OBJECTIVES:
The indication, complications, and outcomes of extracorporeal membrane oxygenation (ECMO) in children with COVID-19–related illnesses remain unelucidated. Our study aimed to investigate the characteristics and outcomes of ECMO in children with COVID-19–related illnesses.
DATA SOURCES:
We searched PubMed and EMBASE databases in March 2022.
STUDY SELECTION:
We retrieved all studies involving children (age ≤ 18 yr) with COVID-19–related illnesses who received ECMO.
DATA EXTRACTION:
Two authors independently extracted data and assessed the risk of bias. Mortality, successful weaning rate, and complications while on ECMO were synthesized by a one-group meta-analysis using a random-effect model. Meta-regression was performed to explore the risk factors for mortality.
DATA SYNTHESIS:
We included 18 observational studies, four case series, and 22 case reports involving 110 children with COVID-19–related illnesses receiving ECMO. The median age was 8 years (range, 10 d to 18 yr), and the median body mass index was 21.4 kg/m2 (range, 12.3–56.0 kg/m2). The most common comorbidities were obesity (11% [7/63]) and congenital heart disease (11% [7/63]), whereas 48% (30/63) were previously healthy. The most common indications for ECMO were multisystem inflammatory syndrome in children (52% [47/90]) and severe acute respiratory distress syndrome (40% [36/90]). Seventy-one percent (56/79) received venoarterial-ECMO. The median ECMO runtime was 6 days (range, 3–51 d) for venoarterial ECMO and 11 days (range, 3–71 d) for venovenous ECMO. The mortality was 26.6% (95% CI, 15.9–40.9), and the successful weaning rate was 77.0% (95% CI, 55.4–90.1). Complications were seen in 37.0% (95% CI, 23.1–53.5) while on ECMO, including stroke, acute kidney injury, pulmonary edema, and thromboembolism. Corticosteroids and IV immunoglobulin therapies were associated with lower mortality.
CONCLUSIONS:
The mortality of children on ECMO for COVID-19 was relatively low. This invasive treatment can be considered as a treatment option for critically ill children with COVID-19.
Keywords: children, COVID-19, extracorporeal life support, extracorporeal membrane oxygenation, mortality, severe acute respiratory syndrome coronavirus-2
RESEARCH IN CONTEXT.
Extracorporeal membrane oxygenation (ECMO) has been widely used to support life-threatening conditions caused by COVID-19. Although evidence of ECMO in adults has been evolving, the literature regarding ECMO for children with COVID-19–related illnesses is lacking.
Reported mortality varies with various backgrounds. Although a range of complications has been identified, the overall frequency is uncertain. Physicians, patients, and families need more information to discuss the risk-benefit balance before the decision to initiate ECMO is made.
This study collects published articles reporting outcomes of children on ECMO for COVID-19–related illnesses and synthesizes mortality, successful weaning rate, and complications. Our findings demonstrate the role and expected outcomes of ECMO in children with COVID-19.
AT THE BEDSIDE.
This meta-analysis synthesized outcomes in children on ECMO for COVID-19–related illnesses and demonstrated the mortality and complications rate, which might be favorable.
In addition to the mortality, understanding the expected recovery timeline according to the indication for ECMO (acute respiratory distress syndrome or multisystem inflammatory syndrome in children) could play an important role.
Future comparative studies with prospective designs will help validate our findings and assess the benefit of ECMO for children with COVID-19 more precisely.
COVID-19 has affected all age groups causing over 6 million deaths worldwide by March 2022 (1). Although studies have reported that children tended to present with milder symptoms after acute infection than adult patients, certain children develop critical illnesses, such as acute respiratory distress syndrome (ARDS), a multisystem inflammatory syndrome in children (MIS-C), and fulminant myocarditis, which may require extracorporeal membrane oxygenation (ECMO) therapy (2, 3). In the wake of the COVID-19 pandemic, ECMO has been widely used to treat severe ARDS in adults (4, 5). Systematic reviews investigating ECMO outcomes in adult COVID-19 patients reported that approximately 5–7% of severe ARDS or ICU-admitted patients received ECMO therapy, with mortality rates ranging between 37% and 62% (4–7). Older age, pre-ECMO cardiac arrest, initial venoarterial mode, shorter runtime, and later in the pandemic have been associated with higher mortality (4, 5).
Compared with adults, evidence of ECMO support for COVID-19 in children is scarce. The European Extracorporeal Life Support Organization (ELSO) prospective survey highlighted that ECMO use for COVID-19 in children is infrequent (8–10). Although various complications, including pneumothorax, gastrointestinal bleeding, and stroke, have been identified, the overall frequency is unknown (8, 10–12). Furthermore, reported mortality in children who required ECMO for severe COVID-19–related illnesses also varies due to the small sample size in each study (8, 9, 11, 13, 14). In this study, we conducted a systematic review and meta-analysis with the primary outcome to describe the mortality rate in this population and the secondary outcomes to describe the rate of successful weaning and the frequency of complications from ECMO.
MATERIALS AND METHODS
This research was conducted under the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines and registered in the international prospective register of systematic reviews (CRD42022316543) (15).
Eligibility Criteria
Studies meeting the following criteria were included in our review: 1) the study was published in a peer-reviewed journal, 2) the study design was either prospective trials, observational studies, case series, or case reports, 3) the study included children (age ≤ 18 yr) with COVID-19 (diagnosed by reverse transcriptase-polymerase chain reaction, antigen, or serum antibody test) who received ECMO (either venovenous or venoarterial), and 4) the study reported the mortality or in-hospital outcomes of the children with ECMO. Articles without original patient data (e.g., guidelines, correspondence, research letters, and reviews) were excluded.
Data Sources and Search
The authors used a two-level strategy to search for all prospective trials, observational studies, case series, and case reports regarding ECMO in children with COVID-19. First, a comprehensive literature search was conducted using PubMed and EMBASE databases on March 14, 2022. The search terms included (“COVID-19” OR “SARS-CoV-2”) AND (“ECMO” OR “Extracorporeal membrane oxygenation” OR “Extracorporeal life support”) AND (“children” OR “pediatric”). Second, we performed an additional manual search of secondary sources, such as references from initially identified studies, to collect relevant articles comprehensively. We did not apply language limitations.
Data Extraction
Two investigators (A.W., J.Y.) reviewed the search results independently to select the articles based on the inclusion and exclusion criteria and assessed the eligibility for the present study. After screening the articles based on title and abstract, the full texts of potentially eligible studies were retrieved for further review. Disagreements were resolved through consensus.
Risk of Bias
For observational studies, we used the assessment of risk of bias in prevalence studies to review the risk of bias in each study (16). A publication bias was assessed by Egger’s test and Funnel plots (17).
Data Items
Baseline characteristics, such as age, sex, body mass index (BMI), and comorbidities, were extracted. Furthermore, indications for ECMO (e.g., ARDS and MIS-C), the time interval between mechanical ventilation (MV) and ECMO initiation, ECMO mode (venoarterial or venovenous), ECMO runtime, other treatment for COVID-19 (e.g., corticosteroids, tocilizumab, and IV immunoglobulin [IVIG]), ECMO-related complications, the rate of successful weaning from ECMO, and mortality data were collected.
Data Synthesis and Analysis
The proportions of males, comorbidities, indications for ECMO, ECMO mode, and other treatments for COVID-19 were calculated by dividing the total number of events by the number of patients in studies wherein the corresponding information was available. To synthesize the data of the primary and secondary outcomes, we pooled the logit of events per total number in each study. We performed a one-group meta-analysis by the Wald method for discrete values using a random-effect model with OpenMetaAnalyst Version 21.11.14 (Brown University; http://www.cebm.brown.edu/openmeta/) and back-transformed the combined logit to the original scale. In addition, to explore the source of heterogeneity and the potential risk factors for mortality, we performed a univariate meta-regression with the following covariates: the proportion of ECMO mode (i.e., venoarterial- and venovenous-), the proportion of children with MIS-C, the proportion of children with ARDS, the proportion of children who received corticosteroids, the proportion of children who received IVIG, and the observational period of each study (i.e., whether a study included patients admitted during either the first-/second-half of 2020 or the first-/second-half of 2021) (18).
Studies that only included one ECMO patient were excluded from the meta-analysis because those data do not yield ses and cannot be synthesized as a meta-analysis. The I2 statistic was used to quantify heterogeneity, with I2 greater than 50% indicating substantial heterogeneity. Publication bias was assessed by Egger’s test and Funnel plot of the primary outcomes in each study using ProMeta 3.0 (IDoStatistics; https://idostatistics.com/prometa3/) (17).
We performed two sensitivity analyses. First, we performed a meta-analysis by excluding studies with less than 10 patients in order to assess the effect of survival reporting bias. Second, we calculated the outcomes by dividing the total event number by the number of all patients in the systematic review (including the studies with only one patient).
RESULTS
Study Selection and Characteristics
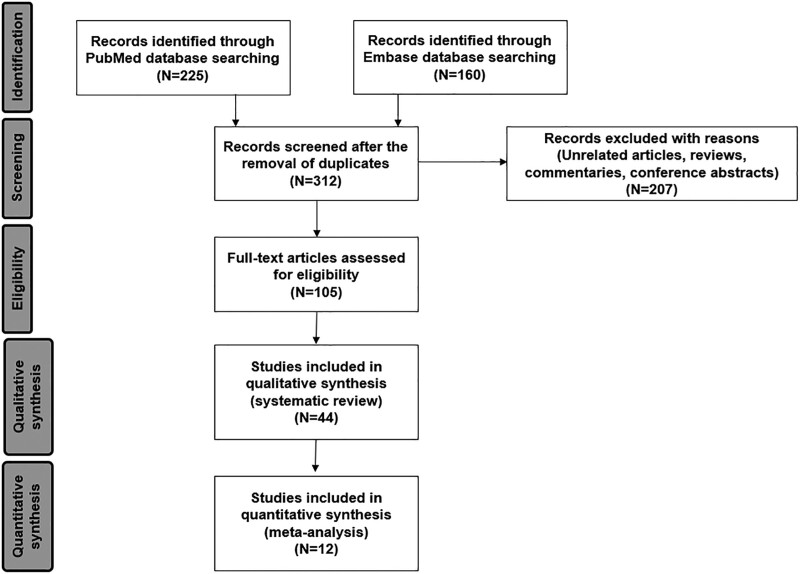
We identified 312 articles through the initial database search and the subsequent manual search. After removing 207 items based on the title and abstract, we reviewed the full text of 105 articles and included 44 studies (total number of ECMO use = 110) in the systematic review (8, 9, 11–14, 19–56). In particular, three studies with relatively large sample sizes (number of ECMO use = 42, 31, and 69) were excluded because they did not provide clinical data of patients on ECMO separately (57–59). Of the 44 articles, 18 were retrospective observational studies (8, 9, 14, 19–33), four were case series (11–13, 34), and 22 were case reports (35–56). Among the total of 22 observational studies and case series, 10 studies included only one ECMO patient (12, 14, 27–34). Therefore, we conducted a meta-analysis of the other 12 studies that included two or more cases of children who received ECMO therapy (Fig. 1) (8, 9, 11, 13, 19–26). There were 24 studies from the United States (n = 46), 13 from Europe (n = 55), four from Turkey (n = 5), and three from other countries (Kuwait, Saudi Arabia, and South Korea). There were no duplicated cases. The risk of bias assessment for the observational studies is shown in Supplemental Figure 1 (http://links.lww.com/PCC/C260).
Figure 1.
Flowchart of study selection.
Baseline Characteristics
Supplemental Table 1 (http://links.lww.com/PCC/C260) summarizes the baseline characteristics and COVID-19 therapies other than ECMO in each study. Age, sex, BMI, and comorbidities were reported in 35, 34, 9, and 30 studies, respectively. The median age was 8 years (range, 10 d to 18 yr), 61% (39/64) were boys, and the median BMI was 21.4 kg/m2 (range, 12.3–56.0 kg/m2). The most frequently reported comorbidities were obesity (11% [7/63]) and congenital heart diseases (e.g., coarctation of the aorta, ventricular septal defects, and tetralogy of Fallot) (11% [7/63]; 3: repaired, 1: unrepaired, 3: unknown), followed by immunodeficiency (e.g., chronic granulomatous diseases and deficiency of adenosine deaminase 2) (6.3% [4/63]) and asthma (4.8% [3/63]). Forty-eight percent of the children (30/63), particularly 82% (18/22) of those with MIS-C, were previously healthy.
Clinical Courses and ECMO Use Details
COVID-19 therapies were described in 35 studies. The most frequently administered agent was corticosteroids (74% [51/69]), followed by IVIG (49% [34/69]), remdesivir (41% [28/69]), anakinra (22% [15/69]), convalescent plasma (13% [9/69]), tocilizumab (13% [9/69]), and infliximab (8.7% [6/69]).
The duration of MV before ECMO initiation was reported in 13 studies (8, 9, 33, 34, 40, 42, 44, 45, 50–53, 55), and 76% (32/42) underwent ECMO within 48 hours of MV initiation, whereas the longest case was 26 days after MV initiation. ECMO use details are summarized in Supplemental Table 2 (http://links.lww.com/PCC/C260). The indications for ECMO were specified in 36 studies (8, 9, 11–13, 20–23, 27, 29–31, 33–48, 50–56). The two major indications for ECMO were MIS-C (52% [47/90]) and ARDS (42% [38/90]). Other indications included septic shock (2/90), Stevens-Johnson syndrome (1/90), massive pulmonary embolism (1/90), and pulmonary hypertensive crisis (1/90).
Among the 31 studies that reported the ECMO mode, 71% (56/79) received venoarterial-ECMO (8, 9, 11–13, 20, 21, 29, 32–41, 44–56). Thirty-four studies reported the ECMO duration; the median days were 7 (interquartile range, 5–13 d), with 71 days being the longest. The median duration of venoarterial-ECMO was shorter than venovenous-ECMO (6 [range, 3–51] vs 11 [range, 7–71]).
Outcomes
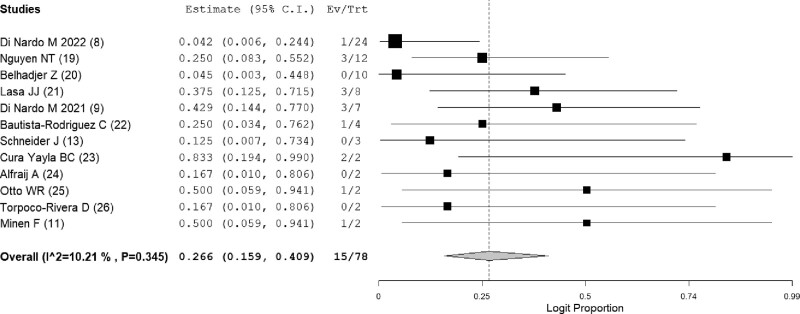
The primary outcome was estimated with the 12 studies (n = 78) that contained two or more cases using a one-group meta-analysis (8, 9, 11, 13, 19–26). A significant publication bias was not detected by Egger’s test (p = 0.329) (Supplemental Fig. 2, http://links.lww.com/PCC/C260). The overall mortality rate among children with COVID-19 who received ECMO was 26.6% (15/78; 95% CI, 15.9–40.9; I2 = 10.2%) (Fig. 2). Five studies reported a mortality rate of 19.8% (4/33; 95% CI, 5.4–51.3; I2 = 40.2%) for children who received venoarterial-ECMO (Supplemental Fig. 3, http://links.lww.com/PCC/C260). The mortality rate of children who received venovenous-ECMO could not be calculated by a meta-analysis because only one study with two or more cases reported the mortality rate of patients on venovenous-ECMO (8). We thus calculated the mortality rate of patients on venovenous-ECMO by summation, which was 10.0% (2/20). The mortality rate of children receiving ECMO for MIS-C and ARDS was 24.0% (3/17; 95% CI, 9.0–50.4; I2 = 0%) (Supplemental Fig. 4, http://links.lww.com/PCC/C260) and 13.4% (2/21; 95% CI, 1.9–55.5; I2 = 44.1%) (Supplemental Fig. 5, http://links.lww.com/PCC/C260), respectively.
Figure 2.
Forest plot showing the mortality of children with COVID-19 who underwent extracorporeal membrane oxygenation. Ev = event, Trt = total.
In the meta-regression analysis, corticosteroids and IVIG were associated with lower mortality. No other covariates were associated with mortality, including the observational periods (Table 1). The sensitivity analysis excluding studies with less than 10 patients showed a mortality of 10.5% (4/46; 95% CI, 2.6–34.2; I2 = 43.4%) (Supplemental Fig. 6, http://links.lww.com/PCC/C260). When studies with only one patient were included in the summation, the mortality was 23.6% (26/110).
TABLE 1.
Univariate Meta-Regression for Mortality Rate
| Covariate | Coefficient | Lower Bound–Upper Bound | p |
|---|---|---|---|
| First-half 2020 | 0.182 | –3.279 to 3.642 | 0.918 |
| Second-half 2020 | 0.692 | –0.805 to 2.190 | 0.365 |
| First-half 2021 | –0.726 | –2.541 to 1.089 | 0.433 |
| Second-half 2021 | 2.443 | –1.290 to 6.176 | 0.200 |
| Venoarterial ECMO | 1.524 | –3.573 to 6.622 | 0.558 |
| Venovenous ECMO | –1.524 | –6.622 to 3.573 | 0.558 |
| Multisystem inflammatory syndrome in children | 1.356 | –1.827 to 4.540 | 0.404 |
| Acute respiratory distress syndrome | –1.899 | –5.318 to 1.520 | 0.276 |
| Corticosteroids | –2.487 | –4.688 to –0.285 | 0.027 |
| IV immunoglobulin | –3.681 | –7.095 to –0.267 | 0.035 |
ECMO = extracorporeal membrane oxygenation.
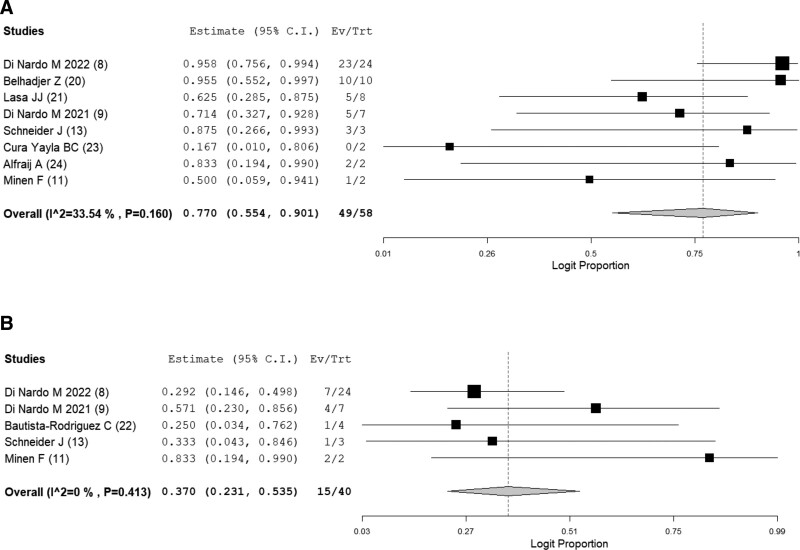
For the secondary outcomes, the successful weaning rate from ECMO and complications while on ECMO was reported in eight (8, 9, 11, 13, 20, 21, 23, 24) and five (8, 9, 11, 13, 22) studies out of the 12, respectively. The successful weaning rate was estimated to be 77.0% (49/58; 95% CI, 55.4–90.1; I2 = 33.5%) (Fig. 3A). Complications were seen in 37.0% (15/40; 95% CI, 23.1–53.5; I2 = 0%) (Fig. 3B). The most prevalent complication while on ECMO was intracranial hemorrhage or infarction (16% [10/62]), followed by acute kidney injury (11% [7/62]), pulmonary edema or hemorrhage (8.1% [5/62]), circuit thrombi (6.5% [4/62]), and venous thromboembolism (6.5% [4/62]). The definitions of complications while on ECMO in each study are shown in Supplemental Table 3 (http://links.lww.com/PCC/C260).
Figure 3.
A, Forest plot showing the successful weaning rate from extracorporeal membrane oxygenation in children with COVID-19. B, Forest plot showing the rates of complication while on extracorporeal membrane oxygenation in children with COVID-19. Ev = event, Trt = total.
The sensitivity analysis excluding studies with less than 10 patients showed a successful weaning rate from ECMO, 95.7% (33/34; 95% CI, 81.3–99.1; I2 = 0%) (Supplemental Fig. 7, http://links.lww.com/PCC/C260) and the frequency of complications, 29.2% (7/24). When studies with only one patient were included in the summation, the successful weaning rate and the frequency of complications were 77.8% (70/90) and 38.7% (24/62), respectively.
DISCUSSION
The present study comprehensively reviewed published articles and described the mortality and outcomes of children receiving ECMO support for COVID-19–related illnesses. Venoarterial-ECMO was more frequently used than venovenous-ECMO in this group, possibly reflecting MIS-C as the most common indication. Venovenous-ECMO tended to be continued longer but was associated with lower mortality than venoarterial-ECMO. In most cases, ECMO was initiated promptly after MV initiation, and the overall mortality was estimated to be 26.6%. Although more than half of the patients were weaned from ECMO successfully, various complications were identified during the ECMO treatment.
The mortality and successful weaning rates reported in previous studies varied. Outcomes of ECMO can be affected by many factors, including the indication for ECMO, the severity of the COVID-19–related illness, the presence of underlying comorbidities, and complications during ECMO treatment (8, 9, 13, 19, 20, 23). Although some observational studies highlighted the favorable outcomes of children receiving ECMO for COVID-19 with a 0% mortality rate (13, 20, 26), others reported no survival of children on ECMO (19, 23). However, we integrated those studies and provided a crude estimate of mortality rates in patients with various backgrounds. According to the last 5 years of ELSO data, the survival to discharge or transfer in neonatal and pediatric patients on ECMO was 61% and 59%, respectively (60). In addition, an international registry and a meta-analysis regarding COVID-19 showed that the adult mortality rate of those receiving ECMO was 37–62% (4, 5, 10). Compared with these reports, our study showed relatively favorable outcomes of ECMO in children with COVID-19 and may endorse ECMO as a treatment option for severe COVID-19 and COVID-19–related illnesses in children.
Our meta-regression showed that corticosteroids and IVIG were associated with lower mortality. This finding is in line with previous retrospective studies that, using a propensity score matching, demonstrated a lower risk of cardiac dysfunction or shorter stay in PICUs among children who received corticosteroids plus IVIG therapy than those who received IVIG alone (61, 62). Due to the nature of our study design, where we extracted data from published articles, our analysis could not assess the impact of corticosteroids and IVIG separately. However, our study involved sicker children than the aforementioned studies and may suggest using corticosteroids and/or IVIG in this population.
In contrast, the observational periods were not associated with mortality in our study. Adult studies showed that the mortality of patients on ECMO was higher later in the pandemic, even after adjusting for known and potential risk factors (4, 5). As the large number of patients with critical COVID-19 necessitated many institutes to treat them with ECMO, the widespread implementation of ECMO for adults at low-volume centers may explain this trend (4, 63). Conversely, since the number of children who progress to the severe condition of COVID-19 is lower than adults (2, 3), ECMO for children may still be used only at high-volume centers throughout the pandemic. Nevertheless, the statistical power of our meta-regression might be insufficient; further investigations are warranted.
ECMO may be required in severe MIS-C and ARDS in addition to anti-inflammatory treatment to provide cardiopulmonary rest while the therapies take effect (64). Our study revealed a higher proportion of venoarterial-ECMO use in children with COVID-19 compared with what has been reported in adults (< 5%) (5, 65), possibly reflecting the most common indication in children (i.e., MIS-C). MIS-C remains one of the most severe COVID-19–related illnesses that affect children, often presenting with severe cardiovascular manifestations, including significant left ventricular dysfunction and cardiogenic shock that may require venoarterial-ECMO to maintain adequate systemic blood flow (3). Interestingly, this severe cardiovascular impairment often recovers, resulting in a relatively short duration of support to allow for end-organ recovery (66). This may explain our finding of a shorter median venoarterial-ECMO runtime than venovenous-ECMO. Although some patients with MIS-C recovered promptly and were decannulated within 1 week after venoarterial-ECMO initiation (11, 13, 29, 33, 34, 39, 51, 52, 54), one patient with ARDS remained on venovenous-ECMO for 71 days (35). It is not uncommon that pulmonary function takes a month or more to recover in adult COVID-19 cases as well (67, 68). In the face of critically ill children with COVID-19 necessitating ECMO support, recognizing the difference in possible recovery timelines according to ECMO indications and modes will play a crucial role in assessing the treatment courses appropriately.
This study had several limitations. First, due to the one-group design, we could not assess the survival benefit of ECMO or the effective timing of ECMO initiation. Since initiating ECMO in critically ill patients is always a challenging decision, further studies are needed to identify the factors affecting those patients’ outcomes. Second, each study contained a small number of patients, potentially leading to heterogeneity. However, we performed several sensitivity analyses, which showed that our primary analysis was not underestimating the outcomes due to survival reporting bias. Given these results, the relatively low mortality of children on ECMO for COVID-19 should be informed to patients, families, and clinicians to discuss the expected prognosis and share the decision-making process. Third, the retrospective designs of the included studies might have underestimated the complications. In an adult systematic review, 35% (559/1,583) experienced renal complications, as opposed to 11% (7/62) in this study (69). Furthermore, reported complications, such as acute kidney injury or pulmonary edema, can simply be conditions associated with severe illness, whereas stroke, bleeding, and limb ischemia are generally considered complications due to ECMO (70). Since these complications can happen due to multifactorial reasons and it is difficult to confirm if a complication is attributable to ECMO, prespecified protocols will help identify complications related to ECMO more accurately and assess its risk-benefit balance. Fourth, several variables were not available. For example, whereas ECMO duration and BMI were significantly associated with mortality in an adult study (69), these variables were not obtainable across the included studies; the influence of these factors in COVID-19 children remains uncertain.
In conclusion, our systematic review and meta-analysis described the overall clinical courses and outcomes in children receiving ECMO for COVID-19–related illnesses, which might be favorable. Comparative studies to examine the ECMO’s benefit in children with severe COVID-19 are warranted.
Supplementary Material
Footnotes
*See also p. 430.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/pccmjournal).
Drs. Watanabe and Yasuhara contributed equally as cofirst authors.
Drs. A. Watanabe and Yasuhara conceptualized and designed the study, conducted the screening of records, collected data, carried out the initial analyses, drafted the initial article, and reviewed and revised the article. Drs Karube, K. Watanabe, and Shirasu critically reviewed and revised the article. Drs. Takagi, Sumitomo, and Lee coordinated and supervised data collection, and critically reviewed and revised the article for important intellectual content. Dr. Kuno conceptualized and designed the study, coordinated and supervised data collection, and reviewed and revised the article. All authors approved the final article as submitted and agree to be accountable for all aspects of the work.
The authors have disclosed that they do not have any potential conflicts of interest.
Data that support the findings of this study are available from the corresponding author upon reasonable requests.
REFERENCES
- 1.World Health Organization: WHO Coronavirus (COVID-19) Dashboard [Internet]. WHO Coronavirus (COVID-19) Dashboard, 2022. Available at https://covid19.who.int/. Accessed March 10, 2022 [Google Scholar]
- 2.Yasuhara J, Kuno T, Takagi H, et al. : Clinical characteristics of COVID-19 in children: A systematic review. Pediatr Pulmonol 2020; 55:2565–2575 [DOI] [PubMed] [Google Scholar]
- 3.Yasuhara J, Watanabe K, Takagi H, et al. : COVID-19 and multisystem inflammatory syndrome in children: A systematic review and meta-analysis. Pediatr Pulmonol 2021; 56:837–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbaro RP, MacLaren G, Boonstra PS, et al. : Extracorporeal membrane oxygenation for COVID-19: Evolving outcomes from the international extracorporeal life support organization registry. Lancet 2021; 398:1230–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling RR, Ramanathan K, Sim JJL, et al. : Evolving outcomes of extracorporeal membrane oxygenation during the first 2 years of the COVID-19 pandemic: A systematic review and meta-analysis. Crit Care 2022; 26:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertini P, Guarracino F, Falcone M, et al. : ECMO in COVID-19 patients: A systematic review and meta-analysis. J Cardiothorac Vasc Anesth 2022; 36:2700–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cho HJ, Heinsar S, Jeong IS, et al. : ECMO use in COVID-19: Lessons from past respiratory virus outbreaks-a narrative review. Crit Care 2020; 24:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Nardo M, De Piero ME, Hoskote A, et al. : Extracorporeal membrane oxygenation in children with COVID-19 and PIMS-TS during the second and third wave. Lancet Child Adolesc Health 2022; 6:e14–e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Nardo M, Hoskote A, Thiruchelvam T, et al. : Extracorporeal membrane oxygenation in children with coronavirus disease 2019: Preliminary report from the collaborative European chapter of the extracorporeal life support organization prospective survey. ASAIO J 2021; 67:121–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Extracorporeal Life Support Organization: Registry Dashboard of ECMO-Supported COVID-19 Patient Data [Internet]. Extracorporeal Membrane Oxygenation. Available at: https://www.elso.org/Registry/FullCOVID-19RegistryDashboard.aspx?goHash=1&sO=1&all=true&NA=false&Eur=false&Asia=false&La=false&Africa=false&AA=false&Neo=true&Ped=true&Adlt=false&AllDts=true&YTD=false#TheFilter. Accessed May 7, 2022
- 11.Minen F, Hands C, Mustafa MR, et al. : Thrombophilia in pediatric patients with multisystem inflammatory syndrome in children secondary to coronavirus disease 2019 supported on extracorporeal membrane oxygenation. ASAIO J 2021; 67:7–11 [DOI] [PubMed] [Google Scholar]
- 12.Stubbs LA, Szafron V, Forbes LR, et al. : Severe pediatric COVID-19 pneumonia treated with adjuvant anakinra. Hosp Pediatr 2022; 12:e162–e170 [DOI] [PubMed] [Google Scholar]
- 13.Schneider J, Tilford B, Safa R, et al. : Extracorporeal membrane oxygenation for multisystem inflammatory syndrome in children. Perfusion 2021; 37:639–642 [DOI] [PubMed] [Google Scholar]
- 14.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. : Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr 2020; 174:868–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, Moher D, Bossuyt PM, et al. : PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021; 372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoy D, Brooks P, Woolf A, et al. : Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012; 65:934–939 [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, et al. : Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO: Global excess deaths associated with COVID-19, January 2020 - December 2021 [Internet]. World Health Organization, 2022. Available at: https://www.who.int/data/stories/global-excess-deaths-associated-with-covid-19-january-2020-december-2021/. Accessed June 30, 2022 [Google Scholar]
- 19.Nguyen NT, Sullivan B, Sagebin F, et al. : Analysis of COVID-19 patients with acute respiratory distress syndrome managed with extracorporeal membrane oxygenation at US academic centers. Ann Surg 2021; 274:40–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belhadjer Z, Méot M, Bajolle F, et al. : Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation 2020; 142:429–436 [DOI] [PubMed] [Google Scholar]
- 21.Lasa JJ, Alali A, Anders M, et al. : Cardiovascular sequelae from COVID-19: Perspectives from a paediatric cardiac ICU. Cardiol Young 2022 Feb 24. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Bautista-Rodriguez C, Sanchez-de-Toledo J, Clark BC, et al. : Multisystem inflammatory syndrome in children: An international survey. Pediatrics 2021; 147:e2020024554. [DOI] [PubMed] [Google Scholar]
- 23.Cura Yayla BC, Özsürekçi Y, Aykaç K, et al. : Characteristics and management of children with COVID-19 in Turkey. Balkan Med J 2020; 37:341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfraij A, Bin Alamir AA, Al-Otaibi AM, et al. : Characteristics and outcomes of coronavirus disease 2019 (COVID-19) in critically ill pediatric patients admitted to the intensive care unit: A multicenter retrospective cohort study. J Infect Public Health 2021; 14:193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otto WR, Geoghegan S, Posch LC, et al. : The epidemiology of severe acute respiratory syndrome coronavirus 2 in a pediatric healthcare network in the United States. J Pediatric Infect Dis Soc 2020; 9:523–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torpoco-Rivera D, Misra A, Sanil Y, et al. : Vitamin D and morbidity in children with multisystem inflammatory syndrome related to COVID-19. Prog Pediatr Cardiol 2022; 66:101507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oualha M, Bendavid M, Berteloot L, et al. : Severe and fatal forms of COVID-19 in children. Arch Pediatr 2020; 27:235–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyts I, Bucciol G, Quinti I, et al. : Coronavirus disease 2019 in patients with inborn errors of immunity: An international study. J Allergy Clin Immunol 2021; 147:520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaushik S, Aydin SI, Derespina KR, et al. : Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): A multi-institutional study from New York City. J Pediatr 2020; 224:24–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee H, Choi S, Park JY, et al. : Analysis of critical COVID-19 cases among children in Korea. J Korean Med Sci 2022; 37:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salman H, Aslan N, Akçam M, et al. : COVID-19-associated multisystem inflammatory syndrome in children: Experiences of three centres in Turkey. Mod Rheumatol 2022; 32:460–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katlan B, Kesici S, Karacanoğlu D, et al. : Early is superior to late plasma exchange for severe multisystem inflammatory syndrome in children. J Clin Apher 2022; 37:281–291 [DOI] [PubMed] [Google Scholar]
- 33.Naber CE, Fernandes ND, Lahoud-Rahme M, et al. : Operational innovation in the provision of pediatric extracorporeal membrane oxygenation for multisystem inflammatory syndrome in children. Health Secur 2022; 20:50–57 [DOI] [PubMed] [Google Scholar]
- 34.Heidemann SM, Tilford B, Bauerfeld C, et al. : Three cases of pediatric multisystem inflammatory syndrome associated with COVID-19 due to SARS-CoV-2. Am J Case Rep 2020; 21:e925779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koh W, Malik P, Whitehead J, et al. : Successful use of veno-venous extracorporeal membrane oxygenation for acute chest syndrome in a child with sickle cell disease and SARS-CoV-2. Pediatr Pulmonol 2022; 57:1096–1099 [DOI] [PubMed] [Google Scholar]
- 36.Flood SM, Osborne CM, Martin B, et al. : Severe SARS-CoV-2 infection in a pediatric patient requiring extracorporeal membrane oxygenation. Case Rep Pediatr 2020; 2020:8885022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz SP, Walker TC, Kihlstrom M, et al. : Extracorporeal membrane oxygenation for COVID-19-associated multisystem inflammatory syndrome in a 5-year-old. Am Surg 2022; 88:174–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cicek M, Onalan MA, Yurtseven N: COVID-19 and ECMO support after neonatal congenital heart surgery: A case report. Cardiol Young 2022; 32:150–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burger MW, Moore MA, Wilburn JM: Case report: Pediatric patient with COVID-19 and multisystem inflammatory syndrome in children. Clin Pract Cases Emerg Med 2020; 4:513–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alfoudri H, Shamsah M, Yousuf B, et al. : Extracorporeal membrane oxygenation and extracorporeal cardiopulmonary resuscitation for a COVID-19 pediatric patient: A successful outcome. ASAIO J 2021; 67:250–253 [DOI] [PubMed] [Google Scholar]
- 41.Zalle I, Barthelemy Y, Piperata A, et al. : Low fetal age is not a contraindication for extracorporeal membranous oxygenation in COVID-19-related ARDS. J Card Surg 2022; 37:1059–1062 [DOI] [PubMed] [Google Scholar]
- 42.Menger J, Apostolidou S, Edler C, et al. : Fatal outcome of SARS-CoV-2 infection (B1.1.7) in a 4-year-old child. Int J Legal Med 2022; 136:189–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Apostolidou S, Harbauer T, Lasch P, et al. : Fatal COVID-19 in a child with persistence of SARS-CoV-2 despite extensive multidisciplinary treatment: A case report. Children 2021; 8:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moscatelli A, Pezzato S, Buratti S, et al. : COVID-19 pneumomediastinum: Possible role of transesophageal echo in bedside percutaneous bicaval double-lumen ECMO cannulation in children. A case report. Front Pediatr 2021; 9:740853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bemtgen X, Klingel K, Hufnagel M, et al. : Case report: Lymphohistiocytic myocarditis with severe cardiogenic shock requiring mechanical cardiocirculatory support in multisystem inflammatory syndrome following SARS-CoV-2 infection. Front Cardiovasc Med 2021; 8:716198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mustafa MR, Carter MJ, Wong J, et al. : Coronary aneurysms, myocardial dysfunction, and shock in a COVID-19 child, role of ECMO, immunomodulation, and cardiac CT. Cardiol Young 2021; 31:1043–1047 [DOI] [PubMed] [Google Scholar]
- 47.Kakuturu J, McCluskey C, Casey FL, 3rd, et al. : Extracorporeal membrane oxygenation to treat a 15-year-old patient with severe coronavirus disease 2019 (COVID-19) respiratory failure. JTCVS Tech 2021; 7:265–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hays BS, Northrop MS, Shetty AK, et al. : Critical COVID-19 complicating recovery from surgical repair of congenital heart disease. Ann Thorac Surg 2022; 113:e119–e121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sainathan S, Heal ME, Frantz E, et al. : Use of VA ECMO and percutaneous palliation of ductal dependent coarctation in a neonate with trisomy 21 and COVID-19 pneumonia. Indian J Thorac Cardiovasc Surg 2021; 37:698–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buitrago DH, Munoz J, Finkelstein ER, et al. : A case of fulminant myocarditis due to COVID-19 in an adolescent patient successfully treated with venous arterial ECMO as a bridge to recovery. J Card Surg 2022; 37:1439–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salik I, Jacoby M: Carotid artery dissection and hemorrhagic stroke in the setting of multisystem inflammatory syndrome in children. Cureus 2021; 13:e13640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaushik S, Ahluwalia N, Gangadharan S, et al. : ECMO support in SARS-CoV2 multisystem inflammatory syndrome in children in a child. Perfusion 2021; 36:524–528 [DOI] [PubMed] [Google Scholar]
- 53.Lewis D, Fisler G, Schneider J, et al. : Veno-venous extracorporeal membrane oxygenation for COVID-19-associated pediatric acute respiratory distress syndrome. Perfusion 2020; 35:550–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tseng Y-S, Herron C, Garcia R, et al. : Sustained ventricular tachycardia in a paediatric patient with acute COVID-19 myocarditis. Cardiol Young 2021; 31:1510–1512 [DOI] [PubMed] [Google Scholar]
- 55.Visveswaran GK, Morparia K, Narang S, et al. : Severe acute respiratory syndrome coronavirus 2 infection and thrombosis: Phlegmasia cerulea dolens presenting with venous gangrene in a child. J Pediatr 2020; 226:281–284.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edwards JJ, Harris MA, Toib A, et al. : Asymmetric septal edema masking as hypertrophy in an infant with COVID-19 myocarditis. Prog Pediatr Cardiol 2022; 64:101464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin B, DeWitt PE, Russell S, et al. : Characteristics, outcomes, and severity risk factors associated with SARS-CoV-2 infection among children in the US national COVID cohort collaborative. JAMA Netw Open 2022; 5:e2143151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin B, DeWitt PE, Russell S, et al.: Children with SARS-CoV-2 in the national COVID cohort collaborative (N3C). medRxiv 2021.07.19.21260767 [Google Scholar]
- 59.Miller AD, Zambrano LD, Yousaf AR, et al. : Multisystem inflammatory syndrome in children-United States, February 2020-July 2021. Clin Infect Dis 2022; 75:e1165–e1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Extracorporeal Life Support Organization: ELSO Live Registry Dashboard of ECMO Patient Data [Internet]. Extracorporeal Membrane Oxygenation Available at: https://www.elso.org/Registry/ELSOLiveRegistryDashboard.aspx. Accessed May 7, 2022
- 61.Son MBF, Murray N, Friedman K, et al. : Multisystem inflammatory syndrome in children - Initial therapy and outcomes. N Engl J Med 2021; 385:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ouldali N, Toubiana J, Antona D, et al. : Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA 2021; 325:855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riera J, Roncon-Albuquerque R, Jr, Fuset MP, et al. : Increased mortality in patients with COVID-19 receiving extracorporeal respiratory support during the second wave of the pandemic. Intensive Care Med 2021; 47:1490–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jurado Hernández JL, Álvarez Orozco IF: COVID-19 in children: Respiratory involvement and some differences with the adults. Front Pediatr 2021; 9:622240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barbaro RP, MacLaren G, Boonstra PS, et al. : Extracorporeal membrane oxygenation support in COVID-19: An international cohort study of the extracorporeal life support organization registry. Lancet 2020; 396:1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.García-Salido A, de Carlos Vicente JC, Belda Hofheinz S, et al. : Severe manifestations of SARS-CoV-2 in children and adolescents: From COVID-19 pneumonia to multisystem inflammatory syndrome: A multicentre study in pediatric intensive care units in Spain. Crit Care 2020; 24:666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.West JL, Nutting A, Daughtry B, et al. : Coronavirus 2019 (COVID-19) venovenous extracorporeal oxygenation: Single community hospital results and insights. J Card Surg 2022; 37:2009–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mustafa AK, Alexander PJ, Joshi DJ, et al. : Extracorporeal membrane oxygenation for patients with COVID-19 in severe respiratory failure. JAMA Surg 2020; 155:990–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ramanathan K, Shekar K, Ling RR, et al. : Extracorporeal membrane oxygenation for COVID-19: A systematic review and meta-analysis. Crit Care 2021; 25:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hadaya J, Benharash P: Extracorporeal membrane oxygenation. JAMA 2020; 323:2536. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.