Abstract
Vibrio vulnificus is an opportunistic pathogen that contaminates oysters harvested from the Gulf of Mexico. In humans with compromising conditions, especially excess levels of iron in plasma and tissues, consumption of contaminated seafood or exposure of wounds to contaminated water can lead to systemic infection and disfiguring skin infection with extremely high mortality. V. vulnificus-associated diseases are noted for the rapid replication of the bacteria in host tissues, with extensive tissue damage. In this study we examined the virulence attributes of three virulent clinical strains and three attenuated oyster or seawater isolates in mouse models of systemic disease. All six V. vulnificus strains caused identical skin lesions in subcutaneously (s.c.) inoculated iron dextran-treated mice in terms of numbers of recovered CFU and histopathology; however, the inocula required for identical frequency and magnitude of infection were at least 350-fold higher for the environmental strains. At lethal doses, all strains caused s.c. skin lesions with extensive edema, necrosis of proximate host cells, vasodilation, and as many as 108 CFU/g, especially in perivascular regions. These data suggest that the differences between these clinical and environmental strains may be related to growth in the host or susceptibility to host defenses. In non-iron dextran-treated mice, strains required 105-fold-higher inocula to cause an identical disease process as with iron dextran treatment. These results demonstrate that s.c. inoculation of iron dextran-treated mice is a useful model for studying systemic disease caused by V. vulnificus.
Vibrio vulnificus is a gram-negative, halophilic, marine bacterium that is the most common cause of seafood-related deaths in the United States (17, 18). Infection by V. vulnificus occurs through the consumption of raw oysters or contamination of wounds. Individuals with high levels of circulating iron, for example, people suffering from hemochromatosis or cirrhosis, are at highest risk for developing disease after contact with V. vulnificus (7, 9, 26, 50). V. vulnificus causes a rapid and severe disease process resulting in extensive tissue damage. In fact, death can occur within 24 h after contact with the bacterium. Typically, individuals infected by V. vulnificus exhibit fever, chills, hypotension, and characteristic bullous skin lesions, which are rapidly progressive. The rapid nature of the disease process makes intervention difficult, yielding mortality rates of greater than 50% in septicemic individuals (17). Using pulsed-field gel electrophoresis, more than 100 strains of V. vulnificus have been identified in individual oysters (8). However, not all strains of V. vulnificus have equal ability to cause disease. Single strains of V. vulnificus were recovered from the blood of septicemic individuals who consumed oysters contaminated with numerous strains of V. vulnificus (19).
Several putative virulence factors have been proposed for V. vulnificus, including metalloprotease, hemolysin-cytolysin, polysaccharide capsules, and mechanisms for iron acquisition (28, 45). However, only the polysaccharide capsule and the siderophore produced by V. vulnificus have been confirmed as virulence factors (29, 54). The polysaccharide capsule mediates resistance of bacteria to complement-mediated bacteriolysis, as well as resistance to phagocytosis (1, 42, 47, 48, 56). Capsule production by V. vulnificus is associated with an opaque-to- translucent colony variation in which opaque colonies are encapsulated and translucent colonies have little or no capsule production. Therefore, all virulent strains are opaque, but all opaque strains are not necessarily virulent. V. vulnificus also produces a type IV leader peptidase-N-methyltransferase that is involved in the formation of adherence pili and type II extracellular protein secretion and is essential for virulence (36). However, the mutation of the type IV leader peptidase-N-methyltransferase causes pleiotropic effects which can attenuate virulence, so the precise attenuating event for the type IV leader peptidase mutant is unknown. In contrast, the hemolysin and metalloprotease are not necessary for virulence in animal models (21, 41, 51) although injection of either of these purified components can reproduce many aspects of pathogenesis of infection (12, 24, 31, 37). The exact role of these latter two factors as virulence determinants is therefore not clear.
Although several in vivo studies examining the pathogenic properties of V. vulnificus have been completed, most have either focused on the injection of purified toxins (12, 14, 24, 25, 30–33, 37) or determination of the 50% lethal dose (LD50) (2, 6, 19, 22, 43, 44, 53, 54, 57). However, some detailed analyses of pathogenesis have been done using subcutaneous (s.c.) or intradermal inoculation of animals (5, 13, 51, 56). Since the major damage and pathology in both sepsis and wound infection occur in skin tissues, we used the s.c. route of inoculation in iron dextran-treated mice to examine virulence and show here that this model is useful for studying the pathophysiology of clinical and environmental strains of V. vulnificus.
MATERIALS AND METHODS
Bacterial strains and media.
We studied three clinical isolates of V. vulnificus (LL728, 2400112, and VV1009) from patients who died of sepsis following ingestion of contaminated oysters and three environmental V. vulnificus strains isolated from oysters (MLT403) or seawater (MLT365 and MLT367) (19, 46). These clinical and environmental strains were selected from a collection based on their relative virulence and attenuation, respectively. The results of these initial analyses of virulence in intraperitoneally (i.p.) inoculated iron dextran-treated mice are reported herein. V. vulnificus strains were grown in Luria-Bertani broth containing 0.85% (wt/vol) NaCl (LB-N) or on LB-N plates containing 1.5% (wt/vol) agar. Strains were stored at −70°C in LB-N with 35% (vol/vol) glycerol. For routine use in infection experiments, a static overnight starter culture of the bacteria was grown in culture tubes at room temperature. Before infection, the starter culture was diluted 1:10 into prewarmed LB-N and then shaken at 37°C until the optical density at 420 nm (OD420) reached approximately 0.8 (exponential growth phase). The bacteria were harvested by centrifugation at 13,800 × g for 10 min at room temperature and suspended in phosphate-buffered saline (PBS) containing 0.01% (wt/vol) gelatin (BSG) (10) to approximately 109 CFU/ml. Vibrios were diluted in BSG to an appropriate concentration for use. CFU per milliliter were measured by dilution and plating.
Infection of mice.
Unless noted otherwise, 7- to 10-week-old female ICR mice (Harlan Sprague-Dawley, Indianapolis, Ind.) housed under specific-pathogen-free conditions were used for all experiments. For experiments involving pretreatment of mice with iron dextran, mice were injected i.p. with 250 μg of iron dextran (Sigma Aldrich, St. Louis, Mo.) per g of body weight, usually 30 min before inoculation, but as early as 2 h preinoculation in initial experiments. Mice were euthanized by either carbon dioxide asphyxiation or cervical dislocation.
Initial i.p. LD50 experiments.
Fifty milliliters of brain heart infusion broth was inoculated with bacteria from a tryptic soy agar plate and shaken at 37°C for 4 to 6 h. Bacteria were harvested by centrifugation at 9,000 × g for 20 min at room temperature and washed twice with PBS and suspended in PBS to approximately 109 CFU/ml, as determined by OD420 analysis. The bacteria were diluted to yield 106 CFU per 0.5-ml inoculum volume in 10-fold dilutions and were injected i.p. into groups of five mice (conventionally housed ICR). Mice were pretreated with iron dextran at 2 h preinfection. Mice were observed for up to 48 h postinfection with recording of deaths or moribund animals. Mice were considered moribund and were euthanized when the rectal temperature measured with a temperature probe (model 15-078-2C; Fisher Scientific) dropped below 33°C. The LD50 was quantitated using the method of Reed and Muench (40).
s.c. inoculation.
Mice were s.c. injected in the right lower flank with bacteria suspended in 0.1 ml of BSG. Mice were sacrificed at between 15 and 20 h postinoculation.
Oral inoculation.
Oral inoculation of mice followed the procedure of Gulig and Curtiss (15) used for Salmonella enterica serovar Typhimurium. In brief, mice were deprived of food and water for at least 4 h before inoculation. Immediately before inoculation, mice were fed 50 μl of 10% (wt/vol) sodium bicarbonate, followed by 10 μl of the bacterial inoculum suspended in BSG. Food and water were returned at 30 min postinoculation. Mice were sacrificed at between 15 and 72 h postinoculation.
Use of Evan's blue dye to observe vascular leakage.
A volume of 0.1 ml of Evan's blue dye at a concentration of 10 mg/ml diluted in PBS was injected into the lateral tail vein 30 min to 1 h before sacrifice of mice for analysis of vascular leakage.
Quantitative analysis of bacteria in tissues.
Spleen, liver, and s.c. skin lesions were aseptically removed from mice, homogenized in 5 ml of BSG using glass tissue homogenizers, diluted, and plated. For orally inoculated mice, salivary glands and three rinsed, 1-cm-length sections of small intestine containing a Peyer's patch were homogenized, diluted, and plated. When a mouse died before it could be euthanized and examined, the presence of a typical skin lesion was confirmed, and the levels of tissue infection were estimated to be the same as the highest among surviving mice in the same group for statistical analysis. Mice with no observable infection were not included in calculating mean CFU recovered, but the proportion of infected mice at each inoculum was noted.
Histological analysis.
Specimens of lesions resulting at inoculation sites were collected immediately after sacrifice and fixed in buffered 10% (vol/vol) formalin, as previously described (16). Formalin-fixed tissues were embedded in paraffin and cut into 5-μm sections at the University of Florida Department of Pathology, Immunology, and Laboratory Medicine Diagnostic Referral Laboratory. Histological sections were routinely stained with hematoxylin-eosin and occasionally with Giemsa. To guard against bias in interpretation, initial examination by the pathologist (T.R.S.) for lesion characteristics and severity was conducted without knowledge of the experimental treatments.
RESULTS
Microbiological characterization of V. vulnificus strains.
The three clinical isolates of V. vulnificus from lethal cases of oyster-associated sepsis (LL728, 2400112, and VV1009) and the three environmental isolates (MLT365, MLT367, and MLT 403) all demonstrated opaque-colony phenotypes on LB-N agar, suggesting that they all were encapsulated. However, environmental strain MLT365 possessed approximately 50% translucent colonies. As discussed below, this characteristic was stable even with in vitro or mouse passaging of this particular strain. As a functional test of encapsulation, we examined the serum resistance of the strains to 95% (vol/vol) normal rat serum. All of the strains except clinical strain 2400112 were fully resistant to complement-mediated bacteriolysis by fresh rat serum. CFU of strain 2400112 dropped by 1,000-fold after a 2-h incubation in rat serum, and this serum sensitivity was abolished by heat treating the serum at 56°C. Furthermore, the other two virulent strains replicated in the serum by as much as sixfold over the 2-h incubation period.
All six V. vulnificus strains possessed hemolytic activity on horse, sheep, and rabbit blood agar plates, in decreasing order of sensitivity. Similarly, all six strains exhibited caseinolytic protease activity on LB-N agar containing 15% (vol/vol) skim milk. Therefore, all of the strains expressed hemolysin and protease, most likely the metalloprotease. The doubling times of the strains in LB-N were approximately 10 min.
i.p. LD50s of clinical and environmental isolates of V. vulnificus in iron dextran-treated mice.
Iron dextran-treated ICR mice were inoculated i.p. with 1 to 106 CFU of the three clinical V. vulnificus strains, LL728, VV1009, and 2400112, or the environmental isolates MLT365, MLT367, and MLT403. Mice with rectal temperatures below 33°C were considered moribund and were euthanized. The clinical-isolate-inoculated mice had rectal temperatures below 33°C within 6 h after infection, and most died or were moribund within 24 h, yielding LD50s below 10 CFU (Table 1). In contrast, mice that succumbed to infection with the environmental isolates died or were moribund mostly at between 36 and 48 h postinfection, yielding i.p. LD50s of 103 to 105 CFU. Therefore, the three clinical isolates of V. vulnificus exhibited significantly higher virulence than the three environmental isolates by the i.p. route.
TABLE 1.
Quantitative infection after s.c. inoculation of iron dextran-treated mice
| V. vulnificus strain | i.p. LD50 (CFU)a | CFU of inoculumb | Infection inc:
|
||
|---|---|---|---|---|---|
| Skin | Spleen | Liver | |||
| LL728 | 4 | 220 (10/10) | 7.1 ± 1.3 | <3.6 ± 1.2e | <3.9 ± 0.9e |
| 2400112 | 1 | 300 (5/5)d | 7.8 ± 0.29 | 5.8 ± 0.66 | 6.9 ± 0.46 |
| VV1009 | 1 | 191 (5/5) | 8.1 ± 0.35 | 2.8 ± 1.2 | 4.3 ± 1.5 |
| MLT403 | 2.1 × 105 | 2.8 × 105 (5/5) | 7.9 ± 0.38 | <2.1 ± 0.55e | <2.8 ± 0.78e |
| 9.9 × 104 (3/5) | 8.2 ± 0.26 | ND | 4.1 ± 1.1 | ||
| 9.9 × 103 (4/5) | 7.7 ± 1.5 | ND | 3.0 ± 0.76 | ||
| 9.9 × 102 (1/5) | 5.5 | ND | <2.4e | ||
| MLT367 | 4.3 × 104 | 2.6 × 105 (5/5) | 7.6 ± 0.32 | 3.7 ± 0.60 | 5.2 ± 0.36 |
| 4.1 × 104 (3/5) | 8.4 ± 0.30 | ND | 5.5 ± 1.3 | ||
| 4.1 × 103 (2/6) | 4.4 ± 1.8 | ND | <2.4e | ||
| 4.1 × 103 (1/6) | 1.2 × 107 | ND | <2.3e | ||
| MLT365 | 2.6 × 103 | 1 × 105 (5/5)d | 8.2 ± 0.37 | 4.2 ± 0.72 | 5.2 ± 0.48 |
| 7 × 104 (5/5) | 7.9 ± 0.46 | ND | 5.2 ± 0.34 | ||
| 7 × 103 (2/5) | 6.4 ± 2.0 | ND | 5.4 ± 3.8 | ||
| 7 × 102 (2/5) | 4.9 ± 2.6 | ND | <2.4 ± 0.04e | ||
LD50 experiments were done using i.p. inoculation of groups of five mice.
Values in parentheses are numbers of detectably infected mice/numbers inoculated.
Values are means ± standard deviations for CFU recovered from homogenized tissues of mice with visible signs of infection, and values are reported in the following units: for skin and liver, in log10 CFU per gram, and for spleen, in log10 CFU total. ND, not done.
Inoculum based on OD420 of cells injected. Based on plate count, CFU could have been as much as 10-fold lower.
Minimum detectable level was used for tissues without recovered CFU, even though mice were visibly infected.
Attempts at developing oral inoculation of iron dextran-treated mice.
Since most infections of humans with V. vulnificus follow consumption of raw oysters, we attempted to develop an iron dextran-treated mouse model with oral inoculation using procedures developed for S. enterica serovar Typhimurium in mice (15). An inoculum of 108 CFU of V. vulnificus clinical isolate LL728 killed all of the mice within 18 h. Some of the mice had swollen heads caused by inflammation and infection of the salivary glands with approximately 107 CFU of vibrios/g. Gross analysis of the intestines was unremarkable, and culture of intestinal homogenates revealed very few if any recoverable V. vulnificus cells (data not shown). None of the other five V. vulnificus strains consistently caused disease even when inoculated at over 108 CFU. Therefore, we did not further pursue this model.
Virulence of V. vulnificus strains after s.c. inoculation of iron dextran-treated mice.
As an intermediate to i.p. inoculation, which caused death too rapidly, and oral inoculation, which was not sensitive enough, we s.c. inoculated iron dextran-treated mice. Although complete LD50 experiments were not performed, the three clinical isolates caused lethal infection within 20 h postinfection in iron dextran-treated mice at doses of 102 CFU. Symptoms included scruffy fur, lethargy, unilateral hind limb paralysis on the side of the bacterial injection, and occasional hemorrhagic conjunctivitis. Rectal temperatures routinely dropped below 33°C from 15 to 20 h postinfection. Upon necropsy of infected mice at between 15 and 20 h postinoculation, a large s.c. lesion was observed surrounding the site of injection that suggested extensive edema, vasodilation, hemorrhage, and necrosis (Fig. 1B). The lesion material was rust colored; however, no frank blood was visible. The lesions notably lacked pus; however, inflammation of the lymphatics and regional lymph node was observed (Fig. 1B, arrow). The vasculature adjacent to the lesion was dilated.
FIG. 1.
Dorsal view of gross pathology of s.c. lesions caused by infection with clinical and environmental isolates of V. vulnificus. Mice were injected i.p. with iron dextran. Immediately afterwards, mice were injected s.c. into the lower right dorsal quadrant with 102 CFU of virulent clinical isolate V. vulnificus LL728 (B) or 105 CFU of environmental strain MLT403 (C). Control mice (A) received no further injections after iron dextran. Between 15 and 24 h later, mice were euthanized, and the skin was peeled back from head to tail to reveal s.c. tissues. In panels B and C, a large, edematous, hemorrhagic lesion is visible in the area surrounding the injection site. The regional lymph node is inflamed (white arrows), and the localized vasculature is dilated. Some lesion material is adherent to the underlying musculature over the lower back as well.
Upon quantitative culture of the s.c. lesion material, very high numbers (107 to 108 CFU/g of tissue) of vibrios were recovered with the three clinical strains (Table 1). Mean recoveries of vibrios from deep tissues such as the spleen and liver were lower and more variable, with 102.8 to as high as 105.8 CFU recovered per spleen and less than 103.9 to as high as 106.9 CFU/g recovered from livers (Table 1).
In contrast, the environmental strains required from 58- to 500-fold-higher CFU levels in inocula to cause similar levels of infection in a similar time course after s.c. inoculation of iron-dextran-treated mice (Table 1). However, with inocula containing less than 105 CFU for the environmental strains, detectable infection of mice was more sporadic. To be able to cause equivalent frequency and magnitude of infection, the environmental strains had to be inoculated at doses approximately 350- to 1500-fold higher than those of the clinical strains. However, all three environmental isolates resulted in a gross pathogenic process in a similar time frame of lethal disease (15 to 20 h) that was apparently identical to that of the clinical strains when inoculated s.c. at doses of 104 to 105 CFU (Fig. 1C and Table 1).
By comparing the total yield of bacteria recovered from mice with the inoculum over the infection period, we calculated that the doubling times of the clinical isolates in mouse tissues were 47 to 57 min. If significant killing of the bacteria by host defenses occurred in vivo, the doubling times could be shorter. In contrast, the calculated doubling times for the three environmental isolates, given their higher CFU levels in inocula, were calculated to be between 72 and 89 min, with the same consideration for possible killing by host defenses.
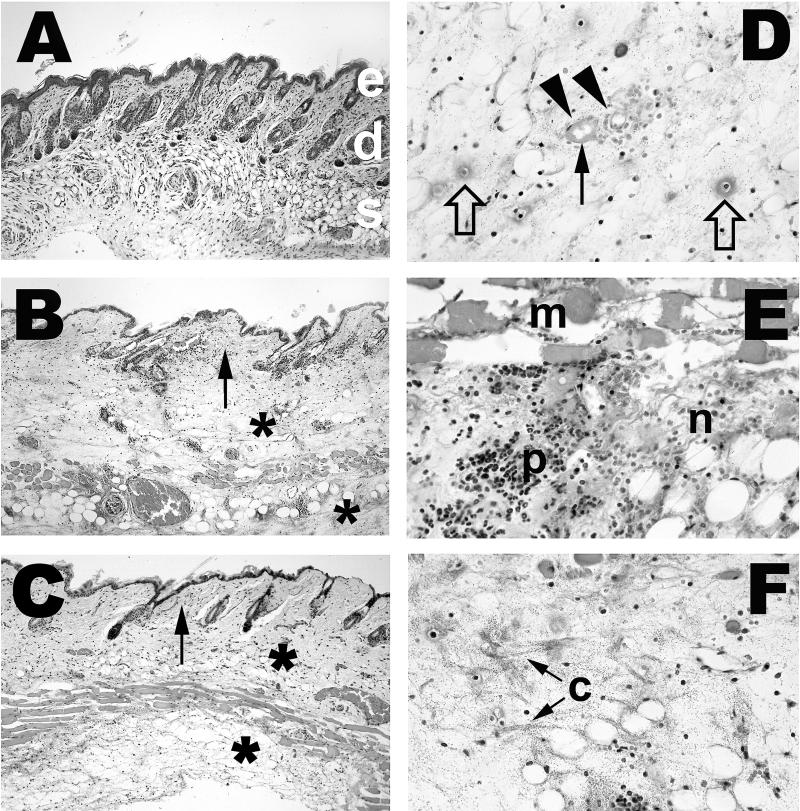
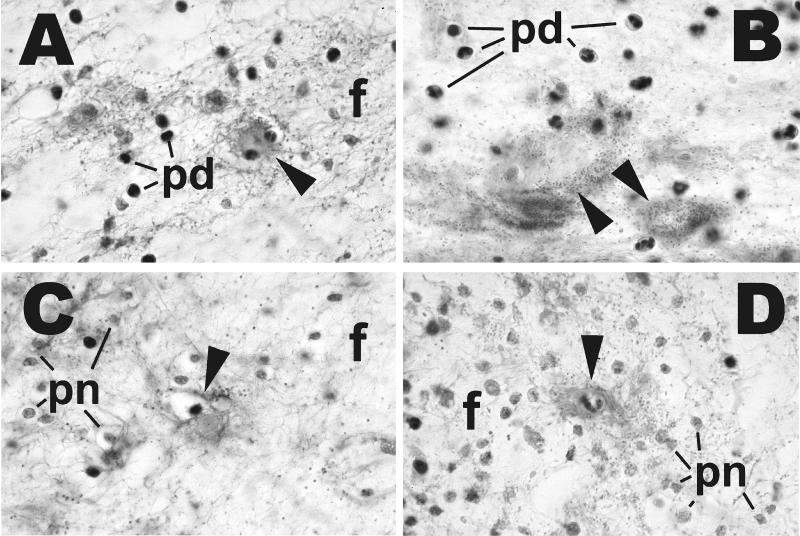
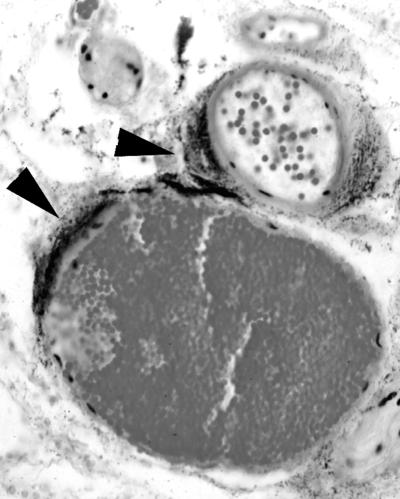
We examined the histology underlying the gross pathology of the s.c. lesions described above. As observed for gross pathology, the histopathologies were similar in general characteristics between the clinical and environmental isolates of V. vulnificus (Fig. 2 and 3). The subcutis was edematous, as evidenced by wide separation of collagen fibers (Fig. 2B, C, and F) and adipocytes, and mild deposition of fibrin was observed (Fig. 2D and 3A). In many cases, the overlying dermis also was affected (Fig. 2B and C). Intralesional bacteria having a coccoid to coccobacillary morphology were extremely numerous and readily visible (Fig. 2, 3, and 4). Small to moderate numbers of neutrophils were present; some were diffusely scattered throughout the edematous areas, but most appeared in collections around small blood vessels. Except at the margins of lesions in a few mice, there was extensive degeneration and necrosis of neutrophils, as indicated by cells with shrunken, condensed nuclei and amorphous eosinophilic cytoplasm or by faintly stained, amorphous remnants (Fig. 2D, E, and F). Other cells within affected areas, including adipocytes, fibroblasts, histiocytes, mast cells, and mammary gland epithelium, also were necrotic, as evidenced by nuclear pyknosis and loss of cytoplasmic staining and structural detail. Vascular lesions were common in affected areas and included neutrophil margination and emigration in venules; neutrophil-laden thrombi in both arterioles and venules; and arteriolar, venular, and capillary necrosis, with formation of fibrin thrombi. Presence of bacteria within the s.c. muscle layer was accompanied by acute myofiber degeneration and necrosis, as evidenced by swelling, loss of striations, and sarcoplasmic fragmentation (Fig. 2E). A typical characteristic of severe lesions was the upward extension into the overlying skin characterized by edema and by acute necrosis of fibroblasts and epithelial cells of hair follicles and sebaceous glands (Fig. 2B and C, arrows). In less severe cases, the lesions appeared to be confined to the subcutis. In nearly all mice, the boundaries of the lesions were indistinct, and there was little evidence of formation of a margin of inflammatory cells. We noted extensive infection of the perivascular region caused by all six strains (that with clinical strain LL728 is shown in Fig. 4).
FIG. 2.
Histopathology of infection with virulent clinical and attenuated environmental V. vulnificus strains. Mice were injected i.p. with iron dextran immediately before s.c. inoculation with 102 CFU of virulent clinical strains or 105 CFU of attenuated environmental strains. Tissues were collected from each mouse 14 to 20 h after inoculation, fixed in buffered formalin, embedded in paraffin, and cut into 5-μm sections. Sections were stained with hematoxylin and eosin. Magnifications, ×100 (A through C) and ×400 (D through F). (A) Normal mouse skin. Epidermis (e), dermis with hair follicles and sebaceous glands (d), and subcutis with adipocytes, blood vessels, and muscle layer (s) are shown. (B) Skin of mouse inoculated with 500 CFU of virulent clinical strain LL728. Shown is severe edema of the subcutis (∗), extending into dermis (arrow). (C) Skin of mouse inoculated with 105 CFU of attenuated environmental strain MLT403. Shown are edema and inflammatory cell accumulation in subcutis (∗), extending into dermis (arrow). (D) Subcutis of mouse inoculated with 500 CFU of virulent clinical strain 2400112. Shown is severe edema with numerous bacteria. Degenerated small vessels with adjacent necrotic neutrophils are indicated by arrowheads, and fibrin deposition or thrombosis is indicated by a closed arrow. Cellular debris and fibrin probably representing necrotic capillaries are indicated by open arrows. (E) Subcutis of mouse inoculated with 105 CFU of attenuated environmental strain MLT367. m, necrotic s.c. muscle; p, edematous subcutis with moderate numbers of neutrophils. Many neutrophils are necrotic (n). (F) Subcutis of mouse inoculated with 500 CFU of virulent clinical strain LL728. Shown are s.c. edema with numerous bacteria as in panel D. Severity of edema is indicated by wide separation of collagen fibers (c).
FIG. 3.
Effects of iron dextran on histopathology of V. vulnificus infection. Mice receiving iron dextran before infection were treated exactly as described in the legend to Fig. 2. Non-iron-dextran-treated mice were inoculated with 107 CFU of clinical strain LL728 or 108 CFU of environmental strain MLT403. Tissues were prepared as described for Fig. 2 and stained with hematoxylin and eosin. Magnification, ×1,000 (all panels). (A and B) Subcutis of mice inoculated with clinical strain LL728 with iron (A) and without iron (B). (C and D) Subcutis of mice inoculated with environmental strain MLT403 with iron (C) and without iron (D). There is edema with deposition of fibrin fibrils (f), degenerated but identifiable neutrophils (pd), and necrotic neutrophils represented by amorphous remnants (pn). In panels A, C, and D, there are necrotic capillaries accompanied by fibrin deposits (arrowheads). In panel B, the structures identified by arrowheads probably represent necrotic small vessels.
FIG. 4.
Perivascular infection by V. vulnificus. Iron-dextran-treated mice were injected s.c. with 102 CFU of clinical strain LL728. Mice were sacrificed 15 to 20 h later, and histopathology of s.c. lesions was examined as described in the legend for Fig. 2, except that this section was stained with Giemsa. Magnification, ×400. The arrowheads indicate the extensive accumulation of dark staining vibrios adjacent to the blood vessels.
When Evan's blue dye was injected intravenously (i.v.) before sacrifice of s.c. inoculated mice, we observed extensive vascular leakage in the region of the s.c. lesion, as well as accumulation of dye within the lesion itself, for both clinical and environmental isolates (data not shown). Additionally, there was leakage in the surrounding tissues accompanied by dilated blood vessels.
s.c. inoculation of non-iron-dextran-treated mice.
We considered the possibility that the differences in virulence between the clinical and environmental isolates were related to their acquisition of iron from the host, especially as related to the iron dextran treatment commonly employed to predispose mice to V. vulnificus infection. In contrast to lethal disease being caused by as few as 102 CFU of the clinical isolates within 18 to 20 h in s.c. inoculated, iron-dextran-treated mice, non-iron-dextran-treated mice required 106 to 107 CFU to cause lethal infection in the same time course (Table 2). A dose of 105 CFU of strain LL728 did not cause observable disease in non-iron-dextran-treated mice. With the larger inocula, the mortality and lesions caused by the clinical isolates in non-iron-dextran-treated mice were indistinguishable from those of the smaller inocula with iron dextran treatment, with the exception that the lesion material was less red (data not shown). The lighter color of lesions in non-iron-dextran-treated mice was most likely due to the lower availability of free iron in the vasculature. Quantitatively, the levels of CFU of clinical isolates recovered from lesions and tissues in non-iron-dextran-treated mice were similar to those seen with iron- dextran-treated mice inoculated with 104- to 105-fold fewer CFU (Table 2). The histopathology of s.c. damage by clinical strains in non-iron-dextran-treated mice was indistinguishable from that of iron-dextran-treated mice (Fig. 3A and B).
TABLE 2.
Quantitative infection after s.c. inoculation of non-iron-dextran-treated mice
| V. vulnificus strain | CFU of inoculuma | Infection inb:
|
||
|---|---|---|---|---|
| Skin | Spleen | Liver | ||
| LL728 | 1.9 × 106 | 7.6 ± 0.36 | 3.6 ± 1.8 | 4.6 ± 1.7 |
| 2400112 | 1.4 × 107 | 7.4 ± 0.33 | <3.8 ± 2.0d | <4.7 ± 2.1d |
| VV1009 | 1.4 × 106 | 7.8 ± 0.44 | 4.7 ± 1.4 | 6.2 ± 0.86 |
| MLT403 | 4.3 × 107 | 7.6 ± 0.29 | <2.8 ± 1.0d | <3.7 ± 1.4d |
| MLT367 | 1.5 × 108 | —c | — | — |
| MLT365 | 1.5 × 108 | — | — | — |
n = 5 for all groups except strain LL728, for which n = 4.
Values are means ± standard deviations for CFU recovered from homogenized tissues of mice with visible signs of infection, and values are reported in the following units: for skin and liver, in log10 CFU per gram, and for spleen, in log10 CFU total.
—, mice inoculated with MLT365 and MLT367 did not exhibit any symptoms and had no recoverable CFU from homogenized tissues.
Minimum detectable level was used for tissues without recovered CFU, even though mice were visibly infected.
Only one of the environmental isolates, MLT403, was able to cause disease in non-iron-dextran-treated mice (Table 2). As seen with the clinical strains, the gross pathology for MLT403 in non-iron-dextran-treated mice at the higher dose was similar to that in iron-dextran-treated mice at a lower dose (Fig. 3C and D). However, strains MLT365 and MLT367 failed to cause observable disease even when mice were observed for as long as 5 days after inoculation with as high as 1.5 × 108 CFU. Therefore, the differences in virulence between the clinical and environmental isolates of V. vulnificus were not due to the ability of the clinical strains to acquire and utilize the exogenously provided iron.
Mouse passaging of environmental isolates to enrich for virulence.
In light of reports that V. vulnificus can undergo phase variation in virulence (42, 43, 56), we considered the possibility that the relative attenuation of the three environmental isolates demonstrated above was due to the accumulation of mutants or phase variants during growth in vitro. Each of the three environmental strains was s.c. inoculated into two iron-dextran-treated mice at doses of 105 CFU, and the following day the livers and s.c. lesions were harvested, homogenized, plated, and used to inoculate static overnight cultures in LB-N. Typical levels of infection were observed. The next day, the overnight culture from the liver with the highest infection was used to inoculate a second group of iron-dextran-treated mice, except that doses of 102 and 105 CFU were administered. For strains MLT403 and MLT367, there were no observable differences in the virulence of the initial inoculum and the mouse-passaged inoculum, with disease occurring only in the mice that received 105 CFU. This result indicates that for strains MLT403 and MLT367, the attenuation is a stable element of the genotype. In contrast, for strain MLT365, inoculation with both 102 and 105 CFU of mouse-passaged bacteria resulted in death and disease in the mice in two of three separate mouse-passaging experiments. In one experiment, growing the passaged bacteria in vitro, freezing at −70°C, thawing, and growing again in vitro for inoculation into mice caused the reversion to the original low level of virulence. However, a second mouse-passaged culture retained a level of virulence consistent with that of clinical strains after passage in LB-N for 27 generations as well as being frozen and thawed. Total cellular protein profiles of the original MLT365 isolate and the freeze-thawed, mouse-passaged culture confirmed that the strain had not been contaminated with another virulent strain (data not shown). Therefore, passaging MLT365 once through mice could significantly increase the virulence of this strain. This result suggests that the attenuation of MLT365 was due to the accumulation in the stock culture of either phase variants or mutants that were reduced for virulence or that passaging MLT365 through mice resulted in the induction of virulence genes that enabled the inoculum to be of a higher virulence. We noticed that upon plating MLT365 on LB-N agar, there was visible heterogeneity among numerous colonies with opaque and translucent forms. However, this variation in colony morphology was observed whenever MLT365 was plated, even directly from animal tissues. The reason for the sporadic nature of increasing virulence of MLT365 by mouse passaging is not clear.
DISCUSSION
Although the numbers of diseased patients are low, infection caused by V. vulnificus is notable because of the rapidly fulminating and severe nature of the disease, which results in an extremely high mortality rate. Additionally, this opportunistic pathogen has a predilection for humans with a limited set of predisposing conditions, namely, high levels of iron and liver disease (7, 9, 26, 50). A hallmark of the two major clinical manifestations, postingestion sepsis and wound infection, is extensive tissue damage with high numbers of bacteria in tissues (4). The ability of this free-living marine bacterium to cause such devastating illness in what is presumed to be an unnatural, dead-end host is intriguing. The virulence mechanisms that enable the bacterium to replicate so rapidly in the human host and to cause such extensive damage are unknown, although much speculation has been made because of the plethora of secreted toxins and enzymes of V. vulnificus. Having characterized the genetic diversity and multitude of different strains of V. vulnificus that contaminate oysters (46), we set out to use the epidemiological tools of clinical and environmental strains to characterize virulence determinants of this pathogen.
Although contamination of oysters harvested from the Gulf of Mexico during the peak summer season with V. vulnificus can be extremely high (17), the rate of human disease is still very low, even among the susceptible populations (17, 18). We previously determined that in three cases of fatal sepsis following ingestion of contaminated oysters, the patients had single strains in their blood, even though the contaminated oysters contained as many as 18 different V. vulnificus strains. All of these data suggest that among the numerous strains of V. vulnificus contaminating oysters, only a subset has the potential to cause disease, even in predisposed humans. Most likely, the low rate of infection is based on a combination of virulence attributes of the vibrios as well as different states of predisposing conditions among the human population. However, it should be noted that wound infection commonly occurs in otherwise healthy people (17). To better understand the bacterial factors involved in the disease process and to contribute to developing a screen for highly virulent versus less virulent V. vulnificus strains contaminating seafood, we performed these studies using the widely used iron-treated mouse model (2, 6, 20, 22, 23, 29, 35, 36, 44, 51, 53, 54). In our previous study (19) and those of many others, iron-treated mice were injected i.p. with the bacteria. Although i.p. inoculation resulted in a measurable difference in virulence between clinical isolates and environmental isolates (19), we feel that the extremely rapid progression of the disease process and the extremely low LD50s of virulent strains (less than 10 CFU), make this model less than optimal for differentiating strains and elucidating the disease process. For example, by injecting the mice i.p., the bacteria do not have to cross as many tissue barriers or replicate within skin tissues to cause disease. As discussed below, we found that the s.c. route of inoculation was optimal for examining virulence.
To begin to dissect the differences between virulent clinical and possibly less virulent environmental strains of V. vulnificus, we chose three clinical isolates from our previous study (19) which had been shown to be virulent in mice and three seawater- or oyster-derived strains which were less virulent (19, 46). We confirmed by opaque-colony morphology that the strains were encapsulated; however, one of the seawater strains, MLT365, consisted of a heterogeneous population of opaque and translucent colonies, even when plated from single colonies. This result suggested that an encapsulated to unencapsulated phase variation was occurring, as had been reported for other strains by others (43, 52, 56). All six strains were hemolytic on sheep, horse, and rabbit blood agar plates. Similarly, all six strains possessed protease (caseinase) activity on skim milk-LB-N agar plates. To assay for susceptibility to host defenses, we examined the resistance to rat serum complement. All of the strains were resistant to rat complement except one of the clinical isolates, 2400112. How a human clinical isolate could be complement sensitive is puzzling. It is possible that the patient from which 2400112 was isolated was complement deficient; however, this fact will never be known because the patient died. It is possible that a complement-sensitive strain is virulent in mice because of the diminished complement activity known for mice in general. Others have reported that virulent V. vulnificus strains could be killed by normal human serum complement as much as 99% over a 1-h period (44). The summary of the initial in vitro characterization of the six strains was that the environmental strains were not defective for any known and easily assayed putative virulence attributes of V. vulnificus.
To inoculate mice with bacteria in a manner that required replication within host tissues and invasion through host tissues to cause systemic disease, we chose the s.c. route. We found that iron-dextran-treated ICR mice were very susceptible to relatively low doses of all three virulent clinical V. vulnificus strains. Of equal importance, s.c. inoculation yielded at least a 350-fold difference in the virulence potential of our clinical versus environmental V. vulnificus strains. Most importantly, the host-pathogen interaction and damage to s.c. inoculated mice mimics damage observed in humans suffering from either postingestion sepsis or wound infection caused by V. vulnificus (3, 4, 27). It should be noted that even though the two primary diseases caused by V. vulnificus in humans, septicemia after ingestion and wound infection, utilize different routes of entry, both infections are significantly manifested in the skin (4). We therefore feel that s.c. inoculation is a reasonable model for examining the pathogenesis of V. vulnificus disease in host tissues beyond the intestines for both wound infection and septicemia. At the gross level of pathology, large, edematous lesions were observed in the s.c. tissues with occasional evidence of hemorrhage but little pus. This is similar to the pathology observed on the extremities of human patients with V. vulnificus sepsis; however, as noted by Gray and Kreger (14), bullous lesions were not observed after s.c. inoculation of mice. The reason for the absence in mice of this most distinctive symptom of human disease is unknown. Gray and Kreger (14) speculated that it could represent a difference in anatomy between mice and humans; however, the elaboration of different virulence factors by the vibrios in the different hosts or the reaction of the hosts to common virulence factors could be involved. Bowdre et al. (5) estimated that edema in s.c. inoculated, non-iron-treated mice was approximately 1 ml per mouse. Although we did not perform similar quantitative studies, we estimate that the severe edematous lesions observed in our studies were of about the same size (see Fig. 1).
Our histopathologic findings were consistent with the gross appearance, clinical progression, and results of cultures of the experimental lesions in the mice, as well as with results of previous studies in experimental animals (5, 6, 14, 39, 56) and with findings in human cases (3, 27). The salient characteristics of the lesions were profound edema, presence of numerous bacteria, especially in perivascular regions, and necrosis of cells of all types within affected tissues. Furthermore, as noted above by the relative lack of pus in lesions, histological examination confirmed that, considering the enormous number of bacteria in tissues, there was a paucity of inflammatory cells. Similar observations have been made in human infections (3). These observations indicate an aggressive, rapidly progressing process in which the host defenses are unable to effectively limit the multiplication and spread of the vibrios. Inflammatory edema is an important defense against infection with most bacteria. The influx of fluid normally dilutes bacterial toxins and brings complement, transferrin, and immunoglobulins into the affected area, and fibrin deposition helps restrict bacterial spread. In the case of infection with V. vulnificus, however, tissue invasion appears to be facilitated by the resulting edema. Furthermore, the vibrios may promote fluid accumulation by direct vascular damage. As demonstrated in Fig. 2 and 3, V. vulnificus also appears to be able to kill responding neutrophils very effectively in vivo. Although there was some variation among mice in severity and extent of lesions, the pathologies were similar for both virulent clinical and attenuated environmental strains, whether or not the mice were pretreated with iron dextran, as long as sufficient numbers of organisms were injected. This result suggests that the major difference between these virulent clinical and attenuated environmental strains is the ability to either replicate in host tissues or resist the host defenses.
One aspect of our study which might appear to be at variance with results of others is the lack of virulence in all of our environmental V. vulnificus strains examined. Three earlier reports examining virulence of environmental versus clinical strains found that most environmental isolates were virulent in mice (22, 44, 49). However, since our aim was to compare the pathogenesis of highly virulent strains with that of less virulent strains, we chose our three environmental strains from a collection based on elevated LD50 as well as distinctive ribotype and pulsed-field gel electrophoresis profiles (46; see also http://www.foodsafety.ufl.edu/menu/vv2.htm). Since the most important predictor of virulence among V. vulnificus strains is the opaque- versus translucent-colony morphotype (43), we ensured that all of our strains were opaque. We noted that environmental isolate MLT365 consistently plated approximately 50% opaque versus translucent colonies, even after single-colony passaging in vitro and passage through mice. Therefore, the reason for attenuation of the environmental strains was not based on the opaque-translucent morphotype. In any case, our intention was not to determine the relative frequency of virulent environmental isolates but to examine the disease process in an animal model to elucidate mechanisms of virulence.
A major factor in human disease caused by V. vulnificus is the rapid, fulminating course of infection. Consistent with this clinical observation, we determined the net doubling time of clinical isolates in mice to be between 47 and 57 min. This value could be lower if the bacteria are undergoing appreciable killing by host defenses, which would require more rapid replication to cause the same net yield. This is an extremely rapid replication by a pathogen in host tissues. The calculated doubling times for the three environmental strains in iron-dextran-treated mice were between 72 and 89 min, still relatively rapid yet slower than those of the clinical strains. Our studies of splenic infection of mice by orally inoculated S. enterica serovar Typhimurium revealed a net in vivo doubling time of approximately 8.5 h (15). We also extrapolated data from published results of others and estimated the following doubling times for other bacterial pathogens in mouse tissues: for Listeria monocytogenes, 8 h in spleen, 3.5 h in liver, and 3.8 h in brain (11); for Streptococcus pneumoniae, 5.9 h in blood and 11 h in lung (55); and for Mycobacterium tuberculosis, 57 to 87 h in lung (34, 38). The mechanisms by which V. vulnificus replicates so rapidly in host tissues are unknown; however, they undoubtedly, contribute to the acute and fulminating nature of the disease in both humans and animals.
A major question arising from this observation is whether the difference in virulence between the clinical and environmental strains is completely attributable to the difference in in vivo replication rate or whether resistance to host defenses and the ability to cause damage to host tissues are also factors in the attenuation of the environmental strains. Of course, these factors may not be completely separate. We are currently pursuing the dissection of growth rates versus susceptibility to host defenses using both in vivo and in vitro assays. We doubt that the ability to cause tissue damage is a key differential virulence factor among our six strains, since all of the strains had the ability to cause histologically indistinguishable pathology; the inocula required to achieve the same pathology were different by at least 350-fold. Furthermore, as discussed above, all of the strains shared expression of hemolysin and metalloprotease activities. One striking observation in histopathology was the propensity for all of the V. vulnificus strains to cause infection of the perivascular regions (Fig. 4), which is also noted in human disease (3). We speculate that when the bacteria increase vascular permeability, most likely by the action of secreted enzymes or toxins, nutrient-rich plasma becomes more available for the bacteria within the surrounding tissues. The apparent perivascular infection might be the result of either proliferation in situ or the migration, by chemotactic means, of the bacteria to the perivascular region.
In summary, we used s.c. inoculated, iron-dextran-treated mice as a model to study the host-pathogen interaction of V. vulnificus disease of humans. s.c. inoculation demonstrates the necessity of predisposing conditions such as excess iron, commonly seen in human disease. The gross and histological pathologies are also very similar between mouse and human infections, and the s.c. model enables the differentiation of virulent clinical from more attenuated environmental strains of V. vulnificus. Although this model is not so simple as to serve as a screening assay for contamination of oysters with highly virulent versus less virulent V. vulnificus strains, the model will be useful in dissecting the disease process and hopefully will lead to the identification of critical virulence attributes that might be differentially present or expressed between virulent and attenuated V. vulnificus strains.
ACKNOWLEDGMENTS
This work was supported by USDA-NRICGP grant 9802757 to M.L.T. and P.A.G. and a Focused Giving Award to P.A.G., who was an American Heart Association Established Investigator with funds provided in part by the American Heart Association—Florida Affiliate. A.M.S. was supported by NIH training grant T32 AI07110.
REFERENCES
- 1.Amako K, Okada K, Miake S. Evidence for the presence of a capsule in Vibrio vulnificus. J Gen Microbiol. 1984;130:2741–2743. doi: 10.1099/00221287-130-10-2741. [DOI] [PubMed] [Google Scholar]
- 2.Amaro C, Biosca E G, Fouz B, Toranzo A E, Garay E. Role of iron, capsule, and toxins in the pathogenicity of Vibrio vulnificus biotype 2 for mice. Infect Immun. 1994;62:759–763. doi: 10.1128/iai.62.2.759-763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckman E N, Leonard G L, Castillo L E, Genre C F, Pankey G A. Histopathology of marine vibrio wound infections. Am J Clin Pathol. 1981;76:765–772. doi: 10.1093/ajcp/76.6.765. [DOI] [PubMed] [Google Scholar]
- 4.Blake P A, Merson M H, Weaver R E, Hollis D G, Heublein P C. Disease caused by a marine vibrio. Clinical characteristics and epidemiology. N Engl J Med. 1979;300:1–5. doi: 10.1056/NEJM197901043000101. [DOI] [PubMed] [Google Scholar]
- 5.Bowdre J H, Poole M D, Oliver J D. Edema and hemoconcentration in mice experimentally infected with Vibrio vulnificus. Infect Immun. 1981;32:1193–1199. doi: 10.1128/iai.32.3.1193-1199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennaman B, Soucy D, Howard R J. Effect of iron and liver injury on the pathogenesis of Vibrio vulnificus. J Surg Res. 1987;43:527–531. doi: 10.1016/0022-4804(87)90126-0. [DOI] [PubMed] [Google Scholar]
- 7.Brennt C E, Wright A C, Dutta S K, Morris J G., Jr Growth of Vibrio vulnificus in serum from alcoholics: association with high transferrin iron saturation. J Infect Dis. 1991;164:1030–1032. doi: 10.1093/infdis/164.5.1030. [DOI] [PubMed] [Google Scholar]
- 8.Buchrieser C, Gangar V V, Murphree R L, Tamplin M L, Kaspar C W. Multiple Vibrio vulnificus strains in oysters as demonstrated by clamped homogeneous electric field gel electrophoresis. Appl Environ Microbiol. 1995;61:1163–1168. doi: 10.1128/aem.61.3.1163-1168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bullen J J, Spalding P B, Ward C G, Gutteridge J M. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch Intern Med. 1991;151:1606–1609. [PubMed] [Google Scholar]
- 10.Curtiss R., III . Gene transfer. In: Gerhardt P, Murray R G E, Costilow R, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general bacteriology. Washington, D.C.: American Society for Microbiology; 1981. pp. 243–265. [Google Scholar]
- 11.Gaillot O, Pellegrini E, Bregenholt S, Nair S, Berche P. The ClpP serine protease is essential for the intracellular parasitism and virulence of Listeria monocytogenes. Mol Microbiol. 2000;35:1286–1294. doi: 10.1046/j.1365-2958.2000.01773.x. [DOI] [PubMed] [Google Scholar]
- 12.Gray L D, Kreger A S. Purification and characterization of an extracellular cytolysin produced by Vibrio vulnificus. Infect Immun. 1985;48:62–72. doi: 10.1128/iai.48.1.62-72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray L D, Kreger A S. Detection of anti-Vibrio vulnificus cytolysin antibodies in sera from mice and a human surviving Vibrio vulnificus disease. Infect Immun. 1986;51:964–965. doi: 10.1128/iai.51.3.964-965.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray L D, Kreger A S. Mouse skin damage caused by cytolysin from Vibrio vulnificus and by V. vulnificus infection. J Infect Dis. 1987;155:236–241. doi: 10.1093/infdis/155.2.236. [DOI] [PubMed] [Google Scholar]
- 15.Gulig P A, Curtiss R., III Plasmid-associated virulence of Salmonella typhimurium. Infect Immun. 1987;55:2891–2901. doi: 10.1128/iai.55.12.2891-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulig P A, Doyle T J, Hughes J A, Matsui H. Analysis of host cells associated with the Spv-mediated increased intracellular growth rate of Salmonella typhimurium in mice. Infect Immun. 1998;66:2471–2485. doi: 10.1128/iai.66.6.2471-2485.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hlady W G, Klontz K C. The epidemiology of vibrio infections in Florida, 1981–1993. J Infect Dis. 1996;173:1176–1183. doi: 10.1093/infdis/173.5.1176. [DOI] [PubMed] [Google Scholar]
- 18.Hlady W G, Mullen R C, Hopkin R S. Vibrio vulnificus from raw oysters. Leading cause of reported deaths from foodborne illness in Florida. J Fla Med Assoc. 1993;80:536–538. [PubMed] [Google Scholar]
- 19.Jackson J K, Murphree R L, Tamplin M L. Evidence that mortality from Vibrio vulnificus infection results from single strains among heterogeneous populations in shellfish. J Clin Microbiol. 1997;35:2098–2101. doi: 10.1128/jcm.35.8.2098-2101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayalakshmi S, Venugopalan V K. Role of iron in the virulence of Vibrio vulnificus isolated from Cuddalore coastal waters (India) Indian J Med Res. 1992;95:294–296. [PubMed] [Google Scholar]
- 21.Jeong K C, Jeong H S, Rhee J H, Lee S E, Chung S S, Starks A M, Escudero G M, Gulig P A, Choi S H. Construction and phenotypic evaluation of a Vibrio vulnificus vvpE mutant for elastolytic protease. Infect Immun. 2000;68:5096–5106. doi: 10.1128/iai.68.9.5096-5106.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaysner C A, Abeyta C, Jr, Wekell M M, DePaola A, Jr, Stott R F, Leitch J M. Virulent strains of Vibrio vulnificus isolated from estuaries of the United States West Coast. Appl Environ Microbiol. 1987;53:1349–1351. doi: 10.1128/aem.53.6.1349-1351.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaysner C A, Wekell M M, Abeyta C., Jr Enhancement of virulence of two environmental strains of Vibrio vulnificus after passage through mice. Diagn Microbiol Infect Dis. 1990;13:285–288. doi: 10.1016/0732-8893(90)90018-q. [DOI] [PubMed] [Google Scholar]
- 24.Kook H, Lee S E, Baik Y H, Chung S S, Rhee J H. Vibrio vulnificus hemolysin dilates rat thoracic aorta by activating guanylate cyclase. Life Sci. 1996;59:PL41–PL47. doi: 10.1016/0024-3205(96)00292-5. [DOI] [PubMed] [Google Scholar]
- 25.Kothary M H, Kreger A S. Purification and characterization of an elastolytic protease of Vibrio vulnificus. J Gen Microbiol. 1987;133:1783–1791. doi: 10.1099/00221287-133-7-1783. [DOI] [PubMed] [Google Scholar]
- 26.Kraffert C A, Hogan D J. Vibrio vulnificus infection and iron overload. J Am Acad Dermatol. 1992;26:140. doi: 10.1016/s0190-9622(08)80542-7. [DOI] [PubMed] [Google Scholar]
- 27.Kumamoto K S, Vukich D J. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61–66. doi: 10.1016/s0736-4679(97)00230-8. [DOI] [PubMed] [Google Scholar]
- 28.Linkous D A, Oliver J D. Pathogenesis of Vibrio vulnificus. FEMS Microbiol Lett. 1999;174:207–214. doi: 10.1111/j.1574-6968.1999.tb13570.x. [DOI] [PubMed] [Google Scholar]
- 29.Litwin C M, Rayback T W, Skinner J. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect Immun. 1996;64:2834–2838. doi: 10.1128/iai.64.7.2834-2838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyoshi N, Miyoshi S, Sugiyama K, Suzuki Y, Furuta H, Shinoda S. Activation of the plasma kallikrein-kinin system by Vibrio vulnificus protease. Infect Immun. 1987;55:1936–1939. doi: 10.1128/iai.55.8.1936-1939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyoshi S, Shinoda S. Role of the protease in the permeability enhancement by Vibrio vulnificus. Microbiol Immunol. 1988;32:1025–1032. doi: 10.1111/j.1348-0421.1988.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 32.Miyoshi S, Shinoda S. Inhibitory effect of alpha 2-macroglobulin on Vibrio vulnificus protease. J Biochem (Tokyo) 1989;106:299–303. doi: 10.1093/oxfordjournals.jbchem.a122848. [DOI] [PubMed] [Google Scholar]
- 33.Molla A, Yamamoto T, Akaike T, Miyoshi S, Maeda H. Activation of hageman factor and prekallikrein and generation of kinin by various microbial proteinases. J Biol Chem. 1989;264:10589–10594. [PubMed] [Google Scholar]
- 34.North R J, LaCourse R, Ryan L, Gros P. Consequence of Nramp1 deletion to Mycobacterium tuberculosis infection in mice. Infect Immun. 1999;67:5811–5814. doi: 10.1128/iai.67.11.5811-5814.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oliver J D, Bockian R. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol. 1995;61:2620–2623. doi: 10.1128/aem.61.7.2620-2623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paranjpye R N, Lara J C, Pepe J C, Pepe C M, Strom M S. The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to HEp-2 cells and virulence in iron-overloaded mice. Infect Immun. 1998;66:5659–5668. doi: 10.1128/iai.66.12.5659-5668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J W, Ma S N, Song E S, Song C H, Chae M R, Park B H, Rho R W, Park S D, Kim H R. Pulmonary damage by Vibrio vulnificus cytolysin. Infect Immun. 1996;64:2873–2876. doi: 10.1128/iai.64.7.2873-2876.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedrosa J, Saunders B M, Appelberg R, Orme I M, Silva M T, Cooper A M. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577–583. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole M D, Oliver J D. Experimental pathogenicity and mortality in ligated ileal loop studies of the newly reported halophilic lactose-positive Vibrio sp. Infect Immun. 1978;20:126–129. doi: 10.1128/iai.20.1.126-129.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 41.Shao C P, Hor L I. Metalloprotease is not essential for Vibrio vulnificus virulence in mice. Infect Immun. 2000;68:3569–3573. doi: 10.1128/iai.68.6.3569-3573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shinoda S, Kobayashi M, Yamada H, Yoshida S, Ogawa M, Mizuguchi Y. Inhibitory effect of capsular antigen of Vibrio vulnificus on bactericidal activity of human serum. Microbiol Immunol. 1987;31:393–401. doi: 10.1111/j.1348-0421.1987.tb03102.x. [DOI] [PubMed] [Google Scholar]
- 43.Simpson L M, White V K, Zane S F, Oliver J D. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect Immun. 1987;55:269–272. doi: 10.1128/iai.55.1.269-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stelma G N, Jr, Reyes A L, Peeler J T, Johnson C H, Spaulding P L. Virulence characteristics of clinical and environmental isolates of Vibrio vulnificus. Appl Environ Microbiol. 1992;58:2776–2782. doi: 10.1128/aem.58.9.2776-2782.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strom M S, Paranjpye R N. Epidemiology and pathogenesis of Vibrio vulnificus. Microbes Infect. 2000;2:177–188. doi: 10.1016/s1286-4579(00)00270-7. [DOI] [PubMed] [Google Scholar]
- 46.Tamplin M L, Jackson J K, Buchrieser C, Murphree R L, Portier K M, Gangar V, Miller L G, Kaspar C W. Pulsed-field gel electrophoresis and ribotype profiles of clinical and environmental Vibrio vulnificus isolates. Appl Environ Microbiol. 1996;62:3572–3580. doi: 10.1128/aem.62.10.3572-3580.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamplin M L, Specter S, Rodrick G E, Friedman H. Differential complement activation and susceptibility to human serum bactericidal action by Vibrio species. Infect Immun. 1983;42:1187–1190. doi: 10.1128/iai.42.3.1187-1190.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamplin M L, Specter S, Rodrick G E, Friedman H. Vibrio vulnificus resists phagocytosis in the absence of serum opsonins. Infect Immun. 1985;49:715–718. doi: 10.1128/iai.49.3.715-718.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tison D L, Kelly M T. Virulence of Vibrio vulnificus strains from marine environments. Appl Environ Microbiol. 1986;51:1004–1006. doi: 10.1128/aem.51.5.1004-1006.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vollberg C M, Herrera J L. Vibrio vulnificus infection: an important cause of septicemia in patients with cirrhosis. South Med J. 1997;90:1040–1042. doi: 10.1097/00007611-199710000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Wright A C, Morris J G., Jr The extracellular cytolysin of Vibrio vulnificus: inactivation and relationship to virulence in mice. Infect Immun. 1991;59:192–197. doi: 10.1128/iai.59.1.192-197.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright A C, Powell J L, Tanner M K, Ensor L A, Karpas A B, Morris J G, Jr, Sztein M B. Differential expression of Vibrio vulnificus capsular polysaccharide. Infect Immun. 1999;67:2250–2257. doi: 10.1128/iai.67.5.2250-2257.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wright A C, Simpson L M, Oliver J D. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun. 1981;34:503–507. doi: 10.1128/iai.34.2.503-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wright A C, Simpson L M, Oliver J D, Morris J G., Jr Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect Immun. 1990;58:1769–1773. doi: 10.1128/iai.58.6.1769-1773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yesilkaya H, Kadioglu A, Gingles N, Alexander J E, Mitchell T J, Andrew P W. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect Immun. 2000;68:2819–2826. doi: 10.1128/iai.68.5.2819-2826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida S, Ogawa M, Mizuguchi Y. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect Immun. 1985;47:446–451. doi: 10.1128/iai.47.2.446-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zuppardo A B, Siebeling R J. An epimerase gene essential for capsule synthesis in Vibrio vulnificus. Infect Immun. 1998;66:2601–2606. doi: 10.1128/iai.66.6.2601-2606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]