Abstract
Host protection against Candida albicans infection in a model of oral candidiasis involving infection-prone [DBA/2 (H-2d)] and less infection-prone [BALB/c (H-2d)] mouse strains was analyzed in terms of antibody and cellular responses, and in terms of cytokine patterns from regional lymph node cells. There was a selective expansion of γ/δ+ T-cell receptor cells, which correlated with the patterns of colonization in both mouse strains, with higher numbers of γ/δ T cells detected in BALB/c mice. Antigen-induced T-cell proliferation was significantly higher in BALB/c mice than in DBA/2 mice. Higher levels of serum immunoglobulin G (IgG) and salivary IgA antibodies were detected in BALB/c mice than in DBA/2 mice, but only after the infection was cleared. The cervical lymph node cells from infected mice were assessed for interleukin-4 (IL-4), IL-12, and gamma interferon (IFN-γ) mRNA gene expression by reverse transcription-PCR and protein production in the culture supernatants following restimulation in vitro. In BALB/c mice, an early increase in levels of IL-4, IFN-γ, and IL-12 correlated with rapid elimination of C. albicans. In DBA/2 mice, where resolution of infection was delayed, IL-4 message expression was delayed and the IL-4 secretion level was lower. Neutralization of IL-4 by multiple injections of an anti-IL-4 monoclonal antibody in BALB/c mice resulted in increased carriage rate and delayed clearance of the yeasts. Collectively, the data suggest that the T-cell response to C. albicans in the regional lymph nodes which correlates best with rapid oral clearance of C. albicans is a balanced Th0 cytokine response involving early secretion of both IFN-γ and IL-4.
Candida albicans is a yeast-like fungus that colonizes human mucosal surfaces of the mouth, vagina, and gastrointestinal tract as part of the normal microbial flora. It is also an opportunistic pathogen that can cause stomatitis and vaginitis. Under certain conditions it can invade tissues and cause systemic infection (6, 17, 19, 32, 43). Both clinical and animal studies indicate that containment within the oral cavity is in part determined by CD4+ T lymphocytes (7, 12–14), while recurrent vaginal infection is predicated by a reduction in the circulating Candida-reactive T-cell pool (8). Invasive infection is uncommon in subjects with T-cell deficiency but is common in subjects with neutropenia (2) or neutrophil dysfunction (2), suggesting that different mechanisms operate to contain mucosal spread compared to those responsible for systemic immunity. In murine studies to determine the exact mechanism of protection using genetically manipulated mice or contrived experimental conditions, conflicting results have been obtained as to the cytokine mix most relevant to protection (24, 27, 33–39). Resistance to systemic infection in some murine strains identifies Th1 effector cells producing gamma interferon (IFN-γ) and interleukin-12 (IL-12) as mediators of protection (9, 37, 41, 42). By contrast, in other studies, susceptibility to infection has been linked to Th2 cells producing IL-4 and IL-10, which in turn downregulate Th1 effector cells (38, 40). A valuable natural model of oral infection has been the development of an oral infection in the BALB/c and DBA/2 mouse strains, which share the same H-2d major histocompatibility locus complex but which show different rates of spontaneous clearance from the buccal cavity (12). In this model the local cellular immune response is characterized by the recruitment of CD4+, CD8+, and γ/δ T cells within the mucosa (12, 38). The molecular mechanisms mediating protection, however, remain unclear.
To clarify the mechanisms of protection in this model of oral candidiasis, we have examined the patterns of cytokine and antibody response in both naive and primed animals. The results support the proposal that a balanced (Th0) cytokine response is important in mucosal protection in this model of oral infection.
MATERIALS AND METHODS
Mice.
Male BALB/c (H-2d) and DBA/2 (H-2d) mice, 6 to 8 weeks old, were purchased from the Animal Resource Center, Perth, Western Australia. They were housed in groups of three to five and provided with food and water ad libitum. All mice were used after 1 week of acclimatization.
Fungal culture.
C. albicans isolate 3630 was obtained from the National Reference Laboratory, Royal North Shore Hospital, Sydney, Australia. The yeast cells were cultured in Sabouraud dextrose broth (Oxoid, Basingstoke, Hampshire, United Kingdom) for 48 h at 25°C in a shaking water bath. The blastospores were transferred into fresh medium and cultured at 25°C for a further 18 h. Then the blastospores were collected by centrifugation, washed twice with phosphate-buffered saline (PBS), and adjusted to 108 blastospores per ml in PBS until use.
Candida antigen.
Freshly cultured C. albicans isolate 3630 organisms were resuspended in PBS at 1010 cells/ml and then sonicated in an MSE Soniprep set at an amplitude of 10 for 30 cycles with intermittent cooling and sonication. The sonicate was centrifuged for 10 min at 2,000 × g, after which the supernatant was collected and dialyzed against PBS. After protein estimation, the solution was filter sterilized and stored in aliquots at −20°C until use.
Oral infection.
Mice were anesthetized by intraperitoneal injection with 75 μl of ketamine-Xylazil (100 mg/ml and 20 mg/ml, respectively). They were orally inoculated with the blastospores by the method described by Chakir et al. (12). Briefly, 108 blastospores/ml in PBS were centrifuged at 14,000 × g for 5 min. The pellet was recovered on a fine-tip sterile swab (Corsham, MW & E, Wiltshire, United Kingdom) which was then used for oral inoculation by topical application.
Quantitation of oral infection.
Groups of mice (three to five per group) were sacrificed at various time points to determine the number of C. albicans organisms in the oral mucosa. The oral cavity (i.e., cheek, tongue, and soft palate), was completely swabbed using a fine-tip cotton swab. After swabbing, the cotton end was cut off and then placed in an Eppendorf tube containing 1 ml of PBS. The yeast cells were resuspended by mixing on a vortex mixer before culture in serial 10-fold dilutions on Sabouraud dextrose agar (Oxoid) supplemented with chloramphenicol (0.05 g/liter) for 48 h at 37°C. For histological studies, oral tissues were fixed in 10% formalin and embedded in paraffin. Tissue sections 5 mm thick were cut, mounted on glass slides, and then stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) stain for fungi. The numbers of blastospores and hyphal forms were enumerated by light microscopy. The results were expressed as the mean count of five fields at a magnification of ×40.
Cell separation and flow cytometry.
The cervical lymph nodes (CLN) were excised from three to five C. albicans-infected mice for each time point after infection, and single-cell suspensions were prepared (18). Pooled CLN populations were analyzed in two-color mode using Lysis 2 software and FACSan cytometry (Becton Dickinson, Mountain View, Calif.). The monoclonal antibodies (MAbs) used for staining were fluorescein isothiocyanate (FITC) conjugated (H129.19 anti-CD4 and H57-597 anti-α/β T-cell receptor [TCR]) or phycoerythrin (PE) conjugated (H53-6.7 anti-CD8α, ID3 anti-CD19, and GL3 anti-α/δ TCR). FITC- or PE-conjugated isotype-matched antibodies were used as negative controls. All MAbs were purchased from PharMingen (San Diego, Calif.). At least 10,000 viable cells from each preparation were used for analysis.
Lymphoproliferation assay.
Pooled CLN cells in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) were cultured in triplicate at 0.2 × 106 cells per well in wells of a 96-well round-bottom microtiter plate (Nunc, Roskilde, Denmark). C. albicans antigen was added to each well at a final concentration of 2.5 μg/ml. The cultures were incubated for 72 h under an atmosphere of 5% CO2 in a humidified incubator. Thymidine incorporation was measured by pulsing the cells with 1 μCi of 3H-labeled thymidine (Amersham, Aylesbury, United Kingdom) for the final 6 h of incubation before harvesting and counting. The results were expressed as mean counts per minute ± standard errors of the means (SEM).
Antibody assay.
A microplate enzyme-linked immunosorbent assay (ELISA) was used to quantitate specific antibody in the saliva and serum (37, 38). Immunopolysorb microtiter (Nunc) wells were coated with 50 μl of C. albicans antigen/ml in 0.1 M sodium borate-buffered saline (pH 8.4). Appropriate serial dilutions of the serum and saliva samples were added to each well. Bound antibodies were detected by the addition of biotinylated goat anti-mouse immunoglobulin G (IgG) or IgA (Sigma-Aldrich) followed by alkaline phosphatase-conjugated streptavidin (AMRAD, Melbourne, Australia). After addition of the substrate solution, the optical density of duplicate samples was read at 450 nm with an ELISA plate reader (Bio-Rad, Richmond, Va.).
RT-PCR.
RNA extraction and amplification of synthesized cDNA from lymphoid cells have been described elsewhere (29, 39). Briefly, 10 μl of total RNA extracted from 4 × 106 CLN cells/ml was added to 20 μl of reverse transcriptase (RT) mix containing 6 μl of 5× RT reaction buffer (250 mM Tris-HCl, 375 mM KCl, and 15 mM MgCl2), 3 μl of 100 mM dithiothreitol, 1.5 μl of deoxynucleotides (10 mM), 1 μl of RNase inhibitor (40 U/ml), 0.5 μl of Moloney murine leukemia virus (MMLV) RT (200 U/ml), 3 μl of oligo(dT)15′, 3 μl of acetylated bovine serum albumin (BSA; 1 mg/ml), and 2 μl of diethyl pyrocarbonate (DEPC)-treated water. The cDNA synthesis was carried out at 42°C for 1 h followed by heating at 72°C for 10 min. PCR amplification was carried out by adding 5 μl of the first-strand cDNA to the PCR mix containing 1 μM each primer (20 μM), 1 μl of 4 mM deoxynucleoside triphosphate (dNTP) mix, 5 μl of 10× PCR buffer, 1.2 μl of 1.5 mM MgCl2, 0.2 μl of Taq DNA polymerase (50 U/ml), and 31 μl of DEPC-treated water. The mixture was subjected to amplification using a thermal cycler (Hybaid, Ashford, Middlesex, United Kingdom) set at 94°C for 1 min (IL-4 and G3DPH) and 30 s (for IFN-γ), 60°C for 2 min (IL-4 and G3DPH) and 62°C for 1 min (IFN-γ), and 72°C for 3 mins (IL-4 and G3DPH) and 90 s (for IFN-γ), with a final elongation step at 72°C for 10 min. PCR amplification was carried out for 35 to 40 cycles. PCR fragments were separated by 2% agarose gel electrophoresis, stained with ethidium bromide, and then viewed under a UV transilluminator. For IL-4, the sense primer was GAA TGT ACC AGG AGC CAT ATC and the antisense primer was CTC AGT ACT ACG AGT ATT CCA; for IFN-γ, the sense primer was TCT CTC CTG CCT GAA GGA C and the antisense primer was ACA CAG TGA TCC TGT GGA A. The amplified DNA products for IL-4 and IFN-γ were 399 and 460 bp, respectively.
Cytokine assay.
CLN cells in RPMI 1640 medium supplemented with 10% FCS were cultured at 4 × 106 cells per well in the presence of 2.5 μg of C. albicans antigen/ml in a 24-well plate for 3 days (as described above). The culture supernatants were collected and then assayed for IL-4, IL-12, and IFN-γ by ELISA using matched-antibody pairs and recombinant cytokines as standards (PharMingen). Briefly, Immuno-polysorb microtiter plates (Nunc) were coated with a capture rat monoclonal anti-IL-4 (IgG1), anti-IL-12 (IgG2a), or anti-IFN-γ (IgG1) antibody at 1 μg/ml in sodium bicarbonate buffer (pH 9.6) overnight at 4°C. The wells were washed and then blocked with 1% BSA before the culture supernatants and the appropriate standard were added to each well. Biotinylated rat monoclonal anti-IL-4, anti-IL-12, or anti-IFN-γ antibody at 2 μg/ml was added as the second antibody. Detection was done with streptavidin peroxidase (AMRAD) and TMB (Sigma-Aldrich). The sensitivity of the cytokine ELISAs was 31 pg/ml. The results were expressed as net Candida-induced counts from which the background was subtracted.
Infection and treatment with anti-IL-4 MAb.
Mice were injected intraperitoneally (i.p.) with 30 μg of rat anti-recombinant IL-4 (rIL-4) (29) (clone 11B11; PharMingen) or with the purified rat IgG1 matched isotype in 200 μl of PBS per mouse at days 1, 3, and 5 after oral infection with 108 yeast cells. The number of yeasts in the oral cavity was determined as described above.
Statistical analysis.
The data were compared using the nonparameteric Mann-Whitney U test. P values of < 0.05 were considered significant. All calculations were performed using a statistical software program (StatView; Abacus Concepts, Berkeley, Calif.).
RESULTS
Kinetics of oral infection by C. albicans in BALB/c and DBA/2 mice.
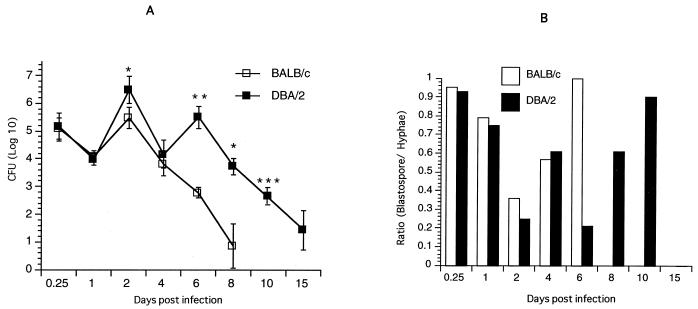
The oral mucosae of BALB/c and DBA/2 mice were infected with 108 C. albicans blastospores on day 0, after which time the level of colonization was examined over 28 days. As shown in Fig. 1A, the levels of colonization 6 h after infection were similar in BALB/c and DBA/2 mice. However, resistance to infection in BALB/c mice was evident at day 2 after an initial reduction in colonization at day 1 after inoculation, compared with a 1-log-unit increase in the number of yeasts in DBA/2 mice (P < 0.05). While there was a decrease in colonization in BALB/c and DBA/2 mice on day 4, a 2-log-unit increase in the number of yeasts occurred on day 6 in DBA/2 mice (P < 0.001), compared with BALB/c mice. By day 8, the BALB/c mice had no yeasts in the oral cavity, whereas in DBA/2 mice the number of yeasts was above 3 log units, which gradually declined to background level by day 15. Cultures of fecal pellets from mice after inoculation of C. albicans showed no growth or <3 CFU per fecal pellet, thus excluding the possibility that the repeat cycle of infection in DBA/2 mice was due to coprophagia in the mice.
FIG. 1.
Patterns of colonization with C. albicans in BALB/c and DBA/2 mice. (A) Mice were infected by swabbing the oral mucosa with C. albicans (108 CFU/mL). At various times indicated, the level of colonization was assessed by swabbing the oral cavity. Data shown are means ± standard errors for three to five mice. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.0001 (for values from BALB/c versus DBA/2 mice). (B) The numbers of blastospore and hyphae in oral tissues were counted by light microscopy (magnification, ×40) after staining with H&E and PAS stains. Data are means ± standard errors for three to five mice.
To determine whether the pattern of infection was characterized by different morphological forms of C. albicans, the proportions of blastospores and hyphal forms in oral tissues were enumerated. Figure 1B represents the ratios of blastospores to hyphal forms of Candida in tissue sections of the oral mucosa in BALB/c and DBA/2 mice. After inoculation, the ratios of blastospores to hyphal forms were about similar in DBA/2 and BALB/c mice. By day 2, the ratio was lower in DBA/2 than in BALB/c mice. On day 4, about equal ratios of blastospores to hyphae were detected in the two mice strains. In BALB/c mice, the ratios of blastospores to hyphae continued to rise over time; when 100% of yeasts present in the oral mucosa were blastospores on day 6 before they were cleared by day 8. In marked contrast, a low blastospore-to-hypha ratio was detected in DBA/2 mice on day 6; then it rose by day 10 before the yeasts, consisting predominantly of blastospores, were cleared on day 15.
Cellular response in the CLN.
The mean number of cells recovered from the CLN increased from 9.8 × 106 to 22 × 106 cells per mouse, and from 9.5 × 106 to 18 × 106 cells per mouse 4 days after infection with C. albicans in BALB/c and DBA/2 mice, respectively (Table 1). A drop in cell counts on day 6 followed the clearance of C. albicans in both BALB/c and DBA/2 mice, but in DBA/2 mice it was followed by a rise in cell counts after reinfection before decline on day 15. While the relative proportions of CD19+ B cells and the various T-cell subsets remained constant, there was a significant increase in the percentage of γ/δ T cells above the background level during the course of infection. In BALB/c mice, the number of γ/δ T cells increased five- to sixfold on day 6 and then declined thereafter when the infection was cleared. In contrast, in DBA/2 mice, the increase in the numbers of γ/δ T cells was cyclical, with maximum levels occurring on days 8 and 10 before falling to background levels on day 15, when the infection was cleared.
TABLE 1.
γ/δ T cells post-oral infection in BALB/c and DBA/2 micea
| Time (days) | (BALB/c)
|
(DBA/2)
|
||
|---|---|---|---|---|
| % γδ T cells | LN count (106) | % γδ T cells | LN count (106) | |
| 0 | 0.98 | 9.75 | 0.89 | 10.47 |
| 0.25 | 0.98 | 12.03 | 0.90 | 10.63 |
| 1 | 1.25 | 15.47 | 0.90 | 13.57 |
| 2 | 1.65 | 18.30 | 2.00 | 14.83 |
| 4 | 4.25* | 21.87 | 3.54* | 16.97 |
| 6 | 6.50** | 17.87 | 2.20 | 12.17 |
| 8 | 3.25* | 15.00 | 4.23* | 13.47 |
| 10 | 0.94 | 17.27 | 5.68** | 14.50 |
| 15 | 0.97 | 14.47 | 1.00 | 12.33 |
| 28 | 0.88 | 11.93 | 0.92 | 11.93 |
Cell numbers are expressed as counts per mouse, and the percentages of γ/δ T cells were significantly different above background. ∗, P < 0.05; ∗∗, P < 0.01 (compared with background levels).
In vitro stimulation of CLN cells.
The effect of C. albicans colonization on T-cell proliferation was determined in culture of CLN cells stimulated with C. albicans antigens. As shown in Fig. 2, there was a significantly higher antigen-stimulated T-cell proliferative response, which peaked at day 4 (P < 0.05) and day 10 (P < 0.05), in DBA/2 mice than in unstimulated controls. In contrast, a lower (but significant) increase in the proliferative response in BALB/c mice occurred at day 4 (P < 0.05) and was maintained thereafter at a similar level after a peak response at day 6 (P < 0.01). The proliferative response in DBA/2 mice, however, continued to decline to control levels by day 28.
FIG. 2.
Lymphocyte proliferation. CLN cells were stimulated or not with C. albicans antigen for 3 days, after which time thymidine incorporation was assessed. The results shown are mean counts per minute ± standard errors for three mice. ∗, P < 0.05; ∗∗, P < 0.01 (compared with values from unstimulated cells).
Serum and local IgG and IgA antibody responses.
As shown in Fig. 3, an increase in serum IgG antibody levels was detected in both BALB/c and DBA/2 mice 10 days after infection, with maximum levels detected on day 15. The levels of IgG antibody were significantly higher in BALB/c mice compared to DBA/2 mice at days 10 and 15 (P < 0.05) and at day 28 (P < 0.01). Similarly, significantly higher levels of IgA antibody were detected in the saliva of BALB/c mice compared to DBA/2 mice at all time points from day 8, with maximum levels at day 15 (P < 0.05), before dropping at day 28 (P < 0.05).
FIG. 3.
Serum IgG and salivary IgA antibody levels. IgG and IgA antibody levels were measured in serum and saliva from infected mice by ELISA. Time zero represents uninfected mice. The results shown are means ± SEM for three mice. ∗, P < 0.05; ∗∗, P < 0.01 (for values from BALB/c or DBA/2 mice).
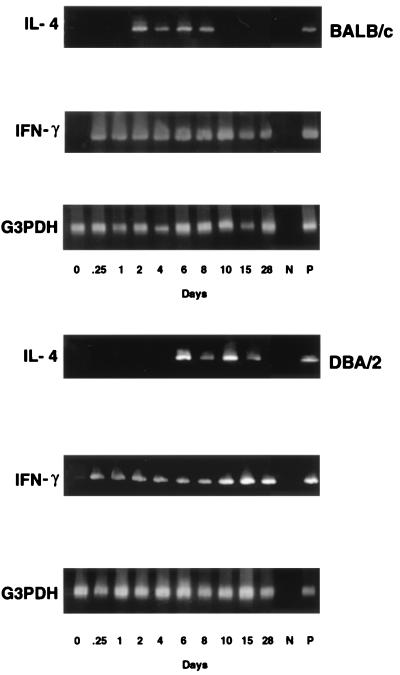
Effect of infection on IL-4 and IFN-γ mRNA gene expression.
The effect of colonization on mRNA expression of IL-4 and IFN-γ in CLN cells was examined by RT-PCR. As shown in Fig. 4, IL-4 gene expression was detected on day 2 in BALB/c mice, whereas it was not expressed until day 6 in DBA/2 mice. While IL-4 gene expression disappeared by day 10 in BALB/c mice, it continued in DBA/2 mice at day 15. In contrast, IFN-γ mRNA gene expression was first detected at 6 h after infection and then gradually declined in BALB/c mice, whereas it continued strongly in DBA/2 mice over the 28 days.
FIG. 4.
IL-4 and IFN-γ mRNA gene expression in CLN cells. Total RNAs were extracted from CLN cells of mice infected with C. albicans and analyzed by RT-PCR using cytokine-specific primers. Equivalent loading of each sample was determined by the G3DPH message.
IL-4, IL-12, and IFN-γ production by CLN cells stimulated with C. albicans antigen.
To determine the pattern and the kinetics of cytokine production following infection, CLN cells were stimulated with C. albicans antigen for 72 h, after which time the levels of IL-4 and IFN-γ in the culture supernatants were measured. As shown in Fig. 5, significantly higher levels of IL-4 were produced at day 2, with maximum levels occurring at days 4 and 6, in BALB/c mice than in DBA/2 mice at those times (P < 0.01 and (P < 0.05). In contrast, increases in IFN-γ levels were observed in both BALB/c and DBA/2 mice, but with significantly higher levels produced in DBA/2 mice at 6 h and at day 2 after infection than were seen in BALB/c mice at those times (P < 0.05 and (P < 0.01). By days 4 and 6, IFN-γ production was at its highest level in BALB/c mice compared to DBA/2 mice, where IFN-γ production was at background levels by day 6 (P < 0.01). While the production of IFN-γ declined, except for a small increase at day 15, in BALB/c mice, a marked increase in production was detected in DBA/2 mice at days 8 (P < 0.05) and 10 (P < 0.01). By day 28, the levels of IFN-γ returned to background in both mouse strains.
FIG. 5.
IL-4, IL-12, and IFN-γ production by CLN cells stimulated in vitro. CLN cells from infected mice were stimulated with C. albicans antigen for 3 days, after which time the culture supernatants were assayed for cytokines by ELISA. Time zero represents uninfected mice. The results shown are means ± SEM for three to five mice. ∗, P < 0.05; ∗∗, P < 0.01 (values from BALB/c versus DBA/2 mice).
To determine whether the different levels of IL-4 and IFN-γ production are related to IL-12 production, CLN cells were isolated at various times from BALB/c and DBA/2 mice that were infected and then stimulated with C. albicans antigen for 3 days, after which IL-12 was measured in the culture supernatant. As shown in Fig. 5, significantly higher production of IL-12 was detected as early as 2 days after infection in DBA/2 mice (P < 0.05). In BALB/c mice, an increase in IL-12 production was detected at day 6 and day 8 (P < 0.05). Following a further increase in DBA/2 mice, IL-12 was maintained at similar levels for 28 days in both mice strains.
Effect of multiple injections of anti-IL-4 MAb on susceptibility to Candida infection in BALB/c mice.
To determine whether the higher production of IL-4 in BALB/c mice was associated with rapid clearance of the yeasts, the effect of anti-IL-4 administration was assessed. Figure 6 demonstrates that BALB/c mice infected with the yeasts followed by administration of 30 μg of anti-IL-4 on days 1, 3, and 5 after oral infection had a higher carriage rate, with a delayed clearance of the yeasts, compared with untreated controls. However, there was no detectable difference in the amounts of IFN-γ in CLN cell culture supernatants between anti-IL-4 MAb-treated and control C. albicans-infected mice (data not shown).
FIG. 6.
Effect of treatment with an anti-IL-4 MAb on resistance to acute infection with C. albicans. BALB/c mice were injected i.p. with 30 μg of rat anti-IL-4 or with purified rat IgG1 matched isotype on days 1, 3, and 5 after challenge with yeast cells. On various days, the number of yeasts in the oral cavity was determined and the results were expressed as mean CFU ± SEM for three to five mice. ∗, P < 0.05.
Anti-IL-4 MAb-treated C. albicans-infected mice had no detectable IL-4 in CLN cell culture supernatants in the first 8 days; thereafter, small amounts of IL-4 could be detected (data not shown).
DISCUSSION
The results of this study demonstrate that host resistance to C. albicans infection in the oral mucosa in a murine model is linked to a particular pattern of cytokine response and an accumulation of γ/δ T cells in the regional lymph nodes. The differences between the colonization patterns of C. albicans in “infection-resistant” BALB/c mice and “infection-prone” DBA/2 mice following infection correlated with both T-cell proliferation and the secretion pattern of the cytokines IL-4, IL-12, and IFN-γ. Colonization patterns for both blastospore and hyphal forms of C. albicans were cyclical, with higher levels of colonization in DBA/2 mice. The more “infection-resistant” BALB/c strain showed a single peak, with lower levels of colonization and more rapid clearance of C. albicans from the oral cavity. There was a selective expansion of γ/δ T cells in the regional lymph node, which correlated in time with the clearance of infection in both mouse strains. Sustained antigen-specific T-cell proliferation was produced only in the infection-resistant BALB/c mouse strain. Higher levels of serum IgG and salivary IgA antibodies followed resolution of infection in BALB/c mice, but levels of these antibodies were lower in DBA/2 mice. In DBA/2 mice, a cyclic colonization pattern with high numbers of fungi, and relatively delayed clearance of infection, correlated with high levels of IFN-γ and IL-12 soon after infection and a delayed and blunted IL-4 response. In contrast, the infection-resistant BALB/c strain showed a lower and more transient colonization with C. albicans, which correlated with early production of both IL-4 and IFN-γ. Neutralization of IL-4 in these mice with an anti-IL-4 MAb (11B11) directly demonstrated that IL-4 contributed to protection. Collectively, these results indicate that the induction of a balanced Th1 and Th2 helper cell response characterized by the production of both IFN-γ and IL-4, and the proliferation of γ/δ T cells, contributes to host resistance to C. albicans infection in oral candidiasis.
The mechanisms of host protection against C. albicans infection have been extensively studied in murine models of candidiasis in terms of the impact of T-cell cytokines operating through various effector mechanisms of immunity (10, 11, 29, 31). In invasive candidiasis, neutrophils and macrophages are involved in host defense (2, 3, 22). A link between resistance and susceptibility, and T-cell cytokine profiles, has been demonstrated in these models in terms of mortality or survival (reviewed in reference 2). For instance, IFN-γ is rapidly produced following infection in both resistant and susceptible mice (27, 41). Neutralizing IFN-γ increased the susceptibility of resistant mice to infection (37), an outcome also achieved by overproduction of IFN-γ mediated by IL-12 (27). In a study of IFN-γ-deficient mice, IFN-γ-induced activation of macrophages was essential for survival (24). Yet other studies have shown that IFN-γ is not essential for host defense against systemic candidiasis (34). It is important in such studies to distinguish among mechanisms that limit mucosal colonization, those that prevent systemic invasion, and those essential for survival. Studies involving manipulation of single components of the host response, while valuable, must be interpreted with caution. The present study examined mechanisms of host resistance and susceptibility in a natural model of self-limited oral mucosal candidiasis. Different patterns of colonization and IFN-γ and IL-4 production were compared in an “infection-resistant” strain (BALB/c) and an “infection-prone” strain (DBA/2). While IFN-γ transcripts were detected early (at 6 h) in both BALB/c and DBA/2 mice following initial infection with C. albicans, the production of IFN-γ did not on its own prevent more-protracted colonization in DBA/2 mice. Whether deficiency of the fifth component of complement in DBA/2 contributes to the protracted colonization in oral candidiasis is unclear. However, several studies with congenic mice, including those bred from different genetic backgrounds of the DBA/2 strain, have reported that C5 deficiency is not an essential factor contributing to the pathogenesis of invasive candidiasis (1, 2, 28).
The present study showed that high levels of IL-12 and IFN-γ, together with a delayed message expression and lower production of IL-4, correlated with higher levels of colonization and delayed clearance of C. albicans in DBA/2 mice. This is consistent with the observation which showed that C. albicans infection of the gastric mucosa in susceptible DBA/2 mice correlates with decreased expression of IL-4 in Peyer's patches (10). By contrast, the lower levels of IL-12 and IFN-γ, together with earlier and higher production of IL-4, correlated with a lower colonization and more-rapid clearance of C. albicans in BALB/c mice, suggesting that the degree, the kinetics, and the mix of cytokines may be critical factors in determining the level of protection after challenge. Both Th1 and Th2 cytokines, albeit in different amounts with different kinetics of production, were present in DBA/2 and BALB/c mice recovering from oral candidiasis, as was seen in gastric candidiasis (10). Thus, resistance to primary infection with C. albicans in the oral mucosa is associated with Th1 and Th2 responses. Support for a role for IL-4 in clearance was directly demonstrated by the increased carriage rate and delayed clearance of C. albicans from the oral mucosae of BALB/c mice following treatment with anti-IL-4 antibody.
The mechanism of IL-4-enhanced resistance to C. albicans infection in the oral mucosa is unclear. In primary systemic candidiasis, IL-4 may limit C. albicans infection through promoting effector mediators of immunity, including the differentiation of effector Th1 cells (29, 30). In particular, IL-4 promotes the development of a protective Th1 response in systemic and gastric candidiasis (10, 30). Other studies have shown that mice deficient in IL-4 were more susceptible to acute systemic infection than normal controls (30, 45), though no difference in susceptibility to orogastric candidiasis after challenge was noted (45). These paradoxical findings may be explained by different experimental models, different mouse strains, and different routes of challenge and doses of C. albicans to induce systemic or mucosal candidiasis. For example, intragastric challenge with C. albicans induced a more severe gastric candidiasis in BALB/c mice than in DBA/2 mice, whereas the reverse was true for systemic candidiasis (10). In the present model, acute oral candidiasis was induced by topical application of C. albicans, as opposed to an intragastric bolus of C. albicans to induce gastric candidiasis (30, 45). Furthermore, topical application of C. albicans to the oral mucosa restricts the supply of antigen to the gut-associated lymphoid tissue (GALT) compartment, limiting modification of the course of infection via activation of the common mucosal immune system (14). Little or no fungus was recovered from fecal pellets.
In the present study both BALB/c and DBA/2 mice cleared infection before the onset of antibody production, indicating that production of serum IgG and secretory IgA antibodies did not play a significant role in mucosal clearance, in agreement with findings in murine gastric candidiasis (10). In the latter model, enhanced production of secretory IgA antibody did not accelerate the resolution of infection (10). Despite an increase in cell counts in the CLN after infection, the relative proportions of CD4+, CD8+, and α/β T cells and B cells remained constant, suggesting cell recruitment rather than antigen-induced proliferation of cells. However, there was a selective expansion of γ/δ T cells, which correlated with the elimination of C. albicans. While the numbers were low, the increase was significant, considering the paucity of γ/δ T cells in peripheral lymphoid tissues (21). Increased numbers of γ/δ T cells have been reported after bacterial, viral, and parasitic infections, suggesting a role for γ/δ T cells in the first line of host defense (25, 26). It has previously been reported that γ/δ T cells enhance resistance to mucosal candidiasis (16, 23), and increased numbers of γ/δ T cells in the oral mucosa correlated with the pattern of colonization in BALB/c and DBA/2 mice infected with C. albicans (12). It is not clear, however, whether γ/δ T cells are a source of IL-4. Although it has been reported that γ/δ T cell clones and cell lines are capable of secreting IL-4 (4, 46, 47), we could not demonstrate significant amounts of IL-4 in γ/δ T cells in these mice (data not shown). It has recently been reported that γ/δ T cells can enhance nitric oxide (NO) production by macrophages in mice injected i.p. with C. albicans (23), further linking γ/δ T cells with potential mechanisms of resistance. Furthermore, NO can enhance IL-4 expression in T cells (15), further influencing the balance of cytokine secretion. Thus, mucosal containment of C. albicans may depend on the interaction between macrophages and T cells through the release of NO and IL-4, mediators which have been reported to enhance the killing of yeast cells by both neutrophils (5) and macrophages (20, 35) bearing IL-4 surface receptors. γ/δ T cells can secrete IFN-γ and IL-4, which both activate macrophages to act directly on C. albicans (35, 44). Preliminary studies with the current model show high levels of NO in saliva to support this hypothesis (unpublished results).
In summary, analysis of regional lymph node cell populations provides data consistent with current ideas about cytokine function in experimental models of infection. The findings presented in this study of a model of oral candidiasis indicate that the production of IL-4 and IFN-γ is critical to the resolution of mucosal infection in the intact animal. The early appearance of IL-4 production suggests the importance of this cytokine in enhancing immunity against C. albicans infection in the oral mucosa, a correlation directly supported by data obtained following treatment with anti-IL-4 antibody. The concurrent presence of high levels of IL-12 and IFN-γ supports the concept of a balanced Th1 and Th2 response as being an efficient host defense mechanism in clearing oral mucosal infection.
REFERENCES
- 1.Ashman R B, Bolitho E M, Papadimitriou J M. Patterns of resistance to Candida albicans in inbred mouse strains. Immunol Cell Biol. 1993;71:221–225. doi: 10.1038/icb.1993.25. [DOI] [PubMed] [Google Scholar]
- 2.Ashman R B. Genetic determination of susceptibility and resistance in the pathogenesis of Candida albicans infection. FEMS Immunol Med Microbiol. 1997;19:183–189. doi: 10.1111/j.1574-695X.1997.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 3.Ashman R B. Candida albicans: pathogenesis, immunity and host defence. Res Immunol. 1998;149:281–288. doi: 10.1016/s0923-2494(98)80752-9. [DOI] [PubMed] [Google Scholar]
- 4.Azuara V, Levraud J P, Lembezat M P, Pereira P. A novel subset of adult γδ thymocytes that secretes a distinct pattern of cytokines and expresses a very restricted T cell receptor repertoire. Eur J Immunol. 1997;27:544–553. doi: 10.1002/eji.1830270228. [DOI] [PubMed] [Google Scholar]
- 5.Bobert L A, Waters T A, Pugliese-Sivo C C, Sullivan L M, Narula S K, Grace M J. IL-4 induces neutrophilic maturation of HL-60 cells and activation of human peripheral blood neutrophils. Exp Immunol. 1995;99:129–136. doi: 10.1111/j.1365-2249.1995.tb03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodey G P. Infection in cancer patients. Am J Med. 1986;81A:11–25. doi: 10.1016/0002-9343(86)90510-3. [DOI] [PubMed] [Google Scholar]
- 7.Cantorna M T, Balish E. Role of CD+ lymphocytes in resistance to mucosal candidiasis. Infect Immunol. 1991;59:2447–2455. doi: 10.1128/iai.59.7.2447-2455.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrigan E M, Clancy R L, Dunkley M L, Eyers F M, Beagley K W. Cellular immunity in recurrent vulvovaginal candidiasis. Clin Exp Immunol. 1998;111:574–578. doi: 10.1046/j.1365-2249.1998.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cenci E, Romani L, Mencacci A, Spaccapolo R, Schiaffella E, Puccetti P, Bistoni F. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur J Immunol. 1993;23:1034–1038. doi: 10.1002/eji.1830230508. [DOI] [PubMed] [Google Scholar]
- 10.Cenci E, Mencacci A, Spaccapelo R, Tonnetti L, Mosci P, Enssle K H, Puccetti P, Romani L, Bistoni F. T helper cell type 1 (Th-1) and Th-2-like responses are present in mice with gastric candidiasis, but protective immunity is associated with Th1 development. J Infect Dis. 1995;171:1279–1288. doi: 10.1093/infdis/171.5.1279. [DOI] [PubMed] [Google Scholar]
- 11.Cenci E, Mencacci A, Del Sero G, d'Ostiani C F, Mosci P, Bacci A, Montagnali C, Kopf M, Romani L. Interferon-γ is required for IL-12 responsiveness in mice with Candida albicans infection. J Immunol. 1998;161:3543–3550. [PubMed] [Google Scholar]
- 12.Chakir J, Cote L, Coulombe C, Deslauriers N. Differential pattern of infection and immune response during experimental oral candidiasis in BALB/c and DBA/2 (H-2d) mice. Oral Microbiol Immunol. 1994;9:88–94. doi: 10.1111/j.1399-302x.1994.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 13.Challacombe S J. Immunological aspects of oral candidiasis. Oral Surg Oral Med Oral Pathol. 1994;78:202–210. doi: 10.1016/0030-4220(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 14.Challacombe S J, Rahman D. Oral immunization against mucosal candidiasis in a mouse model. In: Mestecky J, editor. Advances in mucosal immunology. New York, N.Y: Plenum Press; 1995. [PubMed] [Google Scholar]
- 15.Chang R, Linfeng M H, Liu W H, Lai M Z. Nitric oxide increased IL-4 expression in T lymphocytes. Immunology. 1997;90:364–369. doi: 10.1111/j.1365-2567.1997.00364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng S H, Penninger J, Ferrick D, Molina T, Wallace V, Mak T W. Biology of murine γδ T cells. Crit Rev Immunol. 1991;11:145–166. [PubMed] [Google Scholar]
- 17.Cho Y S, Choi H Y. Opportunistic fungal infection among cancer patients. A ten-year autopsy study. Am J Clin Pathol. 1979;72:617–625. doi: 10.1093/ajcp/72.4.617. [DOI] [PubMed] [Google Scholar]
- 18.Deslauriers N, Cote L, Montplaisir S, deRepentigny L. Oral carriage of Candida albicans in murine AIDS. Infect Immun. 1997;65:661–667. doi: 10.1128/iai.65.2.661-667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epstein J, Truelove E, Izutau K. Oral candidiasis: pathogenesis and host defense. Rev Infect Dis. 1984;6:96–106. doi: 10.1093/clinids/6.1.96. [DOI] [PubMed] [Google Scholar]
- 20.Feldman G M, Finbloom D S. Induction and regulation of IL-4 receptor expression on murine macrophage cell lines and bone marrow-derived macrophages by IFN-γ. J Immunol. 1990;145:854–859. [PubMed] [Google Scholar]
- 21.Gerber D J, Azuara V, Levraud J P, Huang S Y, Lembezat M P, Pereira P. IL-4-producing γδ T cells that express a very restricted TCR repertoire are preferentially localized in liver and spleen. J Immunol. 1999;163:3076–3082. [PubMed] [Google Scholar]
- 22.Hurtrel B, Lagrange P H, Michel J C. Systemic candidiasis in mice. II. Main role of polymorphonuclear leukocytes in resistance to infection. Ann Immunol. 1980;131:105–113. [PubMed] [Google Scholar]
- 23.Jones-Carson J, Vazquez-Torres A, van der Heyde H C, Warner T, Wagner R, Balish E. γδ T cell-induced nitric oxide production enhances resistance to mucosal candidiasis. Nat Med. 1995;1:552–557. doi: 10.1038/nm0695-552. [DOI] [PubMed] [Google Scholar]
- 24.Kaposzta R, Tree P, Marodi L, Gordon S. Characteristics of invasive candidiasis in gamma interferon- and interleukin-4 deficient mice: role of macrophages in host defense against Candida albicans. Infect Immun. 1998;66:1708–1717. doi: 10.1128/iai.66.4.1708-1717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufmann S. γδ and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci USA. 1996;93:2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein J. Whence the intestinal intraepithelial lymphocyte? J Exp Med. 1996;184:1203–1206. doi: 10.1084/jem.184.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavigne L M, Schopf L R, Chung C L, Maylor R, Sypek J P. The role of recombinant murine IL-12 and IFN-γ in the pathogenesis of a murine systemic Candida albicans infection. J Immunol. 1998;160:284–292. [PubMed] [Google Scholar]
- 28.Lyon F L, Hector R F, Domer J E. Innate and acquired immune responses against Candida albicans in congenic B10.D2 mice with deficiency of the C5 complement component. J Med Vet Mycol. 1996;24:359–367. doi: 10.1080/02681218680000551. [DOI] [PubMed] [Google Scholar]
- 29.Mencacci A, Spaccapelo R, Del Sero G, Enssle K H, Cassone A, Bistoni F, Romani L. CD4+ T helper cell responses in mice with low-level Candida albicans infection. Infect Immun. 1996;64:4907–4914. doi: 10.1128/iai.64.12.4907-4914.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mencacci A, Del Sero G, Cenci E, d'Ostiani C F, Bacci A, Montagnoli C, Kopf M, Romani L. Endogenous interleukin 4 is required for development of protective CD4+ T helper type 1 cell responses to Candida albicans. J Exp Med. 1998;187:307–317. doi: 10.1084/jem.187.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mencacci A, Cenci E, Del Sero G, d'Ostiani C F, Mosci P, Trinchieri G, Adorini L, Romani L. IL-10 is required for development of protective Th1 responses in IL-12-deficient mice upon Candida albicans infection. J Immunol. 1998;161:6228–6237. [PubMed] [Google Scholar]
- 32.Odds F C. Candida and candidosis. Baltimore, Md: University Park Press; 1988. pp. 104–110. [Google Scholar]
- 33.Puccetti P, Mencacci A, Cenci E, Spaccapolo R, Mosci P, Enssle K H, Romani L, Bistoni F. Cure of murine candidiasis by recombinant soluble IL-4 receptor. J Infect Dis. 1994;169:1325–1331. doi: 10.1093/infdis/169.6.1325. [DOI] [PubMed] [Google Scholar]
- 34.Qian Q, Cutler J E. Gamma interferon is not essential in host defense against disseminated candidiasis in mice. Infect Immun. 1997;65:1748–1753. doi: 10.1128/iai.65.5.1748-1753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Redmond H, Schuchter L, Shou J, Hofmann K L, Leon P, Marodi L, Johnston R, Daly J. Interleukin 4 (IL-4) enhances macrophage microbicidal function. J Leukoc Biol. 1990;48:24–31. [Google Scholar]
- 36.Romani L, Mocci S, Bietta C, Lanfaloni L, Puccetti P, Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991;59:4647–4654. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romani L, Cenci E, Mencacci A, Spaccapelo R, Grohmann U, Puccetti P, Bistoni F. Gamma interferon modifies CD4+ subset expression in murine candidiasis. Infect Immun. 1992;60:4950–4952. doi: 10.1128/iai.60.11.4950-4952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romani L, Mencacci A, Grohmann U, Mocci S, Puccetti P, Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romani L, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Puccetti P, Bistoni F. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J Immunol. 1993;150:925–931. [PubMed] [Google Scholar]
- 40.Romani L, Puccetti P, Mencacci A, Cenci E, Spaccapelo R, Tonnetti L, Grohmann U, Bistoni F. Neutralization of IL-10 up-regulates nitric oxide production and protects susceptible mice from challenge with Candida albicans. J Immunol. 1994;152:3514–3521. [PubMed] [Google Scholar]
- 41.Romani L, Mencacci A, Tonnetti L, Spaccapelo R, Cenci E, Puccetti P, Wolf S F, Bistoni F. Interleukin-12 is both required and prognostic in vivo for T helper type-1 differentiation in murine candidiasis. J Immunol. 1994;152:5167–5175. [PubMed] [Google Scholar]
- 42.Romani L, Bistoni F, Mencacci A, Conci E, Spaccapolo R, Puccetti P. IL-12 in Candida albicans infections. Res Immunol. 1996;146:532–538. doi: 10.1016/0923-2494(96)83028-8. [DOI] [PubMed] [Google Scholar]
- 43.Samaranayake L P. Host factors and oral candidiasis. London, United Kingdom: Wright; 1990. pp. 66–103. [Google Scholar]
- 44.Vazquez-Torres A, Jones-Carson J, Wagner R, Warner T, Balish E. Candidal activity of macrophages from immunocompetent and congenitally immunodeficient mice. J Infect Dis. 1994;170:180–188. doi: 10.1093/infdis/170.1.180. [DOI] [PubMed] [Google Scholar]
- 45.Vazquez-Torres A, Jones-Carson J, Wagner R, Warner T, Balish E. Early resistance of IL-10 knockout mice to acute systemic candidiasis. Infect Immun. 1999;67:670–674. doi: 10.1128/iai.67.2.670-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vicari A P, Mocci S, Openshaw P, O'Garra A, Zlotnik A. Mouse γδ TCR+ NK1.1+ thymocytes specifically produce IL-4, are major histocompatibility complex class I independent, and are developmentally related to αβ TCR+ NK1.1+ thymocytes. Eur J Immunol. 1996;26:1424–1433. doi: 10.1002/eji.1830260704. [DOI] [PubMed] [Google Scholar]
- 47.Zlotnik A, Godfrey D I, Fischer M, Suda T. Cytokine production by mature and immature CD4− CD8− T cells: αβ-T cell receptor+ CD− CD8− T cells produce IL-4. J Immunol. 1992;149:1211–1218. [PubMed] [Google Scholar]