Abstract
Background:
The effect of sleep medications on cognition in older adults is controversial, possibly dependent upon sleep quality, and may differ by race.
Objective:
To determine the longitudinal association between sleep medication use and incident dementia over 15 years, and to explore whether the association is independent of nighttime sleep disturbances and if it differs by race.
Methods:
We examined 3,068 community-dwelling older adults (aged 74.1 ± 2.9 years, 41.7% Black, 51.5% female) without dementia. Sleep medication use was recorded three times by asking “Do you take sleeping pills or other medications to help you sleep?” with the response options: “Never (0)”, “Rarely (≤1/month)”, “Sometimes (2–4/month)”, “Often (5–15/month)”, or “Almost Always (16–30/month)”. Incident dementia was defined using hospitalization records, dementia medication prescription or clinically significant decline in global cognition.
Results:
138 (7.71%) of Whites and 34 (2.66%) of Blacks reported taking sleep medications “often or almost always”. Whites were almost twice as likely to take all prescription hypnotics. 617 participants developed dementia over the follow-up. After adjustment for all covariates, participants who reported taking sleep medications ≥ 5/month versus ≤1/month were significantly more likely to develop dementia, and the association was only observed among Whites (HR = 1.79,1.21–2.66) but not Blacks (HR = 0.84,0.38–1.83); p for interaction = 0.048. Further adjustment for nighttime sleep did not appreciably alter the results. The association was similar for the cumulative frequency of sleep medication use and remained after introducing a time lag of 3 years.
Conclusion:
Frequent sleep medication use was associated with an increased risk of dementia in White older adults. Further research is needed to determine underlying mechanisms.
Keywords: Dementia, epidemiology, medication, race, sleep
INTRODUCTION
Sleep medications or aids are one of the most commonly used medications in older adults. Despite the growing concern over the short-term adverse events related to sleep medication use in older adults, such as increased risk of falls and acute memory loss [1, 2], the long-term effects of sleep medications on cognitive decline and risk of dementia in older adults of different ethnicities remain controversial. Indeed, some have hypothesized that sleep medications might benefit cognition by improving sleep quality, given the growing evidence on the link between poor sleep and increased risk of dementia [3]. Meanwhile, some epidemiological studies have suggested an increased risk of dementia associated with benzodiazepines use [4, 5], whereas others suggested that trazodone use might benefit cognition [6].
Furthermore, while Black older adults are often less likely to report hypnotics use, yet more likely to develop dementia than Whites [7, 8], almost nothing is known about whether the association between hypnotics use and risk of dementia might differ by race. Studies have suggested that some risk factors for dementia might differ among African Americans and non-Hispanic Whites [9]. It is hypothesized that since participants and especially Blacks who have greater access to sleep medications might have higher socio-economic status and thus greater cognitive reserve, Blacks who use sleep medications more frequently might have a lower risk of dementia compared to Whites. The types of sleep medications used by Blacks and Whites could also be very different given the different physician prescribing practices, i.e., racial biases in the prescription of controlled substances [10, 11]. Given the staggering amount of sleep aids consumed by elderly in general, understanding the long-term cognitive effects of sleep medication use in Black and White elderly has important public health implications. Furthermore, knowledge about the specific types of sleep aids consumed by US elderly and particularly among individuals of different ethnicities would help clarify underlying pathways.
In a longitudinal cohort of community-dwelling Black and White elderly, we examined the race-specific association between the frequency of sleep medication use and risk of incident dementia over 15 years. We also explored whether the association is independent of sleep disturbances and examined the types of sleep medications consumed by Blacks and Whites.
MATERIALSANDMETHODS
Participants
We examined participants from the Health, Aging and Body Composition (Health ABC) study, a prospective cohort study of 3,075 community-dwelling Black and White men and women (aged 70–79 years) without dementia in Memphis, Tennessee, and Pittsburgh, Pennsylvania. Potential participants were identified and contacted based on a random sample of White and all Black Medicare-eligible older adults within designated zip code areas. Details of the study protocol have been described previously [12]. The study was approved by the institutional review boards at the University of Pittsburgh, University of Tennessee (Memphis), and University of California (San Francisco). All participants provided written informed consent.
Measures
At baseline (1997–1998), the frequency of sleep medication use was recorded by asking the participants “Do you take sleeping pills or other medications to help you sleep?” with the following response options: “Never (0)”, “Rarely (1/month or less)”, “Sometimes (2–4/month)”, “Often (5–15/month)” or “Almost Always (16–30/month)”. This same question was asked again at Year 3 (1999–2000) and at Year 5 (2000–2002). Participants were also asked to report their sleep duration and the frequency of sleep disturbances including trouble falling asleep and waking up from sleep or too early in the morning with difficulty going back to sleep.
We then verified the types of medications by asking participants to bring in the clinics their medicines and supplements. The “brown bag” review method was used where a trained interviewer recorded the drug name, strength, and frequency of use in the previous two weeks [13]. All medications were coded using the Iowa Drug Information System (IDIS) Drug Vocabulary and Thesaurus [14]. We included both OTC [including antihistamine (diphenhydramine or doxylamine), melatonin, and valerian] and prescription drugs [including trazodone, antidepressant (doxepin, amitriptyline, imipramine, and mirtazapine), antipsychotic (hydroxyzine), benzodiazepines (flurazepam, estazolam, triazolam, temazepam, lormetazepam, loprazolam, nitrazepam, quazepam, alprazolam, lorazepam, diazepam, clonazepam, and oxazepam), z-drug (zolpidem)] that are known to have hypnotic indications.
As with previous studies in the Health ABC cohort [7], the onset of incident dementia was defined as the date a participant first met any of the following criteria: 1) hospitalization record indicating dementia as a primary or secondary diagnosis (participants were asked every 6 months about hospital admissions, and records related to the hospitalizations were reviewed); 2) prescription for dementia medication (participants were asked to bring in their medications to each clinic visit); or 3) evidence of clinically significant decline in global cognitive function (change of at least 1.5 standard deviations on the race-stratified Modified Mini-Mental State examination (3MS) score). We defined time to event as the time between study baseline (year 0) and when the participant was classified as having incident dementia or censored from observation at the last available contact with follow-up available for 15 years.
All participants completed questionnaires and clinic examinations, which included information on demographics, smoking, alcohol consumption, physical activity, and body mass index (BMI). Depressive symptoms were examined using the Center for Epidemiologic Studies-Depression Scale [15], with higher score indicating more depressive symptoms. History of stroke, hypertension, diabetes, and myocardial infarction was determined using a combination of self-report data, physician diagnosis, medication use, and laboratory values. Apolipoprotein E genotype was assessed using standard methods, and participants were coded as 4 carriers or non-carriers [16].
Statistical analysis
We first compared baseline characteristics by the frequency of sleep medication use, i.e., “never or rarely”, “sometimes”, or “often or almost always”, using ANOVA or Kruskal Wallis tests for continuous variables and chi-square tests for categorical variables. We used Cox proportional hazard models to examine the association between frequency of hypnotics use at baseline and risk of incident dementia over 15 years, adjusting first for sociodemographic factors and then further for other covariates that were significantly related (p < 0.10) to either sleep medication use or dementia. These included smoking, alcohol, physical activity, BMI, depressive symptoms, comorbidities, and APOE4 genotype. We further adjusted for nighttime sleep duration and experience of sleep disturbances and examined the interaction between sleep medication use and race. To explore the effects of cumulative use of sleep medications over years, we added up the frequency of sleep medication use at baseline, Year 3, and Year 5 and repeated the analysis to examine the association between the cumulative frequency of sleep medication use and risk of dementia. To minimize the effects of protopathic bias and reverse causality, we introduced a lag time of 3 years, only including dementia cases identified after three years after the report of sleep medication use. Results are reported as Hazard ratios (HR) with 95% confidence intervals. All statistical tests were two-sided, and a p-value of less than.05 was considered significant. We performed the analysis using Stata, version 14.1 (Stata Corp LP, College Station, TX).
RESULTS
Among the 3,068 participants (aged 74.1 ± 2.9 years) who had complete information on sleep medication use at baseline, 1279 (41.7%) were Black and 1579 (51.5%) were female. A total of 172 (5.6%) reported taking sleep medications often or almost always (≥5/month). White participants were three times as likely to be taking sleep medications often or almost always: 138 (7.7%) of Whites and 34 (2.7%) of Blacks. Table 1 shows baseline characteristics by frequency of sleep medication use. More frequent users of sleep medications were more likely to be women, had higher education and more depressive symptoms and were less likely to have diabetes. This was similar for Blacks and Whites. Figure 1 describes the types of sleep aids used by Blacks and Whites. 4.1% of Blacks and 5.9% of Whites used OTC hypnotics, and 6.1% of Blacks and 10.2% of Whites took prescription hypnotics. Whites were almost twice as likely to take benzodiazepines and were ten times as likely to use trazadone compared to Blacks.
Table 1.
Baseline characteristics by frequency of sleep medication use in Black and White older adults
| Characteristics mean ± SD or N (%) | Frequency of sleep medication use |
p | ||
|---|---|---|---|---|
| Never or rarely (N = 2749) |
Sometimes (N = 147) |
Often or almost always (N = 172) |
||
|
| ||||
| Age | 74.1 ± 2.9 | 74.5 ± 2.8 | 74.1 ± 2.7 | 0.37 |
| Sex (female) | 1384 (50.4) | 93 (63.3) | 102 (59.3) | 0.001 |
| Education < high school | 714 (26.0) | 30 (20.4) | 30 (17.4) | 0.02 |
| Lower socioeconomic status | 1637 (62.4) | 92 (65.3) | 106 (63.9) | 0.75 |
| Current smoking | 284 (10.4) | 20 (13.6) | 14 (8.1) | 0.28 |
| >1 alcoholic drink/day | 201 (7.3) | 11 (7.5) | 14 (8.1) | 0.93 |
| Stroke history | 211 (7.8) | 15 (10.2) | 19 (11.2) | 0.18 |
| Coronary heart disease | 317 (11.5) | 17 (11.6) | 22 (12.8) | 0.88 |
| Hypertension | 1671 (60.8) | 86 (58.5) | 111 (64.5) | 0.52 |
| Diabetes | 530 (19.3) | 21 (14.3) | 22 (12.8) | 0.04 |
| Body Mass Index | 27.4 ± 4.9 | 26.8 ± 4.4 | 27.0 ± 4.6 | 0.16 |
| Energy expenditure (cal/kg/week) | 83.4 ± 69.6 | 76.3 ± 58.5 | 79.6 ± 65.9 | 0.65 |
| Depression score | 4.4 ± 5.0 | 7.9 ± 7.1 | 7.6 ± 7.3 | <0.001 |
| APOE4 genotype | 750 (28.8) | 43 (31.9) | 42 (26.1) | 0.55 |
| Sleep duration (h) | 6.9 (1.4) | 6.5 (1.4) | 6.7 (1.5) | 0.51 |
Fig. 1.
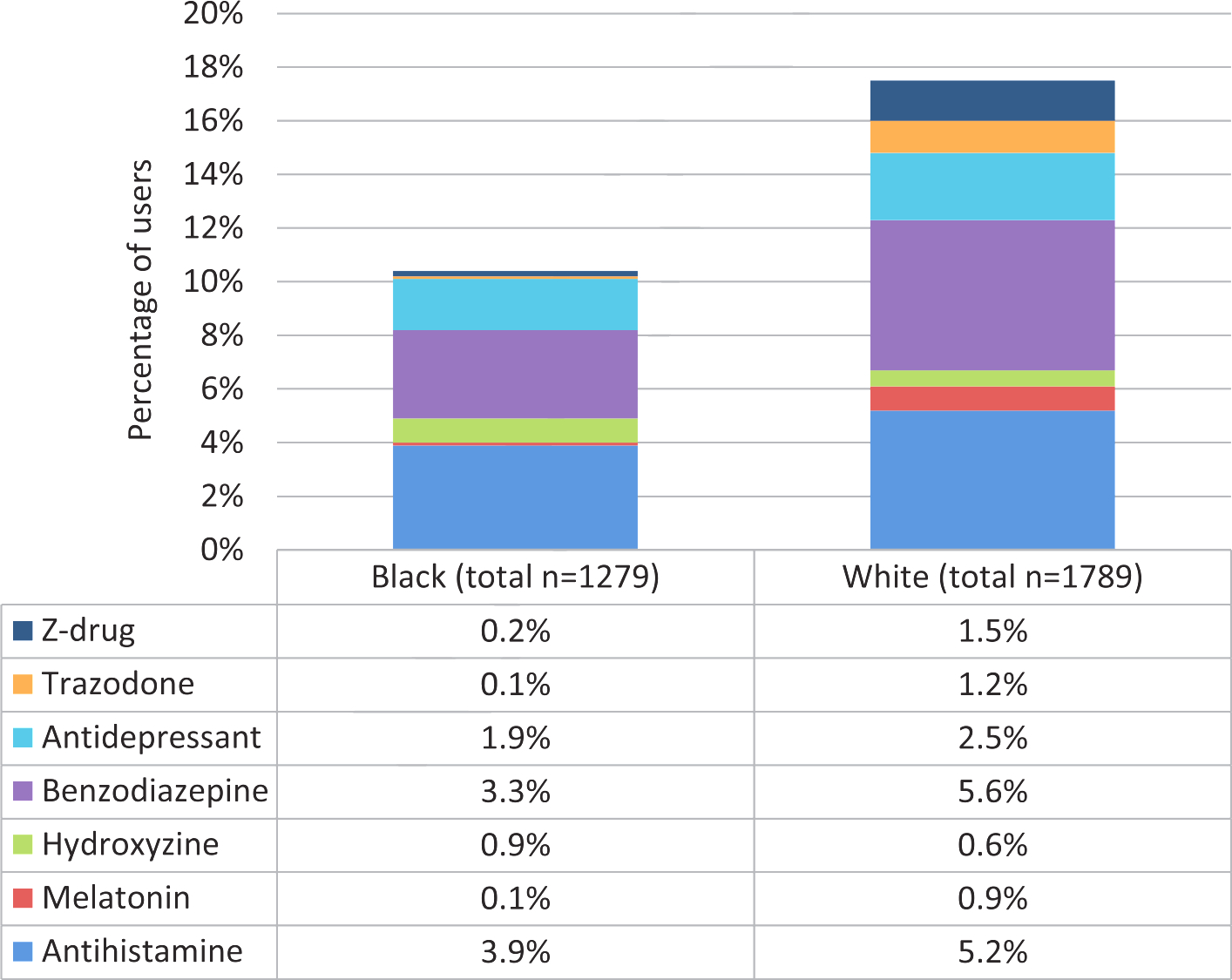
Use of different types of sleep medications in Black and White older adults. Whites were more likely to take sleep medications, especially prescription drugs or melatonin.
Overall, 617 (20%) participants developed dementia over 9.2 ± 4.6 years. After adjustment for sociodemographic variables, compared to the “never or rarely” users, the HRs (95% CI) of dementia for “sometimes” and “often or almost always” were 1.01 (0.61–1.68) and 1.83 (1.28–2.61) among Whites; and 0.70 (0.36–1.37) and 1.32 (0.68–2.56) among Blacks. Figure 2 shows the multivariable-adjusted race-specific HRs (95% CI) of incident dementia associated with sleep medication use. After adjustment for all covariates, we observed a robust association between frequent use of sleep medications and increased risk of dementia only among Whites: the HRs (95% CI) of incident dementia for “often or almost always” compared to the “never or rarely” users were 1.79 (1.21–2.66) for White participants and 0.84 (0.38–1.83) for Black participants; p for interaction = 0.048. There was no increased risk of dementia for those who took sleep medications sometimes. Further adjustment for sleep duration and disturbances did not appreciably alter the results (for the most frequent user group: HR = 1.78, 95% CI: 1.18–2.70 for Whites and HR = 0.85, 95% CI:0.38–1.89 for Blacks). The cumulative frequency of sleep medication use was associated with risk of dementia in a similar manner (for the most frequent user group: HR = 1.60, 95% CI: 1.04–2.46 for Whites and HR = 0.26, 95% CI: 0.04–1.88 for Blacks). 3-year time lag analysis did not appreciably alter the results (for the most frequent user group: HR = 1.82, 95% CI: 1.22–2.71 for Whites and HR = 0.93, 95% CI:0.42–2.02 for Blacks). Given the small number of participants that have taken each type of sleep medications, we did not perform further analysis on the types of sleep medications and risk of dementia.
Fig. 2.
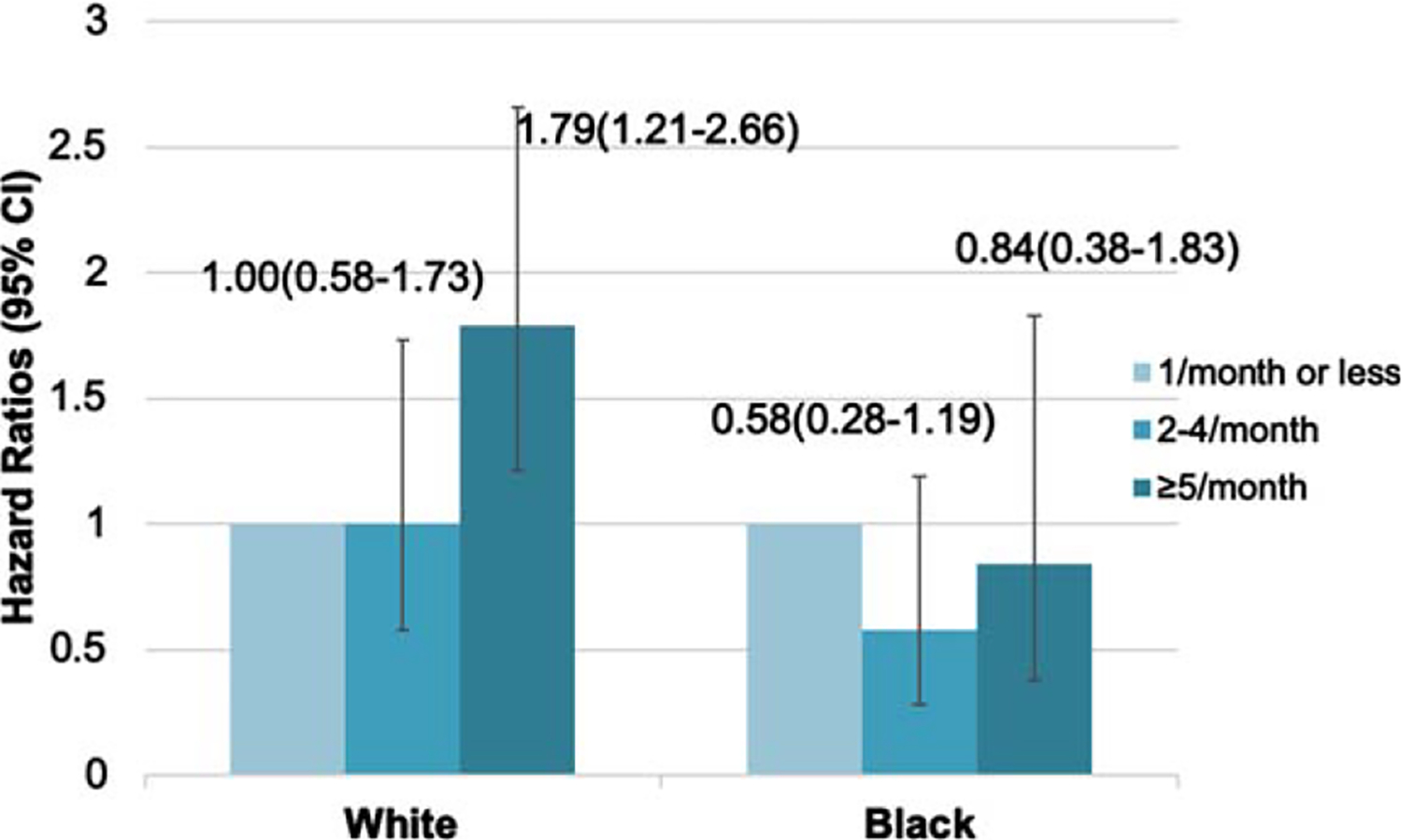
Hazard ratios* (95% CI) of dementia associated with sleep medication use in Black and White older adults. *Adjusted for age, sex, education, socio-economic status, smoking, alcohol drinking, physical activity, depression, comorbidities, and APOE4 genotype. There was a robust association between frequent (i.e., often or almost always) use of sleep medications and increased risk of dementia only among Whites.
DISCUSSION
In this longitudinal cohort of Black and White older men and women, Whites were more frequent users of sleep medications, particularly prescription hypnotics such as benzodiazepine, trazadone, and z-drugs. Frequent use of sleep medications was associated with an increased risk of developing dementia in Whites but not among Black participants. This association was independent of a number of factors including sleep duration and disturbances. The association was similar for the cumulative frequency of sleep medication use and remained after introducing a lag time of 3 years.
To our knowledge, this is the first study to show race difference in the association between hypnotics use and risk of dementia. It is unclear why the association was only observed among Whites. In this study, White participants were three times as likely to be taking sleep medications often or almost always compared to Blacks. One possibility is that Blacks who have access to sleep medications are a selected group of participants who had greater cognitive reserve and were less susceptible to dementia risk. Indeed, participants who reported taking sleep medications more frequently had higher education level. Moreover, Blacks are known to have worse sleep quality [17], and thus frequent hypnotics use in Blacks might have helped mitigate dementia risk more by improving the sleep quality of this population. It should also be noted that there might be racial biases in the prescription of controlled substances [10], and thus the different types of sleep medications received by Blacks and Whites could have contributed to the different risk of dementia in these populations. Indeed, this study and others have shown that Black older adults were significantly less likely to receive prescriptions for benzodiazepines as compared to non-Hispanic whites [11]. Interestingly, it has been reported that proneness to psychological distress was associated with risk of Alzheimer’s disease only in Whites but not in African Americans [18] and that treatment of vascular diseases could have a greater impact on AD incidence in African Americans [9].
It remains controversial whether sleep medications are good or bad for cognition in the long term. Since sleep disturbances have been found to increase the risk of dementia [19], it is plausible that hypnotics use might benefit cognition through improvement of sleep quality. For example, both animal and human studies suggested that benzodiazepine use might help reduce amyloid-β (Aβ) and prevent Aβ-neurotoxicity [20, 21]. Prolonged use of trazodone has also been found to delay cognitive decline by enhancing slow-wave sleep [6]. Meanwhile, epidemiological studies have suggested an increased risk of dementia associated with the use of hypnotics, including benzodiazepine, the ‘Z-drugs’, antidepressants, and anticholinergic drugs [4, 22–24]. One potential explanation for these observed associations is that these drugs might have been used to treat prodromal features of dementia rather than causing dementia themselves. However, the association remained after introducing a time lag of 3 years, which has helped to address this issue of protopathic bias. Our study suggested that those who reported more frequent hypnotics use were more likely to suffer from more depressive symptoms, which itself could be associated with an increased risk of dementia if not managed well. Notably, we found that the association between sleep medication use and increased dementia risk was independent of sleep duration and disturbances, which argues against the use of hypnotics among individuals at high risk for cognitive impairment. Given the increasing trends in the prescription of certain hypnotics such as trazodone [25], future studies are needed to investigate the effects of different types of hypnotics on cognition.
Our study has several strengths, including a representative sample of Black and White community-dwelling elders, followed up for a long period. We considered a number of potential confounders, including sleep duration and quality, and examined the influence of race on the association. We also had comprehensive data of recent medication use and were able to compare the types of sleep medications taken by Black and White participants. There are a few limitations. Participants were aged 70–79 years at baseline, and thus these findings might not be generalizable to those of other ages. Given the growing evidence that suggested the importance of midlife risk factors for dementia, further research is needed to extend our findings to earlier in life [26]. We focused on studying the frequency of sleep medication use, which was reported through questionnaire, while the dose or duration of medication use was not accounted for in this study. The association between specific types of sleep medications and risk of dementia was not examined due to limited statistical power. Given the racial biases in the prescription of certain controlled substances, the small number of participants that have taken each type of sleep medications in each race group and the different cognitive effects of specific types of sleep medications, larger studies that examine the association between specific types of sleep medications and dementia will provide further insights into the observed race differences. Although our method for dementia diagnosis is believed to be sensitive to capturing those with dementia, it is not based on a formal clinical interview, and we did not have information on type of dementia.
Conclusion
In a longitudinal cohort of Black and White community-dwelling older adults, we found that those who reported taking sleep medication often or almost always had a significantly increased risk of developing dementia 15 years later. This association was only found among Whites and was independent of sleep duration and quality. Further study of type of sleep medications will help to understand potential mechanisms, especially for the observed race differences. Future sleep intervention trials in older adults should closely monitor cognition as an endpoint.
ACKNOWLEDGMENTS
Research reported in this publication was supported by the UCSF Claude D. Pepper Older Americans Independence Center funded by National Institute on Aging, P30 AG044281. Dr. Yue Leng is supported by the National Institute on Aging (NIA) (R00 AG056598). The sponsor had no role in the design, methods, subject recruitment, data collections, analysis, or preparation of paper.
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/22-1006r1).
REFERENCES
- [1].Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE (2005) Sedative hypnotics in older people with insomnia: Meta-analysis of risks and benefits. BMJ 331, 1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].De Crescenzo F, D’Alò GL, Ostinelli EG, Ciabattini M, Di Franco V, Watanabe N, Kurtulmus A, Tomlinson A, Mitrova Z, Foti F, Del Giovane C, Quested DJ, Cowen PJ, Barbui C, Amato L, Efthimiou O, Cipriani A (2022) Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: A systematic review and network meta-analysis. Lancet 400, 170184. [DOI] [PubMed] [Google Scholar]
- [3].Kaufmann CN, Bondi MW, Thompson WK, Spira AP, Ancoli-Israel S, Malhotra A (2022) Cognitive performance trajectories before and after sleep treatment initiation in middle-aged and older adults: Results from the Health and Retirement Study. J Gerontol A Biol Sci Med Sci 77, 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gray SL, Dublin S, Yu O, Walker R, Anderson M, Hubbard RA, Crane PK, Larson EB (2016) Benzodiazepine use and risk of incident dementia or cognitive decline: Prospective population based study. BMJ 352, i90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].He Q, Chen X, Wu T, Li L, Fei X (2019) Risk of dementia in long-term benzodiazepine users: Evidence from a meta-analysis of observational studies. J Clin Neurol 15, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].La AL, Walsh CM, Neylan TC, Vossel KA, Yaffe K, Krystal AD, Miller BL, Karageorgiou E (2019) Long-term trazodone use and cognition: A potential therapeutic role for slow-wave sleep enhancers. J Alzheimers Dis 67, 911–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, Ayonayon H, Simonsick E, for the Health ABC Study (2013) Effect of socioeconomic disparities on incidence of dementia among biracial older adults: Prospective study. BMJ 347, f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Allen KD, Renner JB, DeVellis B, Helmick CG, Jordan JM (2008) Racial differences in sleep medication use: A cross-sectional study of the Johnston County Osteoarthritis Project. Ann Pharmacother 42, 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barnes LL, Bennett DA (2014) Alzheimer’s disease in African Americans: Risk factors and challenges for the future. Health Aff (Millwood) 33, 580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Friedman J, Kim D, Schneberk T, Bourgois P, Shin M, Celious A, Schriger DL (2019) Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med 179, 469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cook B, Creedon T, Wang Y, Lu C, Carson N, Jules P, Lee E, Alegría M (2018) Examining racial/ethnic differences in patterns of benzodiazepine prescription and misuse. Drug Alcohol Depend 187, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rooks RN, Simonsick EM, Miles T, Newman A, Kritchevsky SB, Schulz R, Harris T (2002) The association of race and socioeconomic status with cardiovascular disease indicators among older adults in the health, aging, and body composition study. J Gerontol B Psychol Sci Soc Sci 57, S247–256. [DOI] [PubMed] [Google Scholar]
- [13].Marcum ZA, Peron EP, Hanlon JT (2012) Medication use in older adults. In The Epidemiology of Aging, Newman AB, Cauley JA, eds. Springer Netherlands, Dordrecht, pp. 317–326. [Google Scholar]
- [14].Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P (1994) Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol 10, 405–411. [DOI] [PubMed] [Google Scholar]
- [15].Radloff LS (1977) The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Measure 1, 385–401. [Google Scholar]
- [16].Livak KJ (2003) SNP genotyping by the 5’-nuclease reaction. Methods Mol Biol 212, 129–147. [DOI] [PubMed] [Google Scholar]
- [17].Johnson DA, Jackson CL, Williams NJ, Alcántara C (2019) Are sleep patterns influenced by race/ethnicity - a marker of relative advantage or disadvantage? Evidence to date. Nat Sci Sleep 11, 79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wilson RS, Barnes LL, Bennett DA, Li Y, Bienias JL, Mendes de Leon CF, Evans DA (2005) Proneness to psychological distress and risk of Alzheimer disease in a biracial community. Neurology 64, 380–382. [DOI] [PubMed] [Google Scholar]
- [19].Sexton CE, Sykara K, Karageorgiou E, Zitser J, Rosa T, Yaffe K, Leng Y (2020) Connections between insomnia and cognitive aging. Neurosci Bull 36, 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chung JK, Nakajima S, Shinagawa S, Plitman E, Chakravarty MM, Iwata Y, Caravaggio F, Pollock BG, Gerretsen P, Graff-Guerrero A, Alzheimer’s Disease Neuroimaging Initiative (2016) Benzodiazepine use attenuates cortical β-amyloid and is not associated with progressive cognitive decline in nondemented elderly adults: A pilot study using F18-Florbetapir positron emission tomography. Am J Geriatr Psychiatry 24, 1028–1039. [DOI] [PubMed] [Google Scholar]
- [21].Tampellini D, Capetillo-Zarate E, Dumont M, Huang Z, Yu F, Lin MT, Gouras GK (2010) Effects of synaptic modulation on beta-amyloid, synaptophysin, and memory performance in Alzheimer’s disease transgenic mice. J Neurosci 30, 14299–14304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Leng Y, Diem SJ, Stone KL, Yaffe K (2018) Antidepressant use and cognitive outcomes in very old women. J Gerontol A Biol Sci Med Sci 73, 1390–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coupland CAC, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J (2019) Anticholinergic drug exposure and the risk of dementia: A nested case-control study. JAMA Intern Med 179, 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brandt J, Leong C (2017) Benzodiazepines and Z-drugs: An updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D 17, 493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wong J, Murray Horwitz M, Bertisch SM, Herzig SJ, Buysse DJ, Toh S (2020) Trends in dispensing of zolpidem and low-dose trazodone among commercially insured adults in the United States, 2011–2018. JAMA 324, 2211–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yaffe K, Vittinghoff E, Hoang T, Matthews K, Golden SH, Zeki Al Hazzouri A (2021) Cardiovascular risk factors across the life course and cognitive decline: A pooled cohort study. Neurology 96, e2212. [DOI] [PMC free article] [PubMed] [Google Scholar]


