Abstract
Purpose
The purpose of this study was to evaluate the epidemiology, etiology, clinical assessment, investigation, management, and visual consequences of high myopia (≤−6 diopters [D]) in infants and young children.
Findings
High myopia is rare in pre-school children with a prevalence less than 1%. The etiology of myopia in such children is different than in older children, with a high rate of secondary myopia associated with prematurity or genetic causes. The priority following the diagnosis of high myopia in childhood is to determine whether there is an associated medical diagnosis that may be of greater overall importance to the health of the child through a clinical evaluation that targets the commonest features associated with syndromic forms of myopia. Biometric evaluation (including axial length and corneal curvature) is important to distinguishing axial myopia from refractive myopia associated with abnormal development of the anterior segment. Additional investigation includes ocular imaging, electrophysiological tests, genetic testing, and involvement of pediatricians and clinical geneticists is often warranted. Following investigation, optical correction is essential, but this may be more challenging and complex than in older children. Application of myopia control interventions in this group of children requires a case-by-case approach due to the lack of evidence of efficacy and clinical heterogeneity of high myopia in young children.
Conclusions
High myopia in infants and young children is a rare condition with a different pattern of etiology to that seen in older children. The clinical management of such children, in terms of investigation, optical correction, and use of myopia control treatments, is a complex and often multidisciplinary process.
Keywords: high myopia, secondary myopia, syndromic myopia, myopia control, myopia genetics
There is a growing interest in active management of myopia progression in children, with a range of behavioral, pharmacological, and optical interventions available.1,2 Such interventions have generally been tested on children between 6 and 16 years old who show a conventional pattern of myopia onset and progression. In such cases, myopia develops after the age of 5 or 6 years and progresses for a variable duration until stabilizing in the teenage or early adult years.3 More rarely, children are found to have high levels of myopia in infancy or early childhood, often reaching the −6.0 diopter (D) threshold of high myopia.4 In such cases, there are a wide range of diagnostic, systemic health, and visual challenges that need to be considered. The management of such issues, at least initially, may represent a higher clinical priority than treating any observed myopic progression.
High myopia in infants and young children creates issues in relation to optical correction to ensure normal visual development and the avoidance of amblyopia. High myopia in combination with behavioral or developmental issues can be particularly challenging in children intolerant to wearing spectacles or contact lenses, with the resulting poor vision often compounding behavioral issues. In addition to behavior, visual impairment from uncorrected high myopia can adversely impact child development. For example, the inability to see faces and hence understand the emotion of others can be profoundly isolating. The term “visual autism” has been used to describe the impact of visual isolation in such children.5
The aim of this paper is to provide an overview of this complex, multifaceted topic, and give recommendations regarding the identification, evaluation, and management of high myopia in infants and young children. Such patients may present initially to primary eye care services where specialist multidisciplinary investigation and management services are not available. In such settings, a good clinical history, thorough clinical examination, and recognition of the features that mark such children as “out of the ordinary” are critical. This will facilitate prompt and appropriate onward referral.
This group of patients is commonly referred to hospital-based eye clinics. In such settings, the initial challenge is to correctly identify those cases that merit more detailed investigation, such as to separate secondary, monogenic, and syndromic forms of early onset high myopia from typical or normal high myopia, so that the appropriate range of clinical and diagnostic services are brought to bear. The scope of such management may be very wide-ranging and includes advanced ocular imaging, electrophysiology, genetic investigations, involvement of pediatricians and clinical geneticists, genetic counseling, customized optical correction strategies, and appropriate follow-up protocols for high-risk children.
Prevalence of High Myopia in Children
Despite the rising global numbers of myopic adults and children, the prevalence of high myopia (worse than −6.0 D) in children remains low. Even in high prevalence countries, such as China, population surveys have shown that the prevalence of high myopia is low (0.03 to 0.2%) before 7 years of age.6,7 Singapore has a reported prevalence of high myopia below 6 years old of 0.2%.8 In Western countries, the prevalence is very low. In a population survey of 728 children ages 6 to 7 years old, the most myopic cycloplegic refraction was −5.0 D, with no children identified with high myopia.9 In a large US report from a single health insurer, the rate of high myopia at 5 to 7 years was 0.6%.10 Two US population surveys found a prevalence of myopia worse than −4.0 D at 5 to 6 years old of 0.6% to 0.8%.11,12 As with all forms of myopia, high myopia increases with age, particularly in Asia, and this relationship is highly nonlinear. In China, the prevalence of high myopia in 15-year-olds was found to be increasing over 10 years from 3.96% to 6.69% and rising very rapidly to 15.1% (95% confidence interval [CI] = 6.4–23.8) by age 16 to 18 years.13,14 In a retrospective report from the United Kingdom, 4.6% of children less than 17 years old had high myopia,15 and in the United States a rate of 4.8% for high myopia was found in 14 to 16-year-old children.10 These two latter studies were clinic based and hence they likely overestimate population levels of myopia.
Etiology of High Myopia in Children
High myopia in children can result from both environmental and genetic causes. Such environmental factors are often distinct from the risk factors identified in epidemiological studies,16 with prematurity being the most notable environmental factor in infants. There are two distinct genetic mechanisms that can cause high myopia, first, via the interaction of the hundreds of known genetic risk factors with environmental factors, such as near work and outdoor exposure. Such cases can be considered extreme examples of typical or normal myopia, often with a higher polygenic risk score of known myopia alleles.17,18 Second, early-onset high myopia can result from mutations in a single gene that have a large impact on refractive development, independent of the usual environmental myopia risk factors, such as monogenic high myopia. In monogenic forms, high myopia can be isolated, or it can be accompanied by a wide spectrum of ocular and extraocular features, a combination that is called syndromic myopia.19 Myopia of prematurity and monogenic forms of myopia fall within the category of “secondary myopia,” which has been defined as, “A myopic refractive state for which a single, specific cause (for example drug, corneal disease or systemic clinical syndrome) can be identified that is not a recognized population risk factor for myopia development.”4
Studies of highly myopic children indicate that they represent a distinct population. High myopia present in children before 10 years of age is associated with a much higher risk of other ocular and systemic disorders than in children without high myopia. In a hospital-based survey in 2001, 54% of the highly myopic children younger than 10 years of age were born prematurely, had a neurodevelopmental delay, or had an underlying systemic disorder (including Marfan syndrome, Stickler syndrome, Noonan syndrome, and Down syndrome). In addition, 38% of the sample had associated ocular pathology.20 The authors found that severe developmental delay was the most common association at 12%. Although such patients may be uncommon in some community settings, a study in the United Kingdom showed that 44% of community-identified children with high myopia (defined as worse than −5.0 D) younger than 10 years of age had either associated ocular (25%) or undiagnosed systemic conditions (18%), including Stickler syndrome, Weill-Marchesani syndrome, and homocystinuria.21 High myopia in young children (worse than −6 D before 10 years of age) is also associated with high rates of reduced best-corrected visual acuity (78%), strabismus (32%), and anisometropia (35%).22 Both high myopia and high hyperopia are risk factors for retinal abnormalities in children, with cone-rod dystrophies and inner retinal dysfunction being the most common retinal disorders in children with high myopia.23
With increasing age there are likely fewer children with secondary high myopia and more with rapidly progressive “normal” high myopia. There is a lack of evidence to quantify these proportions by age. Very large sample sizes are necessary to identify adequate numbers of children with high myopia and such population studies are less likely to include comprehensive genetic and clinical investigations of secondary myopia. One informative study, involving 36,000 school children in Beijing,24 reported that the rate of high myopia was low in children less than 6 years old and rose only slowly up to age 10 years. After 10 years of age, the prevalence of high myopia accelerated, in line with the overall levels of myopia. Thus, it is reasonable to propose that any high myopia in a child younger than 10 years old is worthy of investigation in relation to possible underlying ocular, genetic, or systemic causes. For lower levels of myopia, a high level of clinical suspicion is warranted for children who are more myopic in diopters than their age in years. This proposal would help to identify many such cases, but by no means all. It is therefore important that clinicians should be aware of the other clinical features that can be helpful in distinguishing common forms of high myopia from monogenic and syndromic myopia.
Premature children, especially those with retinopathy of prematurity (ROP), are at high risk of developing myopia and merit appropriate monitoring to ensure timely identification and correction of visually impactful early-onset myopia.25,26 Syndromic forms of myopia, particularly in the absence of a clear family history, represent a far more challenging clinical problem because they may carry specific risks to vision (for example, inherited retinal disease and Stickler syndrome) and impact on general health to the point of being life-threatening (for example, connective tissues disorders, such as Marfan syndrome, and some metabolic disorders).19,27
Myopia of Prematurity
Premature infants (defined as those born before 37 completed weeks of gestation), both with and without ROP, are at high risk for myopia, even in the first year of life. Myopia associated with prematurity is a complex phenomenon with multiple interacting mechanisms.28,29 These include arrested anterior segment development and impaired emmetropization, with both mechanisms influenced by the disease process of ROP and the impact of treatment on ocular structures. Myopia of prematurity (MOP) has specific biometric features that differ from typical myopia, specifically steeper corneas, shallower anterior chambers, thicker lenses, and shorter axial lengths than full-term infants.30,31 The characteristic biometric features of MOP have also been reported to persist into adolescence.32,33 A proportion of premature infants without ROP display myopia that resolves in the first few months or years of life due to emmetropization, in a similar manner as what occurs in the much smaller proportion of myopic full-term infants.30,34 However, emmetropization appears to be impaired in premature infants, often resulting in persistent myopia.35
Premature Infants With ROP
The presence of ROP greatly increases the prevalence of myopia in premature infants. In very low birth weight infants (<1250 g), the prevalence of myopia at 2 years of age was 19% in eyes with any degree of ROP and only 6% in eyes without ROP.36 The prevalence increases markedly in eyes with more severe disease. About 70% of high-risk pre-threshold ROP eyes, which were treated with ablative therapy, were myopic at age 4 to 6 years. High risk eyes that resolved without treatment showed a myopia prevalence of 45%. The prevalence of high myopia (defined as <−5.0 D, in the Early Treatment for Retinopathy of Prematurity trial [ETROP]) is far more common in premature infants with ROP than those without. In the ETROP trial, 38% of treated high-risk pre-threshold ROP eyes were highly myopic by age 4 years compared with 19% of eyes with regressed ROP.26 In the presence of ROP, myopia can reach very high levels, in excess of −20.0 D, yet this myopia is not primarily axial in nature but mostly attributable to corneal, anterior chamber depth, and lenticular changes.37,38 A comparison of highly myopic premature infants with ROP and full-term highly myopic children showed that those with ROP had a mean refraction of −12.4 D and a mean axial length of 23.36 mm, compared with full-term children who had a mean refraction of −11.7 D and a mean axial length of 27.02 mm.37 The refractive difference in this study was almost entirely lenticular in etiology. In addition to myopia, such infants also display high rates of astigmatism, anisometropia, and ROP-associated posterior segment changes.39 Aggressive posterior ROP has been found to be a particularly strong risk factor for high myopia, especially in Asian countries.40–42
Myopia associated with ROP is further complicated by the impact of treatment on eye growth, with cryotherapy leading to more myopia than laser treatment.43,44 Bevacizumab appears to have less impact on refraction than laser treatment.45 In keeping with earlier studies, the variation in refractive outcomes of eyes treated for ROP is not correlated with axial length. At 2 years of age, eyes treated with bevacizumab had a mean refraction of −0.98 D, as compared with −14.38 D in eyes treated with lens sparing vitrectomy, but the axial lengths were not significantly different (21.30 mm vs. 21.85 mm).46 Another study found 14% of bevacizumab treated eyes developed spherical equivalent myopia worse than −5.0 D at 1 year, supporting the concept that VEGF inhibitor treatment leads to less myopia, but some eyes still develop high myopia.47
Monogenic Forms of Myopia
Monogenic forms of myopia can be broadly categorized into four groups: (1) ametropic retinal dystrophies; (2) connective tissue disorders; (3) monogenic isolated high myopia; and (4) other disorders.
Ametropic Retinal Dystrophies
High myopia, and refractive errors in general, can be a first or accompanying feature of an inherited retinal disease.23,48 An analysis of a large group of genetically characterized patients with inherited retinal disease has demonstrated that four genetic subtypes, blue cone monochromacy, Bornholm eye disease (both associated with OPN1LW and OPN1MW or their regulatory regions), and retinal dystrophies caused by RPGR and RPE65 mutations were associated with increased axial length.49 Two genes associated with albinism (such as OCA2 and TYR) also increased the risk of longer axial length. Short axial lengths are also seen in certain gene variants. In terms of refraction, the range of retinal dystrophies that predispose to high myopia include retinitis pigmentosa (RP) and cone-rod dystrophies caused by mutations in the RPGR gene (15% of male patients with non-syndromic RP,50 congenital stationary night blindness (commonly associated with genes NYX and CACNA1F),51 and rare conditions, such as blue cone monochromacy.52
Metabolic disorders can also lead to high myopia. Gyrate atrophy (ornithine amino transferase [OAT] deficiency) leads to chorioretinal atrophy and myopia. Long chain 3-hydroxyacyl-CoA dehydrogenase deficiency (due to mutations in HADHA), is a mitochondrial fatty acid oxidation disorder associated with chorioretinal atrophy, myopia, and staphyloma formation.53 Homocystinuria is caused by mutations in cystathionine beta-synthase (CBS) and can present with myopia, with or without obvious lens dislocation.54 Although rare, the clinical significance of these metabolic disorders lies in the fact that dietary measures may improve outcomes.55,56 Early diagnosis and active management in homocystinuria can greatly improve cognitive outcomes and reduce the risk of the life-threatening thromboembolic complications, most notably in countries without a neonatal screening program.57
High myopia may represent the initial presenting feature of an inherited retinal disease, placing a burden on the first eye care practitioner who sees the child to consider such a diagnosis. In addition to the immediate benefits to vision from identifying and correcting high myopia, there are several benefits of early diagnosis of a myopia-associated retinal dystrophy. The variable effects of this class of conditions on visual acuity, color vision, contrast sensitivity, visual fields, and night vision have huge implications for a child's education and their safe navigation. In addition, an accurate genetic diagnosis provides valuable prognostic information, and there is now an approved therapy for at least one of the retinal dystrophy genes associated with increased axial length (RPE65).58
Myopia-Associated Connective Tissue Disorders
High myopia is a characteristic feature in connective tissue disorders. Stickler syndrome (incidence 1:7500–1:9000) and Marfan syndrome (incidence 1:5000–1:10000) are two relatively common heritable conditions associated with high myopia in children that can have a range of severe ocular and extra-ocular manifestations. Stickler syndrome can include ocular findings, such as cataract and retinal detachment, and extraocular features, such as hearing loss (both conductive and sensorineural), midfacial underdevelopment and cleft palate (either alone or as part of the Robin sequence), and mild spondyloepiphyseal dysplasia and/or precocious arthritis.59
Marfan syndrome is characterized by its cardinal features involving the ocular (ectopia lentis, retinal detachment, glaucoma, and cataracts), skeletal (bone overgrowth and joint laxity, disproportionately long extremities, pectus abnormalities, and scoliosis), and cardiovascular system (aortic dilatation predisposing to aortic tear and rupture, and mitral and/or tricuspid valve prolapse).60 Marfan syndrome is a dominantly inherited abnormality of fibrillin-1 (FBN1) with high penetrance where intrafamilial phenotypic variation is very typical. Family members without myopia may carry the defective gene and be at risk of life-threatening but treatable cardiac complications. Diagnosis of Marfan syndrome in a patient without a family history therefore merits onward referral of the entire family to a clinical genetics service.
Ehlers-Danlos is another connective tissue disorder that is characterized by joint hypermobility, skin hyperextensibility, and tissue fragility, with associated ocular disorders that include high myopia, lens opacifications, and convergence insufficiency.61 Knobloch syndrome is also worth noting as a rare but commonly missed diagnosis associated with very high levels of myopia.62 Features of all these syndromes are highly variable, even within the same family, and can also be absent or subtle. Some phenotypes have primarily ocular signs, thus placing the onus on eye care practitioners who may be the first health professionals in a position to make a diagnosis, which can have serious implications for a patient's general health, risk for offspring, reproductive options, and potentially for undiagnosed family members.
Stickler Syndrome and Allied Collagen Vitreoretinopathies
The Stickler syndromes are an important group of connective tissue disorders within syndromic myopia because they are relatively common and have sight-threatening complications from retinal detachment for which prophylactic treatments are available.63 They form part of the spectrum of inherited vitreoretinopathies usually resulting from congenital disorders of type II, IX, and XI collagen which are major structural components of the extracellular matrix in vitreous and cartilage.64 The various subtypes of Stickler syndrome are listed in Table 1.
Table 1.
Known and Suspected Genetic Causes of Stickler Syndrome
| Inheritance | Gene | Clinical Features | MIM No.* | |
|---|---|---|---|---|
| 1 | AD | COL2A1 | Membranous congenital vitreous anomaly, congenital megalophthalmos, deafness, arthropathy, cleft palate | 108300 |
| 2 | AD | COL2A1 | Ocular only. Membranous congenital vitreous anomaly (usually), congenital megalophthalmos. No systemic features. | 609508 |
| 3 | AD | COL11A1 | Ocular only. Membranous congenital vitreous anomaly (usually), congenital megalophthalmos. No systemic features. | 604841 |
| 4 | AR | COL11A1 | Beaded congenital vitreous anomaly, congenital megalophthalmos, arthropathy, cleft palate, profound severe congenital deafness | MIM not yet assigned128 |
| 5 | AD | COL11A2 | Normal vitreous and ocular phenotype, deafness, arthropathy, cleft palate | 184840 |
| 6 | AR | COL9A1 | Sensorineural deafness, myopia, vitreoretinopathy, epiphyseal dysplasia | 614134 |
| 7 | AR | COL9A2 | Sensorineural deafness, myopia, vitreoretinopathy, epiphyseal dysplasia | 614284 |
| 8 | AR | COL9A3 | Sensorineural deafness, myopia, vitreoretinopathy, epiphyseal dysplasia | 620022 |
| 9 | AD | BMP4 | Hypoplastic vitreous, deafness, arthropathy, cleft palate | MIM not yet assigned129 |
| 10 | AR | LOXL3 | sensorineural deafness, myopia, vitreoretinopathy, epiphyseal dysplasia | MIM not yet assigned130 |
| 11 | AR | LRP2 | sensorineural deafness, myopia, vitreoretinopathy, epiphyseal dysplasia | MIM not yet assigned131 |
Online Mendelian Inheritance in Man, OMIM. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD), {date}. World Wide Web URL: https://omim.org/.
The clinical features may be considered under four categories:
1. Ophthalmic
The classic and pathognomonic ophthalmic feature of Stickler syndromes is embryological arrest or disruption of vitreous embryogenesis. This is usually in association with congenital myopia. In contrast to normal developmental myopia, the refractive error may be high, but it is usually nonprogressive.65
Other recognized ophthalmic features are congenital lamellar cataract, and a high risk of retinal detachment and giant retinal tear. Although the congenital (refractive) myopia is common, it is important to recognize that approximately 15% of patients exhibit no significant refractive error, although many of these may still have congenital megalophthalmos but with associated cornea plana rendering them refractively emmetropic – “crypto myopia.” Axial length measurement is therefore particularly important. Recent research has identified a variety of genetic Stickler syndrome mutations that may be predominantly ocular or only affect the eye, with minimal or no systemic involvement. In such cases, identification of the typical membranous (COL2A1 mutations) or beaded (COL11A1 mutations) vitreous syneresis is key to making the clinical diagnosis.66
2. Auditory
Conductive and sensorineural hearing loss (often combined) may be congenital and subclinical. If present, the sensorineural hearing loss tends to be nonprogressive and the conductive hearing loss which is common in childhood becomes less prevalent with age.
3. Oro-facial
All subgroups of Stickler syndrome may be associated with cleft or high arch palate and/or Pierre-Robin sequence. Other features include micrognathia, mid-facial hypoplasia, nasal hypoplasia, anteverted nares, and long philtrum. As with other features, the variability of the phenotype means that the lack of these features does not rule out Stickler syndrome.
4. Musculo-skeletal
Patients with Stickler syndrome can suffer from joint hypermobility leading to secondary premature arthropathy (typically but not exclusively affecting hips, knees, and lumbar spine in young adulthood). The musculo-skeletal features of the allelic disorders Kniest dysplasia and spondyloepiphyseal dysplasia congenita (SEDC) are more severe and associated with rhizomelic (proximal) limb shortening, kyphosis, and scoliosis, but similarly associated with congenital myopia and megalophthalmos. An association between Stickler syndrome and mitral valve prolapse has been reported, but this was on a cohort with a clinical not genetic diagnosis.67
A cohort of 78 genetically confirmed cases of Stickler syndrome found no clinical or echocardiographic evidence of mitral valve prolapse, suggesting that earlier studies may have included other phenotypically similar connective tissue disorders.68
Monogenic Forms of Myopia
Over the past few years, many genes have been identified as causing isolated monogenic high myopia, but greater progress has been made in identifying monogenic syndromic forms of myopia.69–71 Whole exome sequencing of a cohort of patients with high myopia has demonstrated that 20% of such cases had a causal pathogenic mutation in a known myopia-associated gene. A genetic diagnosis was more likely in cases with ocular or systemic syndromic features, being found in 35% of such cases as compared with only 14% of cases of isolated myopia. This shows the diagnostic value of identifying associated ocular and systemic features in early-onset high myopia. Although most of these disorders display conventional inheritance patterns, such as autosomal dominant (30%), autosomal recessive (30%), or X-linked (33%), more unusual inheritance patterns have been observed. The X-linked female limited inheritance pattern seen in ARR3 mutations, with affected female carriers and unaffected male carriers, can make diagnosis challenging.72,73
Other Disorders Associated With Myopia
Other forms of syndromic myopia can be related to corneal or lens malformations. Keratoconus, a cause of predominantly non-axial myopia, is usually isolated but linked with over 20 genes and 49 syndromes, including Down Syndrome. Microspherophakia, characterized by a lens with reduced equatorial diameter and increased optical power, is associated with Weill-Marchesani syndrome (WMS) and linked to mutations in ADAMTS10 and FBN1 genes, but it may appear in isolation or in association with a range of other syndromic conditions. Another cause of myopia in newborns is congenital glaucoma, which can cause buphthalmos and progressive myopia, and is associated mutations in the CYP1B1, LTBP2, and TEK genes.
Clinical Evaluation of a Highly Myopic Child
There are many aspects to the clinical evaluation of high myopia in children. In addition to the normal considerations of optical correction and managing myopia progression, a primary goal of investigating highly myopic children is to identify syndromic forms of myopia, as discussed above, that may have additional implications for their vision and general health.
Recommended Assessment Procedures
Due to the increased risk of associated ocular and systemic comorbidities, high myopia in children merits a very detailed clinical evaluation. The clinical history needs to be tailored to identify possible monogenic inheritance patterns and the symptoms of the most significant syndromic forms of myopia. In addition to a detailed ocular examination of the cornea, lens, vitreous, and retina, attention must be given to the non-ocular clinical signs of connective disorders. Following cycloplegic refraction, biometric analysis is essential to determine which ocular components are contributing to the child's myopia. This will help to distinguish corneal, lenticular, axial, or mixed pattern forms of myopia. Depending on the history and examination findings, additional investigations are often merited. These may include specialized ocular investigations, such as electrophysiology and retinal imaging. Where a systemic medical condition is diagnosed clinically, or suspected, the involvement of clinical geneticists and pediatricians may be warranted.
History
The clinical history should start with a family history, inquiring about refractive status and potentially significant ocular and non-ocular medical conditions. Ocular factors that may suggest genetic retinal disease include family members with poor vision despite optical correction, color vision deficits that include tritanopia, and poor night vision. Previous retinal detachment surgery (especially giant retinal tears or bilateral detachments) in any family member, familial deafness or hearing loss, and cleft palate are useful markers for Stickler syndrome. A history of heart valve surgery within the extended family can point to connective tissues disorders, such as Marfan syndrome. Birth history is important in relation to the possible contribution of prematurity, and assessment of milestones provides a simple test for developmental delay which is a common finding in childhood high myopia.20
General Clinical Examination
Prior to the ocular examination, a general examination of a child by the eye care practitioner can reveal useful information. Assessment of their general psychomotor development, facial morphology, and limbs can indicate the need for review by a pediatrician or clinical geneticist. Facial features suggestive of connective disorders, such as Stickler syndrome, include micrognathia, mid-facial hypoplasia, long philtrum, and a flattened nasal contour. This is often best appreciated from a lateral viewpoint.
Screening for joint hypermobility and skin elasticity is useful for identifying potential cases of Ehlers–Danlos syndrome, Marfan syndrome, and Stickler syndrome. The standardized method for assessing joint hypermobility is the Beighton score. A quick clinical screening can be done in a minute or less by looking for little finger/pinkie hyperextension, testing if the thumb can touch the forearm, elbow/knee hyperextension, and checking for elbow skin elasticity. Examination of the palate for midline clefting, previous cleft palate surgery, bifid uvula, or a high arched palate is easily done with light from a direct or indirect ophthalmoscope.
Ocular Examination
Age-specific visual acuity testing and cycloplegic refraction are essential starting points of the clinical examination. Reduced best-corrected visual acuity is rare in uncomplicated myopia but an important marker of retinal disease in children with high myopia.23 Color vision testing is a useful screening test for cone dystrophies provided it tests for tritan (blue-yellow) discrimination (for example, Hardy-Rand-Ritter [HRR] plates or the 100-hue test). Paradoxical pupillary reaction is a screening test for retinal diseases, such as congenital stationary night blindness and achromatopsia, and is characterized by initial pupillary constriction to darkness. In older children, automated perimetry can be useful to detect peripheral field loss from inherited retinal disease.
The presence of nystagmus in combination with high myopia strongly suggests the possibility of retinal dystrophy. Clinical evaluation of the cornea, iris (for transillumination as a pointer to albinism), anterior chamber depth, lens (curvature, size and dislocation), vitreous, and retina can be diagnostic in certain cases, allowing identification of keratoconus, anterior lens dislocation, microspherophakia, lenticonus, or the classical membranous or beaded features of the vitreous in Stickler syndrome. Retinal examination can reveal features of inherited retinal disease or neonatal treatments for ROP that may not have been revealed during history taking. Older highly myopic children may demonstrate a range of structural changes, including optic nerve changes, myopic maculopathy, and posterior staphyloma, which carry implications for future visual impairment.
Even though intraocular pressure can be difficult to measure in infants, this is an essential step in any myopic infant to exclude the possibility of congenital or early-onset glaucoma. Non-contact tonometry techniques can be performed in the clinic without the need for sedation or anesthesia.
Ocular Biometry
Ocular biometry is essential in the evaluation of high myopia in children because there are many conditions, such as keratoconus and ROP, where the associated myopia is not primarily axial. In addition to assessment of axial length, keratometry, corneal topography, corneal thickness, anterior chamber depth, and lens thickness are valuable measurements for assessing and monitoring corneal disorders (for example, keratoconus) and conditions affecting the lens shape and location, such as microspherophakia and early lens dislocation in Marfan syndrome.
Ocular Imaging
Ocular imaging using techniques, such as wide-angle fundus photography, fundus autofluorescence imaging, optical coherence tomography (OCT), and optical coherence tomography angiography,74 can all provide critical diagnostic information regarding possible inherited retinal diseases. Wide field fundus autofluorescence imaging is particularly useful in children because the characteristic retinal pigmentary changes seen in adults with inherited retinal disease are often absent in children, but changes in fundus autofluorescence are present from a young age in such cases.75 In older children, analysis of the ellipsoid zone (specifically photoreceptor outer segment length) with OCT can be an effective method of objectively diagnosing and monitoring retinal dystrophies.
Electrophysiology
Visual electrophysiology tests are used to help diagnose a variety of visual disorders, and are essential for detecting retinal dystrophies.76 Such testing is indicated in highly myopic children where there is a family history of inherited retinal disease, in patients with reduced best-corrected visual acuity with or without nystagmus, color vision deficits, poor night vision, abnormal retinal autofluorescence, or suspicious retinal findings. Full-field electroretinogram (ERG) remains the most useful single test,77 but pattern ERG, multi-focal ERG,78 on-off ERG,79 and S-cone ERG80 are valuable additions when available. The electrooculogram, which assesses retinal pigment epithelial function, is also very helpful in specific disorders, such as Best disease, but this condition is unlikely to present as high myopia.
Role of Clinical Geneticists and Genetic Testing
If the clinical evaluation suggests a monogenic or syndromic form of myopia, the involvement of other medical professionals may be warranted. These may include ophthalmologists who specialize in inherited disease, clinical geneticists, genetic counselors, and/or pediatricians. Ideally, this multidisciplinary evaluation should be done within the context of a well-defined care pathway.81 Traditionally, genetic testing has been based on testing genes known to be involved in the suspected condition. The diagnostic yield is higher in the context of known or suspected ocular or systemic features, but the advent of whole exome sequencing using large panels of ocular disease genes has provided a route to a rapid genetic diagnosis in childhood high myopia. In the future, reduced cost and increased availability of such approaches may simplify the clinical evaluation of early-onset high myopia, with molecular genetic testing replacing other investigations, such as electrophysiology and detailed retinal imaging as the initial approach to diagnosis.
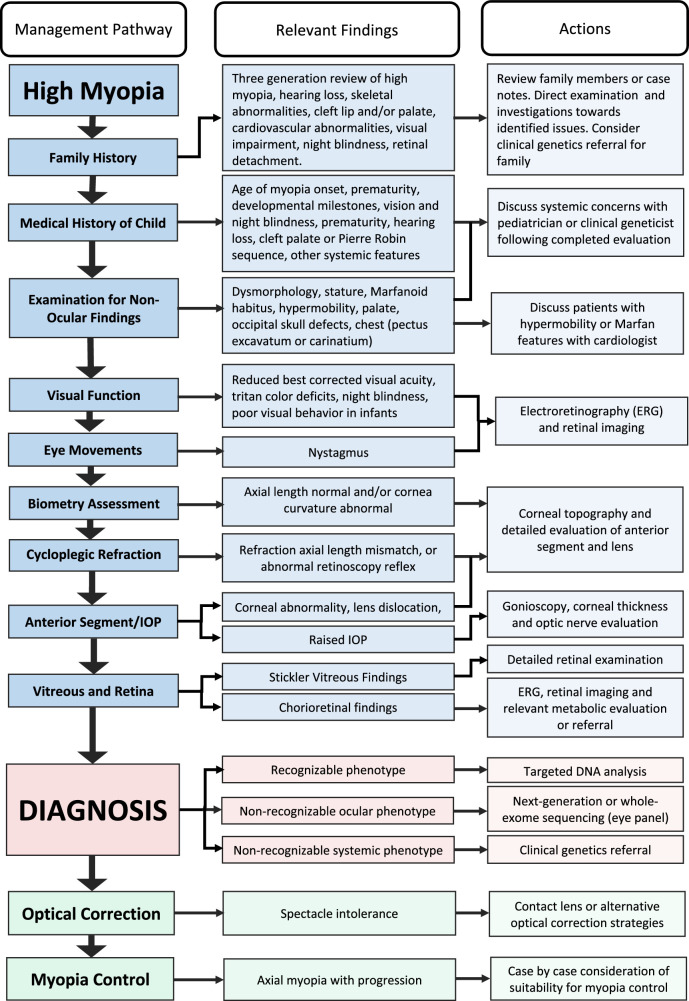
Figure 1 provides a flow chart for the suggested clinical evaluation, diagnosis, and management process and Table 2 provides additional details regarding the various steps. Depending on the clinical setting and age of the patient, this may occur over one or more visits in a single tertiary referral site or be broken down into smaller steps that are performed by multiple health professionals in different sites.
Figure 1.
A comprehensive guide to the assessment, investigation, diagnosis, and management of high myopia in a hospital setting.
Table 2.
Clinical Diagnostic Approach
| Medical and family history |
| - Age of myopia onset |
| - Environmental risk factors: prematurity, outdoor exposure, near work |
| - Vision: night blindness and color blindness |
| - Hearing loss |
| - Cleft lip and/or palate (including Pierre Robin sequence) |
| - Joint hypermobility and skeletal abnormalities |
| - Cardiovascular abnormalities (aortic root dilatation or dissection) |
| - Developmental milestones and cognitive abilities |
| - Three-generation family tree, with specific enquiry about (high) myopia, hearing loss, skeletal abnormalities, cleft lip and/or palate, cardiovascular abnormalities, visual impairment and/or blindness, retinal detachment. |
| Ophthalmological examination |
| - Visual acuity |
| - Cycloplegic refraction |
| - Color vision testing (including Tritan deficits) |
| - Eye movements (for nystagmus) |
| - Biometry including keratometry and axial length measurement at a minimum |
| - Fundoscopy |
| - Wide field fundus photography |
| - Fundal auto-fluorescence (if a retinal dystrophy is suspected clinically) |
| - Electroretinography (if a retinal dystrophy is suspected clinically) |
| Clinical examination for extraocular features |
| - Dysmorphological assessment (e.g. midfacial underdevelopment, Marfanoid habitus) |
| - Stature; arm span to height ratio |
| - Occipital skull defects, cutis aplasia |
| - Wrist sign, thumb sign |
| - Beighton hypermobility score |
| - Skeletal abnormalities (e.g. pectus excavatum or carinatum) |
| Genetic Testing and/or onward referral |
| - In case of a recognizable phenotype: targeted DNA analysis. |
| - In case of a non-recognizable ocular phenotype or broader differential diagnosis, consider next-generation sequencing or whole-exome sequencing using gene panel for vision disorders (where available). |
| - In case of suspected ocular and systemic phenotype, refer to pediatric or clinical genetics services. |
| - Consider referral to a clinical geneticist: in case of extraocular features, when a variant of unknown significance is found, complex pedigrees, segregation analysis. |
| Diagnoses to consider |
| - Common myopia |
| - Connective tissue disorder (e.g. Marfan syndrome, Stickler syndrome, Knobloch syndrome) |
| - Retinal dystrophy (e.g. RPGR/RPE65-related retinitis pigmentosa, congenital stationary night blindness, blue cone monochromacy, Bornholm eye disease) |
| - Monogenic isolated high myopia |
| - Non-axial high myopia (e.g. myopia of prematurity, spherophakia, keratoconus) |
Role of Primary Eye Care Practitioners
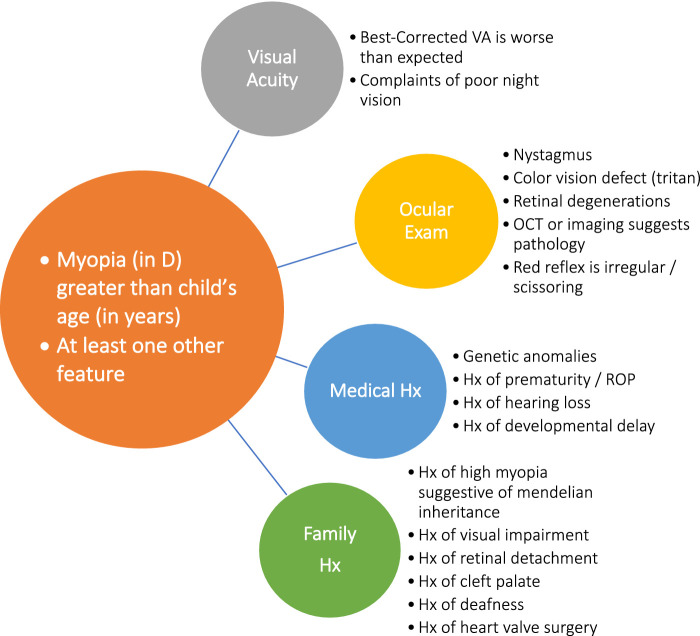
Primary eye care providers are the first line of defense following a failed vision screening by a pediatrician or school program. With the advent of instrument-based vision screenings, these referrals can happen as early as 12 months of age. The American Association of Pediatric Ophthalmology and Strabismus (AAPOS) recommends that infants and toddlers undergoing instrument-based vision screenings be referred if myopia worse than −3.0 D is detected in children younger than 48 months of age or myopia worse than −2.0 D in children 48 months of age or older.82 In addition, the primary eye care community has been at the forefront of introducing myopia control treatments, with many primary eye care practices offering pediatric myopia control services. Therefore, the primary eye care community needs to be on heightened alert in recognizing the risk factors for syndromic forms of myopia in children so that timely and appropriate referrals for further investigation can be made when applicable. Once the initial diagnosis of high myopia is made, the priority is to determine whether there is an associated systemic or ocular disorder (as outlined in Fig. 2). The presence of one or more of the history and clinical features noted in Figure 2, indicates that additional investigation and multidisciplinary clinical evaluation are warranted, which may require referral to a pediatric tertiary eye care center.
Figure 2.
Guide for identification of cases that may represent secondary or syndromic myopia in a primary eye care setting and hence merit referral.
Although a comprehensive evaluation is unlikely to be possible in a primary care setting, a clinical history (as described above) that targets the commonest features of syndromic myopia can be performed in any clinical setting and should be considered mandatory in assessing high myopia in young children. The clinical tests for joint hypermobility in children are easy to learn and apply in any clinical environment (see https://www.ehlers-danlos.com/assessing-joint-hypermobility [accessed January 2023] for a simple guide). Other valuable screening tests, such as looking for reduced best-corrected visual acuity, tritan color vision deficits, or visual field defects in older children capable of doing automated perimetry, can be performed in a primary care setting.
High myopia has been identified as a significant risk factor for having associated ocular and systemic conditions, but the converse is not always true. Many significant ocular conditions typically associated with myopia (for example, Stickler syndrome), show marked variability and some cases may not display high levels of myopia and the absence of myopia cannot be taken as proof that such systemic conditions are not present. Myopia levels may also be lower at the early stages of some systemic disorders.
Challenges of Optical Correction of High Myopia in Children
High myopia in young children creates unique issues relating to optical correction to ensure normal visual development and the avoidance of amblyopia. Although spectacles will be the primary form of optical correction, contact lenses may be more appropriate for children with significant anisometropia (such as in high anisomyopia) or where craniofacial abnormalities make the wearing of conventional spectacles challenging.83 Contact lenses may be useful in improving quality of life and even visual acuity because highly myopic spectacle lenses cause minification of the retinal images.20 Optical correction can be particularly challenging in children with neuro-behavioral abnormalities, as the visual impairment associated with uncorrected myopia can adversely impact both their behavior and development. In such cases, surgical refractive correction may be a viable intervention when performed at centers with extensive pediatric experience, although this remains a controversial topic.84
Conventional Optical Correction of High Myopia in Childhood
Whereas full correction of myopia in school-aged children is appropriate based on the maximizing distance visual acuity and avoiding accelerated myopic progression, in preschool children, it is common practice for lower levels of myopia not to be corrected. The revised American Academy of Ophthalmology Preferred Practice Guidelines published in 2022 (https://www.aao.org/preferred-practice-pattern/pediatric-eye-evaluations-ppp-2022 Accessed January 2023) do not explicitly address the issue of high myopia in young children, but recommend that the following levels of bilateral myopia merit optical correction, noting that smaller amounts of refractive error may also warrant correction depending on the clinical situation.
-
•
refractions < −5.0 D in <1 year of age
-
•
refractions < −4.0 D from 1 year and <2 years
-
•
refractions < −3.0 D from 2 years and <3 years
-
•
refractions < −2.5 D from 3 years and <4 years
Under these guidelines, all high myopes (worse than −6.0 D) and most significantly myopic children under 4 years of age merit correction. It is noted that “these values were generated by consensus and are based solely on professional experience and clinical impressions because there are no scientifically rigorous published data for guidance.” In the absence of such evidence, an analytical approach can be a helpful guide. Leat provided a valuable analysis and a set of questions that can be applied to a specific patient.85
-
1.
Is the refractive error within the normal range for the child's age?
-
2.
Will this child's refractive error emmetropize?
-
3.
Will this level of refractive error disrupt normal visual development or functional vision?
-
4.
Will prescribing spectacles improve visual function or functional vision?
-
5.
Will prescribing spectacles interfere with the normal process of emmetropization?
Considering these questions for highly myopic preschool children points to the need for optical correction.
-
•
Question 1: Based on the prevalence of high myopia in this age group, their refraction is certainly outside the normal range.
-
•
Question 2: Although low myopic errors can emmetropize, there is little evidence regarding high myopia86 and early onset highly myopic eyes have already demonstrated a failure to emmetropize.
-
•
Question 3: Uncorrected, or significantly undercorrected, high myopia could certainly disrupt visual function and normal visual development.
-
•
Question 4: Correcting high myopia will undoubtedly improve visual function.
-
•
Question 5: In lower levels of myopia undercorrection does not slow down progression or promote emmetropization.87 There is no evidence to suggest any benefit for undercorrection in high myopia.
Overall, until better evidence is available, such analysis would appear to favor full correction of high myopia in young children to optimize functional vision and avoid disrupting visual development.
Management of High Anisomyopia
High anisomyopia is a challenging condition that can be associated with significant unilateral visual impairment from amblyopia, particularly when associated with myelinated retinal nerve fibers (Straatsma syndrome).88,89 Optical correction is an essential part of the management of anisomyopia, usually combined with amblyopia treatment. Despite the traditional view that amblyopia in the presence of myelinated nerve fibers and anisomyopia responds poorly to occlusion therapy, some patients respond extremely well, making a therapeutic trial worthwhile.90,91
The intraocular difference in refraction in anisomyopia is often dramatic; a mean value of 9.4 D was reported in one of the larger series,92 leading to aniseikonia with spectacle correction. As a result, contact lens correction of the myopic eye is the preferred option. Corneal rigid lenses do not normally have a significant impact on normal myopia progression but, in high anisomyopia, several case studies suggest a possible beneficial effect on myopic progression in the more myopic eye.93,94 If contact lenses prove impossible, full or partial optical correction should be attempted, especially in young children to minimize amblyopia. Children are often capable of surprisingly large amounts of neuroadaptation to retinal size differences.95
Attempts have been made to directly reduce anisomyopia with myopia control interventions in the myopic eye. A comparison of low-dose atropine and orthokeratology indicated that orthokeratology had a more beneficial effect, but this was a retrospective series, not a controlled trial.96 A meta-analysis of multiple cohort studies came to the same conclusion, but definitive trials are required to clarify whether myopia control interventions can meaningfully alter the natural refractive history of anisomyopia.97
Surgical Correction of High Myopia in Childhood
Most children with high myopia wear their refractive correction because it greatly improves their vision, but compliance with spectacles or contact lenses is not guaranteed in young children. Patience and perseverance by parents will overcome many of the challenges with conventional optical corrections, but there are certain children with high myopia who refuse or are unable to wear a refractive correction and consideration should be given to possible surgical solutions.
Such children will broadly fall into one of three categories:
-
1.
Children with bilateral high ametropia who are noncompliant with or intolerant of optical correction due to neurobehavioral problems or intellectual disability. Such children often display a profound tactile aversion to both spectacles and insertion of contact lenses.
-
2.
Children with severe anisomyopia who are spectacle intolerant due to aniseikonia/binocular vision issues and who are also contact lens intolerant.
-
3.
Children with high ametropia or anisometropia, who have other special circumstances, such as craniofacial anomalies, ear deformities, or neck hypotonia, that creates issues for frame fitting. Contact lens fitting can be challenging in such cases due to shallow orbits, associated lagophthalmos, and corneal exposure.98
In all three groups, the absence of optical correction can have a negative impact on their quality of life and or visual development. Untreated high refractive error in young children can result in severe levels of blur-induced amblyopia akin to that found with a dense congenital cataract or corneal opacity. We should therefore approach the amblyopia of conventionally uncorrectable refractive errors as we would these other treatable causes of form vision deprivation, where surgical interventions are now routinely used. One difference is the higher proportion of associated systemic issues in this group of patients (compared, for example, to congenital cataracts), which requires an individualized risk-benefit assessment with multidisciplinary input from a range of healthcare professionals.
Outcomes of Refractive Surgery in Children
Both corneal and intraocular procedures can correct refractive errors. Excimer laser refractive surgery for high myopia associated with amblyopia has been performed for over 2 decades in children, with good visual acuity and refractive results and low levels of complications (see Tables 3, 4). Currently, both corneal and intraocular refractive procedures are utilized “off-label” in children in most jurisdictions and are not approved by the US Federal Drug Administration (FDA) in the United States.
Table 3.
Refractive Surgery for High Bilateral Myopia
| Author(s) | Year | Procedure | No. of Patients | Age Range (Years) | Preoperative SE (D)* | Postoperative SE (D)* | Mean Preoperative BCVA* | Mean Postoperative UCVA† | Follow-Up (mo) | Behavior | Complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Astle et al.132,† | 2002 | PRK | 10 | 1–6 | −10.7 | −1.4 | 20/70 | NR | 12 | Improved in 2/3 of patients | 40% mild to moderate haze |
| Astle et al.133,† | 2004 | LASEK | 11 | 1–17 | −8 | −1.2 | 20/80 | NR | 12 | Improved in 50% to 83% of patients | 22% mild haze |
| Astle et al.133,† | 2006 | LASEK | 1 | 7 | −7.5 | −3.3 | 3/200 | NR | 12 | Improved | None |
| Tychsen et al.134 | 2006 | RLE/CLE | 13 | 1–18 | −19.1 | 0.8 | 20/76 | 20/38 | 54 | Improved by questionnaire | One dislocated IOL and one posterior capsule opacity |
| Tychsen and Hoekel5 | 2006 | LASEK | 9 | 3–16 | Range: −3.8 to −11.5 | 89% within 1 D of goal | 20/133 | 20/60 | 17 | Improved in 88% of patients | 35% mild haze |
Mean unless range is given.
These studies include patients treated for anisometropia in the same study.
BCVA, best corrected visual acuity; IOL, intraocular lens; LASEK, laser-assisted subepithelial keratectomy; NR, not reported; PRK, photorefractive keratectomy; SE, spherical equivalent; UCVA, uncorrected visual acuity.
Table 4.
Refractive Surgery for High Myopic Anisometropia
| Author(s) | Year | Procedure | Age Range (Years) | No. of Patients | Preoperative SE (D)* | Postoperative SE (D)* | Preoperative BCVA* | Postoperative UCVA† | Mean Follow-Up (mo) | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Singh135 | 1995 | PRK | 10–15 | 6 | −12.1 | −2.9 | 20/82 | 20/44 | 1 | Haze |
| Nano et al.136 | 1997 | PRK | 11–14 | 5 | −8 | −1.6 | 20/400 | 20/72 | 12 | Mild haze |
| Alio et al.137 | 1998 | PRK | 5–7 | 6 | −9.6 | −2 | 20/114 | 20/35 | 24 | Severe haze (1) |
| Rashad138 | 1999 | LASIK | 7–12 | 14 | −7.9 | −0.6 | 20/50 | 20/25 | 12 | None |
| Agarwal et al.139 | 2000 | LASIK | 5–11 | 16 | −14.9 | −1.4 | 20/37 | 20/37 | 12 | Free Flap |
| Nucci and Drack140 | 2001 | PRK/LASIK | 9–14 | 14 | −8 | −0.07 | 20/125 | 20/121 | 20 | None |
| Nassaralla and Nassaralla141 | 2001 | LASIK | 8–15 | 9 | −7.2 | −0.2 | NR | NR | 12 | None |
| Astle et al.132,† | 2002 | PRK | 1–6 | 27 | −10.7 | −1.4 | 20/70 | 20/40 | 12 | Mild haze |
| Autrata and Rehurek142 | 2004 | PRK/LASEK | 4–7 | 27 | −8.3 | −1.6 | 20/95 | 20/26 | 24 | Mild haze (3) |
| Astle et al.143,† | 2004 | LASEK | 1–17 | 13 | −8 | −1.2 | 20/80 | 20/50 | 12 | Minimal haze |
| Phillips et al.144 | 2004 | LASIK | 8–19 | 17 | −9.06 | −3.74 | 20/25 | 20/20 | 18 | None |
| Tychsen et al.145 | 2005 | PRK/LASIK | 4–16 | 35 | −11.5 | −3 | 20/87 | 20/47 | 29 | Minimal haze (3) |
| Paysse et al.146 | 2006 | PRK | 2–11 | 11 | −13.8 | −3.6 | 20/316 | 20/126 | 31 | Minimal haze |
| Yin et al.147 | 2007 | LASIK | 6–14 | 32 | −10.1 | −2.2 | 20/50 | 20/33 | 17 | Minimal haze |
| Ali et al.148 | 2007 | RLE/CLE | 4–20 | 7 | −16.7 | NR | NR (UCVA 20/2550) | NR (UCVA 20/130 | 4 | Posterior capsule fibrosis (1) |
| Pirouzian and Ip149 | 2010 | phIOL | 5–11 | 7 | −14.28 | −1.1 | 20/4000 | NR (BCVA 20/40 | 35 | None |
| Lesueur and Arne150 | 2002 | phIOL | 3–16 | 11 | −12.7 | NR (range −0.75 to +2.00) | NR (range CF to 20/63) | NR (BCVA 20/63) | 21 | None |
Mean unless range is given.
These data include patients treated for bilateral high myopia and high myopia after penetrating keratoplasty in the same study.
BCVA, best corrected visual acuity; UCVA, uncorrected visual acuity; LASEK, laser-assisted subepithelial keratectomy; LASIK, laser in situ keratomileusis; NR, not reported; PRK, photorefractive keratectomy; RLE/CLE, refractive lens exchange/clear lens extraction; phIOL, phakic intraocular lens; SE, spherical equivalent.
Improvements in Visual Function and General Development
Refractive surgery can be effective in children with high refractive errors and amblyopia unresponsive to standard therapy. Substantial gains in visual acuity have been reported with refractive surgery in children, with a mean improvement of visual acuity of 1.6 lines (range = 0 to 7 lines, n = 28) and no loss of best corrected visual acuity in one series.99 A recent study reported that children aged 3 to 7 years with severe anisomyopia treated with patching and excimer laser treatment (n = 27) or phakic IOL (n = 16) achieved significantly better visual acuity than a similar group (n = 37) of compliant children treated with contact lenses and patching.100 Development improvements have also been observed in highly myopic children with intellectual disability unable to use conventional optical corrections.101
Management of Myopia Progression
Management of myopic progression is currently the primary goal of myopia control in most myopic children. For the highly myopic children discussed in this paper, tackling myopic progression is only a small part of their overall management. However, early onset of myopia is associated with increased risk of high myopia in later childhood.102 It is therefore natural that clinicians will want to try and address myopia progression in such cases. A note of caution is, however, required on the basis that many of the forms of myopia described in this paper have been excluded from myopia progression trials, based on the degree of myopia, age, prematurity, or as a syndromic form of myopia.103
The lack of evidence regarding efficacy in these cases makes it difficult to provide evidence-based recommendations. In addition, secondary forms of myopia are inherently a very heterogenous group. Online Mendelian Inheritance in Man (OMIM)104 lists over 400 conditions that include myopia as a clinical feature and the genes that contribute to syndromic myopia are involved in a wide variety of biological processes.19 This heterogeneity makes it very likely that the responses to myopia progression interventions will be highly variable. Clinical trials are unlikely to be feasible for these rare conditions and pooling of outcome data between clinical sites in disease registries may be the best option for developing an evidence-based approach to myopia management in this complex subtype of myopia. Assessing pharmacological myopia treatments, such as atropine, in animal models of human syndromic myopia represents another promising paradigm.105
Myopia Progression Treatments in Myopia of Prematurity
As previously noted, high myopia associated with prematurity and ROP is not primarily axial. In such cases, there is little justification for routine use of currently available optical or pharmacological treatments that have been developed to slow axial growth. Evidence of axial elongation should therefore be regarded as a prerequisite for any myopia control intervention in a child with a history of prematurity. Animal studies involving infant primates have also raised the possibility that topical atropine may lead to arrested development of the anterior segment,106 suggesting caution over the use of high concentrations of atropine for myopia control in the first year or two of life until further clinical data are available. This presents a particular challenge in ROP, because myopic progression appears to be most rapid in the first 3 or 4 years of life in eyes with ROP.36,41 If excessive axial growth is observed in myopia associated with prematurity, active myopia management would be a reasonable approach, but because intervention trials have generally excluded such cases, parents should be informed of the lack of evidence of efficacy in this situation.
Myopia Progression Treatments in Syndromic Myopia
Several forms of syndromic myopia are also primarily lenticular or corneal (for example, Cohen syndrome).107 Biometric analysis of corneal curvature, anterior chamber depth, lens morphology, and axial length are therefore essential in determining whether the observed myopia is refractive, axial, or a combination of both. Where an axial contribution to the myopia is suspected, monitoring of axial length is essential to determine that there is faster than normal axial progression prior to considering treatment.108
The pattern of myopia onset and progression is often very different in syndromic myopia compared to typical myopia. In many forms of syndromic myopia, high levels of myopia are present by the age of 5 years and there is little progression thereafter.65,109 A study of preschool myopic children in China found that those children with a spherical equivalent refraction worse than −6.0 D showed a mean progression of −0.32 D/year, compared with −0.85 D/year for children with lower levels of myopia between −0.5 D and −2.0 D.110 A low rate of progression in highly myopic children has been identified in specific syndromes, such as Stickler syndrome and congenital stationary night blindness, with many cases that show little or no progression while others do progress.65,109 In light of the lack of evidence for effectiveness of treatments and the variability in progression rates in preschool children with high myopia, ensuring that there is myopia progression and axial elongation prior to intervention is warranted.111
An additional complexity is that, in certain syndromic forms of myopia, the associated abnormalities may accentuate the side effects of certain treatments. Photophobia is a common feature of cone dystrophies; hence such patients may be intolerant of even the minimal levels of mydriasis seen with low dose atropine. In conditions with potential cardiac complications, such as Marfan syndrome, reducing cardiac output with beta-blockers is standard practice and any treatment that could increase heart rate (for example higher dose of atropine) should be avoided.
The goals of reducing myopic progression also need to be considered in the overall context of the patient. The primary justification for reducing myopia progression is maintaining good visual acuity into old age by reducing retinal and other ocular complications associated with myopia.112 In some forms of syndromic myopia, the major threat to vision comes from direct retinal involvement of the condition itself, not the myopia, but the two factors may interact. In retinal dystrophies associated with RPGR mutations, high myopia is associated with a faster rate of decline of visual acuity with age.113 Limiting progression of high myopia may also potentially reduce the myopia-related surgical risks of subretinal gene therapy.114 Syndromic myopia can be associated with behavioral abnormalities and looking after such children already places a heavy challenge on parents and carers. This may make eye drops, contact lenses, and even specialized spectacle lenses not feasible. Sadly, some syndromic forms of myopia are also life-shortening and quality of life in the first decades is the priority; hence the burden of additional hospital or clinic visits without a clear long-term benefit needs to be considered.
When considering myopia control interventions in highly myopic children, a case-by-case approach is required. Where myopic progression is observed and the axial length is greater than expected for the age,108 it is reasonable to consider myopia control interventions provided no adverse interactions are anticipated in syndromic cases. Optical interventions are likely to have less potential for such interactions, but their availability may be limited in high refractive errors. The assessment of suitability for myopia control interventions also needs disclosure of the lack of evidence for efficacy in syndromic cases. As a low-risk intervention, advice on increased outdoor activities for all children with myopia or at risk of myopia from an identified syndrome is appropriate.
Complications of High Myopia in Children
It is now well recognized that the structural complications of myopia, as embodied in the term “pathologic myopia”4 increase with age. In 1864, Donders wrote, “it is rare at 60 years of age to find a tolerably useful eye, with myopia of 1:2.5 (−15.75 D in modern units) or even 1:3 (−13.0 D).”115 This adage has been amply confirmed in recent studies. Visual impairment associated with high myopia is rare before 50 years of age and increases rapidly after 60 years of age.112 Ocular complications specifically associated with pathologic myopia, such as myopic maculopathy, posterior staphyloma, and myopic traction maculopathy, show a very similar age profile. This raises two interesting clinical questions. First, are there structural changes present in highly myopic children that could predict visual loss in later years? Second, will children presenting with high myopia not display any structural issues or complications associated with myopia until late adulthood?
A recent retrospective study examined the medical records of children with high myopia who were followed for at least 20 years, looking for evidence of myopic maculopathy.116 Among 35 eyes with signs of myopic maculopathy in adult life, 83% had shown parapapillary diffuse choroidal atrophy confined to the area temporal to the optic nerve head as children. The presence of parapapillary diffuse choroidal atrophy in children with high axial myopia might therefore be a biomarker for pathologic myopia and associated visual impairment in adulthood.
Choroidal Changes in Highly Myopic Children
The choroid, long considered a passive vascular layer, is now thought to play an important role in the regulation of eye growth and refractive error development.117,118 In non-myopic children, the choroid shows a yearly increase in thickness of 12 to 14 µm/year, whereas in myopic children the choroid decreases in thickness with age as the axial length of the eye elongates and myopia progresses.118,119 In highly myopic children, the presence of parapapillary diffuse choroidal atrophy is associated with a marked thinning of the temporal parapapillary choroid and the subfoveal choroid.120
Posterior Staphyloma
Although posterior staphylomas have been generally considered to be features of pathologic myopia that develop in later life, a recent study using wide field OCT in highly myopic children aged 6 to 19 years old, revealed that 12.7% had the initial features of posterior staphyloma.121 Similar to adults, these early-onset staphylomas were characterized by choroidal thinning toward the staphyloma edge with the posterior displacement of the sclera. These results show that posterior staphylomas can be present at a much younger age than generally believed. Statistical comparisons also showed that the eyes with a staphyloma had diffuse chorioretinal atrophy, including parapapillary diffuse choroidal atrophy, significantly more frequently than those without a staphyloma.
Further work is required to establish the causative nature of these associations among choroidal changes, myopic maculopathy, and posterior staphyloma, and to separate early-onset environmental high myopia from high secondary myopia4 from genetic or syndromic causes. However, the findings to date suggest that choroidal thickness is an important initial biomarker for future development of pathologic myopia in highly myopic children. Monitoring choroidal changes in young children with high myopia may therefore be a valuable prognostic tool.
Retinal Detachment Risk in Childhood High Myopia
Retinal detachment is rare in childhood, representing only 2 to 6% of all cases,122 with most cases arising from trauma, ocular syndromes, ROP, and pediatric cataract surgery.122,123 In an Asian pediatric series, myopia worse than −4.0 D was the most frequent risk factor, but a high baseline prevalence of myopia may have influenced this result.124
Several syndromic forms of high myopia carry an increased risk of rhegmatogenous retinal detachment associated with retinal breaks or giant tears. These include Stickler syndrome (type 1 and type 2), Marshall syndrome (allelic with Stickler type 2), Knobloch syndrome, Kniest dysplasia, Donnai-Barrow syndrome, Wagner vitreoretinopathy, spondyloepiphyseal dysplasia congenita, and Ehlers-Danlos syndrome. In many of these syndromes, there are identified structural abnormalities in the vitreous which may represent the primary etiological factor for retinal detachment, as a proportion (10 %) of retinal detachments in Stickler syndrome occur in eyes without myopia.125 This is an important clinical finding, emphasizing that absence of myopia in a child from a family affected by Stickler syndrome does not rule the diagnosis or its sight-threatening complications.
Myopia worse than −10.0 D in children has been found to be associated with reduced success of retinal detachment surgery when compared with surgery for eyes with myopia of −6.0 to −10.0 D.126 Retinal detachment associated with ROP is primarily tractional rather than rhegmatogenous, and although such eyes may be myopic, the myopia is not typically axial in nature. In ROP, such tractional retinal detachments are the consequence of the underlying pathophysiology of the condition rather than a consequence of the degree of myopia.127
Conclusion
The priority following the diagnosis of high myopia in childhood is to determine whether there is an associated medical diagnosis that may be of greater overall importance to the health of the child. A clinical history that targets the common features of syndromic forms of myopia can be performed in any clinical setting and should be considered mandatory in assessing high myopia in young children. Biometric evaluation is important in distinguishing axial myopia from refractive myopia associated with abnormal development of the anterior segment. Where suspicion has been raised during history taking and examination, further specialized investigation and multidisciplinary clinical evaluation are indicated. Where a child has been diagnosed in a primary care setting, this may require referral to a tertiary care facility.
Although it is reasonable to use low risk interventions for reducing myopia progression in childhood myopia with proven axial progression, practitioners should consider the lack of evidence of efficacy for children with high myopia and syndromic forms of myopia. Considering the highly heterogenous nature of high myopia in childhood it is likely that the responses to interventions will be highly variable. When a therapeutic intervention is used, close monitoring of refraction and axial length is recommended. The genetic heterogeneity of syndromic and non-syndromic monogenic myopia makes randomized clinical trials of myopia interventions both unpractical and unlikely. Pooling of outcome data between clinical sites in disease registries may be the best option for developing an evidence-based approach to myopia management in this complex subtype of myopia.
Acknowledgments
Supported by the International Myopia Institute. The publication and dissemination costs of the International Myopia Institute reports were supported by donations from the Brien Holden Vision Institute, Carl Zeiss Vision, CooperVision, EssilorLuxottica, Hoya, Thea, Alcon, and Oculus. A special acknowledgement to Mark Bullimore for harmonization of this white paper.
In preparing this document, the authors received valuable feedback and support from the wide range of scientists and clinicians who have contributed to this IMI initiative and staff of the IMI. Specifically acknowledged Contributors:
Annechien E.H. Haarman1,4, Jan Roelof Polling1,3, J. Willem Tideman1,4, Alberta A.H.J Thiadens1, Anneke J.A. Kievit2, and Tae Igarashi-Yokoi5
1Department of Ophthalmology, Erasmus Medical Center, Rotterdam, The Netherlands
2Department of Clinical Genetics, Erasmus Medical Center, Rotterdam, The Netherlands
3Department of Optometry and Orthoptics, Hogeschool Utrecht, University of Applied Science, Utrecht, The Netherlands
4Department of Epidemiology, Erasmus Medical Center, Rotterdam, The Netherlands
5Tokyo Medical and Dental University, Japan.
Disclosure: I. Flitcroft, CooperVision (C), EssilorLuxottica (C), Johnson & Johnson Vision (C), Vyluma (C), Thea (C), Ocumetra (O), Myopia control monitoring tools and devices (P); J. Ainsworth, None; A. Chia, None; S. Cotter, None; E. Harb, None; Z.-B. Jin, None; C.C.W. Klaver, Bayer (C), Novartis (C), Optos (C), Topcon (F), Thea (C); A.T. Moore, None; K.K. Nischal, Essilor Luxoittica (C), Ocumension (C), Graybug (C), Santen (C); K. Ohno-Matsui, Santen (C), CooperVision (C); E.A. Paysse, None; M.X. Repka, Alcon (C), Luminopia (C); I.Y. Smirnova, None; M. Snead, None; V.J.M. Verhoeven, None; P.K. Verkicharla, Essilor India (C)
References
- 1. Gifford KL, Richdale K, Kang P, et al.. IMI—Clinical management guidelines report. Invest Ophthalmol Vis Sci. 2019; 60(3): M184–M203. [DOI] [PubMed] [Google Scholar]
- 2. Németh J, Tapasztó B, Aclimandos WA, et al.. Update and guidance on management of myopia. European Society of Ophthalmology in cooperation with International Myopia Institute. Eur J Ophthalmol. 2021; 31: 853–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Verkicharla PK, Kammari P, Das AV. Myopia progression varies with age and severity of myopia. PLoS One. 2020; 15: e0241759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flitcroft DI, He M, Jonas JB, et al.. IMI—Defining and classifying myopia: A proposed set of standards for clinical and epidemiologic studies. Invest Ophthalmol Vis Sci. 2019; 60: M20–M30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tychsen L, Hoekel J. Refractive surgery for high bilateral myopia in children with neurobehavioral disorders: 2. Laser-assisted subepithelial keratectomy (LASEK) . J AAPOS. 2006; 10: 364–370. [DOI] [PubMed] [Google Scholar]
- 6. Lan W, Zhao F, Lin L, et al.. Refractive errors in 3-6 year-old Chinese children: A very low prevalence of myopia? PLoS One. 2013; 8: e78003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma Y, Qu X, Zhu X, et al.. Age-specific prevalence of visual impairment and refractive error in children aged 3-10 years in Shanghai, China. Invest Ophthalmol Vis Sci. 2016; 57: 6188–6196. [DOI] [PubMed] [Google Scholar]
- 8. Dirani M, Chan YH, Gazzard G, et al.. Prevalence of refractive error in Singaporean Chinese children: The strabismus, amblyopia, and refractive error in young Singaporean Children (STARS) study. Invest Ophthalmol Vis Sci. 2010; 51: 1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrington SC, Stack J, Saunders K, O'Dwyer V. Refractive error and visual impairment in Ireland schoolchildren. Br J Ophthalmol. 2019; 103: 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Theophanous C, Modjtahedi BS, Batech M, Marlin DS, Luong TQ, Fong DS. Myopia prevalence and risk factors in children. Clin Ophthalmol. 2018; 12: 1581–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Multi-Ethnic Pediatric Eye Disease Study Group. Prevalence of myopia and hyperopia in 6- to 72-month-old African American and hispanic children: The multi-ethnic pediatric eye disease study. Ophthalmology. 2010; 117: 140–147.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Giordano L, Friedman DS, Repka MX, et al.. Prevalence of refractive error among preschool children in an urban population: The Baltimore pediatric eye disease study. Ophthalmology. 2009; 116: 739–746.e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dong L, Kang YK, Li Y, Wei W, Jonas JB. Prevalence and time trends of myopia in children and adolescents in China: A systemic review and meta-analysis. Retina. 2020; 40: 399–411. [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Liu J, Qi P. The increasing prevalence of myopia in junior high school students in the Haidian district of Beijing, China: A 10-year population-based survey. BMC Ophthalmol. 2017; 17: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong K, Dahlmann-Noor A. Myopia and its progression in children in London, UK: A retrospective evaluation. J Optom. 2020; 13: 146–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morgan IG, Wu PC, Ostrin LA, et al.. IMI risk factors for myopia. Invest Ophthalmol Vis Sci. 2021; 62: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghorbani Mojarrad N, Plotnikov D, Williams C, Guggenheim JA. Association between polygenic risk score and risk of myopia. JAMA Ophthalmol. 2020; 138: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lanca C, Kassam I, Patasova K, et al.. New polygenic risk score to predict high myopia in Singapore Chinese children. Transl Vis Sci Technol. 2021; 10: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Flitcroft DI, Loughman J, Wildsoet CF, Williams C, Guggenheim JA. Novel myopia genes and pathways identified from syndromic forms of myopia. Invest Ophthalmol Vis Sci. 2018; 59: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marr JE, Halliwell-Ewen J, Fisher B, Soler L, Ainsworth JR. Associations of high myopia in childhood. Eye. 2001; 15(Pt 1): 70–74. [DOI] [PubMed] [Google Scholar]
- 21. Logan NS, Gilmartin B, Marr JE, Stevenson MR, Ainsworth JR. Community-based study of the association of high myopia in children with ocular and systemic disease. Optom Vis Sci. 2004; 81: 11–13. [DOI] [PubMed] [Google Scholar]
- 22. FitzGerald DE, Chung I, Krumholtz I. An analysis of high myopia in a pediatric population less than 10 years of age. Optometry. 2005; 76: 102–114. [DOI] [PubMed] [Google Scholar]
- 23. Flitcroft DI, Adams GGW, Robson AG, Holder GE. Retinal dysfunction and refractive errors: An electrophysiological study of children. Br J Ophthalmol. 2005; 89: 484–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guo Y, Duan JL, Liu LJ, et al.. High myopia in greater Beijing school children in 2016. PLoS One. 2017; 12: e0187396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nissenkorn I, Yassur Y, Mashkowski D, Sherf I, Ben-Sira I. Myopia in premature babies with and without retinopathy of prematurity. Br J Ophthalmol. 1983; 67: 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quinn GE, Dobson V, Davitt BV, et al.. Progression of myopia and high myopia in the early treatment for retinopathy of prematurity study: Findings at 4 to 6 years of age. J AAPOS. 2013; 17: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tedja MS, Haarman AEG, Meester-Smoor MA, et al.. IMI—Myopia genetics report. Invest Ophthalmol Vis Sci. 2019; 60: M89–M105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laws D, Shaw DE, Robinson J, Jones HS, Ng YK, Fielder AR. Retinopathy of prematurity: A prospective study. Review at six months. Eye (Lond). 1992; 6(Pt 5): 477–483. [DOI] [PubMed] [Google Scholar]
- 29. Repka MX. Refraction and keratometry in premature infants. Br J Ophthalmol. 2004; 88: 853–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cook A, White S, Batterbury M, Clark D. Ocular growth and refractive error development in premature infants with or without retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2008; 49: 5199–5207. [DOI] [PubMed] [Google Scholar]
- 31. Zha Y, Zhu G, Zhuang J, Zheng H, Cai J, Feng W. Axial length and ocular development of premature infants without ROP. J Ophthalmol. 2017; 2017: 6823965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fledelius HC. Ophthalmic changes from age of 10 to 18 years. A longitudinal study of sequels to low birth weight. IV. Ultrasound oculometry of vitreous and axial length. Acta Ophthalmol. 1982; 60: 403–411. [DOI] [PubMed] [Google Scholar]
- 33. Fledelius HC. Pre-term delivery and subsequent ocular development. A 7-10 year follow-up of children screened 1982-84 for ROP. 3) refraction. Myopia of prematurity. Acta Ophthalmol Scand. 1996; 74: 297–300. [DOI] [PubMed] [Google Scholar]
- 34. Ehrlich DL, Atkinson J, Braddick O, Bobier W, Durden K. Reduction of infant myopia: A longitudinal cycloplegic study. Vision Res. 1995; 35: 1313–1324. [DOI] [PubMed] [Google Scholar]
- 35. Saunders KJ, Mcculloch DL, Shepherd AJ, Wilkinson AG. Emmetropisation following preterm birth. British Journal of Ophthalmology. 2002; 86: 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Quinn GE, Dobson V, Kivlin J, et al.. Prevalence of myopia between 3 months and 5 1/2 years in preterm infants with and without retinopathy of prematurity. Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology. 1998; 105: 1292–1300. [DOI] [PubMed] [Google Scholar]
- 37. Garcia-Valenzuela E, Kaufman LM. High myopia associated with retinopathy of prematurity is primarily lenticular. J AAPOS. 2005; 9: 121–128. [DOI] [PubMed] [Google Scholar]
- 38. Gordon RA, Donzis PB. Myopia associated with retinopathy of prematurity. Ophthalmology. 1986; 93: 1593–1598. [DOI] [PubMed] [Google Scholar]
- 39. Quinn GE, Dobson V, Repka MX, et al.. Development of myopia in infants with birth weights less than 1251 grams. The cryotherapy for retinopathy of prematurity cooperative group. Ophthalmology. 1992; 99: 329–340. [DOI] [PubMed] [Google Scholar]
- 40. Ruan L, Shan H-D, Liu X-Z, Huang X. Refractive status of Chinese with laser-treated retinopathy of prematurity. Optom Vis Sci. 2015; 92: S3–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kaur S, Sukhija J, Katoch D, Sharma M, Samanta R, Dogra MR. Refractive and ocular biometric profile of children with a history of laser treatment for retinopathy of prematurity. Indian J Ophthalmol. 2017; 65: 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005; 123: 991–999. [DOI] [PubMed] [Google Scholar]
- 43. Algawi K, Goggin M, O'Keefe M. Refractive outcome following diode laser versus cryotherapy for eyes with retinopathy of prematurity. Br J Ophthalmol. 1994; 78: 612–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ling CS, Fleck BW, Wright E, Anderson C, Laing I. Diode laser treatment for retinopathy of prematurity: Structural and functional outcome. Br J Ophthalmol. 1995; 79(7): 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Geloneck MM, Chuang AZ, Clark WL, et al.. Refractive outcomes following bevacizumab monotherapy compared with conventional laser treatment: A randomized clinical trial. JAMA Ophthalmol. 2014; 132: 1327–1333. [DOI] [PubMed] [Google Scholar]
- 46. Chen YH, Chen SN, Lien RI, et al.. Refractive errors after the use of bevacizumab for the treatment of retinopathy of prematurity: 2-year outcomes. Eye (Long). 2014; 28: 1080–1086; quiz 1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Crouch ER, Kraker RT, Wallace DK, et al.. Secondary 12-month ocular outcomes of a phase 1 dosing study of bevacizumab for retinopathy of prematurity. JAMA Ophthalmol. 2020; 138: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hendriks M, Verhoeven VJM, Buitendijk GHS, et al.. Development of refractive errors-What can we learn from inherited retinal dystrophies? Am J Ophthalmol. 2017; 182: 81–89. [DOI] [PubMed] [Google Scholar]
- 49. Williams KM, Georgiou M, Kalitzeos A, et al.. Axial length distributions in patients with genetically confirmed inherited retinal diseases. Invest Ophthalmol Vis Sci. 2022; 63: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang J, Zhou L, Ouyang J, et al.. Genotype-phenotype analysis of RPGR variations: Reporting of 62 Chinese families and a literature review. Front Genet. 2021; 12: 600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zeitz C, Robson AG, Audo I. Congenital stationary night blindness: An analysis and update of genotype-phenotype correlations and pathogenic mechanisms. Prog Retin Eye Res. 2015; 45: 58–110. [DOI] [PubMed] [Google Scholar]
- 52. Tsang SH, Sharma T. Blue cone monochromatism. Adv Exp Med Biol. 2018; 1085: 65–66. [DOI] [PubMed] [Google Scholar]
- 53. Tyni T, Kivelä T, Lappi M, Summanen P, Nikoskelainen E, Pihko H. Ophthalmologic findings in long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency caused by the G1528C mutation: A new type of hereditary metabolic chorioretinopathy. Ophthalmology. 1998; 105: 810–824. [DOI] [PubMed] [Google Scholar]
- 54. Gus PI, Donis KC, Marinho D, et al.. Ocular manifestations in classic homocystinuria. Ophthalmic Genet. 2021; 42: 71–74. [DOI] [PubMed] [Google Scholar]
- 55. Elnahry AG, Elnahry GA. Gyrate atrophy of the choroid and retina: A review. Eur J Ophthalmol. 2022; 32: 1314–1323. [DOI] [PubMed] [Google Scholar]
- 56. Karall D, Brunner-Krainz M, Kogelnig K, et al.. Clinical outcome, biochemical and therapeutic follow-up in 14 Austrian patients with long-chain 3-hydroxy acyl CoA dehydrogenase deficiency (LCHADD). Orphanet J Rare Dis. 2015; 10: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yamada K, Yokoyama K, Aoki K, Taketani T, Yamaguchi S. Long-term outcomes of adult patients with homocystinuria before and after newborn screening. Int J Neonatal Screen. 2020; 6: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Russell S, Bennett J, Wellman JA, et al.. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet. 2017; 390: 849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Robin NH, Moran RT, Ala-Kokko L. Stickler Syndrome. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews [Internet] - NCBI Bookshelf. Seattle, WA: University of Washington, Seattle; 2021; 1993-2023. Available at: https://www.ncbi.nlm.nih.gov/books/NBK1302/. [Google Scholar]
- 60. Sakai LY, Keene DR, Renard M, de Backer J. FBN1: The disease-causing gene for Marfan syndrome and other genetic disorders. Gene. 2016; 591: 279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Malfait F, Francomano C, Byers P, et al.. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017; 175: 8–26. [DOI] [PubMed] [Google Scholar]
- 62. Hull S, Arno G, Ku CA, et al.. Molecular and clinical findings in patients with knobloch syndrome. JAMA Ophthalmol. 2016; 134: 753–762. [DOI] [PubMed] [Google Scholar]
- 63. Fincham GS, Pasea L, Carroll C, et al.. Prevention of retinal detachment in stickler syndrome: The Cambridge prophylactic cryotherapy protocol. Ophthalmology. 2014; 121: 1588–1597. [DOI] [PubMed] [Google Scholar]
- 64. Snead M. Retinal detachment in childhood. In Paediatric Ophthalmology and Strabismus (eds. Lyons C., Hoyt C.) New York, NY: Elsevier Saunders; 2016. [Google Scholar]
- 65. Wilson MC, McDonald-McGinn DM, Quinn GE, et al.. Long-term follow-up of ocular findings in children with Stickler's syndrome. Am J Ophthalmol. 1996; 122: 727–728. [DOI] [PubMed] [Google Scholar]
- 66. Snead MP, McNinch AM, Poulson AV, et al.. Stickler syndrome, ocular-only variants and a key diagnostic role for the ophthalmologist. Eye. 2011; 25: 1389–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Liberfarb RM, Goldblatt A, Opitz JM, Reynolds JF. Prevalence of mitral-valve prolapse in the Stickler syndrome. Am J Med Genet. 1986; 24: 387–392. [DOI] [PubMed] [Google Scholar]
- 68. Ahmad N, Richards AJ, Murfett HC, et al.. Prevalence of mitral valve prolapse in Stickler syndrome. Am J Med Genet A. 2003; 116A: 234–237. [DOI] [PubMed] [Google Scholar]
- 69. Tedja MS, Haarman AEG, Meester-Smoor MA, et al.. IMI—Myopia genetics report. Invest Ophthalmol Vis Sci. 2019; 60: M89–M105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haarman AEG, Thiadens AAHJ, van Tienhoven M, et al.. Whole exome sequencing of known eye genes reveals genetic causes for high myopia. Hum Mol Genet. 2022; 31: 3290–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Flitcroft DI, Loughman J, Wildsoet CF, Williams C, Guggenheim JA. Novel myopia genes and pathways identified from syndromic forms of myopia. Invest Ophthalmol Vis Sci. 2018; 59: 338–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xiao X, Li S, Jia X, Guo X, Zhang Q. X-linked heterozygous mutations in ARR3 cause female-limited early onset high myopia. Mol Vis. 2016; 22: 1257. [PMC free article] [PubMed] [Google Scholar]
- 73. van Mazijk R, Haarman AEG, Hoefsloot LH, et al.. Early onset X-linked female limited high myopia in three multigenerational families caused by novel mutations in the ARR3 gene. Hum Mutat. 2022; 43: 380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ong SS, Patel TP, Singh MS. Optical coherence tomography angiography imaging in inherited retinal diseases. J Clin Med. 2019; 8: 2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Butt DK, Gurbaxani A, Kozak I. Ultra-wide-field fundus autofluorescence for the detection of inherited retinal disease in difficult-to-examine children. J Pediatr Ophthalmol Strabismus. 2019; 56: 383–387. [DOI] [PubMed] [Google Scholar]
- 76. Robson AG, Nilsson J, Li S, et al.. ISCEV guide to visual electrodiagnostic procedures. Doc Ophthalmol. 2018; 136: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Robson AG, Frishman LJ, Grigg J, et al.. ISCEV standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol. 2022; 144: 165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hoffmann MB, Bach M, Kondo M, et al.. ISCEV standard for clinical multifocal electroretinography (mfERG) (2021 update). Doc Ophthalmol. 2021; 142: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sustar M, Holder GE, Kremers J, et al.. ISCEV extended protocol for the photopic on-off ERG. Doc Ophthalmol. 2018; 136: 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Perlman I, Kondo M, Chelva E, Robson AG, Holder GE. ISCEV extended protocol for the S-cone ERG. Doc Ophthalmol .2020; 140: 95–101. [DOI] [PubMed] [Google Scholar]
- 81. Zhu J, Stephenson KAJ, Dockery A, et al.. Electrophysiology-guided genetic characterisation maximises molecular diagnosis in an irish paediatric inherited retinal degeneration population. Genes (Basel). 2022; 13: 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Donahue SP, Arthur B, Neely DE, et al.. Guidelines for automated preschool vision screening: A 10-year, evidence-based update. J AAPOS. 2013; 17: 4–8. [DOI] [PubMed] [Google Scholar]
- 83. O'Brien DA, Phillips AJ. Stickler syndrome. Clin Exp Optom. 2000; 83: 330–332. [DOI] [PubMed] [Google Scholar]
- 84. Daoud YJ, Hutchinson A, Wallace DK, Song J, Kim T. Refractive surgery in children: Treatment options, outcomes, and controversies. Am J Ophthalmol. 2009; 147: 573–582.e2. [DOI] [PubMed] [Google Scholar]
- 85. Leat SJ. To prescribe or not to prescribe? Guidelines for spectacle prescribing in infants and children. Clin Exp Optom. 2011; 94: 514–527. [DOI] [PubMed] [Google Scholar]
- 86. Ehrlich DL, Atkinson J, Braddick O, Bobier W, Durden K. Reduction of infant myopia: A longitudinal cycloplegic study. Vis Res. 1995; 35: 1313–24. [DOI] [PubMed] [Google Scholar]
- 87. Logan NS, Wolffsohn JS. Role of un-correction, under-correction and over-correction of myopia as a strategy for slowing myopic progression. Clin Exp Optom. 2020; 103: 133–137. [DOI] [PubMed] [Google Scholar]
- 88. Tarabishy AB, Alexandrou TJ, Traboulsi EI. Syndrome of myelinated retinal nerve fibers, myopia, and amblyopia: A review. Surv Ophthalmol. 2007; 52: 588–596. [DOI] [PubMed] [Google Scholar]
- 89. Straatsma BR, Heckenlively JR, Foos RY, Shahinian JK. Myelinated retinal nerve fibers associated with ipsilateral myopia, amblyopia, and strabismus. Am J Ophthalmol. 1979; 88: 506–510. [DOI] [PubMed] [Google Scholar]
- 90. Kee C, Hwang JM. Visual prognosis of amblyopia associated with myelinated retinal nerve fibers. Am J Ophthalmol. 2005; 139: 259–265. [DOI] [PubMed] [Google Scholar]
- 91. Summers CG, Romig L, Lavoie JD. Unexpected good results after therapy for anisometropic amblyopia associated with unilateral peripapillary myelinated nerve fibers. J Pediatr Ophthalmol Strabismus. 1991; 28: 134–136. [DOI] [PubMed] [Google Scholar]
- 92. Weiss AH. Unilateral high myopia: Optical components, associated factors, and visual outcomes. Br J Ophthalmol. 2003; 87: 1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li Z, Sun L, Song H, Guo Y. Clinical effect of rigid gas permeable (RGP) contact lens in improving vision and controlling myopia progression of unilateral high myopic children. Int Ophthalmol. 2022; 42: 3511–3520. [DOI] [PubMed] [Google Scholar]
- 94. Wang B, Naidu RK, Qu X. The use of rigid gas permeable contact lenses in children with myopic amblyopia: A case series. Cont Lens Anterior Eye. 2018; 41: 224–228. [DOI] [PubMed] [Google Scholar]
- 95. Heczko J, Sierpina D. Rapid neuroadaptation to surgically-induced aniseikonia in a 17-year-old patient with high preoperative anisometropia: A case report. Am J Ophthalmol Case Rep. 2018; 9: 75–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tsai WS, Wang JH, Chiu CJ. A comparative study of orthokeratology and low-dose atropine for the treatment of anisomyopia in children. Sci Rep. 2020; 10: 14176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tsai HR, Wang JH, Chiu CJ. Effect of orthokeratology on anisometropia control: A meta-analysis. J Formos Med Assoc. 2021; 120: 2120–2127. [DOI] [PubMed] [Google Scholar]
- 98. Nischal KK. Ocular aspects of craniofacial disorders. Am Orthopt J. 2002; 52: 58–68. [DOI] [PubMed] [Google Scholar]
- 99. Astle WF, Fawcett SL, Huang PT, Alewenah O, Ingram A. Long-term outcomes of photorefractive keratectomy and laser-assisted subepithelial keratectomy in children. J Cataract Refract Surg. 2008; 34: 411–416. [DOI] [PubMed] [Google Scholar]
- 100. Autrata R, Krejčiřová I, Griščiková L, Doležel Z. [Refractive surgery in children with myopic anisometropia and amblyopia in comparison with conventional treatment by contact lenses]. [Article in Czech]. Cesk Slov Oftalmol. 2016; 72: 12–19. [PubMed] [Google Scholar]
- 101. Paysse EA, Kong L, Achim C, et al.. Developmental improvement in children with intellectual disability after photorefractive keratectomy for severe isoametropia. Am J Ophthalmol. 2022; 235: 15–23. [DOI] [PubMed] [Google Scholar]
- 102. Chua SYL, Sabanayagam C, Cheumg Y-B, et al.. Age of onset of myopia predicts risk of high myopia in later childhood in myopic Singapore children. Ophthalmic Physiol Opt. 2016; 36: 388–394. [DOI] [PubMed] [Google Scholar]
- 103. Walline JJ, Lindsley KB, Vedula SS, et al.. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2020; 1(1): CD004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Online Mendelian Inheritance in Man, OMIM. McKusick-Nathans Institute of Genetic Medicine. Baltimore, MD: Johns Hopkins University; 2022. Available at: https://omim.org/. [Google Scholar]
- 105. van der Sande E, Polling JR, Tideman JWL, et al. Myopia control in mendelian forms of myopia. Ophthalmic Physiol Opt. 2023; 43: 494–504. [DOI] [PubMed] [Google Scholar]
- 106. Whatham AR, Lunn D, Judge SJ. Effects of monocular atropinization on refractive error and eye growth in infant new world monkeys. Invest Opthalmol Vis Sci. 2019; 60: 2623. [DOI] [PubMed] [Google Scholar]
- 107. Summanen P, Kivitie-Kallio S, Norio R, Raitta C, Kivelä T. Mechanisms of myopia in Cohen syndrome mapped to chromosome 8q22. Invest Ophthalmol Vis Sci. 2002; 43: 1686–1693. [PubMed] [Google Scholar]
- 108. Tideman JWL, Polling JR, Vingerling JR, et al.. Axial length growth and the risk of developing myopia in European children. Acta Ophthalmol. 2018; 96: 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bijveld MM, van Genderen MM, Riemslag FC, et al.. Genotype and phenotype of patients with congenital stationary night blindness (CSNB). ARVO Annual Meeting Abstract April 2011. Invest Ophthalmol Vis Sci. 2011; 52: 2377. [Google Scholar]
- 110. Hu Y, Ding X, Long W, He M, Yang X. Longitudinal changes in spherical equivalent refractive error among children with preschool myopia. Invest Ophthalmol Vis Sci. 2019; 60: 154–160. [DOI] [PubMed] [Google Scholar]
- 111. Klaver C, Polling JR. Myopia management in the Netherlands. Ophthalmic Physiol Opt. 2020; 40: 230–240. [DOI] [PubMed] [Google Scholar]
- 112. Tideman JWL, Snabel MCC, Tedja MS, et al.. Association of axial length with risk of uncorrectable visual impairment for Europeans with myopia. JAMA Ophthalmol. 2016; 134: 1355–1363. [DOI] [PubMed] [Google Scholar]
- 113. Talib M, van Schooneveld MJ, Thiadens AA, et al.. Clinical and genetic characteristics of male patients with RPGR-associated retinal dystrophies: A long-term follow-up study. Retina. 2019; 39: 1186–1199. [DOI] [PubMed] [Google Scholar]
- 114. Nuzbrokh Y, Kassotis AS, Ragi SD, Jauregui R, Tsang SH. Treatment-emergent adverse events in gene therapy trials for inherited retinal diseases: A narrative review. Ophthalmol Ther. 2020; 9: 709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Donders FC On the Anomalies of Accommodation and Refraction of the Eye With a Preliminary Essay on Physiological Dioptrics. Sydenham, London: The New Sydenham Society; 1864. [Google Scholar]
- 116. Yokoi T, Zhu D, Bi HS, et al.. Parapapillary diffuse choroidal atrophy in children is associated with extreme thinning of parapapillary choroid. Invest Opthalmol Vis Sci. 2017; 58: 901–906. [DOI] [PubMed] [Google Scholar]
- 117. Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010; 29: 144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Read SA, Fuss JA, Vincent SJ, Collins MJ, Alonso-Caneiro D. Choroidal changes in human myopia: Insights from optical coherence tomography imaging. Clin Exp Optom. 2019; 102: 270–285. [DOI] [PubMed] [Google Scholar]
- 119. Hoseini-Yazdi H, Vincent SJ, Collins MJ, Read SA, Alonso-Caneiro D. Wide-field choroidal thickness in myopes and emmetropes. Sci Rep. 2019; 9: 3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yokoi T, Jonas JB, Shimada N, et al.. Peripapillary diffuse chorioretinal atrophy in children as a sign of eventual pathologic myopia in adults. Ophthalmology. 2016; 123: 1783–1787. [DOI] [PubMed] [Google Scholar]
- 121. Tanaka N, Shinohara K, Yokoi T, et al.. Posterior staphylomas and scleral curvature in highly myopic children and adolescents investigated by ultra-widefield optical coherence tomography. PLoS One. 2019; 14: e0218107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Nuzzi R, Lavia C, Spinetta R. Paediatric retinal detachment: A review. Int J Ophthalmol. 2017; 10: 1592–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rumelt S, Sarrazin L, Averbukh E, Halpert M, Hemo I. Paediatric vs adult retinal detachment. Eye (Lond). 2007; 21: 1473–1478. [DOI] [PubMed] [Google Scholar]
- 124. Chang PY, Yang CM, Yang CH, et al.. Clinical characteristics and surgical outcomes of pediatric rhegmatogenous retinal detachment in Taiwan. Am J Ophthalmol. 2005; 139: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 125. Alshahrani ST, Ghazi NG, Al-Rashaed S. Rhegmatogenous retinal detachments associated to Stickler syndrome in a tertiary eye care center in Saudi Arabia. Clin Ophthalmol. 2015; 10: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang NK, Chen YP, Lai CC, et al.. Paediatric retinal detachment: Comparison of high myopia and extreme myopia. Br J Ophthalmol. 2009; 93: 650–655. [DOI] [PubMed] [Google Scholar]
- 127. Barry GP, Yu Y, Ying GS, et al.. Retinal detachment after treatment of retinopathy of prematurity with laser versus intravitreal anti–vascular endothelial growth factor. Ophthalmology. 2021; 128: 1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Nixon T, Richards AJ, Lomas A, et al.. Inherited and de novo biallelic pathogenic variants in COL11A1 result in type 2 Stickler syndrome with severe hearing loss. Mol Genet Genomic Med. 2020; 8: e1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nixon TRW, Richards A, Towns LK, et al.. Bone morphogenetic protein 4 (BMP4) loss-of-function variant associated with autosomal dominant Stickler syndrome and renal dysplasia. Eur J Hum Genet. 2019; 27: 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Alzahrani F, al Hazzaa SA, Tayeb H, Alkuraya FS. LOXL3, encoding lysyl oxidase-like 3, is mutated in a family with autosomal recessive Stickler syndrome. Hum Genet. 2015; 134: 451–453. [DOI] [PubMed] [Google Scholar]
- 131. Schrauwen I, Sommen M, Claes C, et al.. Broadening the phenotype of LRP2 mutations: A new mutation in LRP2 causes a predominantly ocular phenotype suggestive of Stickler syndrome. Clin Genet. 2014; 86: 282–286. [DOI] [PubMed] [Google Scholar]
- 132. Astle WF, Huang PT, Ells AL, Cox RG, Deschenes MC, Vibert HM. Photorefractive keratectomy in children. J Cataract Refract Surg. 2002; 28: 932–941. [DOI] [PubMed] [Google Scholar]
- 133. Astle WF, Papp A, Huang PT, Ingram A. Refractive laser surgery in children with coexisting medical and ocular pathology. J Cataract Refract Surg. 2006; 32: 103–108. [DOI] [PubMed] [Google Scholar]
- 134. Tychsen L, Packwood E, Hoekel J, Lueder G. Refractive surgery for high bilateral myopia in children with neurobehavioral disorders: Clear lens extraction and refractive lens exchange. J AAPOS. 2006; 10: 357–363. [DOI] [PubMed] [Google Scholar]
- 135. Singh D. Photorefractive keratectomy in pediatric patients. J Cataract Refract Surg. 1995; 21: 630–632. [DOI] [PubMed] [Google Scholar]
- 136. Nano Jr, Muzzin S, Irigaray LF. Excimer laser photorefractive keratectomy in pediatric patients. J Cataract Refract Surg. 1997; 23: 736–739. [DOI] [PubMed] [Google Scholar]
- 137. Alió JL, Artola A, Claramonte P, Ayala MJ, Chipont E. Photorefractive keratectomy for pediatric myopic anisometropia. J Cataract Refract Surg. 1998; 24: 327–330. [DOI] [PubMed] [Google Scholar]
- 138. Rashad KM. Laser in situ keratomileusis for myopic anisometropia in children. J Refract Surg. 1999; 15: 429–435. [DOI] [PubMed] [Google Scholar]
- 139. Agarwal A, Agarwal A, Agarwal T, Siraj AA, Narang P, Narang S. Results of pediatric laser in situ keratomileusis. J Cataract Refract Surg. 2000; 26: 684–689. [DOI] [PubMed] [Google Scholar]
- 140. Nucci P, Drack AV. Refractive surgery for unilateral high myopia in children. J AAPOS. 2001; 5: 348–351. [DOI] [PubMed] [Google Scholar]
- 141. Nassaralla BR, Nassaralla JJ Jr. Laser in situ keratomileusis in children 8 to 15 years old. J Refract Surg. 2001; 17: 519–524. [DOI] [PubMed] [Google Scholar]
- 142. Autrata R, Rehurek J. Laser-assisted subepithelial keratectomy and photorefractive keratectomy versus conventional treatment of myopic anisometropic amblyopia in children. J Cataract Refract Surg. 2004; 30: 74–84. [DOI] [PubMed] [Google Scholar]
- 143. Astle WF, Huang PT, Ingram AD, Farran RP. Laser-assisted subepithelial keratectomy in children. J Cataract Refract Surg. 2004; 30: 2529–2535. [DOI] [PubMed] [Google Scholar]
- 144. Phillips CB, Prager TC, McClellan G, Mintz-Hittner HA. Laser in situ keratomileusis for treated anisometropic amblyopia in awake, autofixating pediatric and adolescent patients. J Cataract Refract Surg. 2004; 30: 2522–2528. [DOI] [PubMed] [Google Scholar]
- 145. Tychsen L, Packwood E, Berdy G. Correction of large amblyopiogenic refractive errors in children using the excimer laser. J AAPOS. 2005; 9: 224–233. [DOI] [PubMed] [Google Scholar]
- 146. Paysse EA, Coats DK, Hussein MA, Bowes Hamill M, Koch DD. Long-term outcomes of photorefractive keratectomy for anisometropic amblyopia in children. Ophthalmology. 2006; 113: 169–176. [DOI] [PubMed] [Google Scholar]
- 147. Yin ZQ, Wang H, Yu T, Ren Q, Chen L. Facilitation of amblyopia management by laser in situ keratomileusis in high anisometropic hyperopic and myopic children. J AAPOS. 2007; 11: 571–576. [DOI] [PubMed] [Google Scholar]
- 148. Ali A, Packwood E, Lueder G, Tychsen L. Unilateral lens extraction for high anisometropic myopia in children and adolescents. J AAPOS. 2007; 11: 153–158. [DOI] [PubMed] [Google Scholar]
- 149. Pirouzian A, Ip KC. Anterior chamber phakic intraocular lens implantation in children to treat severe anisometropic myopia and amblyopia: 3-year clinical results. J Cataract Refract Surg. 2010; 36: 1486–1493. [DOI] [PubMed] [Google Scholar]
- 150. Lesueur LC, Arne JL. Phakic intraocular lens to correct high myopic amblyopia in children. J Refract Surg. 2002; 18: 519–523. [DOI] [PubMed] [Google Scholar]