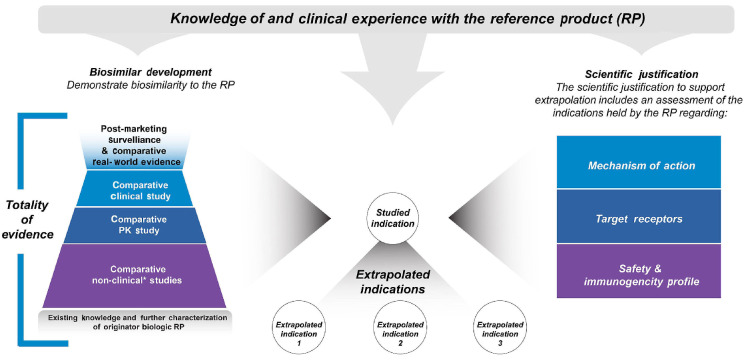
Figure 4.
Extrapolation of indications for a biosimilar: scientific justification. A biosimilar may be approved for an indication without direct studies of the biosimilar in that indication. Regulatory agencies may allow for extrapolation of indications approved for the reference product (RP) based on adequate scientific justification supported by the biosimilar totality of evidence, the previous finding of safety and effectiveness for the RP in the indications sought for approval, and adequately addressing several key scientific factors. PK: pharmacokinetics. *Non-human studies including analytical, in vitro, in vivo (animal), ex vivo studies.