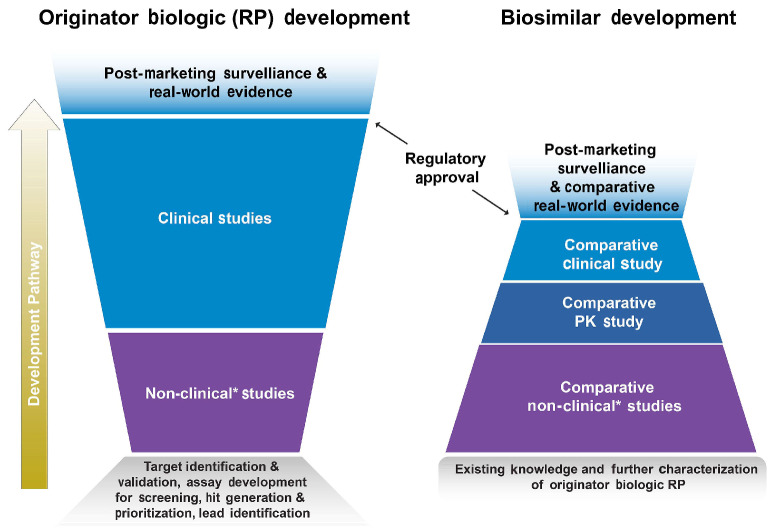
Figure 3.
Comparison of the development pathway for biosimilars versus originator biologics. The development of an originator biologic typically begins with target identification and validation, assay development for screening, and hit generation and prioritization. Optimization, characterization, and candidate drug selection is followed by broad clinical, dose-ranging, pharmacokinetics (PK) / pharmacodynamics (PD), efficacy, safety, and immunogenicity studies. After regulatory approval, the product undergoes post-marketing surveillance, and on occasion, real-world studies. The development of a biosimilar is a stepwise process that begins with the gathering of existing knowledge about the reference product (RP). Following the development of a candidate biosimilar, it and the RP are then comparatively assessed in terms of their structure, mechanism of action, and PK/PD profile. Comparative assessments of efficacy, safety, and immunogenicity are also performed. After regulatory approval, the biosimilar undergoes post-marketing surveillance and is often compared to the RP in real-world studies. *Nonhuman studies including analytical, in vitro, in vivo (animal), ex vivo studies.