Abstract
The actions of estrogens and related estrogenic molecules are complex and multifaceted in both sexes. A wide array of natural, synthetic, and therapeutic molecules target pathways that produce and respond to estrogens. Multiple receptors promulgate these responses, including the classical estrogen receptors of the nuclear hormone receptor family (estrogen receptors α and β), which function largely as ligand-activated transcription factors, and the 7-transmembrane G protein–coupled estrogen receptor, GPER, which activates a diverse array of signaling pathways. The pharmacology and functional roles of GPER in physiology and disease reveal important roles in responses to both natural and synthetic estrogenic compounds in numerous physiological systems. These functions have implications in the treatment of myriad disease states, including cancer, cardiovascular diseases, and metabolic disorders. This review focuses on the complex pharmacology of GPER and summarizes major physiological functions of GPER and the therapeutic implications and ongoing applications of GPER-targeted compounds.
Keywords: cancer, cardiovascular, endocrine, estrogen, immunity, metabolism
INTRODUCTION
Estrogens elicit a multitude of effects throughout the body in virtually every organ, tissue, and physiological system. Although predominantly recognized as the female sex hormone, regulating sexual development at puberty, the menstrual cycle and pregnancy during the reproductive years, and, through the cessation of its synthesis, menopause, estrogen also has important and diverse roles in cardiovascular, metabolic, and neurologic functions as well as in many other systems. As a result of its critical functions in reproductive tissues (predominantly the uterus but also the breast), estrogen and its derivatives are employed in contraceptives, hormone replacement therapies for menopause, and the treatment of hormone-responsive (i.e., ER-positive) breast cancer. The diverse roles of estrogen are perhaps best exemplified by symptomatic and physiological changes experienced by women following menopause that include loss of periods, vaginal dryness, urinary incontinence, loss of breast fullness, hot flashes/chills/night sweats, sleep difficulties, mood changes, weight gain/slowed metabolism, thinning hair, and dry skin (1). However, additional roles for estrogen are revealed by the increased risk following menopause, and the decreased risk following hormone replacement, of a multitude of diseases, including cardiovascular diseases (e.g., coronary artery disease, hypertension, stroke), osteoporosis, obesity and dyslipidemia, diabetes, and neurological changes (e.g., depression and dementia) (2, 3). Estrogen also plays a critical role in about 80% of breast cancers, in which tumor growth is stimulated by and often dependent upon estrogen. This estrogen dependence has led to diverse therapeutic approaches to treat breast cancer that include inhibiting the production of estrogen via the enzyme aromatase and targeting one of its receptors (ERα) through either inhibition or degradation (4, 5).
The pharmacology surrounding estrogen receptors is diverse and complex (6, 7). In addition to the multiple forms of estrogen produced in the human body [predominantly estrone (E1), 17β-estradiol (E2), estriol (E3), and estetrol (E4) (8)], natural and manmade xenoestrogens elicit estrogenic activity (9, 10). The definition of stimulating effects in the uterus (imbibition and proliferation as standardized end points), as E2 does, is practical but neglects broader effects, with little consideration of actions in other tissues. Natural plant- or fungus-derived and manmade xenoestrogens, also referred to as environmental estrogens or endocrine-disrupting compounds, are ubiquitous in the environment and diet and have impacts on biology and human health (9, 11). Drugs targeting estrogen levels/synthesis and receptor activity play a role in the treatment of many conditions and diseases (12, 13), but particularly cancer (5, 14). Thus, understanding the mechanisms of action with respect to the multiple estrogen receptors is of critical importance. In this review, we describe the pharmacology and therapeutic implications of these diverse compounds with particular reference to their actions via the 7-transmembrane G protein–coupled estrogen receptor (GPER).
ESTROGEN RECEPTORS: ERα/β AND GPER
Two distinct receptor families mediate estrogen’s diverse transcriptional (i.e., genomic) and rapid signaling (i.e., nongenomic) activities (6, 7). Although early experimentation identified estrogen-induced rapid signaling [e.g., cyclic adenosine monophosphate (cAMP) production and Ca2+ uptake], the transcriptional activities of ER soon dominated the field. Continued reports of the rapid actions of estrogen and other steroids led to the hypothesis of membrane-associated forms of ER in the 1990s (15). In 1996, a receptor homologous to ER was cloned and functionally shown to be a second ER, leading to the current nomenclature of ERα and ERβ (16, 17), while concurrently an orphan 7-transmembrane-spanning G protein–coupled receptor (GPCR) was cloned and termed GPR30 (18). In 2000, GPR30 was shown to mediate rapid activation of extracellular signal-regulated kinase (ERK) in response to estrogen, providing the first evidence for its actions as a functional estrogen receptor (19). This discovery was followed by the demonstration of specific estrogen binding, employing both tritiated (20) and fluorescent derivatives (21) in 2005, leading to the official designation of GPR30 as GPER by the International Union of Basic and Clinical Pharmacology in 2007 (22). Demonstration of its activity as a classical GPCR was provided by the effect of guanosine-5′-triphosphate (GTP) (specifically GTPγS, via activation and dissociation of heterotrimeric G proteins) on reducing ligand binding through conversion of the receptor to a lower affinity state as well as by increased GTPγS binding in the presence of estrogen (20).
As a GPCR, GPER’s primary site of subcellular localization, the endoplasmic reticulum and Golgi apparatus (21), is unusual although not unique (23). In some cells, detectable GPER is found at the plasma membrane, although even in such cells, most is present in intracellular membranes at steady state (23). As estrogens are cell permeable (24, 25) and activate ERs intracellularly, and as most ERα is localized within the nucleus at steady state (26), studies with permeable and non-permeable estrogen derivatives suggest that GPER signals predominantly from an intracellular location(s) (27). Receptor trafficking studies suggest that GPER expressed at the cell surface is constitutively internalized in a ligand-independent manner, consistent with the majority of the receptor being observed intracellularly at steady state (23).
Signaling initiated by GPER occurs through a multitude of pathways. Coupling occurs through multiple heterotrimeric G proteins, primarily Gαs (28) and Gαi (21), as well as Gβγ-mediated signaling (19). In addition, much if not all signaling initiated by GPER activation involves transactivation of the epidermal growth factor receptor (EGFR) (19), a pathway described for many GPCRs (29). This pathway involves Gβγ-mediated activation of Src, leading to α5β1 recruitment and matrix metalloproteinase (MMP)-mediated release of heparan-binding EGF-like growth factor, which then transactivates EGFR, with ensuing activation of multiple additional pathways such as ERK and PI3K/Akt (29). Whereas ERK activation leads to proliferative signaling and Elk-1-mediated transcriptional regulation (30), Akt activation leads to phosphorylation of both eNOS (31), leading to NO production, and Foxo3 (32), leading to prosurvival signals. GPER activation also leads to adenylyl cyclase activation, producing cAMP, which in turn activates protein kinase A (PKA), and transcriptional events via cAMP response element-binding protein (CREB) (33, 34). Thus, although signaling via GPER is widely considered to mediate rapid nongenomic signaling, the downstream events of these early signaling events include extensive genomic regulation, much as ER-mediated signaling involves rapid events in addition to its classical transcriptional regulation.
GPER LIGANDS AND PHARMACOLOGY
Promiscuous ligand binding with many different structural classes and diverse pharmacology are characteristics of both classical (nuclear) ERα/β and GPER. The most potent estrogenic hormone, E2, is a lipophilic phenol, and compounds featuring this functionality are frequently cross-reactive ligands. The identification and characterization of pharmacologically active GPER ligands were extensively reviewed in 2015 (7), and recent developments and discoveries with the potential of impacting human health and clinical applications are the focus of this review. The scope of compounds with recognized biological effects through GPER continues to grow and includes US Food and Drug Administration (FDA)-approved drugs and chemicals ingested in food, nutritional supplements, and other environmental exposures. It is important to recognize that ligands with widely disparate GPER-binding affinities can regulate diverse nongenomic signaling pathways that ultimately impact genomic outcomes. This scenario presents challenges for interpreting gene expression and toxicological effects that may be observed at doses that are significantly lower than measured affinities or activities would predict, in alignment with observations that endocrine disruptors frequently exhibit nonmonotonic dose-response relationships (35).
GPER Pharmacology with Natural Steroids and Derivatives
Competitive binding assays with radiolabeled or fluorescent probes revealed that E2 has the highest GPER binding affinity (3–6 nM) and greater than 1,000-fold selectivity compared to other steroid hormones such as progesterone, testosterone, and cortisol (20). Whether and how aldosterone may act in concert with or through GPER remain complex and controversial questions (36, 37), particularly given the demonstrated lack of binding (38). The physiologically relevant estrogen E1 has much lower affinity for GPER (>10 μM) (20). The 16α-hydroxy analog E3 (20) and the catechol metabolite 2-hydroxy-17β-estradiol (39) have relatively low GPER binding affinities (>1 μM and 0.1–1 μM) but function as weak antagonists (Figure 1). In contrast, the more lipophilic metabolite 2-methoxyestradiol exhibits relatively high affinity (10 nM) and functions as an agonist (40–44). The oxysterol 27-hydroxycholesterol has recently been demonstrated to bind GPER (with an affinity of approximately 1 μM) and function as an agonist in ER-negative breast cancer cells (45). The 17β-d-glucuronide metabolite of E2 has low GPER binding affinity (>50 μM) and reported agonist activity (46), but interpretations of results from these types of conjugates are complicated by the susceptibility to chemical or enzymatic hydrolysis releasing E2. Similar cautions are appropriate using dehydroepiandrosterone (DHEA) in cells and particularly in vivo studies where biosynthesis to produce E2 can occur (47). The synthetic estrogen derivative fulvestrant [a selective estrogen receptor downregulator/degrader (SERD)] functions as a pure ERα antagonist but also induces ERα degradation due to conformational changes induced by the extended 7α appendage in the ligand-bound structure. This drug is FDA approved for advanced ER-positive breast cancer but also acts as a GPER agonist (19), illustrating the need for including GPER when profiling receptor selectivity to develop more selective drugs with fewer potential off-target effects. ERα and GPER binding, functional responses, and ligand localization of E2 conjugates with fluorescent dyes or chelates have been employed to quantitate, characterize, and visualize ligand binding and function at the subcellular/cellular (21, 27) and organismal levels (48), respectively. Proteolysis-targeting chimeras (PROTACs), based on small molecules linked to an E3 ubiquitin ligase ligand that degrades the target, are under evaluation as a strategy for the development of novel cancer therapeutics (49), with nuclear receptors offering an important target (50). Estrogen chimerae (E2-PROTACs), first described in 2005 (51), have recently been reported to bind both GPER and ER with relatively high affinity (~ 30 nM and 10–20 nM, respectively), resulting in the degradation of ERα/β as well as GPER in MCF7 and SKBR3 cell lines without affecting progesterone receptor levels (52). PROTACs provide an alternative approach for targeting plasma membrane and intracellular estrogen receptors that could enable receptor-selective degradation based on selective receptor ligands.
Figure 1.
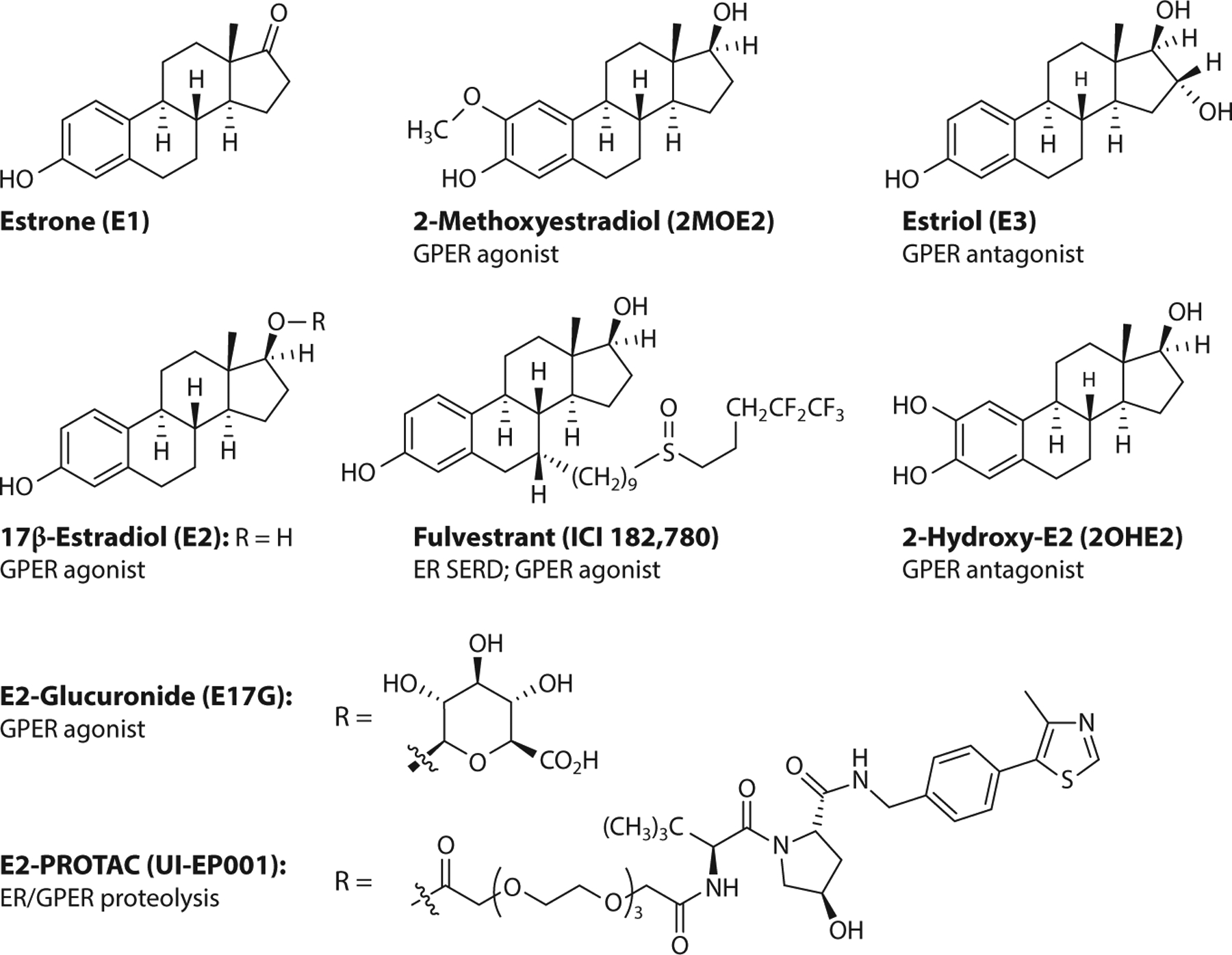
Steroidal ligands of GPER. Abbreviations: ER, estrogen receptor; GPER, G protein–coupled estrogen receptor; PROTAC, proteolysis-targeting chimera; SERD, selective estrogen receptor downregulator/degrader.
Xenoestrogens as GPER Ligands
There is growing recognition of the role of GPER in endocrine disruption through exposure to natural and synthetic xenoestrogens originating from dietary intake, health and nutritional supplements, and environmental exposures to agrochemicals and industrial compounds, including polymers and their degradation products. The number of recognized xenoestrogens is staggering, and while previous studies have primarily focused on ERα/β, many of these compounds activate GPER, with possible consequences on neurogenic processes (53), and cancers of the breast (54), prostate (55) and digestive system (56). The breadth of possible GPER ligands was made apparent by a study that virtually screened a database of 30,926 natural products and identified 500 compounds, representing diverse structural classes that included flavonoids, isoflavonoids, chalcones, coumestans, stilbenes, lignans, ginsenosides, and tetrahydrofurandiols (57). The presence of a phenol and hydrophobic scaffold is a characteristic feature of many xenoestrogenic compounds.
The isoflavones genistein and daidzein are phytoestrogens that are widely consumed directly from soy products and often taken as medicinal supplements with the intent of easing menopausal symptoms, improving metabolism, reducing cardiovascular disease, or preventing certain hormone-related cancers (Figure 2). These compounds possess a phenol group at the 3-position of the 4H-chromen-4-one core and bind to GPER with high affinity. The crystalline sodium salt dihydrate of genistein, designated AXP107–11, sensitized gemcitabine chemotherapy in pancreatic ductal adenocarcinoma patient-derived xenografts synergistically through activation of GPER and mitogen-activated protein kinase (MAPK) signaling (58). GPER has been implicated in studies demonstrating that genistein attenuates inflammation in a model of Parkinson’s disease, inhibiting microglial activation and protecting dopaminergic neurons (59); protects against oxidative stress in hepatocytes (60); and improves glucose tolerance and white adipose tissue thermogenesis (61). Daidzein is converted to S-(−)-equol by mammalian gut bacteria, and individual metabolic variations result in widely ranging exposures. S-equol targeted GPER to promote glucose-induced insulin secretion from pancreatic β cells and prevented glucagon-like peptide-1 secretion from enteroendocrine L cells (62); activated GPER signaling, with effects on vascular smooth muscle cells (63); inhibited nitric oxide production and reduced expression of inducible NO synthase in lipopolysaccharide-stimulated astrocytes (64); and induced cell proliferation and migration in astrocytes that were attenuated by the GPER antagonist G15 but not by the SERD/GPER agonist fulvestrant (65). Genistein, daidzein and S-(−)-equol increased glial cell migration through activation of GPER signaling, and molecular docking studies suggest that these three compounds may bind to GPER at the same position as E2 (65).
Figure 2.
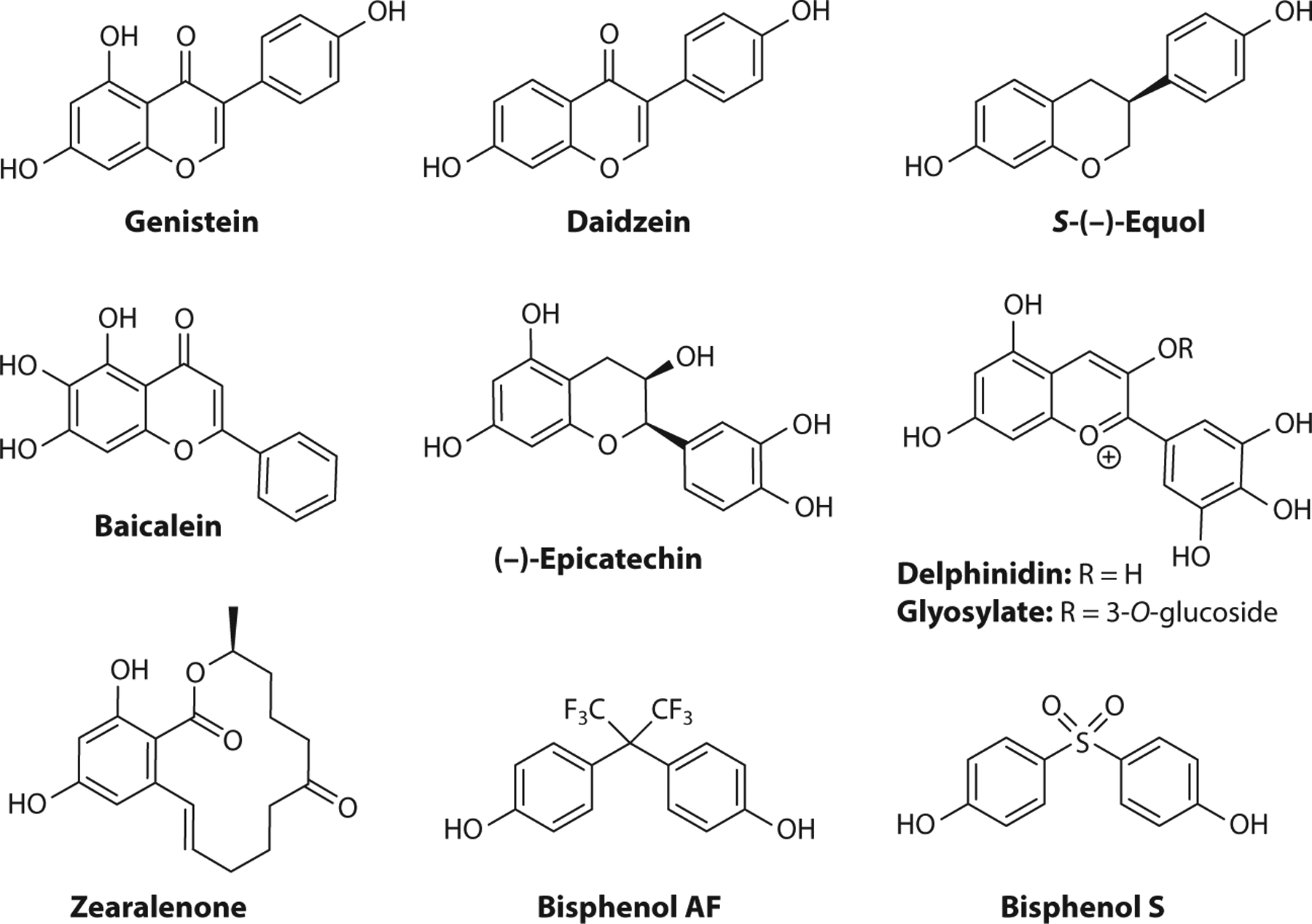
Xenoestrogen ligands of G protein–coupled estrogen receptor (GPER).
The isomeric flavones feature 2-aryl substituents on the 4H-chromen-4-one core, and these compounds are further metabolized to various phytoestrogens. Baicalein occurs in an herb used in traditional Chinese medicine and, with a simple 2-phenyl substituent, functioned as a GPER antagonist to reduce E2-induced migration, adhesion, and invasion in breast cancer cells (66) and suppressed E2-induced cell invasion and matrix metalloproteinase-9 expression and activation (67).
Polyphenolic catechins such as (−)-epicatechin occur in green tea, cacao, and pome fruits and have attracted significant attention regarding their potential health benefits. (−)-Epicatechin activated GPER signaling pathways for vasodilation similarly to G-1 (68) and stimulated mitochondrial biogenesis in mouse skeletal muscle (69). Synthetic propargylic ether derivatives of (−)-epicatechin retained activity in the eNOS/NO pathway and, when immobilized, functioned as an affinity column to pull down GPER from protein extracts of endothelial cells, further validating (−)-epicatechin as a GPER ligand (70).
Anthocyanins are a diverse class of highly colored flavonoids found in fruits and red wine that have been of interest for their nutraceutical value and potential benefits for vascular disease. The aglycone delphinidin and the glycosylate delphinidin 3-glucoside were equipotent in eliciting rapid NO-mediated vasodilator responses in male rats, and this response was mimicked by tissue perfusion with either G-1 or E2 and significantly reduced by treatment with G36, which implicated GPER in this pathway (71).
Zearalenone is a phenolic macrolactone produced by mycotoxins in grains and cereals and is metabolized to the epimeric alcohols α- and β-zearalenol. With widespread occurrence, these compounds are consumed by animals and humans, raising concerns of estrogenic effects on the reproductive system, other toxicities, and potential roles in the development of hormone-dependent cancers. Zearalenone is a GPER agonist, and exposure in pig pituitary cells and glands caused increased expression of GPER messenger RNA, but not ERα/β, along with activation of GPER/PKC/p38 pathways to upregulate the microRNA miR-7, which targets the FOS gene, leading to inhibition of follicle stimulating hormone synthesis and secretion and resulting in reproductive defects (72, 73). In colon cancer cell lines, which are not typically considered hormone sensitive, zearalenone promoted anchorage-independent cell growth and cell cycle progression, which was suppressed by G15, via MAPK and Hippo pathway effector YAP1, providing a mechanism for promoting colon cancer growth (74).
Multiple structural classes of synthetic endocrine-disrupting chemicals are ligands for GPER, and a pharmacological screening approach was developed to distinguish GPER agonist/antagonist activities. This method used live-cell imaging to monitor changes in the morphology of MR5C human fibroblast cells in response to G-1 and G15 to evaluate GPER activity (75). Bisphenols are produced industrially and incorporated into many consumer products on a massive scale worldwide and are one of the most important classes of endocrine-disrupting compounds. These analogs generally exhibit higher relative binding affinities for GPER than ER and initiate extranuclear signaling pathways at low doses that challenge their classical designation as weak estrogens (76, 77). The fluorinated bisphenol analog BPAF had ninefold higher affinity for GPER than the parent compound, determined using a fluorescent competitive binding assay in GPER-expressing SKBR3 cells, and GPER-mediated nongenomic effects were observed at 10-nM concentrations (78). As manufacturers move to BPA-free alternatives with analogs such as the sulfone BPS, the concerns for estrogenic activity remain and warrant further study in the broad context of involved receptors and increased diligence for monitoring and risk assessment.
Synthetic GPER-Targeted Compounds
The discovery that GPER could play a role in the activities of estrogenic compounds (19–21, 79) established the critical need for new pharmacological tools to distinguish between the activities of ERα/β and GPER. The challenges of obtaining atomic-resolution structures for membrane-bound GPCRs, and the absence of such a structure of GPER, have been significant impediments for structure-based approaches seeking to design and optimize GPER-targeted compounds. Combined virtual and biomolecular screening approaches resulted in the discovery of the first, and to date the most widely studied, GPER agonist, G-1 (80) (Figure 3). This strategy employed a ligand-based computational screen of a 10,000-member compound library for structural similarity with E2 to rank compounds for cell-based flow cytometry competitive binding assays, which employed a fluorescent synthetic E2-probe to distinguish compounds exhibiting selective GPER binding with respect to the nuclear receptor subtypes (80). Subsequent application of synthetic medicinal chemistry for structure-activity studies of the tetrahydro-3H-cyclopenta[c]quinoline scaffold resulted in the identification of the first GPER antagonist, G15 (81), and the improved analog, G36 (82). The activity, selectivity, and GPER dependence of G-1 have been demonstrated in a number of ER-negative cell lines, including SKBR3 (breast cancer) (19), Hec50 (endometrial cancer) (83), and MCF10A (normal breast epithelium) (84) cells, employing small interfering RNA knockdown approaches (32, 83, 84) as well as in multiple systems in GPER knockout (KO) mice (85). To date, the activities of these compounds, where examined, are absent in cells and mice lacking GPER (85), with the exception of reported effects on tubulin at high concentrations (3–50 μM) (86, 87). These validated GPER-selective G-series compounds are commercially available as racemic mixtures and have enabled the application of molecular biology approaches and a wide variety of in vitro and in vivo studies for characterizing new ligands and distinguishing GPER from ERα/β in multiple pathways in diverse cell, tissue, and organ types. The (S,R,R)-enantiomer of G-1 [1-((3aS,4R,9bR)-4-(6-bromobenzo[d][1,3]dioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolin-8-yl)ethan-1-one] was obtained by chiral (high-performance liquid) chromatography and has advanced as the first GPER-targeted investigational new drug (IND), LNS8801 (88), to enter human clinical trials (https://clinicaltrials.gov/ct2/show/NCT04130516).
Figure 3.
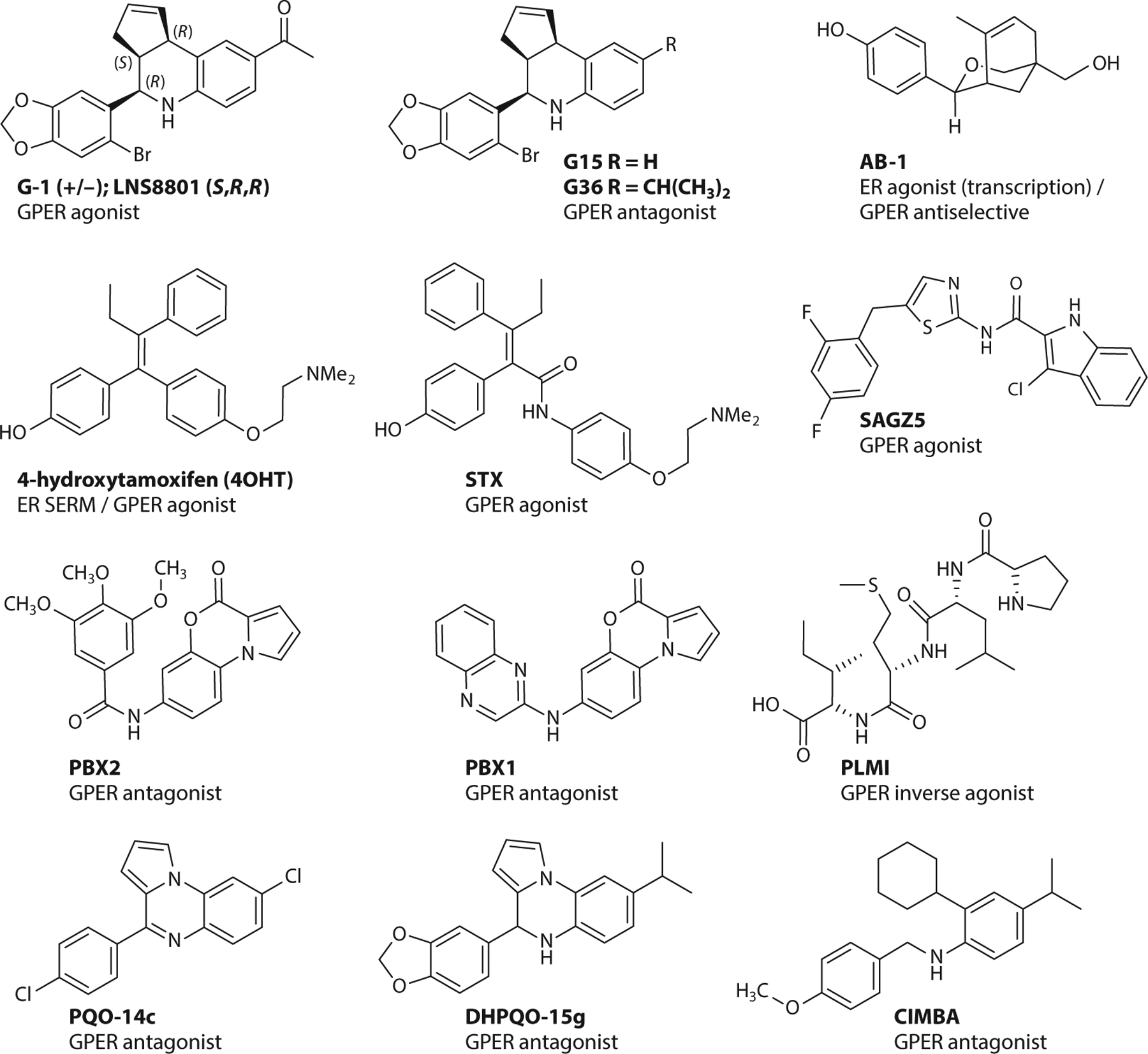
Synthetic heterocycles with selectivity for GPER. Abbreviations: ER, estrogen receptor; GPER, G protein–coupled estrogen receptor; SERM, selective estrogen receptor modulator.
The approach used to identify the G-series compounds also led to the discovery of the oxabicyclic compound AB-1, which exhibits a unique and inverse selectivity profile compared to G-series compounds, not binding to or affecting the activity of GPER, while acting as an agonist of ERα/β classical genomic responses/transcription (and antagonizing rapid nonclassical signaling pathways mediated by ER) (89). This selectivity is particularly notable considering that many ER-targeted compounds also interact with GPER; for example, the selective estrogen receptor modulator (SERM) (4-hydroxy)tamoxifen (the active metabolite of tamoxifen employed in in vitro experiments) is a potent agonist of GPER (20, 21, 90). The structurally related diphenylacrylamide tamoxifen-raloxifene hybrid, STX, also activated GPER in mHippoE-18 hippocampal clonal cells (37, 91).
Considerable effort has focused on the development of computational homology models for GPER, which have enabled molecular docking studies, molecular dynamics simulations, and virtual screening approaches, as described in recent reviews (92–101). While in-depth coverage of this subject is beyond the scope of this review, and characterization of many of the compounds remains incomplete, it is instructive to survey select new ligands that have been identified and summarize accompanying insights with respect to binding and function.
The structural significance of the G-1 scaffold as a pharmacophore has been established through several synthetic programs generating derivatives that retain GPER binding. The cyclopentene group has been saturated and replaced with tetrahydrofuranyl and tetrahydropyranyl groups (98, 102, 103). The methylketone group has been replaced by carboxylate and carboxamide functional groups (97, 104), and biaryl derivatives of the 5-bromobenzo[1,3]dioxole group have been prepared by Suzuki–Miyaura cross-coupling (94). In another example, a fluorescent borondipyrromethene difluoride (BODIPY) dye conjugated to the 6-position of a 5-bromobenzo[1,3]dioxole group mimicked the structure of G-1 and exhibited competitive ligand binding to GPER with 3H-E2 and G15 in SKBR3 cells (105). The amide-linked indole-thiazole SAGZ5 was identified by virtual screening of a 3D pharmacophore model and found to be a GPER agonist that activated adenylate cyclase and subsequent cAMP formation in HL60 cells with EC50 values similar to those of G-1 (106). The docking model proposed that SAGZ5 binds in the same hydrophobic site as modeled for G-1.
Several additional GPER antagonists with some structural similarities to G15 and G36 have been identified. The pyrrolobenzoxazinone compounds PBX1 and PBX2 were identified as GPER ligands by competitive binding studies and at 10-μM concentrations inhibited SKBR3 cell proliferation and cell migration of cancer-associated fibroblasts induced by 100 nM E2 and G-1 (107). Additional structurally related compounds such as pyrrolo[1,2-a]quinoxaline and dihydopyrrolo[1,2-a]quinoxaline (PQO-14c and DHPQO-15g) were identified as GPER antagonists using a homology model based on the chemokine receptor CXCR4 and by virtually screening for compounds with binding modes similar to the G-series of compounds (108). These compounds induced cell death in GPER-expressing MCF7 and SKBR3 cells, with structural analogs exhibiting differential effects on the expression of p53 and p21. Structurally related analogs of this scaffold inhibited cell proliferation in TNBC cells, with increased activity of a dihydropyrrolo derivative observed (109).
A homology modeling approach was used to design the benzylic aniline CIMBA that inhibited G-1-induced calcium mobilization (110). The structure of CIMBA can be considered as an acyclic analog of G36, providing increased conformational flexibility and aqueous solubility compared with the quinoline scaffold. Intraperitoneal injection of CIMBA in an ovariectomized mouse model prevented E2-induced cholesterol gallstones in a dose-dependent manner. These results encourage further study of GPER antagonists for the development of new drugs for treating cholesterol gallstone disease in women.
A peptide corresponding to residues 295–311 from the hinge region/AF2 domain of ERα (termed ERα17p) induced apoptosis in breast cancer cells and promoted regression in an ERα-negative tumor xenograft model (111). This peptide was suggested to be an inverse agonist of GPER, decreasing phosphorylation of EGFR and ERK1/2, decreasing c-fos expression, and inducing the proteasome-dependent downregulation of GPER (112). This activity was replicated by the short synthetic tetrapeptide PLMI, which is based on the N terminus of the larger peptide, and while at first consideration these peptides appear strikingly different from the other heterocyclic scaffolds, molecular docking studies suggested a correlation in the predicted GPER binding sites of the heterocyclic antagonist PBX-1 compound (112).
THERAPEUTIC OPPORTUNITIES FOR GPER-SELECTIVE LIGANDS
Cancer
GPER is expressed in a wide range of human cancers, suggesting possible roles for diagnosis, prognosis, or targeting its activity or expression as therapeutic interventions. GPER expression has been documented in human cancers (or cell lines) such as breast, endometrial, ovarian, prostate, pancreatic, thyroid, colon, lung, renal, and melanoma, among many others (reviewed in 113). In many cancer cell lines, including breast (84), endometrial (114), thyroid (115), and ovarian (116), G-1 promotes proliferation and associated signaling pathways (Figure 4). However, inhibition of proliferation has also been reported in breast (117), melanoma (118), prostate (119, 120), pancreatic (121), and other cancer cell lines. In a murine xenograft prostate cancer model encompassing both androgen-sensitive and castration-resistant cancer, G-1 inhibited cancer progression but only in castration-resistant disease (119, 120). Differences in mechanisms of cellular proliferation as well as G-1 concentrations used may account for these in vitro differences.
Figure 4.
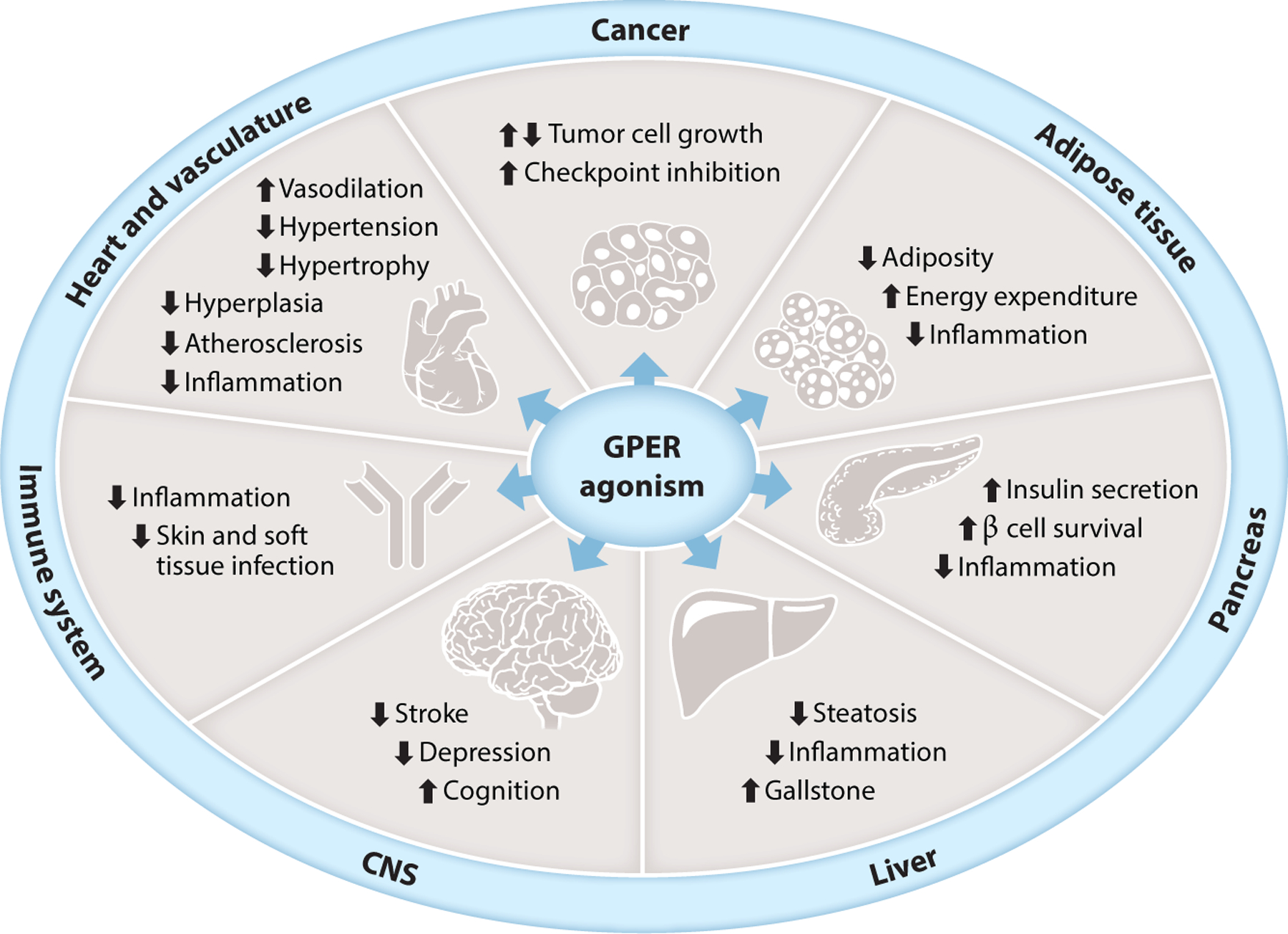
Examples of physiological effects resulting from selective GPER agonism. Abbreviations: CNS, central nervous system; GPER, G protein–coupled estrogen receptor.
In humans, GPER expression correlates with poor outcome in breast (122–124), endometrial (125), and ovarian (126) cancers. GPER expression is increased in breast cancer metastases compared to matched primary tumors (127, 128) but, interestingly, only in women treated with tamoxifen (128). GPER expression also correlates with decreased tumor growth inhibition in primary ER-/GPER-positive breast tumors treated with tamoxifen compared to aromatase inhibition. This difference is absent in primary ER-positive breast tumors that do not express GPER (123, 124). The role of global GPER expression has been evaluated in the MMTV-PyMT murine model of spontaneous mammary tumorigenesis. Compared to wild-type mice, GPER KO mice yielded smaller tumors with reduced metastasis, which suggests that, in vivo, GPER has a protumorigenic function (129). Whether this finding is due to expression in the tumor cells or stromal cells (e.g., immune cells or fibroblasts) remains unknown.
As an agonist of GPER, (4-hydroxy)tamoxifen’s effects on breast cancer (cells) have been widely examined and are complex. Tamoxifen-resistant MCF7 cells proliferated in response to tamoxifen through a GPER-dependent pathway (127, 130), which was blocked by either GPER knockdown or G15 treatment (81, 127). Tamoxifen elicited cytoplasmic translocation of the proapoptotic transcription factor Foxo3, which may in turn contribute to resistance mechanisms (32, 90). Tamoxifen also induced breast cancer cell migration (131) and increased aromatase expression in tamoxifen-resistant cells (132) via GPER. In vivo, tamoxifen-resistant MCF7 xenografts regained sensitivity to tamoxifen upon treatment with G15 (127). G15 sensitized breast cancer cells to doxorubicin by inhibiting epithelial–mesenchymal transition (133). Finally, G-1 (as well as tamoxifen and fulvestrant) increased natural killer cell–mediated killing of both ER-negative and ER-positive breast cancer cells, suggesting yet another possible role for GPER in immune regulation (134).
In vivo effects of GPER agonists and antagonists are complicated by the widespread expression of GPER beyond tumor cells, including in tumor-associated immune and stromal cells (such as fibroblasts, adipocytes, and vascular cells). Anti-inflammatory effects of GPER and G-1 likely affect cancer initiation and early progress, as evidenced by accelerated inflammation-driven liver tumorigenesis in GPER-deficient mice (135). GPER expression in breast cancer–associated fibroblasts also suggests a role in cancer progression (136–138), where it promoted migration and invasion of cancer cells (139–141). Adipocytes in fat-rich tissues such as the breast and in the obese (142) also contribute to carcinogenesis of multiple cancers (143). Adipocytes express aromatase, yielding increased local estrogen levels as well as many adipokines and generally proinflammatory cytokines and hormones that can promote tumorigenesis. As G-1 reduces obesity and metabolic dysfunction (144), inflammation (113, 145), and chemotherapy-induced cardiotoxicity (146), it may reduce the incidence of or improve outcomes in breast and other cancers through diverse mechanisms.
GPER also plays important roles in many other cancer types. G-1 reduced liver tumorigenesis, in part through inhibiting inflammation and fibrosis (135). In contrast, in non-small-cell lung cancer, tumor burden increased with E2 or G-1 treatment and decreased with G15 treatment (147, 148). In melanoma cells, G-1 (as well as tamoxifen) inhibited proliferation in vitro (149), and when combined with anti-PD-1 antibody therapy, G-1 priming led to reduced tumor growth, substantially improving survival of melanoma-bearing mice (118). G-1 combination with immune checkpoint inhibition therapy also showed efficacy in pancreatic cancer models (121). These combined therapies led to immune memory, protecting against tumor rechallenge, suggesting broad effects in tumor and immune cells (118). These studies led to IND approval for G-1 in cancer and subsequent initiation of the first Phase I clinical trial of G-1 in 2019 (https://clinicaltrials.gov/ct2/show/NCT04130516).
Cardiovascular System
Estrogens play important roles in the regulation of cardiovascular function, and their receptors therefore represent potential targets for therapeutic interventions in multiple cardiovascular diseases, including myocardial infarction (coronary heart disease), atherosclerosis, arterial and pulmonary arterial hypertension, and heart failure. The role(s) of estrogen is exemplified by the lower incidence of hypertension and coronary artery disease in premenopausal women compared to age-matched men and the substantial increase in both diseases following menopause (150, 151). Roles for GPER in the regulation of cardiovascular function and disease have been widely demonstrated using G-1 and include the regulation of blood pressure, angiogenesis, myocardial function, and inflammation (152).
G-1, like E2, induced vasorelaxation largely through nitric oxide production within multiple vessels (of rodent, porcine, and human origin) and acutely lowered blood pressure in mice, an effect that was absent in GPER KO mice (31, 71, 153–155). In salt-dependent hypertension with early diastolic dysfunction (heart failure with preserved ejection fraction), employing the mRen2.Lewis rat, chronic G-1 treatment improved myocardial relaxation in ovary-intact and ovariectomized females and reduced cardiac myocyte hypertrophy and wall thickness, in the absence of overt changes in blood pressure (156, 157). Similar therapeutic effects of G-1 occurred in aged rats (158) and in AngII-induced hypertensive rats (159). G-1 treatment (for 2 weeks at 14 months of age) also reversed hypertension in female intrauterine growth–restricted offspring (i.e., low birth weight) rats that occurs with advanced age (160). In addition to the effects of GPER agonism via G-1, G36 prevented AngII-induced hypertension in mice through a unique mechanism resulting from the downregulation of Nox1 with the subsequent lack of reactive oxygen species production involved in AngII-induced vasoconstriction and thus hypertension (161). In a rat model of diabetic cardiomyopathy, mean arterial pressure, cardiac weight, and atherogenic and cardiovascular risk indices were improved by E2 and G-1 treatment, with the salutary effects of E2 inhibited by G15 (162). In addition to arterial hypertension, G-1 was effective in treating pulmonary arterial hypertension, reversing both cardiac and skeletal muscle functional aberrations in ovariectomized female (163) and male (164) mice.
Atherosclerosis, which can lead to coronary artery disease, results from elevated lipid levels in the blood and a chronic inflammatory state. G-1 protected against the development of atherosclerosis through multiple actions in both diet-induced (165) and genetic models (166). First, G-1 reduced plasma cholesterol levels (see below) (144). Second, G-1 induced differentiation and inhibited coronary smooth muscle cell proliferation (167). Third, G-1 reduced inflammation in a diet-induced model of atherosclerosis in mice (165). Consistent with an anti-inflammatory role for GPER, GPER KO mice exhibited increased accumulation of inflammatory cells as well as atherosclerosis in both ovary-intact and ovariectomized mice (165). Fourth, G-1, as well as E2, induces nitric oxide production in human endothelial cells (both inhibited by G36) (165) and enhanced vasodilation (166). Endothelial dysfunction and reduced NO production are hallmarks of atherosclerosis and vascular disease (151, 168).
Endocrinology and Metabolism
Metabolic homeostasis is differentially regulated in males and females (169, 170), with premenopausal women exhibiting a lower incidence of obesity and diabetes compared to age-matched men. These protective effects, presumably a result of estrogen, are lost following menopause (171, 172). This sex difference, as well as the effects of estrogen deprivation, is also present in mice (173, 174). Estrogen replacement therapy in postmenopausal women, as well as in ovariectomized mice, can alleviate weight gain and its associated adverse metabolic effects (173–176).
GPER expression is associated with body weight, energy expenditure, and glucose homeostasis. This is evidenced by the fact that GPER KO mice exhibited increased body weight and adiposity (in both the visceral and subcutaneous depots), dyslipidemia, and insulin resistance and glucose intolerance (153, 177–180). That GPER modulates basal metabolism was concluded from the fact that no changes in either steady-state daily food intake or locomotor activity were observed, but energy expenditure decreased in GPER KO mice, consistent with the observation of decreased brown adipose tissue expression of the thermogenic genes uncoupling protein 1 and β3-adrenergic receptor (177, 179). Interestingly, although there was no overall difference in food intake, female GPER KO mice exhibited a lower sensitivity to the short-term feeding inhibition of leptin and cholecystokinin (179). Consistent with this effect, G-1 treatment of ovariectomized rats led to an acute transient decrease in food intake (181). Employing models of obesity through either estrogen deprivation (i.e., ovariectomy) or a high-fat diet (HFD), chronic G-1 treatment following weight gain led to weight and adipose tissue loss, improved levels of circulating lipids, and increased energy expenditure with no changes in food consumption or locomotion (144). Similarly, no changes in either lean mass or bone density/mineral content were observed. In both white and brown adipose tissue, as well as skeletal muscle, G-1 treatment increased the expression of genes involved in mitochondrial biogenesis and fatty acid oxidation while reducing expression of many genes involved in inflammation, hypoxia, and angiogenesis (144). Importantly, as previously observed (165), G-1 treatment of ovariectomized mice did not lead to uterine imbibition (144), as occurs with estrogen supplementation (182).
GPER KO mice also exhibited higher plasma glucose and impaired insulin sensitivity and glucose tolerance as well as defective glucose- and estrogen-stimulated insulin secretion (177–179). In a streptozotocin-induced model of type 1 diabetes, female GPER KO mice displayed decreased pancreatic insulin and pancreatic β cell content as well as higher blood glucose (183). In addition to promoting islet survival (183), GPER mediated insulin secretion in isolated islets in response to E2 and G-1, both of which were reduced by GPER inhibition with G15 or in islets from GPER KO mice (184). Lastly, ovariectomized wild-type but not GPER KO mice responded to acute and chronic estrogen treatment with improved glucose homeostasis, further revealing the role of GPER in estrogen function in vivo (178, 179). The models described above also resulted in metabolic dysfunction, including insulin resistance and glucose intolerance. Treatment with G-1 also led to improvements in glucose homeostasis, as revealed by glucose- and insulin-tolerance tests and reduced fasting glucose and insulin concentrations (144). Employing ovariectomy, streptozotocin, and a HFD to create a rat model of severe postmenopausal type 2 diabetes, one study found that estrogen and G-1 treatment improved fasting blood glucose and HOMA-IR (homeo-static model assessment for insulin resistance), with estrogen’s salutary effects reversed by G15 (162). That GPER also functions to enhance insulin secretion in humans has been demonstrated in pancreatic islets isolated from type 2 diabetic patients where glucose-stimulated insulin secretion increased, while glucagon and somatostatin secretion decreased, upon G-1 stimulation (185, 186).
Actions of GPER-Selective Ligands in Other Systems
GPER is expressed in and plays multiple roles in the skin. GPER’s role in estrogen-induced melanogenesis suggests GPER modulators could find applications in chloasma and other skin pigmentation disorders (187, 188). In skin and soft tissue infection resulting from Staphylococcus aureus, G-1 decreased dermonecrosis, likely through reduced overall neutrophil accumulation, and increased bacterial clearance in the absence of direct bactericidal effects (189). The observed sex difference and role of estrogen in wound healing (190, 191), along with reduced healing in GPER KO mice (R. Ko, O. Davidson, K. Ahmed, R. Clark, J. Brandenburg, et al., unpublished results), suggest additional opportunities for GPER agonist therapy in skin conditions and wound healing.
With respect to the hepatobiliary system, estrogen has multiple functions in both the liver and gall bladder, protecting liver function and reducing steatohepatitis, while promoting gallstone formation. Estrogen and genistein, in part through GPER, protected hepatocytes from mitochondrial dysfunction and triglyceride accumulation (192). DHEA, through conversion to estrogen, which then acts through GPER, reduced murine nonalcoholic steatohepatitis (47). Estrogen-promoted formation of gallstones involves both GPER and ERα, with distinct cholesterol crystallization pathways for the two receptors described (193). Furthermore, in GPER KO mice, gallstone formation was absent, whereas it was increased by treatment of wild-type mice with estrogen and G-1 (193, 194). Conversely, gallstone formation was reduced by selective GPER antagonists such as the G36 analog, CIMBA, suggesting that targeting GPER with antagonists may represent a therapeutic opportunity for this condition (110).
In the gastrointestinal tract, G-1 reduced motor function (i.e., muscle contractility and therefore motility), visceral pain (195, 196), and reperfusion injury following intestinal ischemia/reperfusion through decreased colonic crypt cell injury (197). G-1 also reduced mortality and tissue damage in a model of Crohn’s disease (198), and GPER activation also attenuated intestinal inflammation in a model of acute colitis, resulting in improved intestinal mucosal barrier function (199, 200). Intestinal expression of GPER appears to be increased in Crohn’s disease (198), ulcerative colitis (201), and irritable bowel syndrome (202).
GPER regulates multiple aspects of kidney function, including renal artery and interlobular artery vascular tone (203, 204). Estrogen and G-1, through GPER activation, stimulated H+-ATPase activity in renal tubular intercalated cells (205) and regulated Na+ excretion in vivo (206). Icariin, a GPER agonist, protected kidney podocytes from apoptosis (207), and in hypertensive nephropathy, G-1 reduced proteinuria, without accompanying changes in blood pressure (208, 209). G-1 also reduced renal cell injury resulting from methotrexate treatment (210). Interestingly, GPER KO mice exhibited greatly reduced age-associated renal fibrosis and kidney disease, likely through the regulation of Nox1, as observed in the heart and vasculature, suggesting a therapeutic role for GPER antagonists in chronic kidney disease (161, 211).
Many salutary effects of estrogen in nonreproductive tissues and diseases appear to involve anti-inflammatory effects that are at least in part mediated through GPER, which is broadly expressed in immune cells (145). Consistent with this, GPER KO mice exhibited increased inflammation in many models (135, 165, 177, 179, 212), whereas G-1 administration reduced inflammation in multiple murine models, including lung allergy with airway hyperresponsiveness (213), chronic obesity and diabetes (144), inflammatory bowel diseases (198, 214), and chronic neurological diseases (212, 215–218). Among its actions, G-1 promoted production of the anti-inflammatory cytokine IL-10 in Th17 cells (219, 220) and reduced the production of lipopolysaccharide-induced cytokines in macrophages (217). GPER activity was also sufficient to protect fetal development and neonatal viability in times of maternal infection and inflammation of the placenta, suggesting a therapeutic avenue through G-1 (221).
GPER plays extensive roles in the central and peripheral nervous system, as demonstrated by the array of protective G-1 actions in acute and chronic neurological/neurodegenerative diseases (218). In a multiple sclerosis model (experimental autoimmune encephalomyelitis), G-1 both reduced the severity and delayed the onset of symptoms through reductions in immune reactivity (212, 217). In models of Alzheimer’s and Parkinson’s disease and after traumatic brain injury, G-1 improved multiple measures of neurological functions in part through reducing neuroinflammation (222–225). In traumatic brain and spinal cord injury models, G-1 provided protection (223, 226, 227). In stroke models, G-1 reduced infarct size, blood-brain barrier permeability, and stroke-induced immunosuppression (228–232) through improved neuronal survival signaling (228, 233) and improved cerebral microvascular function (231), restoring autophagy in astrocytes (234) or inhibiting TL4-mediated microglial inflammation (229). GPER activation with G-1 also exhibited antidepressant and anxiolytic effects (81, 235), supported by studies in GPER KO rats (236). Cognition, learning, memory, and other behavioral effects (e.g., lordosis) are also associated with GPER via the actions of G-1 (222, 224, 237–241).
CONCLUSIONS AND FUTURE DIRECTIONS
Since our last review on the pharmacology of GPER in 2015 (7), significant advances have been made in understanding the functions of GPER and the potential applications of GPER-targeted ligands (both agonists and antagonists). New natural and synthetic compounds have been identified as GPER agonists and antagonists, suggesting roles for GPER in the beneficial effects of phytoestrogens as well as the harmful effects of endocrine disruptors. The continuing identification of novel GPER actions and applications, frequently demonstrated through the use of GPER-targeted ligands, in virtually every system of the body, portends opportunities for therapeutic development of such targeted ligands and the necessity of assessing GPER effects of current and developing drugs. With the advancement of G-1 to Phase I/II clinical trials in 2020 for advanced cancers (https://clinicaltrials.gov/ct2/show/NCT04130516), and intriguing opportunities in metabolic disorders and cardiovascular, kidney, and hepatobiliary diseases, not to mention immune, neurological, gastrointestinal, and infectious diseases, GPER-targeted compounds may find broad use in the pharmacopoeia.
ACKNOWLEDGMENTS
We would like to thank all those who have contributed knowledge in this field and apologize to those whose work could not be cited due to length constraints. The authors were supported by National Institutes of Health R01 grants CA127731, CA163890, and CA194496; Dialysis Clinic Inc.; the Center of Biomedical Research Excellence in Autophagy, Inflammation and Metabolism (P20 GM121176); and the University of New Mexico Comprehensive Cancer Center (P30 CA118100).
Footnotes
DISCLOSURE STATEMENT
The authors are inventors on US patents related to GPER-selective compounds (7,875,721 and 8,487,100) and their applications (10,251,870; 10,471,047; 10,561,648; 10,682,341; and 10,980,785), which have been licensed to Sandia Biotech, GPER G-1 Development Group, and Linnaeus Therapeutics. The authors are entitled to royalties as managed by university policies for inventors but have no equity interests in any licensing companies.
LITERATURE CITED
- 1.Santoro N, Epperson CN, Mathews SB. 2015. Menopausal symptoms and their management. Endocrinol. Metab. Clin. North Am 44:497–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta J, Kling JM, Manson JE. 2021. Risks, benefits, and treatment modalities of menopausal hormone therapy: current concepts. Front. Endocrinol 12:564781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.North Am. Menopause Soc. Hormone Ther. Position Statement Advis. Panel. 2018. The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 25:1362–87 [DOI] [PubMed] [Google Scholar]
- 4.McDonnell DP, Wardell SE, Chang CY, Norris JD. 2021. Next-generation endocrine therapies for breast cancer. J. Clin. Oncol 39:1383–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haines CN, Wardell SE, McDonnell DP. 2021. Current and emerging estrogen receptor-targeted therapies for the treatment of breast cancer. Essays Biochem. 65:985–1001 [DOI] [PubMed] [Google Scholar]
- 6.Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, et al. 2006. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacol. Rev 58:773–81 [DOI] [PubMed] [Google Scholar]
- 7.Prossnitz ER, Arterburn JB. 2015. International Union of Basic and Clinical Pharmacology. XCVII. G protein-coupled estrogen receptor and its pharmacologic modulators. Pharmacol. Rev 67:505–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fruzzetti F, Fidecicchi T, Montt Guevara MM, Simoncini T. 2021. Estetrol: a new choice for contraception. J. Clin. Med 10:5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, et al. 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev 30:293–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorand T, Vigh E, Garai J. 2010. Hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds: phytoestrogens and xenoestrogens. Curr. Med. Chem 17:3542–74 [DOI] [PubMed] [Google Scholar]
- 11.Frye CA, Bo E, Calamandrei G, Calza L, Dessi-Fulgheri F, et al. 2012. Endocrine disrupters: a review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocrinol 24:144–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song S, Guo Y, Yang Y, Fu D. 2022. Advances in pathogenesis and therapeutic strategies for osteoporosis. Pharmacol. Ther 237:108168. [DOI] [PubMed] [Google Scholar]
- 13.Yang F, Li N, Gaman MA, Wang N. 2021. Raloxifene has favorable effects on the lipid profile in women explaining its beneficial effect on cardiovascular risk: a meta-analysis of randomized controlled trials. Pharmacol. Res 166:105512. [DOI] [PubMed] [Google Scholar]
- 14.Nabieva N, Fasching PA. 2021. Endocrine treatment for breast cancer patients revisited—history, standard of care, and possibilities of improvement. Cancers 13:5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wehling M 1994. Nongenomic actions of steroid hormones. Trends Endocrinol. Metab 5:347–53 [DOI] [PubMed] [Google Scholar]
- 16.Mosselman S, Polman J, Dijkema R. 1996. ERβ: identification and characterization of a novel human estrogen receptor. FEBS Lett. 392:49–53 [DOI] [PubMed] [Google Scholar]
- 17.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. 1996. Cloning of a novel receptor expressed in rat prostate and ovary. PNAS 93:5925–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barton M, Filardo EJ, Lolait SJ, Thomas P, Maggiolini M, Prossnitz ER. 2018. Twenty years of the G protein-coupled estrogen receptor GPER: historical and personal perspectives. J. Steroid Biochem. Mol. Biol 176:4–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr. 2000. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol 14:1649–60 [DOI] [PubMed] [Google Scholar]
- 20.Thomas P, Pang Y, Filardo EJ, Dong J. 2005. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 146:624–32 [DOI] [PubMed] [Google Scholar]
- 21.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. 2005. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307:1625–30 [DOI] [PubMed] [Google Scholar]
- 22.Alexander SPH, Mathie A, Peters JA. 2008. Guide to Receptors and Channels (GRAC), 3rd edition. Br. J. Pharmacol 153(Suppl. 2):S1–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaudet HM, Cheng SB, Christensen EM, Filardo EJ. 2015. The G-protein coupled estrogen receptor, GPER: the inside and inside-out story. Mol. Cell. Endocrinol 418(Part 3):207–19 [DOI] [PubMed] [Google Scholar]
- 24.Giorgi EP, Stein WD. 1981. The transport of steroids into animal cells in culture. Endocrinology 108:688–97 [DOI] [PubMed] [Google Scholar]
- 25.Muller RE, Johnston TC, Traish AM, Wotiz HH. 1979. Studies on the mechanism of estradiol uptake by rat uterine cells and on estradiol binding to uterine plasma membranes. Adv. Exp. Med. Biol 117:401–21 [DOI] [PubMed] [Google Scholar]
- 26.Maruvada P, Baumann CT, Hager GL, Yen PM. 2003. Dynamic shuttling and intranuclear mobility of nuclear hormone receptors. J. Biol. Chem 278:12425–32 [DOI] [PubMed] [Google Scholar]
- 27.Revankar CM, Mitchell HD, Field AS, Burai R, Corona C, et al. 2007. Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem. Biol 2:536–44 [DOI] [PubMed] [Google Scholar]
- 28.Filardo EJ, Quinn JA, Frackelton AR Jr., Bland KI. 2002. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol 16:70–84 [DOI] [PubMed] [Google Scholar]
- 29.Kilpatrick LE, Hill SJ. 2021. Transactivation of G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs): recent insights using luminescence and fluorescence technologies. Curr. Opin. Endocr. Metab. Res 16:102–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vivacqua A, De Marco P, Santolla MF, Cirillo F, Pellegrino M, et al. 2015. Estrogenic GPER signaling regulates miR144 expression in cancer cells and cancer-associated fibroblasts (CAFs). Oncotarget 6:16573–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fredette NC, Meyer MR, Prossnitz ER. 2018. Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. J. Steroid Biochem. Mol. Biol 176:65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zekas E, Prossnitz ER. 2015. Estrogen-mediated inactivation of FOXO3a by the G protein-coupled estrogen receptor GPER. BMC Cancer 15:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Lu S, Xu R, Tang Y, Liu J, et al. 2020. Mechanisms of estradiol-induced EGF-like factor expression and oocyte maturation via G protein-coupled estrogen receptor. Endocrinology 161:bqaa190. [DOI] [PubMed] [Google Scholar]
- 34.Yu T, Yang G, Hou Y, Tang X, Wu C, et al. 2017. Cytoplasmic GPER translocation in cancer-associated fibroblasts mediates cAMP/PKA/CREB/glycolytic axis to confer tumor cells with multidrug resistance. Oncogene 36:2131–45 [DOI] [PubMed] [Google Scholar]
- 35.Lagarde F, Beausoleil C, Belcher SM, Belzunces LP, Emond C, et al. 2015. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environ. Health 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dinh QN, Vinh A, Kim HA, Saini N, Broughton BRS, et al. 2021. Aldosterone-induced hypertension is sex-dependent, mediated by T cells and sensitive to GPER activation. Cardiovasc. Res 117:960–70 [DOI] [PubMed] [Google Scholar]
- 37.Evans PD. 2019. Rapid signalling responses via the G protein-coupled estrogen receptor, GPER, in a hippocampal cell line. Steroids 152:108487. [DOI] [PubMed] [Google Scholar]
- 38.Cheng SB, Dong J, Pang Y, LaRocca J, Hixon M, et al. 2014. Anatomical location and redistribution of G protein-coupled estrogen receptor-1 during the estrus cycle in mouse kidney and specific binding to estrogens but not aldosterone. Mol. Cell. Endocrinol 382:950–59 [DOI] [PubMed] [Google Scholar]
- 39.Chourasia TK, Pang Y, Thomas P. 2015. The catecholestrogen, 2-hydroxyestradiol-17beta, acts as a G protein–coupled estrogen receptor 1 (GPER/GPR30) antagonist to promote the resumption of meiosis in zebrafish oocytes. Biol. Reprod 92:69. [DOI] [PubMed] [Google Scholar]
- 40.Koganti S, Snyder R, Gumaste U, Karamyan VT, Thekkumkara T. 2014. 2-Methoxyestradiol binding of GPR30 down-regulates angiotensin AT1 receptor. Eur. J. Pharmacol 723:131–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thekkumkara T, Snyder R, Karamyan VT. 2016. Competitive binding assay for the G-protein-coupled receptor 30 (GPR30) or G-protein-coupled estrogen receptor (GPER). Methods Mol. Biol 1366:11–17 [DOI] [PubMed] [Google Scholar]
- 42.Ogola B, Zhang Y, Iyer L, Thekkumkara T. 2018. 2-Methoxyestradiol causes matrix metalloproteinase 9-mediated transactivation of epidermal growth factor receptor and angiotensin type 1 receptor down-regulation in rat aortic smooth muscle cells. Am. J. Physiol. Cell Physiol 314:C554–68 [DOI] [PubMed] [Google Scholar]
- 43.Singh P, Song CY, Dutta SR, Gonzalez FJ, Malik KU. 2020. Central CYP1B1 (Cytochrome P450 1B1)-estradiol metabolite 2-methoxyestradiol protects from hypertension and neuroinflammation in female mice. Hypertension 75:1054–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pingili AK, Davidge KN, Thirunavukkarasu S, Khan NS, Katsurada A, et al. 2017. 2-Methoxyestradiol reduces angiotensin II-induced hypertension and renal dysfunction in ovariectomized female and intact male mice. Hypertension 69:1104–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Avena P, Casaburi I, Zavaglia L, Nocito MC, La Padula D, et al. 2022. 27-Hydroxycholesterol binds GPER and induces progression of estrogen receptor-negative breast cancer. Cancers 14:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zucchetti AE, Barosso IR, Boaglio AC, Basiglio CL, Miszczuk G, et al. 2014. G-protein-coupled receptor 30/adenylyl cyclase/protein kinase A pathway is involved in estradiol 17β-d-glucuronide-induced cholestasis. Hepatology 59:1016–29 [DOI] [PubMed] [Google Scholar]
- 47.Li L, Wang H, Yao Y, Cao J, Jiang Z, et al. 2021. The sex steroid precursor dehydroepiandrosterone prevents nonalcoholic steatohepatitis by activating the AMPK pathway mediated by GPR30. Redox Biol. 48:102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramesh C, Bryant B, Nayak T, Revankar CM, Anderson T, et al. 2006. Linkage effects on binding affinity and activation of GPR30 and estrogen receptors ERα/β with tridentate pyridin-2-yl hydrazine tricarbonyl-Re/99mTc(I) chelates. J. Am. Chem. Soc 128:14476–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C, Zhang Y, Wang J, Xing D. 2022. VHL-based PROTACs as potential therapeutic agents: recent progress and perspectives. Eur. J. Med. Chem 227:113906. [DOI] [PubMed] [Google Scholar]
- 50.Flanagan JJ, Neklesa TK. 2019. Targeting nuclear receptors with PROTAC degraders. Mol. Cell. Endocrinol 493:110452. [DOI] [PubMed] [Google Scholar]
- 51.Bargagna-Mohan P, Baek SH, Lee H, Kim K, Mohan R. 2005. Use of PROTACs as molecular probes of angiogenesis. Bioorg. Med. Chem. Lett 15:2724–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu AS, Rouhimoghadam M, Arnatt CK, Filardo EJ, Salem AK. 2021. Proteolytic targeting chimeras with specificity for plasma membrane and intracellular estrogen receptors. Mol. Pharmacol 18:1455–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bustamante-Barrientos FA, Mendez-Ruette M, Ortloff A, Luz-Crawford P, Rivera FJ, et al. 2021. The impact of estrogen and estrogen-like molecules in neurogenesis and neurodegeneration: beneficial or harmful? Front. Cell. Neurosci 15:636176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molina L, Bustamante FA, Bhoola KD, Figueroa CD, Ehrenfeld P. 2018. Possible role of phytoestrogens in breast cancer via GPER-1/GPR30 signaling. Clin. Sci 132:2583–98 [DOI] [PubMed] [Google Scholar]
- 55.Lacouture A, Lafront C, Peillex C, Pelletier M, Audet-Walsh E. 2022. Impacts of endocrine-disrupting chemicals on prostate function and cancer. Environ. Res 204:112085. [DOI] [PubMed] [Google Scholar]
- 56.Qiu YA, Xiong J, Yu T. 2021. Role of G protein-coupled estrogen receptor in digestive system carcinomas: a minireview. OncoTargets Ther. 14:2611–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khan SU, Ahemad N, Chuah LH, Naidu R, Htar TT. 2022. Natural bioactive compounds as a new source of promising G protein-coupled estrogen receptor (GPER) modulators: comprehensive in silico approach. J. Biomol. Struct. Dyn 40:1617–28 [DOI] [PubMed] [Google Scholar]
- 58.Mesmar F, Dai B, Ibrahim A, Hases L, Jafferali MH, et al. 2019. Clinical candidate and genistein analogue AXP107–11 has chemoenhancing functions in pancreatic adenocarcinoma through G protein-coupled estrogen receptor signaling. Cancer Med. 8:7705–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du ZR, Gu Y, Xie XM, Zhang M, Jiang GY, Chen WF. 2021. GPER and IGF-1R mediate the anti-inflammatory effect of genistein against lipopolysaccharide (LPS)-induced nigrostriatal injury in rats. J. Steroid Biochem. Mol. Biol 214:105989. [DOI] [PubMed] [Google Scholar]
- 60.Surico D, Ercoli A, Farruggio S, Raina G, Filippini D, et al. 2017. Modulation of oxidative stress by 17 β-estradiol and genistein in human hepatic cell lines in vitro. Cell. Physiol. Biochem 42:1051–62 [DOI] [PubMed] [Google Scholar]
- 61.Vasquez-Reyes S, Vargas-Castillo A, Noriega LG, Velazquez-Villegas LA, Perez B, et al. 2022. Genistein stimulation of white adipose tissue thermogenesis is partially dependent on GPR30 in mice. Mol. Nutr. Food Res 66:e2100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harada K, Sada S, Sakaguchi H, Takizawa M, Ishida R, Tsuboi T. 2018. Bacterial metabolite S-equol modulates glucagon-like peptide-1 secretion from enteroendocrine L cell line GLUTag cells via actin polymerization. Biochem. Biophys. Res. Commun 501:1009–15 [DOI] [PubMed] [Google Scholar]
- 63.Wu H, Nie P, Zhou Z, Hu J, Li G, et al. 2020. S-(−)-equol alleviates stenosis of the injured carotid artery in Sprague Dawley rats by preventing the vascular smooth muscle cell phenotypic switch via inhibition of the MAPKp38-NFkBp65 signaling. Mater. Express 10:1237–48 [Google Scholar]
- 64.Moriyama M, Hashimoto A, Satoh H, Kawabe K, Ogawa M, et al. 2018. S-Equol, a major isoflavone from soybean, inhibits nitric oxide production in lipopolysaccharide-stimulated rat astrocytes partially via the GPR30-mediated pathway. Int. J. Inflam 2018:8496973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ariyani W, Miyazaki W, Koibuchi N. 2019. A novel mechanism of S-equol action in neurons and astrocytes: the possible involvement of GPR30/GPER1. Int. J. Mol. Sci 20:5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shang D, Li Z, Zhu Z, Chen H, Zhao L, et al. 2015. Baicalein suppresses 17-β-estradiol-induced migration, adhesion and invasion of breast cancer cells via the G protein-coupled receptor 30 signaling pathway. Oncol. Rep 33:2077–85 [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Hong DY, Wang J, Ling-Hu J, Zhang YY, et al. 2017. Baicalein, unlike 4-hydroxytamoxifen but similar to G15, suppresses 17β-estradiol-induced cell invasion, and matrix metalloproteinase-9 expression and activation in MCF-7 human breast cancer cells. Oncol. Lett 14:1823–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moreno-Ulloa A, Mendez-Luna D, Beltran-Partida E, Castillo C, Guevara G, et al. 2015. The effects of (−)-epicatechin on endothelial cells involve the G protein-coupled estrogen receptor (GPER). Pharmacol. Res 100:309–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreno-Ulloa A, Miranda-Cervantes A, Licea-Navarro A, Mansour C, Beltran-Partida E, et al. 2018. (−)-Epicatechin stimulates mitochondrial biogenesis and cell growth in C2C12 myotubes via the G-protein coupled estrogen receptor. Eur. J. Pharmacol 822:95–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarmiento V, Ramirez-Sanchez I, Moreno-Ulloa A, Romero-Perez D, Chavez D, et al. 2018. Synthesis of novel (−)-epicatechin derivatives as potential endothelial GPER agonists: evaluation of biological effects. Bioorg. Med. Chem. Lett 28:658–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Calfio C, Donoso F, Huidobro-Toro JP. 2021. Anthocyanins activate membrane estrogen receptors with nanomolar potencies to elicit a nongenomic vascular response via NO production. J. Am. Heart Assoc 10:e020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He J, Zhang J, Wang Y, Liu W, Gou K, et al. 2018. MiR-7 mediates the zearalenone signaling pathway regulating FSH synthesis and secretion by targeting FOS in female pigs. Endocrinology 159:2993–3006 [DOI] [PubMed] [Google Scholar]
- 73.He J, Wei C, Li Y, Liu Y, Wang Y, et al. 2018. Zearalenone and alpha-zearalenol inhibit the synthesis and secretion of pig follicle stimulating hormone via the non-classical estrogen membrane receptor GPR30. Mol. Cell. Endocrinol 461:43–54 [DOI] [PubMed] [Google Scholar]
- 74.Lo EKK, Lee JC, Turner PC, El-Nezami H. 2021. Low dose of zearalenone elevated colon cancer cell growth through G protein-coupled estrogenic receptor. Sci. Rep 11:7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perian S, Cerutti C, Forcet C, Tribollet V, Vanacker JM. 2020. A cell-based method to detect agonist and antagonist activities of endocrine-disrupting chemicals on GPER. Front. Endocrinol 11:547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herz C, Tran HTT, Schlotz N, Michels K, Lamy E. 2017. Low-dose levels of bisphenol A inhibit telomerase via ER/GPR30-ERK signalling, impair DNA integrity and reduce cell proliferation in primary PBMC. Sci. Rep 7:16631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nadal A, Fuentes E, Ripoll C, Villar-Pazos S, Castellano-Munoz M, et al. 2018. Extranuclear-initiated estrogenic actions of endocrine disrupting chemicals: Is there toxicology beyond paracelsus? J. Steroid Biochem. Mol. Biol 176:16–22 [DOI] [PubMed] [Google Scholar]
- 78.Cao LY, Ren XM, Li CH, Zhang J, Qin WP, et al. 2017. Bisphenol AF and bisphenol B exert higher estrogenic effects than bisphenol A via G protein–coupled estrogen receptor pathway. Environ. Sci. Technol 51:11423–30 [DOI] [PubMed] [Google Scholar]
- 79.Thomas P, Dong J. 2006. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol 102:175–79 [DOI] [PubMed] [Google Scholar]
- 80.Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, et al. 2006. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol 2:207–12 [DOI] [PubMed] [Google Scholar]
- 81.Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, et al. 2009. In vivo effects of a GPR30 antagonist. Nat. Chem. Biol 5:421–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, et al. 2011. Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity. J. Steroid Biochem. Mol. Biol 127:358–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petrie WK, Dennis MK, Hu C, Dai D, Arterburn JB, et al. 2013. G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth. Obstet. Gynecol. Int 2013:472720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scaling AL, Prossnitz ER, Hathaway HJ. 2014. GPER mediates estrogen-induced signaling and proliferation in human breast epithelial cells and normal and malignant breast. Horm. Cancer 5:146–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Prossnitz ER, Hathaway HJ. 2015. What have we learned about GPER function in physiology and disease from knockout mice? J. Steroid Biochem. Mol. Biol 153:114–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gui Y, Shi Z, Wang Z, Li JJ, Xu C, et al. 2015. The GPER agonist G-1 induces mitotic arrest and apoptosis in human vascular smooth muscle cells independent of GPER. J. Cell. Physiol 230:885–95 [DOI] [PubMed] [Google Scholar]
- 87.Holm A, Grande PO, Luduena RF, Olde B, Prasad V, et al. 2012. The G protein-coupled oestrogen receptor 1 agonist G-1 disrupts endothelial cell microtubule structure in a receptor-independent manner. Mol. Cell. Biochem 366:239–49 [DOI] [PubMed] [Google Scholar]
- 88.Natale CA, Garyantes T. 2021. IND-enabling characterization of the selective GPER agonist, LNS8801. Cancer Res. 81(Suppl. 13):1282 [Google Scholar]
- 89.Revankar CM, Bologa CG, Pepermans RA, Sharma G, Petrie WK, et al. 2019. A selective ligand for estrogen receptor proteins discriminates rapid and genomic signaling. Cell Chem. Biol 26:1692–702.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pepermans RA, Prossnitz ER. 2019. ERα-targeted endocrine therapy, resistance and the role of GPER. Steroids 152:108493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Evans PD. 2019. Aldosterone, STX and amyloid-β1–42 peptides modulate GPER (GPR30) signalling in an embryonic mouse hippocampal cell line (mHippoE-18). Mol. Cell. Endocrinol 496:110537. [DOI] [PubMed] [Google Scholar]
- 92.Lappano R, Rosano C, Santolla MF, Pupo M, De Francesco EM, et al. 2012. Two novel GPER agonists induce gene expression changes and growth effects in cancer cells. Curr. Cancer Drug Targets 12:531–42 [DOI] [PubMed] [Google Scholar]
- 93.Arnatt CK, Zhang Y. 2013. G protein-coupled estrogen receptor (GPER) agonist dual binding mode analyses toward understanding of its activation mechanism: a comparative homology modeling approach. Mol. Inform 32:647–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mendez-Luna D, Morelos-Garnica LA, Garcia-Vazquez JB, Bello M, Padilla M II, et al. 2021. Modifications on the tetrahydroquinoline scaffold targeting a phenylalanine cluster on GPER as antiproliferative compounds against renal, liver and pancreatic cancer cells. Pharmaceuticals 14:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosano C, Ponassi M, Santolla MF, Pisano A, Felli L, et al. 2016. Macromolecular modelling and docking simulations for the discovery of selective GPER ligands. AAPS J. 18:41–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bruno A, Aiello F, Costantino G, Radi M. 2016. Homology modeling, validation and dynamics of the G protein-coupled estrogen receptor 1 (GPER-1). Mol. Inform 35:333–39 [DOI] [PubMed] [Google Scholar]
- 97.Martinez-Munoz A, Prestegui-Martel B, Mendez-Luna D, Fragoso-Vazquez MJ, Garcia-Sanchez JR, et al. 2018. Selection of a GPER1 ligand via ligand-based virtual screening coupled to molecular dynamics simulations and its anti-proliferative effects on breast cancer cells. Anticancer Agents Med. Chem 18:1629–38 [DOI] [PubMed] [Google Scholar]
- 98.Khan SU, Ahemad N, Chuah L-H, Naidu R, Htar TT. 2019. Sequential ligand- and structure-based virtual screening approach for the identification of potential G protein-coupled estrogen receptor-1 (GPER-1) modulators. RSC Adv. 9:2525–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grande F, Occhiuzzi MA, Lappano R, Cirillo F, Guzzi R, et al. 2020. Computational approaches for the discovery of GPER targeting compounds. Front. Endocrinol 11:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan SU, Ahemad N, Chuah LH, Naidu R, Htar TT. 2020. G protein-coupled estrogen receptor-1: homology modeling approaches and application in screening new GPER-1 modulators. J. Biomol. Struct. Dyn 40:3325–35 [DOI] [PubMed] [Google Scholar]
- 101.D’Arrigo G, Gianquinto E, Rossetti G, Cruciani G, Lorenzetti S, Spyrakis F. 2021. Binding of androgen- and estrogen-like flavonoids to their cognate (non)nuclear receptors: a comparison by computational prediction. Molecules 26:1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burai R, Ramesh C, Shorty M, Curpan R, Bologa C, et al. 2010. Highly efficient synthesis and characterization of the GPR30-selective agonist G-1 and related tetrahydroquinoline analogs. Org. Biomol. Chem 8:2252–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cerra B, Mostarda S, Custodi C, Macchiarulo A, Gioiello A. 2016. Integrating multicomponent flow synthesis and computational approaches for the generation of a tetrahydroquinoline compound based library. MedChemComm 7:439–46 [Google Scholar]
- 104.Zacarias-Lara OJ, Mendez-Luna D, Martinez-Ruiz G, Garcia-Sanchez JR, Fragoso-Vazquez MJ, et al. 2019. Synthesis and in vitro evaluation of tetrahydroquinoline derivatives as antiproliferative compounds of breast cancer via targeting the GPER. Anticancer Agents Med. Chem 19:760–71 [DOI] [PubMed] [Google Scholar]
- 105.Papalia T, Lappano R, Barattucci A, Pisano A, Bruno G, et al. 2015. A Bodipy as a luminescent probe for detection of the G protein estrogen receptor (GPER). Org. Biomol. Chem 13:10437–41 [DOI] [PubMed] [Google Scholar]
- 106.O’Dea A, Sondergard C, Sweeney P, Arnatt CK. 2018. A series of indole-thiazole derivatives act as GPER agonists and inhibit breast cancer cell growth. ACS Med. Chem. Lett 9:901–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maggiolini M, Santolla MF, Avino S, Aiello F, Rosano C, et al. 2015. Identification of two benzopyrroloxazines acting as selective GPER antagonists in breast cancer cells and cancer-associated fibroblasts. Future Med. Chem 7:437–48 [DOI] [PubMed] [Google Scholar]
- 108.Aiello F, Carullo G, Giordano F, Spina E, Nigro A, et al. 2017. Identification of breast cancer inhibitors specific for G protein-coupled estrogen receptor (GPER)-expressing cells. ChemMedChem 12:1279–85 [DOI] [PubMed] [Google Scholar]
- 109.Perri M, Aiello F, Cione E, Carullo G, Amendola L, et al. 2019. Investigation of TNBC in vitro antiproliferative effects of versatile pirrolo[1,2-a]quinoxaline compounds. Front. Mol. Biosci 6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.DeLeon C, Wang HH, Gunn J, Wilhelm M, Cole A, et al. 2020. A novel GPER antagonist protects against the formation of estrogen-induced cholesterol gallstones in female mice. J. Lipid Res 61:767–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pelekanou V, Kampa M, Gallo D, Notas G, Troullinaki M, et al. 2011. The estrogen receptor alpha-derived peptide ERα17p (P295-T311) exerts pro-apoptotic actions in breast cancer cells in vitro and in vivo, independently from their ERα status. Mol. Oncol 5:36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lappano R, Mallet C, Rizzuti B, Grande F, Galli GR, et al. 2019. The peptide ERα17p is a GPER inverse agonist that exerts antiproliferative effects in breast cancer cells. Cells 8:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pepermans RA, Sharma G, Prossnitz ER. 2021. G protein-coupled estrogen receptor in cancer and stromal cells: functions and novel therapeutic perspectives. Cells 10:672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, et al. 2006. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17β-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol. Endocrinol 20:631–46 [DOI] [PubMed] [Google Scholar]
- 115.Vivacqua A, Bonofiglio D, Albanito L, Madeo A, Rago V, et al. 2006. 17β-Estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the G protein-coupled receptor GPR30. Mol. Pharmacol 70:1414–23 [DOI] [PubMed] [Google Scholar]
- 116.Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, et al. 2007. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 67:1859–66 [DOI] [PubMed] [Google Scholar]
- 117.Ariazi EA, Brailoiu E, Yerrum S, Shupp HA, Slifker MJ, et al. 2010. The G protein-coupled receptor GPR30 inhibits proliferation of estrogen receptor-positive breast cancer cells. Cancer Res. 70:1184–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Natale CA, Li J, Zhang J, Dahal A, Dentchev T, et al. 2018. Activation of G protein-coupled estrogen receptor signaling inhibits melanoma and improves response to immune checkpoint blockade. eLife 7:e31770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chan QK, Lam HM, Ng CF, Lee AY, Chan ES, et al. 2010. Activation of GPR30 inhibits the growth of prostate cancer cells through sustained activation of Erk1/2, c-jun/c-fos-dependent upregulation of p21, and induction of G2 cell-cycle arrest. Cell Death Differ. 17:1511–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lam HM, Ouyang B, Chen J, Ying J, Wang J, et al. 2014. Targeting GPR30 with G-1: a new therapeutic target for castration-resistant prostate cancer. Endocr. Relat. Cancer 21:903–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Natale CA, Li J, Pitarresi JR, Norgard RJ, Dentchev T, et al. 2020. Pharmacologic activation of the G protein-coupled estrogen receptor inhibits pancreatic ductal adenocarcinoma. Cell Mol. Gastroenterol. Hepatol 10(4):868–80.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, et al. 2006. Distribution of GPR30, a seven membrane-spanning estrogen receptor, in primary breast cancer and its association with clinicopatho-logic determinants of tumor progression. Clin. Cancer Res 12:6359–66 [DOI] [PubMed] [Google Scholar]
- 123.Ignatov T, Treeck O, Kalinski T, Ortmann O, Ignatov A. 2020. GPER-1 expression is associated with a decreased response rate to primary tamoxifen therapy of breast cancer patients. Arch. Gynecol. Obstet 301:565–71 [DOI] [PubMed] [Google Scholar]
- 124.Ignatov T, Claus M, Nass N, Haybaeck J, Seifert B, et al. 2019. G-protein-coupled estrogen receptor GPER-1 expression in hormone receptor-positive breast cancer is associated with poor benefit of tamoxifen. Breast Cancer Res. Treat 174:121–27 [DOI] [PubMed] [Google Scholar]
- 125.Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, et al. 2007. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am. J. Obstet. Gynecol 196:386.e1–9 [DOI] [PubMed] [Google Scholar]
- 126.Smith HO, Arias-Pulido H, Kuo DY, Howard T, Qualls CR, et al. 2009. GPR30 predicts poor survival for ovarian cancer. Gynecol. Oncol 114:465–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mo Z, Liu M, Yang F, Luo H, Li Z, et al. 2013. GPR30 as an initiator of tamoxifen resistance in hormone-dependent breast cancer. Breast Cancer Res. 15:R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ignatov A, Ignatov T, Weissenborn C, Eggemann H, Bischoff J, et al. 2011. G-protein-coupled estrogen receptor GPR30 and tamoxifen resistance in breast cancer. Breast Cancer Res. Treat 128:457–66 [DOI] [PubMed] [Google Scholar]
- 129.Marjon NA, Hu C, Hathaway HJ, Prossnitz ER. 2014. G protein-coupled estrogen receptor regulates mammary tumorigenesis and metastasis. Mol. Cancer Res 12:1644–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ignatov A, Ignatov T, Roessner A, Costa SD, Kalinski T. 2010. Role of GPR30 in the mechanisms of tamoxifen resistance in breast cancer MCF-7 cells. Breast Cancer Res. Treat 123:87–96 [DOI] [PubMed] [Google Scholar]
- 131.Li Y, Chen Y, Zhu ZX, Liu XH, Yang L, et al. 2013. 4-Hydroxytamoxifen-stimulated processing of cyclin E is mediated via G protein-coupled receptor 30 (GPR30) and accompanied by enhanced migration in MCF-7 breast cancer cells. Toxicology 309:61–65 [DOI] [PubMed] [Google Scholar]
- 132.Catalano S, Giordano C, Panza S, Chemi F, Bonofiglio D, et al. 2014. Tamoxifen through GPER upregulates aromatase expression: a novel mechanism sustaining tamoxifen-resistant breast cancer cell growth. Breast Cancer Res. Treat 146:273–85 [DOI] [PubMed] [Google Scholar]
- 133.Liu Y, Du FY, Chen W, Fu PF, Yao MY, Zheng SS. 2015. G15 sensitizes epithelial breast cancer cells to doxorubicin by preventing epithelial-mesenchymal transition through inhibition of GPR30. Am. J. Transl. Res 7:967–75 [PMC free article] [PubMed] [Google Scholar]
- 134.Wolfson B, Padget MR, Schlom J, Hodge JW. 2021. Exploiting off-target effects of estrogen deprivation to sensitize estrogen receptor negative breast cancer to immune killing. J. Immunother. Cancer 9:e002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wei T, Chen W, Wen L, Zhang J, Zhang Q, et al. 2016. G protein-coupled estrogen receptor deficiency accelerates liver tumorigenesis by enhancing inflammation and fibrosis. Cancer Lett. 382:195–202 [DOI] [PubMed] [Google Scholar]
- 136.Luo H, Yang G, Yu T, Luo S, Wu C, et al. 2014. GPER-mediated proliferation and estradiol production in breast cancer-associated fibroblasts. Endocr. Relat. Cancer 21:355–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang K, Yao Y. 2019. Mechanism of GPER promoting proliferation, migration and invasion of triple-negative breast cancer cells through CAF. Am. J. Transl. Res 11:5858–68 [PMC free article] [PubMed] [Google Scholar]
- 138.Madeo A, Maggiolini M. 2010. Nuclear alternate estrogen receptor GPR30 mediates 17β-estradiol-induced gene expression and migration in breast cancer-associated fibroblasts. Cancer Res. 70:6036–46 [DOI] [PubMed] [Google Scholar]
- 139.Ren J, Guo H, Wu H, Tian T, Dong D, et al. 2015. GPER in CAFs regulates hypoxia-driven breast cancer invasion in a CTGF-dependent manner. Oncol. Rep 33:1929–37 [DOI] [PubMed] [Google Scholar]
- 140.Santolla MF, Vivacqua A, Lappano R, Rigiracciolo DC, Cirillo F, et al. 2019. GPER mediates a feedfor-ward FGF2/FGFR1 paracrine activation coupling CAFs to cancer cells toward breast tumor progression. Cells 8:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.De Marco P, Lappano R, De Francesco EM, Cirillo F, Pupo M, et al. 2016. GPER signalling in both cancer-associated fibroblasts and breast cancer cells mediates a feedforward IL1β/IL1R1 response. Sci. Rep 6:24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC. 2016. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 18:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Divella R, De Luca R, Abbate I, Naglieri E, Daniele A. 2016. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer 7:2346–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sharma G, Hu C, Staquicini DI, Brigman JL, Liu M, et al. 2020. Preclinical efficacy of the GPER-selective agonist G-1 in mouse models of obesity and diabetes. Sci. Transl. Med 12:aau5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Notas G, Kampa M, Castanas E. 2020. G protein-coupled estrogen receptor in immune cells and its role in immune-related diseases. Front. Endocrinol 11:579420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.De Francesco EM, Rocca C, Scavello F, Amelio D, Pasqua T, et al. 2017. Protective role of GPER agonist G-1 on cardiotoxicity induced by doxorubicin. J. Cell. Physiol 232:1640–49 [DOI] [PubMed] [Google Scholar]
- 147.Liu C, Liao Y, Fan S, Fu X, Xiong J, et al. 2019. G-protein-coupled estrogen receptor antagonist G15 decreases estrogen-induced development of non-small cell lung cancer. Oncol. Res 27:283–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shen Y, Li C, Zhou L, Huang JA. 2021. G protein-coupled oestrogen receptor promotes cell growth of non-small cell lung cancer cells via YAP1/QKI/circNOTCH1/m6A methylated NOTCH1 signalling. J. Cell Mol. Med 25:284–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ribeiro MPC, Santos AE, Custodio JBA. 2017. The activation of the G protein-coupled estrogen receptor (GPER) inhibits the proliferation of mouse melanoma K1735-M2 cells. Chem. Biol. Interact 277:176–84 [DOI] [PubMed] [Google Scholar]
- 150.Barton M, Meyer MR. 2020. Heart failure with preserved ejection fraction in women: new clues to causes and treatment. JACC Basic Transl. Sci 5:296–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Meyer MR, Barton M. 2016. Estrogens and coronary artery disease: new clinical perspectives. Adv. Pharmacol 77:307–60 [DOI] [PubMed] [Google Scholar]
- 152.Feldman RD, Limbird LE. 2017. GPER (GPR30): a nongenomic receptor (GPCR) for steroid hormones with implications for cardiovascular disease and cancer. Annu. Rev. Pharmacol. Toxicol 57:567–84 [DOI] [PubMed] [Google Scholar]
- 153.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, et al. 2009. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ. Res 104:288–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meyer MR, Baretella O, Prossnitz ER, Barton M. 2010. Dilation of epicardial coronary arteries by the G protein-coupled estrogen receptor agonists G-1 and ICI 182,780. Pharmacology 86:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lindsey SH, Liu L, Chappell MC. 2014. Vasodilation by GPER in mesenteric arteries involves both endothelial nitric oxide and smooth muscle cAMP signaling. Steroids 81:99–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jessup JA, Lindsey SH, Wang H, Chappell MC, Groban L. 2010. Attenuation of salt-induced cardiac remodeling and diastolic dysfunction by the GPER agonist G-1 in female mRen2.Lewis rats. PLOS ONE 5:e15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang H, Jessup JA, Lin MS, Chagas C, Lindsey SH, Groban L. 2012. Activation of GPR30 attenuates diastolic dysfunction and left ventricle remodelling in oophorectomized mRen2.Lewis rats. Cardiovasc. Res 94:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Alencar AK, da Silva JS, Lin M, Silva AM, Sun X, et al. 2017. Effect of age, estrogen status, and late-life GPER activation on cardiac structure and function in the Fischer344xBrown Norway female rat. J. Gerontol. A Biol. Sci. Med. Sci 72:152–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ogola BO, Zimmerman MA, Sure VN, Gentry KM, Duong JL, et al. 2019. G protein-coupled estrogen receptor protects from angiotensin II-induced increases in pulse pressure and oxidative stress. Front. Endocrinol 10:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Davis GK, Newsome AD, Cole AB, Ojeda NB, Alexander BT. 2019. Chronic estrogen supplementation prevents the increase in blood pressure in female intrauterine growth-restricted offspring at 12 months of age. Hypertension 73:1128–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Meyer MR, Fredette NC, Daniel C, Sharma G, Amann K, et al. 2016. Obligatory role for GPER in cardiovascular aging and disease. Sci. Signal 9:ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Azizian H, Khaksari M, Asadi Karam G, Esmailidehaj M, Farhadi Z. 2018. Cardioprotective and anti-inflammatory effects of G-protein coupled receptor 30 (GPR30) on postmenopausal type 2 diabetic rats. Biomed. Pharmacother 108:153–64 [DOI] [PubMed] [Google Scholar]
- 163.Alencar AKN, Montes GC, Costa DG, Mendes LVP, Silva AMS, et al. 2018. Cardioprotection induced by activation of GPER in ovariectomized rats with pulmonary hypertension. J. Gerontol. A Biol. Sci. Med. Sci 73:1158–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Alencar AK, Montes GC, Montagnoli T, Silva AM, Martinez ST, et al. 2017. Activation of GPER ameliorates experimental pulmonary hypertension in male rats. Eur. J. Pharm. Sci 97:208–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Meyer MR, Fredette NC, Howard TA, Hu C, Ramesh C, et al. 2014. G protein-coupled estrogen receptor protects from atherosclerosis. Sci. Rep 4:7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jehle J, Becher U, Nöthel M, Adler S, Groll K, et al. 2021. G protein-coupled estrogen receptor GPR30 exerts vasoprotective effects in apolipoprotein E-deficient mice. Arch. Med. Sci 10.5114/aoms/127200 [DOI] [Google Scholar]
- 167.Li F, Yu X, Szynkarski CK, Meng C, Zhou B, et al. 2013. Activation of GPER induces differentiation and inhibition of coronary artery smooth muscle cell proliferation. PLOS ONE 8:e64771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. 2017. Endothelial dysfunction and vascular disease—a 30th anniversary update. Acta Physiol. 219:22–96 [DOI] [PubMed] [Google Scholar]
- 169.Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. 2006. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin. Res. Cardiol 95:136–47 [DOI] [PubMed] [Google Scholar]
- 170.Meyer MR, Clegg DJ, Prossnitz ER, Barton M. 2011. Obesity, insulin resistance and diabetes: sex differences and role of oestrogen receptors. Acta Physiol. 203:259–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kozakowski J, Gietka-Czernel M, Leszczynska D, Majos A. 2017. Obesity in menopause—our negligence or an unfortunate inevitability? Prz. Menopauzalny 16:61–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nakhjavani M, Imani M, Larry M, Aghajani-Nargesi A, Morteza A, Esteghamati A. 2014. Metabolic syndrome in premenopausal and postmenopausal women with type 2 diabetes: loss of protective effects of premenopausal status. J. Diabetes Metab. Disord 13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stubbins RE, Najjar K, Holcomb VB, Hong J, Nunez NP. 2012. Oestrogen alters adipocyte biology and protects female mice from adipocyte inflammation and insulin resistance. Diabetes Obes. Metab 14:58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stubbins RE, Holcomb VB, Hong J, Nunez NP. 2012. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr 51:861–70 [DOI] [PubMed] [Google Scholar]
- 175.Gurney EP, Nachtigall MJ, Nachtigall LE, Naftolin F. 2014. The Women’s Health Initiative trial and related studies: 10 years later: a clinician’s view. J. Steroid Biochem. Mol. Biol 142:4–11 [DOI] [PubMed] [Google Scholar]
- 176.Bonds DE, Lasser N, Qi L, Brzyski R, Caan B, et al. 2006. The effect of conjugated equine oestrogen on diabetes incidence: the Women’s Health Initiative randomised trial. Diabetologia 49:459–68 [DOI] [PubMed] [Google Scholar]
- 177.Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, Prossnitz ER. 2013. GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology 154:4136–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, et al. 2009. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology 150:687–98 [DOI] [PubMed] [Google Scholar]
- 179.Davis KE, Carstens EJ, Irani BG, Gent LM, Hahner LM, Clegg DJ. 2014. Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis. Horm. Behav 66:196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Meoli L, Isensee J, Zazzu V, Nabzdyk CS, Soewarto D, et al. 2014. Sex- and age-dependent effects of Gpr30 genetic deletion on the metabolic and cardiovascular profiles of diet-induced obese mice. Gene 540:210–16 [DOI] [PubMed] [Google Scholar]
- 181.Butler MJ, Hildebrandt RP, Eckel LA. 2018. Selective activation of estrogen receptors, ERα and GPER-1, rapidly decreases food intake in female rats. Horm. Behav 103:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Jablonski EM, McConnell NA, Hughes FM Jr., Huet-Hudson YM. 2003. Estrogen regulation of aqua-porins in the mouse uterus: potential roles in uterine water movement. Biol. Reprod 69(5):1481–87 [DOI] [PubMed] [Google Scholar]
- 183.Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, et al. 2009. Importance of extranuclear estrogen receptor-α and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes 58:2292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sharma G, Prossnitz ER. 2011. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic β-cells. Endocrinology 152:3030–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Balhuizen A, Kumar R, Amisten S, Lundquist I, Salehi A. 2010. Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Mol. Cell. Endocrinol 320:16–24 [DOI] [PubMed] [Google Scholar]
- 186.Kumar R, Balhuizen A, Amisten S, Lundquist I, Salehi A. 2011. Insulinotropic and antidiabetic effects of 17β-estradiol and the GPR30 agonist G-1 on human pancreatic islets. Endocrinology 152:2568–79 [DOI] [PubMed] [Google Scholar]
- 187.Sun M, Xie HF, Tang Y, Lin SQ, Li JM, et al. 2017. G protein-coupled estrogen receptor enhances melanogenesis via cAMP-protein kinase (PKA) by upregulating microphthalmia-related transcription factor-tyrosinase in melanoma. J. Steroid Biochem. Mol. Biol 165:236–46 [DOI] [PubMed] [Google Scholar]
- 188.Natale CA, Duperret EK, Zhang J, Sadeghi R, Dahal A, et al. 2016. Sex steroids regulate skin pigmentation through nonclassical membrane-bound receptors. eLife 5:e15104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Castleman MJ, Pokhrel S, Triplett KD, Kusewitt DF, Elmore BO, et al. 2018. Innate sex bias of Staphylococcus aureus skin infection is driven by α-hemolysin. J. Immunol 200:657–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Brandenburg JS, Clark RM, Coffman B, Sharma G, Hathaway HJ, et al. 2020. Sex differences in murine myocutaneous flap revascularization. Wound Repair Regen. 28:470–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Toutain CE, Brouchet L, Raymond-Letron I, Vicendo P, Berges H, et al. 2009. Prevention of skin flap necrosis by estradiol involves reperfusion of a protected vascular network. Circ. Res 104:245–54 [DOI] [PubMed] [Google Scholar]
- 192.Farruggio S, Cocomazzi G, Marotta P, Romito R, Surico D, et al. 2020. Genistein and 17β-estradiol protect hepatocytes from fatty degeneration by mechanisms involving mitochondria, inflammasome and kinases activation. Cell. Physiol. Biochem 54:401–16 [DOI] [PubMed] [Google Scholar]
- 193.de Bari O, Wang TY, Liu M, Portincasa P, Wang DQ. 2015. Estrogen induces two distinct cholesterol crystallization pathways by activating ERα and GPR30 in female mice. J. Lipid Res 56:1691–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang HH, de Bari O, Arnatt CK, Liu M, Portincasa P, Wang DQ. 2020. Activation of estrogen receptor G protein–coupled receptor 30 enhances cholesterol cholelithogenesis in female mice. Hepatology 72:2077–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zielinska M, Fichna J, Bashashati M, Habibi S, Sibaev A, et al. 2017. G protein-coupled estrogen receptor and estrogen receptor ligands regulate colonic motility and visceral pain. Neurogastroenterol. Motil 29:e13025. [DOI] [PubMed] [Google Scholar]
- 196.Li Y, Xu J, Jiang F, Jiang Z, Liu C, et al. 2016. G protein-coupled estrogen receptor is involved in modulating colonic motor function via nitric oxide release in C57BL/6 female mice. Neurogastroenterol. Motil 28:432–42 [DOI] [PubMed] [Google Scholar]
- 197.Chai S, Liu K, Feng W, Liu T, Wang Q, et al. 2019. Activation of G protein-coupled estrogen receptor protects intestine from ischemia/reperfusion injury in mice by protecting the crypt cell proliferation. Clin. Sci 133:449–64 [DOI] [PubMed] [Google Scholar]
- 198.Jacenik D, Zielinska M, Mokrowiecka A, Michlewska S, Malecka-Panas E, et al. 2019. G protein-coupled estrogen receptor mediates anti-inflammatory action in Crohn’s disease. Sci. Rep 9:6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang Q, Li Z, Liu K, Liu J, Chai S, et al. 2021. Activation of the G protein-coupled estrogen receptor prevented the development of acute colitis by protecting the crypt cell. J. Pharmacol. Exp. Ther 376:281–93 [DOI] [PubMed] [Google Scholar]
- 200.Cao J, Lu M, Yan W, Li L, Ma H. 2021. Dehydroepiandrosterone alleviates intestinal inflammatory damage via GPR30-mediated Nrf2 activation and NLRP3 inflammasome inhibition in colitis mice. Free Radic. Biol. Med 172:386–402 [DOI] [PubMed] [Google Scholar]
- 201.Wlodarczyk M, Sobolewska-Wlodarczyk A, Cygankiewicz AI, Jacenik D, Piechota-Polanczyk A, et al. 2017. G protein-coupled receptor 30 (GPR30) expression pattern in inflammatory bowel disease patients suggests its key role in the inflammatory process. A preliminary study. J. Gastrointestin. Liver Dis 26:29–35 [DOI] [PubMed] [Google Scholar]
- 202.Qin B, Dong L, Guo X, Jiang J, He Y, et al. 2014. Expression of G protein-coupled estrogen receptor in irritable bowel syndrome and its clinical significance. Int. J. Clin. Exp. Pathol 7:2238–46 [PMC free article] [PubMed] [Google Scholar]
- 203.Meyer MR, Rosemann T, Barton M, Prossnitz ER. 2017. GPER mediates functional endothelial aging in renal arteries. Pharmacology 100:188–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chang Y, Han Z, Zhang Y, Zhou Y, Feng Z, et al. 2019. G protein-coupled estrogen receptor activation improves contractile and diastolic functions in rat renal interlobular artery to protect against renal ischemia reperfusion injury. Biomed. Pharmacother 112:108666. [DOI] [PubMed] [Google Scholar]
- 205.Hofmeister MV, Damkier HH, Christensen BM, Olde B, Leeb-Lundberg LMF, et al. 2012. 17β-Estradiol induces nongenomic effects in renal intercalated cells through G protein-coupled estrogen receptor 1. Am. J. Physiol. Ren. Physiol 302:F358–68 [DOI] [PubMed] [Google Scholar]
- 206.Gohar EY, Daugherty EM, Aceves JO, Sedaka R, Obi IE, et al. 2020. Evidence for G-protein-coupled estrogen receptor as a pronatriuretic factor. J. Am. Heart Assoc 9:e015110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Qiao C, Ye W, Li S, Wang H, Ding X. 2018. Icariin modulates mitochondrial function and apoptosis in high glucose–induced glomerular podocytes through G protein–coupled estrogen receptors. Mol. Cell. Endocrinol 473:146–55 [DOI] [PubMed] [Google Scholar]
- 208.Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. 2011. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension 58:665–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Gohar EY, Almutlaq RN, Daugherty EM, Butt MK, Jin C, et al. 2021. Activation of G protein-coupled estrogen receptor 1 ameliorates proximal tubular injury and proteinuria in Dahl salt-sensitive female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol 320:R297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kurt AH, Bozkus F, Uremis N, Uremis MM. 2016. The protective role of G protein-coupled estrogen receptor 1 (GPER-1) on methotrexate-induced nephrotoxicity in human renal epithelium cells. Ren. Fail 38:686–92 [DOI] [PubMed] [Google Scholar]
- 211.Meyer MR, Daniel C, Fredette NC, Amann K, Barton M, Prossnitz ER. 2015. Deletion of GPER protects from age-related renovascular dysfunction and tubulo-interstitial injury. Hypertension 66(Suppl. 1):AP607 [Google Scholar]
- 212.Wang C, Dehghani B, Li Y, Kaler LJ, Proctor T, et al. 2009. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through up-regulation of programmed death 1. J. Immunol 182:3294–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Itoga M, Konno Y, Moritoki Y, Saito Y, Ito W, et al. 2015. G-protein-coupled estrogen receptor agonist suppresses airway inflammation in a mouse model of asthma through IL-10. PLOS ONE 10:e0123210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jacenik D, Beswick EJ, Krajewska WM, Prossnitz ER. 2019. G protein-coupled estrogen receptor in colon function, immune regulation and carcinogenesis. World J. Gastroenterol 25:4092–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Du ZR, Feng XQ, Li N, Qu JX, Feng L, et al. 2018. G protein-coupled estrogen receptor is involved in the anti-inflammatory effects of genistein in microglia. Phytomedicine 43:11–20 [DOI] [PubMed] [Google Scholar]
- 216.Guan J, Yang B, Fan Y, Zhang J. 2017. GPER agonist G1 attenuates neuroinflammation and dopaminergic neurodegeneration in Parkinson disease. Neuroimmunomodulation 24:60–66 [DOI] [PubMed] [Google Scholar]
- 217.Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, et al. 2009. Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. J. Neuroimmunol 214:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Roque C, Mendes-Oliveira J, Duarte-Chendo C, Baltazar G. 2019. The role of G protein-coupled estrogen receptor 1 on neurological disorders. Front. Neuroendocrinol 55:100786. [DOI] [PubMed] [Google Scholar]
- 219.Brunsing RL, Owens KS, Prossnitz ER. 2013. The G protein-coupled estrogen receptor (GPER) agonist G-1 expands the regulatory T-cell population under TH17-polarizing conditions. J. Immunother 36:190–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Brunsing RL, Prossnitz ER. 2011. Induction of interleukin-10 in the T helper type 17 effector population by the G protein coupled estrogen receptor (GPER) agonist G-1. Immunology 134:93–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Harding AT, Goff MA, Froggatt HM, Lim JK, Heaton NS. 2021. GPER1 is required to protect fetal health from maternal inflammation. Science 371:271–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kubota T, Matsumoto H, Kirino Y. 2016. Ameliorative effect of membrane-associated estrogen receptor G protein coupled receptor 30 activation on object recognition memory in mouse models of Alzheimer’s disease. J. Pharmacol. Sci 131:219–22 [DOI] [PubMed] [Google Scholar]
- 223.Wang ZF, Pan ZY, Xu CS, Li ZQ. 2017. Activation of G-protein coupled estrogen receptor 1 improves early-onset cognitive impairment via PI3K/Akt pathway in rats with traumatic brain injury. Biochem. Biophys. Res. Commun 482:948–53 [DOI] [PubMed] [Google Scholar]
- 224.Amirkhosravi L, Khaksari M, Soltani Z, Esmaeili-Mahani S, Asadi Karam G, Hoseini M. 2021. E2-BSA and G1 exert neuroprotective effects and improve behavioral abnormalities following traumatic brain injury: the role of classic and non-classic estrogen receptors. Brain Res. 1750:147168. [DOI] [PubMed] [Google Scholar]
- 225.Bai N, Zhang Q, Zhang W, Liu B, Yang F, et al. 2020. G-protein-coupled estrogen receptor activation upregulates interleukin-1 receptor antagonist in the hippocampus after global cerebral ischemia: implications for neuronal self-defense. J. Neuroinflamm 17:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Prossnitz ER. 2012. G protein-coupled estrogen receptor: a new therapeutic target in stroke and traumatic brain/spinal cord injury? Crit. Care Med 40:3323–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hu R, Sun H, Zhang Q, Chen J, Wu N, et al. 2012. G-protein coupled estrogen receptor 1 mediated estrogenic neuroprotection against spinal cord injury. Crit. Care Med 40:3230–37 [DOI] [PubMed] [Google Scholar]
- 228.Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, et al. 2010. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLOS ONE 5:e8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhang Z, Qin P, Deng Y, Ma Z, Guo H, et al. 2018. The novel estrogenic receptor GPR30 alleviates ischemic injury by inhibiting TLR4-mediated microglial inflammation. J. Neuroinflamm 15:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lu D, Qu Y, Shi F, Feng D, Tao K, et al. 2016. Activation of G protein-coupled estrogen receptor 1 (GPER-1) ameliorates blood-brain barrier permeability after global cerebral ischemia in ovariectomized rats. Biochem. Biophys. Res. Commun 477:209–14 [DOI] [PubMed] [Google Scholar]
- 231.Murata T, Dietrich HH, Xiang C, Dacey RG Jr. 2013. G protein-coupled estrogen receptor agonist improves cerebral microvascular function after hypoxia/reoxygenation injury in male and female rats. Stroke 44:779–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang B, Subramanian S, Dziennis S, Jia J, Uchida M, et al. 2010. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J. Immunol 184:4087–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Han ZW, Chang YC, Zhou Y, Zhang H, Chen L, et al. 2019. GPER agonist G1 suppresses neuronal apoptosis mediated by endoplasmic reticulum stress after cerebral ischemia/reperfusion injury. Neural Regen. Res 14:1221–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang XS, Yue J, Hu LN, Tian Z, Zhang K, et al. 2020. Activation of G protein-coupled receptor 30 protects neurons by regulating autophagy in astrocytes. Glia 68:27–43 [DOI] [PubMed] [Google Scholar]
- 235.Wang J, Li HY, Shen SY, Zhang JR, Liang LF, et al. 2021. The antidepressant and anxiolytic effect of GPER on translocator protein (TSPO) via protein kinase a (PKA) signaling in menopausal female rats. J. Steroid Biochem. Mol. Biol 207:105807. [DOI] [PubMed] [Google Scholar]
- 236.Zheng Y, Wu M, Gao T, Meng L, Ding X, et al. 2020. GPER-deficient rats exhibit lower serum corticosterone level and increased anxiety-like behavior. Neural Plast. 2020:8866187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yang S, Yin Z, Zhu G. 2021. A review of the functions of G protein-coupled estrogen receptor 1 in vascular and neurological aging. Eur. J. Pharmacol 908:174363. [DOI] [PubMed] [Google Scholar]
- 238.Oliveira de Souza L, Barroso Machado GD, Souza de Freitas B, Camargo Rodrigues SL, Severo MPA, et al. 2021. The G protein-coupled estrogen receptor (GPER) regulates recognition and aversively-motivated memory in male rats. Neurobiol. Learn. Mem 184:107499. [DOI] [PubMed] [Google Scholar]
- 239.Zhang C, Liu Q, Yu CY, Wang F, Shao Y, et al. 2020. G protein-coupled estrogen receptor 1 knockout deteriorates MK-801-induced learning and memory impairment in mice. Front. Behav. Neurosci 14:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Barroso Machado GD, Souza de Freitas B, Zanetti Florian L, Tavares Monteiro R, Gus H, Schroder N. 2019. G protein-coupled oestrogen receptor stimulation ameliorates iron- and ovariectomy-induced memory impairments through the cAMP/PKA/CREB signalling pathway. J. Neuroendocrinol 31:e12780. [DOI] [PubMed] [Google Scholar]
- 241.Dominguez-Ordonez R, Garcia-Juarez M, Lima-Hernandez FJ, Gomora-Arrati P, Dominguez-Salazar E, et al. 2018. Lordosis facilitated by GPER-1 receptor activation involves GnRH-1, progestin and estrogen receptors in estrogen-primed rats. Horm. Behav 98:77–87 [DOI] [PubMed] [Google Scholar]


