from their reference drugs in potency, dosage, route of administration, safety profile, and indication. In contrast, biologics, including biosimilars, are large proteins with complex physicochemical structures (Figure 1).11 Their manufacture involves a highly intricate process using genetically engineered cell lines and extraction via complex purification techniques.12-14 Biosimilars have the same amino acid sequence and highly similar structural and functional attributes as their corresponding RP, yet there may be small differences in the clinically inactive components.2,9,15 Therefore, a biosimilar is not an identical copy of its RP. In addition, it takes approximately eight years to develop a biosimilar, at a cost of up to $200 million (Figure 2).16
It is also important to distinguish biosimilars from non-comparable biologics (also known as “non-comparable biotherapeutics”, “biocopies”, “biomimics”, “intended copies”, and “non-regulated biologics”). Although non-comparables may contain the same amino acid sequence as the RP, they have not usually been subjected to the same rigorous evaluations mandated by biosimilar regulatory procedures.17,18 For example, Abcertin® (Imiglucerase, ISU Abxis, South Korea), a non-comparable enzyme replacement therapy for Gaucher disease, has been approved in South Korea despite the lack of a direct comparison to the RP or physicochemical, immunological, or structural data.17 As a result, non-comparable products may have clinically significant differences in terms of quality, efficacy, and safety compared with their RP.
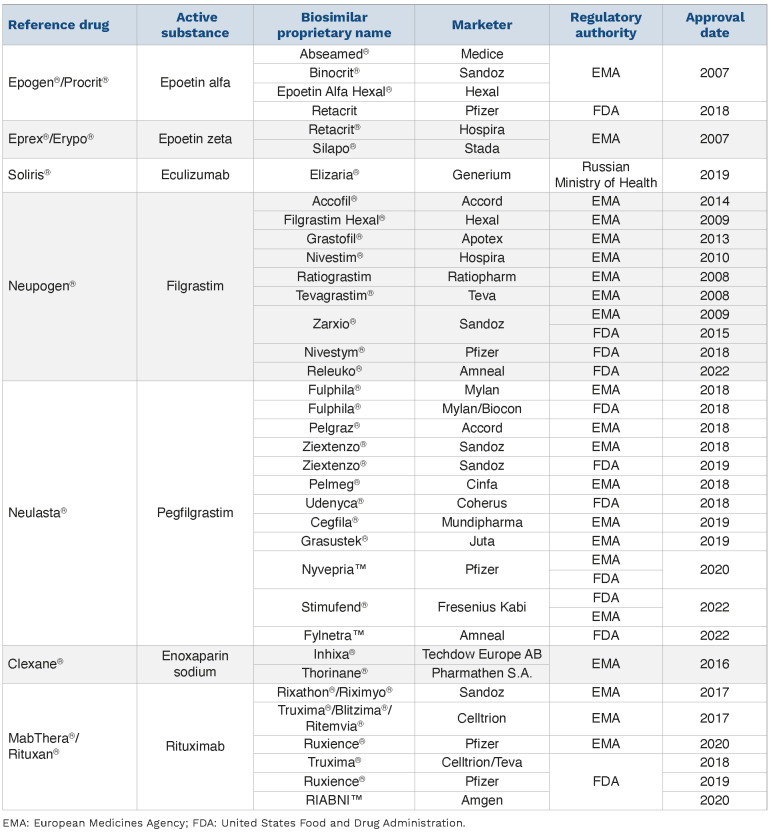
Within the last few years, dozens of biosimilars of interest to hematologists have been approved by regulatory authorities and have been launched in the US, EU, and other countries (Table 1). For example, the first biosimilar to eculizumab RP (Soliris®, Alexion), a monoclonal IgG2/4k antibody, was launched in Russia for the treatment of paroxysmal nocturnal hemoglobinuria (PNH), a rare hematological disease characterized by hemolytic anemia, thrombosis, and peripheral blood cytopenias.19,20 Several other companies are currently developing eculizumab biosimilars (Table 2).21-24
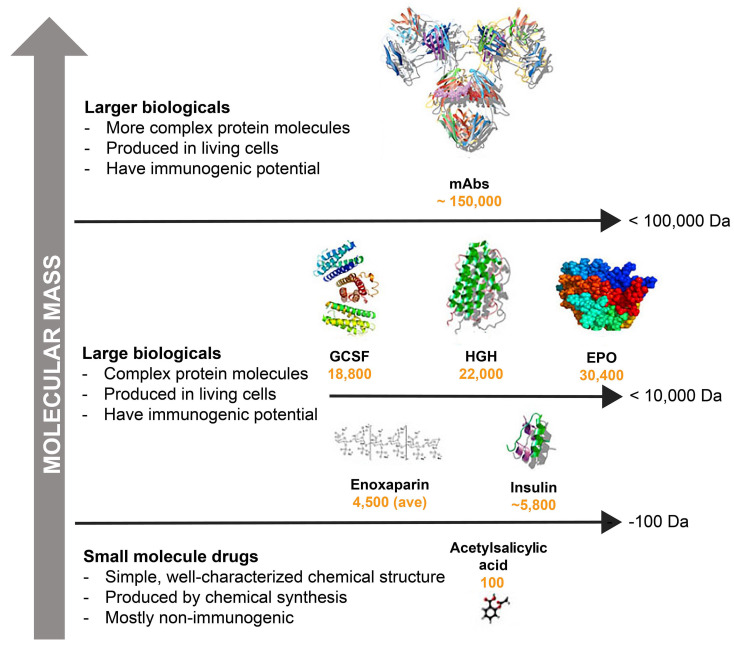
Figure 1.
Molecular mass comparisons: small-molecule drugs versus larger biologics. Adapted from Thill et al.11 ave: average; Da: Daltons; EPO: erythropoietin; GCSF: granulocyte colony-stimulating factor; HGH: human growth hormone; mAbs: monoclonal antibodies. Not drawn to scale.
Figure 2.
Development and manufacturing of biosimilars is more complex than small molecule generics. mln: million; bln: billion.
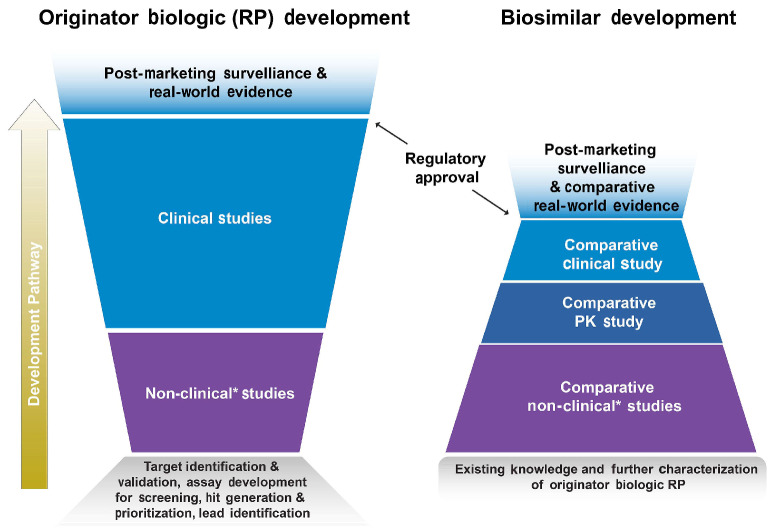
Development of biosimilars
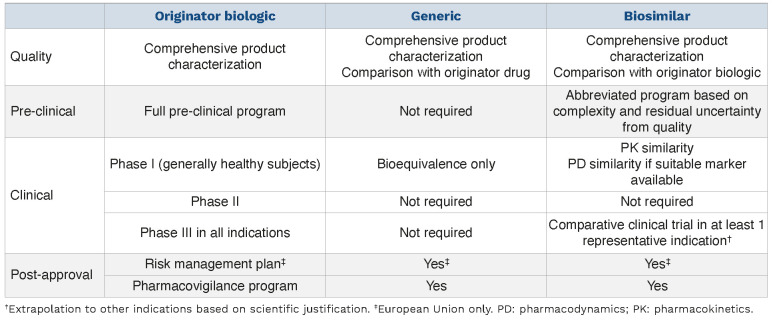
The development of biosimilars differs from originator biologics and generic drugs in many ways (Table 3). Rather than evaluating optimal dosing or patient benefit per se, the biosimilar development process focuses on building a totality of evidence (TOE), which can be defined as the sum of data from comparative analytical, non-clinical, and clinical studies.25 A TOE aims to demonstrate that there are no clinically meaningful differences in safety or efficacy between the biosimilar candidate and its RP.25-27 Biosimilar development uses a stepwise investigational approach which begins with an extensive analytical characterization of the RP to understand structural and functional characteristics such as molecular weight, higher order structure and posttranslational modifications, mechanism of action, and degradation profile denoting stability (Figure 3).25,28,29 These physical and biological critical quality attributes (CQA) are crucial for the function, efficacy, and safety of the RP, and must be clearly described, measured, and monitored.25,30,31 The number of CQA often differs between biologics. For example, based on a scientific understanding of how the attributes of a monoclonal antibody influence safety, efficacy, immunogenicity, and PK/PD, it may have more than 40 CQA (Online Supplementary Figure S1), and these may need to be analyzed using dozens of assays.32
The knowledge gained from these studies is then used to develop a biosimilar product candidate. A series of laboratory-based comparative structural analyses and functional assays are performed, providing an extensive physicochemical and biological profile of the biosimilar candidate. Comparative clinical PK and PD testing is then carried out.26,27 In order to confirm the absence of any clinically meaningful differences between the biosimilar candidate and the RP, regulatory authorities generally recommend at least one comparative clinical study in a representative indication that confirms equivalence with respect to efficacy, safety, and immunogenicity.26,27
Analytical and functional characterization
The structural and functional characterization of a candidate biosimilar is a crucial component of the development process. Although biosimilars have the same amino acid sequence as the RP, different components of the manufacturing process can lead to molecular differences. For example, the structure and stability of a proposed biosimilar can be influenced by the cell line selected, its mutations and culture conditions, as well as the purification method and storage conditions.14 Moreover, post-translational modifications such as glycosylation may yield variants with different function, stability, pharmacologic activity, and immunogenic potential.33-35
Structural and functional characterization entails an analytical evaluation that identifies potential differences between the biosimilar candidate and its RP.6,30 Analytical methods typically include an assessment of CQA such as the amino acid sequence, the primary and higher-order protein structure, disulfide bonds, glycan profile, and potential impurities. Multiple precise, accurate, reproducible, and highly sensitive analytical assays are typically used to evaluate the same quality attribute and maximize the potential for detecting differences.36 For example, the use of complementary analytical techniques in series, such as peptide mapping and capillary electrophoresis combined with mass spectrometry, can provide a meaningful and sensitive comparison of the primary amino acid structure of a candidate biosimilar and RP. Any residual uncertainty regarding a demonstration of similarity between a biosimilar and its RP is reduced if the assessment establishes that the results lie within prespecified criteria based on the knowledge of the RP, method capability, and regulatory guidance.32,36,37
Table 1.
Biosimilars in hematology: recommended for approval or approved in the European Union and/or United States.
Assessment of the candidate biosimilar’s biological activity and mechanism of action follows structural characterization. The goal is to assure the developer that the candidate biosimilar has the same functional activity as its RP. Assays used for functional characterization will depend on the type of molecule, and may include cell-based receptor binding or enzyme kinetics assays. For example, functional assessment of a monoclonal antibody biosimilar candidate involves a clear understanding of the biological effects of the antibody's antigen-binding and complement-binding regions (Online Supplementary Figure S1).25,38 Antibody neutralization and immunogenicity are often mediated via the antigen-binding region. The complement-binding region can impact the PK characteristics, as well as antibody-dependent cell-mediated cytotoxicity and antibody-dependent cellular phagocytosis, both of which are typically important for efficacy.25,38
Non-clinical studies
Once structural and functional similarity has been demonstrated, non-clinical animal studies may be required to assess the safety of the candidate biosimilar prior to conducting clinical studies in humans. Animal studies are typically used to evaluate toxicology and PK to support the safe use of the proposed biosimilar in human subjects; however, studies have generally shown no unexpected findings of safety or toxicity for either the biosimilar candidate or the respective RP when there are minimal structural and functional differences between the molecules.39 Non-clinical animal studies may be skipped if there are minimal analytical variations between the two molecules or if there is no pharmacologically relevant animal species available.27 For example, animal studies were not conducted in preclinical studies of ABP 959, a candidate biosimilar of eculizumab RP, because its target is specific to human complement protein 5.40 Moreover, non-clinical and clinical data from the RP can be used for modeling and simulation to maximize the value of non-clinical studies. Furthermore, modeling and simulation may be used in the design of more efficient comparative clinical studies, which is of particular importance in the development of biosimilars for rare disease indications.41
Clinical studies
The aims of the clinical evaluation are to assess the potential impact of any differences identified during previous steps of the development process and to confirm comparable performance between the candidate biosimilar and the RP. Indeed, the US BPCIA states that an application for a biosimilar must include data from “a clinical study or studies (including an assessment of immunogenicity and pharmacokinetics or pharmacodynamics) that are sufficient to demonstrate safety, purity, and potency in one or more conditions of use for which the reference product is licensed and for which licensure is sought for the biosimilar product”.27
Table 2.
Current status of eculizumab biosimilars.21-24
Table 3.
Differences in regulatory requirements for originator compounds, generics, and biosimilars.
Figure 3.
Comparison of the development pathway for biosimilars versus originator biologics. The development of an originator biologic typically begins with target identification and validation, assay development for screening, and hit generation and prioritization. Optimization, characterization, and candidate drug selection is followed by broad clinical, dose-ranging, pharmacokinetics (PK) / pharmacodynamics (PD), efficacy, safety, and immunogenicity studies. After regulatory approval, the product undergoes post-marketing surveillance, and on occasion, real-world studies. The development of a biosimilar is a stepwise process that begins with the gathering of existing knowledge about the reference product (RP). Following the development of a candidate biosimilar, it and the RP are then comparatively assessed in terms of their structure, mechanism of action, and PK/PD profile. Comparative assessments of efficacy, safety, and immunogenicity are also performed. After regulatory approval, the biosimilar undergoes post-marketing surveillance and is often compared to the RP in real-world studies. *Nonhuman studies including analytical, in vitro, in vivo (animal), ex vivo studies.
The clinical development process begins with an evaluation of the PK/PD profile of the candidate biosimilar.6,27,42 These assessments are a critical part of the TOE demonstrating biosimilarity and can help streamline the design and execution of additional comparative clinical trials.43 PK studies measure parameters such as the area under the curve (AUC) and the maximum observed serum concentration (Cmax). The study population should be the most informative for detecting PK differences between the candidate biosimilar and the RP. Healthy subjects are typically chosen to allow a pertinent and sensitive comparison because they are less likely to produce PK and/or PD variability compared to patients with potential confounding factors such as concomitant disease and medications.6,27 The use of specific patient populations may also be appropriate for various reasons, including potential safety concerns (e.g., known immunogenicity or toxicity from the RP) regarding evaluation in healthy volunteers or if PD biomarkers can only be measured in patients with the relevant disease.
Pharmacokinetic similarity is established when the twosided 90% confidence interval (CI) of the geometric mean ratio of PK values between the candidate biosimilar and the RP lies within a prespecified margin of 80-125% for overall exposure.6,42 The prespecified similarity margin does not denote that the Cmax and AUC, for instance, of the candidate biosimilar may vary from 80% to 125% of the RP. Rather, both sides of the 90% CI must lie within this margin to meet the similarity standard.
Pharmacodynamic assessments examine the biochemical, physiologic, and molecular effects of the proposed biosimilar and RP on the body, such as receptor binding and postreceptor effects. For example, the hemolytic complement activity of eculizumab RP and its biosimilars, which is considered critical to their mechanism of action, was tested using a 50% hemolytic complement assay. This assay is sensitive to the reduction, absence, and/or inactivity of any components of the classical and terminal complement pathway.44 Although PD studies can provide useful evidence of biosimilar function, they are only appropriate when a relevant PD marker is available.
Once structural, functional, and pharmacologic similarity has been established, developers can proceed to an evaluation of comparative clinical efficacy and safety. The goal is to demonstrate that the biosimilar candidate has no clinically meaningful differences compared with the RP.18,27 The extent of the clinical program is determined by any residual uncertainty and the degree of similarity demonstrated in analytical and non-clinical testing. In light of the fact that no biosimilar candidates have ever been rejected for approval due to efficacy differences from their respective RP,45 it should be noted that regulatory agencies are beginning to question whether comparative efficacy trials are routinely necessary if a biosimilar candidate has been wellcharacterized and has demonstrated a highly similar clinical pharmacology profile.46
Assessment of similarity between a candidate biosimilar and its RP in a comparative clinical trial is based on the null hypothesis. Using a two-sided test that demonstrates that efficacy lies within prespecified equivalence margins, the assessment must be able to detect any clinically meaningful difference in efficacy.27 The results are typically expressed as the risk ratio (RR) or risk difference (RD) in efficacy between the candidate biosimilar and its RP. Clinical equivalence is established based on a predetermined two-sided 90%47 or 95%41 CI of the RR or RD since the studies are designed to determine both non-inferiority and non-superiority of the candidate biosimilar. If the CI of the RR or RD lies within the equivalence margin, then a biosimilar candidate can be considered to be clinically equivalent to its RP.
Subjects for comparative clinical trials should be chosen to increase the chance of detecting any possible clinically meaningful differences and to adequately assess safety.27 The development of biosimilars for rare diseases is associated with an additional set of challenges.48 For example, experts specializing in the treatment of rare diseases who are needed to conduct the trials are generally limited. Further, the availability of only a few dedicated treatment facilities around the globe make study participation difficult for some patients. In addition, patient populations are small, and the understanding of the disease process may be limited, making selection and enrollment for comparative clinical trials of biosimilars challenging. This is particularly true of treatment-naïve patients as most patients are often already receiving treatment with the originator product. For example, patients with PNH may be reluctant to participate in a comparative clinical trial because they do not wish to interrupt their current treatment, which further reduces the number of available subjects. Consequently, the ongoing comparative clinical trial for the proposed eculizumab biosimilar ABP 959 recruited patients with PNH who had been previously treated with eculizumab RP.49 In contrast, a comparative clinical trial of Elizaria®, a biosimilar to eculizumab RP available in Russia, included both treatment-naïve patients and patients who had already received eculizumab RP.19 In support of its approval, the Phase 1b open-label study showed acceptable safety and an expected PK/PD profile of Elizaria® in treatmentnaïve patients with PNH during the induction period.50
The identification of endpoints for comparing a biosimilar candidate and its RP must consider how to make a precise comparison of the relevant therapeutic effects while eliminating any confounding factors. Endpoints that are sensitive enough to detect potential differences between the candidate biosimilar and the RP are generally more appropriate than the measures used to demonstrate efficacy in pivotal trials for the RP. The endpoint could be that of clinical outcome, or alternatively, an appropriate surrogate endpoint relevant to clinical outcomes. Studies of eculizumab and its biosimilars, for example, utilized hemolysis as measured by lactase dehydrogenase as a surrogate endpoint. The results from a comparative clinical study can be used to reduce any residual uncertainty regarding whether there are actually any clinically meaningful differences. Since patients in the real world may be switched from an RP to a biosimilar, crossover studies allow developers to better understand comparative efficacy and address potential safety concerns. For example, the DAHLIA study is evaluating the efficacy and safety of ABP 959 compared with eculizumab RP in adult participants with PNH with the use of a crossover design.49 Studies like these may be particularly helpful in alleviating concerns about immunogenicity after a switch from the RP to a biosimilar.
Assessment of immunogenicity
The assessment of immunogenicity is an important component of building the TOE to support biosimilarity and obtain regulatory approval. Due to their antigenic properties, biologics can sometimes trigger unfavorable immune reactions.51 The level of immunogenicity varies between biologics and may increase when they are administered frequently over a long period of time.52 Many factors affect the immunogenicity of biologics, including their structure, primary sequence, and post-translational modifications. The dose, route and frequency of administration, and the product formulation, as well as the patient’s age, sex, genetic profile, and immune status may all also impact a biologic’s immunogenicity.
The presence of antidrug antibodies (ADA) after treatment may decrease the efficacy of the biologic by neutralizing it or decreasing its half-life.53 Although immunogenicity is not a concern for most biologics, some biologics may trigger ADA which impact efficacy and safety. Therefore, biosimilar developers should include at least one clinical study that measures and compares binding and neutralizing antibodies between the candidate biosimilar and the RP.42 It is not advisable to use non-clinical methods for evaluating immunogenicity. Assays used for measuring ADA have become more sensitive and allow specific ADA to be identified.54 Consequently, these sensitive assays may lead to the detection of higher levels of ADA versus those observed in the original studies of the RP.55 Thus, the sensitivity of ADA assays must be considered when comparing results from different trials.
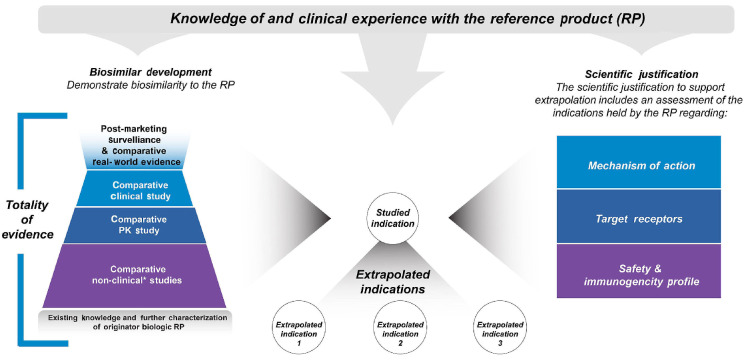
Extrapolation of indications
Rather than conducting clinical trials for every approved indication of a particular RP, biosimilar developers may gain approval in some or all of the indications for which the RP is approved, even if the particular biosimilar candidate was not tested in all of them.27,42 For instance, infliximab RP has been studied in and received approval to treat rheumatoid arthritis, Crohn’s disease, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis. The FDA has approved infliximab biosimilars for the same indications as the RP, even though the biosimilars only underwent clinical testing in a few of the conditions listed above. Similarly, Elizaria® was only tested in a comparative clinical study of patients with PNH, yet it also has indications in Russia for atypical hemolytic uremic syndrome, generalized myasthenia gravis, and neuromyelitis optica spectrum disorder.19,56 Due to the rarity of these diseases and the associated challenges with recruiting patients, extrapolation to additional rare disease indications is critical for the regulatory approval of biosimilars.
Although there is increasing recognition of the value of biosimilars, misunderstandings related to the concept of extrapolation persist, and contribute to the skepticism found among many HCP.57 It is important to emphasize here that extrapolation is not based solely on clinical evidence from one study, nor is it from one indication to another. It is rather that regulatory agencies may allow for extrapolation of indications based on adequate scientific justification supported by the TOE, on the previous finding of safety and effectiveness for the RP in the indications sought for approval, and by adequately addressing several key scientific factors, such as the mechanism of action (Figure 4). Differences in the scientific factors across indications do not preclude extrapolation; however, any differences must be adequately addressed as part of the scientific justification. For example, there may be a difference in the target/receptor between indications, but the comparative functional assessment must demonstrate that binding to all relevant targets/receptors is highly similar between the biosimilar candidate and the RP. If all these factors are adequately addressed, and the study population in the comparative clinical study is sufficiently sensitive so as to allow clinically meaningful differences to be detected, then developers, HCP, and patients can be confident that the candidate biosimilar will have no clinically meaningful differences in efficacy and safety compared with the RP in other approved indications which were not directly studied.
Interchangeability
The emergence of biosimilars has caused many clinicians to reconsider their treatment choices. Based on the law and US FDA draft guidance on interchangeability,58,59 a biosimilar designated as interchangeable “may be substituted for the reference product without the intervention of the healthcare provider who prescribed the reference product” as permitted by state law. In the US, there must be an evidence-based expectation that the biosimilar “can be expected to produce the same clinical result as the reference product in any given patient and, if the biological product is administered more than once to an individual, the risk in terms of safety or diminished efficacy of alternating or switching between the use of the biological product and the reference product is no greater than the risk of using the reference product without such alternation or switch”.59 The FDA guidance on interchangeability indicates that the clinical study should include at least three switches between the biosimilar and RP to support interchangeability.59 The EU and most other countries do not provide regulatory guidance on interchangeability, nor do they evaluate whether biosimilars and RP are interchangeable.
Naming and pharmacovigilance
The FDA recommends the creation of distinguishable names by adding a 4-letter suffix to the “core name” (typically similar to the international non-proprietary name) for a biosimilar.60 For example, specific epoetin alfa and rituximab biosimilars have been given the non-proprietary names epoetin alfa-epbx and rituximab-arrx, respectively. A biosimilar product may not be approved for all the indications approved for the RP for several reasons (e.g., residual regulatory exclusivity protections for the RP). Therefore, the use of unique names is critical for assuring that the appropriate medication is dispensed.61 The adoption of distinguishable names is important to patient safety, and also ensures that specific adverse events are correctly attributed to the appropriate product and manufacturer.60,62
Outside the US, there is no consistent regulatory approach regarding the naming of biosimilars. In the EU, for example, physicians must document the trade name and the batch number for all biologics. However, this is not routinely done in clinical practice, making it challenging for regulators to identify products with safety issues.
Figure 4.
Extrapolation of indications for a biosimilar: scientific justification. A biosimilar may be approved for an indication without direct studies of the biosimilar in that indication. Regulatory agencies may allow for extrapolation of indications approved for the reference product (RP) based on adequate scientific justification supported by the biosimilar totality of evidence, the previous finding of safety and effectiveness for the RP in the indications sought for approval, and adequately addressing several key scientific factors. PK: pharmacokinetics. *Non-human studies including analytical, in vitro, in vivo (animal), ex vivo studies.
Future perspectives
Biologics are a primary treatment option for several cancers and rare diseases; however, their increasing use is one of the main drivers of the growth in healthcare spending. In fact, biologics accounted for 38% of US prescription drug spending in 2015 due to their high cost per dose, and for 70% of drug spending growth between 2010 and 2015.63
Although real-world evaluation of biosimilar-related healthcare cost savings is limited, there is increasing evidence that market entry of biosimilars has a robust impact. For example, a Johns Hopkins study using employer plan data from 13 large companies reported that the prices for infliximab and filgrastim biosimilars were 32% and 26% lower than their RP, respectively.64 Providence St Joseph Health, a US non-profit health system, implemented a biosimilar utilization management program that yielded savings of $26.9 million over 2 years.65 In addition, a recent analysis estimated the cost saving potential of biosimilar use in the US to be $54 billion over 10 years, with a lower- to upperbound range of $25 billion to $150 billion.63 Moreover, a case study by the Pacific Research Institute suggested that the annual cost reductions for US employer-sponsored health plans could be as high as 8.4% (i.e., between $262 million and $315 million in annual cost savings) if biosimilars reach a 50% share for a popular biologic.66 Savings could rise to $7 billion across US federal and commercial programs if biosimilars reach a 75% market share. In Europe, in 2017, sales for the top 10 biologic products were €16.5 billion.67 Most of these biologics have lost exclusivity in Europe and biosimilars are available for clinical use. In a study aimed to assess the cost savings generated by the introduction of anti-TNF biosimilars in French hospitals 5 years ago, a total of €824 million was saved.68 Similarly, a Spanish budget impact analysis estimated that figures for the period 20092019 show biosimilar competition to have resulted in cost savings of €2.3 billion, about half the savings being due to a reduction in list prices, and the other half originating from hospital tender discounts.69 Although the discount on biosimilars may vary from country to country, by 2020, annual savings could be seen to have increased up to €10 billion if they achieve at least a 50% share.70 Biosimilar versions of biologics approved for rare diseases, such as eculizumab RP, could, therefore, offer an important means of generating cost savings and improving access.
The past decade has seen an increase in the scientific evidence supporting the use of biosimilar products. Many countries now have well-defined regulatory standards to ensure that biosimilars are as safe and efficacious as their RP counterparts. Because some clinical practice guidelines have not recommended biosimilars, there continues to be skepticism among HCP about their role in clinical practice.8 Given this, there is a need to explain how to switch patients from an RP to a biosimilar. Biosimilar adoption may be more widely implemented when the data supporting their approval and real-world evidence is available for scrutiny. HCP seem particularly uncertain about extrapolation to other indications.8,71 Promoting a greater understanding of the fact that extrapolation is based on the TOE rather than on clinical evidence from one study may help more physicians to use them. A TOE demonstrating that the biosimilar is comparable to the RP is the best assurance that the two molecules have similar efficacy, safety, and immunogenicity in all approved indications of the RP.
In conclusion, as healthcare costs continue to rise, the availability of biosimilars presents an opportunity to expand the treatment armamentarium and deliver savings to healthcare systems and consumers, just as generics have done for many years. The TOE includes data from analytical studies, non-clinical comparative PK testing, and, in most cases, at least one clinical trial to confirm the absence of any clinically meaningful differences between the biosimilar candidate and the RP. Increased adoption of biosimilars will require robust educational initiatives to help HCP better understand what biosimilars are, how they are developed and approved, and how they can be used in practice. Continuing to educate the HCP community regarding biosimilars will foster informed decision making and help enable the safe use of these potentially cost-effective treatments.
Supplementary Material
Acknowledgments
Editorial and graphics support were provided by Innovation Communications Group, New York, NY, USA, and paid for by Amgen Inc., Thousand Oaks, CA, USA. Medical writing assistance was provided by Alex Romero, PhD (Amgen Inc.), under the direction of Sonya Lehto, PhD (Amgen Inc.).
Funding Statement
Funding: Open Access and Article Processing Charges were funded by Amgen Inc., Thousand Oaks, CA, USA.
References
- 1.Crosson FJ. Addressing the cost of biologic and specialty drugs. JAMA Intern Med. 2021;181(1):22-23. [DOI] [PubMed] [Google Scholar]
- 2.European Medicines Agency. Biosimilars in the EU - Information guide for healthcare professionals. Updated 02/10/2019. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf. Accessed 14 November 2021. [Google Scholar]
- 3.European Medicines Agency. Omnitrope. European public assessment report (EPAR) summary for the public. Updated 28/04/2021. https://www.ema.europa.eu/en/medicines/human/EPAR/omnitrope. Accessed 14 November 2021. [Google Scholar]
- 4.Kang HN, Thorpe R, Knezevic I. noindent1Survey participants from 19 countries. The regulatory landscape of biosimilars: WHO efforts and progress made from 2009 to 2019. Biologicals. 2020;65:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration. Biosimilars action plan: balancing innovation and competition. July 2018. https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/UCM613761.pdf. Accessed 14 November 2021. [Google Scholar]
- 6.US Food and Drug Administration. Guidance for industry: clinical pharmacology data to support a demonstration of biosimilarity to a reference product. December 2016. https://www.fda.gov/media/88622/download. Accessed 14 November 2021. [Google Scholar]
- 7.ZARXIO (filgrastim-sndz) injection [summary of product characteristics]. Sandoz Inc., Princeton NJ, USA. 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125553lbl.pdf. Accessed 1 December 2022. [Google Scholar]
- 8.Kolbe AR, Kearsley A, Merchant L, et al. Physician understanding and willingness to prescribe biosimilars: findings from a US National Survey. BioDrugs. 2021;35(3):363-372. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Guidelines on evaluation of similar biotherapeutic products (SBPs). WHO Technical Report Series No. 977, 2013. https://www.who.int/biologicals/publications/trs/areas/biological_therapeutics/TRS_977_Annex_2.pdf. Accessed 14 November 2021. [Google Scholar]
- 10.Arato T. Japanese regulation of biosimilar products: past experience and current challenges. Br J Clin Pharmacol. 2016;82(1):30-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thill M, Thatcher N, Hanes V, Lyman GH. Biosimilars: what the oncologist should know. Future Oncol. 2019;15(10):1147-1165. [DOI] [PubMed] [Google Scholar]
- 12.Declerck P, Farouk-Rezk M, Rudd PM. Biosimilarity versus manufacturing change: two distinct concepts. Pharm Res. 2016;33(2):261-268. [DOI] [PubMed] [Google Scholar]
- 13.Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19(3):411-419. [DOI] [PubMed] [Google Scholar]
- 14.Nathan JJ, Ramchandani M, Kaur P. Manufacturing of biologics. In: Biologic and Systemic Agents in Dermatology. Yamauchi P. (Ed.). Springer, Cham, Switzerland, 2018. [Google Scholar]
- 15.US Food and Drug Administration. Biosimilars: questions and answers regarding implementation of the Biologics Price Competition and Innovation Act of 2009. https://www.fda.gov/media/119258/download. Accessed 14 November 2021. [Google Scholar]
- 16.Agbogbo FK, Ecker DM, Farrand A, et al. Current perspectives on biosimilars. J Ind Microbiol Biotechnol. 2019;46(9-10):1297-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drelichman G, Castaneda-Hernandez G, Cem Ar M, et al. The road to biosimilars in rare diseases - ongoing lessons from Gaucher disease. Am J Hematol. 2020;95(3):233-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mysler E, Pineda C, Horiuchi T, et al. Clinical and regulatory perspectives on biosimilar therapies and intended copies of biologics in rheumatology. Rheumatol Int. 2016;36(5):613-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulagin AD, Ptushkin VV, Lukina EA, et al. Randomized multicenter noninferiority phase III clinical trial of the first biosimilar of eculizumab. Ann Hematol. 2021;100(11):2689-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804-2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elizaria® (eculizumab) injection [summary of product characteristics]. Generium Pharmaceuticals, Moscow, Russia. 2021. https://www.generium.ru/upload/preparation/elizariya/Elizaria.pdf. Accessed on 1 December 2022. [Google Scholar]
- 22.ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US). ClinicalTrials.gov Identifier: NCT04060264; Clinical Trial of BCD-148 and Soliris® for the treatment of patients with paroxysmal nocturnal hemoglobinuria. https://clinicaltrials.gov/ct2/show/NCT04060264. Accessed 4 October 2022. [Google Scholar]
- 23.Jang JH, Gomez RD, Bumbea H, et al. A phase III randomized clinical trail comparing SB12 (proposed eculizumab biosimilar) with reference eculizumab in patients with paroxysmal nocturnal hemoglobinuria. Presented at European Hematology Association Annual Meeting, June 16-19, 2022, Vienna, Austria. https://library.ehaweb.org/eha/2022/eha2022-congress/357691/jun.ho.jang. Accessed 5 October 2022. [Google Scholar]
- 24.Kulasekararaj A, Lanza F, Arvanitakis A, et al. Efficacy and safety of biosimilar candidate ABP 959 as compared with eculizumab reference product in paroxysmal nocturnal hemoglobinuria. Blood. 2022;140(Suppl 1):8660-8662. [DOI] [PubMed] [Google Scholar]
- 25.Markus R, Liu J, Ramchandani M, Landa D, Born T, Kaur P. Developing the totality of evidence for biosimilars: regulatory considerations and building confidence for the healthcare community. BioDrugs. 2017;31(3):175-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Medicines Agency. Guideline on similar biological medicinal products. 23 October 2014. CHMP/437/04 Rev. 1. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2014/10/WC500176768.pdf. Accessed 14 November 2021. [Google Scholar]
- 27.US Food and Drug Administration. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. April 2015. https://www.fda.gov/media/82647/download. Accessed 14 November 2021. [Google Scholar]
- 28.Abraham I, Sun D, Bagalagel A, et al. Biosimilars in 3D: definition, development and differentiation. Bioengineered. 2013;4(4):203-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCamish M, Woollett G. Worldwide experience with biosimilar development. MAbs. 2011;3(2):209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chow SC, Song F, Bai H. Analytical similarity assessment in biosimilar studies. AAPS J. 2016;18(3):670-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration. Guidance for industry. Q8(R2) Pharmaceutical development. November 2009. https://www.fda.gov/media/71535/download. Accessed 14 November 2021. [Google Scholar]
- 32.Sivendran R, Ramirez J, Ramchandani M, Liu J. Scientific and statistical considerations in evaluating the analytical similarity of ABP 501 to adalimumab. Immunotherapy. 2018;10(11):1011-1021. [DOI] [PubMed] [Google Scholar]
- 33.Barnes HJ, Ragnarrson G, Alvan G. Quality and safety considerations for recombinant biological medicines: a regulatory perspective. Int J Risk Saf Med. 2009;2009(21):13-22. [Google Scholar]
- 34.Kuhlmann M, Covic A. The protein science of biosimilars. Nephrol Dial Transplant. 2006;21 (Suppl 5):v4-8. [DOI] [PubMed] [Google Scholar]
- 35.Colbert RA, Cronstein BN. Biosimilars: the debate continues. Arthritis Rheum. 2011;63(10):2848-2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration. Quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product guidance for industry. April 2015. https://www.fda.gov/media/135612/download. Accessed 14 November 2021. [Google Scholar]
- 37.Hutterer KM, Ip A, Kuhns S, Cao S, Wikstrom M, Liu J. Analytical similarity assessment of ABP 959 in comparison with eculizumab reference product. BioDrugs. 2021;35(5):563-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Declerck PJ. Biosimilar monoclonal antibodies: a science-based regulatory challenge. Expert Opin Biol Ther. 2013;13(2):153-156. [DOI] [PubMed] [Google Scholar]
- 39.Mihalcik L, Chow V, Ramchandani M, Hinkle B, McBride HJ, Lebrec H. Use of nonclinical toxicity studies to support biosimilar antibody development. Regul Toxicol Pharmacol. 2021;122:104912. [DOI] [PubMed] [Google Scholar]
- 40.Chow V, Pan J, Chien D, Mytych DT, Hanes V. A randomized, double-blind, single-dose, three-arm, parallel group study to determine pharmacokinetic similarity of ABP 959 and eculizumab (Soliris®) in healthy male subjects. Eur J Haematol. 2020;105(1):66-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang YC, Wang Y, Schrieber SJ, et al. Role of modeling and simulation in the development of novel and biosimilar therapeutic proteins. J Pharm Sci. 2019;108(1):73-77. [DOI] [PubMed] [Google Scholar]
- 42.European Medicines Agency. Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues. 18 December 2014. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf. Accessed 14 November 2021. [Google Scholar]
- 43.Li J, Florian J, Campbell E, et al. Advancing biosimilar development using pharmacodynamic biomarkers in clinical pharmacology studies. Clin Pharmacol Ther. 2020;107(1):40-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costabile M. Measuring the 50% haemolytic complement (CH50) activity of serum. J Vis Exp. 2010;29(37):1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schiestl M, Ranganna G, Watson K, et al. The path towards a tailored clinical biosimilar development. BioDrugs. 2020;34(3):297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niazi S. Scientific rationale for waiving clinical efficacy testing of biosimilars. Drug Des Devel Ther. 2022;16:2803-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.European Medicines Agency. Biosimilar medicines: overview. 2017. https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview. Accessed 25 May 2021. [Google Scholar]
- 48.Whicher D, Philbin S, Aronson N. An overview of the impact of rare disease characteristics on research methodology. Orphanet J Rare Dis. 2018;13(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.ClinicalTrials.gov. Bethesda, MD: National Library of Medicine (US). ClinicalTrials.gov Identifier: NCT03818607; A study evaluating the efficacy and safety of ABP 959 compared with eculizumab in adult participants with PNH (DAHLIA). https://clinicaltrials.gov/ct2/show/NCT03818607. Accessed 14 November 2021. [Google Scholar]
- 50.Ptushkin VV, Kulagin AD, Lukina EA, et al. [Results of phase Ib open multicenter clinical trial of the safety, pharmacokinetics and pharmacodynamics of first biosimilar of eculizumab in untreated patients with paroxysmal nocturnal hemoglobinuria during induction of therapy]. Ter Arkh. 2020;92(7):77-84. [DOI] [PubMed] [Google Scholar]
- 51.Baker MP, Reynolds HM, Lumicisi B, Bryson CJ. Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self Nonself. 2010;1(4):314-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang BA, Mackey T. Emerging patient safety issues under health care reform: follow-on biologics and immunogenicity. Ther Clin Risk Manag. 2011;7:489-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radstake TR, Svenson M, Eijsbouts AM, et al. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. 2009;68(11):1739-1745. [DOI] [PubMed] [Google Scholar]
- 54.Moxness M, Tatarewicz S, Weeraratne D, et al. Immunogenicity testing by electrochemiluminescent detection for antibodies directed against therapeutic human monoclonal antibodies. Clin Chem. 2005;51(10):1983-1985. [DOI] [PubMed] [Google Scholar]
- 55.Kaur P, Chow V, Zhang N, Moxness M, Kaliyaperumal A, Markus R. A randomised, single-blind, single-dose, three-arm, parallelgroup study in healthy subjects to demonstrate pharmacokinetic equivalence of ABP 501 and adalimumab. Ann Rheum Dis. 2017;76(3):526-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gusarova V, Degterev M, Lyagoskin I, et al. Analytical and functional similarity of biosimilar Elizaria® with eculizumab reference product. J Pharm Biomed Anal. 2022;220:115004. [DOI] [PubMed] [Google Scholar]
- 57.Cohen H, Beydoun D, Chien D, et al. Awareness, knowledge, and perceptions of biosimilars among specialty physicians. Adv Ther. 2017;33(12):2160-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Government Publishing Office [US]. Patient Protection and Affordable Care Act. 23 March 2010. www.gpo.gov/fdsys/pkg/PLAW-111publ148/html/PLAW-111publ148.htm. Accessed 14 November 2021. [Google Scholar]
- 59.US Food and Drug Administration. Guidance for Industry. Considerations in demonstrating interchangeability with a reference product. May 2019. https://www.fda.gov/media/124907/download. Accessed 14 November 2021. [Google Scholar]
- 60.US Food and Drug Administration. Guidance for industry. Nonproprietary naming of biological products. January 2017. https://www.fda.gov/media/93218/download. Accessed 14 November 2021. [Google Scholar]
- 61.Tomaszewski D. Biosimilar naming conventions: pharmacist perceptions and impact on confidence in dispensing biologics. J Manag Care Spec Pharm. 2016;22(8):919-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Felix T, Johansson TT, Colliatie JA, Goldberg MR, Fox AR. Biologic product identification and US pharmacovigilance in the biosimilars era. Nat Biotechnol. 2014;32(2):128-130. [DOI] [PubMed] [Google Scholar]
- 63.Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7(4):3. [PMC free article] [PubMed] [Google Scholar]
- 64.Socal M, Ballreich J, Chyr L, Anderson G. Biosimilar medications savings opportunities for large employers. March 2020. https://www.eric.org/wp-content/uploads/2020/03/JHU-Savings-Opportunities-for-Large-Employers.pdf. Accessed 7 September 2022. [Google Scholar]
- 65.Humphreys SZ. Real-world evidence of a successful biosimilar adoption program. Future Oncol. 2022;18(16):1997-2006. [DOI] [PubMed] [Google Scholar]
- 66.Winegarden W. Pacific Research Institute. Impediments to a stronger biosimilars market: an infliximab case study. June 2018. https://www.pacificresearch.org/wp-content/uploads/2018/06/PolicyObstaclesFweb.pdf. Accessed 14 November 2021. [Google Scholar]
- 67.IQVIA Report. The impact of biosimilar competition in Europe. Presentation by Per Troein, European Commission. London: IQVIA; 2018. [Google Scholar]
- 68.Jarrion Q, Azzouz B, Robinson J, Jolly D, Vallet C, Trenque T. Penetration rate of anti-TNF biosimilars and savings at 5 years after their introduction in French hospitals. Therapie. 2022;77(4):467-475. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Goni M, Rio-Alvarez I, Carcedo D, Villacampa A. Budget impact analysis of biosimilar products in Spain in the period 2009-2019. Pharmaceuticals (Basel). 2021;14(4):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dutta B, Huys I, Vulto AG, Simoens S. Identifying key benefits in European off-patent biologics and biosimilar markets: it is not only about price! BioDrugs. 2020;34(2):159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jacobs I, Singh E, Sewell KL, Al-Sabbagh A, Shane LG. Patient attitudes and understanding about biosimilars: an international cross-sectional survey. Patient Prefer Adherence. 2016;10:937-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.