Abstract
Aims
Neutrophil extracellular trap formation (NETosis) increases atherosclerotic plaque vulnerability and athero-thrombosis. However, mechanisms promoting NETosis during atherogenesis are poorly understood. We have shown that cholesterol accumulation due to myeloid cell deficiency of the cholesterol transporters ATP Binding Cassette A1 and G1 (ABCA1/G1) promotes NLRP3 inflammasome activation in macrophages and neutrophils and induces prominent NETosis in atherosclerotic plaques. We investigated whether NETosis is a cell-intrinsic effect in neutrophils or is mediated indirectly by cellular crosstalk from macrophages to neutrophils involving IL-1β.
Methods and results
We generated mice with neutrophil or macrophage-specific Abca1/g1 deficiency (S100A8CreAbca1fl/flAbcg1fl/fl or CX3CR1CreAbca1fl/flAbcg1fl/fl mice, respectively), and transplanted their bone marrow into low-density lipoprotein receptor knockout mice. We then fed the mice a cholesterol-rich diet. Macrophage, but not neutrophil Abca1/g1 deficiency activated inflammasomes in macrophages and neutrophils, reflected by caspase-1 cleavage, and induced NETosis in plaques. NETosis was suppressed by administering an interleukin (IL)-1β neutralizing antibody. The extent of NETosis in plaques correlated strongly with the degree of neutrophil accumulation, irrespective of blood neutrophil counts, and neutrophil accumulation was decreased by IL-1β antagonism. In vitro, IL-1β or media transferred from Abca1/g1-deficient macrophages increased NETosis in both control and Abca1/Abcg1 deficient neutrophils. This cell-extrinsic effect of IL-1β on NETosis was blocked by an NLRP3 inhibitor.
Conclusion
These studies establish a new link between inflammasome-mediated IL-1β production in macrophages and NETosis in atherosclerotic plaques. Macrophage-derived IL-1β appears to increase NETosis both by increasing neutrophil recruitment to plaques and by promoting neutrophil NLRP3 inflammasome activation.
Keywords: Atherosclerosis, Leukocyte, Inflammation
Graphical Abstract
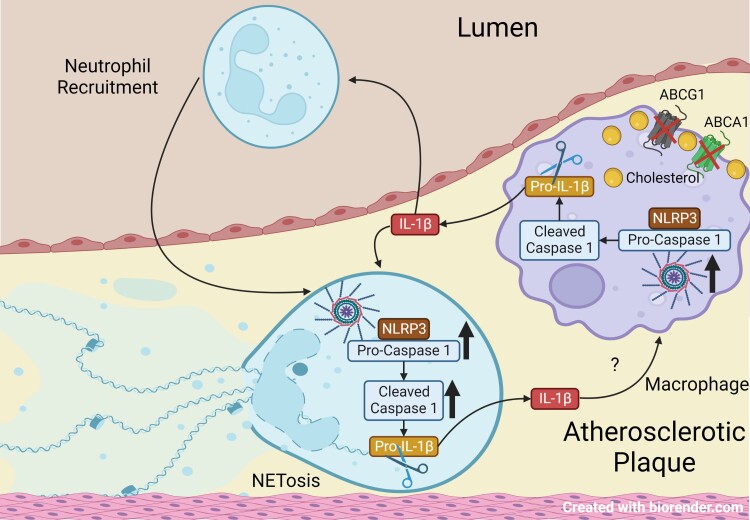
Graphical abstract.
1. Introduction
NLRP3 inflammasome activation leads to the secretion of active IL-1β and IL-18 and contributes to sterile inflammation in several metabolic diseases, including obesity and atherosclerosis.1,2 Antagonism of IL-1β decreased CVD events in the Canakinumab Anti-inflammatory Thrombosis Outcome Study (CANTOS),3 indicating the importance of inflammatory processes and likely inflammasomes in human athero-thrombosis. However, the mechanisms linking inflammasomes and IL-1β to aggravated atherosclerosis remain incompletely understood.
Neutrophil extracellular traps (NETs), originally known for their role in trapping bacteria and inactivating pathogens during inflammation as the first line of host defence,4 also promote atherosclerotic plaque vulnerability and athero-thrombosis.5–8 Several different factors including platelet activation, hyperlipidaemia, and oxidized phospholipids can induce NETosis.6,9–12 Recent studies have shown that activation of NLRP3 and non-canonical inflammasomes in neutrophils can also induce NETosis.13–15 However, the in vivo importance of neutrophil inflammasome activation and its potential role in atherosclerosis remain poorly understood.
Cholesterol efflux pathways exert anti-atherogenic effects by suppressing inflammatory responses in myeloid cells.16–18 The ATP binding cassette transporters A1 and G1 (ABCA1 and ABCG1) mediate cholesterol efflux to apolipoprotein A-1 and HDL.19–21 Low levels of ABCA1/G1 in monocyte/macrophages along with low plasma HDL levels commonly occur in humans with poorly controlled diabetes mellitus,22 chronic kidney disease,23 with myelopoiesis induced by trained immunity24 or simply with ageing.25 Deletion of these transporters by crossing Abca1fl/flAbcg1fl/fl mice with different Cre strains enables an examination of the cell-specific effects of cholesterol accumulation. Recently, we showed increased atherosclerosis and lesional NETosis in Ldlr−/− mice with myeloid deficiency of these transporters (Myl-Abcdko mice), which was mediated through activation of the NLRP3 inflammasome.16 However, whether NETosis was a cell-intrinsic effect in neutrophils or was the result of inflammatory crosstalk between macrophages and neutrophils could not be determined in these studies.
To address this question, we generated mice with neutrophil-specific (S100A8-Cre) or macrophage-specific (CX3CR1-Cre) knockout of Abca1/g1 in the Ldlr−/− background. Deficiency of ABC transporters specifically in macrophages led to inflammasome activation in both macrophages and neutrophils and elevated NETs in plaques whereas neutrophil Abca1/g1 deficiency did not cause inflammasome activation or NETosis. Moreover, IL-1β had a central role in this process since its neutralization with antibodies reduced NETosis in atherosclerotic lesions of Myl-Abcdko Ldlr−/− mice. Our findings identify a new link between macrophage cholesterol accumulation, inflammasome activation, IL-1β, and NETosis in atherosclerotic plaques.
2. Methods
2.1. Animals
All mice except Abca1fl/flAbcg1fl/fl mice were from Jackson Laboratories. Abca1fl/flAbcg1fl/fl mice were generated in our laboratory by crossbreeding Abca1fl/fl mice (kindly provided by Dr. John Parks, Wake Forest University)26 with Abcg1fl/fl mice (generated at Columbia University),17 and Abca1fl/flAbcg1fl/fl mice were deposited at Jackson Laboratories (stock number 021067). Stock numbers of other strains are indicated. S100A8CreAbca1fl/flAbcg1fl/fl, CSF1RCreAbca1fl/flAbcg1fl/fl, and CX3CR1CreAbca1fl/flAbcg1fl/fl mice were generated by crossbreeding Abca1fl/flAbcg1fl/fl with S100A8-Cre (021614), CSF1RCre (029206), or CX3CR1Cre (025524) mice, respectively. LysmCreAbca1fl/flAbcg1fl/fl were generated by crossbreeding Abca1fl/flAbcg1fl/fl with LysmCre mice (004781), as described before.17Abca1fl/flAbcg1fl/flNlrp3−/−, LysmCreAbca1fl/flAbcg1fl/flNlrp3−/−, Abca1fl/flAbcg1fl/flCaspase1−/−11−/−, LysmCreAbca1fl/flAbcg1fl/flCaspase1−/−11−/−, Abca1fl/flAbcg1fl/flCaspase11−/−, and LysmCreAbca1fl/flAbcg1fl/flCaspase11−/− mice were generated by crossbreeding Abca1fl/flAbcg1fl/fl and LysmCreAbca1fl/flAbcg1fl/fl mice with Nlrp3−/− (021302) or Caspase1−/−11−/− mice (016621), as described previously,16 or Caspase11−/− mice (024698). Ldlr−/− mice (002207) were bought. For most experiments, female mice were used since they are more prone to developing atherogenesis. Abca1fl/flAbcg1fl/fl mice are referred to as control mice, LysmCreAbca1fl/flAbcg1fl/fl mice as Myl-Abcdko mice, Abca1fl/flAbcg1fl/flNlrp3−/− mice as Nlrp3−/− mice, Abca1fl/flAbcg1fl/flCaspase1−/−11−/− mice as Caspase1−/−11−/− mice, Abca1fl/flAbcg1fl/flCaspase11−/− mice as Caspase11−/− mice, LysmCreAbca1fl/flAbcg1fl/flNlrp3−/− mice as Myl-AbcdkoNlrp3−/− mice, LysmCreAbca1fl/flAbcg1fl/flCaspase1−/−11−/− mice as Myl-AbcdkoCaspase1−/−11−/− mice, and LysmCreAbca1fl/flAbcg1fl/flCaspase11−/− mice as Myl-AbcdkoCaspase11−/− mice. All mice used for these studies were on a C57BL/6J background and were housed in a specific pathogen-free facility under standard conditions of temperature (about 23°C) with a 12 h light–dark cycle and food available ad lib (humidity was not noted). Cages and water were changed every 14–21 days. For euthanasia, we used CO2 inhalation at a rate of 1.9–4.4 L/min for a minimum of 20 min, followed by cervical dislocation. CO2 is an asphyxiation agent used for endpoint euthanasia in mice. No anaesthetics were used in our studies. All mouse experiments were approved by the Institutional Animal Care and Use Committee of Columbia University and were conducted in accordance with the Institutional Animal Care and Use Committee of Columbia University guidelines.
2.2. Bone marrow transplantation
At 8 weeks of age, female Ldlr−/− mice were lethally irradiated with 1 dose of 9.12 Gy from a cesium g source. At 24 h after irradiation, mice were injected with 5–10*106 bone marrow (BM) cells in DMEM containing 2% FBS from control, S100A8CreAbca1fl/flAbcg1fl/fl, CSF1RCreAbca1fl/flAbcg1fl/fl, CX3CR1CreAbca1fl/flAbcg1fl/fl, Myl-Abcdko, Nlrp3−/−, Myl-AbcdkoNlrp3−/−, Caspase1−/−11−/−, Myl-AbcdkoCaspase1−/−11−/−, Caspase11−/−, or Myl-AbcdkoCaspase11−/− mice. Mice were kept on a chow diet for 5 weeks after BM transplantation to allow for complete reconstitution of the BM, before Western-type diet (WTD; Harlan Teklad TD88137) feeding for 8–8.5 weeks.
2.3. IL-1β antagonism study in vivo
Ldlr−/− mice were transplanted with BM from Myl-Abcdko mice and fed WTD. After 6 weeks of WTD, anti-mouse monoclonal antibodies (IgG2a) that selectively neutralize IL-1β (01BSUR, Novartis), or isotype-matched control IgG2a (Novartis) were administered subcutaneously at a dose of 10 mg/kg weekly.27 Mice received three injections of antibody at 6, 7, and 8 weeks of WTD. At 8.5 weeks of WTD, mice were sacrificed to evaluate the contribution of IL-1β to NETosis.
2.4. Atherosclerosis studies
After the indicated period of time on WTD, mice were sacrificed and hearts were perfused with PBS, isolated, and fixed in phosphate-buffered formalin. Subsequently, hearts were dehydrated and embedded in paraffin, and were cross-sectioned throughout the aortic root area. Haematoxylin-eosin staining was performed on the sections, and the average from six sections for each animal was used to determine lesion size. Lesion size and necrotic area were quantified by morphometric analysis using Image-Pro Plus software (Media Cybernetics, USA).
2.5. Immunofluorescence staining on atherosclerotic plaques
For staining of NETs, paraffin sections were incubated in Tris-Base EDTA at pH 9.0 (15–20 min; pressure cooker) for antigen retrieval. Then, sections were blocked in PBS containing 10% goat serum for 30 min at 4°C. Subsequently, sections were incubated o/n at 4°C with biotinylated myeloperoxidase (MPO) (1:30; R&D systems; BAF3667), Ly6G (1:200; BioLegend; 127602) or Mac-2 (1:10000; Cedarlane; CL8942). When citrullinated histones were stained, sections were concomitantly incubated with Anti-Histone H3 (citrulline R2 + R8 + R17) antibody (1:300; Abcam; ab5103). For MPO staining, the sections were then incubated with Streptavidin Alexa Fluor 488 (1:200; Invitrogen/Life Technologies; S11223). Anti-rat CF 488A (1:200; Thermo Fisher, A11006) was used as a secondary antibody for Ly6G and Mac-2 staining. When citrullinated histones were stained, sections were concomitantly incubated with Alexa-647 (1:200; Invitrogen; A-21447). Sections were mounted using ProLong Gold Antifade Mountant with DAPI (Thermofisher; P3693) and imaged using a Leica DMI6000B microscope running Leica software. The MPO-positive area was quantified using Image-Pro Plus software (Media Cybernetics, USA) and expressed as % of the total lesion area. The overlap of MPO staining with citrullinated histone staining or Ly6G staining with citrullinated histone staining was assessed as NETs and quantified as % of total lesion area using Image-Pro Plus software (Media Cybernetics, USA). The data show the average of three sections from one mouse that were taken at the same location in the aortic root for each animal, adjacent to the sections used for the first and the sixth atherosclerotic lesion measurement, and in between the third and fourth.
2.6. In vitro NETs assay
Bone marrow neutrophils were isolated using Ly6G coated beads (Miltenyi Biotec) according to the manufacturer’s instructions. Cells were plated on Poly-L-Lysine coated plates and allowed to adhere for 30 min at 37°C in DMEM supplemented with 1% pen-strep. Cells were then treated with 100 ng/ml IL-1β (I5271-5UG, Sigma) and/or 100 ng/mL G-CSF (250–05, Peprotech) or conditioned medium for macrophages (see further description below) for 3 h. For NLRP3 inhibition experiments, cells were pre-treated with MCC950 (40 nM, Cayman, 17 510) for 30 min, and subsequently, IL-1β or macrophage-conditioned medium was added for a period of 3 h. For cholesterol loading experiments, neutrophils were treated with acetylated LDL (acLDL) at a concentration of 25 µg/mL for 3 h during incubation with IL-1β. For experiments evaluating the contribution of oxidized lipids to NETosis, neutrophils were treated with 100 µg/mL oxidized LDL (oxLDL) or 20 µg/mL 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine for 3 h. Cells were then washed with PBS, fixed in 4% paraformaldehyde (10 min; RT), washed twice using PBS, permeabilized with PBS-T for 10 min, and incubated overnight at 4°C in PBS 1% BSA containing anti-histone H3 (citrulline R2 + R8 + R17) antibody (1:250; Abcam; ab5103) and biotinylated MPO antibody (1:30; R&D systems; BAF3667). Subsequently, cells were washed and incubated in PBS containing anti-rabbit CF 647 (Sigma; SAB4600184) antibody (1:200) and Streptavidin Alexa Fluor 488 (1:200; Invitrogen/Life Technologies; S11223) for 30 min at RT. Cells were mounted using ProLong Gold Antifade Mountant with DAPI (Thermofisher; P3693) and imaged using a Leica DMI6000B microscope running Leica software. The overlap of citrullinated histones and DAPI was quantified using Image-Pro Plus software (Media Cybernetics, USA) and expressed as % of total cells. For experiments employing acLDL, cholesterol loading was verified using kit from Wako (Cholesterol E; 999–02601) following lipid extraction using the Folch method.
For experiments employing conditioned medium from control and Myl-Abcdko macrophages, BM was isolated from these mice by flushing bones with PBS. Cells were then cultured in DMEM supplemented with 10% FBS, 1% pen-strep, and 20% L929-cell conditioned medium for a period of 5 days, to obtain fully differentiated macrophages. To induce NLRP3 inflammasome activation, cells were stimulated with LPS (20 ng/mL; Sigma-Aldrich, L3024) for 3 h and incubated with ATP (5 mM; Sigma-Aldrich, GE27-2056-01) for 30 min. Cells were then washed with PBS and subsequently incubated for 1 h in DMEM supplemented with 1% pen-strep. The medium was collected and was mixed 1:1 with DMEM containing 1% pen-strep, and incubated with neutrophils (collected as described above) that had been pre-treated with IL-1β antibody, IgG control (both 100 µg/mL) or MCC950 (details stated above) for 30 min. Macrophage-conditioned medium was then added and after 3 h, NETosis was assessed as described above.
2.7. Inflammasome assay
Bone marrow-derived macrophages (BMDMs) were preincubated with LPS (20 ng/mL) for 3 h and then incubated with 2 mM ATP (Sigma-Aldrich, GE27-2056-01) or 10 μg/mL Nigericin (Sigma-Aldrich, SML-1779) for 1 h. To assess inflammasome activation in neutrophils, control and Myl-Abcdko neutrophils were incubated with a conditioned medium from control macrophages treated with LPS or LPS + ATP as described in the in vitro NET assay section above. Net IL-1β secretion from neutrophils was calculated by subtracting IL-1β concentration before incubation with macrophage-conditioned medium from the IL-1β concentration after incubation with macrophage-conditioned medium. IL-1β secretion into the media was measured using ELISA (R&D Systems; DY401). Data were normalized to a protein concentration of neutrophil cell lysates. IL-1β cleavage in neutrophil cell lysates was assessed by western blot using a primary antibody from Abcam (ab9722) and an anti-rabbit secondary antibody from Cell Signaling (7074).
2.8. Flow cytometry
For quantification of blood myeloid cells, blood was collected by tail bleeding into EDTA-coated tubes and immediately put on ice. The samples were kept at 4°C for the whole procedure unless stated otherwise. Red blood cells (RBCs) were lysed (BD Pharm Lyse, BD Bioscience), and white blood cells (WBCs) were centrifuged, washed, and resuspended in FACS buffer. Cells were stained with a cocktail of antibodies against CD45-APC-Cy7, Ly6-C/G-PerCP-Cy5.5 (BD Pharmingen; 557659 and 561103, respectively), and CD115-APC (eBioscience; 17–1152) in FACS buffer. Monocytes were identified as CD45hiCD115hi and further separated into Ly6Chi and Ly6Clo subsets, and neutrophils were identified as CD45hiCD115loLy6C/Ghi (Gr-1).
2.9. Isolation of Ly6G + neutrophils, Ly6G-CD11b + monocytes, Western blot, and qPCR
Spleens were mashed on a 40 μm filter and RBCs were lysed (BD Pharm Lyse, BD Bioscience). First, Ly6G+ neutrophils were isolated, using Ly6G+ (#130-120-337) coated microbeads, and then, from the same sample Ly6G−CD11b+ monocytes were isolated, using CD11b+ (#130-049-601) coated microbeads (Miltenyi Biotec). Caspase-1 or IL-1β cleavage was assessed by Western blot using a primary antibody from eBioscience (14–9832) or Abcam (ab9722) and an anti-rat secondary antibody from Cell Signaling (7077) or anti-rabbit secondary antibody from Cell Signaling (7074), respectively, and Abca1 and Abcg1 by Western blot using antibodies from Novus Biologicals (NB400-105 and NB400-132, respectively).
To assess mRNA expression of Abca1 or Abcg1 in BM myeloid cells, BM was isolated and treated similarly to the splenic homogenate described above to isolate Ly6G+ neutrophils and Ly6G−CD11b+ monocytes. RNA from these cells was isolated using a Qiagen RNeasy kit and cDNA was synthesized using kits from Thermo Scientific (Maxima First Strand cDNA synthesis kit; 1642). Primers for Abca1 were 5′-GTGAATGGGCAATTCGCAAACT-3′ (forward) and 5′-AGATCTCCCCTCCTTGACAATGC-3′ (reverse), for Abcg1 5′-TGTTCAGGAGGCCATGATGGT-3′ (forward) and 5′-TGGCCAGGCGTTTCCG-3′ (reverse), and for Nlrp3 5′- ATTACCCGCCCGAGAAAGG-3′ (forward) and 5′-TCGCAGCAAAGATCCACACAG-3′ (reverse). Initial differences in RNA quantity were corrected by using the housekeeping gene m36B4. SYBR Green Master Mix was from Applied Biosystems (by ThermoScientific; 4385612) and qPCR was run on a StepOne Plus Real-time PCR Systems from Applied Biosystems.
2.10. Plasma cholesterol, IL-18, IL-1β, and G-CSF
Mouse plasma was isolated through centrifugation of blood at 12 000 g for 10 min at 4°C. IL-18, IL-1β, and G-CSF plasma levels were measured by ELISA (MBL International, 7625) and (Abcam, ab229440 and R&D systems MCS00), respectively. Total plasma cholesterol was determined using a cholesterol E assay (Wako, cat. no. 999–02601).
2.11. Power calculations
For all animal studies, power calculations were performed based on NETosis data in atherosclerotic lesions from our previous studies that showed that myeloid Abca1/g1 deficiency enhanced NETosis in plaques of Ldlr−/− mice.16 Effect sizes were estimated at 8-fold increases in NETosis in Myl-Abcdko Ldlr−/− mice vs. controls and standard deviations at 75% of the highest value. Based on these calculations, a minimum of n = 13 Ldlr−/− recipient mice per group were used for NETosis measurements in atherosclerotic plaques for ∼80% chance to detect a difference where P = 0.05.
2.12. Statistical analysis
All data are presented as means ± SEM. The number of mice included in the experiments can be found in the figure legends. All data are based on biological replicates. Outliers were excluded if the ROUT coefficient Q was <1% as determined by the Prism software. The student’s t-test was used to define the differences between two data sets. For three or more data sets, one-way ANOVA coupled with Tukey’s test for multiple comparisons was used. In the case of concomitant comparison of two independent variables, two-way ANOVA coupled with Sidak’s test for multiple comparisons was used. The criterion for significance was set at P < 0.05. Statistical analyses were performed using GraphPad Prism 8 software (San Diego, CA). A two-tailed t-test was performed to test the hypothesis that the slope of the two regression lines was different.
3. Results
3.1. Neutrophil Abca1/g1 deficiency does not promote NETosis in atherosclerotic plaques
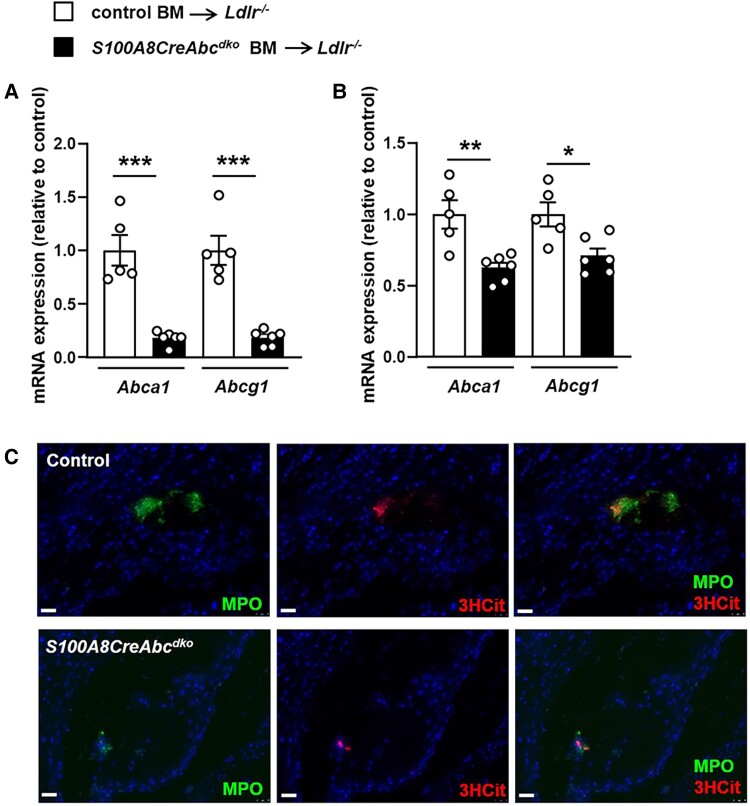
Previously, we showed increased lesional NETosis in female Ldlr−/− mice with myeloid deficiency of Abca1/g1.16 We also observed an increase in NETosis in male Ldlr−/− mice transplanted with Lysm-CreAbca1fl/flAbcg1fl/fl (Myl-Abcdko) BM compared with Abca1fl/flAbcg1fl/fl (control) BM; however, at plasma cholesterol levels similar to our previously published data in females17 (see Supplementary material online, Table S1), the size of lesions was ∼50% smaller in male mice compared with our previously published data in females,16 and many had small or no detectable NETs (see Supplementary material online, Figure S1 A–D), while our previously published data in females showed that all lesions from Myl-Abcdko BMT Ldlr−/− mice had NETs.16 Therefore, we carried out further studies in female mice. To assess the role of neutrophil Abca1 and Abcg1 in inflammasome activation and NETosis during atherogenesis, we generated S100A8-CreAbca1fl/flAbcg1fl/fl mice and Abca1fl/flAbcg1fl/fl littermate controls and transplanted their BM into Ldlr−/− mice. Ldlr−/− mice transplanted with S100A8-CreAbca1fl/flAbcg1fl/fl (S100A8-CreAbcdko) BM showed >90% decrease in Abca1 and Abcg1 mRNA expression in BM neutrophils (Figure 1A) compared with Abca1fl/flAbcg1fl/fl (control) BM transplanted (BMT) Ldlr−/− mice and a modest reduction of Abca1 and Abcg1 mRNA expression in monocytes (Figure 1B), in line with high expression of S100A8-Cre in neutrophils and modest expression in monocytes.28 We refer to S100A8-CreAbca1fl/flAbcg1fl/fl mice as S100A8-CreAbcdko or as neutrophil Abca1/g1-deficient with the caveat that they also show a partial reduction in Abca1/g1 expression in monocytes. After 8 weeks of WTD feeding, we observed no effects of neutrophil Abca1/g1 deficiency on plasma IL-18 levels, while IL-1β plasma levels were low and slightly decreased (see Supplementary material online, Figure S2 A and B). Neutrophil Abca1/g1 deficiency did not affect the cleavage of the caspase-1 p45 pro-form into its active p20 form (see Supplementary material online, Figure S2 C), a marker of inflammasome activation, in splenic neutrophils, indicating that unlike myeloid Abca1/g1 deficiency, neutrophil Abca1/g1 deficiency did not affect inflammasome activation. Plasma G-CSF (see Supplementary material online, Figure S3A) and blood neutrophil numbers but not monocytes (see Supplementary material online, Figure S3B) were slightly elevated in S100A8-CreAbcdko mice, presumably due to the modest reduction of Abca1/g1 in monocytes. Indeed, our previous work has shown that monocyte/macrophage Abca1/g1 deficiency increases G-CSF plasma levels and blood neutrophils.17 Neutrophil Abca1/g1 deficiency did not affect atherosclerotic lesion size (see Supplementary material online, Figure S3C) or Mac-2+ macrophage area in atherosclerotic plaques (see Supplementary material online, Figure S3D) at similar plasma cholesterol levels (see Supplementary material online, Table S1). We evaluated neutrophils in atherosclerotic lesions, by staining for myeloperoxidase (MPO). MPO-positive cells were almost undetectable and were not different between genotypes (Figure 1C and Supplementary material online, Figure S3E). Lesions were co-stained for citrullinated histones 2, 8, and 17 (3HCit), and to assess NETs, the overlap of MPO and 3HCit was quantified. The NET+ area was very low and not affected by neutrophil Abca1/g1 deficiency (Figure 1C and Supplementary material online, Figure S3F). These findings show that neutrophil Abca1/g1 deficiency does not affect inflammasome activation in splenic neutrophils or NETosis in atherosclerotic plaques.
Figure 1.
Neutrophil Abca1/g1 deficiency does not affect NETosis in atherosclerotic plaques of Ldlr−/− mice. Ldlr−/− mice were transplanted with bone marrow (BM) from Abca1fl/flAbcg1fl/fl (control) or S100A8CreAbca1fl/flAbcg1fl/fl (S100A8-Cre-Abcdko) mice and fed Western-type diet (WTD) for 8 weeks. (A–B) Ly6G+ neutrophils (A) and Ly6G−CD11b+ monocytes (B) were isolated from BM and Abca1 and Abcg1 mRNA expression was assessed. n = 5. *P < 0.05, by t-test. (C) Mice were sacrificed and hearts were isolated, sectioned, and neutrophils were stained in atherosclerotic lesions of the aortic root using myeloperoxidase (MPO). Lesions were also stained for citrullinated histones 2, 8, and 17 (3HCit). NETs show an overlap of MPO and 3HCit staining. Representative pictures are shown. Scale bars: 25 μm.
3.2. Macrophage Abca1/g1 deficiency activates macrophage and neutrophil inflammasomes and plaque NETosis
To determine whether macrophage Abca1 and Abcg1 led to inflammasome activation and NETosis in atherosclerotic plaques, we first generated CSF1R-CreAbca1fl/flAbcg1fl/fl (CSF1R-CreAbcdko) mice and Abca1fl/flAbcg1fl/fl littermate controls and transplanted their BM into Ldlr−/− mice that were subsequently fed WTD. CSF1R-CreAbcdko BMT Ldlr−/− mice displayed prominent neutrophil accumulation and NETosis in atherosclerotic plaques (see Supplementary material online, Figure S4A) similar to mice with myeloid Abca1/g1 deficiency,16 while Mac-2+ macrophage area was not affected (see Supplementary material online, Figure S4B), at plasma cholesterol levels that were similar to mice with myeloid Abca1/g1 deficiency17 (see Supplementary material online, Table S1). However, even though the CSF1R-Cre strain was originally developed as a macrophage-specific promoter, high expression in other leukocyte subpopulations has been reported,29 and we found that Abca1 and Abcg1 were efficiently deleted in BM monocytes and neutrophils in CSF1R-CreAbcdko compared to Abca1fl/flAbcg1fl/fl (control) BMT Ldlr−/− mice (see Supplementary material online, Figure S5A). In addition, similar to mice with myeloid Abca1/g1 deficiency,17 percentages of blood monocytes and neutrophils were increased in CSF1R-CreAbcdko BMT Ldlr−/− mice compared with controls (see Supplementary material online, Figure S5B). Previous data we obtained in WTD-fed Ldlr−/− mice with myeloid Abca1/g1 deficiency showed no effects on total WBC (see Supplementary material online, Figure S5C), indicating that effects on percentages of blood CD45+ cells mirror effects on absolute WBC counts in this setting.
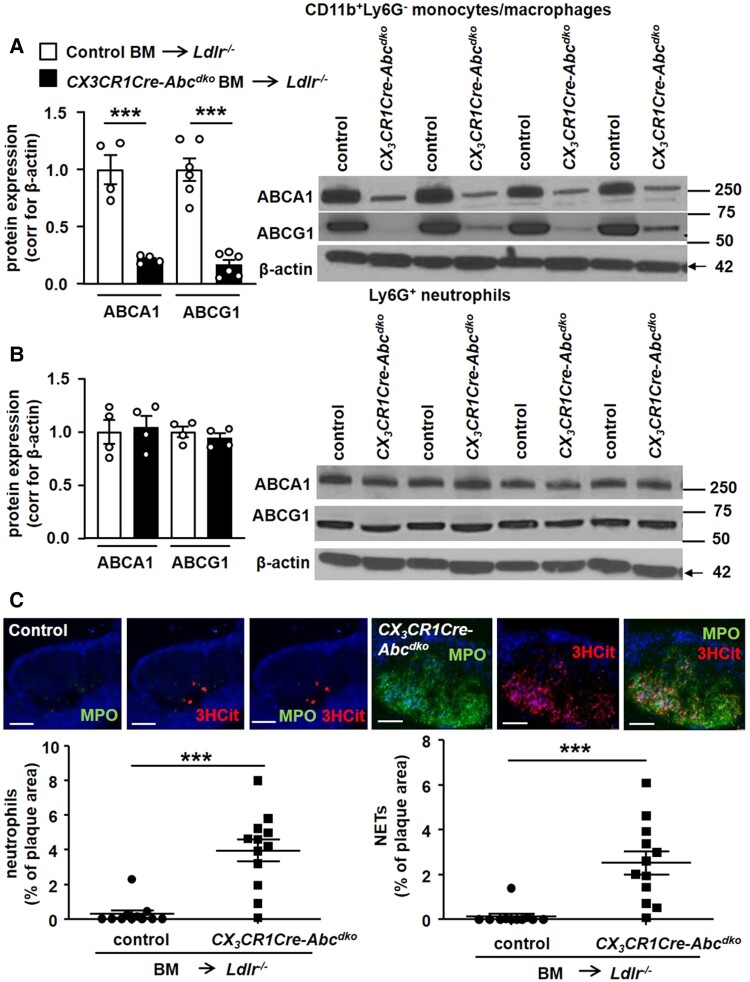
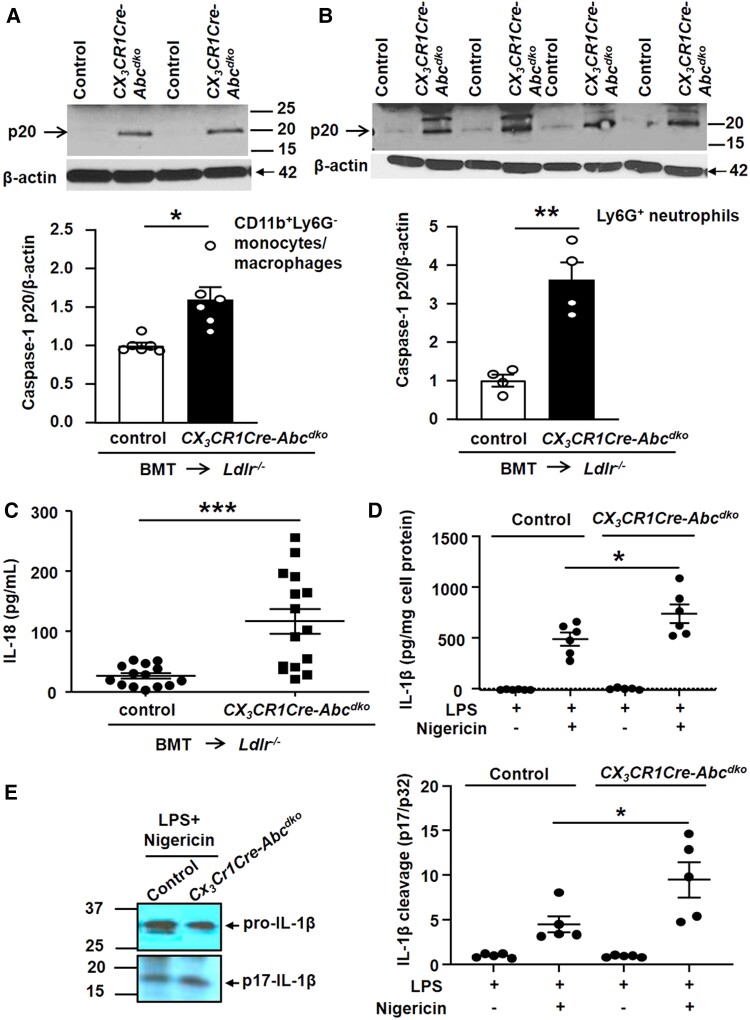
We next generated CX3CR1-CreAbca1fl/flAbcg1fl/fl mice and Abca1fl/flAbcg1fl/fl littermate controls and transplanted their BM into Ldlr−/− mice. Ldr−/− mice transplanted with CX3CR1-CreAbca1fl/flAbcg1fl/fl (CX3CR1-CreAbcdko) BM showed >90% decrease in ABCA1 and ABCG1 protein and mRNA expression in splenic monocytes/macrophages (Figure 2A and Supplementary material online, Figure S6A) but no change in neutrophils (Figure 2B and Supplementary material online, Figure S6A) compared with Abca1fl/flAbcg1fl/fl (control) BMT Ldlr−/− mice. We refer to CX3CR1-CreAbca1fl/flAbcg1fl/fl mice as CX3CR1-CreAbcdko or macrophage Abca1/g1-deficient. After 8 weeks of WTD feeding, macrophage Abca1/g1 deficiency increased blood monocytes and neutrophils (see Supplementary material online, Figure S6B) and plasma G-CSF by twofold (see Supplementary material online, Figure S6C) as well as spleen weight (see Supplementary material online, Figure S6D) in line with previous observations in mice with myeloid Abca1/g1 deficiency.17,30 Similar to WTD-fed Ldlr−/− mice with myeloid Abca1/g1 deficiency, macrophage Abca1/g1 deficiency decreased plasma cholesterol levels by ∼50% (see Supplementary material online, Table S1), and did not affect atherosclerotic lesion size or lesion macrophage content as expected17 (see Supplementary material online, Figure S7A–C), but markedly increased MPO+ neutrophils and NETs in atherosclerotic plaques (Figure 2C). The lack of effect of macrophage Abca1/g1 deficiency on lesion area was expected due to the ∼50% decrease in plasma cholesterol levels, similar to Ldlr−/− mice with myeloid Abca1/g1 deficiency17,31 that likely reflects monocyte/macrophage cholesterol accumulation and decreased sterol regulatory element–binding protein-1c mRNA expression in the liver.17,31 Macrophage Abca1/g1 deficiency increased active caspase-1 (p20) in both Ly6G−CD11b+ splenocytes (monocytes/macrophages) (Figure 3A) and Ly6G+ splenocytes (neutrophils) (Figure 3B) as well as caspase-1 cleavage in these cells (see Supplementary material online, Figure S8), and plasma IL-18 levels (Figure 3C). To further substantiate Nlrp3 inflammasome activation, BMDMs from control or CX3CR1-CreAbcdko mice were treated with LPS and then nigericin. Both IL-1β secretion (Figure 3D) into the media and IL-1β cleavage in cell lysates (Figure 3E) were significantly elevated in BMDMs from CX3CR1-CreAbcdko mice. Together, these findings indicate that macrophage Abca1/g1 deficiency elevates plasma G-CSF, blood, and plaque neutrophils, and NETs in plaques and promotes inflammasome activation in both macrophages and neutrophils.
Figure 2.
Macrophage Abca1/g1 deficiency induces neutrophil accumulation and NETosis in atherosclerotic lesions. Ldlr−/− mice were transplanted with BM from Abca1fl/flAbcg1fl/fl (control) or CX3CR1CreAbca1fl/flAbcg1fl/fl (CX3CR1Cre-Abcdko) mice and fed WTD for 8 weeks. Ly6G−CD11b+ splenic monocytes/macrophages (A) and Ly6G+ splenic neutrophils (B) were isolated and Abca1 and Abcg1 protein expression was assessed by Western blot, corrected for β-actin, and quantified. n = 4. Unedited gels are shown in Supplementary material online, Figure S16. (C) In atherosclerotic lesions, neutrophils were stained using MPO, and the MPO+ percentage of lesion size was quantified. Lesions were also stained for 3HCit. To assess NETs, the overlap of MPO and 3HCit was quantified. Representative pictures are shown. Scale bars: 75 μm. Each datapoint represents an individual mouse. n = 12. ***P < 0.001, by t-test.
Figure 3.
Macrophage Abca1/g1 deficiency induces inflammasome activation in monocytes, macrophages, and neutrophils of Ldlr−/− mice. Ldlr−/− mice were transplanted with BM from Abca1fl/flAbcg1fl/fl (control) or CX3CR1CreAbca1fl/flAbcg1fl/fl (CX3CR1Cre-Abcdko) mice and fed WTD for 8 weeks. Ly6G−CD11b+ splenic monocytes/macrophages (A) and Ly6G+ splenic neutrophils (B) were isolated and caspase-1 cleavage was assessed by Western blot. To quantify caspase-1 cleavage, the p20 cleaved form of caspase-1 was divided by the p45 pro-form. n = 4–6. (C) Plasma IL-18 levels. (D–E) IL-1β secretion from control or CX3CR1Cre-Abcdko BMDMs treated with 20 ng/mL LPS for 3 h, and with 10 µg/mL nigericin for an additional 1 h. (D) IL-1β secretion was assessed by ELISA. (E) Immunoblot of IL-1β cleavage of cell lysates. Unedited gels are shown in Supplementary material online, Figure S17. Each datapoint represents an individual mouse. n = 14. *P < 0.05, ** P < 0.01, ***P < 0.001 (A–C) by t-test and (D–E) by one-way ANOVA.
3.3. IL-1β antagonism reduces neutrophil accumulation and NETosis in atherosclerotic lesions
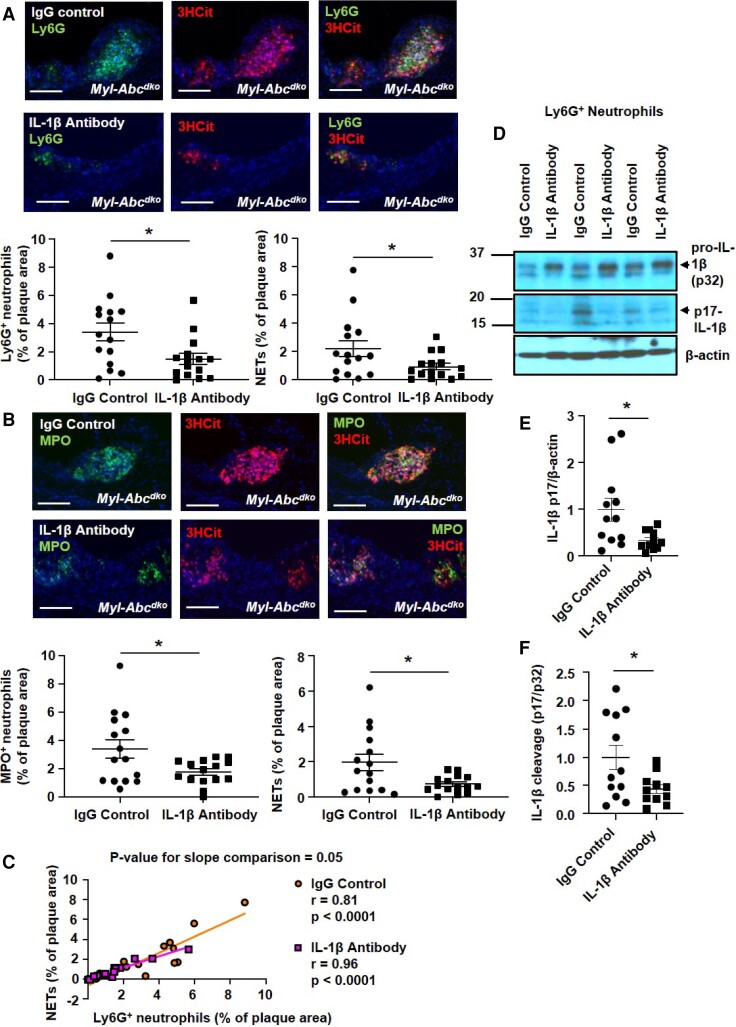
Plaque NETosis in mice with myeloid Abca1/g1 deficiency is dependent on the NLRP3 inflammasome.16 To determine if NETosis is dependent on IL-1β, an active product of the NLRP3 inflammasome, we antagonized IL-1β via the administration of neutralizing antibodies. To simulate a therapeutic scenario, antibodies were administered during the latter phase of diet treatment. Ldlr−/− mice were transplanted with BM from LysmCreAbca1fl/flAbcg1fl/fl (Myl-Abcdko) mice and fed WTD. After 6 weeks of WTD, anti-mouse monoclonal antibodies (IgG2a) that selectively neutralize IL-1β or isotype-matched control IgG2a were administered subcutaneously (three doses of antibody at 6, 7, and 8 weeks of WTD), and mice were sacrificed after 8.5 weeks of WTD. This short-term administration of IL-1β antibodies had no effect on plasma cholesterol levels (see Supplementary material online, Table S1), atherosclerotic lesion area, necrotic core area, plasma IL-18, blood leukocyte or neutrophils, or plasma G-CSF, but slightly decreased spleen weight (see Supplementary material online, Figure S9A–F). In atherosclerotic lesions, IL-1β antagonism significantly decreased both Ly6G+ (Figure 4A) and MPO+ (Figure 4B) neutrophils and NETs measured by Ly6G+/3HCit (Figure 4A) and MPO+/3HCit (Figure 4B and Supplementary material online, Figure S10) co-staining. Neutrophil accumulation in plaques correlated strongly with the extent of NETosis in mice treated with control IgG or IL-1β antibody, suggesting that neutrophil abundance in lesions was a major determinant of the extent of NETosis. However, the regression line for the IL-1β antibody group appeared to have a reduced slope (P = 0.05, by a two-tailed t-test) suggesting an additional mechanism for reducing NETosis (Figure 4C). We considered the possibility that this might reflect reduced neutrophil inflammasome activation. Accordingly, IL-1β antagonism significantly decreased the active form of IL-1β (p17, Figure 4D) in splenic neutrophils. These data show that the IL-1β antibody reduced neutrophil inflammasome activation, which could contribute to decreased NETosis.14,15 Thus, the administration of neutralizing IL-1β antibodies reduced the neutrophil content of atherosclerotic plaques with a parallel effect on NETosis but could also potentially decrease NETosis by reducing neutrophil inflammasome activation.
Figure 4.
IL-1β antagonism decreases neutrophil content and NETosis in atherosclerotic lesions of Ldlr−/− mice with myeloid Abca1/g1 deficiency. Ldlr−/− mice were transplanted with BM from LysmCreAbca1fl/flAbcg1fl/fl (Myl-Abcdko) mice and fed WTD. The anti-mouse monoclonal antibodies (IgG2a) that selectively neutralize IL-1β or isotype-matched control IgG2a were administered subcutaneously at 6, 7, and 8 weeks of WTD feeding (three doses of antibodies in total). At 8.5 weeks of WTD, mice were sacrificed and neutrophils were stained in atherosclerotic lesions using Ly6G (A) or MPO (B), and Ly6G+ (A) or MPO+ (B) percentages of lesion size were quantified. Concomitantly, lesions were stained for 3HCit. To assess NETs, the overlap of Ly6G and 3HCit (A) or MPO and 3HCit (B) was quantified as a percentage of the total lesion area. Representative pictures are shown. Scale bars: 100 μm. (C) Correlation between Ly6G+ and Ly6G + 3HCit+ (NET) area as shown in (A). (D) Ly6G+ splenic neutrophils were isolated and the cleaved and pro-form of IL-1β was assessed by Western blot. Unedited gels are shown in Supplementary material online, Figure S18. Cleaved IL-1β was quantified relative to β-actin (E) and the pro-form of IL-1β (F). n = 12. Each data point represents an individual mouse. n = 15. *P < 0.05, by t-test.
3.4. Macrophage-derived IL-1β induces NETosis by activating the NLRP3 inflammasome in neutrophils
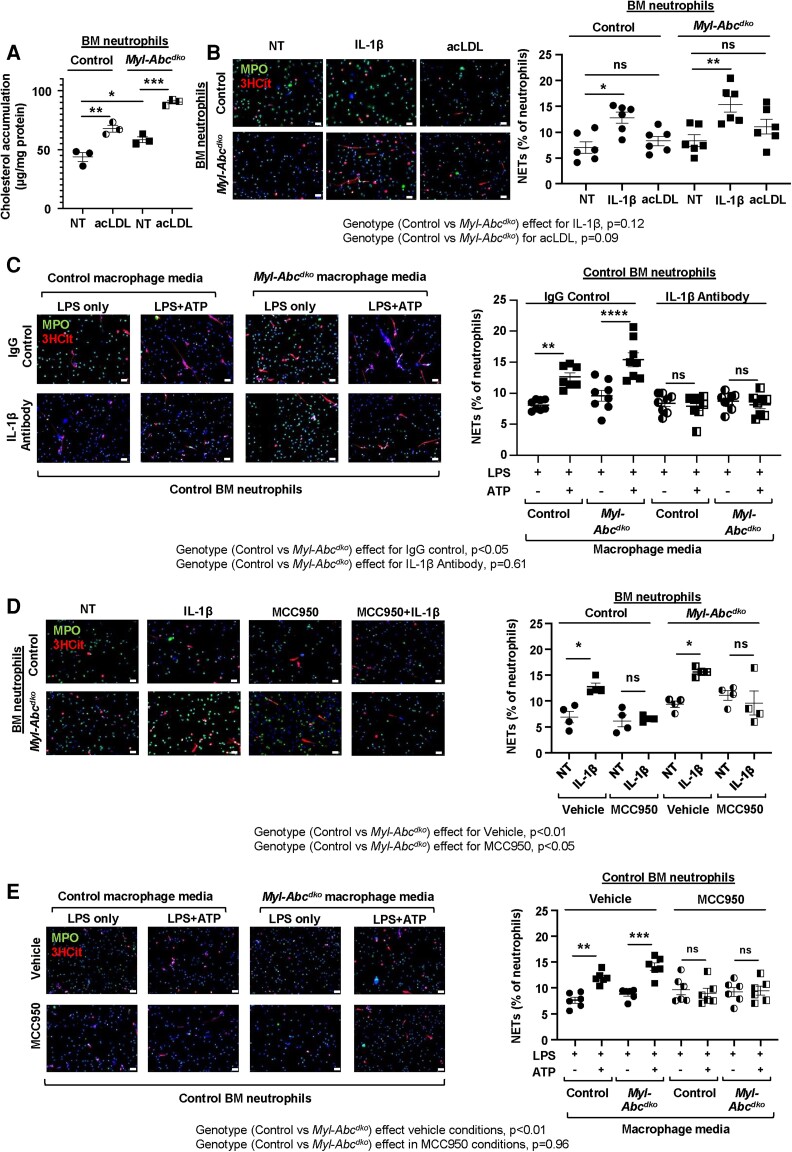
We carried out further experiments with cultured cells to assess the mechanisms of NETosis induced by IL-1β. First, we loaded BM neutrophils isolated from control and Myl-Abcdko mice with acLDL. In the basal state, Myl-Abcdko neutrophils had significantly higher cholesterol levels than control macrophages and this was further increased by acLDL loading in both groups (Figure 5A). However, increased cholesterol content due to acLDL loading did not lead to an increase in NETosis (Figure 5B). In contrast, the addition of IL-1β increased NETosis; the effect was similar in control and Myl-Abcdko neutrophils (Figure 5B and Supplementary material online, Figure S11) suggesting that increased NETosis in Abca1/g1-deficient neutrophils is not a cell-intrinsic process.
Figure 5.
Macrophage-derived IL-1β induces neutrophil inflammasome and NETosis. (A–B) Ly6G+ neutrophils from BM of Abca1fl/flAbcg1fl/fl (control) or LysmCreAbca1fl/flAbcg1fl/fl (Myl-Abcdko) mice were treated with 100 ng/ml IL-1β and/or 25 µg/mL acLDL for 3 h. (A) Total cholesterol in Ly6G+ neutrophils. (B) Neutrophils were stained for DAPI, MPO, and 3HCit. To assess NETs in isolated neutrophils, the overlap of 3HCit and MPO was quantified and expressed as % of total cells. (C) Control or Myl-Abcdko BM-derived macrophages (BMDMs) were treated with LPS and ATP to activate the NLRP3 inflammasome. Cells were washed and incubated for 1 h with medium to allow for cytokine secretion. The medium was collected and mixed 1:1 with DMEM supplemented with 1% pen-strep and then transferred to neutrophils that had been pre-treated with 100 µg/mL IgG control or IL-1β antibodies for 30 min and incubated with these cells for 3 h. (D) Ly6G+ neutrophils from BM of control or Myl-Abcdko were treated with MCC950 for 30 min and then 100 ng/ml IL-1β was added for 3 h. (E) Ly6G+ neutrophils were pre-treated with MCC950 for 30 min, and subsequently with conditioned media as described in (C). For (C–E), NETosis was assessed as in (B). In B–E, representative pictures are shown. Scale bars: 25 µm. ****P < 0.0001, ***P < 0.001, ** P < 0.01, *P < 0.05, by one-way ANOVA with Tukey’s multiple comparison test (A) or by two-way ANOVA with Sidak’s multiple comparison test (B–E). NT, not treated. Genotype effects per condition are indicated for panels B–E.
To test the hypothesis that macrophage inflammasome activation and IL-1β can directly activate NETosis, we treated BM neutrophils isolated from control mice with conditioned media from control and Myl-Abcdko BMDMs. Macrophages were treated with LPS and ATP to activate the NLRP3 inflammasome. Then fresh media not containing LPS/ATP was added for 1 h to allow for secretion of IL-1β and other cytokines, followed by media transfer to control neutrophils, in the presence of 100 µg/mL IgG control or IL-1β antibodies. Media from LPS-/ATP-treated macrophages from both genotypes, but, as shown by two-way ANOVA, especially from Myl-Abcdko BMDMs, induced NETosis, which was completely blocked by IL-1β antibodies (Figure 5C). This indicates a direct effect of IL-1β on NETosis. We next assessed whether IL-1β induces NETosis via activation of the neutrophil NLRP3 inflammasome. To test this, we first assessed inflammasome activation in control or Myl-Abcdko neutrophils treated with media from LPS/ATP-treated control macrophages. Both neutrophil IL-1β cleavage (see Supplementary material online, Figure S12A) and IL-1β secretion (see Supplementary material online, Figure S12B) were increased by macrophage-conditioned media to a similar level in both genotypes. Next, we treated control and Myl-Abcdko BM neutrophils with MCC950, a specific NLRP3 inflammasome inhibitor32,33 for 30 min prior to IL-1β treatment. NLRP3 inflammasome inhibition abolished IL-1β-induced NETosis in neutrophils from mice of both genotypes (Figure 5D). Two-way ANOVA in this experiment suggested a significant effect of neutrophil genotype on NETosis in vehicle and MCC950-treated neutrophils. MCC950 pre-treatment of control neutrophils inhibited NETosis induced by conditioned media from control and Myl-Abcdko BMDMs (Figure 5E). IL-1β treatment or macrophage-conditioned media elevated Nlrp3 mRNA and protein expression in both Myl-Abcdko and control neutrophils (see Supplementary material online, Figure S12C and D), consistent with its role in activating MAP kinase signalling pathways34 and thus increasing inflammasome priming. In conclusion, IL-1β secreted from Myl-Abcdko macrophages activates NET formation by triggering NLRP3 inflammasome activation in neutrophils. This effect appeared to be largely independent of neutrophil genotype; however, a small impact of neutrophil genotype on NETosis cannot be completely excluded.
We previously have shown that myeloid Abca1/g1 deficiency activated the non-canonical inflammasome, reflected by caspase-11 cleavage. However, haematopoietic caspase-11 deficiency did not affect plaque NETosis (see Supplementary material online, Figure S13). Plasma G-CSF is elevated in mice with myeloid or macrophage Abca1/g1 deficiency17,30 and G-CSF is known to prime neutrophils for NET release.35 We, therefore, assessed a potential in vivo role of G-CSF in mediating the effects of myeloid Abca1/g1 deficiency on NETs. However, we found that neither haematopoietic Nlrp3 nor Caspase-1/11 deficiency reduced elevated plasma G-CSF levels in WTD-fed Myl-Abcdko BMT Ldlr−/− mice (see Supplementary material online, Figure S14A), even though these deficiencies virtually abolished plaque NETosis.16 This indicates that although G-CSF increased NET formation similar to IL-1β in vitro (see Supplementary material online, Figure S14B), elevated plasma G-CSF is not sufficient to induce plaque NETosis in Myl-Abcdko BMT Ldlr−/− mice. oxLDL did not induce NETosis, while oxidized phospholipids induced NETosis to a similar extent in neutrophils from control or Myl-Abcdko mice (see Supplementary material online, Figure S15).
4. Discussion
NETosis increases atherosclerotic plaque vulnerability and athero-thrombosis.5–8 However, the mechanisms leading to the NET formation in atherosclerotic plaques remain incompletely understood. Our study provides the first direct evidence that macrophage NLRP3 inflammasome activation and IL-1β release promote plaque NETosis. In our prior studies of Myl-Abcdko mice, with Abca1/g1 deficiency and cholesterol accumulation in both macrophages and neutrophils, we speculated that NETosis was mediated through ‘systemic factors’ related to macrophage inflammasome activation, rather than cell-intrinsic effects of Abca1/g1 deficiency in neutrophils, but did not provide proof of this concept.16 The present study demonstrates unequivocally the lack of a cell-intrinsic effect of Abca1/g1 deficiency on NETosis by showing a lack of NETosis in mice with neutrophil-specific deficiency of Abca1/g1. Most importantly, we clarify a major role of macrophage inflammasome activation and IL-1β in promoting plaque NETosis by showing that macrophage-specific deletion of Abca1/g1 induced NETosis while IL-1β antagonism in Myl-Abcdko mice substantially reduced NETs in plaques.
Our in vivo and cell culture studies define two important mechanisms linking inflammasome activation and IL-1β production to NETosis; both mechanisms involve local effects in plaques rather than systemic effects. The first mechanism is that inflammasome activation in macrophage Abca1/g1 deficiency promotes neutrophil accumulation in plaques, which correlates strongly with the extent of NETosis. Nlrp3 deficiency in our earlier studies16 and IL-1β inhibition in the present study substantially reduced NETosis in Myl-Abcdko mice with only minor or no changes in blood neutrophil counts. Most likely the changes in the neutrophil content of plaques reflect the well-documented effects of IL-1β on the endothelium to promote neutrophil accumulation.36,37
The second more novel mechanism is mediated by cellular crosstalk from macrophages to neutrophils mediated by IL-1β, leading to neutrophil inflammasome activation and NETosis in Myl-Abcdko mice. The evidence for this was that IL-1β added alone or in media transferred from macrophages with prior NLRP3 inflammasome activation increased NETosis. Moreover, NETosis induced by media transfer or exogenous IL-1β was blocked by inhibiting NLRP3 in neutrophils, revealing a role of the neutrophil NLRP3 inflammasome in this process. The mechanism of the IL-1β effect on neutrophils is not completely understood but appears to involve at least in part increased priming of the NLRP3 inflammasome, consistent with the activation of MAPK signalling pathways by IL-1β.34,38In vivo evidence for this mechanism was obtained by showing reduced inflammasome activation in neutrophils from Myl-Abcdko mice treated with IL-1β antibodies. Since IL-1β induced these changes similarly in control neutrophils and in Abca1/g1-deficient neutrophils with or without cholesterol loading, they appear to be largely independent of cell-intrinsic effects of Abca1/g1 deficiency in neutrophils and are consistent with the lack of NETosis in S100A8-Cre-Abcdko mice. Rather the induction of neutrophil inflammasome activation and consequent NETosis is downstream of macrophage inflammasome activation and release of active IL-1β. Why cholesterol accumulation in neutrophils is insufficient to induce neutrophil inflammasome activation and NETosis remains unclear but could be related to relatively low levels of cholesterol accumulation in neutrophils or lack of pathways downstream of cholesterol accumulation that lead to NLRP3 inflammasome activation.
These local mechanisms promoting NETosis in plaque do not preclude an additional role of systemic factors in promoting plaque NETosis. For example, increased plasma G-CSF levels in Myl-Abcdko mice17 likely promote both neutrophilia and NETosis. However, this effect could be dissociated from NETosis in Myl-Abcdko mice with concomitant deficiency of Nlrp3 that had elevated levels of plasma G-CSF but lacked NETs in plaques showing a non-essential role of G-CSF in NETosis.
Previous studies have suggested a possible bi-directional relationship between inflammation, IL-1 production, and NETs; however, this has also been an area of controversy. Mitroulis et al.39 showed using Anakinra, a recombinant IL-1 receptor antagonist, that IL-1 induces NETosis in patients with gout; these studies did not identify the source of IL-1 or differentiate between IL-1α and IL-1β. NETs promote endothelial inflammation via IL-1α and thereby worsen thrombosis due to superficial erosion of plaques.40 IL-1β was associated with increased NETs and worsened abdominal aortic aneurysm formation; however, these studies did not define whether NETs were promoting IL-1β release or vice versa.41 Warnatsch et al.42 have shown that NETs promote inflammasome priming in macrophages in Apoe−/− mice. Consistently, myeloid PAD4 deficiency decreased IL-1β mRNA in the aorta of Apoe−/− mice, indicating decreased inflammasome priming.8 Our study shows that macrophage-derived IL-1β activates the neutrophil Nlrp3 inflammasome leading to caspase-1 cleavage and IL-1β secretion. Since IL-1β can prime the macrophage Nlrp3 inflammasome, this suggests a positive feedback loop from neutrophils to macrophages ultimately inducing NETosis and possibly athero-thrombosis (Graphical Abstract). A limitation of our study is the use of mouse models that do not give rise to thrombus formation on atherosclerotic plaques.
Paulin et al.43 have shown that dsDNA in advanced atherosclerotic plaques of Apoe−/− mice, which may originate from NETs, activates the AIM2 inflammasome. However, these studies seem inconsistent with haematopoietic PAD4 deficiency not affecting atherosclerotic lesion size in Ldlr−/− mice with early lesions.7 Perhaps this is the consequence of the Ldlr−/− background and the relatively small percentage of NETs in atherosclerotic lesions in this particular study.7 We also, in our current and previous study,16 found only a small percentage of NETs in early plaques from Ldlr−/− mice. Nlrp3 deficiency did not affect atherosclerosis in Ldlr−/− mice in our previous study,16 suggesting that this small percentage of NETs was insufficient to activate the NLRP3 inflammasome.
Our findings in macrophage Abca1/g1-deficient mice illustrate how macrophage inflammasome activation and IL-1β release can mediate crosstalk to bystander wild-type cells to promote inflammatory responses. Since macrophage inflammasome activation is prominent in Tet2−/− and JAK2V617F clonal haematopoiesis,37,44 cellular crosstalk from mutant myeloid cells to WT macrophages, neutrophils or SMCs could have an important role in fostering the growth and instability of atherosclerotic plaques. A recent analysis of patients in the CANTOS study suggested that IL-1β inhibition benefited patients with TET2 clonal haematopoiesis more than subjects with other forms of CH or patients without CHIP.45 Our studies define a beneficial role of inhibiting IL-1β that could potentially limit NETosis and other adverse effects of crosstalk from mutant macrophages to other cell types in such patients.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Contributor Information
Mustafa Yalcinkaya, Division of Molecular Medicine, Department of Medicine, Columbia University Irving Medical Center, 630 West 168 Street P&S 8-401, New York, NY 10032, USA.
Panagiotis Fotakis, Division of Molecular Medicine, Department of Medicine, Columbia University Irving Medical Center, 630 West 168 Street P&S 8-401, New York, NY 10032, USA.
Wenli Liu, Division of Molecular Medicine, Department of Medicine, Columbia University Irving Medical Center, 630 West 168 Street P&S 8-401, New York, NY 10032, USA.
Kaori Endo-Umeda, Division of Molecular Medicine, Department of Medicine, Columbia University Irving Medical Center, 630 West 168 Street P&S 8-401, New York, NY 10032, USA; Division of Biochemistry, Department of Biomedical Sciences, Nihon University School of Medicine, 30-1 Oyaguchi-kamicho, Itabashi-ku, Tokyo 173-8610, Japan.
Huijuan Dou, Division of Molecular Medicine, Department of Medicine, Columbia University Irving Medical Center, 630 West 168 Street P&S 8-401, New York, NY 10032, USA.
Sandra Abramowicz, Division of Molecular Medicine, Department of Medicine, Columbia University Irving Medical Center, 630 West 168 Street P&S 8-401, New York, NY 10032, USA.
Tong Xiao, Division of Molecular Medicine, Department of Medicine, Columbia University Irving Medical Center, 630 West 168 Street P&S 8-401, New York, NY 10032, USA.
Peter Libby, Department of Medicine, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA 02115, USA.
Nan Wang, Division of Molecular Medicine, Department of Medicine, Columbia University Irving Medical Center, 630 West 168 Street P&S 8-401, New York, NY 10032, USA.
Alan R Tall, Division of Molecular Medicine, Department of Medicine, Columbia University Irving Medical Center, 630 West 168 Street P&S 8-401, New York, NY 10032, USA.
Marit Westerterp, Division of Molecular Medicine, Department of Medicine, Columbia University Irving Medical Center, 630 West 168 Street P&S 8-401, New York, NY 10032, USA; Department of Pediatrics, University Medical Center Groningen, University of Groningen, Antonius Deusinglaan 1, 9713AV Groningen, The Netherlands.
Conflict of interest
A.R.T. is a consultant for Amgen, CSL Behring, Astra Zeneca, and Foresite Laboratories, and is on the SAB of Staten Biotech, Fortico Biotech, and Beren Therapeutics. P.L. is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Baim Institute, Beren Therapeutics, Esperion Therapeutics, Genentech, Kancera, Kowa Pharmaceuticals, Medimmune, Merck, Norvo Nordisk, Novartis, Pfizer, and Sanofi-Regeneron. P.L. is a member of the scientific advisory board for Amgen, Caristo Diagnostics, Cartesian Therapeutics, CSL Behring, DalCor Pharmaceuticals, Dewpoint Therapeutics, Euclid Bioimaging, Kancera, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Moderna, Novartis, PlaqueTec, TenSixteen Bio, Soley Therapeutics, and XBiotech, Inc. P.L.’s laboratory has received research funding in the last 2 years from Novartis. P.L. is on the Board of Directors of XBiotech, Inc. P.L. has a financial interest in Xbiotech, a company developing therapeutic human antibodies, in TenSixteen Bio, a company targeting somatic mosaicism and CHIP to discover and develop novel therapeutics to treat age-related diseases, and in Soley Therapeutics, a biotechnology company that is combining artificial intelligence with molecular and cellular response detection for discovering and developing new drugs, currently focusing on cancer therapeutics. P.L.'s interests were reviewed and are managed by Brigham and Women's Hospital and Partners HealthCare in accordance with their conflict-of-interest policies.
Funding
This work was supported by 18CVD04 grant from the Leducq Foundation and by National Institutes of Health (NIH) grants HL107653, HL 155431 (to A.R.T.), HL134892, HL163099, the American Heart Association (18CSA34080399), the RRM Charitable Fund, and the Simard Fund (to P.L.), and VIDI (917.15.350) and Aspasia grants from the Netherlands Organization of Scientific Research (NWO), and a Rosalind Franklin Fellowship from the University of Groningen with an European Union Co-Fund attached (to M.W.).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding authors.
Translational perspective
Our studies define a beneficial role of inhibiting macrophage IL-1β secretion limiting NETosis in atherosclerotic plaques by suppressing neutrophil inflammasome activation. These data may have implications for patients showing elevated macrophage IL-1β secretion such as in TET2 or JAK2V617F clonal haematopoiesis, where increased NETosis in atherosclerotic plaques may enhance plaque instability.
References
- 1. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 2011;17:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 Inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010;464:1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 4. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 5. Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, Gallant M, Martinod K, Ten Cate H, Hofstra L, Crijns HJ, Wagner DD, Kietselaer B. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol 2013;33:2032–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doring Y, Libby P, Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ Res 2020;126:1228–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Franck G, Mawson TL, Folco EJ, Molinaro R, Ruvkun V, Engelbertsen D, Liu X, Tesmenitsky Y, Shvartz E, Sukhova GK, Michel JB, Nicoletti A, Lichtman AH, Wagner DD, Croce KJ, Libby P. Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury: implications for superficial erosion. Circ Res 2018;123:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Carmona-Rivera C, Moore E, Seto NL, Knight JS, Pryor M, Yang ZH, Hemmers S, Remaley AT, Mowen KA, Kaplan MJ. Myeloid-Specific deletion of peptidylarginine deiminase 4 mitigates atherosclerosis. Front Immunol 2018;9:1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dou H, Kotini A, Liu W, Fidler T, Endo-Umeda K, Sun X, Olszewska M, Xiao T, Abramowicz S, Yalcinkaya M, Hardaway B, Tsimikas S, Que X, Bick A, Emdin C, Natarajan P, Papapetrou EP, Witztum JL, Wang N, Tall AR. Oxidized phospholipids promote NETosis and arterial thrombosis in LNK(SH2B3) deficiency. Circulation 2021;144:1940–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silvestre-Roig C, Braster Q, Wichapong K, Lee EY, Teulon JM, Berrebeh N, Winter J, Adrover JM, Santos GS, Froese A, Lemnitzer P, Ortega-Gomez A, Chevre R, Marschner J, Schumski A, Winter C, Perez-Olivares L, Pan C, Paulin N, Schoufour T, Hartwig H, Gonzalez-Ramos S, Kamp F, Megens RTA, Mowen KA, Gunzer M, Maegdefessel L, Hackeng T, Lutgens E, Daemen M, von Blume J, Anders HJ, Nikolaev VO, Pellequer JL, Weber C, Hidalgo A, Nicolaes GAF, Wong GCL, Soehnlein O. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 2019;569:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010;16:887–896. [DOI] [PubMed] [Google Scholar]
- 12. Van Avondt K, Maegdefessel L, Soehnlein O. Therapeutic targeting of neutrophil extracellular traps in atherogenic inflammation. Thromb Haemost 2019;119:542–552. [DOI] [PubMed] [Google Scholar]
- 13. Chen KW, Monteleone M, Boucher D, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci Immunol 2018;3:eaar6676. [DOI] [PubMed] [Google Scholar]
- 14. Sollberger G, Choidas A, Burn GL, Habenberger P, Di Lucrezia R, Kordes S, Menninger S, Eickhoff J, Nussbaumer P, Klebl B, Kruger R, Herzig A, Zychlinsky A. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci Immunol 2018;3:eaar6689. [DOI] [PubMed] [Google Scholar]
- 15. Munzer P, Negro R, Fukui S, di Meglio L, Aymonnier K, Chu L, Cherpokova D, Gutch S, Sorvillo N, Shi L, Magupalli VG, Weber ANR, Scharf RE, Waterman CM, Wu H, Wagner DD. NLRP3 Inflammasome assembly in neutrophils is supported by PAD4 and promotes NETosis under Sterile conditions. Front Immunol 2021;12:683803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Westerterp M, Fotakis P, Ouimet M, Bochem AE, Zhang H, Molusky MM, Wang W, Abramowicz S, la Bastide-van Gemert S, Wang N, Welch CL, Reilly MP, Stroes ES, Moore KJ, Tall AR. Cholesterol efflux pathways suppress inflammasome activation, NETosis and atherogenesis. Circulation 2018;138:898–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Westerterp M, Murphy AJ, Wang M, Pagler TA, Vengrenyuk Y, Kappus MS, Gorman DJ, Nagareddy PR, Zhu X, Abramowicz S, Parks JS, Welch C, Fisher EA, Wang N, Yvan-Charvet L, Tall AR. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res 2013;112:1456–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, Ishibashi M, Li R, Wang N, Tall AR. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation 2008;118:1837–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 Has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab 2005;1:121–131. [DOI] [PubMed] [Google Scholar]
- 20. Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A 2004;101:9774–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang N, Silver DL, Costet P, Tall AR. Specific binding of ApoA-I, enhanced cholesterol efflux, and altered plasma membrane morphology in cells expressing ABC1. J Biol Chem 2000;275:33053–33058. [DOI] [PubMed] [Google Scholar]
- 22. Patel DC, Albrecht C, Pavitt D, Paul V, Pourreyron C, Newman SP, Godsland IF, Valabhji J, Johnston DG. Type 2 diabetes is associated with reduced ATP-binding cassette transporter A1 gene expression, protein and function. PloS One 2011;6:e22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ganda A, Yvan-Charvet L, Zhang Y, Lai EJ, Regunathan-Shenk R, Hussain FN, Avasare R, Chakraborty B, Febus AJ, Vernocchi L, Lantigua R, Wang Y, Shi X, Hsieh J, Murphy AJ, Wang N, Bijl N, Gordon KM, de Miguel MH, Singer JR, Hogan J, Cremers S, Magnusson M, Melander O, Gerszten RE, Tall AR. Plasma metabolite profiles, cellular cholesterol efflux, and non-traditional cardiovascular risk in patients with CKD. J Mol Cell Cardiol 2017;112:114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, Chatzigeorgiou A, Schlitzer A, Beyer M, Joosten LAB, Isermann B, Lesche M, Petzold A, Simons K, Henry I, Dahl A, Schultze JL, Wielockx B, Zamboni N, Mirtschink P, Coskun U, Hajishengallis G, Netea MG, Chavakis T. Modulation of myelopoiesis progenitors is an integral component of trained immunity. Cell 2018;172:147–161 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cantuti-Castelvetri L, Fitzner D, Bosch-Queralt M, Weil MT, Su M, Sen P, Ruhwedel T, Mitkovski M, Trendelenburg G, Lutjohann D, Mobius W, Simons M. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 2018;359:684–688. [DOI] [PubMed] [Google Scholar]
- 26. Timmins JM, Lee JY, Boudyguina E, Kluckman KD, Brunham LR, Mulya A, Gebre AK, Coutinho JM, Colvin PL, Smith TL, Hayden MR, Maeda N, Parks JS. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J Clin Invest 2005;115:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vromman A, Ruvkun V, Shvartz E, Wojtkiewicz G, Santos Masson G, Tesmenitsky Y, Folco E, Gram H, Nahrendorf M, Swirski FK, Sukhova GK, Libby P. Stage-dependent differential effects of interleukin-1 isoforms on experimental atherosclerosis. Eur Heart J 2019;40:2482–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abram CL, Roberge GL, Hu Y, Lowell CA. Comparative analysis of the efficiency and specificity of myeloid-cre deleting strains using ROSA-EYFP reporter mice. J Immunol Methods 2014;408:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deng L, Zhou JF, Sellers RS, Li JF, Nguyen AV, Wang Y, Orlofsky A, Liu Q, Hume DA, Pollard JW, Augenlicht L, Lin EY. A novel mouse model of inflammatory bowel disease links mammalian target of rapamycin-dependent hyperproliferation of colonic epithelium to inflammation-associated tumorigenesis. Am J Pathol 2010;176:952–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell 2012;11:195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kappus MS, Murphy AJ, Abramowicz S, Ntonga V, Welch CL, Tall AR, Westerterp M. Activation of liver X receptor decreases atherosclerosis in ldlr(-)/(-) mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells. Arterioscler Thromb Vasc Biol 2014;34:279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O'Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 2015;21:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hochheiser IV, Pilsl M, Hagelueken G, Moecking J, Marleaux M, Brinkschulte R, Latz E, Engel C, Geyer M. Structure of the NLRP3 decamer bound to the cytokine release inhibitor CRID3. Nature 2022;604:184–189. [DOI] [PubMed] [Google Scholar]
- 34. Kulawik A, Engesser R, Ehlting C, Raue A, Albrecht U, Hahn B, Lehmann WD, Gaestel M, Klingmuller U, Haussinger D, Timmer J, Bode JG. IL-1beta-induced and p38(MAPK)-dependent activation of the mitogen-activated protein kinase-activated protein kinase 2 (MK2) in hepatocytes: signal transduction with robust and concentration-independent signal amplification. J Biol Chem 2017;292:6291–6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA, Scadden DT, Wagner DD. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A 2012;109:13076–13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Woodfin A, Voisin MB, Imhof BA, Dejana E, Engelhardt B, Nourshargh S. Endothelial cell activation leads to neutrophil transmigration as supported by the sequential roles of ICAM-2, JAM-A, and PECAM-1. Blood 2009;113:6246–6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, Walsh K. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017;355:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mechelke T, Wittig F, Ramer R, Hinz B. Interleukin-1beta induces tissue factor expression in A549 cells via EGFR-dependent and -independent mechanisms. Int J Mol Sci 2021;22:6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mitroulis I, Kambas K, Chrysanthopoulou A, Skendros P, Apostolidou E, Kourtzelis I, Drosos GI, Boumpas DT, Ritis K. Neutrophil extracellular trap formation is associated with IL-1beta and autophagy-related signaling in gout. PloS One 2011;6:e29318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Folco EJ, Mawson TL, Vromman A, Bernardes-Souza B, Franck G, Persson O, Nakamura M, Newton G, Luscinskas FW, Libby P. Neutrophil extracellular traps induce endothelial cell activation and tissue factor production through interleukin-1alpha and cathepsin G. Arterioscler Thromb Vasc Biol 2018;38:1901–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meher AK, Spinosa M, Davis JP, Pope N, Laubach VE, Su G, Serbulea V, Leitinger N, Ailawadi G, Upchurch GR Jr. Novel role of IL (interleukin)-1beta in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol 2018;38:843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015;349:316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paulin N, Viola JR, Maas SL, de Jong R, Fernandes-Alnemri T, Weber C, Drechsler M, Doring Y, Soehnlein O. Double-Strand DNA sensing Aim2 inflammasome regulates atherosclerotic plaque vulnerability. Circulation 2018;138:321–323. [DOI] [PubMed] [Google Scholar]
- 44. Tall AR, Thomas DG, Gonzalez-Cabodevilla AG, Goldberg IJ. Addressing dyslipidemic risk beyond LDL-cholesterol. J Clin Invest 2022;132:e148559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Svensson EC, Madar A, Campbell CD, He Y, Sultan M, Healey ML, Xu H, D'Aco K, Fernandez A, Wache-Mainier C, Libby P, Ridker PM, Beste MT, Basson CT. TET2-Driven Clonal hematopoiesis and response to canakinumab: an exploratory analysis of the CANTOS randomized clinical trial. JAMA Cardiol 2022;7:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding authors.