Abstract
Our case is a 24-year-old woman who has had abdominal enlargement for eleven months. She had an abdominal mass with an elevated level of CA-125 and imaging studies showed a pelvic cystic mass with a solid part, and thus malignancy was considered in the differential diagnosis. A laparotomy myomectomy was performed. Postoperative histopathological examination results revealed no signs of malignancy. In this case, both ultrasonography and magnetic resonance imaging could not visualize both ovaries and the stalk of the pedunculated fibroid on the posterior uterine corpus. On physical examination and imaging, cystic degeneration of uterine fibroid may present like an ovarian mass. Preoperative diagnosis is challenging. A definitive diagnosis is only feasible postoperatively following histological examination.
Keywords: Fibroid, Leiomyoma, Cystic Degeneration, Ovarian Cystic Malignancy, Diagnosis
CASE REPORT
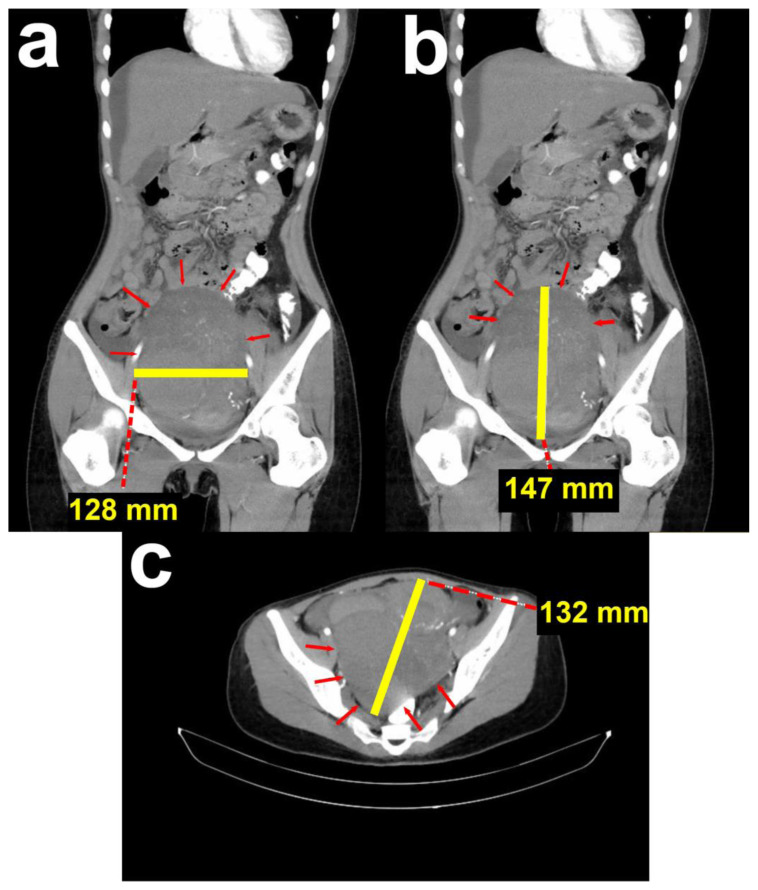
A 24-year-old woman (BMI: 18,5 kg/m2), unmarried, with no history of pregnancy, was referred to the Oncology Gynecology Department. The patient complained of progressive abdominal enlargement for the past eleven months. She had no other symptoms, and her vital signs were within normal limits. Her menstrual cycle was regular, and she had no significant medical history. There was no history of gynecologic malignancies in her family. Upon examination, a smooth-surfaced and fixated mass was palpated, with its superior border at the umbilicus. There were no signs of acute abdomen. An abdominal computed tomography (CT) from the previous hospital showed a partially solid, multilocular cystic mass attached to the uterine corpus and bleeding in the pelvic cavity measuring 132 × 128 × 147 mm (Figure 1). There was also intraperitoneal fluid accumulation corresponding with ascites.
Figure 1. 24-year-old female with cystic degeneration of uterine fibroid.
Findings: CT result from the previous hospital.
(a; left; sagittal view) Mass in the pelvic cavity with a transverse diameter of 10.61 cm.
(b; middle; sagittal view) Mass in the pelvic cavity with a cranial-caudal diameter of 14.78 cm.
(c; right; axial view) Mass in the pelvic cavity with an anterior-posterior diameter of 13.28 cm.
Technique: Contrast enhanced Computed Tomography performed with acquisition of 5 mm section for all three orthogonal planes. Siemens Somatom Perspective 64 Slice scanner, 400 mAs, 120 kV, 1 mm slice thickness. Iodine contrast medium 1.5 mg/kg body weight.
Results of examinations conducted at a previous hospital were suggestive of ovarian neoplasm. The patient was referred to our Oncology Gynecology Department. We performed a physical examination and found a cystic mass suspected of ovarian neoplasm. The tumor marker CA-125 was elevated, at 271.5 U/mL (N: 0–35 U/mL). Other tumor markers measured were AFP (1,55 ng/mL; N: 0–8 ng/mL), LDH (227 IU/L; N: 105–333 IU/L), and β-hCG (<1.20 mIU/mL; N: <5 mIU/mL); all were within normal limits. Thus, a diagnosis of suspected malignant ovarian cyst was made.
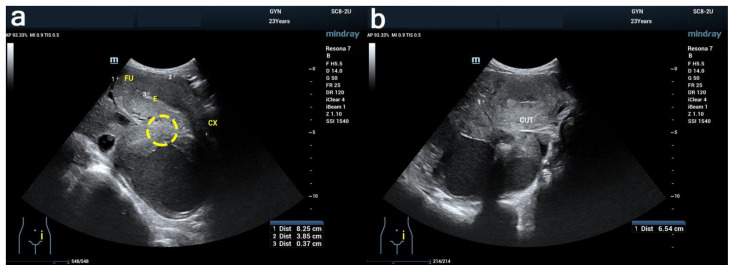
We chose to perform transrectal ultrasonography because the patient was unmarried and had never had sexual intercourse. Transrectal ultrasound (Figure 2) showed an 82 × 38 × 65 mm uterus with homogenous myometrium, partially solid cystic mass from both adnexes, and no free fluid. The patient was uncomfortable during the examination; therefore, we could not further evaluate the mass with ultrasonography (US) and performed magnetic resonance imaging (MRI) instead.
Figure 2. 24-year-old female with cystic degeneration of uterine fibroid.
Findings: Ultrasound result. The yellow marker probably indicates the pedicle that was not successfully identified.
Technique: Transrectal ultrasound with Mindray Resona 7.
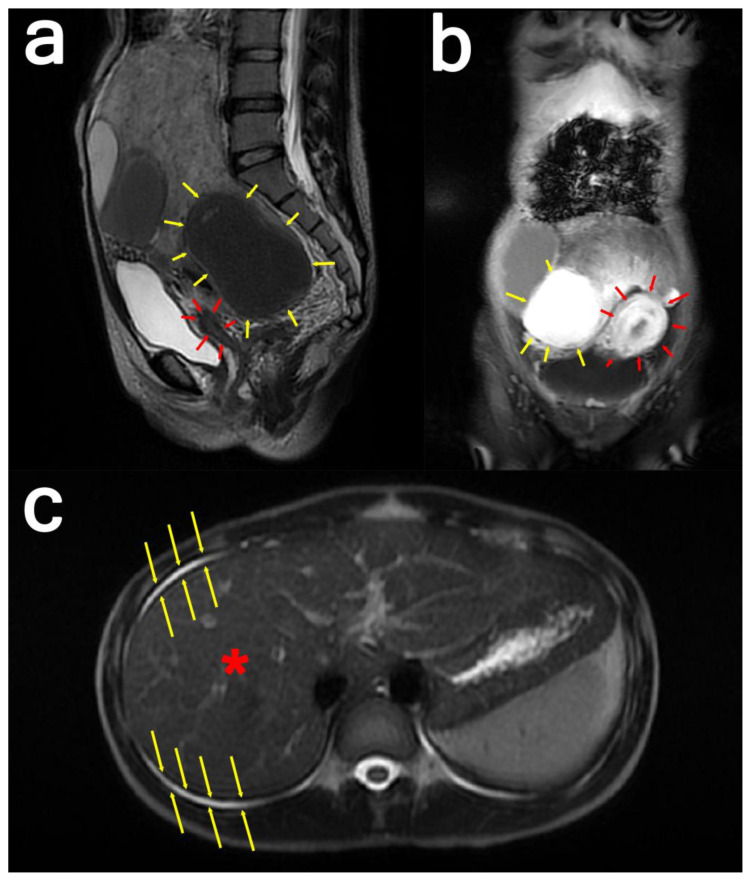
MRI results showed a lobulated mass with solid and cystic components in the lower abdominal cavity, measuring about 94 × 211 × 175 mm (Figure 3). There was no adhesion to the bladder, uterus, or rectum. The standard ovarian structure was not visualized. Thus, a diagnosis of suspected malignant partially solid ovarian cyst was made.
Figure 3: 24-year-old female with cystic degeneration of uterine fibroid.
Findings: (a; left) Sagittal view and (b; middle) coronal view: There is a pathological enhancement and diffusion restriction on the solid component. The cystic part shows the intensity of the blood within it. The mass appears to be pressing the uterus anteriorly.
(c; right) Axial view: The yellow arrows indicate ascites surrounding the liver.
Technique:
(a; left): Sagittal T2-weighted MRI image of the whole abdomen with contrast in a General Electric Healthcare Optima MR450w Scanner (Tesla strength = 1.5T). TR=8130.42, TE=113.40, and slice thickness = 3.0mm
(b; middle): Coronal water sequence with contrast in a General Electric Healthcare Optima MR450w Scanner (Tesla strength = 1.5T). TR=6.44, TE=3.13, and slice thickness = 8.0 mm
(c; right): Axial single-shot fast spin-echo with contrast in a General Electric Healthcare Optima MR450w Scanner (Tesla strength = 1.5T). TR=693.47, TE=92.21, and slice thickness = 8.0 mm.
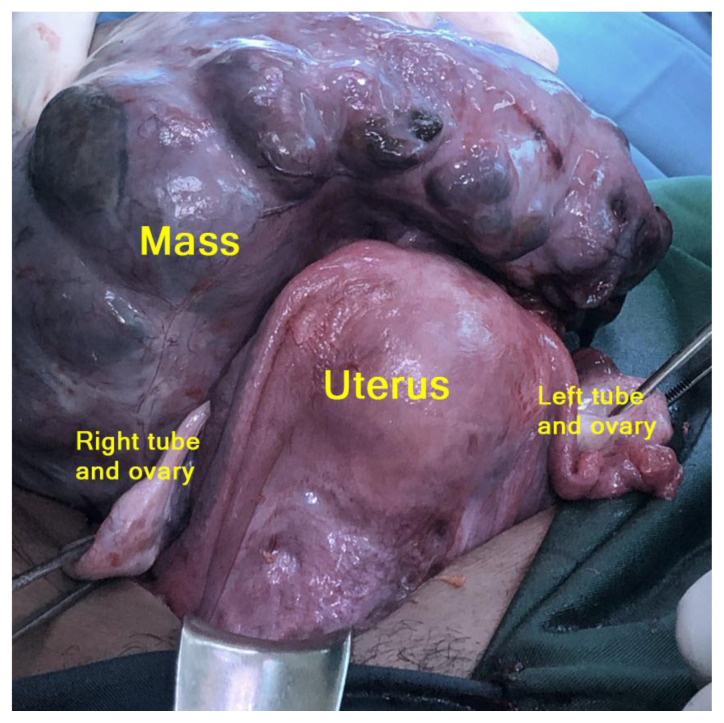
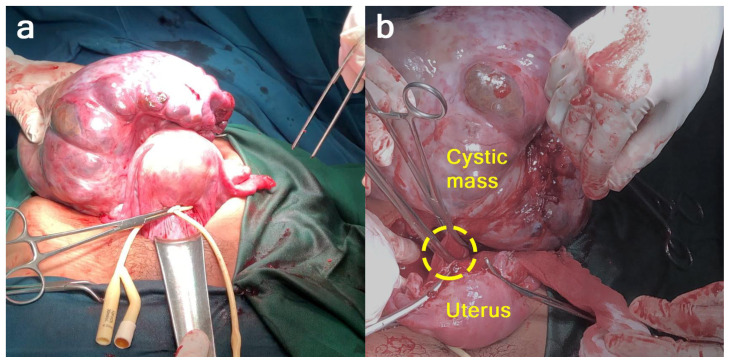
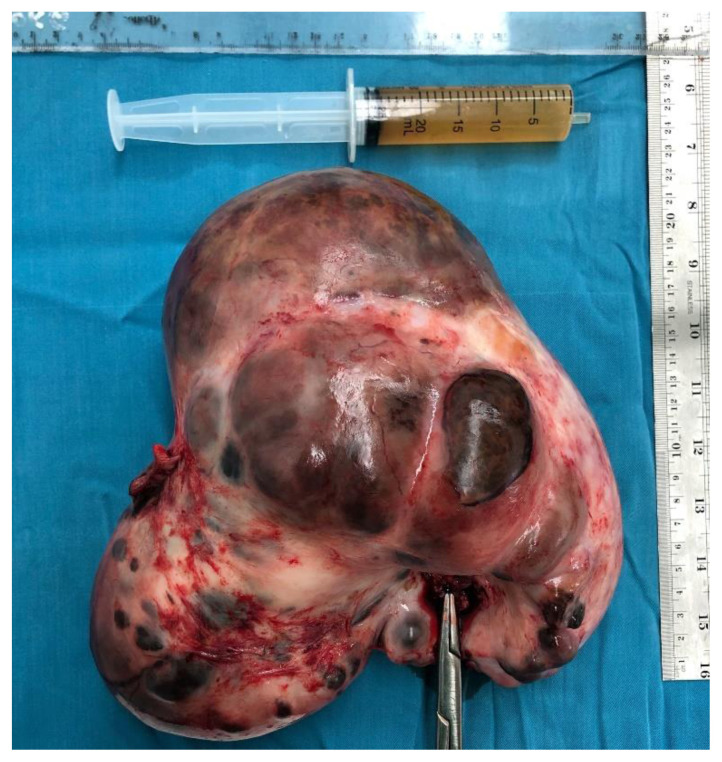
Laparotomy with a midline vertical incision revealed that the cystic mass was a partly solid cystic tumor that originated from the uterus as a myoma with a partial cystic consistency measuring 20 cm in diameter, which stemmed from the uterus at the posterior uterine corpus with a stalk measuring 2 cm in diameter. The tumor mass was attached to the back of the sacrouterine ligament. The uterus, tubes, and ovaries were within normal limits (Figure 4). Postoperative histological examination of the tumor mass revealed it to be a degenerated cystic myoma (malignant degeneration could not be excluded). We performed myoma extirpation (Figure 5). Adhesiolysis and hemostasis sutures were performed on the attachment. 20 cc of ascites fluid was collected for cytology examination (Figure 6).
Figure 4. 24-year-old female with cystic degeneration of uterine fibroid.
Intraoperative findings: Although both ovaries were not identified with imaging, both ovaries were within normal limits. A giant pedunculated uterine fibroid with cystic degeneration was found attached to the posterior corpus.
Figure 5. 24-year-old female with cystic degeneration of uterine fibroid.
Intraoperative finding: Performed myoma extirpation by first placing a tourniquet on the isthmus, identifying the myoma pedicles, clamping, cutting, and suturing.
Figure 6. 24-year-old female with cystic degeneration of uterine fibroid.
Findings: The specimen and ascites fluid.
A histological examination led to the final diagnosis of a pedunculated uterine leiomyoma with marked cystic degeneration.
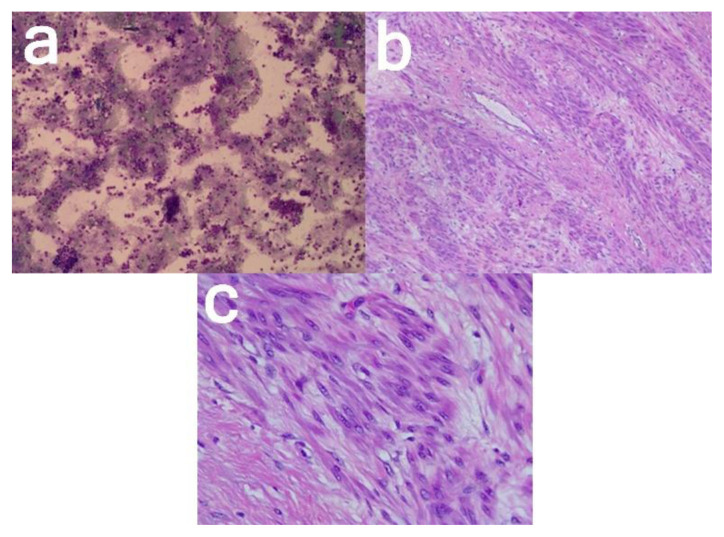
Microscopic examination revealed mesenchymal tumor tissue with rounded edges, composed of cellular smooth muscle cell proliferation, partly loose, and irregularly aligned. The tumor cells are round with oval nucleated, partly cigar-shaped, relatively fine chromatin, and eosinophilic cytoplasm. Mitosis is hard to find. Locally, there are islands of preservation, and a cystically dilated endometrial stroma; a whole part of this endometrium seems to be a lumen filled with eosinophilic material. A hemosiderophage is seen. Histologic signs of malignancy were not found (Figure 7).
Figure 7. 24-year-old female with cystic degeneration of uterine fibroid.
Findings:
(a; left; 100× magnification) Ascites cytology
(b; middle; 100× magnification, and c; right; 400× magnification) Smooth muscle cell proliferation, round with oval nucleated, partly cigar-shaped, relatively fine chromatin.
Technique: Histopathological examination with hematoxylin and eosin stain.
The patient is alive without any complications. The result was not malignancy, and no further follow-up was required.
DISCUSSION
Etiology & Demographics
Uterine fibroids are the most common solid tumor in women [1]. The incidence increases with age; about 20–50% in women over 30 years old [2–4]. Histologically, uterine fibroids consist of smooth muscle and fibrous connective tissue [5]. Other risk factors for uterine fibroids include obesity, family history of uterine fibroids, hypertension, no history of pregnancy, vitamin D deficiency, and African American ethnicity [4,6].
Macroscopically, uterine fibroids appear to have capsules. Cells at the tumor’s edges appear flattened in a concentric pattern that gives it the appearance of a capsule. Although microscopically, no fibrous tissue capsule can be found. The progressive enlargement of the fibroid will eventually outgrow its blood supply. The uterine fibroid’s blood supply enters from the periphery of the mass, leaving the core relatively avascular. This mechanism could explain various types of degeneration and central necrosis of the uterine fibroid, such as hyaline, cystic, myxoid, or red degeneration [5,6].
The incidence of cystic degeneration of uterine fibroid represents 4% of all uterine fibroid cases. Cystic degeneration is most common in post-menopausal women and fibroids of the interstitial type. It is caused by liquefaction which occurs in fibrosis with hyaline changes. Large pedunculated fibroids with cystic degeneration can be difficult to distinguish from ovarian cysts [6,8]. Uterine fibroids can be found in various locations. Although mostly intramural, the tumor can expand and become subserous. The pedunculated subserous fibroid can appear as adnexal organs and is difficult to distinguish from ovarian masses [9]. Few reported uterine fibroid cases have a clinical and imaging examination mimicking ovarian tumors in the literature, thus making the preoperative diagnosis challenging [5].
Clinical and Imaging Findings
Ovarian and uterine mass were considered in the differential diagnosis of the pelvic mass. The diagnosis was mainly an ovarian neoplasm when we discovered a cystic mass. Finding normal ovaries and the pelvic mass as a continuation of the uterus allows ovarian neoplasm to be ruled out. Another way to tell the difference is to find ovarian vascular pedicles on CT and MRI [7].
Ultrasonography remains the primary examination in determining uterine fibroids. Generally, on sonography, the fibroid will appear as a hypoechoic or heterogeneous mass depending on the ratio of connective tissue to smooth muscle and whether degeneration is present. There will be minimal echogenicity, irregular anechoic areas in fibroids, and sometimes a cluster with high echogenicity accompanied by distal acoustic shadowing. Differentiating uterine from an adnexal mass can be done by identifying the “bridging vessel sign” on Doppler sonography or MRI. The presence of tortuous vessels signifies the uterine origin of the mass. Doppler ultrasound typically shows circumferential vascularity, but fibroids that are necrotic or have undergone torsion will show no flow [10–13].
In the case of inconclusive sonographic results, an MRI examination can be performed to determine the origin of the adnexal mass. The sensitivity of MRI examination in uterine fibroid ranges from 88% to 93%, with a specificity of 66% to 91%. Uterine fibroid will show areas of low signal intensity compared to normal myometrium on T2 imaging and areas of isointense to myometrium on T1 imaging [11].
Uterine fibroid with cystic degeneration will show decreased signal intensities in T1 and an increased T2 but no enhancement of the cystic area. MRI examination can also quickly identify normal pedicles or ovaries. If the pelvic mass can be separated from the normal ovary or connected to the round ligament, it is unlikely that the mass will have originated from the ovaries [11,12].
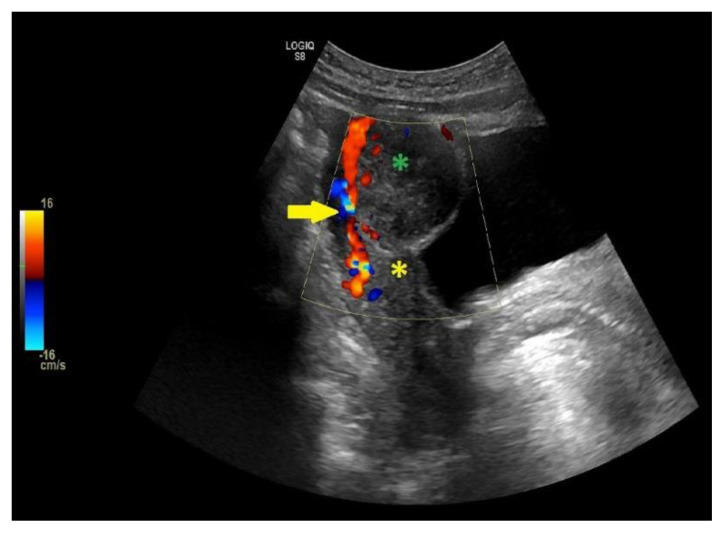
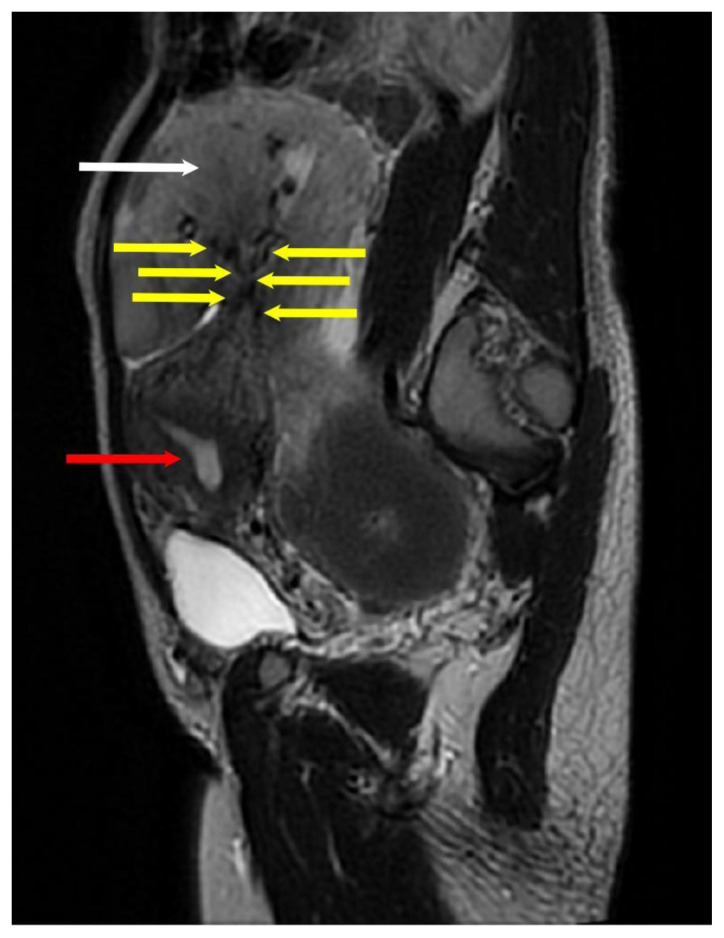
The typical MRI or US finding associated with uterine mass is the presence of a “bridging vessel sign,” indicating blood vessels originating from the uterus supplying the pelvic mass. The bridging vessel can enhance tubular structures on contrast T1 imaging or as flow voids on T2 fast spin-echo sequence. In comparison, Doppler US shows the vessel as a signal flowing from the uterus to the pelvic mass [10,14,15]. Examples of the “bridging vessel sign” can be seen in Figures 8 and 9. In our case, the “bridging vessel sign” could not be identified; therefore, the origin of the mass still could not be confirmed. Although unspecific, the lack of enhancement in the cystic area of the mass was consistent with cystic degeneration.
Figure 8. Example of bridging vascular sign in a 40-year-old female with pedunculated subserosal leiomyoma. Case courtesy of Dr Eid Kakish, Radiopaedia.org; rID: 86109.
Findings: The yellow arrow indicates the bridging vessel sign, which illustrates blood vessels connecting the uterus to a pelvic mass. The green asterisk indicates the pedunculated subserosal fibroid. The yellow asterisk indicates the uterine fundus.
Technique:
Transabdominal longitudinal Doppler ultrasound of the uterus.
Figure 9. Example of bridging vascular sign in a 29-year-old with pedunculated subserous myoma.
Findings: The arrows indicate multiple tortuous vessels with luminal signal voids connects the uterus to the pelvic mass.
Technique:
Sagittal T2-weighted MRI image of the whole abdomen with contrast in a General Electric Healthcare Optima MR450w Scanner (Tesla strength = 1.5T). TR=3409, TE=105.6, and slice thickness = 3.0 mm.
Diffusion-weighted imaging (DWI) can help distinguish between benign and malignant processes in genitourinary imaging. Leiomyoma typically has no restriction diffusion on DWI and apparent diffusion coefficient (ADC) map sequence. Otherwise, leiomyosarcoma typically shows intermediate to high signal intensity on DWI with a lower ADC value.[16]
In the current patient, we could not visualize both ovaries on imaging examination, and we failed to show the stalk of the pedunculated fibroid on the posterior uterine corpus, which is why a Doppler ultrasound was not performed. Additional information in the form of an elevated CA-125 led to the working diagnosis of ovarian malignancy. Uterine fibroids are not associated with any specific tumor marker, but many studies found an increase in several tumor markers in patients with uterine fibroids. An increase in CA-125 levels was found in 20% of patients with leiomyoma. The mean CA-125 level was 27.3 ± 38.1 U/mL. The factors related to elevated levels of CA-125 in patients with uterine fibroids were larger size (> 5 cm), subserosal location, and co-existence with adenomyosis. A large fibroid can cause peritoneal irritation, which may also contribute in elevating CA-125 levels [17].
The most widely used tumor marker for ovarian cancer is CA-125. Elevation of CA-125 is found in 80% of patients with advanced ovarian cancer and 50% in early-stage ovarian cancer. An increase in CA-125 can also be found in other conditions, such as pancreatic cancer, bladder cancer, liver cancer, lung cancer, breast cancer, and benign gynecologic conditions (adenomyosis, endometriosis, pregnancy, menstruation, fibroids, pelvic inflammatory diseases, and ovarian cysts) [18]. AFP and β-hCG are the main markers for diagnosing and monitoring ovarian germ cell tumors, such as yolk sac tumors. However, elevated AFP levels can also be found in other tumors, such as liver and testicular cancer [19,20]. The role of tumor markers is as an addition to clinical and imaging findings in the preoperative, treatment response, and prognostic evaluation of ovarian malignancies [18–20].
Treatment and Prognosis
In published case reports, exploratory laparotomy is the most common and considered the first-line treatment option for uterine fibroid with cystic degeneration. More recently, case reports have successfully conducted laparoscopic excision without lengthening operation time or increasing morbidity. However, laparoscopy was not feasible in this case due to the size of the mass. There are reports which conducted CT-guided aspiration. However, the results were unsatisfactory; patients returned with recurrence and required surgical treatment [21].
The prognosis of cystic degeneration uterine fibroid is similar to uterine fibroids without degeneration. The prognosis in most patients is excellent. Some patients may experience recurrences and require multiple surgeries depending on treatment options. Although rare, complications caused by uterine fibroids during pregnancy can occur, such as pain, preterm labor, uterine rupture, placental abruption, malpresentation, and postpartum hemorrhage. Reduced fertility is also observed in patients with submucosal and interstitial types of uterine fibroids [21].
Differential Diagnoses
Ovarian malignancy
In the discovery of a large partially solid cystic mass and an elevated CA-125 level, the first differential diagnosis would be an ovarian malignancy. The exact etiology of ovarian malignancy is unknown. The strongest risk factor is a family or personal history of breast and/or ovarian cancer, all linked to the mutation of BRCA1 or BRCA2 genes [22].
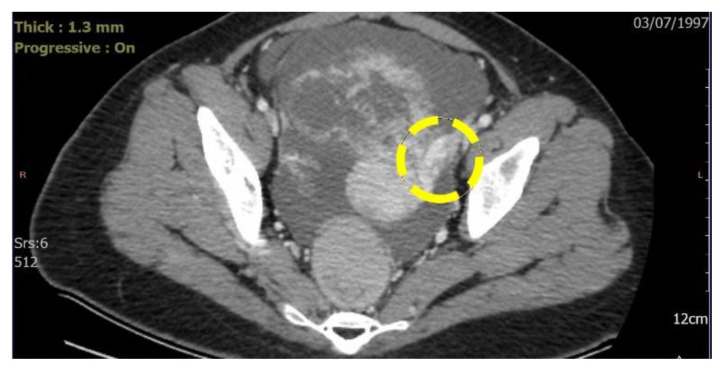
Ruling out ovarian malignancy can be done by visualizing the normal ovaries or identifying the structure connecting the mass and the uterus. On CT, the presence of the ovarian pedicle sign can confirm the mass’s ovarian origin and differentiate it against uterine fibroid. The ovarian pedicle is a collection of ovarian vessels (arteries and veins) supplying and draining blood from the ovaries and branching with uterine vessels. The presence of an ovarian mass may enlarge ipsilateral ovarian vessels. The ovarian pedicle sign is an asymmetrical enlargement of the gonadal veins, which can be detected via retrograde tracing with a single-detector helical CT, optimal contrast, and thin section. An example of the ovarian pedicle sign can be seen in Figure 8 [20]. A less specific finding that can rule out ovarian malignancy is the absence of ascites. However, our case findings showed otherwise [8].
Endometrioma
Endometrioma is caused by bleeding of an ectopically located endometrial tissue in the ovary, which bleeds following the menstrual cycle. Eventually, a hematoma forms a cyst called an endometrioma. On US, the most common finding is a single locule cyst with acoustic enhancement and diffusely spread ground glass echoes. Some less common findings include the presence of multiple locules, a hyperechoic cyst wall, and a partially solid or purely solid mass. Endometriomas are also much smaller than fibroids with cystic degeneration [24].
On MRI, T1 imaging may show hyperintense lesions without signal loss in T1 fat-suppressed sequence. T2 typically shows a hypointense lesion with the occasional finding of dark spot sign, indicating chronic hemorrhage [25].
TEACHING POINT
Uterine fibroids are benign smooth muscle neoplasms that occur in reproductive-aged women. Cystic degeneration is a rare form of uterine fibroid that can mimic other pelvic neoplasms from a clinical and imaging aspect, thus making the preoperative diagnosis challenging. Pedunculated fibroid should always be put as a differential diagnosis in the diagnosis of ovarian cancer.
Figure 10. Example of ovarian pedicle sign in a 25-year-old with malignant ovarian mass. Case courtesy of Dr Trifonia Pingkan Siregar.
Findings:
The dotted circle indicates the ovarian pedicle sign which is characterized by an asymmetrically enlarged left gonadal vein. This finding indicates that the mass originates from the left ovary.
Technique: Contrast enhanced CT on axial view. Contrast enhanced Computed Tomography performed with acquisition of 5 mm section for all three orthogonal planes. Siemens Somatom Sensation 64 Slice scanner, 400 mAs, 120 kV, 1.3 mm slice thickness. Iodine contrast medium 1.5 mg/kg body weight.
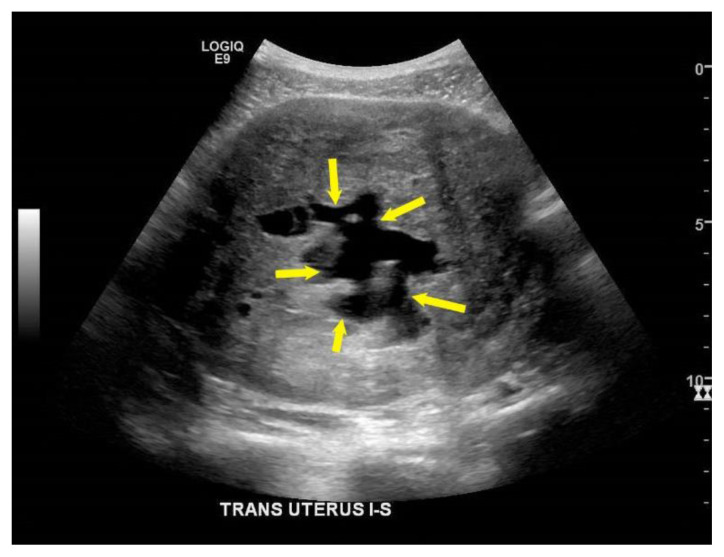
Figure 11. Teaching point. 30-year-old female with cystic degeneration of uterine fibroid. Case courtesy of Dr Michael P Hartung, Radiopaedia.org; rID: 75336.
Findings:
A general transverse view on transabdominal ultrasound finding of a cystic degenerated uterine fibroid may show a heterogeneous mass with low echogenicity and cystic areas (yellow asterisk).
Technique: Transverse transabdominal ultrasound of the uterus
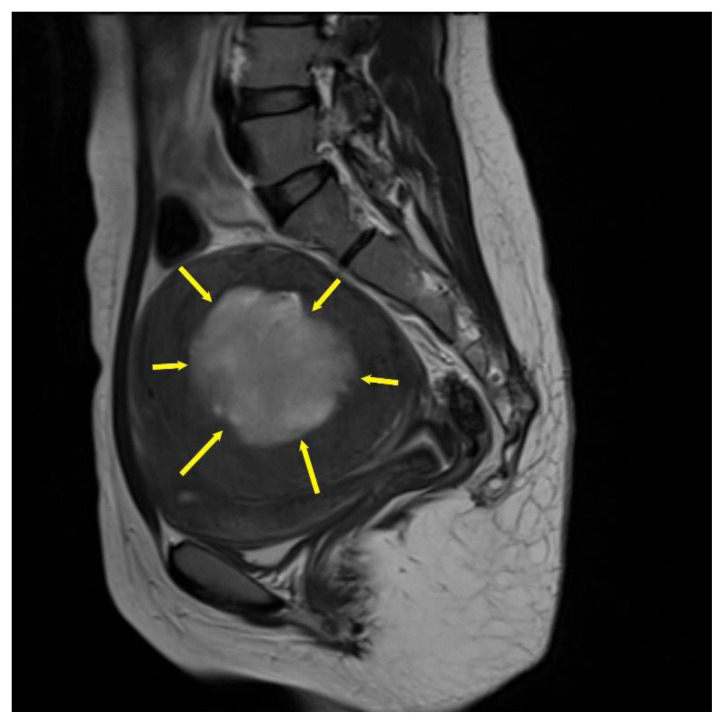
Figure 12. Teaching point. 25-year-old female with cystic degeneration of uterine fibroid Case courtesy of Dr Hidayatullah Hamidi, Radiopaedia.org; rID: 84050.
Findings:
A general MRI finding of cystic degenerated uterine fibroid may present cystic areas with some areas of high T2 signal (yellow asterisk).
Technique: Sagittal T2 weighted MRI of the uterus
Table 1.
Summary table of uterine fibroid with cystic degeneration.
| Etiology | A progressive increase in the size of the uterine fibroid causes its own blood supply to be outgrown, allowing only the periphery to be sufficiently supplied and leaving the core relatively avascular. Thus, leading to various types of degeneration, one of them being cystic. |
| Incidence | Cystic degeneration represents only 4% of all uterine fibroid cases. |
| Gender Ratio | It occurs exclusively in females |
| Age prediction | The incidence of uterine fibroids increases in women above 30 years of age. Cystic degeneration of uterine fibroids is more commonly found in post-menopausal women. |
| Risk factors | Uterine fibroid: Age (>30 years), obesity, family history of uterine fibroids, hypertension, no history of pregnancy, vitamin D deficiency, and African American ethnicity. Cystic degeneration: Age (post-menopausal) and interstitial type of fibroid. |
| Treatment | The most performed procedure is exploratory laparotomy for cystic degenerated uterine fibroid. Recent reports successfully conducted laparoscopic removal of the degenerating fibroid. |
| Prognosis | The prognosis of cystic degenerated uterine fibroids is similar to uterine fibroids without degeneration. Prognosis is excellent in most patients. Although rare, fibroids may cause serious complications in pregnancy, such as pain, preterm labor, uterine rupture, malpresentation, and postpartum hemorrhage. |
| Findings on imaging | Ultrasound:
|
Table 2.
Differential diagnosis table for uterine fibroid with cystic degeneration.
| Diagnosis | Etiology, Mechanism | Imaging |
|---|---|---|
| Ovarian malignancy | The exact etiology is unknown. The strongest risk factor is a family or personal history of ovarian and/or breast cancer. Other studies have suggested environmental factors also play a role. | Plain radiograph, US, CT: The presence of ascites is more often found in malignant tumors, but this is not always the case. CT: The presence of an ovarian vascular pedicle sign is a way to confirm a mass of ovarian origin. |
| Uterine fibroid with cystic degeneration | Progressive enlargement of a fibroid eventually outgrows its own blood supply, leaving its core avascular. Thus, causing degeneration. | US:
|
| Endometrioma | This is caused by the bleeding of an ectopic, hormonally active endometrial tissue located in the ovary following the menstrual cycle. | US:
|
ABBREVIATIONS
- CT
computed tomography
- MRI
magnetic resonance imaging
- N
normal range
- US
ultrasonography
REFERENCES
- 1.Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. Epidemiology of uterine fibroids: a systematic review. BJOG. 2017;124:1501–1512. doi: 10.1111/1471-0528.14640. [DOI] [PubMed] [Google Scholar]
- 2.Padubidri VG, Daftary SN. Textbook of Gynaecology. 3rd ed. Elsevier publishers; New Delhi: 2015. Benign Diseases of the Uterus; pp. 391–408. [Google Scholar]
- 3.Fogota ML, Jain KA. Degenerating cystic uterine fibroid mimics an ovarian cyst in a pregnant patient. J Ultrasound Med. 2006;25(5):671–674. doi: 10.7863/jum.2006.25.5.671. [DOI] [PubMed] [Google Scholar]
- 4.Reshmy JR, Misra B, Rai RK. Giant cystic leiomyoma masquerading as ovarian tumour- case report. Scholars Journal of Medical Case Reports. 2015;3(7):608–610. [Google Scholar]
- 5.Prabhu JK, Samal S, Chandrasekar S, Subramani D, Rajamanickam S. A massive degenerative leiomyoma mimicking an ovarian tumor: a diagnostic dilemma. Journal Of Gynecologic Surgery. 2021;37(1) doi: 10.1089/gyn.2020.0118. doi: [DOI] [Google Scholar]
- 6.Pavone D, Clemenza S, Sorbi F, Fambrini M, Petraglia F. Epidemiology and risk factors of uterine fibroids. Best Practice & Research. Clinical Obstetrics & Gynaecology. 2018;46:3–11. doi: 10.1016/j.bpobgyn.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Rosai J. Rosai and Ackerman’s Surgical Pathology. 10th ed. Vol. 2. London: Mosby; 2011. Female Reproductive System; pp. 1510–1. [Google Scholar]
- 8.Benign Lesions of the Uterus. DC Dutta’s Textbook of Gynecology. 6th ed. Jaypee Brothers Medical Publishers; New Delhi: 2013. pp. 272–88. [Google Scholar]
- 9.Neuwirth R, Moritz J. Leiomyomas of the uterus. Glob libr women’s med. 2008 doi: 10.3843/GLOWM.10007. [DOI] [Google Scholar]
- 10.Khen, et al. Uterine fibroids: current perspectives. International Journal of Women's Health. 2014;6:95–114. doi: 10.2147/IJWH.S51083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maizlin, Vos Zeev V, Patrick M, Cooperberg Peter L. Is it a fibroid? are you sure?: sonography with MRI assistance. Ultrasound Quarterly. 2007;23:55–62. doi: 10.1097/01.ruq.0000263845.23055.f6. [DOI] [PubMed] [Google Scholar]
- 12.Kaushik C, Prasad A, Singh Y, Baruah BP. Case series: cystic degeneration in uterine leiomyomas. Indian Journal Radiology Imaging. 2008 Feb;18(1):69–72. [Google Scholar]
- 13.Ekici E, Vicdan K, Daniman N. Ultrasonographic appearance of cystic uterine leiomyoma. International Journal of Gynecology & Obstetrics. 1994;47(2):171–172. doi: 10.1016/0020-7292(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 14.Babacan A, Kizilaslan C, Gun I, Muhcu M, Mungen E, Atay V. CA 125 and other tumor markers in uterine leiomyomas and their association with lesion characteristics. International Journal of Clinical and Experimental Medicine. 2014;7(4):1078–1083. [PMC free article] [PubMed] [Google Scholar]
- 15.Ueda H, Togashi K, Konishi I, Kataoka ML, Koyama T, Fujiwara T, Kobayashi H, Fujii S, Konishi J. Unusual appearances of uerine leiomyomas: MR imaging findings and their histopathologic backgrounds. RadioGraphics. 1999;19:S131–S145. doi: 10.1148/radiographics.19.suppl_1.g99oc04s131. [DOI] [PubMed] [Google Scholar]
- 16.Sato K, Yuasa N, Fujita M, Fukushima Y. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. American Journal of Obstetrics and Gynecology. 2014;210(4):368.e1–368.e8. doi: 10.1016/j.ajog.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Nusair B, Al-Gudah M, Chodankar R, Abdelazim IA, Abu MA. Uterine fibroid mapping. Curr Obstet Gynecol Rep. 2016;5:73–80. doi: 10.1007/s13669-016-0154-2. [DOI] [Google Scholar]
- 18.Charkhchi P, Cybulski C, Gronwald J, Wong FO, Narod SA, Akbari MR. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers (Basel) 2020;12(12):1–29. doi: 10.3390/cancers12123730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mani R, Jamil K. Specificity of Serum Tumor Markers (CA125, CEA, AFP, Beta HCG) in Ovarian Malignancies. Trends in Medical Research. 2007;2:128–134. doi: 10.17311/tmr.2007.128.134. [DOI] [Google Scholar]
- 20.Bastani A, Asghary A, Heidari MH, Karimi-Busheri F. Evaluation of the sensitivity and specificity of serum level of prostasin, CA125, LDH, AFP, and hCG+? in epithelial ovarian cancer patients. European Journal of Gynaecological Oncology. 2017;38(3):418–24. [PubMed] [Google Scholar]
- 21.Walker C, Banning K, Ritchie C, Kliethermes C. Laparoscopic management of a degenerating cystic leiomyoma imitating an ovarian cyst: a case report. Case Reports in Women's Health. 2020;27:e00205. doi: 10.1016/j.crwh.2020.e00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torre LA, et al. Ovarian cancer statistics, 2018. CA: A Cancer Journal for Clinicians. 2018;68(4):284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JH, Jeong YK, Park JK, Hwang JC. Ovarian Vascular Pedicle. Sign Revealing Organ of Origin of a Pelvic Mass Lesion on Helical CT. 2003;181:131–137. doi: 10.2214/ajr.181.1.1810131. [DOI] [PubMed] [Google Scholar]
- 24.van Holsbeke C, et al. Endometriomas: their ultrasound characteristics. Ultrasound in Obstetrics & Gynecology: The Official Journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2010;35(6):730–740. doi: 10.1002/uog.7668. [DOI] [PubMed] [Google Scholar]
- 25.Bianek-Bodzak A, Szurowska E, Sawicki S, Liro M. The importance and perspective of magnetic resonance imaging in the evaluation of endometriosis. BioMed Research International. 2013 doi: 10.1155/2013/436589. [DOI] [PMC free article] [PubMed] [Google Scholar]