Abstract
Background
Gout incidence is increasing worldwide; appropriate management of serum uric acid levels and a healthy lifestyle may help its prevention. The popularity of electronic cigarettes and the resultant emergence of dual smokers is increasing. Despite many studies on the effects of various health behaviors on serum uric acid levels, the association between smoking and serum uric acid levels remains controversial. This study aimed to investigate the association between smoking and serum uric acid levels.
Methods
In this study, total sample of 27,013 participants (11,924 men and 15,089 women) were analyzed. This study used data from the Korea National Health and Nutrition Examination Survey (2016–2020) and grouped adults into dual smokers, single smokers, ex-smokers, and non-smokers. Multiple logistic regression analyses were performed to investigate the association between smoking behavior and serum uric acid levels.
Results
Compared to male non-smokers, male dual smokers had significantly higher serum uric acid level (odds ratio [OR], 1.43; 95% confidence interval [CI], 1.08–1.88). In female, serum uric acid level was higher among single smokers than non-smokers (OR, 1.68; 95% CI, 1.25–2.25). Higher serum uric acid levels were more likely to be present in male dual smokers with a > 20 pack-year smoking habit (OR, 1.84; 95% CI, 1.06–3.18).
Conclusion
Dual smoking may contribute to high serum uric acid levels in adults. Thus, serum uric acid levels should be properly managed through smoking cessation.
Introduction
Gout is a type of auto-inflammatory arthritis with increasing prevalence and incidence worldwide [1, 2]. Increased incidence and death rate have been reported especially in the United States, Italy, South Korea, Australia, New Zealand, and Taiwan [3, 4]. Serum uric acid (SUA) plays a pivotal role in gout and is an unusual complication of anorexia nervosa [5]. Moreover, SUA is a potential risk factor for the deterioration of kidney function; high SUA levels increase the risk of acute and chronic kidney disease (CKD) [6]. High SUA levels progress to hyperuricemia, which may play a role in the pathogenesis of CKD and damage the vascular lining over time [7]. Additionally, SUA is associated with other health risk factors in daily life, such as hypertension, insulin resistance, and the cardiovascular diseases [8–10]. Given that elevated SUA causes many diseases in the contemporary world, its management is significant for personal health [11].
Smoking is a leading risk factor for premature death worldwide and is one of the primary causes of chronic diseases such as cancer, cardiovascular diseases, and respiratory diseases [12]. Owing to the adverse health outcomes of smoking, people tend to quit conventional smoking and are increasingly turning to electronic cigarettes (e-cigarettes) as an alternative [13, 14]. E-cigarettes heat a liquid that often contains nicotine to produce aerosol, which is subsequently inhaled. Evidence reveals that dual smoking behavior, which involves smoking both e-cigarettes and conventional cigarettes, is as harmful to health as smoking conventional cigarettes alone [15–19]. Although e-cigarette nicotine delivery systems are considered less dangerous than conventional cigarettes, they are associated with a range of complications, including thermal damage, lung damage, cardiovascular outcomes, and psychosocial effects [18]. As of 2020, Korea’s smoking rate is 20.6% (male: 34.0%, female: 6.6%), of which 8.4% for male and 1.9% for female use e-cigarettes. With an increase in the number of dual smokers and decrease in successful smoking cessation observed, dual smoking appears to have the potential to induce tobacco dependence [20–23].
Previous SUA level-related studies have found associations with gout, kidney function, alcohol, tea, coffee, milk, and yogurt [7, 8]. Notably, in the United States, patients with gout are advised to limit the consumption of distilled beverages such as beer and wine and are recommended to consume low-fat or non-fat dairy products [10]. Despite studies conducted by various research groups on the association between smoking and SUA, many conflicting opinions still exist [24]. Additionally, unlike the evidence related to smoking, there is insufficient evidence to clarify the association between e-cigarette or dual smoking and SUA.
Therefore, this study aimed to investigate the association between various smoking behaviors, including dual smoking (both e-cigarettes and conventional cigarettes), single smoking (only conventional cigarettes), and past smoking with respect to SUA, in a representative Korean adult population.
Materials and methods
Data and study population
The data used in this study were obtained from the Korea National Health and Nutrition Examination Survey (KNHANES) conducted from 2016 to 2020. The KNHANES is a cross-sectional, nationwide survey conducted annually by the Korea Disease Control and Prevention Agency (KDCA) of the Ministry of Health and Welfare, South Korea, to evaluate the health status, health behavior, and nutritional status of the South Korean population. The respondents answered the questionnaires, and all the obtained data were anonymized. As the KNHANES complies with the Declaration of Helsinki and provides publicly accessible data, ethical approval was not required.
The total number of respondents from the 2016–2020 survey was 39,738. Information from individuals aged 1–18 years was excluded as they had not been asked regarding smoking behavior (N = 7,610). Additionally, data from participants with missing variables were also excluded (N = 5,105). Finally, 27,013 participants (11,924 men and 15,089 women) were analyzed in this study.
Measures
The dependent variable, SUA was measured by collecting venous blood from participants who had been fasting for > 8 h. SUA was measured by colorimetry with the enzyme uricase using a Hitachi Automatic Biochemical Analyzer 7600–210 (Hitachi, Tokyo, Japan) from 2016 to 2018 in KNHANES. Uricase was also measured using Labospect 008AS (Hitachi, Tokyo, Japan) from 2019 to 2020 in KNHANES. Furthermore, the common cutoff value for SUA level was 7.0 mg/dL (420 μmol/L) for men and 6.0 mg/dL (357 μmol/L) for women [25].
Based on smoking behavior as the independent variable, the study population was divided into four groups: (1) non-smokers, (2) ex-smokers who had been using conventional cigarettes or e-cigarettes in the past, (3) single smokers who used only conventional cigarettes, and (4) dual smokers who used conventional cigarettes and e-cigarettes. This classification was identical to that of the previous studies that investigated smoking behavior using the same investigative tools [16, 17, 26].
The covariates included demographic factors: age (19–29 / 30–39 / 40–49 / 50–59 / 60–69 / ≥ 70), marital status (married / single or widow / divorced or separated), and educational level (middle school or below / high school / college or over); socioeconomic factors: household income (low / mid-low / mid-high / high), region of residence (metropolitan / urban / rural), and occupation (white / pink / blue / inoccupation); health-related factors: body mass index [BMI] (underweight / normal / overweight), hypertension status (normal / pre-hypertension / hypertension), diabetes status (yes / no), and dyslipidemia status (yes / no); and health-related behavioral patterns of alcohol consumption (yes / no).
Statistical analysis
All estimates were calculated using sample weight procedures, clusters, and strata assigned to the study participants. Descriptive analysis was performed to assess the general characteristics of the study population. Subsequently, a multiple logistic regression analysis was performed to evaluate the effect of smoking behavior on SUA levels and perform a subgroup analysis stratified by independent variables. In addition, we calculated the pack-years by the amount and duration of smoking in the past or current smokers and conducted a subgroup analysis by tying it with the smoking behavior. The main results are expressed as odds ratios (ORs) and 95% confidence intervals (CIs). SAS version 9.4 (SAS Institute Inc.; Cary, NC, USA) was used for all analyses, and a p-value <0.05 was considered statistically significant.
Results
Table 1 highlights the general characteristics of the study population. Of the 27,013 participants, 11,924 were men (44.1%) and 15,089 were women (55.9%). Among the men, 357 (3.0%), 3,629 (30.4%), 5,057 (42.4%), and 2,881 (24.2%) were dual smokers, single smokers, ex-smokers, and non-smokers, respectively. Among the women, 72 (0.5%), 697 (4.6%), 938 (6.2%), and 13,382 (88.7%) were dual smokers, single smokers, ex-smokers, and non-smokers, respectively. The relationship between smoking behavior and SUA levels was statistically significant in men and women. Moreover, differences in demographic, socioeconomic, and health status characteristics were primarily significant (p < .0001).
Table 1. General characteristics of the study population.
| Variables | Male | Female | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum Uric Acid Level | Serum Uric Acid Level | ||||||||||||||
| Total | normal (<7) | abnormal (≥7) | P-value | Total | normal (<6) | abnormal (≥6) | P-value | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||||
| Total (N = 27,013) | 11,924 | 100.0 | 9,482 | 79.5 | 2,442 | 20.5 | 15,089 | 100.0 | 13,953 | 92.5 | 1,136 | 7.5 | |||
| Smoking Behavior | < .0001 | < .0001 | |||||||||||||
| Non-smoker | 2,881 | 24.2 | 2,286 | 79.3 | 595 | 20.7 | 13,382 | 88.7 | 12,412 | 92.8 | 970 | 7.2 | |||
| Ex-smoker | 5,057 | 42.4 | 4,069 | 80.5 | 988 | 19.5 | 938 | 6.2 | 862 | 91.9 | 76 | 8.1 | |||
| Single smoker | 3,629 | 30.4 | 2,884 | 79.5 | 745 | 20.5 | 697 | 4.6 | 615 | 88.2 | 82 | 11.8 | |||
| Dual smoker | 357 | 3.0 | 243 | 68.1 | 114 | 31.9 | 72 | 0.5 | 64 | 88.9 | 8 | 11.1 | |||
| Age | < .0001 | < .0001 | |||||||||||||
| 19–29 | 1,636 | 13.7 | 1,169 | 71.5 | 467 | 28.5 | 1,757 | 11.6 | 1,634 | 93.0 | 123 | 7.0 | |||
| 30–39 | 1,858 | 15.6 | 1,327 | 71.4 | 531 | 28.6 | 2,297 | 15.2 | 2,158 | 93.9 | 139 | 6.1 | |||
| 40–49 | 2,146 | 18.0 | 1,653 | 77.0 | 493 | 23.0 | 2,852 | 18.9 | 2,726 | 95.6 | 126 | 4.4 | |||
| 50–59 | 2,204 | 18.5 | 1,857 | 84.3 | 347 | 15.7 | 2,999 | 19.9 | 2,805 | 93.5 | 194 | 6.5 | |||
| 60–69 | 2,126 | 17.8 | 1,833 | 86.2 | 293 | 13.8 | 2,743 | 18.2 | 2,540 | 92.6 | 203 | 7.4 | |||
| ≥70 | 1,954 | 16.4 | 1,643 | 84.1 | 311 | 15.9 | 2,441 | 16.2 | 2,090 | 85.6 | 351 | 14.4 | |||
| Marital status | < .0001 | < .0001 | |||||||||||||
| Married | 8,548 | 71.7 | 6,965 | 81.5 | 1,583 | 18.5 | 10,064 | 66.7 | 9,434 | 93.7 | 630 | 6.3 | |||
| Single, widow | 2,861 | 24.0 | 2,097 | 73.3 | 764 | 26.7 | 4,145 | 27.5 | 3,716 | 89.7 | 429 | 10.3 | |||
| Divorced, Separated | 515 | 4.3 | 420 | 81.6 | 95 | 18.4 | 880 | 5.8 | 803 | 91.3 | 77 | 8.8 | |||
| Educational level | < .0001 | < .0001 | |||||||||||||
| Middle school or below | 2,698 | 22.6 | 2,261 | 83.8 | 437 | 16.2 | 4,994 | 33.1 | 4,459 | 89.3 | 535 | 10.7 | |||
| High school | 4,209 | 35.3 | 3,338 | 79.3 | 871 | 20.7 | 4,730 | 31.3 | 4,416 | 93.4 | 314 | 6.6 | |||
| College or over | 5,017 | 42.1 | 3,883 | 77.4 | 1,134 | 22.6 | 5,365 | 35.6 | 5,078 | 94.7 | 287 | 5.3 | |||
| Household income | 0.0260 | < .0001 | |||||||||||||
| Low | 1,916 | 16.1 | 1,563 | 81.6 | 353 | 18.4 | 2,922 | 19.4 | 2,583 | 88.4 | 339 | 11.6 | |||
| Mid-low | 2,850 | 23.9 | 2,260 | 79.3 | 590 | 20.7 | 3,707 | 24.6 | 3,409 | 92.0 | 298 | 8.0 | |||
| Mid-high | 3,375 | 28.3 | 2,699 | 80.0 | 676 | 20.0 | 4,108 | 27.2 | 3,865 | 94.1 | 243 | 5.9 | |||
| High | 3,783 | 31.7 | 2,960 | 78.2 | 823 | 21.8 | 4,352 | 28.8 | 4,096 | 94.1 | 256 | 5.9 | |||
| Region | 0.1921 | 0.0005 | |||||||||||||
| Metropolitan | 5,196 | 43.6 | 4,125 | 79.4 | 1,071 | 20.6 | 6,756 | 44.8 | 6,293 | 93.1 | 463 | 6.9 | |||
| Urban | 4,421 | 37.1 | 3,492 | 79.0 | 929 | 21.0 | 5,599 | 37.1 | 5,177 | 92.5 | 422 | 7.5 | |||
| Rural | 2,307 | 19.3 | 1,865 | 80.8 | 442 | 19.2 | 2,734 | 18.1 | 2,483 | 90.8 | 251 | 9.2 | |||
| Occupational categories | < .0001 | < .0001 | |||||||||||||
| White | 3,496 | 29.3 | 2,644 | 75.6 | 852 | 24.4 | 3,390 | 22.5 | 3,214 | 94.8 | 176 | 5.2 | |||
| Pink | 1,225 | 10.3 | 946 | 77.2 | 279 | 22.8 | 2,295 | 15.2 | 2,159 | 94.1 | 136 | 5.9 | |||
| Blue | 3,908 | 32.8 | 3,225 | 82.5 | 683 | 17.5 | 2,272 | 15.1 | 2,107 | 92.7 | 165 | 7.3 | |||
| Inoccupation | 3,295 | 27.6 | 2,667 | 80.9 | 628 | 19.1 | 7,132 | 47.3 | 6,473 | 90.8 | 659 | 9.2 | |||
| BMI | < .0001 | < .0001 | |||||||||||||
| Underweight | 284 | 2.4 | 259 | 91.2 | 25 | 8.8 | 718 | 4.8 | 710 | 98.9 | 8 | 1.1 | |||
| Normal | 6,639 | 55.7 | 5,602 | 84.4 | 1,037 | 15.6 | 9,827 | 65.1 | 9,325 | 94.9 | 502 | 5.1 | |||
| Overweight | 5,001 | 41.9 | 3,621 | 72.4 | 1,380 | 27.6 | 4,544 | 30.1 | 3,918 | 86.2 | 626 | 13.8 | |||
| Alcohol consumption | < .0001 | 0.0007 | |||||||||||||
| Yes | 9,883 | 82.9 | 7,762 | 78.5 | 2,121 | 21.5 | 9,792 | 64.9 | 9,107 | 93.0 | 685 | 7.0 | |||
| No | 2,041 | 17.1 | 1,720 | 84.3 | 321 | 15.7 | 5,297 | 35.1 | 4,846 | 91.5 | 451 | 8.5 | |||
| Status of Hypertension | < .0001 | < .0001 | |||||||||||||
| Normal | 4,048 | 33.9 | 3,363 | 83.1 | 685 | 16.9 | 7,585 | 50.3 | 7,237 | 95.4 | 348 | 4.6 | |||
| Pre-Hypertension | 3,647 | 30.6 | 2,839 | 77.8 | 808 | 22.2 | 3,110 | 20.6 | 2,895 | 93.1 | 215 | 6.9 | |||
| Hypertension | 4,229 | 35.5 | 3,280 | 77.6 | 949 | 22.4 | 4,394 | 29.1 | 3,821 | 87.0 | 573 | 13.0 | |||
| Status of Diabetes | < .0001 | < .0001 | |||||||||||||
| Yes | 1,238 | 10.4 | 1,051 | 84.9 | 187 | 15.1 | 1,000 | 6.6 | 850 | 85.0 | 150 | 15.0 | |||
| No | 10,686 | 89.6 | 8,431 | 78.9 | 2,255 | 21.1 | 14,089 | 93.4 | 13,103 | 93.0 | 986 | 7.0 | |||
| Status of Dyslipidemia | 0.3910 | < .0001 | |||||||||||||
| Yes | 2,503 | 21.0 | 1,975 | 78.9 | 528 | 21.1 | 3,955 | 26.2 | 3,565 | 90.1 | 390 | 9.9 | |||
| No | 9,421 | 79.0 | 7,507 | 79.7 | 1,914 | 20.3 | 11,134 | 73.8 | 10,388 | 93.3 | 746 | 6.7 | |||
| Year | 0.0190 | 0.0004 | |||||||||||||
| 2016 | 2,332 | 19.6 | 1,894 | 81.2 | 438 | 18.8 | 3,064 | 20.3 | 2,851 | 93.0 | 213 | 7.0 | |||
| 2017 | 2,430 | 20.4 | 1,959 | 80.6 | 471 | 19.4 | 3,016 | 20.0 | 2,830 | 93.8 | 186 | 6.2 | |||
| 2018 | 2,428 | 20.4 | 1,916 | 78.9 | 512 | 21.1 | 3,126 | 20.7 | 2,872 | 91.9 | 254 | 8.1 | |||
| 2019 | 2,448 | 20.5 | 1,901 | 77.7 | 547 | 22.3 | 3,113 | 20.6 | 2,834 | 91.0 | 279 | 9.0 | |||
| 2020 | 2,286 | 19.2 | 1,812 | 79.3 | 474 | 20.7 | 2,770 | 18.4 | 2,566 | 92.6 | 204 | 7.4 | |||
Table 2 presents the association between smoking behavior and SUA levels in men and women after adjusting for all covariates. In men, dual smokers (OR, 1.43; 95% CI, 1.08–1.88) were statistically associated with SUA, whereas in women, a statistical association was observed in single smokers (OR, 1.68; 95% CI, 1.25–2.25). Compared with non-smokers, both male and female ex-smokers, single smokers, and dual smokers showed higher ORs for abnormal SUA levels, although some were not statistically significant.
Table 2. Results of factors associated between smoking behavior and serum uric acid.
| Variables | Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum Uric Acid ≥7 | Serum Uric Acid ≥6 | ||||||||
| OR | 95% CI | OR | 95% CI | ||||||
| Smoking Behavior | |||||||||
| Non-smoker | 1.00 | 1.00 | |||||||
| Ex-smoker | 1.12 | (0.97 | - | 1.30) | 1.25 | (0.91 | - | 1.71) | |
| Single smoker | 1.03 | (0.88 | - | 1.21) | 1.68 | (1.25 | - | 2.25) | |
| Dual smoker | 1.43 | (1.08 | - | 1.88) | 1.88 | (0.76 | - | 4.65) | |
| Age | |||||||||
| 19–29 | 2.53 | (1.89 | - | 3.40) | 1.72 | (1.15 | - | 2.57) | |
| 30–39 | 2.32 | (1.79 | - | 3.00) | 1.50 | (1.04 | - | 2.16) | |
| 40–49 | 1.60 | (1.26 | - | 2.04) | 0.93 | (0.65 | - | 1.31) | |
| 50–59 | 0.97 | (0.77 | - | 1.22) | 1.13 | (0.87 | - | 1.47) | |
| 60–69 | 0.88 | (0.70 | - | 1.10) | 1.67 | (1.31 | - | 2.14) | |
| ≥70 | 1.00 | 1.00 | |||||||
| Marital status | |||||||||
| Married | 1.00 | 1.00 | |||||||
| Single, widow | 1.17 | (1.00 | - | 1.37) | 1.28 | (1.04 | - | 1.57) | |
| Divorced, Separated | 1.28 | (0.94 | - | 1.75) | 1.05 | (0.77 | - | 1.43) | |
| Educational level | |||||||||
| Middle school or below | 1.15 | (0.95 | - | 1.39) | 1.08 | (0.80 | - | 1.46) | |
| High school | 1.03 | (0.90 | - | 1.18) | 1.08 | (0.87 | - | 1.33) | |
| College or over | 1.00 | 1.00 | |||||||
| Household income | |||||||||
| Low | 1.00 | 1.00 | |||||||
| Mid-low | 1.10 | (0.89 | - | 1.36) | 1.01 | (0.81 | - | 1.26) | |
| Mid-high | 0.95 | (0.77 | - | 1.17) | 0.87 | (0.68 | - | 1.10) | |
| High | 1.03 | (0.83 | - | 1.26) | 1.10 | (0.84 | - | 1.43) | |
| Region | |||||||||
| Metropolitan | 1.00 | 1.00 | |||||||
| Urban | 1.01 | (0.89 | - | 1.14) | 1.02 | (0.86 | - | 1.20) | |
| Rural | 1.06 | (0.89 | - | 1.26) | 1.07 | (0.87 | - | 1.33) | |
| Occupational categories | |||||||||
| White | 1.13 | (0.94 | - | 1.35) | 0.85 | (0.67 | - | 1.07) | |
| Pink | 1.06 | (0.86 | - | 1.31) | 0.70 | (0.55 | - | 0.88) | |
| Blue | 0.90 | (0.75 | - | 1.08) | 0.73 | (0.59 | - | 0.90) | |
| Inoccupation | 1.00 | 1.00 | |||||||
| BMI | |||||||||
| Underweight | 1.00 | 1.00 | |||||||
| Normal | 2.02 | (1.24 | - | 3.30) | 3.77 | (1.80 | - | 7.89) | |
| Overweight | 3.95 | (2.43 | - | 6.43) | 10.30 | (4.85 | - | 21.87) | |
| Alcohol consumption | |||||||||
| Yes | 1.13 | (0.96 | - | 1.33) | 1.12 | (0.96 | - | 1.32) | |
| No | 1.00 | 1.00 | |||||||
| Status of Hypertension | |||||||||
| Normal | 1.00 | 1.00 | |||||||
| Pre-Hypertension | 1.43 | (1.23 | - | 1.65) | 1.52 | (1.21 | - | 1.91) | |
| Hypertension | 1.85 | (1.59 | - | 2.15) | 2.10 | (1.67 | - | 2.64) | |
| Status of Diabetes | |||||||||
| Yes | 0.63 | (0.52 | - | 0.78) | 1.40 | (1.11 | - | 1.76) | |
| No | 1.00 | 1.00 | |||||||
| Status of Dyslipidemia | |||||||||
| Yes | 1.14 | (0.99 | - | 1.31) | 1.16 | (0.97 | - | 1.39) | |
| No | 1.00 | 1.00 | |||||||
| Year | |||||||||
| 2016 | 1.00 | 1.00 | |||||||
| 2017 | 1.11 | (0.93 | - | 1.33) | 0.92 | (0.70 | - | 1.20) | |
| 2018 | 1.21 | (1.00 | - | 1.46) | 1.18 | (0.92 | - | 1.51) | |
| 2019 | 1.35 | (1.12 | - | 1.62) | 1.43 | (1.12 | - | 1.83) | |
| 2020 | 1.18 | (0.99 | - | 1.41) | 1.05 | (0.81 | - | 1.36) | |
Table 3 demonstrates a subgroup analysis performed to evaluate the combined effect of smoking behavior, alcohol consumption, and hypertension on SUA levels. For men, alcohol consumption (OR, 1.43; 95% CI, 1.07–1.91) and hypertension (OR, 1.83; 95% CI, 1.01–3.32) among dual smokers had the strongest associations with SUA compared to those of non-smokers. For women, alcohol consumption (single smokers: OR, 1.68; 95% CI, 1.21–2.32), hypertension (dual smokers, OR: 16.99; 95% CI: 2.70–107.16), and dyslipidemia status (single smokers: OR, 1.83; 95% CI, 1.31–2.58) showed the strongest association with serum uric acid compared to those of non-smokers.
Table 3. Results of subgroup analysis stratified by independent variables.
| Variables† | Serum Uric Acid Level | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoking Behavior | |||||||||||||||
| Non-smoker | Ex-smoker | Single smoker | Dual smoker | ||||||||||||
| OR | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||||||
| Male | BMI | ||||||||||||||
| Underweight | 1.00 | 2.61 | (0.53 | - | 12.87) | 0.50 | (0.12 | - | 1.97) | * | * | * | * | ||
| Normal | 1.00 | 1.21 | (0.98 | - | 1.50) | 1.15 | (0.91 | - | 1.46) | 1.74 | (1.11 | - | 2.71) | ||
| Overweight | 1.00 | 1.06 | (0.87 | - | 1.28) | 0.95 | (0.77 | - | 1.17) | 1.22 | (0.85 | - | 1.75) | ||
| Alcohol consumption | |||||||||||||||
| Yes | 1.00 | 1.12 | (0.96 | - | 1.31) | 1.05 | (0.88 | - | 1.24) | 1.43 | (1.07 | - | 1.91) | ||
| No | 1.00 | 1.10 | (0.77 | - | 1.57) | 0.89 | (0.56 | - | 1.40) | 1.65 | (0.46 | - | 5.95) | ||
| Status of Hypertension | |||||||||||||||
| Normal | 1.00 | 1.05 | (0.82 | - | 1.35) | 1.11 | (0.85 | - | 1.44) | 1.38 | (0.90 | - | 2.12) | ||
| Pre-Hypertension | 1.00 | 1.11 | (0.87 | - | 1.40) | 1.09 | (0.85 | - | 1.39) | 1.25 | (0.80 | - | 1.96) | ||
| Hypertension | 1.00 | 1.22 | (0.97 | - | 1.54) | 0.94 | (0.72 | - | 1.23) | 1.83 | (1.01 | - | 3.32) | ||
| Status of Diabetes | |||||||||||||||
| Yes | 1.00 | 1.01 | (0.61 | - | 1.65) | 0.55 | (0.31 | - | 1.00) | 1.64 | (0.48 | - | 5.58) | ||
| No | 1.00 | 1.13 | (0.97 | - | 1.31) | 1.07 | (0.91 | - | 1.26) | 1.43 | (1.07 | - | 1.90) | ||
| Status of Dyslipidemia | |||||||||||||||
| Yes | 1.00 | 1.29 | (0.91 | - | 1.83) | 1.21 | (0.84 | - | 1.76) | 1.01 | (0.52 | - | 1.97) | ||
| No | 1.00 | 1.08 | (0.92 | - | 1.27) | 1.00 | (0.83 | - | 1.19) | 1.51 | (1.11 | - | 2.06) | ||
| Female | BMI | ||||||||||||||
| Underweight | 1.00 | * | * | * | * | 2.70 | (0.19 | - | 38.94) | * | * | * | * | ||
| Normal | 1.00 | 1.68 | (1.11 | - | 2.55) | 2.14 | (1.41 | - | 3.25) | 2.37 | (0.85 | - | 6.64) | ||
| Overweight | 1.00 | 0.88 | (0.56 | - | 1.38) | 1.20 | (0.79 | - | 1.81) | 1.52 | (0.30 | - | 7.75) | ||
| Alcohol consumption | |||||||||||||||
| Yes | 1.00 | 1.21 | (0.84 | - | 1.74) | 1.68 | (1.21 | - | 2.32) | 2.05 | (0.82 | - | 5.13) | ||
| No | 1.00 | 1.41 | (0.79 | - | 2.52) | 1.50 | (0.72 | - | 3.09) | * | * | * | * | ||
| Status of Hypertension | |||||||||||||||
| Normal | 1.00 | 1.80 | (1.17 | - | 2.76) | 1.76 | (1.15 | - | 2.68) | 2.26 | (0.73 | - | 7.03) | ||
| Pre-Hypertension | 1.00 | 0.66 | (0.31 | - | 1.41) | 1.53 | (0.83 | - | 2.81) | 0.32 | (0.03 | - | 3.07) | ||
| Hypertension | 1.00 | 0.97 | (0.59 | - | 1.62) | 1.44 | (0.87 | - | 2.40) | 16.99 | (2.70 | - | 107.16) | ||
| Status of Diabetes | |||||||||||||||
| Yes | 1.00 | 0.53 | (0.17 | - | 1.60) | 0.46 | (0.15 | - | 1.47) | * | * | * | * | ||
| No | 1.00 | 1.33 | (0.96 | - | 1.85) | 1.90 | (1.41 | - | 2.55) | 2.00 | (0.81 | - | 4.92) | ||
| Status of Dyslipidemia | |||||||||||||||
| Yes | 1.00 | 1.36 | (0.94 | - | 1.95) | 1.83 | (1.31 | - | 2.58) | 1.84 | (0.68 | - | 4.95) | ||
| No | 1.00 | 0.95 | (0.53 | - | 1.71) | 1.37 | (0.81 | - | 2.31) | 2.16 | (0.24 | - | 19.41) | ||
† adjusted for all covariates
* Due to sparsity of the data, OR could not be calculated in the model
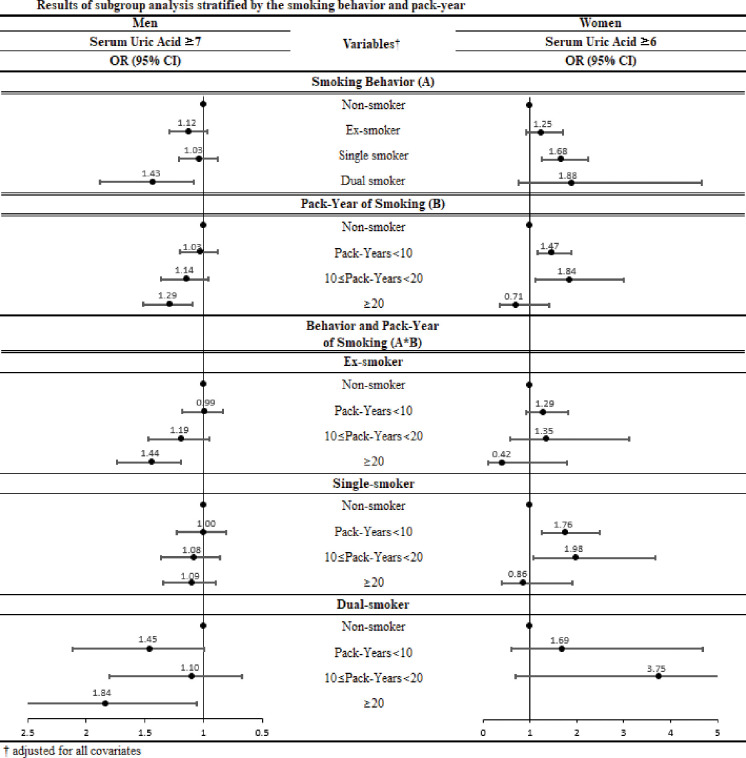
Fig 1 reveals the results of the subgroup analysis depicting changes in ORs according to the pack-years (number of cigarettes smoked and smoking period) smoked. The ORs tended to increase linearly as the pack-years increased. Specifically, male ex-smokers (OR, 1.44; 95% CI, 1.19–1.74) and dual smokers (OR, 1.84; 95% CI, 1.06–3.18) who had > 20 pack-years were more likely to have SUA levels ≥ 7 mg/dL than non-smokers. Female single smokers who had less than 10 pack-years (OR, 1.76; 95% CI, 1.25–2.48) and 10 to 20 pack-years (OR, 1.98; 95% CI, 1.07–3.66) were more likely to have SUA levels ≥ 6 mg/dL than non-smokers.
Fig 1. Results of subgroup analysis stratified by the smoking behavior and pack-year.
Discussion
The World Health Organization has consistently emphasized the importance of quitting smoking and the dangers of smoking, which kills approximately eight million people every year [12]. However, the mechanism explaining how smoking increases SUA levels remains unclear. A study has revealed that current smokers with a BMI > 24.9 have an increased risk of gout over time [27]. This finding can be applied to single or dual smokers, as indirectly implied by the current results. Hyperuricemia is a major risk factor for metabolic syndrome that leads to the development of cardiovascular and cerebrovascular diseases [9]. Most patients with gout have obesity, hypertension, and hyperlipidemia [9, 25, 27]. Therefore, patients with gout may require proper management through smoking cessation to reduce this risk.
Based on this, the present study aimed to validate the association between SUA and various smoking behaviors, including dual smoking, single smoking, and ex-smoking, in a representative Korean adult population. We also conducted a subgroup analysis according to factors related to smoking and SUA, including BMI, alcohol consumption, hypertension, diabetes, and dyslipidemia status. Furthermore, we stratified smoking behavior according to pack-years smoked.
In this study, elevated SUA levels were observed in dual smokers compared to non-smokers. This relationship was especially strong among men who were dual smokers. A strong connection between elevated SUA levels and women who were single smokers was observed. Among men who had more than 20 pack-years, dual smokers and ex-smokers were more strongly associated with SUA. Among women who had less than 10 pack-years and 10 to 20 pack-years, single smoking was significantly associated with SUA. In general, the SUA level linearly increased with the pack-years. Overall, this study found a significant association among men but not among women, which could be considered a result of a recall bias in self-reported data owing to poor perception of women smoking in Korea [28]. The underreporting of women’s smoking is connected to social stigma, which conceals and masks smoking among women more so than men.
A previous study suggested that SUA was only associated with women, not men [24]. Furthermore, based on a previous study, our study considered dual smokers and found that SUA was related to both women and men. With the increase in the use of e-cigarettes, the risk perception of dual smokers has become important [1, 2]. Additionally, as the prevalence and incidence of gout increase [3, 4], research on the association between smoking and SUA is being actively conducted. The increasing effect of smoking on SUA has been observed globally [24, 29–31]. Moreover, one study found that male e-cigarette users have higher levels of SUA than non-smokers and conventional cigarette users [30]. In contrast, some studies suggested that smoking may lower SUA levels [32–34], which was explained by the antioxidant effect on ROS and free radicals produced by cigarettes [32]. No effect of reduction was reported to be found in a large study population considering the amount and duration of smoking [35, 36]. Although the association between smoking and SUA is controversial, generally, low SUA levels in smokers are associated with the depletion of antioxidants [24]. Therefore, these results are consistent with our findings on the adverse effects of e-cigarettes and dual smoking in our study population.
This study has certain limitations. First, it was a cross-sectional study. We found an association between smoking behavior and SUA; however, the causal relationship requires careful interpretation. Therefore, further research is needed to clarify the relationship of smoking behavior and SUA levels. Second, KNHANES data were collected as a self-report survey. Data on smoking behavior and health-related and socioeconomic variables might not have been accurately measured and might not be completely reliable. In particular, it may have resulted in recall bias with underestimated smoking behavior. Therefore, smoking behavior was evaluated on its own, and the exact smoking cessation status of ex-smokers was unclear. Future studies need to clear the smoking cessation period through measurement data to compensate for these limitations. Third, we aimed to consider the pack-years of all participants; however, owing to data limitations, we could not sufficiently reflect information on the pack-years of e-cigarettes. This may lead to uncertainty regarding the relationship between dual smokers and SUA. Therefore, further studies reflecting these data are needed. Fourth, e-cigarettes are more recent than conventional cigarettes, and a limited number of respondents smoked only e-cigarettes. Future research should consider each smoking behavior separately because single smokers who smoke only e-cigarettes were not considered. Finally, although we adjusted for many covariates that might have affected the results, residual confounding factors might not have been measured or considered in our analysis.
In contrast with the limitations, our study had several strengths. First, KNHANES conducted by the KDCA is nationally representative survey based on random cluster sampling, which is reliable and representative. Therefore, our results can be generalized to ordinary Korean adults. Second, blood samples were collected using standardized laboratory procedures, and SUA levels were measured to produce reliable and clear data. Third, few studies have evaluated the association between smoking behaviors, including e-cigarette use, dual smoking, and SUA. Therefore, this study is noteworthy in subgroup analysis by calculating pack-years and smoking behavior, such as dual smoking. In addition, the pack-years of ex-smokers and single and dual smokers were calculated and analyzed.
Conclusion
Smoking behavior, particularly dual smoking, in the male population was associated with SUA. In addition, the higher the pack-years, the greater was the risk of high SUA levels. In particular, the risk of increased SUA levels, particularly in those who are dual smokers (> 20 pack-years), has been reported. Given these results, smoking is related to SUA, and dual and single smoking is harmful to health. These findings should provide the direction of research on the adverse effects of e-cigarettes and dual smoking in future studies and educate people regarding the risk. The current study findings may be significant given that many people believe using e-cigarettes to be safe smoking behaviors, and this could lead to dual smoking.
Acknowledgments
We would like to thank the Korea Centers for Disease Control and Prevention (KDCA) for providing the materials based on the nationwide survey. We would also like to thank our colleagues at the Yonsei University of health research institute for their advice on writing the literature.
Data Availability
The data of KNHANES is publicly available throuth the website (https://knhanes.kdca.go.kr/knhanes/main.do).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Engel B, Just J, Bleckwenn M, Weckbecker K. Treatment Options for Gout. Dtsch Arztebl Int. 2017;114: 215–222. doi: 10.3238/arztebl.2017.0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rock KL, Kataoka H, Lai JJ. Uric acid as a danger signal in gout and its comorbidities. Nat Rev Rheumatol. 2013;9: 13–23. doi: 10.1038/nrrheum.2012.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11: 649–662. doi: 10.1038/nrrheum.2015.91 [DOI] [PubMed] [Google Scholar]
- 4.Singh JA, Edwards NL. Gout management and outcomes during the COVID-19 pandemic: a cross-sectional internet survey. Ther Adv Musculoskelet Dis. 2020;12: 1759720x20966124. doi: 10.1177/1759720X20966124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simeunovic Ostojic M, Maas J. Anorexia nervosa and uric acid beyond gout: An idea worth researching. Int J Eat Disord. 2018;51: 97–101. doi: 10.1002/eat.22817 [DOI] [PubMed] [Google Scholar]
- 6.Giordano C, Karasik O, King-Morris K, Asmar A. Uric Acid as a Marker of Kidney Disease: Review of the Current Literature. Dis Markers. 2015;2015: 382918. doi: 10.1155/2015/382918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joo HJ, Kim GR, Choi DW, Joo JH, Park EC. Uric acid level and kidney function: a cross-sectional study of the Korean national health and nutrition examination survey (2016–2017). Sci Rep. 2020;10: 21672. doi: 10.1038/s41598-020-77702-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Towiwat P, Li ZG. The association of vitamin C, alcohol, coffee, tea, milk and yogurt with uric acid and gout. Int J Rheum Dis. 2015;18: 495–501. doi: 10.1111/1756-185X.12622 [DOI] [PubMed] [Google Scholar]
- 9.Borghi C, Agabiti-Rosei E, Johnson RJ, Kielstein JT, Lurbe E, Mancia G, et al. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur J Intern Med. 2020;80: 1–11. doi: 10.1016/j.ejim.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 10.Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64: 1431–1446. doi: 10.1002/acr.21772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawley WT, Jungels CG, Stenmark KR, Fini MA. U-shaped association of uric acid to overall-cause mortality and its impact on clinical management of hyperuricemia. Redox Biol. 2022;51: 102271. doi: 10.1016/j.redox.2022.102271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Organization WH. WHO Report on the Global Tobacco Epidemic, 2021: Addressing new and emerging products: World Health Organization; 2021. [Google Scholar]
- 13.Callahan-Lyon P. Electronic cigarettes: human health effects. Tob Control. 2014;23 Suppl 2: ii36–40. doi: 10.1136/tobaccocontrol-2013-051470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle RG, Richter S, St Claire AW. Defining adult e-cigarette prevalence: comparing a categorical definition with days of use. Tob Control. 2021;30: 530–533. doi: 10.1136/tobaccocontrol-2020-055641 [DOI] [PubMed] [Google Scholar]
- 15.Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin—Final Report. N Engl J Med. 2020;382: 903–916. doi: 10.1056/NEJMoa1911614 [DOI] [PubMed] [Google Scholar]
- 16.Choi DW, Jeon J, Lee SA, Han KT, Park EC, Jang SI. Association between Smoking Behavior Patterns and Glycated Hemoglobin Levels in a General Population. Int J Environ Res Public Health. 2018;15. doi: 10.3390/ijerph15102260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong SH, Jang BN, Kim SH, Jang SI, Park EC. Investigation of the Association between Smoking Behavior and Metabolic Syndrome Using Lipid Accumulation Product Index among South Korean Adults. Int J Environ Res Public Health. 2021;18. doi: 10.3390/ijerph18084151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherian SV, Kumar A, Estrada YMRM. E-Cigarette or Vaping Product-Associated Lung Injury: A Review. Am J Med. 2020;133: 657–663. doi: 10.1016/j.amjmed.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 19.Glynos C, Bibli SI, Katsaounou P, Pavlidou A, Magkou C, Karavana V, et al. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2018;315: L662–l672. doi: 10.1152/ajplung.00389.2017 [DOI] [PubMed] [Google Scholar]
- 20.Kim CY, Paek YJ, Seo HG, Cheong YS, Lee CM, Park SM, et al. Dual use of electronic and conventional cigarettes is associated with higher cardiovascular risk factors in Korean men. Sci Rep. 2020;10: 5612. doi: 10.1038/s41598-020-62545-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomar SL, Alpert HR, Connolly GN. Patterns of dual use of cigarettes and smokeless tobacco among US males: findings from national surveys. Tob Control. 2010;19: 104–109. doi: 10.1136/tc.2009.031070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piper ME, Baker TB, Benowitz NL, Kobinsky KH, Jorenby DE. Dual Users Compared to Smokers: Demographics, Dependence, and Biomarkers. Nicotine Tob Res. 2019;21: 1279–1284. doi: 10.1093/ntr/nty231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi SH, Ling J, Noonan D, Kim W. Smoking behavior and social contexts associated with smoking among dual-smoker couples. Public Health Nurs. 2020;37: 161–168. doi: 10.1111/phn.12686 [DOI] [PubMed] [Google Scholar]
- 24.Kim SK, Choe JY. Association between smoking and serum uric acid in Korean population: Data from the seventh Korea national health and nutrition examination survey 2016. Medicine (Baltimore). 2019;98: e14507. doi: 10.1097/MD.0000000000014507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu N, Han X, Zhang Y, Huang X, Zhu W, Shen M, et al. Clinical features of gout in adult patients with type Ia glycogen storage disease: a single-centre retrospective study and a review of literature. Arthritis Res Ther. 2022;24: 58. doi: 10.1186/s13075-021-02706-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh SS, Jang JE, Lee DW, Park EC, Jang SI. Cigarette type or smoking history: Which has a greater impact on the metabolic syndrome and its components? Sci Rep. 2020;10: 10467. doi: 10.1038/s41598-020-67524-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurniasari MD, Karwur FF, Rayanti RE, Dharmana E, Rias YA, Chou KR, et al. Second-Hand Smoke and Its Synergistic Effect with a Body-Mass Index of >24.9 kg/m(2) Increase the Risk of Gout Arthritis in Indonesia. Int J Environ Res Public Health. 2021;18. doi: 10.3390/ijerph18084324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang HG, Kwon KH, Lee IW, Jung B, Park EC, Jang SI. Biochemically-verified smoking rate trends and factors associated with inaccurate self-reporting of smoking habits in Korean women. Asian Pac J Cancer Prev. 2013;14: 6807–6812. doi: 10.7314/apjcp.2013.14.11.6807 [DOI] [PubMed] [Google Scholar]
- 29.Fukuhara A, Saito J, Sato S, Saito K, Fukuhara N, Tanino Y, et al. The association between risk of airflow limitation and serum uric acid measured at medical health check-ups. Int J Chron Obstruct Pulmon Dis. 2017;12: 1213–1219. doi: 10.2147/COPD.S126249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moon J, Lee H, Kong M, Kim H, Oh Y. Association Between Electronic Cigarette Use and Levels of High-Sensitivity C-Reactive Protein and Uric Acid. Asia Pac J Public Health. 2020;32: 35–41. doi: 10.1177/1010539519899777 [DOI] [PubMed] [Google Scholar]
- 31.Golli NE, Jrad-Lamine A, Neffati H, Dkhili H, Rahali D, Dallagi Y, et al. Impact of e-cigarette refill liquid exposure on rat kidney. Regul Toxicol Pharmacol. 2016;77: 109–116. doi: 10.1016/j.yrtph.2016.02.012 [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation. 2002;105: 1155–1157. doi: 10.1161/hc1002.105935 [DOI] [PubMed] [Google Scholar]
- 33.Haj Mouhamed D, Ezzaher A, Neffati F, Douki W, Gaha L, Najjar MF. Effect of cigarette smoking on plasma uric acid concentrations. Environ Health Prev Med. 2011;16: 307–312. doi: 10.1007/s12199-010-0198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanna BE, Hamed JM, Touhala LM. Serum uric Acid in smokers. Oman Med J. 2008;23: 269–274 doi: 10.1161/hc1002.105935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okubo Y, Miyamoto T, Suwazono Y, Kobayashi E, Nogawa K. An association between smoking habits and blood pressure in normotensive Japanese men. J Hum Hypertens. 2002;16: 91–96. doi: 10.1038/sj.jhh.1001303 [DOI] [PubMed] [Google Scholar]
- 36.Gordon T, Kannel WB, Dawber TR, McGee D. Changes associated with quitting cigarette smoking: the Framingham Study. Am Heart J. 1975;90: 322–328. doi: 10.1016/0002-8703(75)90320-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of KNHANES is publicly available throuth the website (https://knhanes.kdca.go.kr/knhanes/main.do).