OBJECTIVES:
Extracorporeal membrane oxygenation (ECMO) has been used successfully to support adults with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related cardiac or respiratory failure refractory to conventional therapies. Comprehensive reports of children and adolescents with SARS-CoV-2–related ECMO support for conditions, including multisystem inflammatory syndrome in children (MIS-C) and acute COVID-19, are needed.
DESIGN:
Case series of patients from the Overcoming COVID-19 public health surveillance registry.
SETTING:
Sixty-three hospitals in 32 U.S. states reporting to the registry between March 15, 2020, and December 31, 2021.
PATIENTS:
Patients less than 21 years admitted to the ICU meeting Centers for Disease Control criteria for MIS-C or acute COVID-19.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The final cohort included 2,733 patients with MIS-C (n = 1,530; 37 [2.4%] requiring ECMO) or acute COVID-19 (n = 1,203; 71 [5.9%] requiring ECMO). ECMO patients in both groups were older than those without ECMO support (MIS-C median 15.4 vs 9.9 yr; acute COVID-19 median 15.3 vs 13.6 yr). The body mass index percentile was similar in the MIS-C ECMO versus no ECMO groups (89.9 vs 85.8; p = 0.22) but higher in the COVID-19 ECMO versus no ECMO groups (98.3 vs 96.5; p = 0.03). Patients on ECMO with MIS-C versus COVID-19 were supported more often with venoarterial ECMO (92% vs 41%) for primary cardiac indications (87% vs 23%), had ECMO initiated earlier (median 1 vs 5 d from hospitalization), shorter ECMO courses (median 3.9 vs 14 d), shorter hospital length of stay (median 20 vs 52 d), lower in-hospital mortality (27% vs 37%), and less major morbidity at discharge in survivors (new tracheostomy, oxygen or mechanical ventilation need or neurologic deficit; 0% vs 11%, 0% vs 20%, and 8% vs 15%, respectively). Most patients with MIS-C requiring ECMO support (87%) were admitted during the pre-Delta (variant B.1.617.2) period, while most patients with acute COVID-19 requiring ECMO support (70%) were admitted during the Delta variant period.
CONCLUSIONS:
ECMO support for SARS-CoV-2–related critical illness was uncommon, but type, initiation, and duration of ECMO use in MIS-C and acute COVID-19 were markedly different. Like pre-pandemic pediatric ECMO cohorts, most patients survived to hospital discharge.
Keywords: COVID-19, extracorporeal membrane oxygenation, intensive care unit, pediatric
RESEARCH IN CONTEXT.
-
•
Some children and adolescents with severe acute respiratory syndrome coronavirus 2 infection can develop life-threatening acute COVID-19 or multisystem inflammatory syndrome in children (MIS-C) that may require extracorporeal membrane oxygenation (ECMO) support.
-
•
The characteristics and clinical outcomes of pediatric patients supported on ECMO for acute COVID-19 or MIS-C remain unclear.
-
•
Using a national hospital-based surveillance registry, we describe clinical characteristics of patients with severe COVID-19 or MIS-C admitted to the ICU, with and without ECMO support.
AT THE BEDSIDE.
-
•
Four percent of patients less than 21 years admitted to ICUs in a U.S. public health surveillance registry received ECMO.
-
•
Patients with MIS-C had ECMO initiated early in the hospital course for primary cardiac indications, and had shorter, mostly venoarterial ECMO courses with higher survival to hospital discharge compared with patients with acute COVID-19.
-
•
Overall survival to hospital discharge and need for rehabilitation or for new respiratory support among ECMO survivors were similar or compared favorably with historical, pre-pandemic pediatric ECMO cohorts.
Acute infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in children is usually asymptomatic or mild, with hospitalization rates varying over time with the proportion of hospitalized children requiring ICU admission of 27.8% during the Delta wave and 20.2% during the Omicron wave (1–5). Some young individuals can develop life-threatening COVID-19, mostly from acute hypoxemic respiratory failure. Another rare post-infectious pediatric complication of SARS-CoV-2 is multisystem inflammatory syndrome in children (MIS-C) which can rapidly progress to cardiac dysfunction and/or shock (1). Although most children who require admission to an ICU with severe COVID-19 or MIS-C survive and are discharged home (2, 3, 6, 7), some children with severe cardiac or respiratory failure require extracorporeal membrane oxygenation (ECMO) (8, 9).
ECMO was used successfully in adults with severe refractory SARS-CoV-2–associated respiratory or cardiac failure as described in three large case series with in-hospital mortality ranging from 37% to 62% (10–12). In children and adolescents, ECMO support for severe acute hypoxemic respiratory failure or cardiogenic shock due to MIS-C was described in multiple small case series (8, 13–19). The European Society of Pediatric and Neonatal Intensive Care statement provides recommendations for caring for children with suspected or proven SARS-CoV-2 in ICUs or intermediate care units and suggests using indications and thresholds for ECMO as per currently published Extracorporeal Life Support Organization (ELSO) guidelines (20), noting that specific COVID-19 ECMO data are sparse (21).
Few reports compare MIS-C with severe acute COVID-19 (2, 22, 23), and data examining the differences in clinical characteristics and outcomes of patients requiring ECMO compared with those admitted to the ICU without ECMO remain limited. Using a national hospital-based surveillance registry, we describe clinical characteristics among severe MIS-C and acute COVID-19 patients admitted to the ICU, with and without ECMO. We further present race and ethnicity data, insurance status as well as the Social Vulnerability Index (SVI), in view of previously described health disparities associated with SARS-CoV-2–related critical illness in children in the United States (24–26).
MATERIALS AND METHODS
Study Design and Participants
Patients were identified from the Overcoming COVID-19 network, which performed active surveillance of SARS-CoV-2–related illness in children, adolescents, and young adults hospitalized from March 15, 2020, to December 31, 2021, at 63 hospitals from 32 states in the United States (1, 2). The investigation was approved by the institutional review board at Boston Children’s Hospital (Influenza and Other Emerging Respiratory Pathogens Surveillance Registry; IRB-P00009548; initial approval, September 9, 2013; most recent approval, June 21, 2021). Further, the investigation was reviewed by the Centers for Disease Control and Prevention (CDC) and was conducted consistent with the Declaration of Helsinki and applicable federal law and CDC policy, including a waiver of consent for public health surveillance as defined by 45 CFR 46.102(I)(2) (27). This report conforms to reporting guidelines for uncontrolled case series (28).
Data Collection and Case Definitions
Electronic medical record data were collected and abstracted retrospectively among identified cases. The data collected included demographics, underlying medical conditions, presenting signs and symptoms, hospital clinical course, daily laboratory values, diagnostic imaging, pharmacologic and nonpharmacologic therapeutics, and outcomes. Data were entered into Research Electronic Data Capture (Vanderbilt University, Nashville, TN) hosted at Boston Children’s Hospital.
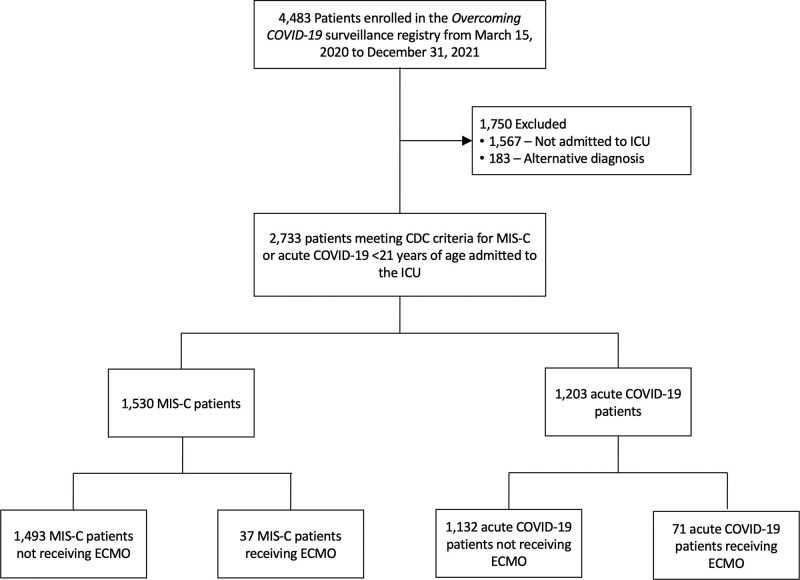
Participating sites aimed to include all patients admitted to the ICU with acute COVID-19 or MIS-C. Patients from the registry were included in our analysis if they were less than 21 years old, admitted to the PICU at a participating site, and met criteria for MIS-C or acute COVID-19. MIS-C criteria were defined by the CDC (29) and included fever lasting for greater than or equal to 24 hours, laboratory evidence of inflammation, multisystem (≥ 2) organ involvement, and laboratory evidence of current or recent SARS-CoV-2 infection. Patients with acute COVID-19 had evidence of acute infection with SARS-CoV-2 via a positive reverse transcription-polymerase chain reaction or antigen test result with symptoms and ICU admission attributed to the acute infection (Fig. 1).
Figure 1.
Eligibility flowchart of hospitalized patients with COVID-19–related illness and extracorporeal membrane oxygenation (ECMO) requirement, March 15, 2020, to December 31, 2021. CDC = Centers for Disease Control and Prevention, MIS-C = multisystem inflammatory syndrome in children.
As previously reported (2), race and ethnicity and insurance status were collected from hospital medical records based upon patient or parental report. The SVI of patients admitted during 2020 was determined based on the first four digits of the patient’s zip code (i.e., county-level SVI only), whereas for patients admitted in 2021, SVI was calculated from the patient’s address of residence using the 2018 data available from the CDC (30). Obesity was classified for patients greater than or equal to 2 years old as greater than or equal to 95th body mass index (BMI) percentile for age and sex based on CDC national reference standards (31). Nonobese patients without chronic underlying diagnoses or use of prescription medications were classified as “previously healthy.” In patients undergoing echocardiography, left ventricular ejection fraction (LVEF) was categorized as normal if the lowest reported LVEF was greater than 55% or noted to be qualitatively normal; LVEF was called depressed if LVEF was less than or equal to 55% or, in cases where LVEF was unavailable, based on the qualitative grade of dysfunction (32). Further, coronary artery aneurysms were defined as at least one body surface area-adjusted z score of the proximal left anterior descending coronary artery or proximal right coronary artery greater than or equal to 2.5, or if an aneurysm was described qualitatively (33). Patients were separated into groups based on their illness (MIS-C or acute COVID-19) and whether they received ECMO. New major morbidities reported after hospital discharge included patients who were discharged with a new tracheostomy, oxygen support, new mechanical ventilation requirement, or with a new neurologic deficit.
SARS-CoV-2 vaccination history among vaccine-eligible children was recorded primarily from the patient’s electronic medical record or the state immunization information system; where possible, patient data were linked to the Overcoming COVID-19 Vaccine Effectiveness study (34), in which robust vaccine verification methods were performed. Patients were categorized as being fully vaccinated if a second dose had been administered at least 14 days before illness onset. Adolescents who had received only one dose of vaccine or who had received a second dose less than 14 days before illness onset were considered to have been partially vaccinated. At the time of this investigation, booster doses had not yet been authorized for administration among children and adolescents.
ECMO Supplemental Data Collection
Cases of ECMO were identified in the registry by the central study team investigators and a supplemental case report form (available in Supplemental Digital Content, http://links.lww.com/PCC/C346) was distributed to site investigators to collect additional data regarding a patient’s ECMO course. Additional variables collected include mode of ECMO (venovenous, venoarterial, or both), reason for ECMO initiation (cardiac, respiratory, other), use of extracorporeal cardiopulmonary resuscitation (ECPR), degree of vasopressor support at ECMO initiation, pre-ECMO initiation lactate, troponin, and measures of oxygenation, ECMO duration, and reason for ECMO discontinuation (recovery, poor prognosis, death, transition to other support, other). ECPR and ECMO discontinuation including reason for discontinuation were defined according to ELSO recommendations (35, 36). In cases where the reasons for ECMO initiation or discontinuation were unclear, the case was adjudicated by the central study team investigators.
Statistical Analyses
Descriptive statistics were used to compare patients receiving ECMO and those not receiving ECMO within a given illness group. Continuous variables included median and interquartile range and categorical variables included counts and percentages. We used chi-square test, Fisher exact test, or Kruskal-Wallis test for between-group differences, where appropriate. p value of less than 0.05 was considered statistically significant. We did not impute missing data. All findings were considered exploratory and adjustment for multiple comparisons was not performed. Analyses were conducted in R Version 4.0.2 (R Project for Statistical Computing, Vienna, Austria).
RESULTS
A total of 2,733 patients less than 21 years old in the Overcoming COVID-19 public health registry were admitted to the PICU during the study period, with 108 patients with SARS-CoV-2–related illness (4%) requiring ECMO support including 37 of 1,530 patients (2.4%) with MIS-C and 71 of 1,203 patients (5.9%) with acute COVID-19. The characteristics of patients with and without ECMO support within each diagnostic group are summarized in Table 1. Patients with MIS-C and acute COVID-19 requiring ECMO were older than those without ECMO support (15.4 vs 9.9 yr [p < 0.001] and 15.3 vs 13.6 yr [p = 0.08], respectively). The BMI percentile was higher in COVID-19 with versus without ECMO groups (98.3 [85.8–99.7] vs 96.5 [60.1–99.3]; p = 0.03) but not different in the MIS-C with versus without ECMO groups (89.9 [68.0–97.8] vs 85.8 [53.2–97.9]; p = 0.22). There were no differences between groups with and without ECMO support within each diagnostic category by race and ethnicity, SVI or insurance status. Among all patients on ECMO support, only four patients with MIS-C were vaccine eligible (all unvaccinated), and 36 patients with acute COVID-19 were vaccine eligible (31 [86%] were unvaccinated and five had unknown vaccination status).
TABLE 1.
Demographics and Baseline Characteristics of 2,733 Patients Less Than 21 Years Admitted to the ICU for Multisystem Inflammatory Syndrome in Children or Acute COVID-19 Stratified by Extracorporeal Membrane Oxygenation Requirementa
| Clinical Characteristics | MIS-C Not Receiving ECMO (n = 1,493) | MIS-C Receiving ECMO (n = 37) | p | Acute COVID-19 Not Receiving ECMO (n = 1,132) | Acute COVID-19 Receiving ECMO (n = 71) | p |
|---|---|---|---|---|---|---|
| Male, n (%) | 906 (61) | 25 (68) | 0.50 | 598 (53) | 36 (51) | 0.82 |
| Median age (IQR), yr | 9.9 (6.5–13.6) | 15.4 (9.0–16.3) | < 0.001 | 13.6 (4.9–16.7) | 15.3 (8.6–17.3) | 0.08 |
| < 1 yr | 31 (2) | 0 (0) | < 0.001 | 154 (14) | 7 (10) | 0.28 |
| 1 to < 5 yr | 209 (14) | 2 (5) | 131 (12) | 8 (11) | ||
| 5 to < 13 yr | 832 (56) | 13 (35) | 240 (21) | 10 (14) | ||
| 13 to < 21 yr | 421 (28) | 22 (60) | 607 (54) | 46 (65) | ||
| Race and ethnic group, n (%)b | ||||||
| White, non-Hispanic | 422 (28) | 8 (22) | 0.77 | 401 (35) | 29 (41) | 0.71 |
| Black, non-Hispanic | 529 (35) | 16 (43) | 295 (26) | 15 (21) | ||
| Hispanic or Latino | 383 (26) | 9 (24) | 320 (28) | 21 (30) | ||
| Other race, non-Hispanic | 102 (7) | 2 (5) | 92 (8) | 6 (9) | ||
| Unknown | 57 (4) | 2 (5) | 24 (2) | 0 (0) | ||
| Insurance status, n (%) | ||||||
| Private/self-pay | 579 (39) | 11 (30) | 0.44 | 333 (29) | 20 (28) | 0.53 |
| Public/government | 852 (57) | 24 (65) | 737 (65) | 45 (63) | ||
| Unknown | 62 (4) | 2 (5) | 62 (5) | 6 (8) | ||
| SVI quartile, n (%)c | ||||||
| Low SVI | 220 (15) | 5 (14) | 136 (12) | 13 (18) | 0.11 | |
| Low/mid SVI | 426 (29) | 7 (19) | 330 (29) | 16 (23) | ||
| Mid/high SVI | 469 (31) | 20 (54) | 0.08 | 365 (32) | 21 (30) | |
| High SVI | 367 (25) | 5 (14) | 257 (23) | 21 (30) | ||
| Unknown SVI | 11 (0.7) | 0 (0) | 44 (4) | 0 (0) | ||
| SVI, median (IQR) | 0.553 (0.369–0.747) | 0.595 (0.442–0.707) | 0.77 | 0.554 (0.377–0.741) | 0.606 (0.340–0.766) | 0.71 |
| Date of admission, n (%) | ||||||
| March 2020 to May 2021 (pre-Delta) | 1,122 (75) | 32 (87) | 0.13 | 678 (60) | 21 (30) | < 0.001 |
| June 2021 to December 2021 (Delta) | 371 (25) | 5 (14) | 454 (40) | 50 (70) | ||
| Presentation, median (IQR) | ||||||
| Days of symptoms before presentation | 4 (3–5) | 4.5 (2–6) | 0.76 | 4 (1–7) | 4 (2–6.5) | 0.51 |
| Organ systems involved | 5 (4–5) | 6 (5–6) | < 0.001 | 3 (2–3) | 5 (4–5.5) | < 0.001 |
| Vaccination status, n (%)d | ||||||
| Vaccine eligible | 222 (15) | 4 (11) | 0.64 | 332 (29) | 36 (51) | < 0.001 |
| Fully vaccinated | 6 (3) | 0 (0) | 1.00 | 3 (1) | 0 (0) | 0.91 |
| Partially vaccinated | 12 (5) | 0 (0) | 5 (2) | 0 (0) | ||
| Unvaccinated | 176 (79) | 4 (100) | 282 (85) | 31 (86) | ||
| Unknown vaccination status | 28 (13) | 0 (0) | 42 (13) | 5 (14) |
ECMO = extracorporeal membrane oxygenation, IQR = interquartile range, MIS-C= multisystem inflammatory syndrome in children, SVI = Social Vulnerability Index.
A full list of underlying conditions is available in Supplemental Table 1 (http://links.lww.com/PCC/C346).
Race and ethnic group were reported by the patient or by the patient’s parent or guardian.
SVI of patients admitted during 2020 was determined based on the first four digits of the patient’s zip code, whereas for patients admitted in 2021, SVI was calculated from the patient’s address of residence using the 2018 data available from the Centers for Disease Control and Prevention (30).
Vaccination eligibility/status was defined as previously reported (29).
Among patients with MIS-C, those supported on ECMO were more likely to have oncologic or immune compromise as underlying conditions and had more organ systems involved at presentation compared with those without ECMO support (Table 1; and Supplemental Table 1, http://links.lww.com/PCC/C346). A significantly higher proportion of patients supported on ECMO had LVEF less than or equal to 35% compared with those without ECMO support (61% vs 8%). Patients with MIS-C who required ECMO had significantly higher in-hospital mortality (27% vs 0.9%), longer ICU and hospital length of stay, and were more likely to be discharged to rehabilitation or chronic care facility compared with those without ECMO support (Table 2).
TABLE 2.
Hospital Characteristics and Outcomes of 2,733 Patients Less Than 21 Years Admitted to the ICU for Multisystem Inflammatory Syndrome in Children or Acute COVID-19 Stratified by Extracorporeal Membrane Oxygenation Requirement
| Clinical Characteristics | MIS-C Not Receiving ECMO (n = 1,493) | MIS-C Receiving ECMO (n = 37) | p | Acute COVID-19 Not Receiving ECMO (n = 1,132) | Acute COVID-19 Receiving ECMO (n = 71) | p |
|---|---|---|---|---|---|---|
| Echocardiogram findings, n (%) | ||||||
| Echocardiogram performed | 1,463 (98) | 36 (97) | 0.54 | 373 (33) | 67 (94) | < 0.001 |
| LVEF > 55% | 629 (43) | 4 (11) | < 0.001 | 267 (72) | 35 (52) | < 0.001 |
| LVEF ≤ 55% and > 35% | 633 (43) | 9 (25) | 42 (11) | 9 (13) | ||
| LVEF ≤ 35% | 119 (8) | 22 (61) | 18 (5) | 12 (18) | ||
| Unknown LVEF | 82 (6) | 1 (3) | 46 (12) | 11 (16) | ||
| Coronary artery aneurysm | 236 (16) | 4 (11) | 0.64 | 11 (3) | 3 (4) | 0.46 |
| Pericarditis or pericardial effusion | 425 (29) | 13 (36) | 0.46 | 45 (12) | 13 (19) | 0.15 |
| Outcomes, n (%)a | . | |||||
| ICU length of stay (d), median (IQR) | 3 (2–5) | 11 (8–17) | < 0.001 | 4 (2–8) | 33 (19.5–47.25) | < 0.001 |
| Hospital length of stay (d), median (IQR) | 7 (5–9) | 20 (17–24.5) | < 0.001 | 7 (4–13) | 52 (35–69) | < 0.001 |
| Died (in hospital) | 13 (0.9) | 10 (27) | < 0.001 | 41 (4) | 26 (37) | < 0.001 |
| Discharged to reha bilitation or chronic care facility | 28 (2) | 4 (11) | 0.006 | 51 (5) | 15 (21) | < 0.001 |
| Discharged with neurologic deficit | 24 (2) | 3 (8) | 0.03 | 29 (3) | 11 (15) | < 0.001 |
| Discharged with new or increased oxygen support | 21 (1) | 0 (0) | 1.00 | 90 (8) | 14 (20) | 0.001 |
| Discharged with new mechanical ventilation requirement | 11 (0.7) | 0 (0) | 1.00 | 27 (2) | 7 (10) | 0.003 |
| Discharged with new tracheostomy | 6 (0.4) | 0 (0) | 1.00 | 19 (2) | 8 (11) | < 0.001 |
ECMO = extracorporeal membrane oxygenation, IQR = interquartile range, LVEF = left ventricular ejection fraction, MIS-C= multisystem inflammatory syndrome in children.
Outcomes are not mutually exclusive.
Among patients with acute COVID-19, those supported on ECMO had similar age and distribution of underlying conditions but more organ systems involved at presentation compared with those without ECMO support (Table 1; and Supplemental Table 1, http://links.lww.com/PCC/C346). A significantly higher proportion of patients supported on ECMO had LVEF less than or equal to 35% compared with those without ECMO support (18% vs 5%). Patients with acute COVID-19 who required ECMO had significantly higher in-hospital mortality (37% vs 4%), longer ICU and hospital length of stay, and were more likely to be discharged to rehabilitation or chronic care facility compared with those without ECMO support (Table 2). In addition, patients with acute COVID-19 who required ECMO were more likely to be discharged with neurologic deficit (16% vs 3%), new or increased oxygen support (20% vs 8%), new mechanical ventilation requirement (10% vs 2%) or new tracheostomy (11% vs 2%), compared with those without ECMO support (Table 2).
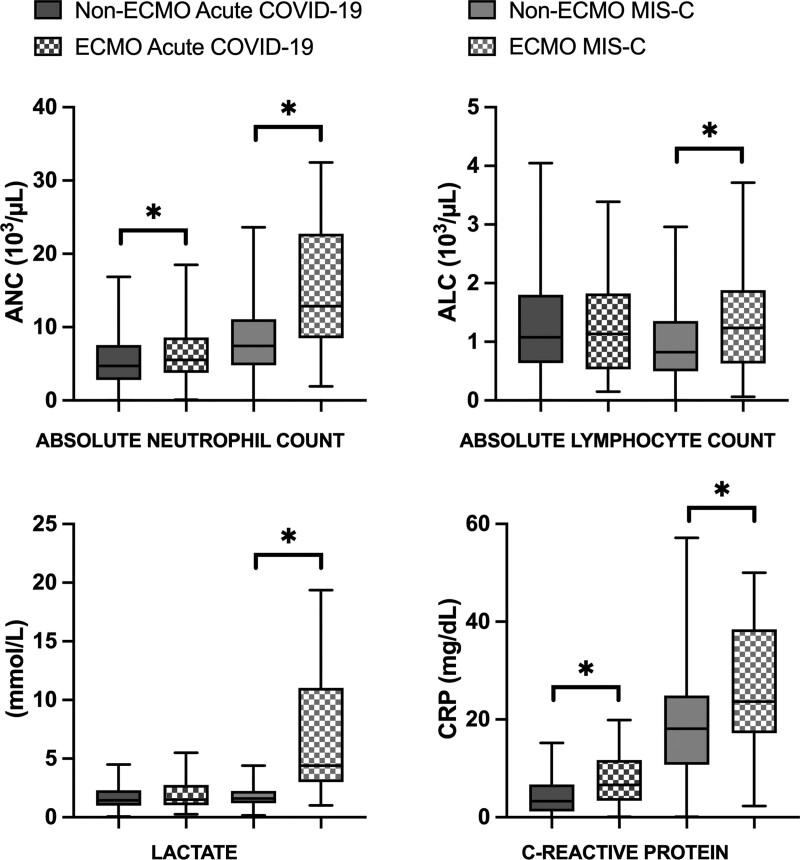
Laboratory values at presentation to the hospital are displayed in Figure 2, Supplemental Figure 1 (http://links.lww.com/PCC/C346), and Supplemental Table 2 (http://links.lww.com/PCC/C346). Overall, patients with MIS-C who required ECMO had significantly higher absolute neutrophil counts, higher absolute lymphocyte counts, WBC counts, lactate, creatinine, and C-reactive protein levels compared with patients with MIS-C without ECMO support. Among acute COVID-19 patients requiring ECMO, absolute neutrophil counts, WBC counts, neutrophil to lymphocyte ratio, creatinine, ferritin, and C-reactive protein levels were significantly greater than acute COVID-19 patients without ECMO support.
Figure 2.
Admission laboratory markers among multisystem inflammatory syndrome in children (MIS-C) and acute COVID-19 patient with and without extracorporeal membrane oxygenation (ECMO) support. *Denotes significant difference between ECMO and non-ECMO patients (p < 0.05). ALC = absolute lymphocyte count, ANC = absolute neutrophil count, CRP = C-reactive protein.
ECMO was initiated earlier in the hospital course in patients with MIS-C versus acute COVID-19 (median 1 vs 5 d from hospital admission) (Table 3). The primary reason for ECMO initiation was cardiac (87%) in the MIS-C group and respiratory (82%) in the acute COVID-19 group. This is reflected in predominantly venoarterial ECMO use in the MIS-C group (89%) and venovenous ECMO use in the acute COVID-19 group (59%) (37). ECPR rates were similar in the MIS-C and acute COVID-19 groups, 19% and 13%, respectively. In line with primary indication for ECMO, there was higher use of vasoactive infusions, higher pre-ECMO lactate (median 4.9 vs 1.8 mmol/L), and higher pre-ECMO troponin (median 0.84 vs 0.05 ng/mL) in the MIS-C group compared with the acute COVID-19 group. Pre-ECMO median Pao2 was higher, and Fio2, Paco2, and mean airway pressure were lower in the MIS-C versus acute COVID-19 group.
TABLE 3.
Characteristics of 108 Patients Less Than 21 Years With Multisystem Inflammatory Syndrome in Children or Acute COVID-19 Requiring Extracorporeal Membrane Oxygenation Support
| Clinical Characteristics | Multisystem Inflammatory Syndrome in Children ECMO (n = 37) | Acute COVID-19 ECMO (n = 71) |
|---|---|---|
| Type of ECMO, n (%) | ||
| Venovenous ECMO only | 3 (8) | 42 (59) |
| Venoarterial ECMO only | 33 (89) | 22 (31) |
| Venovenous and venoarterial ECMOa | 1 (3) | 7 (10) |
| ECMO course | ||
| Hospital day of ECMO start, median (IQR) | 1 (1–2) | 5 (2–10) |
| Total length of ECMO (d), median (IQR) | 3.9 (2.6–5.8) | 14 (6.1–27.4) |
| Multiple ECMO cannulations, n (%) | 0 (0) | 5 (7) |
| Reason for ECMO initiation, n (%) | ||
| Cardiac | 32 (87) | 16 (23) |
| Respiratory | 7 (19) | 58 (82) |
| Septic shock | 0 (0) | 1 (1) |
| Extracorporeal cardiopulmonary resuscitation | 7 (19) | 9 (13) |
| Vasopressor support at ECMO initiation, median (IQR), n (%) | ||
| Dopamine (µg/kg/min) | 12.5 (11.3–13.8), n = 2 (5) | 10 (10–10), n = 1 (1) |
| Dobutamine (µg/kg/min) | 8.25 (4.88–11.63), n = 2 (5) | Not available, n = 0 (0) |
| Epinephrine (µg/kg/min) | 0.13 (0.09–0.29), n = 35 (95) | 0.1 (0.05–0.18), n = 34 (48) |
| Norepinephrine (µg/kg/min) | 0.24 (0.10–0.38), n = 18 (49) | 0.1 (0.05–0.18), n = 20 (28) |
| Milrinone (µg/kg/min) | 0.44 (0.25–0.50), n = 10 (27) | 0.28 (0.25–0.50), n = 8 (11) |
| Vasopressin (U/kg/hr) | 0.07 (0.02–0.84), n = 10 (27) | 0.13 (0.05–0.60), n = 7 (10) |
| Laboratory values, median (IQR) | ||
| Pre-ECMO lactate (mmol/L)b | 4.9 (3.4–10.0) | 1.8 (1.1–4.1) |
| Pre-ECMO troponin (ng/mL)c | 0.84 (0.22–5.68) | 0.05 (0.01–1.10) |
| Highest troponin (ng/mL)d | 0.92 (0.34–8.0) | 0.42 (0.05–5.42) |
| Pre-ECMO oxygenation | ||
| Pao2 (mm Hg)e | 76 (59.3–174) | 68 (58–88.3) |
| Fio2f | 0.8 (0.5–1.0) | 1.0 (0.73–1.0) |
| Paco2 (mm Hg)g | 44 (36–55) | 53.5 (45–68.7) |
| pHh | 7.28 (7.20–7.33) | 7.30 (7.18–7.38) |
| Mean airway pressure (mm Hg)i | 17 (14–22.5) | 22.3 (17.5–26.9) |
| ECMO discontinuation reason, n (%) | ||
| Recovery | 29 (78) | 43 (61) |
| Poor prognosis/redirection of goals of care | 3 (8) | 11 (16) |
| Death on ECMO | 5 (14) | 12 (17) |
| Bleeding complications | 0 (0) | 3 (4) |
| Transition to lung transplant evaluation | 0 (0) | 1 (1) |
| Transition to cardiac supportj | 0 (0) | 1 (1) |
ECMO = extracorporeal membrane oxygenation, IQR = interquartile range.
Multisystem inflammatory syndrome in children (MIS-C) ECMO initial mode: venoarterial, n = 1 (3%); venovenous, n = 0 (0%). Acute COVID-19 ECMO initial mode: venoarterial, n = 2 (3%); venovenous, n = 5 (7%).
Available for 34 patients with MIS-C and 64 with acute COVID-19.
Available for 31 patients with MIS-C and 30 with acute COVID-19.
Available for 31 patients with MIS-C and 30 with acute COVID-19.
Available for 29 patients with MIS-C and 60 with acute COVID-19.
Available for 29 patients with MIS-C and 63 with acute COVID-19.
Available for 30 patients with MIS-C and 62 with acute COVID-19.
Available for 31 patients with MIS-C and 62 with acute COVID-19.
Available for 23 patients with MIS-C and 51 with acute COVID-19.
Patient with underlying cardiomyopathy.
Fourteen percent of patients with MIS-C on ECMO support and 17% of patients with acute COVID-19 on ECMO support died while on ECMO. One patient with acute COVID-19 on ECMO support was transferred to a different center for lung transplant evaluation, and one patient with underlying cardiomyopathy who required ECMO support for acute COVID-19 transitioned to mechanical circulatory support (Table 3). Among MIS-C and acute COVID-19 patients, discharge with one or more new major morbidities was 8% and 31% (p = 0.008), respectively, in survivors (new tracheostomy, oxygen or mechanical ventilation need or neurologic deficit; 0% vs 11%, 0% vs 20%, and 8% vs 15%, respectively).
DISCUSSION
In this multicenter study of critically ill U.S. children and adolescents admitted to the ICU with SARS-CoV-2–related illness including MIS-C and acute COVID-19, ECMO use was uncommon (4% of all ICU admissions), reported mostly in adolescent patients, and the majority of acute COVID-19 patients receiving ECMO were obese. The pattern of initiation, duration of support and type of ECMO used differed between the MIS-C and acute COVID-19 groups, with cardiovascular support more often the trigger for initiation for MIS-C and need for venoarterial ECMO but with shorter ECMO duration and higher frequency of survival without major complications. Although overall survival was over 83% in both groups, almost a third of survivors with acute COVID-19 suffered major morbidities. The great majority of the patients were not COVID-19 vaccine eligible prior to illness, and very few eligible patients were vaccinated. Black non-Hispanic and Hispanic children comprised a disproportionate majority of patients receiving ECMO for MIS-C or acute COVID-19 compared with the U.S. population, another example of severe health disparities during the pandemic.
Symptom duration before presentation was similar for both diagnostic groups regardless of ECMO status; however, ECMO support was initiated on average very soon after hospital admission in patients with MIS-C but later in the disease course of acute COVID-19 (median 5 d). This is in contrast with prior data from a small European case series (n = 24) that reported a median of 8.5 days from onset of symptoms to ECMO without significant differences between children with MIS-C versus acute COVID-19 with acute respiratory distress syndrome (8). The proportions of patients discharged to rehabilitation or chronic care facility among ECMO survivors (11% for MIS-C and 21% of acute COVID-19) were lower compared with reports from pre-COVID-19 pandemic mixed pediatric ECMO populations (34–51%) (38, 39). Among survivors of acute COVID-19 with ECMO support for primary respiratory indications, the proportion of patients who needed new or increased respiratory support at discharge (10% with new mechanical ventilation requirement and 11% with new tracheostomy) was similar to previously published respiratory outcomes in pre-pandemic cohorts of children with acute respiratory failure on ECMO support (9% with new mechanical ventilation requirement and 14% with new tracheostomy) (40, 41).
In-hospital mortality (27% for MIS-C and 37% for acute COVID-19 supported on ECMO) was comparable to the ELSO-reported pre-pandemic mortality in mixed pediatric populations requiring ECMO support (39%) (42). The only published multicenter case series of children supported on ECMO for MIS-C or acute COVID-19 reported higher in-hospital mortality of 43% in the earlier stage of the pandemic (March 2020 to June 2020, n = 7) (43), with much lower in-hospital mortality of 4% in the latter stages of the pandemic (July 2020 to December 2021, n = 24) (8). However, the authors acknowledged that these data may be limited by the survey methods used for data collection and small sample size (8). In-hospital mortality among children and adolescents with acute COVID-19 requiring ECMO in this report was slightly lower (37%) than pooled estimates of mortality reported in a recent systematic review and meta-analysis of studies reporting mortality among adults with COVID-19 receiving ECMO for mixed cardiac and respiratory indications (first half 2020: 41.2%, second half 2020: 46.4%, first half 2021: 62.0%, second half 2021: 46.5%) (44).
The majority of children and adolescents with MIS-C were admitted to U.S. ICUs during the pre-Delta (B.1.617.2) variant period (March 2020 to May 2021) and represented a unique challenge to pediatric providers, with rapidly evolving recommendations for early diagnosis, therapeutic regimens, and initially unknown effect of vaccination on development of MIS-C (45–48). In this Overcoming COVID-19 registry report, the majority of patients (87%) with MIS-C supported on ECMO were admitted in the pre-Delta (B.1.617.2) variant period. Only four patients (11%) were vaccine eligible, and none were vaccinated. In contrast, 70% of patients with acute COVID-19 supported on ECMO were admitted during the Delta variant period (June 2021 to December 2021). Half of these ECMO-supported patients were vaccine eligible but they were mostly unvaccinated. In a vaccine effectiveness case-control study, we reported that COVID-19 vaccination was highly effective at preventing life-threatening illness in children and adolescents (34). We observed higher overall proportion of children identifying as non-Hispanic Black race and Hispanic ethnicity compared with the U.S. general population, more accentuated among those admitted to the PICU with and without ECMO for MIS-C, consistent with previous reports from the Overcoming COVID-19 registry (24). Within the two diagnostic groups of MIS-C and acute COVID-19, there were no differences in distribution of race/ethnicity, insurance status and SVI between those receiving ECMO versus those not receiving ECMO. Should need for ECMO support be considered a marker for severity of illness, these findings are consistent with other cohorts, where lower neighborhood socioeconomic status, higher SVI, Hispanic ethnicity, and Black race were found to be independently associated with MIS-C diagnosis but not with disease severity (49).
While the analyses conducted in this investigation are not adjusted for potential confounders, patterns of risk factors for critical illness requiring ECMO emerge for both diagnostic groups. While relatively rare, oncologic disease or immune compromise were more common among MIS-C patients requiring ECMO compared with the non-ECMO group. Among patients with acute COVID-19 requiring ECMO support, BMI-based obesity was present in almost two-thirds (63%), a higher proportion than had been described in adults with severe acute COVID-19 requiring ECMO (47%) (10), suggesting that obesity may be an important risk factor for severe disease in the pediatric age group and in adolescents in particular. The pediatric hospitals enrolling patients were ECMO referral centers, and it is not possible to assess whether there were any limitations on degree of obesity for ECMO support. However, the upper quartile of BMI percentile was 99.7 for the acute COVID-19 patients. At presentation, patients with MIS-C and acute COVID-19 who required ECMO support had higher levels of inflammatory markers and evidence of end-organ dysfunction compared with their ICU counterparts not requiring ECMO support. Ferritin, a predictor of severe MIS-C (50, 51), was significantly elevated at presentation in patients with acute COVID-19 and was higher in those with MIS-C who required ECMO.
This study has several limitations. First, participating hospitals were geographically diverse but selected for sentinel surveillance of severe disease and more likely to be tertiary or quaternary care centers that were ECMO referral centers. They may not be representative of all U.S. hospitals providing pediatric ECMO support. Second, criteria for ECMO deployment and clinical management during the ECMO course were not standardized. Third, some ECMO-associated complications (42) that may have impacted outcomes may not have been collected as part of the Overcoming COVID-19 registry or the supplemental ECMO data collection form. Fourth, data collection during the Delta variant period may have been limited by the large number of hospital admissions; registry data may be biased toward inclusion of those with higher severity of illness. Fifth, there are several data elements obtained from the medical record based upon patient or parental report, such as the race and ethnicity information, which cannot be independently verified in such a registry report. Sixth, for the patients from 2020, investigators only captured the first four digits of the patient’s zip code, which allows for county-level SVI rather than individual census tract. In 2021, sites entered SVI based on patient’s address; hence, there is a specific census tract SVI for these patients. The SVI reported herein is still determined from the 15 variables as defined by the CDC, although the precision of the information differs between the two groups.
CONCLUSIONS
In this public health registry of patients less than 21 years admitted to U.S. ICUs for SARS-CoV-2–related illness, ECMO support was uncommon and was required mostly for adolescent patients. The type and timing of ECMO use in patients with MIS-C versus acute COVID-19 were markedly different. Patients with MIS-C had ECMO initiated early in the hospital course for primary cardiac indications, and had shorter, mostly venoarterial ECMO courses with higher survival to hospital discharge compared with patients with acute COVID-19. Overall survival to hospital discharge and need for rehabilitation or for new respiratory support among ECMO survivors were similar or compared favorably with historical, pre-pandemic pediatric ECMO cohorts.
ACKNOWLEDGMENTS
We appreciate and thank the many research coordinators at the Overcoming COVID-19 hospitals who assisted in data collection for this investigation. We thank the leadership of the Pediatric Acute Lung Injury and Sepsis Investigator’s Network for their ongoing support.
Supplementary Material
Footnotes
*See also p. 419.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
This work was supported by the U.S. Centers for Disease Control and Prevention and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS106292 (to Dr. Bembea).
Dr. Bembea’s institution received funding from the National Institute of Neurological Disorders and Stroke (R01NS106292) and Grifols Investigator Sponsored Research Grant. Drs. Bembea, Heidemann, Zinter, and Randolph received support for article research from the National Institutes of Health (NIH). Dr. Thiagarjan’s institution received funding from the U.S. Department of Defense (Peer Reviewed Medical Research Project Clinical Trial Award No. W81XWH2210301 Trial of Indication-based Transfusion of Red blood cells in Extracorporeal Membrane Oxygenation); he received funding from Society of Critical Care Medicine and the Extracorporeal Life Support Organization. Drs. Young’s, McCadden’s, Newhams’s, Kucuak’s, Mack’s, Fitzgerald’s, Rowan’s, Maddux’s, Kolmar’s, Heidemann’s, Schwartz’s, Kong’s, Crandall’s, Singh’s, Schuster’s, Hall’s, Wellnitz’s, Maamari’s, Gaspers’s, Nofziger’s, Cullimore’s, Halasa’s, McLaughlin’s, Pannaraj’s, Cvijanovich’s, Coates’s, Horwitz’s, Hobbs’s, Dapul’s, and Randolph’s institutions received funding from the U.S. Centers for Disease Control and Prevention (CDC). Dr. McCadden disclosed work for hire. Dr. Newhams’ institution received funding from the National Institute of Allergy and Infectious Diseases. Drs. Fitzgerald’s, Kong’s, Cullimore’s, Cvijanovich’s, and Randolph’s institutions received funding from the NIH. Dr. Rowan’s institution received funding from the National Heart, Lung, and Blood Institute (NHLBI) (K23HL150244). Dr. Maddux’s institution received funding from the National Institute of Child Health and Human Development (K23HD096018). Drs. Irby, Crandall, Singh, Wellnitz, Nofziger, Bradford, McLaughlin, Coates, Hobbs, and Zambrano received support for article research from the CDC. Dr. Schuster’s institution received funding from Merck. Dr. Hall received funding from Abbvie, Kiadis, and the American Board of Pediatrics. Dr. Wellnitz’s institution received funding from the University of Pennsylvania (prime sponsor NIH) and the University of Nebraska (prime sponsor Administration for Strategic Preparedness and Response). Dr. Gaspers received funding from Abbott Laboratories. Dr. Munoz’s institution received funding from Boston’s Children’s Hospital; he received funding from the University of Texas Health Science Center at Houston. Dr. Halasa’s institution received funding from Sanofi. Dr. McLaughlin received funding from expert witness fees from two entities. Dr. Pannaraj’s institution received funding from AstraZeneca and Pfizer. Dr. Coates’ institution received funding from the NHLBI and the American Lung Association; she received funding from Sobi. Drs. Hobbs and Randolph received funding from UpToDate. Dr. Hobbs received funding from Dynamed.com; she disclosed that she was a speaker for Biomerieux 2021–2022. Drs. Zambrano and Campbell disclosed government work. Dr. Randolph had full access to all the data in the investigation and takes responsibility for the integrity of the data and the accuracy of the data analysis. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Drs. Bembea and Loftis contributed equally as first authors. Drs. Campbell and Randolph contributed equally as senior authors.
A complete list of members and affiliations is provided in the Supplemental Digital Content (http://links.lww.com/PCC/C346).
REFERENCES
- 1.Feldstein LR, Rose EB, Horwitz SM, et al. : Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020; 383:334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldstein LR, Tenforde MW, Friedman KG, et al. : Characteristics and outcomes of US children and adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) compared with severe acute COVID-19. JAMA 2021; 325:1074–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagnoli R, Votto M, Licari A, et al. : Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: A systematic review. JAMA Pediatr 2020; 174:882–889 [DOI] [PubMed] [Google Scholar]
- 4.Marks KJ, Whitaker M, Anglin O, et al. : Hospitalizations of children and adolescents with laboratory-confirmed COVID-19 - COVID-NET, 14 states, July 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marks KJ, Whitaker M, Agathis NT, et al. : Hospitalization of infants and children aged 0-4 years with laboratory-confirmed COVID-19 - COVID-NET, 14 states, March 2020-February 2022. MMWR Morb Mortal Wkly Rep 2022; 71:429–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. : Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr 2020; 174:868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed M, Advani S, Moreira A, et al. : Multisystem inflammatory syndrome in children: A systematic review. EClinicalMedicine 2020; 26:100527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Nardo M, De Piero ME, Hoskote A, et al. : Extracorporeal membrane oxygenation in children with COVID-19 and PIMS-TS during the second and third wave. Lancet Child Adolesc Health 2022; 6:e14–e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yasuhara J, Watanabe K, Takagi H, et al. : COVID-19 and multisystem inflammatory syndrome in children: A systematic review and meta-analysis. Pediatr Pulmonol 2021; 56:837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbaro RP, MacLaren G, Boonstra PS, et al. : Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020; 396:1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall CA, Jacobs JP, Stammers AH, et al. : Multi-institutional analysis of 505 patients with coronavirus disease-2019 supported with extracorporeal membrane oxygenation: Predictors of survival. Ann Thorac Surg 2022; 114:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen NT, Sullivan B, Sagebin F, et al. : Analysis of COVID-19 patients with acute respiratory distress syndrome managed with extracorporeal membrane oxygenation at US academic centers. Ann Surg 2021; 274:40–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riphagen S, Gomez X, Gonzalez-Martinez C, et al. : Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020; 395:1607–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaushik S, Aydin SI, Derespina KR, et al. : Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): A multi-institutional study from New York City. J Pediatr 2020; 224:24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider J, Tilford B, Safa R, et al. : Extracorporeal membrane oxygenation for multisystem inflammatory syndrome in children. Perfusion 2022; 37:639–642 [DOI] [PubMed] [Google Scholar]
- 16.Alfraij A, Bin Alamir AA, Al-Otaibi AM, et al. : Characteristics and outcomes of coronavirus disease 2019 (COVID-19) in critically ill pediatric patients admitted to the intensive care unit: A multicenter retrospective cohort study. J Infect Public Health 2021; 14:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz SP, Walker TC, Kihlstrom M, et al. : Extracorporeal membrane oxygenation for COVID-19-associated multisystem inflammatory syndrome in a 5-year-old. Am Surg 2022; 88:174–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belhadjer Z, Meot M, Bajolle F, et al. : Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020; 142:429–436 [DOI] [PubMed] [Google Scholar]
- 19.Whittaker E, Bamford A, Kenny J, et al. : Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020; 324:259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shekar K, Badulak J, Peek G, et al. : Extracorporeal life support organization coronavirus disease 2019 interim guidelines: A consensus document from an International Group of Interdisciplinary Extracorporeal Membrane Oxygenation Providers. ASAIO J 2020; 66:707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rimensberger PC, Kneyber MCJ, Deep A, et al. : Caring for critically ill children with suspected or proven coronavirus disease 2019 infection: Recommendations by the scientific sections’ collaborative of the European Society of Pediatric and Neonatal Intensive Care. Pediatr Crit Care Med 2021; 22:56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swann OV, Holden KA, Turtle L, et al. : Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: Prospective multicentre observational cohort study. BMJ 2020; 370:m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes DM, Oliveira CR, Guerguis S, et al. : Severe acute respiratory syndrome coronavirus 2 clinical syndromes and predictors of disease severity in hospitalized children and youth. J Pediatr 2021; 230:23–31.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zambrano LD, Ly KN, Link-Gelles R, et al. : Investigating health disparities associated with multisystem inflammatory syndrome in children after SARS-CoV-2 infection. Pediatr Infect Dis J 2022; 41:891–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Havers FP, Whitaker M, Self JL, et al. : Hospitalization of adolescents aged 12-17 years with laboratory-confirmed COVID-19 - COVID-NET, 14 states, March 1, 2020-April 24, 2021. MMWR Morb Mortal Wkly Rep 2021; 70:851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee EH, Kepler KL, Geevarughese A, et al. : Race/ethnicity among children with COVID-19-associated multisystem inflammatory syndrome. JAMA Netw Open 2020; 3:e2030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Office of the Federal Register; Goverment Publishing Office: Electronic Code of Federal Regulations Part 46 – Protection of Human Subjects. 2017. Available at: https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46#46.102. Accessed July 19, 2022
- 28.Kempen JH: Appropriate use and reporting of uncontrolled case series in the medical literature. Am J Ophthalmol 2011; 151:7–10.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention: Multisystem Inflammatory Syndrome in Children (MIS-C) Associated With Coronavirus Disease 2019 (COVID-19). 2020. Available at: https://emergency.cdc.gov/han/2020/han00432.asp. Accessed July 19, 2022
- 30.Flanagan BE, Hallisey EJ, Adams E. et al: Measuring community vulnerability to natural and anthropogenic hazards: The Centers for Disease Control and Prevention’s social vulnerability index. J Environ Health 2018; 80:34–36 [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention: Defining Childhood Weight Status. 2022. Available at: https://www.cdc.gov/obesity/basics/childhood-defining.html. Accessed July 19, 2022
- 32.Lopez L, Colan SD, Frommelt PC, et al. : Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 2010; 23:465–495; quiz 576–577 [DOI] [PubMed] [Google Scholar]
- 33.McCrindle BW, Rowley AH, Newburger JW, et al. : Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation 2017; 135:e927–e999 [DOI] [PubMed] [Google Scholar]
- 34.Olson SM, Newhams MM, Halasa NB, et al. : Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents. N Engl J Med 2022; 386:713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conrad SA, Broman LM, Taccone FS, et al. : The Extracorporeal Life Support Organization Maastricht Treaty for Nomenclature in extracorporeal life support. A position paper of the extracorporeal life support organization. Am J Respir Crit Care Med 2018; 198:447–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Extracorporeal Life Support Organization: ELSO Registry Data Definitions. 2022. Available at: https://www.elso.org/Portals/0/Files/PDF/ELSO%20Registry%20Data%20Definitions%2005_17_22.pdf. Accessed August 8, 2022
- 37.Makdisi G, Wang IW: Extra corporeal membrane oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis 2015; 7:E166–E176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bembea MM, Felling RJ, Caprarola SD, et al. : Neurologic outcomes in a two-center cohort of neonatal and pediatric patients supported on extracorporeal membrane oxygenation. ASAIO J 2020; 66:79–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence AE, Sebastiao YV, Deans KJ, et al. : Beyond survival: Readmissions and late mortality in pediatric ECMO survivors. J Pediatr Surg 2021; 56:187–191 [DOI] [PubMed] [Google Scholar]
- 40.Mallory PP, Barbaro RP, Bembea MM, et al. : Tracheostomy and long-term mechanical ventilation in children after veno-venous extracorporeal membrane oxygenation. Pediatr Pulmonol 2021; 56:3005–3012 [DOI] [PubMed] [Google Scholar]
- 41.Kohne JG, MacLaren G, Rider E, et al. : Tracheostomy practices and outcomes in children during respiratory extracorporeal membrane oxygenation. Pediatr Crit Care Med 2022; 23:268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbaro RP, Paden ML, Guner YS, et al. : Pediatric extracorporeal life support organization registry international report 2016. ASAIO J 2017; 63:456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Nardo M, Hoskote A, Thiruchelvam T, et al. : Extracorporeal membrane oxygenation in children with coronavirus disease 2019: Preliminary report from the collaborative European Chapter of the Extracorporeal Life Support Organization Prospective Survey. ASAIO J 2021; 67:121–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling RR, Ramanathan K, Sim JJL, et al. : Evolving outcomes of extracorporeal membrane oxygenation during the first 2 years of the COVID-19 pandemic: A systematic review and meta-analysis. Crit Care 2022; 26:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Son MBF, Murray N, Friedman K, et al. : Multisystem inflammatory syndrome in children - initial therapy and outcomes. N Engl J Med 2021; 385:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McArdle AJ, Vito O, Patel H, et al. : Treatment of multisystem inflammatory syndrome in children. N Engl J Med 2021; 385:11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zambrano LD, Newhams MM, Olson SM, et al. : BNT162b2 mRNA vaccination against COVID-19 is associated with decreased likelihood of multisystem inflammatory syndrome in U.S. children ages 5-18 years. Clin Infect Dis 2023; 76:e90–e100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zambrano LD, Newhams MM, Olson SM, et al. : Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12-18 years - United States, July-December 2021. MMWR Morb Mortal Wkly Rep 2022; 71:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Javalkar K, Robson VK, Gaffney L, et al. : Socioeconomic and racial and/or ethnic disparities in multisystem inflammatory syndrome. Pediatrics 2021; 147:e2020039933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Merckx J, Cooke S, El Tal T, et al. : Predictors of severe illness in children with multisystem inflammatory syndrome after SARS-CoV-2 infection: A multicentre cohort study. CMAJ 2022; 194:E513–E523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abrams JY, Oster ME, Godfred-Cato SE, et al. : Factors linked to severe outcomes in multisystem inflammatory syndrome in children (MIS-C) in the USA: A retrospective surveillance study. Lancet Child Adolesc Health 2021; 5:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.