Covalent, non-reversible Bruton tyrosine kinase inhibitors (BTKi) ibrutinib and acalabrutinib have changed the management landscape of several chronic B-cell malignancies. However, BTKi have demonstrated some challenging off-target toxicities and relatively high rates of discontinuation due to toxicity in non-trial patient populations.1,2 A pooled analysis of the long-term follow-up of 424 patients receiving ibrutinib enrolled in chronic lymphocytic leukemia (CLL) trials3 reported any grade bleeding adverse events (AE) in 55% (using multiple AE terms for bleeding) including grade >3 bleeding events in 5%. A recent pooled analysis of 1,040 patients receiving the more selective, second generation BTKi acalabrutinib reported hemorrhage AE of any grade in 482 patients (46%, grade >3, 3%).4 The high rates of bleeding AE with covalent BTKi have been attributed to inhibition of platelet tyrosine kinases which results in impaired signaling and function downstream of platelet GPVI, integrin aIIbb3 and GPIb.57 Off-target inhibition of TEC and Src family kinases (SFK) expressed by platelets has been implicated in the mechanism of bleeding as patients with X-linked agammaglobulinemia (XLA), that lack functional BTK, do not suffer from increased bleeding.8 Pirtobrutinib (formerly Loxo-305) is a non-covalent oral BTKi9 that demonstrates a greater than 300-fold selectivity for BTK versus 363 (98%) of 370 other kinases reducing the potential risk for off-target toxicities and impressive early clinical activity in mantle cell lymphoma (MCL), chronic lymphocytic leukemia (CLL), Waldenstrom’s macroglobulinemia (WM) and Richter transformation (RT) of CLL in patients resistant or intolerant to covalent BTK inhibition in the BRUIN phase Ib/II trial.9,10 Although follow-up remains relatively short (median, ~9 months), grade 1-2 bruising or bleeding was reported in only 22% (20% grade 1, 2% grade 2, 15% treatment-related) with no grade 3-5 bleeding described.11 In order to establish whether the lower rates of bleeding reported in pirtobrutinib trials corresponded with milder platelet dysfunction relative to covalent BTKi ibrutinib and acalabrutinib, we investigated platelet function in patients with non-Hodgkin lymphoma or CLL receiving treatment with covalent and non-covalent BTKi.
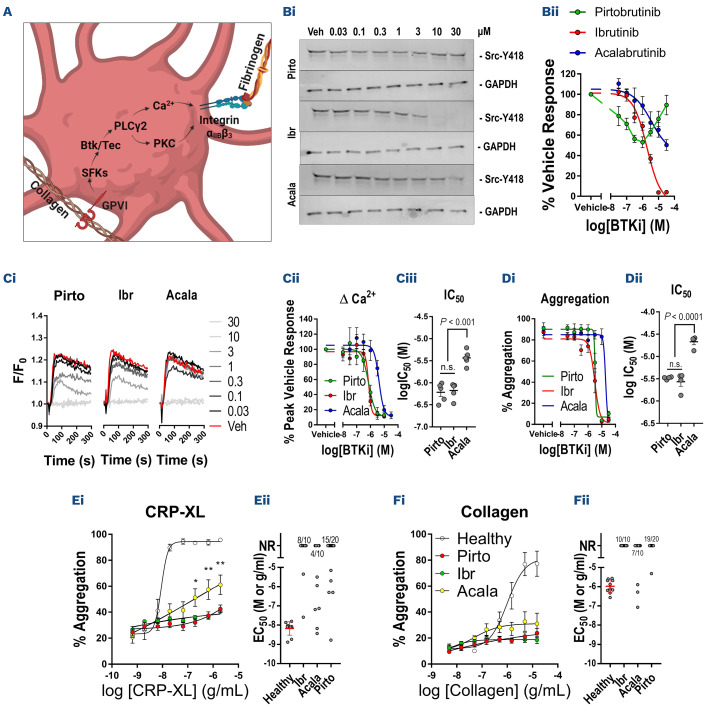
We initially characterized the relative effects of BTKi on platelet signaling and function (Figure 1A) following in vitro treatment of blood samples from healthy donors using procedures approved by the University of Reading Research Ethics Committee. Ibrutinib caused complete inhibition of Src tyrosine autophosphorylation (half maximal inhibitory concentration [IC50]=1.9 µM) stimulated by the specific GPVI agonist, collagen-related peptide (CRP-XL). Acalabrutinib caused partial inhibition at higher concentrations (>1 µM) while pirtobrutinib did not cause dose-dependent inhibition of SFK activity (Figure 1B). Release of Ca2+ from intracellular stores downstream of GPVI is dependent on both BTK and TEC5,12 and was inhibited by ibrutinib (IC50=0.74 µM) and pirtobrutinib (IC50=0.73 µM) with similar potency, but acalabrutinib (IC50=4.2 µM) was less potent (Figure 1C), suggesting that all three inhibitors inhibit both TEC and BTK within the concentration range tested. Future studies could assess the effects of BTKi in the absence of platelet stimulation to elucidate effects on constitutive kinase activity. Ibrutinib and pirtobrutinib inhibited aggregation evoked by CRP-XL with equal potency (IC50=3.3 µM) while acalabrutinib (IC50=19.1 µM) was again less potent (Figure 1D).
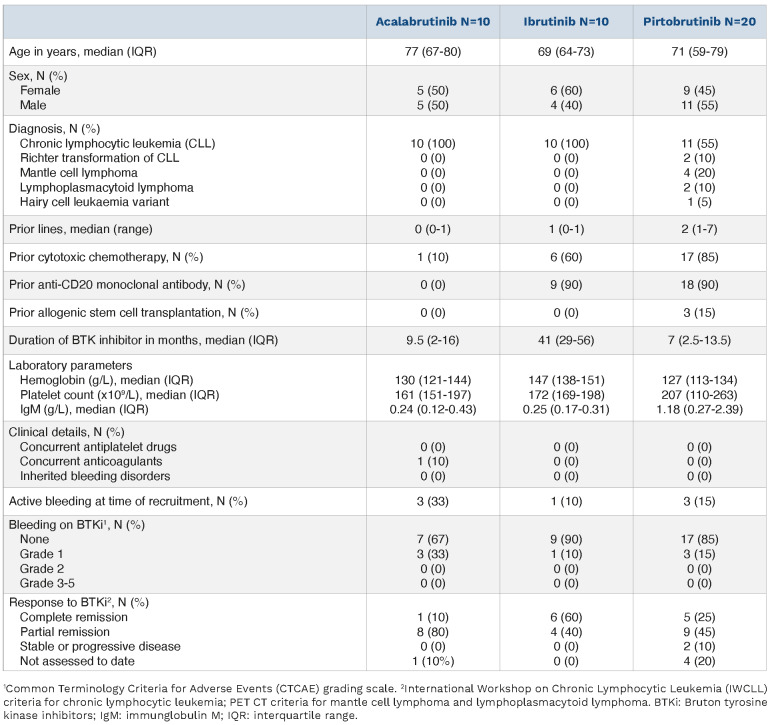
Blood samples were obtained from BRUIN phase Ib/II trial CLL and B-cell non-Hodgkin lymphoma patients (Table 1) receiving pirtobrutinib monotherapy (n=20), and CLL non-clinical trial patients receiving either ibrutinib monotherapy (n=10) or acalabrutinib monotherapy (n=10) under Oxford Radcliffe Biobank research tissue bank ethics, HTA License Number 12217, Oxfordshire C REC:09/H0606/515, project approval code 21/A027 and in accordance with the Declaration of Helsinki. Analysis of platelet function using high throughput plate-based aggregometry (PBA) indicated that aggregation stimulated by CRP-XL was absent in 75% of patients receiving pirtobrutinib, 80% receiving ibrutinib and 40% receiving acalabrutinib (Figure 1E). Responses to 3, 1 and 0.3 µg/mL CRP-XL were significantly higher in patients receiving acalabrutinib relative to ibrutinib or pirtobrutinib. Responses to collagen were absent in 95% of patients receiving pirtobrutinib, 100% receiving ibrutinib and 70% receiving acalabrutinib (Figure 1F). These data indicate that platelet activation downstream of GPVI is impaired by all three BTKi, but that acalabrutinib caused milder inhibition of GPVI signaling, with ibrutinib and pirtobrutinib causing similar levels of platelet inhibition. Small differences in aggregation responses between BTKi treatments were noted following stimulation with other agonists but differences were not significant compared to healthy controls (Online Supplementary Figure 1A-D). Future studies could assess functional and signaling responses to GPCR agonists in blood samples treated with BTKi in vitro to investigate whether the minor differences observed in the patient groups are linked to their pharmacological properties. Impairment of GPVI-evoked platelet aggregation is thought to be more pronounced when measured by PBA relative to classic Born aggregometry,13 and we found that aggregation responses stimulated by GPVI inhibitors were generally delayed rather than entirely ablated using this method (Online Supplementary Figure S1 D, F).
Figure 1.
Pirtobrutinib, ibrutinib and acalabrutinib inhibit platelet signaling and function in vitro and ex vivo. (A) Schematic diagram depicting an abridged platelet GPVI signaling pathway in which stimulation of GPVI results in activation of SFK and BTK/TEC followed by PLCy2 which enables cytosolic Ca2+ release and activation of PKC. Subsequent activation of integrin aIIbb3 enables fibrinogen binding and platelet aggregation. (Bi) Western blot analysis of Src Y418 phosphorylation with GAPDH loading control following stimulation of washed platelets with 3 mg/mL collagen-related peptide (CRP-XL) for 3 minutes in the presence of a range of concentrations of pirtobrutinib, acalabrutinib and ibrutinib (30-0.03 mM) or vehicle and (Bii) graph of mean phosphorylation relative to vehicle treated samples. (Ci) Cytosolic Ca2+ levels after stimulation of fura-2 loaded platelet-rich plasma (PRP) with 3 mg/mL CRP-XL in the presence of Bruton tyrosine kinase inhibitors (BTKi) and (Cii) graphs of mean increase in fura-2 signal relative to vehicle-treated samples and (Ciii) log-half maximal inhibitory concentration (IC50) values for the BTKi. (Di) Graph of % aggregation of PRP measured by PBA after stimulation with 3 mg/mL CRP-XL for 5 minutes in the presence of BTKi and (Dii) mean log-IC50 values. Aggregation of PRP from patients receiving BTKi therapy measured in 96-well plates following stimulation with concentration ranges of (A) CRP-XL, (B) collagen. Plots of (i) concentration-response curves for each agonist in which points represent the mean response to each concentration ± standard error of the mean and (ii) scatter plots of half maximal effective concentration (EC50) values, bars represent the mean ± standard deviation. Failure to induce concentration-dependent aggregation was designated ‘No Response’. The proportion of non-responders is noted at the top of relevant scatter plots. Statistical comparisons were performed by two-way ANOVA with Tukey multiple comparisons test. *P<0.05, **P<0.01.
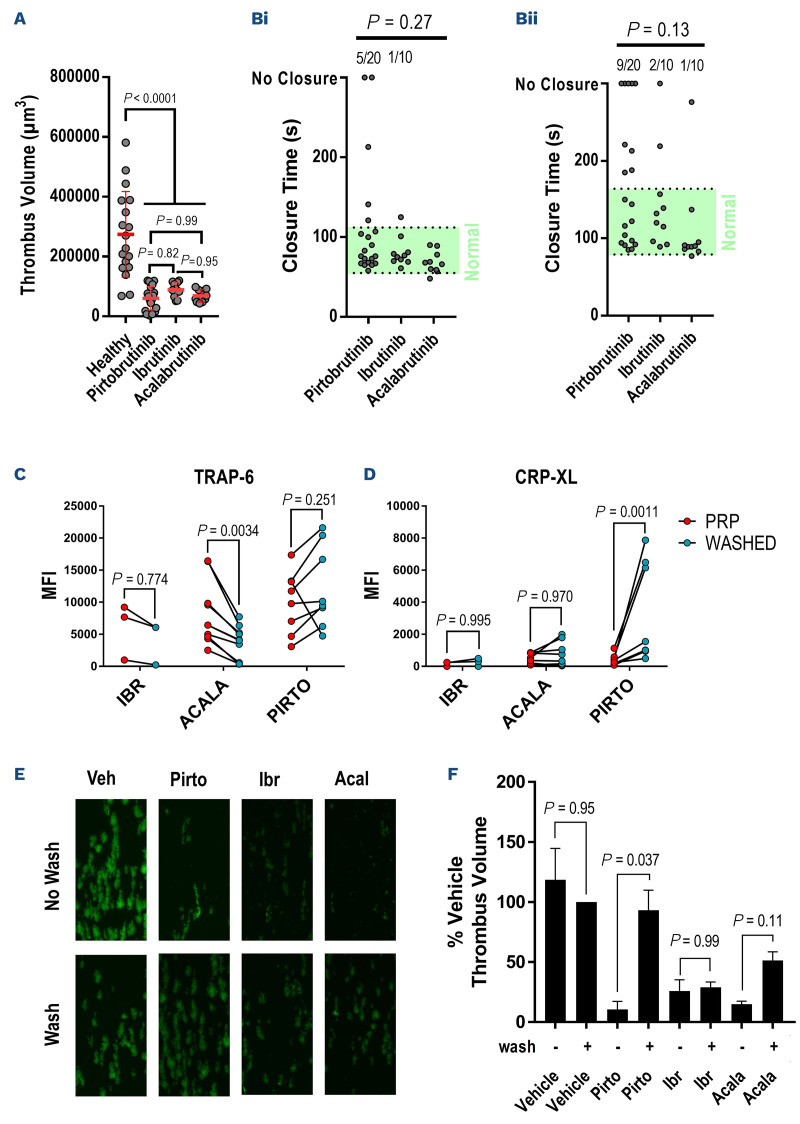
Ibrutinib impairs formation of stable, retracted aggregates under arterial shear conditions and this may underpin higher rates of hemorrhage associated with ibrutinib therapy.5,6 We found that the volumes of thrombi formed on collagen following perfusion of whole blood at arterial shear (1,000 s-1) for 6 minutes were significantly reduced by all three BTKi relative to healthy controls (Figure 2A). No significant differences were observed between the three BTKi groups. We measured closure time (CT) of the PFA-200 assay using both collagen/ADP and collagen/epinephrine cartridges (Figure 2B). The PFA-200 system uses prolonged (>137 coll/ADP, >199 s coll/EPI) or absent CT to identify impairment of hemostasis at very high shear rates. We found that CT in the collagen/ADP cartridges were prolonged in 25% of the pirtobrutinib patients (10% failed to close within 300 s) while only 10% of ibrutinib patients and 0% of acalabrutinib patients had prolonged CT. The CT collagen/epinephrine cartridges was prolonged in 45% of pirtobrutinib patients (25% failed to close within 300 s) while 20% of ibrutinib and 10% of acalabrutinib patients had prolonged CT. Differences in CT between the three BTKi were not significant in either the collagen/ADP or collagen/epinephrine tests. In order to control for the effects of different B-cell malignancies present in the pirtobrutinib group on platelet function, we restricted analysis to patients with CLL only and found that this did not alter the results of aggregation, in vitro thrombus formation or PFA-200 assays (Online Supplementary Figure S2).
Table 1.
Patient information.
Figure 2.
Pirtobrutinib causes similar impairment of platelet function at high shear but the inhibitory effects is rapidly reversed following wash-off. Whole blood from patients receiving Bruton tyrosine kinase inhibitors (BTKi) therapy were perfused through collagen-coated microfluidic chips for 6 minutes at a shear rate of 1,000 s-1 and the volume of resulting thrombi measured by confocal fluorescence microscopy. (A) A scatter plot of thrombus volumes, bars represent the mean ± standard deviation. PFA-200 closure times (CT) were also measured in whole blood samples. Scatter plots CT measured in (Bi) collagen/ADP and (Bii) collagen/epinephrine cartridges, including the normal range (green area) and proportion of CT outside of the range (above plot). The P values were calculated using Fisher’s exact test to compare normal and prolonged CT. Flow cytometry was used to assess a-granule secretion by measuring P-selectin exposure in platelet-rich plasma (PRP) from patients receiving BTKi and then again in washed platelets that had been incubated for 1 hour at room temperature after washing. Scatter plots of P-selectin exposure stimulated by (C) 15 mM TRAP-6 or (D) 3 mg/mL CRP-XL after 20 minutes. Lines connect responses in PRP and washed platelets for each patient. The P values were calculated by matched two-way ANOVA with Tukey’s multiple comparison test. (E) Confocal microscopy images of thrombi formed in type I collagen-coated microfluidic chips after perfusing whole blood from healthy donors treated with vehicle, pirtobrutinib (10 µM), ibrutinib (1 µM) or acalabrutinib (1 µM) for 1 hour with or without inhibitor wash-off. (F) Bar chart of thrombus volumes normalized to the volume of thrombi formed in vehicle-treated whole blood after washing. Bars represent the mean thrombus volume ± standard error of the mean. P values were calculated using one-way ANOVA with Sidak’s multiple comparisons test.
Rates of hemorrhagic AE for patients with B-cell malignancies receiving BTKi are highest when therapy is first initiated.14 Having established that pirtobrutinib therapy induces similar impairment of hemostasis to acalabrutinib and ibrutinib, we investigated the influence of the duration of BTKi therapy on platelet function parameters. We compared the results of aggregation, thrombus formation and PFA-200 assays of patients within the first 12 months of treatment with a BTKi (n=19) to patients with longer treatment durations (n=21). Duration of therapy had no significant effect on aggregation response to any agonist except U46619, but in vitro thrombus formation was significantly impaired and higher proportion of collagen/epinephrine assays failed to close within 300 s in patients with a treatment duration of <12 months (Online Supplementary Figure S3).
Ibrutinib and acalabrutinib irreversibly inhibit BTK by covalently modifying a cysteine in the ATP binding pocket required for enzymatic activity. Recovery from platelet dysfunction caused by irreversible BTKi is dependent on platelet turnover, which has a half-life of 7 to 10 days. Pirtobrutinib also targets the ATP binding pocket but binds non-covalently and inhibition is reversible. Recovery of normal platelet function is therefore likely to be determined by drug wash-out and not platelet turnover. In order to investigate how wash-off of BTKi influenced platelet function, we measured P-selectin exposure as a marker of α-granule secretion evoked by CRP-XL or TRAP-6 in PRP or 1 hour after washing the platelets. There was no difference in P-selectin exposure evoked by TRAP-6, except a small but significant decrease after washing in patients receiving acalabrutinib, possibly caused by a small loss in responsiveness during the washing process (Figure 2C). We found that p-selectin exposure evoked by CRP-XL recovered in washed platelets but not in PRP from patients receiving pirtobrutinib. There was no significant difference for patients receiving acalabrutinib or ibrutinib (Figure 2D). We further investigated reversibility by treating whole blood samples from healthy donors with the BTKi and measuring thrombus formation with or without washing off the inhibitors (Figure 2E, F). After pirtobrutinib was washed off, platelet function was not significantly different to vehicle-treated samples, but thrombus formation in ibrutinib- and acalabrutinib-treated samples remained impaired.
Inhibition of SFK is implicated in bleeding risk, and although we found that pirtobrutinib spared SFK activity to a greater extent that the covalent BTKi, this did not result in reduced platelet dysfunction. This study does not rule out that additional effects on coagulation and platelet procoagulant activity may contribute to observed differences, although it should be noted that prior studies attribute increased rates of hemorrhage during BTKi therapy predominantly to inhibition of primary hemostasis. The comprehensive analysis of platelet functional responses presented in this study indicate that low rates of hemorrhage reported in pirtobrutinib trials might not correspond with milder dysfunction compared to ibrutinib and acalabrutinib. Our findings suggest that rapid reversibility in platelet function rather than reduced platelet dysfunction might play a role in the low rates of hemorrhagic adverse events with pirtobrutinib. Further clinical data will be required to identify if the rapid reversal of platelet inhibition observed with pirtobrutinib may simplify elective peri-operative management, emergency surgical management and the management of unrelated major hemorrhage in patients on this reversible BTKi compared to irreversible BTKi.
Supplementary Material
Acknowledgments
The authors acknowledge the contribution to this study made by the Oxford Center for Histopathology Research and the Oxford Radcliffe Biobank, which are supported by the National Institutes of Health Research Oxford Biomedical Research Center. The schematic diagram was created with BioRender.com.
Funding Statement
Funding: This work was supported by grant RG/20/7/34866 from the British Heart Foundation and a Rosetrees Fellowship StGeorges-21\1 held by APB.
References
- 1.Mato AR, Thompson M, Allan JN, et al. Real-world outcomes and management strategies for venetoclax-treated chronic lymphocytic leukemia patients in the United States. Haematologica. 2018;103(9):1511-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forum UC. Ibrutinib for relapsed/refractory chronic lymphocytic leukemia: a UK and Ireland analysis of outcomes in 315 patients. Haematologica. 2016;101(12):1563-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coutre SE, Byrd JC, Hillmen P, et al. Long-term safety of singleagent ibrutinib in patients with chronic lymphocytic leukemia in 3 pivotal studies. Blood Adv. 2019;3(12):1799-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furman RR, Byrd JC, Owen RG, et al. Pooled analysis of safety data from clinical trials evaluating acalabrutinib monotherapy in mature B-cell malignancies. Leukemia. 2021;35(11):3201-3211. [DOI] [PubMed] [Google Scholar]
- 5.Bye AP, Unsworth AJ, Desborough MJ, et al. Severe platelet dysfunction in NHL patients receiving ibrutinib is absent in patients receiving acalabrutinib. Blood Adv. 2017;1(26):2610-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bye AP, Unsworth AJ, Vaiyapuri S, Stainer AR, Fry MJ, Gibbins JM. Ibrutinib inhibits platelet integrin alphaIIbbeta3 outside-in signaling and thrombus stability but not adhesion to collagen. Arterioscler Thromb Vasc Biol. 2015;35(11):2326-2335. [DOI] [PubMed] [Google Scholar]
- 7.Levade M, David E, Garcia C, et al. Ibrutinib treatment affects collagen and von Willebrand factor-dependent platelet functions. Blood. 2014;124(26):3991-3995. [DOI] [PubMed] [Google Scholar]
- 8.Futatani T, Watanabe C, Baba Y, Tsukada S, Ochs HD. Bruton's tyrosine kinase is present in normal platelets and its absence identifies patients with X-linked agammaglobulinaemia and carrier females. Br J Haematol. 2001;114(1):141-149. [DOI] [PubMed] [Google Scholar]
- 9.Mato AR, Shah NN, Jurczak W, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892-901. [DOI] [PubMed] [Google Scholar]
- 10.Mato AR, Pagel JM, Coombs CC, et al. LOXO-305, a next generation, highly selective, non-covalent BTK inhibitor in previously treated CLL/SLL: results from the phase 1/2 BRUIN study. Blood. 2020;136(Suppl 1):S35-37. [Google Scholar]
- 11.Eyre TA, Shah NN, Le Gouill S, et al. BRUIN MCL-321: a phase 3 open-label, randomized study of pirtobrutinib versus investigator choice of BTK inhibitor in patients with previously treated, BTK inhibitor naïve mantle cell lymphoma (trial in progress). Blood. 2021;138(Suppl 1):S2422. [Google Scholar]
- 12.Atkinson BT, Ellmeier W, Watson SP. Tec regulates platelet activation by GPVI in the absence of Btk. Blood. 2003;102(10):3592-3599. [DOI] [PubMed] [Google Scholar]
- 13.Nicolson PLR, Hughes CE, Watson S, et al. Inhibition of Btk by Btk-specific concentrations of ibrutinib and acalabrutinib delays but does not block platelet aggregation mediated by glycoprotein VI. Haematologica. 2018;103(12):2097-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441-3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.