Abstract
Objective.
The radiologic evaluation of patients with hearing loss includes computed tomography and magnetic resonance imaging (MRI) to highlight temporal bone and cochlear nerve anatomy. The central auditory pathways are often not studied for routine clinical evaluation. Diffusion tensor imaging (DTI) is an emerging MRI-based modality that can reveal microstructural changes in white matter. In this systematic review, we summarize the value of DTI in the detection of structural changes of the central auditory pathways in patients with sensorineural hearing loss.
Data Sources.
PubMed, Embase, and Cochrane.
Review Methods.
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement checklist for study design. All studies that included at least 1 sensorineural hearing loss patient with DTI outcome data were included.
Results.
After inclusion and exclusion criteria were met, 20 articles were analyzed. Patients with bilateral hearing loss comprised 60.8% of all subjects. Patients with unilateral or progressive hearing loss and tinnitus made up the remaining studies. The auditory cortex and inferior colliculus (IC) were the most commonly studied regions using DTI, and most cases were found to have changes in diffusion metrics, such as fractional anisotropy, compared to normal hearing controls. Detectable changes in other auditory regions were reported, but there was a higher degree of variability.
Conclusion.
White matter changes based on DTI metrics can be seen in patients with sensorineural hearing loss, but studies are few in number with modest sample sizes. Further standardization of DTI using a prospective study design with larger sample sizes is needed.
Keywords: diffusion tensor imaging, tractography, hearing loss, tinnitus, fractional anisotropy, auditory brainstem, cochlear nucleus, auditory brainstem implant, inferior colliculus, auditory cortex, MRI, magnetic resonance imaging
Radiologic evaluation of children and adults with sensorineural hearing loss typically consists of computed tomography (CT) and/or magnetic resonance imaging (MRI) to assess for temporal bone and retrocochlear pathology.1,2 While high-resolution MRI is sufficient to diagnose congenital anomalies of the otic capsule, tumors of the internal auditory canal, and anatomy of the cochlear nerve,3–5 information about central auditory pathway is limited.
Recent advancements in MRI technology have led to the emergence of diffusion tensor imaging (DTI).6,7 DTI is an MRI-based modality that can reveal white matter tract microstructure of the central nervous system (CNS).6 DTI is based on the capacity of diffusion-weighted imaging (DWI) to measure the extent and direction of water molecule diffusion within CNS tissue.8 Water molecule diffusion in white matter is hindered by myelin sheaths and is therefore anisotropic, occurring only along directions parallel to fiber tract orientation.9 The directionality of water molecule diffusion in white matter is exploited by DTI processing protocols to (1) identify axonal bundles, (2) estimate axonal orientation, and (3) obtain objective measures of their directionality and myelination status.
Local measurements of diffusion direction and magnitude can be used to calculate what is known as a “tensor.” The diffusion tensor can be represented in 3-dimensional (3D) space by an ellipsoid defined by 3 “eigenvalues”: λ1, λ2, and λ3.8 In the postprocessing setting, a number of scalar values that suggest the directionality and magnitude of diffusion at a particular region of interest can be extracted and quantified. For example, computation of scalar values, such as fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD), from the 3 defining eigenvalues can be used to assess the fidelity of white matter tracts.7 AD, RD, and MD are relatively straightforward measures, representing diffusivity in the direction of the largest eigenvector (AD), in the direction of the smaller eigenvectors (RD), and the mean diffusivity across all 3 eigenvectors (MD). FA is a measure of the fraction of total diffusion within a given tensor that is anisotropic and is widely used as an important parameter to determine the directionality of diffusion. Estimation of neighboring tensors within white matter bundles typically exhibits highly anisotropic diffusion in the same orientation and can be used to create topographical maps of nerve fiber bundles, a technique more commonly known as “tractography”9 (Table 1).
Table 1.
Diffusion Tensor Imaging Definitions.
| Characteristic | Definition |
|---|---|
| Eigenvectors/eigenvalues | Eigenvectors are characteristic vectors of a linear transformation that do not change direction when the transformation is applied to them. The eigenvalue is the scalar value by which the eigenvector is multiplied by when it is subject to the transformation. In the diffusion tensor model, this linear algebraic concept is used to estimate the extent of water diffusion along the x-, y-, and z-axes within a given tensor.7 |
| Diffusion tensor | A 3 × 3 matrix that represents molecular diffusion in a 3-dimensional space. Often represented as an ellipsoid defined by 3 eigenvalues: λ1, λ2, and λ3, where λ1 > λ2 > λ3.6 |
| Diffusion tensor imaging | Magnetic resonance imaging modality that maps the extent and direction of water molecule diffusion within tissue using the tensor model. |
| Diffusion tractography | Technique used to map out white matter bundles. This is done by fitting adjacent tensors to predict the most probable course of fibers. |
| Fractional anisotropy (FA) | Represents the degree of diffusion within a given tensor that is anisotropic. FA = √(3/2) * (√((λ1 − MD)2 + (λ2 − MD)2 + (λ3 − MD)2)/√(λ12 + λ22 + λ32).6 |
| Mean diffusivity (MD) | Represents mean diffusion across all three axes within a given tensor. MD = (λ1 + λ2 + λ3)/3.7 |
| Radial diffusivity (RD) | Represents the magnitude of diffusion along axes perpendicular to the principal direction of diffusion within a given tensor. RD = (λ2 + λ3)/2.30 |
| Axial diffusivity (AD) | Represents magnitude of molecular diffusion along the axis parallel to the principal direction of diffusion within a given tensor. AD = λ1.30 |
DTI is an increasingly important clinical and research tool that is expanding our understanding of white matter changes that occur in the context of neurological and neurodevelopmental disorders. DTI studies in the fields of neurology, neurosurgery, and psychiatry have shown promising results that suggest the clinical usefulness of this novel radiologic approach.10–13 For example, patients with traumatic and ischemic neurologic insults have reduced FA measures at several neuroanatomical regions. Correlational analyses between diffusion measures and long-term cognitive and motor outcomes suggest that DTI can provide important prognostic information in patients afflicted with these conditions. DTI and tractography studies in the visual system have demonstrated changes in the connectivity and integrity of optical white matter tracts in blind patients.14,15
Detailed morphometric analyses of the cochlea and cochlear nerve are possible with CT and MRI. Findings of an enlarged vestibular aqueduct or a dysmorphic, hypoplastic, or absent cochlea or cochlear nerve provide important information for prognostic counseling and therapeutic decision making in patients who are cochlear or auditory brainstem implant candidates.3,4,16 Auditory structures upstream to the cerebellopontine angle, however, are not well defined on contemporary clinical imaging. Over the past 5 years, the literature on the utility of DTI in the evaluation of the auditory white matter tracks has rapidly evolved. In this review, we aim to provide a comprehensive qualitative analysis of DTI studies in patients with sensorineural hearing loss.
Materials and Methods
Search Strategy
We performed a systematic review of the literature to identify articles employing DTI to investigate the auditory pathways in patients with hearing loss. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement checklist was used to inform the design of the review.17 A comprehensive review of PubMed, Embase, and Cochrane was performed in March 2017. Key search terms included the following words: diffusion tensor imaging, tractography, auditory, hearing loss, acoustic, and cochlear. MeSH search terms included the following: “diffusion tensor imaging AND hearing,”“Diffusion Tensor imaging AND hearing loss,”“Diffusion tensor imaging AND auditory,”“Diffusion tensor imaging AND cochlear,”“Tractography AND hearing” OR “Tractography AND hearing loss,”“Tractography AND auditory,” and “Tractography AND cochlear.”
Inclusion/Exclusion Criteria and Variables Assessed
Titles and abstracts of all search results were screened to determine relevance to our topic. Full-text articles of screened studies were reviewed to determine eligibility. All studies in which DTI and diffusion tractography were performed on patients with hearing loss were included regardless of their quality. Studies in which full-text articles were not available or that describe use of DTI in the auditory system outside the context of hearing loss were excluded.
Included articles were assessed for year of publication, population size, regions of auditory pathways investigated, and diffusion indices reported. All patients included in the study were assessed for the following parameters when found: age, degree of hearing loss, side of hearing loss, etiology of hearing loss (congenital, age related), type of hearing loss (prelingual/postlingual, unilateral/bilateral), cochlear implant usage, hearing aid usage, and audiometric testing results.
Level of Evidence/Quality Assessment
Determination of level of evidence for all included studies was guided by the Oxford Center for Evidence-based Medicine Levels of Evidence.18 This grading system categorizes studies into 4 different “levels.” Level 1 is reserved for systematic reviews of randomized trials. Level 2 includes individual high-quality randomized trials that report a dramatic effect. Level 3 includes individual nonrandomized controlled cohort studies. Level 4 includes individual case-control studies, case series reports, and historically controlled studies. A 10-item quality assessment checklist was generated to grade the quality of included studies. Studies received 1 point for meeting each of the items in the checklist. Studies achieving scores of 0 to 4 were considered “low-quality,” those achieving scores of 5 to 7 were considered of “moderate quality,” and studies with scores of 8 to 10 were deemed to be “high-quality” studies (Table 2).
Table 2.
Ten-Item Rating Scale for Quality Assessment of Included Studies.
| 1. | Clear description of study design |
| 2. | Clear description of subject/control inclusion and exclusion criteria |
| 3. | Inclusion of appropriate control group |
| 4. | Clear description of study population demographics |
| 5. | Clear description of hearing loss type, degree, and laterality |
| 6. | Clear description of MRI acquisition parameters and DTI/tractography workflow |
| 7. | Description of regions of interest included with well-formulated reasoning |
| 8. | Scalar diffusion metrics reported |
| 9. | Diffusion tractography performed |
| 10. | Correlation of scalar diffusion metrics with audiometric tools (CAP, audiometric thresholds etc) |
Abbreviations: CAP, categories of auditory performance; DTI, diffusion tensor imaging; MRI, magnetic resonance imaging.
Results
Search Results
Our initial search yielded 623 records after duplicates were removed. Thirty-four full-text articles were reviewed after irrelevant articles were screened out by title/abstract. Thirteen studies were excluded after reviewing full-text articles for the following reasons: patients exhibited normal hearing (n = 11), studies were unclear whether included subjects had hearing loss (n = 2), and DTI analysis was not used (n = 1). Summary of the search strategy is outlined in Figure 1. Included articles were published between 2004 and 2017.
Figure 1.
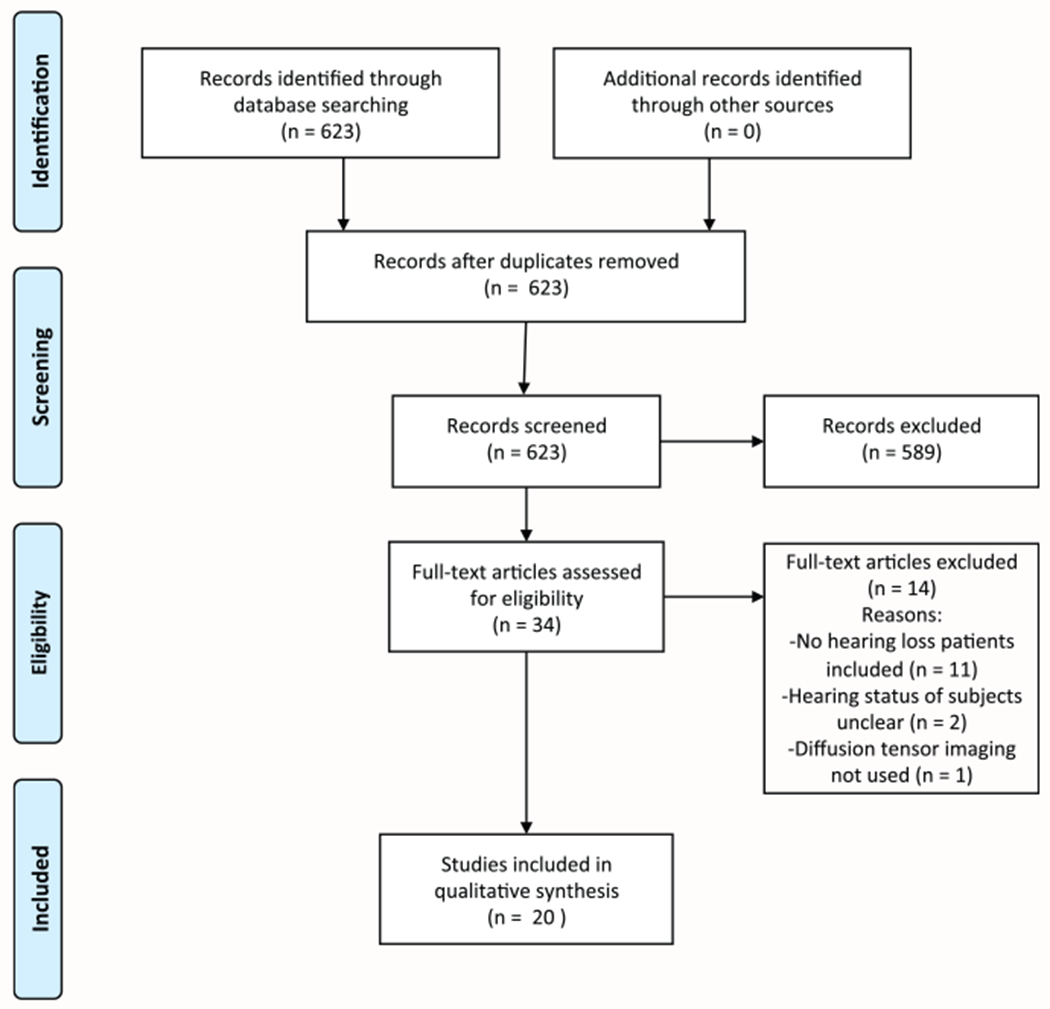
Flowchart demonstrating the study selection process, following the established Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommended guidelines.
Most studies analyzed in this review had a cross-sectional design (90%) and were of moderate quality (80%) according to our quality assessment checklist. Patient demographics and study characteristics are summarized in Table 3.
Table 3.
Study Characteristics and Patient Demographics.
| Reference | Design, Level of Evidence, Quality | No. in Study Group, Mean Age ± SEM (Range) | No. in Control Group, Mean Age (Range), y | DTI/Tractography | Correlation with Audiometric Tool |
|---|---|---|---|---|---|
| BHL | |||||
| Chang et al34 | Cross-sectional, 4, moderate | n = 10, 33.7 (8-85) | n = 10, 26.5 (22-34) | DTI | None |
| Lee et al32 | Cross-sectional, 4, low | n = 12, 4.5 (2-9) | n = 5, 17.8 (3-26) | DTI | None |
| Kim et al21 | Cross-sectional, 4, moderate | n = 13, 29.3 ± 6.8 | n = 29, 26.5 ± 4.5 | DTI | None |
| Chang et al26 | Retrospective, 4, moderate | n = 18, 5.9 (1-14) | None | DTI | FA correlated with CAP scores |
| Miao et al22 | Cross-sectional, 4, moderate | n = 16, 14.56 ± 2.1 (10-18) | n = 16, 14.75 ± 2.38 (10-18) | DTI | None |
| Hribar et al23 | Cross-sectional, 4, moderate | n = 14,35.4 ± 0.6 (23-50) | n = 14, 30.5 ± 5.2 (23-50) | DTI | None |
| Lyness et al36 | Cross-sectional, 4, low | n = 13, 39.08 ± 11.08 | n = 13, 38.7 ± 8.1 | DTI | None |
| Wu35 | Cross-sectional, 4, moderate | n = 92, 4.9 (1-14) | n = 46, 3.7 (1-10) | DTI | FA correlated with CAP scores |
| Huang et al25 | Prospective, 4, high | n = 24, 4.7 ± 1 | n = 20, 4.2 ± 2.9 | DTI | None |
| Karns et al24 | Cross-sectional, 4, high | n = 23, 28 ± 1.4 | n = 26, 25 ± 1 | DTI + tractography | None |
| Lin et al31 | Cross-sectional, 4, moderate | n = 15, 32.4 ± 11.8 | n = 10, 31.1 ± 11.6 | DTI | FA correlated with dB threshold |
| UHL | |||||
| Lin et al31 | Cross-sectional, 4, moderate | n = 12, 30.8 ± 10.1 | n = 10, 31.1 ± 11.6 | DTI | FA correlated with dB threshold |
| Wu30 | Cross-sectional, 4, moderate | n = 19, 24.1 (8-60) | n = 10, 31 (18-58) | DTI | None |
| Wu et al27 | Cross-sectional, 4, moderate | n = 12, 13 (8-29) | n = 12 “age matched” | DTI | None |
| Rachakonda et al37 | Cross-sectional, 4, moderate | n = 29, 12.2 ± 2.35 | n = 20, 12.7 ± 2.93 | DTI | None |
| Vos et al33 | Cross-sectional, 4, moderate | n = 5 (34-67) | n = 5 (29-57) | DTI + tractography | None |
| Presbycusis | |||||
| Profant et al19 | Cross-sectional, 4, moderate | n = 27, 68.04 ± 0.6 | n = 12, 25.3 ± 7.2 | DTI + tractography | None |
| Ma et al20 | Cross-sectional, 4, moderate | n = 15, 63.2 ± 2.6 | n = 14, 62.4 ± 2 | DTI | None |
| Tinnitus | |||||
| Crippa et al29 | Cross-sectional, 4, moderate | n = 10, 49 ± 12 (30-70) | n = 15, 46 ± 16 (26-75) | DTI | None |
| Husain et al38 | Cross-sectional, 4, moderate | n = 8 (42-64) | n = 11 | DTI | None |
| Seydell-Greenwald et al28 | Cross-sectional, 4, moderate | n = 24, 50.13 ± 14.64 (23-66) | n = 19, 48.32 ± 12.04 (27-67) | DTI | None |
Abbreviations: BHL, bilateral hearing loss; CAP, categories of auditory performance; DTI, diffusion tensor imaging; FA, fractional anisotropy; UHL, unilateral hearing loss.
Patient Demographics and Hearing Loss
Of the studies that met inclusion and exclusion criteria, 411 patients with hearing loss and 297 normal hearing controls were included in all studies evaluated. Age ranged from 1 to 85 years in the hearing loss group and 1 to 75 years in the normal hearing control group. Of the patients in the cumulative hearing loss cohort, 60.8% had the diagnosis of bilateral hearing loss, and 18.7% patients were diagnosed with severe to profound unilateral hearing loss. We inferred from description of hearing loss cause and age of onset in the included articles that 32.3% of the unilateral hearing loss group were deaf prelingually. Profant et al19 and Ma et al20 investigated white matter changes in a total of 42 (10.2%) elderly subjects with presbycusis. A total of 10.2% of all patients complained of tinnitus as their primary problem, with pure-tone audiometry revealing mild to moderate hearing loss bilaterally in this cohort. All studies used FA measures. MD, RD, and AD reporting was inconsistent across included studies. Three studies performed diffusion tractography to map their regions of interest (ROIs) (Figure 2).
Figure 2.
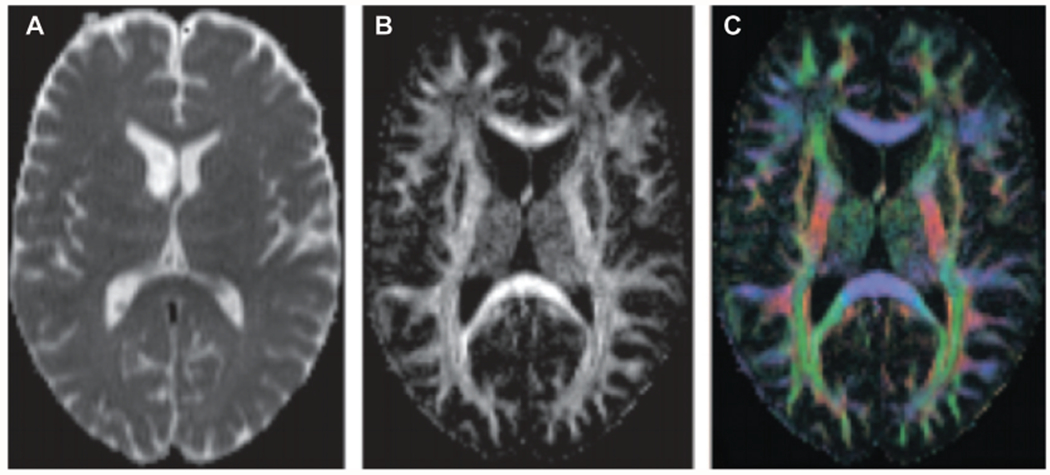
Example of diffusion tensor imaging maps. (A) Gray-scale mean diffusivity (MD) map generated by whole-brain diffusion tensor estimation and MD computation. (B) Gray-scale fractional anisotropy (FA) map generated by whole-brain diffusion tensor estimation and MD computation. (C) Red, blue, and green color map of the 3 eigenvectors of diffusion tensors. Red, green, and blue represent diffusion on the x-, y-, and z-axes, respectively. Republished with permission from: Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(suppl 1):S205-S223. © Radiological Society of North America.
Regions of Interest along the Auditory Pathway
Auditory ROIs studied included structures at several points along the central pathways. Regions associated with the auditory cortex such as Heschl’s gyrus, the superior temporal gyrus, and the auditory radiation were the most extensively imaged regions with 11 of 20 (55%)19–29 articles. The inferior colliculus and lateral lemniscus were less frequently examined in 7 of 20 (35%)25,27–32 and 3 of 20 (15%),27,30,31 respectively. One article detailed DTI and tractography of the cochlear nerve in hearing loss patients.33 The variability in ROIs, types of hearing loss studied, diffusion indices measured, and statistical methods employed made comparisons of findings across studies difficult. An attempt was made to report general trends seen in the literature.
Diffusion Anisotropy Measures in Patients with Bilateral Hearing Loss
Ten of 20 studies included evaluated patients with bilateral hearing loss. All 10 studies used DTI, and 1 reported the use of diffusion tractography. Most patients in this group were diagnosed with prelingual severe to profound bilateral hearing loss. Chang et al34 studied 6 patients with mild to moderate bilateral hearing loss but did not see differences in mean FA compared to controls. Lin et al31 also studied 15 patients with bilateral profound hearing loss but did not provide details regarding the etiology or onset of hearing loss. Congenital deafness, drug toxicity, and meningitis were all reported as causes of hearing loss. Etiology was reported as unknown or not reported at all in many cases. A great degree of variability in anatomical ROIs selected for DTI analysis was noted in both auditory and nonauditory regions of the brain. Auditory ROIs analyzed in this cohort include the inferior colliculus (IC), trapezoid body (TB), lateral lemniscus (LL), superior olivary nucleus (SON), medial geniculate body (MGB), Heschl’s gyrus (HG), the auditory radiation (AR), and the superior temporal gyrus (STG).
Regions of the auditory cortex (STG, HG) were the most frequently implicated ROIs, with 6 of 10 studies reporting significantly lower FA values in the deaf cohort at this ROI.21–25,35 Another region found to be commonly affected in this cohort was the IC, with 3 of 10 studies showing significant reductions in FA measures in deaf participants.31,32,34 Similarly, reductions in FA values were found at the IC, SON, TB, MGB, and AR in other studies as well as a number of nonauditory ROIs (internal capsule, superior longitudinal fasciculus, genu of the corpus callosum). RD was found to be higher at the HG, LL, and IC in patients with profound bilateral sensorineural hearing loss. Finally, Lyness et al36 studied thalamocortical projections in congenitally deaf individuals and found alterations in diffusion measures in tracts connecting the thalamus to motor, somatosensory, parietal, occipital, and frontal cortical targets.
Diffusion Anisotropy and Cochlear Implantation
Three of the 10 studies mentioned above correlated FA measures with cochlear implant outcomes as measured by categories of auditory performance (CAP) scores.25,26,35 Huang et al25 prospectively studied 24 patients with profound prelingual deafness who received a DTI scan before cochlear implantation. Patients were divided into good-outcome (CAP >6) and poor-outcome (CAP <6) groups based on 12-month postoperative CAP scores. FA measures from 6 ROIs (IC, SON, TB, HG, TB, AR) were compared and found to be significantly higher in the good-outcome group.25 No significant difference between groups was seen in MD values. Furthermore, regression analysis revealed that FA measures at all 6 ROIs showed a positive correlation with CAP scores. Similarly, Chang et al26 retrospectively studied a group of 18 cochlear implant (CI) recipients and found significantly higher FA values in good-outcome subjects at the AR. Significant correlations were also noted between CAP scores and FA values at the AR. No difference was seen in FA values at the STG. Furthermore, Wu et al35 studied a subgroup of their hearing loss cohort who received the CI and had 12-month postoperative CAP scores. Similar to the findings by Chang et al,26 higher FA values at the AR were noted in the good-outcome group, with a significant positive correlation between FA values at the AR and CAP scores (Table 4).
Table 4.
Differences in Scalar Values by Location.
| Characteristic | FA | MD | RD | AD |
|---|---|---|---|---|
| BHL vs NH controls | ||||
| Auditory cortex (HG/STG)21–25,35 | Decreased | No difference | Increased | Decreased |
| Inferior colliculus25,31,32 | Decreased | No difference | Increased | No difference |
| Auditory radiation25 | Decreased | No difference | NA | NA |
| Superior olivary nucleus25 | Decreased | No difference | NA | NA |
| Lateral lemniscus31 | Decreased | No difference | Increased | No difference |
| Trapezoid body25 | Decreased | No difference | NA | NA |
| Medial geniculate body25 | Decreased | No difference | NA | NA |
| Good vs poor CI outcome | ||||
| Auditory cortex (HG/STG)25 | Increased | No difference | NA | NA |
| Inferior colliculus25 | Increased | No difference | NA | NA |
| Auditory radiation25,26,35 | Increased | No difference | NA | NA |
| Superior olivary nucleus25 | Increased | No difference | NA | NA |
| Trapezoid body25 | Increased | No difference | NA | NA |
| Medial geniculate body25 | Increased | No difference | NA | NA |
| UHL vs NH controls | ||||
| Inferior colliculus27,30 | Decreased | Increased | Increased | No difference |
| Lateral lemniscus27,30 | Decreased | Increased | Increased | No difference |
| Auditory nerve33 | Decreased | No difference | NA | NA |
| UHL: ipsilateral vs contralateral to affected side | ||||
| Inferior colliculus30 | Increased | No difference | Decreased | No difference |
| Lateral lemniscus30 | Increased | No difference | Decreased | No difference |
| Auditory nerve33 | No difference | No difference | NA | NA |
| Presbycusis vs age-matched NH controls | ||||
| Auditory cortex (HG/STG)20 | No difference | Increased | Increased | Increased |
| Auditory radiation19,20 | Decreased | No difference | No difference | No difference |
| Auditory pathway (IC to HG)19 | No difference | No difference | Increased | No difference |
| Tinnitus vs NH controls | ||||
| Auditory cortex (HG/STG)28,29 | Increased | Decreased | NA | NA |
| Inferior colliculus28,29 | Increased | Decreased | NA | NA |
| Auditory radiation38 | Decreased | NA | NA | NA |
Abbreviations: AD, axial diffusivity; BHL, bilateral hearing loss; CI, cochlear implant; FA, fractional anisotropy; HG, Heschl’s gyrus; IC, inferior colliculus; MD, mean diffusivity; NA, not available; NH, normal hearing; RD, radial diffusivity; STG, superior temporal gyrus; UHL, unilateral hearing loss.
Nonprimary auditory regions found to have significantly lower FA values in this cohort of deaf patients include the following: genu of the corpus collosum, planum polare, planum temporale, external capsule, internal capsule, and Broca’s area.
Diffusion Anisotropy Measures and Tractography in Unilateral Hearing Loss
Wu et al27,30 published reports on 2 series of patients with severe to profound unilateral hearing loss (n = 31). Of these patients, 62.5% were deaf congenitally, trauma and acute myeloid leukemia were the etiology of hearing loss for 2 patients, and the etiology of hearing loss for the remaining 10 patients was unknown. Age at scan time for the congenital hearing loss group ranged from 8 to 60 years. No details regarding interval time between onset of hearing loss and scan time were provided for other etiologies. It is unclear whether there is overlap between the 2 populations. FA, MD, RD, and AD were measured at the LL and the IC and compared to normal hearing controls. FA at the LL and IC on both the healthy and affected sides of unilateral hearing loss patients was significantly lower compared to normal hearing controls in both studies. MD and RD were significantly elevated at the level of the LL and IC in the hearing loss group compared to normal hearing controls. Within the unilateral hearing loss group, FA values on the side of hearing loss were significantly reduced at the IC and elevated at the LL compared to the normal hearing side. In a different study, Vos et al33 reported the use of FA measures and diffusion tractography to study the cochlear nerve in a group of 5 patients with severe to profound unilateral deafness. Diffusion tractography was successfully performed in all patients. FA values at the auditory nerve were significantly lower on both the healthy and affected sides in unilateral hearing loss patients compared to normal hearing controls. No significant differences in FA or MD were seen in comparisons between the healthy and affected sides in this patient group. Tractographic reconstructions were also used to guide “along-tract” analyses of FA/MD values by plotting them as a function of their distance from the brainstem. Along-tract analyses showed high inter- and intrasubject variability and did not provide valuable information. Finally, Rachakonda et al37 studied 29 patients with unilateral hearing loss and found FA values to be significantly lower at the level of the LL and HG compared to normal hearing controls. MD values were also found to be elevated at the level of HG compared to controls (Table 4).
Diffusion Anisotropy Measures in Presbycusis
Two studies investigated the use of DTI to evaluate the white matter of patients with age-related hearing loss/presbycusis. Profant et al19 reported significant reduction in RD at the HG attributable to hearing loss in presbycusis patients. No differences in FA, MD, and AD were appreciated. In the other study, Ma et al20 compared a cohort of presbycusis patients to age-matched normal hearing controls. This study identified decreased FA and increased MD/AD/RD in the right-sided HG in the presbycusis group (Table 4).
Diffusion Anisotropy Measures in Tinnitus
Three studies identified in our literature search used DTI to study structural changes in the auditory pathways of patients whose chief complaint was tinnitus. In an attempt to discern white matter changes associated with tinnitus from those associated with hearing loss, Husain et al38 conducted whole-brain analyses on 3 different groups: (1) mild to moderate hearing loss with tinnitus, (2) mild to moderate hearing loss with no tinnitus, and (3) normal hearing with no tinnitus. FA values at the AR, inferior and superior longitudinal fasciculi, inferior fronto-occipital fasciculus, and the anterior thalamic radiation on the right side were significantly lower in the hearing loss groups (groups 1 and 2) compared to the normal hearing group (group 3). Subgroup analyses revealed that patients without tinnitus had a greater reduction in FA in the mentioned regions compared to those with tinnitus.
Seydell-Greenwald et al28 reported that FA values are increased and MD values are decreased in the left auditory cortex and in the white matter surrounding the IC in tinnitus patients compared to normal hearing controls. Finally, Crippa et al29 performed probabilistic tractography to visualize fiber tracts interconnecting the auditory cortex, IC, and the amygdala (thought to be involved in tinnitus). Mean FA values were computed by averaging FA measures obtained at each voxel along that path. A significant decrease in FA values was seen in tracts connecting the auditory cortex (AC) to the amygdala. Furthermore, the “strength” of each path, defined as the proportion of fibers leaving the origin ROI that reach the target ROI, was measured and was found to be significantly lower in the right hemisphere of tinnitus patients in tracts connecting the AC to the IC and the amygdala.
Discussion
In this report, we reviewed the current state of DTI literature pertaining to individuals with hearing loss. We analyzed the quality and design of individual studies, characteristics of study subjects, diffusion indices used, and neuroanatomical regions studied. Patients with bilateral profound prelingual deafness constituted the most widely studied population in the literature. These studies demonstrated lower diffusion scalar values at several cortical and subcortical auditory structures in the hearing loss cohort compared to normal hearing controls. The IC and the auditory cortex (HG/STG) were most consistently found to have lower FA values in this group. The LL, SON, MGB, AR, and several nonauditory regions were also implicated by a number of different reports. Three studies correlated FA values with audiometric outcomes after cochlear implant surgery and demonstrated a positive correlation between CAP scores and FA values at the AR. One study also showed that CAP scores were positively correlated with FA values at the SON, IC, MGB, TB, Broca’s area, and genu of the corpus collosum (CC). These data are particularly promising, and further work in this context has the potential to provide an objective method to prospectively predict outcomes of cochlear implantation and assist surgical decision making. Only one of these studies went beyond FA measures to include MD, where no difference was seen. Refinement of tractography approaches may enable better characterization of the cochlear nucleus in the future, and this will have implications in the candidacy of auditory brainstem implant (ABI) candidates. This is especially relevant in children with congenital deafness associated with cochlear or cochlear nerve aplasia in whom cochlear nucleus morphology may also be problematic.
In unilateral hearing loss patients, significant differences in FA between the healthy and affected sides were observed at the lateral lemniscus and inferior colliculus. Furthermore, FA values at the lateral lemniscus, inferior colliculus, and auditory nerve were lower on both sides compared to normal hearing controls. There was no difference in FA between the ipsilateral and contralateral cochlear nerves. The latter of these findings is particularly interesting as the cochlear nerve is the only point along the auditory pathways that receives sound input exclusively from the ipsilateral ear and is not involved in binaural processing. If these early observations are true, this suggests the possible influence of contralateral projection activity to the cochlear nucleus on cochlear nerve morphology. However, this finding is based on a limited patient cohort (n = 5) from a single study33 (Figure 3), and more data are needed to support these findings.
Figure 3.
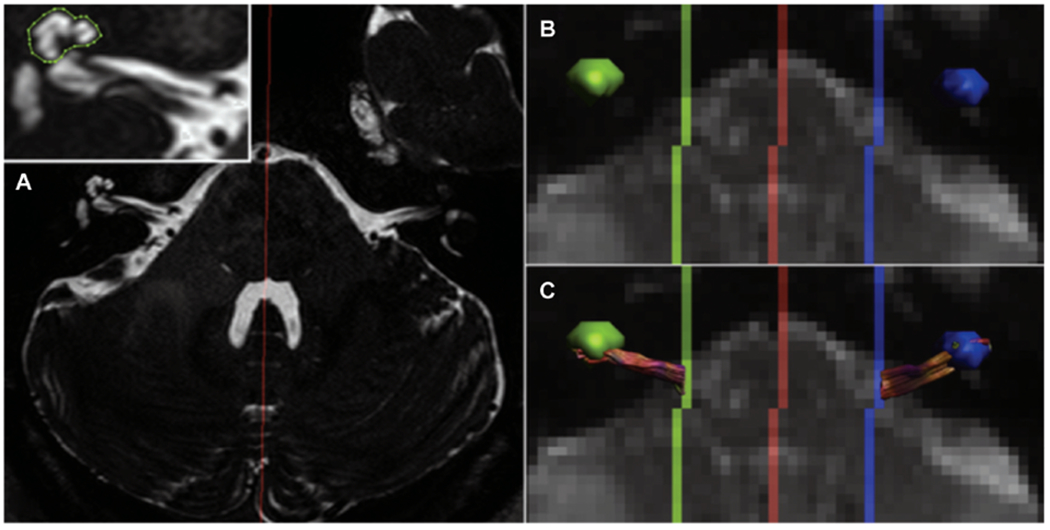
Example of tractography. (A) T2-weighted structural magnetic resonance imaging used to identify the cochlear coordinates to use it as a seed point for tractography of the cochlear nerve. (B, C) Cochlear volume is transposed onto diffusion-weighted imaging, and tractography is used to connect seed points originating in the cochlea to the brainstem. Reprinted from Vos SB, Haakma W, Versnel H, et al. Diffusion tensor imaging of the auditory nerve in patients with long-term single-sided deafness. Hearing Res. 2015;323:1-8. Copyright 2015, with permission from Elsevier.
Patients with progressive hearing loss represented the least well-studied subgroup in our review. This group demonstrated decreased FA at the auditory radiation and increased MD/AD/RD in regions of the auditory cortex. A number of studies in the literature investigate age-related changes in the auditory system that may be relevant to this population of patients.39,40 These studies were excluded from our analysis as hearing status was not documented and the effects of presbycusis on white matter tracts could not possibly be discerned from those of an aging CNS based on the data presented.
The final group of articles that we included in our analysis investigated white matter changes in tinnitus. Work in this field has the promise to identify neurological regions that undergo structural changes that may be used as therapeutic targets for this intractable clinical problem. In contrast to what was seen in other groups, FA values actually experienced an increase in regions of the auditory cortex and the inferior colliculus. This finding is in line with one proposed pathophysiological mechanism stating that tinnitus is primarily a hyperplastic process (disinhibition of central auditory nuclei) driven by deprivation of auditory input.
DTI is an evolving tool in the field of hearing research, and the clinical utility of scalar diffusion metrics remains uncertain. The findings of our review that show promise of becoming clinically useful in the near future are those demonstrating positive correlations between FA values at multiple auditory nuclei and the clinical outcome of cochlear implant surgery. This suggests that further research in the area has the potential to inform and potentially modify cochlear implant candidacy guidelines and predict outcomes. Beyond this finding, the literature is largely binary in its application of DTI to the hearing system and focuses on simply reporting the presence or absence of a difference in diffusion measures between patients and normal hearing controls. Further work that correlates diffusion measures to different objective and subjective auditory tests must be done to enhance the clinical utility of this novel tool.
The main limitation of this review is the lack of comparability among studies due to variations in the methods employed, neurological ROIs studied, and types of hearing loss studied. Differences in MRI acquisition protocols, MRI scanner field strength, and postprocessing strategies of imaging data introduce a lack of uniformity to our review that is difficult to control for. It is also important to note that diffusion tensor imaging performs poorly in regions with multiple fiber populations traveling in a number of different directions. This limitation is problematic when trying to accurately demarcate auditory nuclei in the complex neural framework of the brainstem. The variability in anatomical regions of interest can be attributed in part to the use of automated whole-brain analytic methods that extract voxel clusters found to have significantly different scalar diffusion metrics between 2 data sets. The practice and equipment used by radiology departments also vary widely from center to center and may contribute further to the lack of comparability in our review. Other notable limitations to our study are as follows: (1) literature review may have failed to identify studies that report the use of DTI in the context of hearing loss, (2) otherwise eligible non-English articles that may have yielded important findings were not included, (3) publication bias may have influenced our findings as no attempt was made to access unpublished data, and (4) nonstandardized approaches to reporting outcomes were used in several articles. Regarding the latter of these points, attempts were made when possible to interpret the study design and fit disparate data into comparable data sets.
In summary, DTI is an exciting MRI research tool that is being increasingly used to study microstructural changes in the central auditory pathways of hearing loss patients. Sensorineural hearing loss can be associated with white matter changes in several auditory and nonauditory neurological structures. Further research in this area has the potential to deepen our understanding of subclinical anatomical changes that occur in the auditory pathways of patients with hearing loss.
Conclusion
DTI is an emerging technology to study the auditory system. There are detectable changes associated with hearing loss, but to date, there is a large degree of variability in the regions of interest studied and the diffusion metrics used. Further study and standardization of DTI protocols are needed before DTI can become a robust diagnostic modality in the evaluation of a patient with sensorineural hearing loss.
Funding source:
NIH T32 Training Grant (V.V.K.) and American Hearing Research Foundation Grant (E.D.K., A.K.R., D.J.L.).
Footnotes
Competing interests: None.
References
- 1.Sennaroglu L, Saatci I. A new classification for cochleovestibular malformations. Laryngoscope. 2002;112:2230–2241. [DOI] [PubMed] [Google Scholar]
- 2.Jackler RK, Luxford WM, House WF. Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngoscope. 1987;97:2–14. [DOI] [PubMed] [Google Scholar]
- 3.Glastonbury CM, Davidson HC, Harnsberger HR, Butler J, Kertesz TR, Shelton C. Imaging findings of cochlear nerve deficiency. Am J Neuroradiol. 2002;23:635–643. [PMC free article] [PubMed] [Google Scholar]
- 4.Noij KS, Remenschneider AK, Kozin ED, et al. Direct parasagittal magnetic resonance imaging of the internal auditory canal to determine cochlear or auditory brainstem implant candidacy in children. Laryngoscope. 2015;125:2382–2385. [DOI] [PubMed] [Google Scholar]
- 5.Gentry LR, Jacoby CG, Turski PA, Houston LW, Strother CM, Sackett JF. Cerebellopontine angle-petromastoid mass lesions: comparative study of diagnosis with MR imaging and CT. Radiology. 1987;162:513–520. [DOI] [PubMed] [Google Scholar]
- 6.Hagmann P, Jonasson L, Maeder P, Thiran JP, Wedeen VJ, Meuli R. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26(suppl 1):S205–S223. [DOI] [PubMed] [Google Scholar]
- 7.Soares JM, Marques P, Alves V, Sousa N. A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci. 2013;7:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori S, Barker PB. Diffusion magnetic resonance imaging: its principle and applications. Anat Rec. 1999;257:102–109. [DOI] [PubMed] [Google Scholar]
- 9.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. [DOI] [PubMed] [Google Scholar]
- 10.Subramaniam K, Gill J, Fisher M, Mukherjee P, Nagarajan S, Vinogradov S. White matter microstructure predicts cognitive training-induced improvements in attention and executive functioning in schizophrenia [published online July 6, 2017]. Schizophr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aoki Y, Inokuchi R, Gunshin M, Yahagi N, Suwa H. Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis. J Neurol Neurosurg Psychiatry. 2012;83:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts RM, Mathias JL, Rose SE. Relationship between diffusion tensor imaging (DTI) findings and cognition following pediatric TBI: a meta-analytic review. Dev Neuropsychol. 2016;41:176–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar P, Kathuria P, Nair P, Prasad K. Prediction of upper limb motor recovery after subacute ischemic stroke using diffusion tensor imaging: a systematic review and meta-analysis. J Stroke. 2016;18:50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reislev NH, Dyrby TB, Siebner HR, Lundell H, Ptito M. Thalamocortical connectivity and microstructural changes in congenital and late blindness. Neural Plast. 2017;2017:9807512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reislev NL, Dyrby TB, Siebner HR, Kupers R, Ptito M. Simultaneous assessment of white matter changes in microstructure and connectedness in the blind brain. Neural Plast. 2016;2016:6029241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deep NL, Carlson ML, Weindling SM, et al. Diagnosing large vestibular aqueduct: radiological review of high-resolution CT versus high-resolution volumetric MRI. Otol Neurotol. 2017;38:948–955. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howick J, Chalmers I, Glasziou P, et al. The 2011 Oxford CEBM Levels of Evidence (Introductory Document). Oxford, UK: Oxford Centre For Evidence Based Medicine; 2011. [Google Scholar]
- 19.Profant O, Škoch A, Balogová Z, Tintěra J, Hlinka J, Syka J. Diffusion tensor imaging and MR morphometry of the central auditory pathway and auditory cortex in aging. Neuroscience. 2014;260:87–97. [DOI] [PubMed] [Google Scholar]
- 20.Ma W, Li M, Gao F, et al. DTI analysis of presbycusis using voxel-based analysis. Am J Neuroradiol. 2016;37:2110–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DJ, Park SY, Kim J, Lee DH, Park HJ. Alterations of white matter diffusion anisotropy in early deafness. Neuroreport. 2009;20:1032–1036. [DOI] [PubMed] [Google Scholar]
- 22.Miao W, Li J, Tang M, et al. Altered white matter integrity in adolescents with prelingual deafness: a high-resolution tract-based spatial statistics imaging study. Am J Neuroradiol. 2013;34:1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hribar M, Suput D, Carvalho AA, Battelino S, Vovk A. Structural alterations of brain grey and white matter in early deaf adults. Hear Res. 2014;318:1–10. [DOI] [PubMed] [Google Scholar]
- 24.Karns CM, Stevens C, Dow MW, Schorr EM, Neville HJ. Atypical white-matter microstructure in congenitally deaf adults: a region of interest and tractography study using diffusion-tensor imaging. Hear Res. 2017;343:72–82. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, Zheng W, Wu C, et al. Diffusion tensor imaging of the auditory neural pathway for clinical outcome of cochlear implantation (CI) of pediatric congenital sensorineural hearing loss patients. PLoS One. 2015;10:e0140643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang Y, Lee HR, Paik JS, Lee KY, Lee SH. Voxel-wise analysis of diffusion tensor imaging for clinical outcome of cochlear implantation: retrospective study. Clin Exp Otorhinolaryngol. 2012;5(suppl 1):S37–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu CM, Ng SH, Wang JJ, Liu TC. Diffusion tensor imaging of the subcortical auditory tract in subjects with congenital cochlear nerve deficiency. Am J Neuroradiol. 2009;30:1773–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seydell-Greenwald A, Raven EP, Leaver AM, Turesky TK, Rauschecker JP. Diffusion imaging of auditory and auditory-limbic connectivity in tinnitus: preliminary evidence and methodological challenges. Neural Plast. 2014;2014:145943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crippa A, Lanting CP, van Dijk P, Roerdink JB. A diffusion tensor imaging study on the auditory system and tinnitus. Open Neuroimag J. 2010;4:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu CM, Ng SH, Liu TC. Diffusion tensor imaging of the subcortical auditory tract in subjects with long-term unilateral sensorineural hearing loss. Audiol Neurotol. 2009;14:248–253. [DOI] [PubMed] [Google Scholar]
- 31.Lin Y, Wang J, Wu C, Wai Y, Yu J, Ng S. Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: changes in radial diffusivity and diffusion anisotropy. J Magn Reson Imaging. 2008;28:598–603. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Chang Y, Lee JE, Cho JH. The values of diffusion tensor imaging and functional MRI in evaluating profound sensorineural hearing loss. Cochlear Implants Int. 2004;5:149–152. [DOI] [PubMed] [Google Scholar]
- 33.Vos SB, Haakma W, Versnel H, et al. Diffusion tensor imaging of the auditory nerve in patients with long-term single-sided deafness. Hear Res. 2015;323:1–8. [DOI] [PubMed] [Google Scholar]
- 34.Chang Y, Lee SH, Lee YJ, et al. Auditory neural pathway evaluation on sensorineural hearing loss using diffusion tensor imaging. Neuroreport. 2004;15:1699–1703. [DOI] [PubMed] [Google Scholar]
- 35.Wu C, Huang L, Tan H, et al. Diffusion tensor imaging and MR spectroscopy of microstructural alterations and metabolite concentration changes in the auditory neural pathway of pediatric congenital sensorineural hearing loss patients. Brain Res. 2016;1639:228–234. [DOI] [PubMed] [Google Scholar]
- 36.Lyness RC, Alvarez I, Sereno MI, MacSweeney M. Microstructural differences in the thalamus and thalamic radiations in the congenitally deaf. NeuroImage. 2014;100:347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rachakonda T, Shimony JS, Coalson RS, Lieu JE. Diffusion tensor imaging in children with unilateral hearing loss: a pilot study. Front Syst Neurosci. 2014;8:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Husain FT, Medina RE, Davis CW, et al. Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res. 2011;1369:74–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz J, Hemminger F, Stahl R, et al. Evidence of subcortical and cortical aging of the acoustic pathway: a diffusion tensor imaging (DTI) study. Acad Radiol. 2007;14:692–700. [DOI] [PubMed] [Google Scholar]
- 40.Moseley M. Diffusion tensor imaging and aging—a review. NMR Biomed. 2002;15:553–560. [DOI] [PubMed] [Google Scholar]


