Abstract
Prenatal exposure to endocrine disrupting chemicals (EDCs) from personal care products may be associated with birth outcomes including preterm birth and low birth weight. There is limited research examining the role of personal care product use during pregnancy on birth outcomes. Our pilot study consisted of 164 participants in the Environmental Reproductive and Glucose Outcomes (ERGO) study (Boston, MA), with data on self-reported personal care product use at four study visits throughout pregnancy (product use in the 48 hours before a study visit and hair product use in the month before a study visit). We used covariate-adjusted linear regression models to estimate differences in mean gestational age at delivery, birth length, and sex-specific birth weight-for-gestational age (BW-for-GA) Z-score based on personal care product use. Hair product use in the past month prior to certain study visits was associated with decreased mean sex-specific BW-for-GA Z-scores. Notably, hair oil use in the month prior to study visit 1 was associated with a lower mean BW-for-GA Z-score (V1: −0.71, 95% confidence interval: −1.12, −0.29) compared to non-use. Across all study visits (V1-V4), increased mean birth length was observed among nail polish users vs. non-users. In comparison, decreased mean birth length was observed among shave cream users vs. non-users. Liquid soap, shampoo, and conditioner use at certain study visits were significantly associated with higher mean birth length. Suggestive associations were observed across study visits for other products including hair gel/spray with BW-for-GA Z-score and liquid/bar soap with gestational age. Overall, use of a variety of personal care products throughout pregnancy was observed to be associated with our birth outcomes of interest, notably hair oil use during early pregnancy. These findings may help inform future interventions/clinical recommendations to reduce exposures linked to adverse pregnancy outcomes.
Keywords: endocrine disrupting chemicals, personal care products, hair products, pregnancy, birth outcomes
1. INTRODUCTION
Gestational age at delivery and infant anthropometric measurements at birth are important indicators of perinatal health. Infants born preterm (less than 37 weeks of gestation) or low birth weight (LBW) (less than 2,500 g) are at a higher risk of short-term mortality and morbidity1,2 and subsequent health outcomes later in life, including growth and neurodevelopmental delays3-5 and increased risk of cardiometabolic outcomes.6,7 Birth length and weight are positively associated with height in adulthood,8 which has been hypothesized to be linked to health outcomes.9 Racial and ethnic disparities in these pregnancy outcomes are not only persistent but have been increasing in recent years.10-13 Non-Hispanic Black women are more likely to give birth to preterm and/or LBW infants compared to non-Hispanic White women.14-17 While a variety of exposures, stressors, and individual-level risk factors underlie these disparities,18 personal care product use represents a potential modifiable source of exposure to environmental chemicals linked to these adverse pregnancy outcomes and therefore may be an important area for future interventions.
Personal care products often contain endocrine disrupting chemicals (EDCs)—synthetic or natural chemicals that can interfere with the body’s hormonal processes19—such as phthalates and parabens. Specifically, phthalate metabolites and parabens have been shown to interfere with hormonal pathways and bind to nuclear receptors.20,21 Racial and ethnic disparities in urinary phthalate metabolite and paraben concentrations have been reported among pregnant people,22 indicating potential differences in personal care product use patterns.22-24 Importantly, the prenatal period is a sensitive window of development where early life exposures may impact health later in life. For example, studies have observed that higher exposure to EDCs during pregnancy may be associated with LBW infants and preterm birth (PTB).25,26 Since personal care product use can be a modifiable source of EDC exposure, research assessing specific product types that may contribute to pregnancy health outcomes is important for clinical recommendations and interventions.
Given that EDC exposure may contribute to adverse perinatal outcomes, along with associated health disparities,22,23,27 additional research is needed to examine the role of personal care product use on pregnancy outcomes, including, gestational age at delivery, birth weight, and birth length. In previous work, we conducted a proof-of-concept pilot study to evaluate the association between a racially and ethnically disparate pregnancy outcome (i.e. shortened gestational age) and a single type of EDC-associated personal care product that has been shown to be racially and ethnically patterned (i.e. hair product use). From that analysis, we reported associations between hair oil use and shorter gestational age at delivery.28 In a subsequent exposure assessment, we observed higher urinary concentrations of multiple phthalate metabolites, including monoethyl phthalate (MEP), among hair oil users compared to non-users.28,29 Thus, building on this work and based on our proposed translational epidemiologic framework for environmental health disparities,30 we now examine associations of an expanded suite of personal care products throughout pregnancy with gestational age at delivery, birth length, and sex-specific birth weight-for-gestational age (BW-for-GA) Z-scores within a pregnancy cohort in Boston, MA.
2. METHODS
2.1. Study Population
The study population consisted of pregnant participants enrolled in the Environmental Reproductive and Glucose Outcomes (ERGO) study. In 2016, ERGO began enrolling participants during routine prenatal visits in early pregnancy (median: 12 weeks of gestation). Participants were eligible if they were at least 18 years old, less than 15 weeks of gestation at enrollment, English speaking, and planning to receive care and deliver at either Brigham and Women’s Hospital or Beth Israel Deaconess Medical Center (BIDMC) in Boston, MA. Participants could also be actively participating in the Massachusetts General Hospital Study of Pregnancy Regulation of Insulin and Glucose (SPRING), a cohort study assessing subforms of gestational diabetes.31 Ineligible participants included those with pre-existing diabetes, pregnancies with more than two fetuses, and those who were not able to complete oral glucose tolerance tests. ERGO participants completed up to four study visits throughout pregnancy at a median of 12, 19, 26, and 36 weeks of gestation, during which they completed demographic and behavioral questionnaires. For purposes of the present analysis, we only analyzed data from a single site of the ERGO study (BIDMC) due to the inclusion of the specific study questions on hair and personal care product use across the four study visits. BIDMC was added as a site to the ERGO study in April 2018 and recruitment was completed for all of the ERGO study in November 2020.
Of the 175 participants from the BIDMC site, 171 had data on self-reported product use from at least one study visit. Of those, 164 participants had data on at least one birth outcome measure (n=7 lost-to-follow up; these participants either transferred care or moved out of MA during their pregnancy). Three participants were missing data on birth length. The final analytic study population included 164 participants. All participants provided informed consent, and study protocols were approved by Harvard Longwood Campus Institutional Review Board and the Committee on Clinical Investigations for BIDMC.
2.2. Personal Care Product Use
The ERGO study collected data on self-reported product use at each of the four study visits during pregnancy. Product use was assessed using two previously validated questionnaires, which have been used in prior epidemiologic and exposure assessment research.32-34 Participants were asked to report use (yes or no) of 12 categories of products—including deodorant, crème rinse/conditioner, shampoo, hair gel, perfume, bar soap, liquid soap/body wash, hand/body lotion, shave cream, nail polish, other hair products, and colored cosmetics—within the 48 hours prior to the prenatal study visit. For this analysis, we excluded the categories “other hair products,” as the participants were asked more specific questions about categories of hair product use, and “colored cosmetics,” as this question’s wording resulted in an overly broad and potentially unrelated group of products (e.g., mascara, blush, hair dye, etc.). Using a second validated questionnaire, participants were asked about their use (yes or no) of eight categories of hair products (hair oil, hair lotion, leave-in conditioner, non-lye perm/relaxer, lye perm/relaxer, natural products, prescription, and other) within the month prior to the prenatal study visit. Additionally, participants reported how often they used each of the eight hair product categories (i.e., daily, 1+ times per week, 1+ times per month, every six months or more, and other). Due to the low use of some of the hair product categories in this subset, we only included hair oil, hair lotion, and leave-in conditioner in this analysis.28 We modeled product use individually as “yes” or “no” at each pregnancy visit and as “ever” or “never” during pregnancy, where “ever” use represented self-reported use at one or more pregnancy visits. We did not model product use as “ever”/“never” use for the following products due to near-universal usage in the population: deodorant (94%), conditioner (94%), shampoo (99%), liquid soap/body wash (98%), and lotion (95%). For hair products only, we additionally modeled product use frequency (daily, less than daily, or no use) at each study visit (hair oil only), as well as “ever daily use”, “less than daily use”, and “never use” in pregnancy (hair oil, hair lotion, leave-in conditioner).
2.3. Birth Outcome Assessment
Data on gestational age at delivery, birth length, and birth weight were abstracted from medical records. Gestational age at delivery (weeks) was estimated using self-reported last menstrual period and confirmed with the earliest prenatal ultrasound. Birth length (cm) and birth weight (g) were measured at delivery. For our analyses, we calculated the sex-specific BW-for-GA Z-scores and percentiles for each infant based on a recently updated U.S. reference population and methods described in Aris et al. 201935 and used in previous research.36,37 BW-for-GA Z-scores were used in our primary analyses and percentiles were used to categorize infants as small for gestational age (SGA, <10th percentile) and large for gestational age (LGA, >90th percentile) only for descriptive statistics due to the small sample sizes. Gestational age (days), birth length (cm), and BW-for-GA Z-score were modeled continuously for all analyses.
2.4. Covariates
The ERGO study collected data on sociodemographic characteristics, including education, parity, and race and ethnicity, via a baseline questionnaire at the first prenatal study visit. For the analyses, we categorized parental educational level as a bachelor’s degree or higher versus no bachelor’s degree and self-reported race and ethnicity as non-Hispanic White, non-Hispanic Black, Hispanic, and “Other” race. The “Other” racial and ethnic category included participants self-identifying as Asian, non-Hispanic Haitian/Caribbean, American Indian/Alaskan Native, Native Hawaiian, “Other” Pacific Islander, or more than one race. Race and ethnicity are indicators of sociocultural differences in product use patterns that potentially drive exposure differences, as well as structural racism factors that drive adverse pregnancy health disparities. Parental age at enrollment (years) was calculated from the date of birth and date of study consent and body mass index (BMI, kg/m2) was calculated from the first prenatal visit weight (kg) and height (meters). Infant sex was obtained from medical records.
2.5. Statistical Analysis
We calculated descriptive statistics (mean ± SD, median (range), or n (%)) for the participants’ sociodemographic characteristics, birth outcomes measures, and personal care product use.
2.5.1. Linear Regression Models of Individual Product Use and Birth Outcomes
We fit separate linear regression models for each personal care product category and birth outcome (i.e., gestational age at delivery (days), birth length (cm), and BW-for-GA Z-score) to evaluate the associations of self-reported personal care product use at each pregnancy visit and “ever” use in pregnancy with each birth outcome. The associations between hair product use and frequency of use with gestational age at delivery are presented among a similar subset of ERGO participants in Preston et al. 2021.28 For hair products with sufficient frequency use data, we ran additional models estimating associations of frequency of product use (daily, < daily, never) at each visit (hair oil) and “ever” use during pregnancy (hair oil, hair lotion, leave-in conditioner) with birth length and BW-for-GA Z-scores. Additionally, we plotted estimated probability distribution functions for BW-for-GA Z-scores to visually depict the observed shift in mean Z-scores and differences in distribution shapes comparing daily, < daily, and non-users of hair oil, which are reported to be hormonally active and associated with EDCs in our previous analyses.29,38 We included the following covariates as potential confounders selected a priori: parental age at enrollment (years, continuous), self-reported education (bachelor’s degree or higher versus no bachelor’s degree), parity (0, 1, 2+), and parental BMI at visit 1 (kg/m2, continuous) in our models of gestational age at delivery and sex-specific BW-for-GA Z-scores. For our analyses examining birth length, we additionally adjusted for gestational age at delivery (days, continuous) and infant sex.
2.5.2. Sensitivity Analysis
Race and ethnicity, particularly the non-Hispanic Black race, are associated with both gestational age and birth weight, as well as the use of certain personal care products (e.g., high hair oil use by non-Hispanic Black participants).33,38 Thus, we were interested in evaluating if any of the observed associations between product use and birth outcomes were driven by the usage patterns of these participants. We did not have a large enough sample size to stratify our associations by race and ethnicity or to adjust for race and ethnicity; therefore, we performed a sensitivity analysis excluding non-Hispanic Black participants (n=18) from our linear regression models examining the associations of personal care product use within the 48 hours and hair product use within the past month prior to pregnancy study visits and “ever” use in pregnancy, with our birth outcome measures. Statistical analyses were completed in SAS version 9.4 (Cary, NC).
3. RESULTS
Among the 164 participants in our analytic pilot study population, the mean age at enrollment (SD) was 32.5 (4.4) years with a mean BMI of 25.6 (5.3) kg/m2 (Table 1). Sixty-two percent of participants were non-Hispanic White, 19% were categorized as “Other” race, 11% were non-Hispanic Black, and 8% were Hispanic. Seventy-nine percent held a bachelor’s degree or higher. Of the infants, 6% were born preterm (<37 weeks), and 17% were classified as SGA. Mean (SD) gestational age at delivery was 39.2 (1.9) weeks, mean birth length was 48.8 (3.8) cm, and mean infant birth weight was 3,297 (566) g. Excluded participants (n=11) were more likely to be parous (86%), of “Other” race (37.5%), and were slightly younger (mean 31.7 years) with higher BMI at visit 1 (mean 26.5 kg/m2) (Supplemental Table S1).
Table 1.
Participant characteristics and birth outcome measures (n=164)
| Characteristic/outcome | mean ± SD, median (range)a, or n (%) |
|---|---|
| Gestational age at study visits (weeks) | |
| Visit 1 | 11 (6, 16) |
| Visit 2 | 19 (15, 23) |
| Visit 3 | 26 (23, 35) |
| Visit 4 | 36 (32, 39) |
| Parental characteristics | |
| Age at consent (years) | 32.5 ± 4.4 |
| BMI at visit 1 (kg/m2) | 25.6 ± 5.3 |
| Bachelor's degree or higher | 130 (79) |
| Race and ethnicity | |
| Non-Hispanic White | 101 (62) |
| Non-Hispanic Black | 18 (11) |
| Hispanic | 13 (8) |
| “Other” race | 32 (19) |
| Parity | |
| 0 | 84 (51) |
| 1 | 51 (31) |
| 2+ | 29 (18) |
| Infant outcomes | |
| Gestational age at delivery (weeks) | 39.2 ± 1.9 |
| Birth length (cm)b | 48.8 ± 3.8 |
| Birth weight (g) | 3,297 ± 566 |
| Sex-specific BW-for-GA Z-score | −0.20 ± 1.17 |
| Preterm birth (<37 weeks) | 10 (6) |
| Small for gestational age (SGA) | 28 (17) |
| Large for gestational age (LGA) | 11 (7) |
| Female infant | 88 (54) |
| Mode of delivery | |
| Vaginal | 125 (76) |
| Cesarean | 39 (24) |
Median (range) presented for gestational age at study visits (weeks)
n=3 missing birth length data
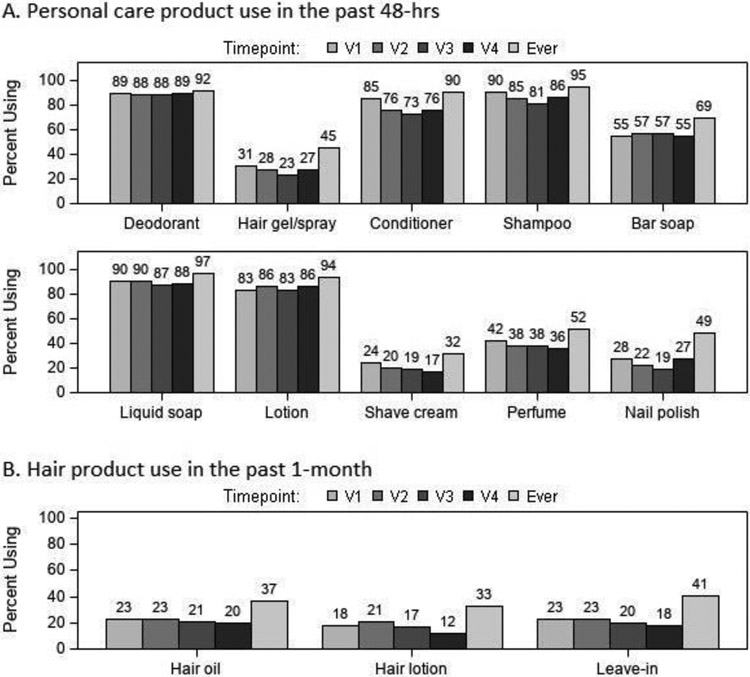
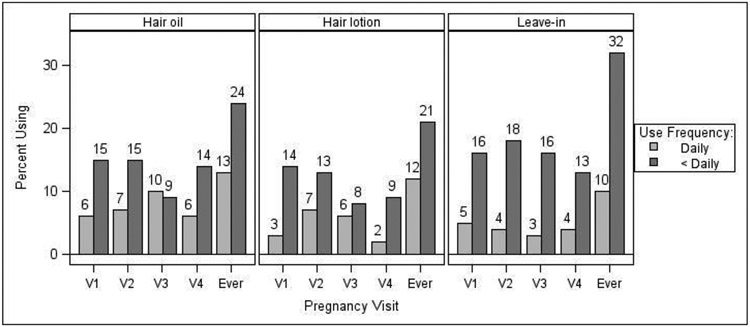
The products with the highest reported use during the 48 hours prior to any study visit (“ever” use during pregnancy) were liquid soap/body wash (97%), shampoo (95%), lotion (94%), deodorant (92%), and crème rinse/conditioner (90%) (Figure 1A). For hair products, use within one month prior to any study visit during pregnancy (“ever” use) was highest for leave-in conditioner (41%), followed by hair oil (37%) and hair lotion (33%) (Figure 1B). Self-reported frequency of hair product use is displayed in Figure 2. Self-reported daily use of hair products at one or more study visits was highest for hair oil (13%), followed by hair lotion (12%), and leave-in conditioner (10%). Flair oil had the highest percentage of daily users across pregnancy visits (range 6-10%).
Figure 1.
Self-reported use of (A) personal care products within the past 48 hours and (B) hair products within the past month prior to each pregnancy study visit (V1-4) and “ever” in pregnancy (n=164*). “Ever” represents self-reported use at any of the four pregnancy study visits. *Sample size varied across visits and by product category due to missing data (range: n=137 to n=164).
Figure 2.
Self-reported frequency of hair product use (daily, <daily, no use) within the past month prior to each pregnancy study visit (V1-4) and “ever” in pregnancy (n=164*). “Ever” represents self-reported use at any of the four pregnancy study visits. Values may vary from those in Figure 1 due to missing frequency data for products and/or timepoints. *Sample size varied across visits and by hair product due to missing data (range: n=137 to n=164).
3.1. Personal Care Product Use and Gestational Age at Delivery (days)
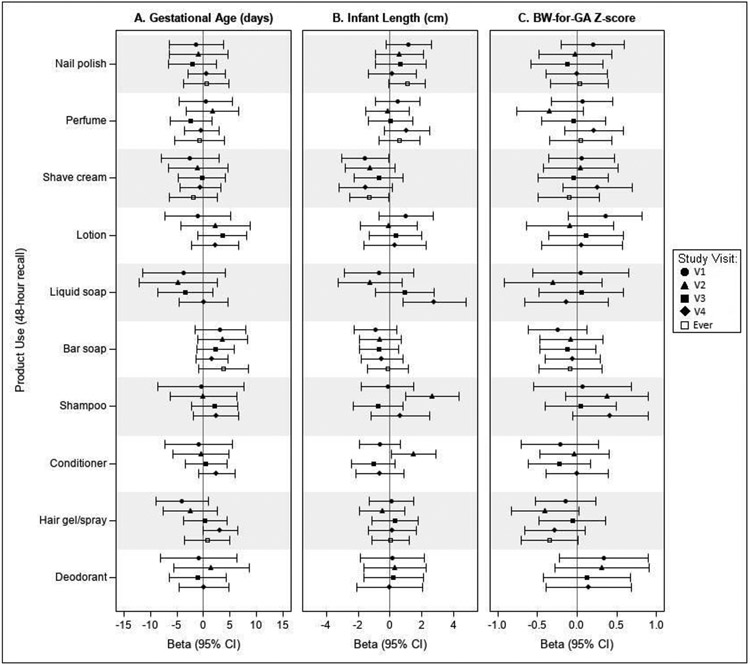
Results from covariate-adjusted regression models estimating associations of personal care product use (48 hours prior to each visit) with gestational age at delivery (days) (Figure 3A) were inconsistent without clear patterns of associations for most products across visits. We observed suggestive associations of shorter mean gestational age at delivery among liquid soap/body wash users compared to non-users at visits 1 through 3 (V1: −3.8 days, 95% Confidence Interval (CI): −11.4, 4.0; V2: −4.8 days, 95% CI: −12.2, 2.6; V3: −3.5 days, 95% CI: −8.6, 1.7). We were unable to model associations with “ever” use of liquid soap/body wash due to a lack of sufficient “never” users. Conversely, we observed suggestive associations of longer mean gestational age at delivery in bar soap users compared to non-users at all time points and “ever” use (V1: 3.2 days, 95% CI: −1.6, 7.9; V2: 3.6 days, 95% CI: −1.1, 8.3; V3: 2.3 days, 95% CI: −1.2, 5.7; V4: 1.6 days, 95% CI: −1.4, 4.7; Ever: 3.8 days, 95% CI: −0.9, 8.4).
Figure 3.
Covariate-adjusted differences in mean birth outcomes associated with use of personal care products compared to non-use within the 48 hours prior to each pregnancy visit (n=164). Figures depict the estimated mean difference in (A) gestational age at delivery (days), (B) birth length (cm), and (C) sex-specific BW-for-GA Z-score in product users compared to non-users at each study visit and “ever” users compared to “never” users during pregnancy. Models were adjusted for the following covariates: (A) parental age at consent (years), bachelor’s degree or higher (yes vs. no), BMI at visit 1 (kg/m2), and parity (0, 1, 2+); (B) parental age at consent (years), bachelor’s degree or higher (yes vs. no), BMI at visit 1 (kg/m2), parity (0, 1, 2+), infant sex, and gestational age at delivery (weeks); (C) parental age at consent (years), bachelor’s degree or higher (yes vs. no), BMI at visit 1 (kg/m2), and parity (0, 1, 2+). N=3 participants missing birth length data.
3.2. Personal Care Product Use and Birth Length (cm)
In models estimating associations of product use (48 hours prior to each visit) and birth length (cm), we observed consistently lower mean birth length (cm) in shave cream users compared to non-users (Figure 3B). For example, “ever” users of shave cream gave birth to infants with −1.3 cm (95% CI: −2.6, −0.1) lower mean length at birth (cm). A suggestive pattern of bar soap use and lower mean birth length was also observed across study visits during pregnancy. Conversely, nail polish users had consistently higher mean birth length compared to non-users, with “ever” use associated with a 1.1 cm (95% CI: −0.1, 2.2) higher mean birth length compared to “never” users. Associations with other products were more inconsistent. We observed a significantly higher mean birth length in liquid soap/body wash users compared to non-users, but only at visit 4 (V4: 2.8 cm, 95% CI: 0.8, 4.8). Similarly, we observed significantly higher mean birth length in shampoo and conditioner users compared to non-users at visit 2 (Shampoo V2: 2.6 cm, 95% CI: 1.0, 4.3; Conditioner V2: 1.5 cm, 95% CI: 0.1, 2.9), but did not observe similar associations at the other pregnancy visits. We were not able to assess “ever” versus “never” use of liquid soap/body wash, shampoo, or conditioner due to insufficient numbers of “never” users for these products. Associations with other personal care products were inconsistent and primarily null. We did not observe any notable associations between hair product use or frequency of use in the past month with birth length (Supplemental Figure S1).
3.3. Personal Care Product Use and Sex-Specific BW-for-GA Z-score
3.3.1. Personal care product use reported 48 hours prior to study visits
Figure 3C presents the covariate-adjusted differences in mean sex-specific BW-for-GA Z-score associated with personal care product use compared to non-use (48 hours prior to each visit) and “ever” versus “never” use in pregnancy. We observed a suggestive pattern of associations between hair gel/spray use and lower mean BW-for-GA Z-scores at visits 2 and 4 compared to non-use and “ever” compared to “never” in pregnancy. Estimates at visits 1 and 3 were smaller in magnitude and less precise. At visit 2, hair gel/spray users had a lower mean BW-for-GA Z-score compared to non-users (V2: −0.41, 95% CI: −0.84, 0.02). Associations with other products were more inconsistent. We observed positive associations between shampoo use (visits 2 and 4), lotion use (visit 1), and deodorant use (visits 1 and 2) and higher mean BW-for-GA Z-score compared to non-users, but these associations were not consistent across visits and should be interpreted with caution.
3.3.2. Hair product use reported one month prior to study visits
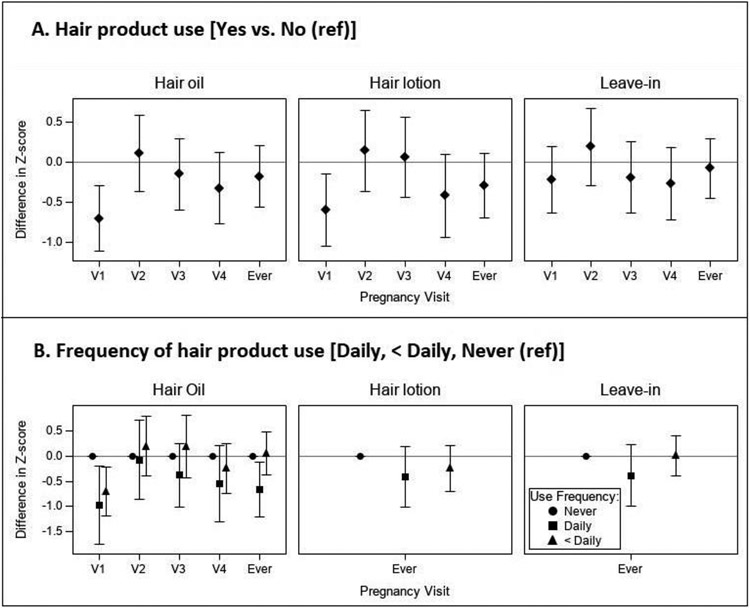
Figure 4A presents the covariate-adjusted differences in mean sex-specific BW-for-GA Z-score associated with hair product use (one month prior to each visit) and “ever” in pregnancy. Hair oil use was consistently associated with lower BW-for-GA Z-score across visits except for visit 2. The strongest association was observed for visit 1, where hair oil users had a lower mean BW-for-GA Z-score of −0.71 (95% CI: −1.12, −0.29) compared to non-users. We observed a similar pattern for hair lotion, with hair lotion use at pregnancy visits 1 and 4, and “ever” in pregnancy associated with lower mean BW-for-GA Z-score (e.g., V1: −0.60, 95% CI: −1.06, −0.15). Use of leave-in conditioner at visits 1, 3, and 4 was associated with lower mean BW-for-GA Z-score compared to non-use, but not at visit 2 or “ever” in pregnancy (e.g., V4: −0.27, 95% CI: −0.72, 0.18).
Figure 4.
Covariate-adjusted differences in mean sex-specific BW-for-GA Z-score associated with (A) hair product use and (B) frequency of hair product use within the month prior to each pregnancy visit (n=149*). All models were adjusted for parental participant age at consent (years), bachelor’s degree or higher (yes vs. no), BMI at visit 1 (kg/m2), and parity (0, 1, 2+). (B) “Ever” use categories represent report of daily use or less than daily use at one or more pregnancy visits compared to “never” users. Visit-specific estimates for frequency of hair lotion and leave-in conditioner use were not modeled due to insufficient numbers of daily users at individual study visits. *Due to missing frequency data at some individual visits sample sizes ranged from n=137 to n=149.
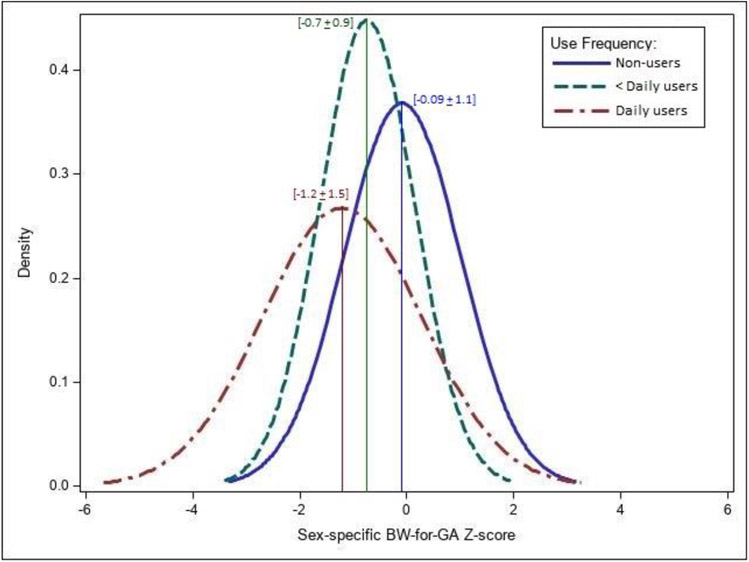
Figure 4B displays estimates for associations of frequency of hair product use (one month prior to each visit) with BW-for-GA Z-score, comparing daily or less than daily users to “never” users at all visits for hair oil and “ever” daily, less than daily, or “never” use in pregnancy for all three hair products. Participants reporting daily use of any of the three products during pregnancy (“Ever” daily use) had lower mean BW-for-GA Z-scores compared to “never” users (Hair oil: −0.67, 95% CI: −1.22, −0.12; Hair lotion: −0.41, 95% CI: −1.01, 0.20; Leave-in conditioner: −0.38, 95% CI: −1.01, 0.24). For hair oil, daily use at visits 1, 3, 4, and “ever” in pregnancy was associated with lower mean BW-for-GA Z-scores, with the strongest association seen at visit 1 (V1: −0.98, 95% CI: −1.76, −0.20). The differences in BW-for-GA Z-score between the hair oil user groups are further illustrated in Figure 5, which depicts estimated probability density functions for normal distributions in each user group. Compared to non-users, the estimated normal Z-score distribution for daily hair oil users at visit 1 shifted to the left with a visual widening of the distribution tails. Mean (SD) BW-for-GA Z-scores for hair oil user groups were −1.2 (1.5) for daily users, −0.7 (0.9) for less than daily users, and −0.09 (1.1) for non-users. Compared to non-users, the Z-score distribution for daily hair oil users at visit 1 shifted left with a −1.0 (95% CI: −1.8, −0.2) lower mean BW-for-GA Z-score and a visual widening of the distribution tails.
Figure 5.
Estimated normal probability density functions of sex-specific BW-for-GA Z-scores in daily, less than daily, and non-users of hair oil at Visit 1 in pregnancy (n=144). Vertical lines depict the mean BW-for-GA Z-scores for daily users (mean ± SD: −1.2 ± 1.5), less than daily users (−0.7 ± 0.9) non-users (−0.09 ± 1.1).
3.4. Sensitivity Analysis
In sensitivity analyses, excluding non-Hispanic Black participants (n=18), we observed similar results for associations of personal care product use (48 hours prior to each visit) (Supplemental Table S2) and hair product use (one month prior to each visit) (Supplemental Table S3) with birth outcomes. For personal care product use, gestational age model estimates were generally consistent in the direction and magnitude of the associations, but some associations were strengthened (e.g., Bar soap, “ever” use: 4.5 days, 95% CI: −0.1, 9.1; Shave cream, “ever” use: −3.2 days, 95% CI: −7.8, 1.4). Results for birth length (cm) were very similar to those in the full cohort. For BW-for-GA Z-scores models, results excluding non-Hispanic Black participants were generally similar to those in the full cohort, however, some estimates were attenuated (e.g., hair gel/spray) while others were strengthened (e.g., lotion). As in the full cohort, we did not observe notable or consistent associations between hair product use and birth length (cm). Associations between hair product use and sex-specific BW-for-GA Z-scores were similar in the direction and magnitude of the associations, although some estimates for hair oil were weakened (e.g., V1: −0.46, 95% CI: −0.90, −0.02) and some estimates for hair lotion were strengthened (e.g., V4: −0.35, 95% CI: −0.78, 0.08).
4. DISCUSSION
In this pilot study of pregnant individuals, we observed that the use of certain categories of personal care products was associated with our birth outcomes of interest. Hair oil and hair lotion use (most notably in the month prior to the first study visit), and leave-in conditioner use (in the month prior to visits 1, 3, and 4) were associated with lower mean sex-specific BW-for-GA Z-scores. Daily use of all three hair products reported in the past month prior to study visits (specifically, “ever” daily use, and also daily use at visits 1 and 4 for hair oil) was also observed to be associated with lower mean sex-specific BW-for-GA Z-scores. Consistent and suggestive associations across pregnancy were observed for a number of other product categories with all three birth outcomes. In general, many of the reported model estimates had wide confidence intervals, likely due to the small sample size in this pilot study.
4.1. Personal Care Product Use and Birth Outcomes
A small number of studies have investigated the association between personal care product use during pregnancy and perinatal outcomes, including gestational age at delivery, PTB, and size for gestational age. A Chinese cohort study (n=9,710) that recruited from September 2016 to August 2017 reported that cosmetics use during pregnancy was associated with a higher risk of SGA, but not for PTB, LBW, LGA, and macrosomia.39 They also reported a positive association between the frequency of cosmetic use during pregnancy and risk of SGA. However, this study only considered facial hygiene care products and make-up products and did not include hair or other personal care products. Conversely, a U.S. case-control study in North Carolina (n= 188 preterm cases, n=156 LBW cases, n=304 controls) that recruited between September 1988 and April 1991 found that use of permanent hair straightening and curling products among Black women during pregnancy was not associated with an increased risk of delivering a preterm or LBW infant.40 Similarly, a separate U.S.-based case-control study among participants from the Black Women’s Health Study (n=5,633 controls, n=497 cases) which recruited in 1995 with follow-up information collected in 1997, 1999, and 2001, reported no association between “ever” versus “never” use of hair relaxers and odds of PTB.41 However, differences in the measurement of personal care product use, such as the included product categories/types, timing of assessment during pregnancy, recall time period, and frequency data, as well as the country/region of study, can impact both product formulation and product use patterns and make it challenging to compare results across studies. For example, two of the three aforementioned studies focused on chemical hair straightening and curling products, which are used on average over several months compared to leave-in maintenance hair products which can be used daily (e.g., leave-in conditioner, hair oil, curl cream, gel, edge control, pomade).33
Additionally, these U.S.-based case-control studies recruited participants prior to 2000, which may represent a different exposure distribution to environmental chemicals from personal care products compared to current day exposures. Specifically, exposure to EDCs has changed over time.42,43 For example, an analysis examining temporal trends (2001-2010) in phthalate exposures using National Health and Nutrition Examination Survey (NHANES) data reported a decrease in concentrations of certain phthalate metabolites including MEP and monobenzyl phthalate, while increased concentrations of other phthalate metabolites such as monoisobutyl phthalate (MiBP) and monocarboxynonyl phthalate.42 These differences may be driven by changes in personal care product use patterns as well as changes in the chemical composition of personal care and consumer products over time.44,45 As an example, U.S. market research has observed a temporal decline in hair relaxer sales.44 These changes in hair product use patterns may contribute to the frequent use of leave-in maintenance hair products such as hair oil, leave-in conditioner, and hair gel reported among Black women.38,46,47 Previous research has reported differences in chemical composition between leave-in and rinse-out products as well as differences in urinary concentrations of personal care product-associated EDCs among more frequent users of leave-in versus rinse-out products.48-50 These products are modifiable sources of EDC exposure that may contribute to racial and ethnic disparities in birth outcomes.16-18 Thus, recent U.S.-based studies focusing on personal care product use, such as ours, are important to not only fill the gap in the literature on the association between personal care product use and birth outcomes but also document current personal care product use patterns and related chemical exposure.
In our previous research examining hair product use and frequency of use during pregnancy among a similar overlapping subset of the BIDMC participants in ERGO (n=154), we found that participants reporting daily use of hair oil in late pregnancy (month prior to study visit 4; median: 36 weeks of gestation) had lower mean gestational age at delivery compared to non-users (β: −8.3 days, 95% CI: −14.9, −1.6).51 In subsequent work using data from a smaller subset of the present study population, we observed that hair oil use in the month prior to a participant’s last prenatal study visit was associated with 125% higher mean urinary MEP concentrations (95% CI: −0.1, 408).29 Additionally, shampoo users had higher mean urinary monobutyl phthalate (MBP) and MiBP concentrations, and hair gel users had higher mean MiBP concentrations. Hair oil was also identified in one of our previous analyses as one of the two products to present hormonal activity on all four hormone assays conducted (estrogen, androgen, progesterone, and glucocorticoid).38 Furthermore, in a separate exposure assessment examining hair products commonly used by Black women from the Greater New York Hair Products Study,32 hair oil was observed to contain benzophenone, a potential EDC that also may impact perinatal health outcomes.46 In our present study, we observed lower mean sex-specific BW-for-GA Z-scores among participants who reported using hair products at different time points during pregnancy compared to non-users and suggestive associations with the use of other personal care product categories (i.e., shave cream, nail polish, liquid soap/body wash, and bar soap) and our outcomes of interest. The present study adds to our previous work by examining a broader suite of personal care product categories and evaluating associations with the additional birth outcomes of birth length and sex-specific BW-for-GA Z-scores, in addition to gestational age at delivery. Through these successive analyses, we have generated evidence for the complex narrative surrounding the role of EDC-associated personal care product use and pregnancy outcomes, where racial and ethnic inequities persist.
Among the other personal care product categories evaluated, suggestive, yet some imprecise, associations with birth outcomes were observed, including among shave cream, nail polish, and soap (liquid and bar) users versus non-users across pregnancy visits. Shave cream has previously been identified to contain EDCs or suspected EDCs, including multiple phthalates, bisphenol A (BPA), diethanolamine, glycol ethers, cyclosiloxane, and UV filters (such as benzophenone-3).52,53 Both bar soap and liquid soaps have also been observed to contain EDCs, notably, phthalates, BPA, and antimicrobials (triclocarban and triclosan).52,54 Furthermore, nail polish use has previously been positively associated with higher urinary MBP concentrations55 and reported to contain di(2-ethylhexyl)phthalate and BPA.52 Thus, personal care product use during pregnancy, where numerous products can contain a number of EDCs or suspected EDCs, may contribute to a variety of pregnancy outcomes, such as those evaluated in this analysis.
While there is limited research examining the effects of personal care product use during pregnancy on perinatal outcomes in the general population, adverse pregnancy outcomes (including PTB, LBW, and SGA) among individuals with occupational exposure to personal care product chemicals (cosmetologists, manicurists, and hair stylists) have been reported.56,57 For example, one retrospective study compared birth outcomes among cosmetologists in New York State (n= 15,003) to licensed realtors (n=4,246) and the general population (n=12,171).58 Compared to realtors, cosmetologists had increased odds of giving birth to a LBW infant (adjusted OR: 1.36, 95% CI: 1.09, 1.70). The observed associations were larger for non-White participants compared to White participants, which could indicate differences in treatments or products used by non-White participants (personally and/or professionally), other EDC exposure routes/sources, or increased vulnerability to the effects of personal care product-associated exposures due to concomitant risk factors. While these studies focused on occupational exposure, which likely represents greater and more continuous exposure to certain personal care products and associated EDCs, their findings may be applicable to the same or similar products used routinely by the general population.
4.2. Potential Mechanisms
We observed the strongest association between hair oil use at visit 1 and lower BW-for-GA Z-score, which may indicate that exposures during early pregnancy impact fetal growth. We hypothesize that this finding may be driven by underlying pregnancy physiology where the placenta is being formed during early pregnancy. Thus, the association between product use during early pregnancy and lower BW-for-GA Z-score could be attributed to chemical exposures during this period impacting the placental formation and resulting nutrient delivery.59 We also observed associations with use of other personal care products including nail polish use at any visit with birth length; however, the clinical relevance and pathophysiology of this mechanism are unclear. Future studies will need to replicate these findings and further explore potential mechanisms on the associations between nail polish use, among other personal care products identified in this study, and birth outcomes.
Furthermore, we hypothesize that exposure to phthalates and other EDCs contained in personal care products is the underlying pathway for the observed associations between the use of certain products during pregnancy and adverse birth outcomes including shorter gestational age and lower birth weight. However, while exposure to phthalates and other EDCs have been associated with shorter gestational age and lower birth weight in some studies, the exact biological mechanisms behind these associations are unclear. Studies suggest that phthalate exposure may alter inflammatory pathways60,61 and placental function.61,62 Previous research has identified inflammation as a potential contributor to preterm parturition63,64 and reported associations between inflammation and altered placental function with fetal growth restriction—resulting in lower birth weight.65,66 Additionally, phthalate exposure has been shown to alter placental essential fatty acid homeostasis,61,67,68 which is essential for proper fetal growth and development.69 Altered maternal and fetal fatty acid profiles have been associated with LBW and fetal growth restriction.70-73 These may represent potential mechanisms for the observed associations between exposure to some phthalates with shorter gestational age and lower birth weight. In addition to phthalates, other chemical classes of EDCs may impact perinatal health. For example, parabens are commonly used as preservatives in personal care products and may contribute to PTB risk by impacting normal placental development through their ability to alter progesterone and estrogen receptors.74,75 Additionally, personal care products often contain a combination of different chemical ingredients resulting in exposure to EDC mixtures with potential joint effects.
4.3. Limitations and Strengths
Our analysis has some limitations. First, while we asked about categories of personal care products used in the past month and 48 hours prior to study visits, we did not collect data on the frequency of use for the majority of products, specific product brands, the total number of products used within each category, or the ingredients of products. Therefore, we are unable to account for these factors that contribute to individual-level exposure to personal care product-associated EDCs. Second, we used self-reported data on personal care product use, and there may be misclassification due to poor recall or improper categorization of products. Next, this was a pilot study among a small subset of ERGO participants who were predominately non-Hispanic White with relatively low use of many of the queried hair products. Due to the sample size of our pilot study, we did not have sufficient numbers to perform an analysis of the change in product use across pregnancy in association with our outcomes. Additionally, based on our smaller sample size, we were unable to assess potential windows of susceptibility to personal care product-associated chemicals across prenatal study visits. Furthermore, based on our study population in Boston (predominately non-Hispanic White and English-speaking), our results may not be generalizable to other populations due to potential differences in patterns of product use based on sociodemographic, cultural, and geographic factors.76-78 Additionally, our study results reflect current day exposures to personal care products and may not reflect future exposures due to changes in the cosmetic composition of products. Previous research has reported notable differences in patterns of personal care products across racial and ethnic groups.76,78-80 As race and ethnicity are important sociocultural factors that can drive both product use and chemical exposures, the use of certain personal care products is often highly correlated with race and their individual effects can be difficult to separate. We were unable based on our relatively small sample size to adjust for race and ethnicity due to model convergence issues. Additionally, based on our sample size, we were unable to stratify our models by categories of race and ethnicity or to explore associations with birth outcomes such as PTB and SGA. However, results from our sensitivity analysis excluding non-Hispanic Black participants from our models were generally comparable with our main model estimates, indicating that the observed associations, particularly with hair product use, were not driven by the inclusion of these individuals. Future studies should consider the associations between personal care product use and personal care product-associated EDCs with gestational age at delivery, birth length, and BW-for-GA Z-scores among a larger, more diverse cohort to confirm these findings.
This analysis also has several notable strengths. To our knowledge, this is one of the few analyses to examine personal care product use on perinatal health outcomes. Previous research has mainly examined the association between certain urinary chemical metabolites (e.g., phthalates) with pregnancy outcomes. Examining personal care product use specifically allows for a solution-oriented approach for EDC exposure sources and perinatal health outcomes relevant to possible reduction strategies and clinical recommendations. Next, we used two validated questionnaires to examine personal care product use, which has been used in prior research demonstrating higher concentrations of personal care product-associated EDCs with the use of certain products and associations between product use and health endpoints.32-34 Additionally, our use of these validated questionnaires at four prenatal study visits allowed us to examine the associations between personal care product use and birth outcomes across pregnancy. Lastly, we included the use of leave-in maintenance hair products, which are most frequently used by non-Hispanic Black women, have documented hormonal activity,38 are associated with higher phthalate exposure,29 but are often classified broadly as “other hair products” in previous research.
4.4. Conclusions
In this pilot study, we found evidence that the use of hair products, particularly hair oil, during pregnancy may be associated with lower mean BW-for-GA Z-score. Associations for birth length and gestational age at delivery were also observed for certain product categories. Since personal care product use represents a modifiable risk factor for EDC exposure, these findings may inform future recommendations and interventions aiming to reduce adverse perinatal health outcomes, particularly those linked to racial and ethnic disparities in personal care product use and associated EDC exposures.
Supplementary Material
HIGHLIGHTS.
Personal care products (PCPs) often contain endocrine disrupting chemicals.
We estimated associations of product use at 4 visits in pregnancy with birth outcomes.
Lower birth weight-for-gestational age (BW-for-GA) was reported among hair product users.
The strongest association with lower BW-for-GA was observed for daily hair oil use.
Use of liquid soaps, shampoos, conditioners were associated with longer infant length.
Acknowledgements
The authors would like to thank the participants of the ERGO study, as well as Marissa Grenon, Francesca Yi, Autumn Hoyt, Rasha Baig, Jorja Kahn, Michaiah Parker, and Ayanna Coburn-Sanderson for their assistance with the ERGO Study.
Funding
This work was supported by the National Institutes of Health (R01ES026166, T32ES007069, P30ES000002), the March of Dimes (MOD Research Grant #6-FY19-367), and the National Science Foundation Graduate Research Fellowships Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement
MC: Writing – Original Draft; EVP: Writing – Original Draft, Formal Analysis, Visualization, Data curation VF: Writing – Original Draft, Formal Analysis; MRQ: Project administration, Writing – Review & Editing, Investigation, Data curation; MRH: Project administration, Writing – Review & Editing, Investigation; BJW: Project administration, Writing – Review & Editing, Investigation; KO: Project administration, Writing – Review & Editing; PLW: Writing – Review & Editing; RH: Conceptualization, Supervision, Writing – Review & Editing; TJT: Conceptualization, Methodology, Supervision, Project administration, Funding acquisition, Writing – Review & Editing; SM: Conceptualization, Methodology, Supervision, Project administration, Funding acquisition, Writing – Review & Editing
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Basso O, Wilcox AJ, Weinberg CR. Birth Weight and Mortality: Causality or Confounding? Am J Epidemiol. 2006;164(4):303–311. doi: 10.1093/aje/kwj237 [DOI] [PubMed] [Google Scholar]
- 2.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The Contribution of Preterm Birth to Infant Mortality Rates in the United States. Pediatrics. 2006;118(4):1566–1573. doi: 10.1542/peds.2006-0860 [DOI] [PubMed] [Google Scholar]
- 3.Wood-Bradley RJ, Henry SL, Vrselja A, Newman V, Armitage JA. Maternal dietary intake during pregnancy has longstanding consequences for the health of her offspring. Can J Physiol Pharmacol. 2013;91(6):412–420. doi: 10.1139/cjpp-2012-0352 [DOI] [PubMed] [Google Scholar]
- 4.Aylward GP. Neurodevelopmental Outcomes of Infants Born Prematurely. Journal of Developmental & Behavioral Pediatrics. 2014;35(6):394–407. doi: 10.1097/01.DBP.0000452240.39511.d4 [DOI] [PubMed] [Google Scholar]
- 5.Guerra CC, Barros MC de M, Goulart AL, Fernandes LV, Kopelman BI, Santos AM dos. Premature infants with birth weights of 1500–1999 g exhibit considerable delays in several developmental areas. Acta Paediatrica. 2014;103(1):e1–e6. doi: 10.1111/apa.12430 [DOI] [PubMed] [Google Scholar]
- 6.Street ME, Bernasconi S. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int J Mol Sci. 2020;21(4). doi: 10.3390/ijms21041430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crump C An overview of adult health outcomes after preterm birth. Early Human Development. 2020;150:105187. doi: 10.1016/j.earlhumdev.2020.105187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eide MG, Øyen N, Skjærven R, Nilsen ST, Bjerkedal T, Tell GS. Size at Birth and Gestational Age as Predictors of Adult Height and Weight. Epidemiology. 2005;16(2):175–181. doi: 10.1097/01.ede.0000152524.89074.bf [DOI] [PubMed] [Google Scholar]
- 9.Perkins JM, Subramanian SV, Davey Smith G, Özaltin E. Adult height, nutrition, and population health. Nutr Rev. 2016;74(3):149–165. doi: 10.1093/nutrit/nuv105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin AJ, Hamilton BE, Osterman MJK. Births in the United States, 2018. NCHS Data in Brief, No 346. National Center for Health Statistics; 2019. [PubMed] [Google Scholar]
- 11.Burris HH, Hacker MR. Birth outcome racial disparities: A result of intersecting social and environmental factors. Semin Perinatol. 2017;41(6):360–366. doi: 10.1053/j.semperi.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thoma ME, Drew LB, Hirai AH, Kim TY, Fenelon A, Shenassa ED. Black-White Disparities in Preterm Birth: Geographic, Social, and Health Determinants. Am J Prev Med. 2019;57(5):675–686. doi: 10.1016/j.amepre.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 13.Manuck TA. Racial and ethnic differences in preterm birth: A complex, multifactorial problem. Semin Perinatol. 2017;41(8):511–518. doi: 10.1053/j.semperi.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin J, Hamilton B, Osterman M, Driscoll A. Births: Final Data for 2018. 2019;68.https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_13-508.pdf [PubMed] [Google Scholar]
- 15.Ratnasiri AWG, Parry SS, Arief VN, et al. Recent trends, risk factors, and disparities in low birth weight in California, 2005–2014: a retrospective study. Maternal Health, Neonatology and Perinatology. 2018;4(1):15. doi: 10.1186/s40748-018-0084-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaaf JM, Liem SMS, Mol BWJ, Abu-Hanna A, Ravelli ACJ. Ethnic and racial disparities in the risk of preterm birth: a systematic review and meta-analysis. Am J Perinatol. 2013;30(6):433–450. doi: 10.1055/s-0032-1326988 [DOI] [PubMed] [Google Scholar]
- 17.Culhane JF, Goldenberg RL. Racial disparities in preterm birth. Semin Perinatol. 2011;35(4):234–239. doi: 10.1053/j.semperi.2011.02.020 [DOI] [PubMed] [Google Scholar]
- 18.Manuck TA. Racial and ethnic differences in preterm birth: A complex, multifactorial problem. Semin Perinatol. 2017;41(8):511–518. doi: 10.1053/j.semperi.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr Rev. 2009;30(4):293–342. doi: 10.1210/er.2009-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler W, Numtip W, Grote K, Csanády GA, Chahoud I, Filser JG. Blood burden of di(2-ethylhexyl)phthalate and its primary metabolite mono(2-ethylhexyl) phthalate in pregnant and nonpregnant rats and marmosets. Toxicol Appl Pharmacol. 2004;195(2):142–153. doi: 10.1016/j.taap.2003.11.014 [DOI] [PubMed] [Google Scholar]
- 21.Gomez E, Pillon A, Fenet H, et al. Estrogenic Activity of Cosmetic Components in Reporter Cell Lines: Parabens, UV Screens, and Musks. Journal of Toxicology and Environmental Health, Part A. 2005;68(4):239–251. doi: 10.1080/15287390590895054 [DOI] [PubMed] [Google Scholar]
- 22.Chan M, Mita C, Bellavia A, Parker M, James-Todd T. Racial/Ethnic Disparities in Pregnancy and Prenatal Exposure to Endocrine-Disrupting Chemicals Commonly Used in Personal Care Products. Curr Environ Health Rep. 2021;8(2):98–112. doi: 10.1007/s40572-021-00317-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.James-Todd TM, Chiu YH, Zota AR. Racial/ethnic disparities in environmental endocrine disrupting chemicals and women’s reproductive health outcomes: epidemiological examples across the life course. Curr Epidemiol Rep. 2016;3(2):161–180. doi: 10.1007/s40471-016-0073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen VK, Kahana A, Heidt J, et al. A comprehensive analysis of racial disparities in chemical biomarker concentrations in United States women, 1999-2014. bioRxiv. Published online August 28, 2019:746867. doi: 10.1101/746867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson KK, Rosen EM, Rosario Z, et al. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environment International. 2019;132:105099. doi: 10.1016/j.envint.2019.105099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smarr MM, Grantz KL, Sundaram R, Maisog JM, Kannan K, Louis GMB. Parental urinary biomarkers of preconception exposure to bisphenol A and phthalates in relation to birth outcomes. Environ Health. 2015;14. doi: 10.1186/s12940-015-0060-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. The Lancet Diabetes & Endocrinology. 2020;8(8):703–718. doi: 10.1016/S2213-8587(20)30129-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Preston EV, Fruh V, Quinn MR, et al. Endocrine disrupting chemical-associated hair product use during pregnancy and gestational age at delivery: a pilot study. Environmental Health. 2021;20(1):86. doi: 10.1186/s12940-021-00772-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fruh V, Preston EV, Quinn MR, et al. Urinary phthalate metabolite concentrations and personal care product use during pregnancy – Results of a pilot study. Science of The Total Environment. 2022;835:155439. doi: 10.1016/j.scitotenv.2022.155439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellavia A, Zota AR, Valeri L, James-Todd T. Multiple mediators approach to study environmental chemicals as determinants of health disparities. Environmental Epidemiology. 2018;2(2):e015. doi: 10.1097/EE9.0000000000000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edelson PK, James KE, Leong A, et al. Longitudinal Changes in the Relationship Between Hemoglobin A1c and Glucose Tolerance Across Pregnancy and Postpartum. The Journal of Clinical Endocrinology & Metabolism. 2020;105(5):e1999–e2007. doi: 10.1210/clinem/dgaa053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James-Todd T, Terry MB, Rich-Edwards J, Deierlein A, Senie R. Childhood Hair Product Use and Earlier Age at Menarche in a Racially Diverse Study Population: A Pilot Study. Ann Epidemiol. 2011;21(6):461–465. doi: 10.1016/j.annepidem.2011.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James-Todd T, Senie R, Terry MB. Racial/ethnic differences in hormonally-active hair product use: a plausible risk factor for health disparities. J Immigr Minor Health. 2012;14(3):506–511. doi: 10.1007/s10903-011-9482-5 [DOI] [PubMed] [Google Scholar]
- 34.Braun JM, Just AC, Williams PL, Smith KW, Calafat AM, Hauser R. Personal care product use and urinary phthalate metabolite and paraben concentrations during pregnancy among women from a fertility clinic. J Expo Sci Environ Epidemiol. 2014;24(5):459–466. doi: 10.1038/jes.2013.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aris IM, Kleinman KP, Belfort MB, Kaimal A, Oken E. A 2017 US Reference for Singleton Birth Weight Percentiles Using Obstetric Estimates of Gestation. Pediatrics. 2019;144(1):e20190076. doi: 10.1542/peds.2019-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann N Y Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x [DOI] [PubMed] [Google Scholar]
- 37.Howe CG, Claus Henn B, Eckel SP, et al. Prenatal Metal Mixtures and Birth Weight for Gestational Age in a Predominately Lower-Income Hispanic Pregnancy Cohort in Los Angeles. Environ Health Perspect. 2020;128(11):117001. doi: 10.1289/EHP7201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.James-Todd T, Connolly L, Preston EV, et al. Hormonal activity in commonly used Black hair care products: evaluating hormone disruption as a plausible contribution to health disparities. J Expo Sci Environ Epidemiol. 2021;31(3):476–486. doi: 10.1038/s41370-021-00335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, Zheng J, Wang H, et al. Maternal cosmetics use during pregnancy and risks of adverse outcomes: a prospective cohort study. Sci Rep. 2019;9(1):8030. doi: 10.1038/s41598-019-44546-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blackmore-Prince C, Harlow SD, Gargiullo P, Lee MA, Savitz DA. Chemical hair treatments and adverse pregnancy outcome among Black women in central North Carolina. Am J Epidemiol. 1999;149(8):712–716. doi: 10.1093/oxfordjournals.aje.a009879 [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg L, Wise LA, Palmer JR. Hair-relaxer use and risk of preterm birth among African-American women. Ethn Dis. 2005;15(4):768–772. [PubMed] [Google Scholar]
- 42.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ Health Perspect. 2014;122(3):235–241. doi: 10.1289/ehp.1306681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim K, Shin HM, Busgang SA, et al. Temporal Trends of Phenol, Paraben, and Triclocarban Exposure in California Pregnant Women during 2007-2014. Environ Sci Technol. 2021;55(16):11155–11165. doi: 10.1021/acs.est.1c01564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mintel. Hair relaxer sales decline 26% over the past five years. Mintel. Published 2013. Accessed January 16, 2022. https://www.mintel.com/press-centre/beauty-and-personal-care/hairstyle-trends-hair-relaxer-sales-decline [Google Scholar]
- 45.Rodríguez-Carmona Y, Ashrap P, Calafat AM, et al. Determinants and characterization of exposure to phthalates, DEHTP and DINCH among pregnant women in the PROTECT birth cohort in Puerto Rico. J Expo Sci Environ Epidemiol. 2020;30(1):56–69. doi: 10.1038/s41370-019-0168-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Helm JS, Nishioka M, Brody JG, Rudel RA, Dodson RE. Measurement of endocrine disrupting and asthma-associated chemicals in hair products used by Black women. Environmental Research. 2018;165:448–458. doi: 10.1016/j.envres.2018.03.030 [DOI] [PubMed] [Google Scholar]
- 47.Gaston SA, James-Todd T, Riley NM, et al. Hair Maintenance and Chemical Hair Product Usage as Barriers to Physical Activity in Childhood and Adulthood among African American Women. Int J Environ Res Public Health. 2020;17(24):9254. doi: 10.3390/ijerph17249254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo Y, Kannan K. A Survey of Phthalates and Parabens in Personal Care Products from the United States and Its Implications for Human Exposure. Environ Sci Technol. 2013;47(24):14442–14449. doi: 10.1021/es4042034 [DOI] [PubMed] [Google Scholar]
- 49.Hsieh CJ, Chang YH, Hu A, et al. Personal care products use and phthalate exposure levels among pregnant women. Science of The Total Environment. 2019;648:135–143. doi: 10.1016/j.scitotenv.2018.08.149 [DOI] [PubMed] [Google Scholar]
- 50.Lu S, Yu Y, Ren L, Zhang X, Liu G, Yu Y. Estimation of intake and uptake of bisphenols and triclosan from personal care products by dermal contact. Science of The Total Environment. 2018,’621:1389–1396. doi: 10.1016/j.scitotenv.2017.10.088 [DOI] [PubMed] [Google Scholar]
- 51.Preston EV, Fruh V, Quinn MR, et al. Endocrine disrupting chemical-associated hair product use during pregnancy and gestational age at delivery: a pilot study. Environ Health. 2021;20(1):86. doi: 10.1186/s12940-021-00772-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect. 2012;120(7):935–943. doi: 10.1289/ehp.1104052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Philippat C, Bennett D, Calafat AM, Picciotto IH. Exposure to select phthalates and phenols through use of personal care products among Californian adults and their children. Environmental Research. 2015;140:369–376. doi: 10.1016/j.envres.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Perencevich EN, Wong MT, Harris AD. National and regional assessment of the antibacterial soap market: A step toward determining the impact of prevalent antibacterial soaps. American Journal of Infection Control. 2001;29(5):281–283. doi: 10.1067/mic.2001.115469 [DOI] [PubMed] [Google Scholar]
- 55.Buckley JP, Palmieri RT, Matuszewski JM, et al. Consumer product exposures associated with urinary phthalate levels in pregnant women. Journal of Exposure Science & Environmental Epidemiology. 2012;22(5):468–475. doi: 10.1038/jes.2012.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quach T, Von Behren J, Goldberg D, Layefsky M, Reynolds P. Adverse birth outcomes and maternal complications in licensed cosmetologists and manicurists in California. Int Arch Occup Environ Health. 2015;88(7):823–833. doi: 10.1007/s00420-014-1011-0 [DOI] [PubMed] [Google Scholar]
- 57.Halliday-Bell JA, Gissler M, Jaakkola JJK. Work as a hairdresser and cosmetologist and adverse pregnancy outcomes. Occupational Medicine. 2009;59(3):180–184. doi: 10.1093/occmed/kqp017 [DOI] [PubMed] [Google Scholar]
- 58.Herdt-Losavio ML, Lin S, Druschel CM, Hwang SA, Mauer MP, Carlson GA. The Risk of Having a Low Birth Weight or Preterm Infant among Cosmetologists in New York State. Matern Child Health J. 2009;13(1):90–97. doi: 10.1007/s10995-008-0324-6 [DOI] [PubMed] [Google Scholar]
- 59.van den Dries MA, Keil AP, Tiemeier H, et al. Prenatal Exposure to Nonpersistent Chemical Mixtures and Fetal Growth: A Population-Based Study. Environ Health Perspect. 2021;129(11):117008. doi: 10.1289/EHP9178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferguson KK, Loch-Caruso R, Meeker JD. Exploration of oxidative stress and inflammatory markers in relation to urinary phthalate metabolites: NHANES 1999-2006. Environ Sci Technol. 2012;46(1):477–485. doi: 10.1021/es202340b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Latini G, Del Vecchio A, Massaro M, Verrotti A, DE Felice C. In utero exposure to phthalates and fetal development. Curr Med Chem. 2006;13(21):2527–2534. doi: 10.2174/092986706778201666 [DOI] [PubMed] [Google Scholar]
- 62.Xu Y, Agrawal S, Cook TJ, Knipp GT. Maternal di-(2-ethylhexyl)-phthalate exposure influences essential fatty acid homeostasis in rat placenta. Placenta. 2008;29(11):962–969. doi: 10.1016/j.placenta.2008.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bastek JA, Gómez LM, Elovitz MA. The role of inflammation and infection in preterm birth. Clin Perinatol. 2011;38(3):385–406. doi: 10.1016/j.clp.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 64.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168(9):980–989. doi: 10.1093/aje/kwn202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kamai EM, McElrath TF, Ferguson KK. Fetal growth in environmental epidemiology: mechanisms, limitations, and a review of associations with biomarkers of non-persistent chemical exposures during pregnancy. Environ Health. 2019;18:43. doi: 10.1186/s12940-019-0480-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sundrani DP, Karkhanis AR, Joshi SR. Peroxisome Proliferator-Activated Receptors (PPAR), fatty acids and microRNAs: Implications in women delivering low birth weight babies. Syst Biol Reprod Med. 2021;67(1):24–41. doi: 10.1080/19396368.2020.1858994 [DOI] [PubMed] [Google Scholar]
- 67.Xu Y, Cook TJ, Knipp GT. Effects of di-(2-ethylhexyl)-phthalate (DEHP) and its metabolites on fatty acid homeostasis regulating proteins in rat placental HRP-1 trophoblast cells. Toxicol Sci. 2005;84(2):287–300. doi: 10.1093/toxsci/kfi083 [DOI] [PubMed] [Google Scholar]
- 68.Xu Y, Knipp GT, Cook TJ. Effects of di-(2-ethylhexyl)-phthalate and its metabolites on the lipid profiling in rat HRP-1 trophoblast cells. Arch Toxicol. 2006;80(5):293–298. doi: 10.1007/s00204-005-0047-z [DOI] [PubMed] [Google Scholar]
- 69.Bobiński R, Mikulska M. The ins and outs of maternal-fetal fatty acid metabolism. Acta Biochim Pol. 2015;62(3):499–507. doi: 10.18388/abp.2015_1067 [DOI] [PubMed] [Google Scholar]
- 70.Gómez-Vilarrubla A, Mas-Parés B, Díaz M, et al. Fatty acids in the placenta of appropiate- versus small-for-gestational-age infants at term birth. Placenta. 2021;109:4–10. doi: 10.1016/j.placenta.2021.04.009 [DOI] [PubMed] [Google Scholar]
- 71.Alvino G, Cozzi V, Radaelli T, Ortega H, Herrera E, Cetin I. Maternal and fetal fatty acid profile in normal and intrauterine growth restriction pregnancies with and without preeclampsia. Pediatr Res. 2008;64(6):615–620. doi: 10.1203/PDR.0b013e31818702a2 [DOI] [PubMed] [Google Scholar]
- 72.Bobiński R, Mikulska M, Mojska H, Simon M. Comparison of the fatty acid composition of maternal blood and cord blood of mothers who delivered healthy full-term babies, preterm babies, and full-term small for gestational age infants. J Matern Fetal Neonatal Med. 2013;26(1):96–102. doi: 10.3109/14767058.2012.722717 [DOI] [PubMed] [Google Scholar]
- 73.Vilbergsson G, Samsioe G, Wennergren M, Karlsson K. Essential fatty acids in pregnancies complicated by intrauterine growth retardation. Int J Gynaecol Obstet. 1991;36(4):277–286. doi: 10.1016/0020-7292(91)90481-j [DOI] [PubMed] [Google Scholar]
- 74.Błędzka D, Gromadzińska J, Wąsowicz W. Parabens. From environmental studies to human health. Environ Int. 2014;67:27–42. doi: 10.1016/j.envint.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 75.Kiyama R, Wada-Kiyama Y. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ Int. 2015;83:11–40. doi: 10.1016/j.envint.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 76.Preston EV, Chan M, Nozhenko K, et al. Socioeconomic and racial/ethnic differences in use of endocrine-disrupting chemical-associated personal care product categories among pregnant women. Environmental Research. 2021;198:111212. doi: 10.1016/j.envres.2021.111212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang VA, Chu MT, Chie L, et al. Acculturation and endocrine disrupting chemical-associated personal care product use among US-based foreign-born Chinese women of reproductive age. Journal of Exposure Science & Environmental Epidemiology. Published online November 24, 2020:1–9. doi: 10.1038/s41370-020-00279-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dodson RE, Cardona B, Zota AR, Robinson Flint J, Navarro S, Shamasunder B. Personal care product use among diverse women in California: Taking Stock Study. J Expo Sci Environ Epidemiol. 2021;31(3):487–502. doi: 10.1038/s41370-021-00327-3 [DOI] [PubMed] [Google Scholar]
- 79.Wu XM, Bennett DH, Ritz B, Cassady DL, Lee K, Hertz-Picciotto I. Usage pattern of personal care products in California households. Food Chem Toxicol. 2010;48(11):3109–3119. doi: 10.1016/j.fct.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 80.Branch F, Woodruff TJ, Mitro SD, Zota AR. Vaginal douching and racial/ethnic disparities in phthalates exposures among reproductive-aged women: National Health and Nutrition Examination Survey 2001–2004. Environ Health. 2015;14. doi: 10.1186/s12940-015-0043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.