Abstract
The manner in which clinical trial investigators present their findings to healthcare providers and the public can have a substantial influence on their impact. For example, if a heart attack occurs in 2% of those in the placebo group and in 1% of those in the drug-treated group, the benefit to the treated population is only one percentage point better than no treatment. This finding is unlikely to generate much enthusiasm from the study sponsors and in the reporting of the findings to the public. Instead, trial directors can amplify the magnitude of the appearance of the treatment benefit by using the relative risk (RR) value of a 50% reduction of the risk of a heart attack, since one is 50% of two. By using the RR type of data analysis, clinical trial directors can promote the outcome of their trial in their publication and to the media as highly successful while minimizing or disregarding entirely the absolute risk (AR) reduction of only one percentage point. The practice of expressing the RR without the AR has become routinely deployed in the reporting of findings in many different areas of clinical research. We have provided a historical perspective on how this form of data presentation has become commonplace in the reporting of findings from randomized controlled trials (RCTs) on coronary heart disease (CHD) event monitoring and prevention over the past four decades. We assert that the emphasis on RR coupled with insufficient disclosure of AR in the reporting of RCT outcomes has led healthcare providers and the public to overestimate concerns about high cholesterol and to be misled as to the magnitude of the benefits of cholesterol-lowering therapy. The goal of this review is to prompt the scientific community to address this misleading approach to data presentation.
Keywords: statistics, statins, hypercholesterolemia, absolute risk, relative risk
Introduction and background
Richard Horton, editor of the Lancet, expressed the opinion that “much of the scientific literature, perhaps half, may simply be untrue” [1]. A similar sentiment was expressed by John Ioannidis, professor of medicine, epidemiology, and population health at Stanford, who stated, “There is increasing concern that in modern research, false findings may be the majority or even the vast majority of published research” [2]. Marcia Angell, former editor of The New England Journal of Medicine (NEJM), disclosed, “It is simply no longer possible to believe much of the clinical research that is published, or to rely on the judgment of trusted physicians or authoritative medical guidelines” [3]. The skepticism over the credibility of much of the published research may have occurred, in part, from decades of misleading presentations of research findings by clinical trial directors.
While these eminent leaders of medical establishments have lamented over a range of flaws in the conduct of medical research, we have focused on one aspect of data analysis that has been deployed by many trial directors to promote their agenda, rather than to present their findings in the most objective manner possible. Specifically, directors have deployed a statistical strategy that can amplify a modest benefit of drug treatment to appear as if the effect is of great clinical significance. This statistical strategy focuses on the use of relative risk (RR) reduction and the exclusion or minimization of absolute risk (AR) reduction, which are two different ways to express the same raw data. The data format and clinical relevance of this issue were addressed by Meleady and Graham [4] over two decades ago: “If an 80 per cent mortality occurs in the placebo group and 40 per cent in the treatment group, this intervention has a 50 per cent relative mortality reduction and a 40 per cent absolute mortality reduction, which is both clinically and statistically important. If 2 per cent mortality occurs in the placebo and 1 per cent in the treatment group, again a 50 per cent relative mortality reduction has occurred but the absolute mortality reduction is 1 per cent, a clinically trivial reduction.”
Skolbekken [5] also illustrated this issue with a more extreme example in his critique of how the benefits of cholesterol-lowering therapy had been portrayed. The following is our summary of the data from hypothetical studies in his Table 1: In one randomized controlled trial (RCT), 2,000 people die out of 10,000 in a placebo group, and 1,000 people die out of 10,000 in a treated group, resulting in an AR reduction of 10% (1,000/10,000=10%). In another RCT, two people die out of 10,000 in a placebo group, and one person dies out of 10,000 in a treated group, resulting in an AR reduction of 0.01% (1/10,000=0.01%). Despite the vast difference in the ARs between the two studies (10% and 0.01%), in both, the RR reduction was 50% (1,000 is 50% of 2,000, and one is 50% of two).
Skolbekken asserted that the “real impact of treatment … can only be seen by also reviewing the absolute risk reduction.” He also noted that reporting only the relative risk gives “a more favourable impression of the effectiveness of a drug than absolute risk estimates.” Numerous investigators have emphasized the importance of this issue; surveys have shown that lay people, as well as healthcare providers, overestimate the benefit of a treatment, such as cholesterol reduction, when the findings are presented solely as the RR [6-16].
In this review, we have provided a historical perspective on the miscommunication of risk and treatment benefit in cholesterol-heart disease research with an in-depth analysis of the following five landmark randomized controlled trials (RCTs) that assessed coronary event monitoring and prevention over the past four decades: 1984, “Lipid Research Clinics Coronary Primary Prevention Trial” (LRC-CPPT) [17,18]; 1986, “Multiple Risk Factor Intervention Trial” (MRFIT) [19]; 2008, “Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin” (JUPITER) [20]; 2017, “Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk” (FOURIER) [21]; and 2023, “Cholesterol Lowering via Bempedoic Acid [ECT1002], an ACL-Inhibiting Regimen” (CLEAR) [22].
These five clinical trials have been selected because they played a key role in implicating high total serum cholesterol, in general, and low-density lipoprotein cholesterol (LDL-C), in particular, in causing heart disease and in promoting the pharmacological reduction of cholesterol to prevent coronary heart disease (CHD). Our review addresses how the findings in each of these clinical trials were presented to all audiences primarily in the RR format, which exaggerated the role of cholesterol in CHD and amplified the modest benefits of lipid reduction.
Review
LRC-CPPT: Miscommunication of the benefits of lipid-lowering therapy
LRC-CPPT addressed the hypothesis that “long-term reduction of serum cholesterol in hypercholesterolemic men initially free of CHD will lead to a lowered incidence of coronary heart disease” [17]. Approximately half a million middle-aged males were screened to find those with the highest (top 5%) cholesterol levels (n=3,806). These hypercholesterolemic males were put on a low-cholesterol diet for 7.4 years. In addition, about half of the males were given a placebo (n=1,900), and the remainder (n=1906) were given a bile-sequestering agent (cholestyramine), which reduced cholesterol levels [18].
The authors reported that cholesterol reduction resulted in “a 24% reduction in definite CHD death and a 19% reduction in nonfatal myocardial infarction.” The investigators emphasized the importance and clinical relevance of their findings by stating, “The consistency of the reductions in CHD manifestations observed with cholestyramine in this controlled trial … leaves little doubt of the benefit of cholestyramine therapy” [18].
There was widespread praise for the LRC-CPPT findings, as exemplified by an editorial in the British Medical Journal (BMJ), which stated, “At long last we have clear evidence that reducing very high plasma concentrations of cholesterol and low density lipoprotein (LDL) cholesterol lowers the incidence of coronary heart disease” [23]. Similar views were expressed in a lead article in the Medical Journal of Australia entitled “The lipid hypothesis is proven,” which declared “the incidence of death from definite CHD was reduced by 24% in the cholestyramine group” [24]. This commentary concluded, “The LRC-CPPT has given a new respectability and credibility to the dietary and pharmacological management of hypercholesterolemia.”
In the same year in which the LRC-CPPT findings were published, the National Institutes of Health (NIH) convened a panel of experts on diet, cholesterol, and heart disease. The panel published a consensus statement, which was based, in large part, on the LRC-CPPT findings [25]. The NIH panel concluded, “It has been established beyond a reasonable doubt that lowering definitely elevated blood cholesterol levels … will reduce the risk of heart attacks caused by coronary heart disease. This has been demonstrated most conclusively in men with elevated blood cholesterol levels ….”
The NIH consensus panel failed to address the ensuing controversy over numerous irregularities in the LRC-CPPT statistical methodology. Experts in the field objected to multiple aspects of the data analysis, including concerns with a post hoc relaxation of the threshold with which to determine statistical significance and an apparent post hoc change to the primary endpoint analysis [26-30]. Specifically, commentators accused the LRC-CPPT investigators of changing how they analyzed the data because the initial data analysis, based on their prespecified method paper [17], failed to support the hypothesis that cholesterol reduction would reduce CHD events or mortality. According to Kronmal [27], the statistically significant effect of cholestyramine treatment was “based on a change in criteria that apparently took place after analyzing the data.” Similarly, L’Abbe et al. [26] stated that the LRC-CPPT investigators failed “to mention and justify their post-hoc choice of a more liberal α-level.” And Mann [30] wrote the following: “the study managers, after seeing the result, used an inappropriate ‘one-tailed test’.”
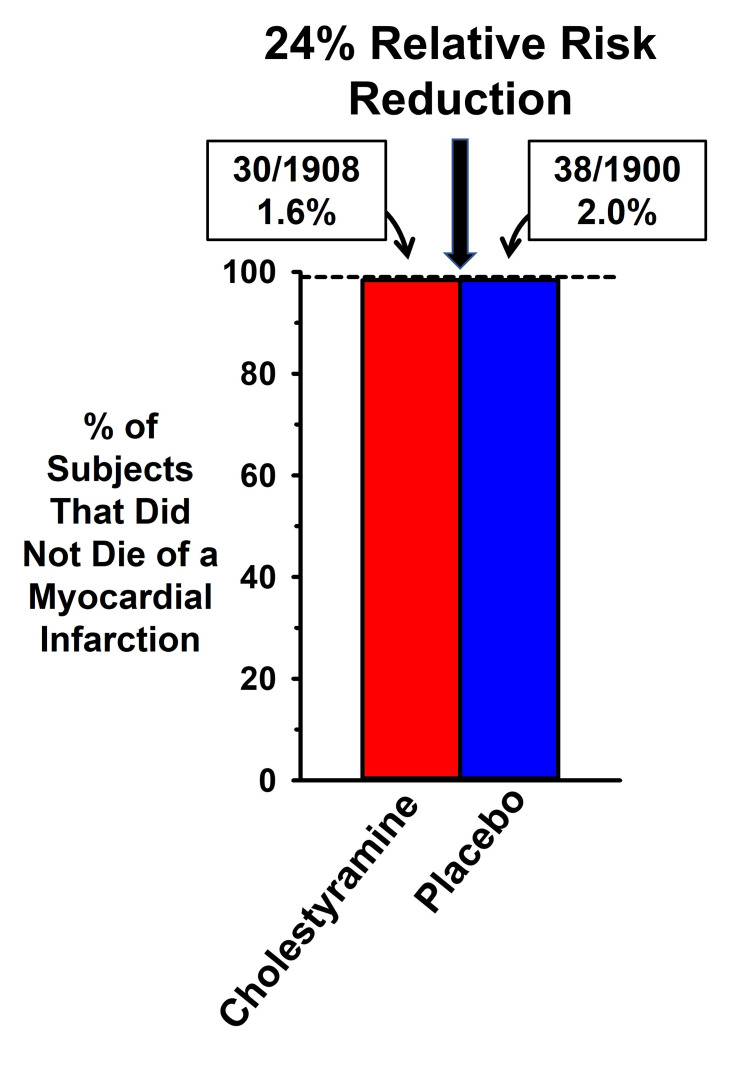
A second area of contention involved how the data were portrayed. In Figure 1, we have illustrated the data in the original publication (from their Table 3) to highlight the difference between the magnitudes of the absolute risk reduction and the relative risk reduction. The figure shows that the absolute CHD death rates were 2.0% (38/1,900) for the placebo group and 1.6% (30/1,906) for the experimental group, an absolute difference of 0.4 percentage points. In addition, there were 68 deaths from any cause in the treatment group and 71 in the placebo group (3.7% versus 3.6%), a difference that was not statistically significant.
Figure 1. Comparison of absolute and relative risk benefits of cholesterol reduction in LRC-CPPT.
Data from the Lipid Research Clinics Coronary Primary Prevention Trial (LRC-CPPT) [18]. The 0.4 percentage point absolute risk difference in heart disease mortality between the cholestyramine and placebo groups is actually a 20% relative risk reduction (0.4/2.0) but was converted into a 24% relative risk reduction in events in the LRC-CPPT publication.
Praise for the findings of this trial stated that cholesterol reduction resulted in “a 24% reduction in definite CHD death and a 19% reduction in nonfatal myocardial infarction.” How can the CHD mortality rate be reported as a 24% reduction in the publication, as well as in the medical journals that praised the findings, when the difference in CHD mortality between the two groups was only 0.4%? The explanation is that the AR mortality reduction of 0.4% was transformed into an RR reduction by dividing 0.4% by 2.0%, which resulted in a far more impressive 24% effect.
Expressing the data in the RR format provided strong support for the conclusion of the authors that cholesterol reduction was successful at reducing the rate of coronary mortality. The magnitude of this treatment benefit is quantified as the number needed to treat (NNT), which is the inverse of the AR. in this case, the NNT is 250. That is, one would need to treat 250 subjects with this drug for over seven years to delay a single CHD death from occurring, with no effect on overall mortality.
The same RR data transformation was applied to the composite measure of all CHD deaths combined with nonfatal myocardial infarction (MI), which was a 1.6 percentage point difference in events (8.6% {placebo} versus 7% {drug}). The 1.6% (AR) divided by 8.6% results in an RR reduction of 19%. In their summary and in the NIH consensus publication [25], the authors presented only the 19% RR reduction of combined endpoints, without mentioning the AR reduction of 1.6%. It was the 19% RR measure for the composite endpoint, as well as the 24% RR reduction in CHD mortality, that propelled this trial to be the centerpiece of the 1985 NIH consensus report on the benefit of cholesterol reduction for heart health.
Given the negligible effects of cholesterol reduction on coronary events and mortality, LRC-CPPT could well have been dismissed by the steering committee as a negative trial. Instead, the trial directors focused only on the RR, which resulted in the study findings promoted on the cover of TIME magazine [31], with the message that “the cholestyramine group … suffered 19% fewer heart attacks. Their cardiac death rate was a remarkable 24% lower than that of the placebo group.” The Time magazine article quoted Project Director Basil Rifkind, who stated that the LRC-CPPT findings are “a turning point in cholesterol-heart-disease research.”
More objective assessments of flaws with the LRC-CPPT and the NIH consensus panel were provided at the time by two acknowledged experts. The first was from Dr. Thomas Chalmers of Mount Sinai Medical School, who commented, “I think they made an unconscionable exaggeration of all the data” [32]. The second was from Dr. George Mann, professor of biochemistry and medicine at Vanderbilt University Medical School [30]: “They have held repeated press conferences bragging about this cataclysmic breakthrough which the study directors claim shows that lowering cholesterol lowers the frequency of coronary disease. They have manipulated the data to reach the wrong conclusion. This is plain for any student of elementary statistics to see. The managers at NIH have used Madison Avenue hype to sell this failed trial in the way media people sell an underarm deodorant. The (NIH Consensus Panel) has failed to acknowledge that the LRC trial, like so many before it, is saying firmly and loudly ‘No, … the drug you generously tested for a pharmaceutical house does not work …’.”
MRFIT: Miscommunication of risk in the portrayal of CHD mortality
MRFIT had two components, an interventional trial [33] and an observational study [19]. We have focused on the observational component, which assessed the hypothesis that there would be a positive association between serum cholesterol and CHD deaths in middle-aged males. Its independent variable was total cholesterol and not LDL cholesterol (LDL-C). Despite the flaw that total cholesterol is of lesser value in assessing CHD risk [34-39], MRFIT was promoted as demonstrating that small increments, across the entire physiological range of total cholesterol, increased one’s risk of dying of CHD.
MRFIT involved the screening of 356,222 American males, 35-57 years of age, initially free of CHD. The authors reported that they found a “continuous, graded, strong relationship between serum cholesterol and six-year age-adjusted CHD.” The study concluded that “of all CHD deaths, 46% were estimated to be excess deaths attributed (solely) to serum cholesterol 180 mg/dl or greater.”
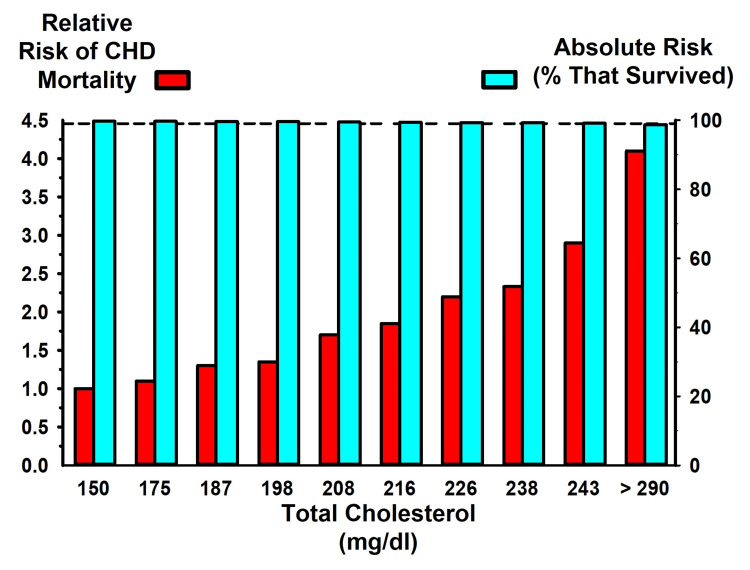
The data from Table 3 of the original publication are provided here as Figure 2, which illustrates the relation between serum cholesterol levels (in deciles) and CHD mortality. The same raw data generated the RR (red bars) and the AR (blue bars) for CHD death rates. The data presented as RR seem alarming, since they appear to strongly support the authors’ conclusion that small increments in cholesterol are associated with substantial increases in CHD risk. The graph illustrates that people with total cholesterol levels of 216 mg/dl, 246 mg/dl, and >290 mg/dl have two, three, and four times the risk, respectively, of dying of CHD, compared to people whose total cholesterol level is 150 mg/dl. This dramatic increase in CHD death supported the authors’ conclusion that cholesterol levels above 180 mg/dl “powerfully affects risk for the great majority of middle-aged American men.”
Figure 2. MRFIT findings expressed as the relative and absolute risk of CHD.
Data from the Multiple Risk Factor Intervention Trial (MRFIT) trial [19] expressed as relative risk (red bars, left y-axis) and as absolute risk (blue bars, right y-axis)
CHD: coronary heart disease
A less alarming view of the MRFIT findings is provided by the overlapping AR data in Figure 2. The blue bars illustrate the AR of CHD in terms of the rate of survival in relation to cholesterol levels (the dashed line provides a visual guide at 99%, which indicates that at every level of cholesterol, the survival rate was approximately 99%). Thus, at the lowest level of cholesterol, CHD survival was 99.7%, and at the highest level of cholesterol, CHD survival was 98.7%. Thus, across the entire physiological range of cholesterol levels, the difference in the rate of six-year CHD mortality was only about one percentage point.
The difference in CHD mortality across the physiological range of cholesterol levels was notably less for the nonsmoking, non-hypertensive subgroup (45% of all subjects). Only 0.3% of all the subjects in this subgroup died of CHD. The AR of CHD death in this group from the highest to lowest cholesterol levels was only 0.48 percentage points (0.64% versus 0.16%). Despite the absence of a clinically relevant AR relation between cholesterol levels and CHD mortality in this subgroup, the authors reported that CHD mortality was four times greater for those with the highest cholesterol compared to those with the lowest cholesterol. Again, the authors reported their findings as the RR (0.64% is four times greater than 0.16%), without mention of the AR, to support their conclusion that elevated levels of cholesterol are implicated “in the causation of premature CHD.”
To put the MRFIT findings into historical perspective, by the 1980s, the hypothesis that cholesterol caused heart disease was falling out of favor. Clinical trials of different agents, such as corn oil [40], clofibrate [41], and cholestyramine [18], had all failed to demonstrate a significant clinical benefit of cholesterol reduction on coronary events and mortality. Had the trial directors focused on AR instead of RR, MRFIT should have served as the death knell for the cholesterol hypothesis. Cardiovascular research would have shifted its focus in subsequent decades on more traditional CHD risk factors, including smoking, stress, hypertension, hyperglycemia, and insulin resistance. Instead, MRFIT, as well as LRC-CPPT, provided the impetus for the expansion of cardiovascular disease (CVD) treatments to new approaches to reduce cholesterol, including the development of statins.
JUPITER: Miscommunication of lipid-lowering benefits in the statin era
Statins reduce cholesterol levels by blocking the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-controlling enzyme in cholesterol synthesis. Statins have been considered so successful in preventing coronary events that William Roberts, MD, editor of the American Journal of Cardiology, described statins as “miracle drugs,” which “are to atherosclerosis what penicillin was to infectious diseases” [42]. Praise for statins has been so strong that critics have been labeled as members of a “statin denial cult” [43], who disseminate “fake medical news and fearmongering … through relentless attacks on statins” [44].
We suggest that statins are promoted as “miracle drugs” largely because statin advocates have often publicized their RR reduction while failing to highlight their modest AR reduction. Moreover, the adverse effects of statins have been minimized or ignored entirely. Diamond and Ravnskov [45] provided a critique of this strategy in their assessment of clinical trial outcomes for statin treatment in primary and secondary prevention of CHD.
Here, we have focused on how the findings of the JUPITER trial were presented in the medical literature and to the public. JUPITER included nearly 18,000 subjects, which was designed to test the hypothesis that treatment with rosuvastatin for primary prevention would reduce vascular events in relatively healthy people with high-sensitivity C-reactive protein (CRP) but without hyperlipidemia [20].
JUPITER was a landmark trial with findings that were embraced by many healthcare providers because it appeared to demonstrate substantial benefits for people without high cholesterol and with a low risk for CHD. In two representative comments on JUPITER outcomes, Dr. Steven E. Nissen, director of cardiology at the Cleveland Clinic, proclaimed, “It’s a breathtaking study. It’s a blockbuster. It’s absolutely paradigm-shifting,” and Dr. W. Douglas Weaver, president of the American College of Cardiology, was equally emphatic: “This takes prevention to a whole new level. Yesterday you would not have used a statin for a patient whose cholesterol was normal. Today you will” [46]. The JUPITER findings appeared so impressive that the trial was terminated prematurely on an ethical basis because in only 1.9 years, there was “a 44% reduction in the trial primary end point of all vascular events, a 54% reduction in myocardial infarction, a 48% reduction in stroke, a 46% reduction in need for arterial revascularization, and a 20% reduction in all-cause mortality” [47].
The finding that medication can reduce the likelihood of a coronary event by 50% may well seem like a miracle treatment, as it would appear that half of all statin-treated people are protected from having a heart attack. However, as with LRC-CPPT and MRFIT, the magnitude of benefits with cholesterol reduction in the original publication [20], as well as in the summary by Ridker [47], emphasized the RR benefit, with little to no mention of the AR.
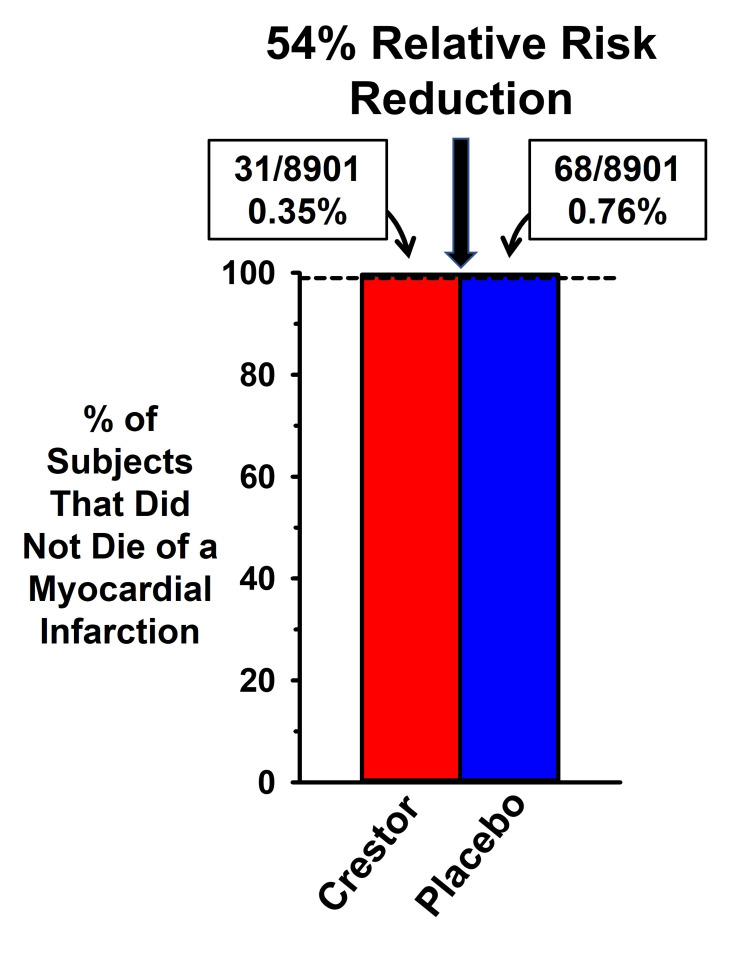
Figure 3 is based on the data in Table 3 of the original publication, which illustrates the great disparity between how the AR and RR represent the magnitude of rosuvastatin effects on coronary events. The dashed line at 99% is a visual aid to illustrate that over 99% of rosuvastatin (Crestor) and placebo subjects did not suffer from a fatal myocardial infarction (MI). If more than 99% of all subjects did not die as a result of an MI, how was it that the authors could claim that rosuvastatin reduced the rate of a fatal MI by 54%? As with the LRC-CPPT and MRFIT trials, the data in JUPITER were presented as the RR. The incidence of a fatal MI occurred in 0.76% of the subjects in the placebo group and in 0.35% of the rosuvastatin-treated subjects. Thus, the AR reduction was only 0.41 percentage points, but 0.41 is 54% of 0.76%. It is difficult to understand how an improvement in coronary outcomes in less than one-half of 1% of subjects treated with rosuvastatin generated such unbridled enthusiasm by experts in the field.
Figure 3. Comparison of absolute and relative risk benefits of cholesterol reduction in the JUPITER RCT.
Data from the “Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin” (JUPITER) randomized controlled trial (RCT) [20], expressed as the percent of subjects in the rosuvastatin group (Crestor) compared to the placebo group without myocardial infarction (MI) mortality. The 0.41 percentage point absolute risk difference in MI mortality between the Crestor and placebo groups was converted into a 54% relative risk reduction in events
Although the AR reduction with rosuvastatin treatment was modest, a small benefit could be of value if statins had very few and only minor adverse effects. However, the adverse effects of statins are extensive [45,48-59], including an increased risk of new-onset type 2 diabetes [20,60-65], an increase in fasting blood glucose in patients with and without diabetes [66], mitochondrial dysfunction [67-69], tendinopathy [70], myopathy [71,72], acute kidney injury/renal failure [73-75], and cognitive deficits [54,76-83].
The JUPITER trial documented a significant increase in the incidence of new-onset diabetes with rosuvastatin treatment compared to placebo (their Table 4). In reporting this adverse effect of statins, the authors presented the data only in terms of its AR, without transforming it into the RR. According to Gigerenzer et al. [84], the portrayal of the RR without including the AR provides “incomplete and misleading information,” which they referred to as “mismatched framing.”
Had the authors of the JUPITER trial used the RR, they would have reported a statistically significant 25% increase in new-onset diabetes with rosuvastatin treatment. In addition to the fact that the authors did not report the diabetogenic effect in terms of its RR, they downplayed its clinical relevance in the original publication, as well as in subsequent work. In a paper addressing the controversy over JUPITER, the trial director considered the increase in diabetes as only a “play of chance” [47]. However, the significant increase in the incidence of diabetes with statin treatment has been reported in numerous subsequent publications [60-64,85], including an RCT that characterized the mechanistic basis as to how statins increase the susceptibility of users to develop diabetes [65]. This finding is relevant to why RCTs have demonstrated an increase in fasting blood glucose in patients with and without diabetes [66], as well as a meta-analysis that suggested females are more susceptible than males to develop type 2 diabetes with statin treatment [60].
The findings of the JUPITER trial were subject to widespread criticism, similar to the controversy generated in response to LRC-CPPT. According to Curtiss and Fairman [86], “Criticism of the JUPITER trial results began immediately” and developed into “an avalanche” of published critiques [87-90]. Criticisms were raised at multiple levels, including questionable justification for terminating JUPITER in less than two years despite the plan to run the trial for four years. There were also concerns that the premature termination was influenced by the pharmaceutical company sponsoring the trial. There was also criticism of the efforts by the authors to downplay the increased incidence of diabetes with drug treatment. It is noteworthy that Serebruany [91] asserted that “the medical community does not uniformly accept the results of the JUPITER trial, engaging in heavy, somewhat personal debates over the validity of the JUPITER findings” including “commercial interests of the principal investigator” and a “fundamental problem with trial integrity.”
Another criticism of JUPITER is also the focus of our commentary. Critics commented that the study outcomes emphasized the RR and ignored the AR reduction. For example, Vaccarino et al. [90] expressed concerns regarding the lack of attention to the modest AR benefit in JUPITER. These authors stated that the trial was terminated prematurely based on the 44% RR reduction in events, but they echoed the concerns of others by stating, “what really matters for dictating changes in clinical practice is the absolute risk reduction.” According to Vaccarino et al., the AR for unambiguous hard events (nonfatal and fatal MI) was just 0.20% per year, which translates into “500 persons need to be treated for 1 year to prevent 1 event.” Despite the appearance of an impressive 54% RR, the modest AR led Vaccarino et al. to conclude that “the treatment benefits achieved in the JUPITER trial are not large enough to advocate an expansion in the clinical indications for statins.”
Nevertheless, despite the unimpressive benefits and evidence of a diabetogenic effect, the FDA granted approval for the use of rosuvastatin for the primary prevention of heart disease in people without high cholesterol but with high CRP and otherwise at a low risk for CHD.
FOURIER and CLEAR: Miscommunication of lipid-lowering benefits in contemporary research
At the American College of Cardiology meeting in 2017, Dr. Marc Sabatine, the principal investigator of the FOURIER trial, was extremely positive in reporting the findings that assessed coronary event outcomes with evolocumab treatment. Unlike statins, which block cholesterol production, evolocumab reduces LDL-C by inhibiting the activity of proprotein convertase subtilisin/kexin type 9 (PCSK9), which is an LDL receptor (LDL-R)-degrading enzyme [92]. In FOURIER, evolocumab reduced LDL-C by 59%, achieving one of the lowest levels of LDL-C reported in any RCT [21].
According to Sabatine, “Evolocumab reduced the risk of cardiovascular events, a 15% reduction in the primary endpoint, a 20% reduction in the risk of cardiovascular death, MI or stroke” [92]. The 15%-20% benefits of evolocumab treatment were highlighted, as well, in the opening paragraph of the Discussion section of the publication [21].
Sabatine’s approach to data presentation followed the now routine practice of mentioning only the RR, without including the AR. The impact of the FOURIER findings may have been less impressive had the reduction in the risk of the composite of cardiovascular death, MI, or stroke been expressed as the AR, which was only a 1.5 percentage point difference between treatment and placebo (5.9% versus 7.4%).
It is also notable that the 20% benefit that Sabatine mentioned and stated in the Discussion section of the publication was based on a composite of heterogeneous outcomes in multiple CVD categories, which included significant, as well as non-significant, effects. Of clinical importance is the finding that there was no statistically significant effect on CVD death (or death due to MI, stroke, or “other CVD death”). In addition, there was no effect of treatment on all-cause mortality.
Of the three CVD event categories in the composite measure reported to have a 20% benefit (CVD death, MI, or stroke), only one category exhibited an AR benefit greater than a single percentage point; there was a 0.4% AR reduction in the incidence of stroke (1.9% versus 1.5%), a non-significant 0.1% increase in the incidence of CVD death (1.7% versus 1.8%), and a 1.2% AR reduction in the incidence of MI (4.6% versus 3.4%), placebo versus drug, respectively. Thus, the 20% RR and 1.5% AR reduction in the composite measure of CVD death, MI, or stroke was carried almost entirely by the 1.2% AR in MI.
A recently published paper provided an update on long-term effects of evolocumab treatment on coronary event rate [93]. In the Clinical Perspective section of the paper, which highlighted the most important findings of the study, the authors stated, “patients who were originally randomized to evolocumab had a 15-20% lower risk of major adverse cardiovascular events and 23% lower risk of cardiovascular death than those randomized to placebo.” As before, the RR reduction was highlighted, and the AR reduction was not mentioned in the paper.
The reporting of the FOURIER findings in reviews followed the established practice of portraying the benefits of lipid-lowering therapy in the RR format, without addressing the AR. In one example, Libby and Tokgözoğlu [94] stated that evolocumab reduced cardiovascular events by 15%-20%, without mentioning the AR. Their review failed to note that there were no statistically significant effects of evolocumab treatment on a range of CVD measures, including CVD death or hospitalization from heart failure, death from any cause, or hospitalization for unstable angina. In fact, the 1.2 percentage point difference in AR reduction of MI (from 4.6% to 3.4%, placebo versus treatment, respectively) was the only hard event category, that is, MI, death, and stroke, of 13 individual categories, in which the AR difference between evolocumab and placebo exceeded 0.5 percentage points. Despite the negligible overall benefit of evolocumab on most CVD outcomes, Libby and Tokgözoğlu [94] concluded that PCSK9 medication serves as a model “in the quest to conquer cardiovascular diseases.”
In the most recent RCT on lipid-lowering therapy, statin-intolerant patients were administered a placebo or bempedoic acid [22], which is similar to statins in that it reduces hepatic cholesterol synthesis and raises LDL receptor expression, thereby increasing the clearance of LDL cholesterol from the circulation. Unlike statins, bempedoic acid is activated in the liver and not in most peripheral tissues, including skeletal muscle. Bempedoic acid, therefore, was expected to have less of an adverse effect on muscles than statins.
The group-administered bempedoic acid exhibited a 26.1% reduction in LDL-C levels (compared to a 10.6% reduction in the placebo group). The AR reduction with bempedoic acid for combined major coronary adverse events was 1.5 percentage points (13.3% versus 11.7%), with no benefit in fatal or nonfatal stroke or death from any cause, including cardiovascular causes. Bempedoic acid treatment produced adverse effects not seen with statins, including a significant increase in hyperuricemia (10.9% versus 5.6%), gout (3.1% versus 2.1%) and cholelithiasis (gall stones) (2.2% versus 1.2%), renal impairment (11.5% versus. 8.6%), and elevated hepatic enzyme level (4.5% versus. 3.0%).
In a discussion of the CLEAR outcomes, Dr. Ann Marie Navar did not mention the AR 1.6 percentage point difference in the benefits of bempedoic acid treatment. Instead, she presented the benefits of bempedoic acid in terms of the RR data, by stating that there was a 13% RR reduction in events [95]. In contrast, when Dr. Navar addressed the adverse effects of the treatment, she mentioned only the AR data, stating that there was only a 1% increase in the incidence of gout and cholelithiasis (the formation of gallstones), with no mention of the increased incidence of renal impairment, elevated hepatic enzyme level, and near doubling of the incidence of hyperuricemia with bempedoic acid treatment versus placebo. Gigerenzer et al. [84] considered this form of data presentation to be the second “sin” against transparent reporting, that is, deliberately reporting benefits as relative risk reductions while reporting harms as absolute risk increases.
Our perspective
We are not the first to object to the preferential use of RR, without sufficient attention to AR, in clinical trial reporting. For decades, academicians have deplored the strategy taken by some clinicians to promote their findings by emphasizing RR to the exclusion of reporting the AR [6-16]. In one example, Gigerenzer et al. [84] considered this form of data presentation to be “the first ‘sin’ against transparent reporting.”
We have considered two consequences of the preferential use of RR, without sufficient attention to AR, in clinical trial reporting and in the perceived risk of elevated LDL-C. First, the emphasis on promoting relative risk benefits in different areas of clinical trial findings appears to have become an accepted practice in major medical journals. In a study of bias toward presenting RR in favor over AR, Schwartz et al. [96] reported that in a five-year review of four medical journals (Lancet, BMJ, Journal of the American Medical Association (JAMA), and NEJM), 68% of the papers in these journals reported only the RR in the abstracts and only about 50% reported the AR in the entire paper. These authors concluded that the focus on RR and the exclusion of AR in publications “can easily exaggerate readers’ perceptions of benefit or harm” (of a treatment).
As an example of how healthcare providers can be misled by an exclusive presentation of data as RR, Bucher et al. [97] demonstrated that physicians were more inclined to prescribe cholesterol-lowering medication for hypercholesterolemia when the trial results for identical endpoints were expressed as RR reduction, compared to AR reduction. Sackett and Cook [98] commented on this finding by stating that “restricting the reporting of efficacy to just relative risk reductions can lead to greater - and at times - excessive zeal in decisions about treatment for patients with low susceptibilities.”
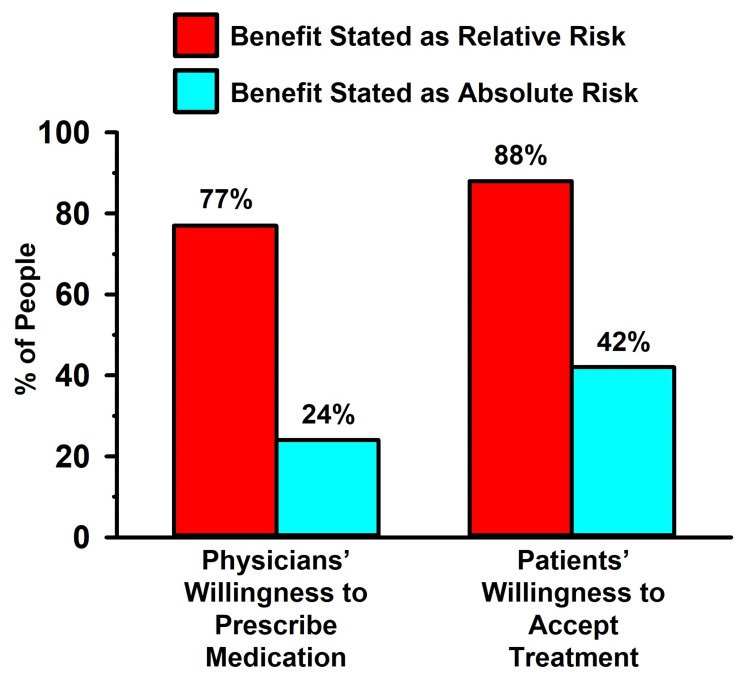
The bias of physicians toward prescribing lipid-lowering medication, as well as lay people accepting treatment, when trial results were reported solely in the RR format was addressed empirically by Bobbio et al. [11] and Hux and Naylor [9]. In these two studies, the investigators presented real-world data from a lipid-lowering RCT (the Helsinki Heart Study [99]) to physicians [11] and to patients [9]. The subjects of both studies were informed of the AR (a 1.4% difference in cardiac events between treated and untreated individuals) and the RR (a 34% cardiac event reduction) of a treatment. They were then asked if they would prescribe the medication (in the physician study [11]) or if they would take the medication (in the patient study [9]). Unbeknownst to the subjects of both studies, the AR and RR were based on the same raw data (coronary events occurred in 4.1% of the subjects in the placebo group and in 2.7% of the subjects in the lipid-lowering group; an AR reduction of 1.4% and an RR reduction of 34%).
The findings of the Bobbio et al. [11] and Hux and Naylor [9] studies are illustrated in Figure 4. When the lipid-lowering effects were presented as RR, more than three-quarters of the clinicians were amenable to prescribing the medication, and even more of the patients were amenable to taking the medication. By contrast, when the data were presented as AR, only a quarter of the physicians were amenable to prescribing the medication, and less than half of the patients were amenable to taking the medication. The findings of this study, as well as others with similar outcomes [6-16,100-102], illustrate how presenting RCT outcomes in the AR versus the RR form can influence people as to their confidence in the effectiveness of an intervention.
Figure 4. Influence of data presented as relative risk versus absolute risk in patient and physician decision-making.
Data from Bobbio et al. [11] (left) and Hux and Naylor [9] (right) trials
Stegenga [103] emphasized that “Effectiveness always should be measured and reported in absolute terms (using measures such as ‘absolute risk reduction’)” and he further stated that “people’s comparative understanding of relative versus absolute outcome measures is dubious. Relative measures … fundamentally mislead patients into overestimating effectiveness.” Nevertheless, peer-reviewed medical publications and the media exhibit a high prevalence of bias toward reporting the RR and ignoring the AR.
In our view, this strategy by clinical trial investigators to amplify the appearance of the magnitude of their intervention is unacceptable scientific behavior. We concur with Gigerenzer et al. [84] that journal editors should enforce transparent reporting of data, including the requirement that papers include the AR and RR in the abstracts. In addition, an education on the miscommunication of risk and benefits in research should be included in the training of healthcare workers and students of public health.
Second, we suggest that the exaggeration of the putative harms caused by elevated LDL-C [104] and the benefits of lipid-lowering medications has detracted from an appreciation of the physiological relevance of elevated levels of LDL-C in optimal health. For example, LDL-C is an important component of the immune system [105-107]. Chronically elevated LDL-C levels may enhance aspects of immune functioning, which is potentially relevant to the finding that elderly people with familial hypercholesterolemia (FH) have lower rates of mortality from cancer and infection compared to the general population [108-110].
The importance of LDL-C to overall health may explain why LDL-C is such a poor marker of risk for CVD [34-38], as well as cardiovascular and all-cause mortality [39]. Coronary artery calcification (CAC), in contrast to LDL-C, is the single best predictor of future fatal and nonfatal coronary events [111-121]. Moreover, among those with genetically confirmed FH, approximately half showed no detectable CAC and had a favorable prognosis, despite significantly elevated LDL-C levels [122]. These observations help to explain why FH individuals do not face an increased risk of CVD mortality with advanced age, as well as the greater longevity of people in the general population with high LDL-C, compared to those with low LDL-C [39].
It is also important to note that what is often overlooked in the discussion about LDL-C as a cardiovascular risk factor is the heterogeneity of different LDL particles. That is, the “total LDL-C” reported in a conventional lipid panel represents the sum of a heterogeneous population of different low-density lipoprotein particles [38]. Specifically, LDL-C is contained in heterogeneous particles that range in size and composition from a small, dense LDL (sdLDL) to a large, buoyant LDL (lbLDL). Circulating sdLDL, unlike lbLDL, readily undergoes atherogenic modifications in plasma, including glycation, which is associated with heightened inflammation, hyperglycemia, insulin resistance, and an increased incidence of CVD in the general population [123-126] and in FH individuals [127-131].
Finally, the overestimation of the involvement of LDL-C in producing CHD based on RR statistics is confirmed by the modest AR benefits of statins, as reflected by the substantial number needed to treat (NNT), that is, the number of individuals that needed to be treated to prevent one event from occurring. This finding was quantified recently by Byrne et al. [132] in a systematic review and meta-analysis of statin RCTs. These investigators reported that the AR reduction of statins was only 0.6% for all-cause mortality, 0.7% for MI, and 0.3% for stroke and 0.9%, 2.2%, and 0.7%, respectively, in primary and secondary prevention. These modest AR benefits of statin therapy yield NNTs of 50-200 to reduce the occurrence of a single coronary event in an average of 4.4 years of treatment. This finding of limited benefits of statins is further confirmed by the work of Kristensen et al. [133] who reported that overall, statin treatment delayed death in primary and secondary prevention trials by only 3.2 and 4.1 days, respectively. These findings support the conclusions of Byrne et al. [132] that “when considering the ARR of statins, the benefits are quite modest, and most trial participants who took statins derived no clinical benefit.”
Hence, the pejorative view of LDL-C as the “bad cholesterol,” which has been perpetuated by the disproportionate emphasis on RR statistics, is not supported by a balanced review of the literature. The characteristic of this perspective is the opinion that “evidence falsifying the hypothesis that LDL drives atherosclerosis has been largely ignored” [134], and the opinion of three cardiologists that “LDL cholesterol risk has been exaggerated” [135] (see also Ravnskov et al. [34] and Diamond et al. [136] for related reviews and discussion).
Conclusions
We have reviewed the findings of five landmark clinical trials conducted over the past four decades that have supported the current consensus that high LDL cholesterol causes CVD and that the pharmacological reduction of LDL produces substantial CVD benefits. Our assessment of these trials demonstrates that the association of cholesterol with CVD is far more modest than has been portrayed. We also assert that the promotion of statins as “miracle drugs” has been based on an emphasis on their RR reduction, which amplifies the appearance of their modest AR benefits.
The biased approach to data analysis in the five trials we have reviewed is representative of the now common practice of highlighting RR over AR in data presentation and in the media, which has been referred to as a “miscommunication of risk.” A consequence of this biased approach to the reporting of CVD clinical trial findings explains in large part why healthcare providers and the public have overestimated the purported hazards of high cholesterol and the benefits of cholesterol reduction.
In conclusion, for the past four decades, academics have repeatedly asserted that the portrayal of clinical trial findings as the RR, while disregarding the AR, is a deceptive practice. In the cardiovascular disease field, a consequence of this strategy has resulted in the exaggerated appearance of the purported hazards of high cholesterol and an amplification of the magnitude of benefits of cholesterol-lowering medications. This strategy appears to have been deployed for the first time with the publication of the LRC-CPPT trial in 1984 but has now become commonplace. To counter this trend of scientific misconduct, we assert that publications and media reports of clinical trial findings should always portray the benefits, as well as harms, of interventions in terms of both absolute and relative risks.
Acknowledgments
We thank Dr. Douglas Schocken for providing commentary and editing suggestions on the manuscript.
The authors have declared that no competing interests exist.
References
- 1.Offline: what is medicine’s 5 sigma? Horton R. Lancet. 2015;385:1380. [Google Scholar]
- 2.Why most published research findings are false. Ioannidis JP. PLoS Med. 2005;2:0. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The New York Review: the truth about the drug companies. [ Apr; 2023 ]. 2004. https://www.nybooks.com/articles/2004/07/15/the-truth-about-the-drug-companies/ https://www.nybooks.com/articles/2004/07/15/the-truth-about-the-drug-companies/
- 4.The benefit of lowering cholesterol: relative risk can be absolutely misleading! Meleady R, Graham IM. Ir J Med Sci. 1998;167:92–93. doi: 10.1007/BF02937945. [DOI] [PubMed] [Google Scholar]
- 5.Communicating the risk reduction achieved by cholesterol reducing drugs. Skolbekken JA. BMJ. 1998;316:1956–1958. doi: 10.1136/bmj.316.7149.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prevention. How much harm? How much benefit? 1. Influence of reporting methods on perception of benefits. Marshall KG. https://www.ncbi.nlm.nih.gov/pubmed/8624999. CMAJ. 1996;154:1493–1499. [PMC free article] [PubMed] [Google Scholar]
- 7.Relative risk versus absolute risk: one cannot be interpreted without the other. Noordzij M, van Diepen M, Caskey FC, Jager KJ. Nephrol Dial Transplant. 2017;32:0–8. doi: 10.1093/ndt/gfw465. [DOI] [PubMed] [Google Scholar]
- 8.Statistical illiteracy undermines informed shared decision making. Gaissmaier W, Gigerenzer G. Z Evid Fortbild Qual Gesundhwes. 2008;102:411–413. doi: 10.1016/j.zefq.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Communicating the benefits of chronic preventive therapy: does the format of efficacy data determine patients' acceptance of treatment? Hux JE, Naylor CD. Med Decis Making. 1995;15:152–157. doi: 10.1177/0272989X9501500208. [DOI] [PubMed] [Google Scholar]
- 10.Outcomes, outcomes, every where, nor any stop to think? West CP. J Gen Intern Med. 2011;26:1239–1240. doi: 10.1007/s11606-011-1852-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Completeness of reporting trial results: effect on physicians' willingness to prescribe. Bobbio M, Demichelis B, Giustetto G. Lancet. 1994;343:1209–1211. doi: 10.1016/s0140-6736(94)92407-4. [DOI] [PubMed] [Google Scholar]
- 12.The framing effect of relative and absolute risk. Malenka DJ, Baron JA, Johansen S, Wahrenberger JW, Ross JM. J Gen Intern Med. 1993;8:543–548. doi: 10.1007/BF02599636. [DOI] [PubMed] [Google Scholar]
- 13.GPs' and physicians' interpretation of risks, benefits and diagnostic test results. Heller RF, Sandars JE, Patterson L, McElduff P. Fam Pract. 2004;21:155–159. doi: 10.1093/fampra/cmh209. [DOI] [PubMed] [Google Scholar]
- 14.Why does framing influence judgment? Gigerenzer G. J Gen Intern Med. 2003;18:960–961. doi: 10.1046/j.1525-1497.2003.30901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Same information, different decisions: the influence of evidence on the management of hypertension in the elderly. Cranney M, Walley T. https://www.ncbi.nlm.nih.gov/pubmed/8978112. Br J Gen Pract. 1996;46:661–663. [PMC free article] [PubMed] [Google Scholar]
- 16.Helping doctors and patients make sense of health statistics. Gigerenzer G, Gaissmaier W, Kurz-Milcke E, Schwartz LM, Woloshin S. Psychol Sci Public Interest. 2007;8:53–96. doi: 10.1111/j.1539-6053.2008.00033.x. [DOI] [PubMed] [Google Scholar]
- 17.The coronary primary prevention trial: design and implementation: the Lipid Research Clinics Program. J Chronic Dis. 1979;32:609–631. doi: 10.1016/0021-9681(79)90092-4. [DOI] [PubMed] [Google Scholar]
- 18.The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA. 1984;251:351–364. doi: 10.1001/jama.1984.03340270029025. [DOI] [PubMed] [Google Scholar]
- 19.Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT) Stamler J, Wentworth D, Neaton JD. JAMA. 1986;256:2823–2828. [PubMed] [Google Scholar]
- 20.Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. Ridker PM, Danielson E, Fonseca FA, et al. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 21.Evolocumab and clinical outcomes in patients with cardiovascular disease. Sabatine MS, Giugliano RP, Keech AC, et al. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 22.Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. Nissen SE, Lincoff AM, Brennan D, et al. N Engl J Med. 2023;388:1353–1364. doi: 10.1056/NEJMoa2215024. [DOI] [PubMed] [Google Scholar]
- 23.Hypercholesterolaemia and coronary heart disease: an answer. Oliver MF. Br Med J (Clin Res Ed) 1984;288:423–424. doi: 10.1136/bmj.288.6415.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The lipid hypothesis is proven. Simons LA. Med J Aust. 1984;140:316–317. doi: 10.5694/j.1326-5377.1984.tb104080.x. [DOI] [PubMed] [Google Scholar]
- 25.Lowering blood cholesterol to prevent heart disease. JAMA. 1985;253:2080–2086. [PubMed] [Google Scholar]
- 26.The Lipid Research Clinics Coronary Primary Prevention Trial. L'Abbe KA, Detsky AS, Logan AG. JAMA. 1985;253:3091. [PubMed] [Google Scholar]
- 27.Commentary on the published results of the Lipid Research Clinics Coronary Primary Prevention Trial. Kronmal RA. JAMA. 1985;253:2091–2093. [PubMed] [Google Scholar]
- 28.Lowering cholesterol and the incidence of coronary heart disease. Adams DD. JAMA. 1985;253:3090–3091. [PubMed] [Google Scholar]
- 29.Dietary lipids and heart disease. The contriving of a relationship. Smith RL. Am Clin Lab. 1989;26:33. [Google Scholar]
- 30.Coronary heart disease— “doing the wrong things”. Mann GV. https://journals.lww.com/nutritiontodayonline/Citation/1985/07000/Coronary_Heart_Disease___Doing_the_Wrong_Things_.2.aspx Nutr Today. 1985;12:14. [Google Scholar]
- 31.TIME: hold the eggs and butter. [ Mar; 2023 ]. 1984. https://content.time.com/time/subscriber/article/0,33009,921647,00.html https://content.time.com/time/subscriber/article/0,33009,921647,00.html
- 32.Heart panel's conclusions questioned. Kolata G. Science. 1985;227:40–41. doi: 10.1126/science.3880617. [DOI] [PubMed] [Google Scholar]
- 33.Multiple risk factor intervention trial. Risk factor changes and mortality results. Multiple Risk Factor Intervention Trial Research Group. https://www.ncbi.nlm.nih.gov/pubmed/7050440. JAMA. 1982;248:1465–1477. [PubMed] [Google Scholar]
- 34.LDL-C does not cause cardiovascular disease: a comprehensive review of the current literature. Ravnskov U, de Lorgeril M, Diamond DM, et al. Expert Rev Clin Pharmacol. 2018;11:959–970. doi: 10.1080/17512433.2018.1519391. [DOI] [PubMed] [Google Scholar]
- 35.A critical review of the consensus statement from the European Atherosclerosis Society consensus panel 2017. Okuyama H, Hamazaki T, Hama R, Ogushi Y, Kobayashi T, Ohara N, Uchino H. Pharmacology. 2018;101:184–218. doi: 10.1159/000486374. [DOI] [PubMed] [Google Scholar]
- 36.LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study - implications for LDL management. Cromwell WC, Otvos JD, Keyes MJ, et al. J Clin Lipidol. 2007;1:583–592. doi: 10.1016/j.jacl.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiding unhealthy heart outcomes in a low-fat diet trial: the Women's Health Initiative Randomized Controlled Dietary Modification Trial finds that postmenopausal women with established coronary heart disease were at increased risk of an adverse outcome if they consumed a low-fat 'heart-healthy' diet. Noakes TD. Open Heart. 2021;8:0. doi: 10.1136/openhrt-2021-001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Apolipoprotein B is associated with carotid atherosclerosis progression independent of individual cholesterol measures in a 9-year prospective study of multi-ethnic study of Atherosclerosis participants. Steffen BT, Guan W, Remaley AT, Stein JH, Tattersall MC, Kaufman J, Tsai MY. J Clin Lipidol. 2017;11:1181–1191. doi: 10.1016/j.jacl.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review. Ravnskov U, Diamond DM, Hama R, et al. http://dx.doi.org/10.1136/bmjopen-2015-010401. BMJ Open. 2016;6:0. doi: 10.1136/bmjopen-2015-010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corn oil in treatment of ischaemic heart disease. Rose GA, Thomson WB, Williams RT. Br Med J. 1965;1:1531–1533. doi: 10.1136/bmj.1.5449.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.A co-operative trial in the primary prevention of ischaemic heart disease using clofibrate. Report from the committee of principal investigators. Br Heart J. 1978;40:1069–1118. doi: 10.1136/hrt.40.10.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The underused miracle drugs: the statin drugs are to atherosclerosis what penicillin was to infectious disease. Roberts WC. Am J Cardiol. 1996;78:377–378. doi: 10.1016/s0002-9149(96)00441-9. [DOI] [PubMed] [Google Scholar]
- 43.Statin denial: an Internet-driven cult with deadly consequences. Nissen SE. Ann Intern Med. 2017;167:281–282. doi: 10.7326/M17-1566. [DOI] [PubMed] [Google Scholar]
- 44.Fear-based medical misinformation and disease prevention: from vaccines to statins. Navar AM. JAMA Cardiol. 2019;4:723–724. doi: 10.1001/jamacardio.2019.1972. [DOI] [PubMed] [Google Scholar]
- 45.How statistical deception created the appearance that statins are safe and effective in primary and secondary prevention of cardiovascular disease. Diamond DM, Ravnskov U. Expert Rev Clin Pharmacol. 2015;8:201–210. doi: 10.1586/17512433.2015.1012494. [DOI] [PubMed] [Google Scholar]
- 46.McGill: the JUPITER statin trial. [ Mar; 2023 ]. 2017. https://www.mcgill.ca/oss/article/science-science-everywhere/jupiter-statin-trial https://www.mcgill.ca/oss/article/science-science-everywhere/jupiter-statin-trial
- 47.The JUPITER trial: results, controversies, and implications for prevention. Ridker PM. Circ Cardiovasc Qual Outcomes. 2009;2:279–285. doi: 10.1161/CIRCOUTCOMES.109.868299. [DOI] [PubMed] [Google Scholar]
- 48.Formal comment on "systematic review of the predictors of statin adherence for the primary prevention of cardiovascular disease". Diamond DM, de Lorgeril M, Kendrick M, Ravnskov U, Rosch PJ. PLoS One. 2019;14:0. doi: 10.1371/journal.pone.0205138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Statin effects on aggression: results from the UCSD statin study, a randomized control trial. Golomb BA, Dimsdale JE, Koslik HJ, et al. PLoS One. 2015;10:0. doi: 10.1371/journal.pone.0124451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Statin adverse effects : a review of the literature and evidence for a mitochondrial mechanism. Golomb BA, Evans MA. Am J Cardiovasc Drugs. 2008;8:373–418. doi: 10.2165/0129784-200808060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Effects of statins on energy and fatigue with exertion: results from a randomized controlled trial. Golomb BA, Evans MA, Dimsdale JE, White HL. Arch Intern Med. 2012;172:1180–1182. doi: 10.1001/archinternmed.2012.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amyotrophic lateral sclerosis-like conditions in possible association with cholesterol-lowering drugs: an analysis of patient reports to the University of California, San Diego (UCSD) statin effects study. Golomb BA, Kwon EK, Koperski S, Evans MA. Drug Saf. 2009;32:649–661. doi: 10.2165/00002018-200932080-00004. [DOI] [PubMed] [Google Scholar]
- 53.Amyotrophic lateral sclerosis associated with statin use: a disproportionality analysis of the FDA’s Adverse Event Reporting System. Golomb BA, Verden A, Messner AK, Koslik HJ, Hoffman KB. Drug Saf. 2018;41:403–413. doi: 10.1007/s40264-017-0620-4. [DOI] [PubMed] [Google Scholar]
- 54.The effect of HMG-CoA reductase inhibitors on cognition in patients with Alzheimer's dementia: a prospective withdrawal and rechallenge pilot study. Padala KP, Padala PR, McNeilly DP, Geske JA, Sullivan DH, Potter JF. Am J Geriatr Pharmacother. 2012;10:296–302. doi: 10.1016/j.amjopharm.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Pleiotropic and adverse effects of statins-do epigenetics play a role? Allen SC, Mamotte CD. J Pharmacol Exp Ther. 2017;362:319–326. doi: 10.1124/jpet.117.242081. [DOI] [PubMed] [Google Scholar]
- 56.The impact of statins on biological characteristics of stem cells provides a novel explanation for their pleiotropic beneficial and adverse clinical effects. Izadpanah R, Schächtele DJ, Pfnür AB, Lin D, Slakey DP, Kadowitz PJ, Alt EU. Am J Physiol Cell Physiol. 2015;309:0–31. doi: 10.1152/ajpcell.00406.2014. [DOI] [PubMed] [Google Scholar]
- 57.Pharmacogenomics of statins: understanding susceptibility to adverse effects. Kitzmiller JP, Mikulik EB, Dauki AM, Murkherjee C, Luzum JA. Pharmgenomics Pers Med. 2016;9:97–106. doi: 10.2147/PGPM.S86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Should people at low risk of cardiovascular disease take a statin? Abramson JD, Rosenberg HG, Jewell N, Wright JM. BMJ. 2013;347:0. doi: 10.1136/bmj.f6123. [DOI] [PubMed] [Google Scholar]
- 59.Cholesterol lowering in intermediate-risk persons without cardiovascular disease. Yusuf S, Bosch J, Dagenais G, et al. N Engl J Med. 2016;374:2021–2031. doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 60.Relationship of sex to diabetes risk in statin trials. Goodarzi MO, Li X, Krauss RM, Rotter JI, Chen YD. Diabetes Care. 2013;36:0–1. doi: 10.2337/dc13-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Statin induced diabetes and its clinical implications. Aiman U, Najmi A, Khan RA. J Pharmacol Pharmacother. 2014;5:181–185. doi: 10.4103/0976-500X.136097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Statins are associated with increased insulin resistance and secretion. Abbasi F, Lamendola C, Harris CS, et al. Arterioscler Thromb Vasc Biol. 2021;41:2786–2797. doi: 10.1161/ATVBAHA.121.316159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mechanisms of insulin resistance by simvastatin in C2C12 myotubes and in mouse skeletal muscle. Sanvee GM, Panajatovic MV, Bouitbir J, Krähenbühl S. Biochem Pharmacol. 2019;164:23–33. doi: 10.1016/j.bcp.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 64.Statin use is associated with insulin resistance in participants of the Canadian multicentre osteoporosis study. Rees-Milton KJ, Norman P, Babiolakis C, et al. J Endocr Soc. 2020;4:0. doi: 10.1210/jendso/bvaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow-up study of the METSIM cohort. Cederberg H, Stančáková A, Yaluri N, Modi S, Kuusisto J, Laakso M. Diabetologia. 2015;58:1109–1117. doi: 10.1007/s00125-015-3528-5. [DOI] [PubMed] [Google Scholar]
- 66.Effect of statins on fasting plasma glucose in diabetic and nondiabetic patients. Sukhija R, Prayaga S, Marashdeh M, et al. J Investig Med. 2009;57:495–499. doi: 10.2310/JIM.0b013e318197ec8b. [DOI] [PubMed] [Google Scholar]
- 67.Toxicity of atorvastatin on pancreas mitochondria: a justification for increased risk of diabetes mellitus. Sadighara M, Amirsheardost Z, Minaiyan M, et al. Basic Clin Pharmacol Toxicol. 2017;120:131–137. doi: 10.1111/bcpt.12656. [DOI] [PubMed] [Google Scholar]
- 68.Mitochondrial dysfunction induced by statin contributes to endothelial dysfunction in patients with coronary artery disease. Dai YL, Luk TH, Siu CW, et al. Cardiovasc Toxicol. 2010;10:130–138. doi: 10.1007/s12012-010-9071-1. [DOI] [PubMed] [Google Scholar]
- 69.Atorvastatin induces mitochondria-dependent ferroptosis via the modulation of Nrf2-xCT/GPx4 axis. Zhang Q, Qu H, Chen Y, et al. Front Cell Dev Biol. 2022;10:806081. doi: 10.3389/fcell.2022.806081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Statin treatment increases the clinical risk of tendinopathy through matrix metalloproteinase release - a cohort study design combined with an experimental study. Eliasson P, Dietrich-Zagonel F, Lundin AC, Aspenberg P, Wolk A, Michaëlsson K. Sci Rep. 2019;9:17958. doi: 10.1038/s41598-019-53238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Statin-associated autoimmune myopathy: review of the literature. Barrons R. J Pharm Pract. 2023;36:383–393. doi: 10.1177/08971900211040291. [DOI] [PubMed] [Google Scholar]
- 72.Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 73.Statins of high versus low cholesterol-lowering efficacy and the development of severe renal failure. Chung YH, Lee YC, Chang CH, Lin MS, Lin JW, Lai MS. Pharmacoepidemiol Drug Saf. 2013;22:583–592. doi: 10.1002/pds.3433. [DOI] [PubMed] [Google Scholar]
- 74.Use of high potency statins and rates of admission for acute kidney injury: multicenter, retrospective observational analysis of administrative databases. Dormuth CR, Hemmelgarn BR, Paterson JM, et al. BMJ. 2013;346:0. doi: 10.1136/bmj.f880. [DOI] [PubMed] [Google Scholar]
- 75.High-potency statins increase the risk of acute kidney injury: evidence from a large population-based study. Corrao G, Soranna D, Casula M, Merlino L, Porcellini MG, Catapano AL. Atherosclerosis. 2014;234:224–229. doi: 10.1016/j.atherosclerosis.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 76.Effects of lovastatin on cognitive function and psychological well-being. Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, Manuck SB. Am J Med. 2000;108:538–546. doi: 10.1016/s0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- 77.Cognition, statins, and cholesterol in elderly ischemic stroke patients: a neurologist’s perspective. Jurcau A, Simion A. Medicina (Kaunas) 2021;57:616. doi: 10.3390/medicina57060616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Association of cognitive impairment in patients on 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors. Roy S, Weinstock JL, Ishino AS, et al. J Clin Med Res. 2017;9:638–649. doi: 10.14740/jocmr3066w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Examination of the FDA warning for statins and cognitive dysfunction. Sahebzamani FM, Munro CL, Marroquin OC, Diamond DM, Keller E, Kip KE. J Pharmacovigilance. 2014;2:1000141. [Google Scholar]
- 80.Effects of high-dose statin therapy on cognitive functions and quality of life in very high cardiovascular risk patients. Kobalava ZD, Villevalde SV, Vorobyeva SV. Kardiologiia. 2018;57:34–41. doi: 10.18087/cardio.2017.9.10024. [DOI] [PubMed] [Google Scholar]
- 81.Cognitive function assessment in patients on moderate- or high-intensity statin therapy. Roy S, Hyman D, Ayyala S, et al. J Clin Med Res. 2020;12:255–265. doi: 10.14740/jocmr4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evidence and mechanisms for statin-induced cognitive decline. Tan B, Rosenfeldt F, Ou R, Stough C. Expert Rev Clin Pharmacol. 2019:1–10. doi: 10.1080/17512433.2019.1606711. [DOI] [PubMed] [Google Scholar]
- 83.Simvastatin impairs hippocampal synaptic plasticity and cognitive function in mice. Guo Y, Zou G, Qi K, Jin J, Yao L, Pan Y, Xiong W. Mol Brain. 2021;14:41. doi: 10.1186/s13041-021-00758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Misleading communication of risk. Gigerenzer G, Wegwarth O, Feufel M. BMJ. 2010;341:0. doi: 10.1136/bmj.c4830. [DOI] [PubMed] [Google Scholar]
- 85.Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Sattar N, Preiss D, Murray HM, et al. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 86.Tough questions about the value of statin therapy for primary prevention: did JUPITER miss the moon? Curtiss FR, Fairman KA. J Manag Care Pharm. 2010;16:417–423. doi: 10.18553/jmcp.2010.16.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.By Jove! What is a clinician to make of JUPITER? Kaul S, Morrissey RP, Diamond GA. Arch Intern Med. 2010;170:1073–1077. doi: 10.1001/archinternmed.2010.189. [DOI] [PubMed] [Google Scholar]
- 88.Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. de Lorgeril M, Salen P, Abramson J, et al. Arch Intern Med. 2010;170:1032–1036. doi: 10.1001/archinternmed.2010.184. [DOI] [PubMed] [Google Scholar]
- 89.Statins and primary prevention of cardiovascular events. Donner-Banzhoff N, Sönnichsen A. BMJ. 2008;337:0. doi: 10.1136/bmj.a2576. [DOI] [PubMed] [Google Scholar]
- 90.JUPITER: a few words of caution. Vaccarino V, Bremner JD, Kelley ME. Circ Cardiovasc Qual Outcomes. 2009;2:286–288. doi: 10.1161/CIRCOUTCOMES.109.850404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Extreme all-cause mortality in JUPITER requires reexamination of vital records. Serebruany VL. Cardiology. 2011;120:84–88. doi: 10.1159/000330507. [DOI] [PubMed] [Google Scholar]
- 92.MedPage Today: ACC video conference reporter: ACC 2017. ACC Video Conference Reporter: ACC. [ Mar; 2023 ]. 2017. https://www.medpagetoday.com/video/meetingcoverage/acc/acc-video-conference-reporter-2017/139/2131 https://www.medpagetoday.com/video/meetingcoverage/acc/acc-video-conference-reporter-2017/139/2131
- 93.Long-term evolocumab in patients with established atherosclerotic cardiovascular disease. O'Donoghue ML, Giugliano RP, Wiviott SD, et al. Circulation. 2022;146:1109–1119. doi: 10.1161/CIRCULATIONAHA.122.061620. [DOI] [PubMed] [Google Scholar]
- 94.Chasing LDL cholesterol to the bottom — PCSK9 in perspective. Libby P, Tokgözoğlu L. https://doi.org/10.1038/s44161-022-00085-x. Nat Cardiovasc Res. 2022;1:554–561. doi: 10.1038/s44161-022-00085-x. [DOI] [PubMed] [Google Scholar]
- 95.Medscape: is bempedoic acid the clear answer to statin intolerance? [ Mar; 2023 ]. 2023. https://www.medscape.com/viewarticle/988228 https://www.medscape.com/viewarticle/988228
- 96.Ratio measures in leading medical journals: structured review of accessibility of underlying absolute risks. Schwartz LM, Woloshin S, Dvorin EL, Welch HG. BMJ. 2006;333:1248. doi: 10.1136/bmj.38985.564317.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Influence of method of reporting study results on decision of physicians to prescribe drugs to lower cholesterol concentration. Bucher HC, Weinbacher M, Gyr K. BMJ. 1994;309:761–764. doi: 10.1136/bmj.309.6957.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Understanding clinical trials. Sackett DL, Cook RJ. BMJ. 1994;309:755–756. doi: 10.1136/bmj.309.6957.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. Frick MH, Elo O, Haapa K, et al. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 100.Informing people about the risks and benefits of medicines: implications for the safe and effective use of medicinal products. Berry DC. Curr Drug Saf. 2006;1:121–126. doi: 10.2174/157488606775252638. [DOI] [PubMed] [Google Scholar]
- 101.Expressing medicine side effects: assessing the effectiveness of absolute risk, relative risk, and number needed to harm, and the provision of baseline risk information. Berry DC, Knapp P, Raynor T. Patient Educ Couns. 2006;63:89–96. doi: 10.1016/j.pec.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 102.Communicating clinical trial outcomes: effects of presentation method on physicians' evaluations of new treatments. Marcatto F, Rolison JJ, Ferrante D. https://doi.org/10.1017/S1930297500004472 Judgm Decis Mak. 2013;8:29–33. [Google Scholar]
- 103.Measuring effectiveness. Stegenga J. Stud Hist Philos Biol Biomed Sci. 2015;54:62–71. doi: 10.1016/j.shpsc.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 104.Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society consensus panel. Borén J, Chapman MJ, Krauss RM, et al. Eur Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cytokine response by monocytes and macrophages to free and lipoprotein-bound lipopolysaccharide. Cavaillon JM, Fitting C, Haeffner-Cavaillon N, Kirsch SJ, Warren HS. Infect Immun. 1990;58:2375–2382. doi: 10.1128/iai.58.7.2375-2382.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.High cholesterol may protect against infections and atherosclerosis. Ravnskov U. QJM. 2003;96:927–934. doi: 10.1093/qjmed/hcg150. [DOI] [PubMed] [Google Scholar]
- 107.SA-11 rotavirus binding to human serum lipoproteins. Superti F, Seganti L, Marchetti M, Marziano ML, Orsi N. Med Microbiol Immunol. 1992;181:77–86. doi: 10.1007/BF00189426. [DOI] [PubMed] [Google Scholar]
- 108.Mortality among patients with familial hypercholesterolemia: a registry-based study in Norway, 1992-2010. Mundal L, Sarancic M, Ose L, et al. J Am Heart Assoc. 2014;3:0. doi: 10.1161/JAHA.114.001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mortality over two centuries in large pedigree with familial hypercholesterolaemia: family tree mortality study. Sijbrands EJ, Westendorp RG, Defesche JC, de Meier PH, Smelt AH, Kastelein JJ. BMJ. 2001;322:1019–1023. doi: 10.1136/bmj.322.7293.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reductions in all-cause, cancer, and coronary mortality in statin-treated patients with heterozygous familial hypercholesterolaemia: a prospective registry study. Neil A, Cooper J, Betteridge J, et al. Eur Heart J. 2008;29:2625–2633. doi: 10.1093/eurheartj/ehn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Coronary artery calcium score and risk classification for coronary heart disease prediction. Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. JAMA. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quantification of coronary atherosclerosis and inflammation to predict coronary events and all-cause mortality. Mohlenkamp S, Lehmann N, Moebus S, et al. J Am Coll Cardiol. 2011;57:0. doi: 10.1016/j.jacc.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 113.Utility of nontraditional risk markers in atherosclerotic cardiovascular disease risk assessment. Yeboah J, Young R, McClelland RL, et al. J Am Coll Cardiol. 2016;67:139–147. doi: 10.1016/j.jacc.2015.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Evaluation of newer risk markers for coronary heart disease risk classification: a cohort study. Kavousi M, Elias-Smale S, Rutten JH, et al. Ann Intern Med. 2012;156:438–444. doi: 10.7326/0003-4819-156-6-201203200-00006. [DOI] [PubMed] [Google Scholar]
- 115.Low short-term and long-term cardiovascular and all-cause mortality in absence of coronary artery calcium: A 22-year follow-up observational study from large cohort. Shaikh K, Li D, Nakanishi R, et al. J Diabetes Complications. 2019;33:616–622. doi: 10.1016/j.jdiacomp.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 116.Coronary artery calcium score prediction of all cause mortality and cardiovascular events in people with type 2 diabetes: systematic review and meta-analysis. Kramer CK, Zinman B, Gross JL, Canani LH, Rodrigues TC, Azevedo MJ, Retnakaran R. BMJ. 2013;346:0. doi: 10.1136/bmj.f1654. [DOI] [PubMed] [Google Scholar]
- 117.Absence of coronary artery calcification and all-cause mortality. Blaha M, Budoff MJ, Shaw LJ, et al. JACC Cardiovasc Imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 118.All-cause mortality by age and gender based on coronary artery calcium scores. Nakanishi R, Li D, Blaha MJ, et al. Eur Heart J Cardiovasc Imaging. 2016;17:1305–1314. doi: 10.1093/ehjci/jev328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.A 15-year warranty period for asymptomatic individuals without coronary artery calcium: a prospective follow-up of 9,715 individuals. Valenti V, Ó Hartaigh B, Heo R, et al. JACC Cardiovasc Imaging. 2015;8:900–909. doi: 10.1016/j.jcmg.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Association of coronary plaque with low-density lipoprotein cholesterol levels and rates of cardiovascular disease events among symptomatic adults. Mortensen MB, Caínzos-Achirica M, Steffensen FH, et al. JAMA Netw Open. 2022;5:0. doi: 10.1001/jamanetworkopen.2021.48139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Clinical significance of zero coronary artery calcium in individuals with LDL cholesterol ≥190 mg/dL: the multi-ethnic study of atherosclerosis. Sandesara PB, Mehta A, O'Neal WT, et al. Atherosclerosis. 2020;292:224–229. doi: 10.1016/j.atherosclerosis.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 122.Coronary artery calcium and cardiovascular events in patients with familial hypercholesterolemia receiving standard lipid-lowering therapy. Miname MH, Bittencourt MS, Moraes SR, et al. JACC Cardiovasc Imaging. 2019;12:1797–1804. doi: 10.1016/j.jcmg.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 123.Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Oxid Med Cell Longev. 2017;2017:1273042. doi: 10.1155/2017/1273042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Glycated apolipoprotein B-A surrogate marker of subclinical atherosclerosis. Dev K, Sharma SB, Garg S, Aggarwal A, Madhu SV. Diabetes Metab Syndr. 2016;10:78–81. doi: 10.1016/j.dsx.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 125.Susceptibility of LDL and its subfractions to glycation. Soran H, Durrington PN. Curr Opin Lipidol. 2011;22:254–261. doi: 10.1097/MOL.0b013e328348a43f. [DOI] [PubMed] [Google Scholar]
- 126.Small dense LDL is more susceptible to glycation than more buoyant LDL in type 2 diabetes. Younis NN, Soran H, Pemberton P, Charlton-Menys V, Elseweidy MM, Durrington PN. Clin Sci (Lond) 2013;124:343–349. doi: 10.1042/CS20120304. [DOI] [PubMed] [Google Scholar]
- 127.Evaluation of coronary risk factors in patients with heterozygous familial hypercholesterolemia. Hopkins PN, Stephenson S, Wu LL, Riley WA, Xin Y, Hunt SC. Am J Cardiol. 2001;87:547–553. doi: 10.1016/s0002-9149(00)01429-6. [DOI] [PubMed] [Google Scholar]
- 128.Familial hypercholesterolemia and triglyceride metabolism. Kolovou GD, Kostakou PM, Anagnostopoulou KK. Int J Cardiol. 2011;147:349–358. doi: 10.1016/j.ijcard.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 129.Atherogenic dyslipidemia: cardiovascular risk and dietary intervention. Musunuru K. Lipids. 2010;45:907–914. doi: 10.1007/s11745-010-3408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Insulin resistance, small LDL particles, and risk for atherosclerotic disease. Toth PP. Curr Vasc Pharmacol. 2014;12:653–657. doi: 10.2174/15701611113119990125. [DOI] [PubMed] [Google Scholar]
- 131.Diet, lipids, and cardiovascular disease. Siri-Tarino PW, Krauss RM. Curr Opin Lipidol. 2016;27:323–328. doi: 10.1097/MOL.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 132.Evaluating the association between low-density lipoprotein cholesterol reduction and relative and absolute effects of statin treatment: a systematic review and meta-analysis. Byrne P, Demasi M, Jones M, Smith SM, O'Brien KK, DuBroff R. JAMA Intern Med. 2022;182:474–481. doi: 10.1001/jamainternmed.2022.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.The effect of statins on average survival in randomised trials, an analysis of end point postponement. Kristensen ML, Christensen PM, Hallas J. BMJ Open. 2015;5:0. doi: 10.1136/bmjopen-2014-007118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.The mainstream hypothesis that LDL cholesterol drives atherosclerosis may have been falsified by non-invasive imaging of coronary artery plaque burden and progression. Ware WR. Med Hypotheses. 2009;73:596–600. doi: 10.1016/j.mehy.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 135.Saturated fat does not clog the arteries: coronary heart disease is a chronic inflammatory condition, the risk of which can be effectively reduced from healthy lifestyle interventions. Malhotra A, Redberg RF, Meier P. Br J Sports Med. 2017;51:1111–1112. doi: 10.1136/bjsports-2016-097285. [DOI] [PubMed] [Google Scholar]
- 136.Statin therapy is not warranted for a person with high LDL-cholesterol on a low-carbohydrate diet. Diamond DM, Bikman BT, Mason P. Curr Opin Endocrinol Diabetes Obes. 2022;29:497–511. doi: 10.1097/MED.0000000000000764. [DOI] [PubMed] [Google Scholar]