Abstract
Autoantibodies against myosin are associated with myocarditis and rheumatic heart disease. In this study, the antigenic cross-reactivity of myosin and N-acetyl-glucosamine (GlcNAc), the dominant epitope of Group A streptococcal polysaccharide, was examined. Six antimyosin monoclonal antibodies (MAbs) derived from mice with cardiac myosin-induced myocarditis were characterized. All MAbs cross-reacted with GlcNAc, mimicking a subset of MAbs derived from rheumatic carditis patients that bind both myosin and streptococcal polysaccharide. Variable (V) region gene usage was diverse, with five of six MAb heavy-chain V regions encoded by distinct members of the J558 family and the sixth encoded by a member of the VGAM3.8 family. Light-chain V-region segments were derived from the Vk1, Vk4/5, Vk10, and Vk21 families. These antimyosin, anti-GlcNac MAbs demonstrated several T-cell-dependent features: they were predominantly immunoglobulin G, were encoded by V-region genes expressed late in development, and displayed somatic mutation. A direct correlation between the extent of somatic mutation and the affinity for myosin was observed. Affinity for GlcNAc also increased with the frequency of mutation, demonstrating that affinity maturation can occur simultaneously for both self antigen and foreign antigen. Based on these observations, we immunized mice with GlcNAc coupled to bovine serum albumin and demonstrated that a T-cell-dependent response to GlcNAc leads to antimyosin reactivity. We speculate that the pathogenic antibody response in rheumatic carditis may reflect the conversion of a T-cell-independent response to GlcNAc to a T-cell-dependent cross-reactive response to GlcNAc and myosin.
Autoantibodies to heart antigens are frequently present in patients with inflammatory carditis (6, 16, 22). Both clinical and experimental studies have suggested that these antibodies (Abs) can mediate cardiac myocyte injury (reviewed in references 4 and 11). In murine coxsackievirus B3-induced myocarditis, the majority of antiheart reactivity recognizes the heavy chain of cardiac myosin (3), and immunization of susceptible mouse strains with cardiac myosin is a well-established model of autoimmune myocarditis (21). Our laboratory has previously demonstrated that antimyosin monoclonal antibodies (MAbs) derived from mice with cardiac myosin-induced myocarditis can cause disease in naive DBA/2 mice and thereby established a direct role of antimyosin Abs in the pathogenesis of autoimmune myocarditis (17). Elevated levels of autoantibodies against myosin have also been detected in humans with myocardial inflammation (16). Ab-mediated myocarditis in mice and rheumatic carditis in humans share several histopathological features, including infiltration of the myocardium by inflammatory cells, myocyte necrosis, Aschoff bodies, and valvulitis. These similarities suggest that the two diseases may share common molecular mechanisms. Here we examine the specificity and molecular origin of antimyosin MAbs derived from mice with cardiac myosin-induced myocarditis, three of which MAbs have been previously shown to be pathogenic, and of serum antibodies from N-acetyl-glucosamine (GlcNAc) immunized mice.
All the antimyosin MAbs were found to cross-react with keratin and GlcNAc, in a manner similar to that of a subset of murine antistreptococcal, antimyosin MAbs and a subset of antistreptococcal, antimyosin MAbs derived from rheumatic carditis patients (1, 29). GlcNAc is the immunodominant epitope of the group A streptococcal carbohydrate, and reactivity against GlcNAc following streptococcal infection is associated with valvular damage (8). Recently, molecular self-targets for anti-GlcNAc reactivity were identified, and they include cytoskeletal and heart proteins, such as keratin and myosin (29). The cross-reactive MAbs that are elicited following myosin immunization utilize an array of variable (V)-region genes, despite their similarity in antigenic specificity and despite the restricted V-region gene usage seen when GlcNAc is the immunogen (18). Based on the characterization of the cross-reactive antimyosin, anti-GlcNAc response, we immunized mice with GlcNAc coupled to a protein carrier and demonstrated that a T-cell-dependent response to GlcNAc results in antimyosin reactivity. These observations suggest a mechanism for the upregulation of the autoreactive response that occurs in both rheumatic carditis and myocarditis.
MATERIALS AND METHODS
Hybridomas and purification of MAbs.
Three of the murine antimyosin MAbs (2D6-B1, 11C6-E3, and 10D4-A9) have been previously described (17); the other three MAbs (6G14-F6, 16B2-A1, and 14G2-B12) were produced by immunization of a BALB/c mouse with cardiac myosin. All MAbs were purified as described previously (17), except MAb 6G14-F6, which was purified by anti-immunoglobulin M (IgM) affinity chromatography (Zymed Laboratories, Inc., San Francisco, Calif.). Purity was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the concentration was determined by enzyme-linked immunosorbent assay (ELISA).
Immunization of mice.
Six-to-eight-week-old BALB/c female mice were obtained from the Jackson Laboratory (Bar Harbor, Maine) and immunized with 100 μg of GlcNAc-bovine serum albumin (BSA) in ImJect Alum (Pierce, Rockford, Ill.) subcutaneously at four sites on days 0, 7, and 21. Serum was obtained by a retroorbital bleed on days 0 and 28.
Antigens.
Cardiac myosin was prepared from BALB/c mouse hearts according to a protocol of Pollack et al. (24). Keratin from human epidermis, mouse laminin, tropomyosin from rabbit muscle, actin from rabbit muscle, BSA, and calf thymus double-stranded and single-stranded DNA were obtained from Sigma (St. Louis, Mo.). Collagen type IV from human placenta was obtained from Fluka Biochemical Corp. (Ronkonkoma, N.Y.). GlcNAc was conjugated to BSA in a two-step reaction (29). A conjugate with a 50:1 molar ratio of GlcNAc to BSA was used.
ELISAs.
Purified MAbs were titrated in phosphate-buffered saline (PBS) (pH 8.0) and tested on cardiac myosin by ELISA using a modification of the protocol described by Neu et al. (20). Briefly, Falcon microtiter plates (Becton Dickinson Labware, Lincoln Park, N.J) were adsorbed with 10 μg of myosin/ml in buffer (13 mM sodium carbonate, 35 mM sodium bicarbonate, and 50 mM sodium pyrophosphate [pH 9.6], blocked with 2% BSA–PBS, and incubated with samples for 2 h at 37°C. The secondary Ab was an alkaline-phosphatase-conjugated anti-mouse IgG or IgM Ab (Southern Biotechnology Associates, Inc., Birmingham, Ala.) diluted 1:1,000 in 0.5% BSA–PBS, and the substrate was p-nitrophenyl phosphate (Sigma).
Purified MAbs were also tested on a panel of antigens using the following protocol. Ten micrograms of each antigen/ml in 0.1 M sodium carbonate buffer, pH 9.0, was adsorbed on Immulon-4 microtiter plates (Dynatech, Alexandria, Va.) overnight at 4°C. The plates were blocked in 1% BSA–PBS and incubated with 25 μg of purified MAbs/ml. The secondary Ab was alkaline phosphatase-conjugated goat anti-mouse Ig (Sigma) diluted 1:250, and the substrate used was p-nitrophenyl phosphate (Sigma). Results were calculated from triplicate measurements, and experiments were repeated three times. The criteria for antigen binding were a twofold increase in binding over that with BSA and an optical density at 405 nm greater than 0.2.
Sera from preimmune and immunized mice were titrated in 0.1 M β-mercaptoethanol in PBS (to dissociate IgM pentameters) and tested for IgG titers against cardiac myosin as described above. Serum IgG titers against GlcNAc-BSA were tested by the same protocol using microtiter plates coated with 2.5 μg of GlcNAc-BSA/ml in 0.1 M sodium carbonate buffer.
Competitive ELISAs.
A constant concentration of the MAbs or serum was incubated with increasing amounts of soluble antigen on antigen-coated plates for 2 h at 37°C, and the usual ELISA protocol was followed. All sera were diluted in 0.1 M β-mercaptoethanol. The percent inhibition was calculated by comparing wells with MAb and inhibitor to wells with MAb without inhibitor.
Immunoslot blot.
The BALB/c myosin heavy-chain-α fragments were prepared as described previously (17). Head fragments MF1 (amino acids 1 to 562) and MF2 (amino acids 562 to 1102) and rod fragments MF3 (amino acids 1102 to 1542) and MF4 (amino acids 1542 to 1972) were used. Ten micrograms of each sample was loaded directly onto nitrocellulose (Schleicher & Schuell, Inc., Keene, N.H.) using a slot blot apparatus (Life Technologies, Gaithersburg, Md.). The membrane was blocked in 5% milk in PBS overnight at 4°C. Strips were incubated with purified MAbs at a concentration of 0.1 μg/ml for 1 h at room temperature, washed with PBS–0.05% Tween 20, and incubated with a 1:2,000 dilution of peroxidase-labeled goat anti-mouse IgG or IgM (Southern Biotechnology, Inc.) for 1 h at room temperature. The ECL Plus Western blotting detection system (Amersham Pharmacia Biotech, Piscataway, N.J.) was used as a substrate.
cDNA synthesis and purification.
Total hybridoma mRNA was isolated by the Ultraspec method (Biotecx Laboratories, Inc., Houston, Tex.). First-strand synthesis of the heavy chains was performed using either an IgG (5′-TGGACAGGG(A/C)TCCA(G/T) AGTTC-3′) or an IgM (5′-TCAGTGTTGTTCTGGTAGTTCAC-3′) 3′ constant region primer proximal to the V region and Superscript II reverse transcriptase (Life Technologies). First-strand synthesis of the light chains was performed using an Igk 3′ constant region primer (5′-ACACTCATTCCTGTTGAA-3′) and Moloney murine leukemia virus reverse transcriptase (Life Technologies). PCR amplification of the heavy chains was performed using one of the 3′ constant region primers and one of two sets of previously described degenerate 5′ heavy-chain V-region (VH) primers (VHfr1a–e or VHfrf–j) (13) and Vent polymerase (New England Biolabs, Beverly, Mass.). PCR amplification of the light chains was performed using the 3′ constant region primer and previously described degenerate 5′ kappa light chain V-region (Vk) primers (Vkfr1a–g) (13). PCR products were purified from a 1.5% low-melt agarose preparative gel by SpinBind (FMC Bioproducts, Rockland, Maine).
Sequencing.
The PCR products were sequenced using the dsDNA Cycle Sequencing System (Life Technologies). To sequence in the 3′ direction, an IgG (5′-GGGGGCAGTGGATAGAC-3′), an IgM (5′-GCAGGAGACGAGGGGGA-3′), or an IgK (5′-TGGATGGTGGGAAGATG3′) 3′ constant region primer and an internal 3′ V-region primer were used. To sequence in the opposite direction, a degenerate 5′ VH or Vk primer was chosen. For each sequence, 1 pmol of primer and 20 ng of cDNA were used. The sequencing reactions were run on a 6% acrylamide gel using Sequagel-6 and Complete Buffer (National Diagnostics, Atlanta, Ga.) per the manufacturer's instructions.
Since cycle sequencing continuously resulted in the amplification of the aberrantly rearranged myeloma partner light chain of the 10D4-A9 hybridoma, the productive light chain was sequenced directly from RNA, using a modification of the protocol described by Geliebter et al. (10).
Random replacement/silent mutation (R:S) ratios were calculated as nucleotide changes resulting in missense mutations divided by nucleotide changes resulting in neutral mutations. The program used was Mutability (http://www.hgu.mrc.ac.uk/cgi-bin/mutable), written by Alastair Brown, Medical Research Council Human Genetics Unit, Edinburgh, Scotland.
Nucleotide sequence accession numbers.
VH and Vk sequences were submitted to GenBank and assigned accession numbers AF206021 through AF206032.
RESULTS
Affinity and specificity of anti-myosin MAbs.
All MAbs were derived from mice immunized with cardiac myosin and were selected for binding to cardiac myosin. Apparent affinities for myosin were determined and are shown in Table 1. Using genetically engineered BALB/c myosin heavy-chain fragments on an immunoslot blot, we found that all MAbs reacted with MF1, a fragment spanning amino acids 1 through 562 in the S1 domain of the head region (data not shown). The MAbs were further tested for cross-reactivity to a panel of self and foreign antigens. All displayed cross-reactivity to keratin, also an α-helical protein, and GlcNAc-BSA (Fig. 1).
TABLE 1.
Apparent affinity constants of MAbs to myosin and GlcNAc-BSA
| Hybridoma | Affinity toa:
|
|
|---|---|---|
| Myosin (M−1) | GlcNAc-BSA (M−1) | |
| 10D4-A9 | 2.50 × 107 | 1.17 × 106 |
| 14G2-B12 | 8.55 × 106 | 9.52 × 105 |
| 11C6-E3 | 3.77 × 106 | 8.85 × 105 |
| 2D6-B1 | 1.03 × 106 | 2.64 × 105 |
| 16B2-A1 | 9.35 × 105 | 4.74 × 105 |
| 6G14-F6 | 5.99 × 105 | 1.89 × 105 |
Affinity constants were calculated as the inverse of the 50% inhibitory concentrations of the MAbs by competitive ELISAs for solid-phase myosin or GlcNAc-BSA as described in Materials and Methods.
FIG. 1.
Profiles of cross-reactivity of MAbs 10D4-A9, 2D6-B1, 16B2-A1, 14G2-B12, 11C6-E3, and 6G14-F6, as determined by ELISA, are shown in panels A through F, respectively. ssDNA, single-stranded DNA; dsDNA, double-stranded DNA.
Anti-GlcNAc reactivity in rheumatic patients arises as a consequence of streptococcal infection, and cross-reactivity between GlcNAc and myosin has been demonstrated in antibodies from patients with rheumatic carditis. We speculated that cross-reactive anti-myosin, anti-GlcNAc B cells might escape tolerance if they had a high affinity for GlcNAc and a low affinity for self-myosin. However, each MAb exhibited a higher apparent affinity to myosin than to GlcNAc-BSA. Most MAbs displayed a direct correlation between apparent affinity to myosin and apparent affinity to GlcNAc-BSA. At one end, 10D4-A9 and 14G2-B12 had the highest apparent affinities to myosin and GlcNAc-BSA, while at the other end, 6G14-F6, the only IgM, had the lowest apparent affinities to both antigens.
Cross-inhibitions of MAbs.
To determine whether the cross-reactivity to myosin and GlcNAc occurs through a common binding site, cross-inhibition assays were performed. Myosin inhibited GlcNAc-BSA binding of all MAbs (data not shown). GlcNAc-BSA at the same molar concentrations did not significantly inhibit myosin binding, presumably reflecting an up to 10-fold-lower apparent affinity of the MAbs for GlcNAc-BSA than for myosin. It is also possible that the two antigens are bound within proximal but distinct binding sites and that myosin sterically inhibits GlcNAc binding.
V-region genes.
The anti-GlcNAc response that is protective against streptococcal infection is highly restricted with respect to V-gene usage (19). To determine the heterogeneity of V-gene usage among the cross-reactive MAbs, we sequenced heavy- and light-chain genes. The nucleotide sequences and corresponding deduced amino acid sequences for the MAb heavy-chain V regions were compared to those of the most homologous germline genes and their products. A summary of VH gene segment usage and nucleic acid and amino acid sequence homologies to the most homologous germline genes and gene products is shown in Table 2.
TABLE 2.
Summary of heavy-chain V-region gene usage among MAbs
| Hybridoma | Isotype | VH family | Homologous germline gene | % Homologya
|
Gene segment used
|
||
|---|---|---|---|---|---|---|---|
| Nucleic acid | Amino acid | D region | JH region | ||||
| 2D6-B1 | IgG2bb | J558 | VH104B | 97.4 | 94.4 | NKc | 4 |
| 11C6-E3 | IgG2b | VGAM3.8 | VFM1 | 98.0 | 95.2 | Q52 | 3 |
| 10D4-A9 | IgG2a | J558 | H10 | 97.0 | 93.3 | FL16.1 | 2 |
| 6G14-F6 | IgM | J558 | A5d | 99.5 | 98.5 | FL16.1 | 2 |
| 16B2-A1 | IgG2b | J558 | J558 Liv | 99.6 | 100 | SP.2.5/.7/.8 | 2 |
| 14G2-B12 | IgG2b | J558 | 43Y | 89.3 | 83.3 | Q52 | 3 |
Sequences of the MAb heavy-chain V regions and their deduced products were compared to those of the most homologous germline genes and their products.
Previously characterized as IgG2a.
NK, not known.
Only a partial sequence was available.
Heavy-chain V-region usage was unrestricted. Although five hybridomas expressed J558 VH family genes, they were encoded by distinct VH germline segments (7, 26–28, 32). The sixth hybridoma, 11C6–E3, expressed a VGAM3.8 VH family gene and was encoded by the BALB/c VFM1 germline gene (30). The heavy-chain V regions of the antimyosin MAbs showed an average of 96.8% nucleotide homology to their germline counterparts, with homology ranging from 89.3 to 99.5%. A variety of D and JH genes were used. The D region of 2D6–B1 was unidentifiable. All JH segments were identical to germline segments, except that 10D4-A9 had a single replacement substitution at codon 112. The diversity of D and JH segments used, the variety of splice sites used for joining, and nucleotide additions resulted in complementarity-determining region 3s (CDR3s) that were completely different and ranged from 8 to 13 amino acids.
The nucleotide sequences and corresponding deduced amino acid sequences for the MAb light-chain V regions were compared to those of the most homologous germline genes and their products. A summary of Vk gene segment usage and nucleic acid and amino acid sequence homology to the most homologous germline genes and their products is shown in Table 3.
TABLE 3.
Summary of light-chain V-region gene usage among MAbs
| Hybridoma | Vk family | Homologous germline gene | % Homologya
|
Jk gene segment used | |
|---|---|---|---|---|---|
| Nucleic acid | Amino acid | ||||
| 2D6-B1 | 4/5 | am4 | 96.5 | 96.5 | 2 |
| 11C6-E3 | 4/5 | kf4 | 98.5 | 98.9 | 2 |
| 10D4-A9 | 10 | IgK-V10.2 | 93.0 | 88.4 | 1 |
| 6G14-F6 | 21 | Vk21G | 100 | 100 | 1 |
| 16B2-A1 | 1 | Vk Ser | 99.6 | 98.9 | 1 |
| 14G2-B12 | 1 | Vk1A | 98.6 | 97.8 | 2 |
Sequences of the MAb light-chain V regions and their deduced products were compared to those of the most homologous germline genes and their products.
Similar to heavy-chain V-region usage, light-chain V-region usage appeared to be unbiased. Four Vk families were represented. Two MAbs were encoded by Vk4/5-family genes, two by Vk1-family genes (5, 23), and one each by Vk10- (14) and Vk21-family genes (2). Overall, the Vk segments were slightly less mutated than the VH segments. They showed an average of 97.7% nucleic acid homology to their germline counterparts, with homology ranging from 93 to 100%. 2D6-B1, 11C6-E3, and 14G2-B12 utilized the Jk2 segment, while 10D4-A9, 6G14-F6, and 16B2-A1 utilized the Jk1 segment. All the J region sequences were identical to germline gene sequences, except that 16B2-A1 had a single replacement substitution in codon 96.
Somatic mutation.
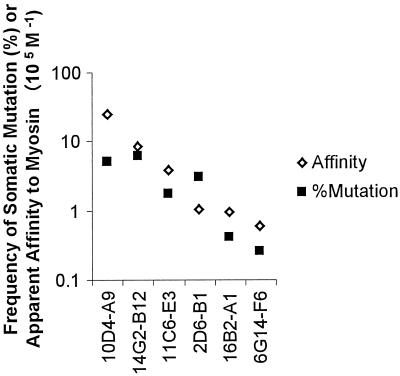
A summary of the somatic mutations observed in the V regions of antimyosin MAbs is shown in Table 4. Higher R:S ratios in CDRs, relative to those of framework regions (FRs), and nonrandom R:S ratios in CDRs are considered evidence of antigen-driven selection. By these criteria, only the light chain of 2D6-B1 appears to have undergone antigen-driven selection. It is possible, however, to have affinity maturation without higher-than-random R:S ratios, since a single mutation can alter the affinity. We found that the degree of somatic mutation was positively correlated with the degree of affinity for myosin (Fig. 2), providing evidence suggestive of affinity maturation. Whether or not antigen-driven selection actually occurred cannot be firmly established without determining the affinities of Abs back-mutated to their germline counterparts.
TABLE 4.
Summary of somatic mutation in V regions of antimyosin MAbs
| Hybridoma | VH R:S ratiosa
|
VK R:S ratiosa
|
||||
|---|---|---|---|---|---|---|
| Total | FR | CDR | Total | FR | CDR | |
| 14G2-B12 | 19:10 | 0.85 (2.96)b | 4.33 (4.61) | 2:2 | 0 (3.01) | 2 (3.41) |
| 16B2-A1 | 0:1 | 0 (3.04) | 0 (4.81) | 2:0 | (3.05) | (3.78) |
| 2D6-B1 | 5:2 | 0.5 (2.77) | 4:0 (5.68) | 7:2 | 0 (2.93) | 7:0 (3.62) |
| 6G14-F6 | 1:0 | 0 (2.86) | 0 (5.71) | 0 | 0 (3.22) | 0 (3.66) |
| 10D4-A9 | 6:2 | 3:0 (2.97) | 3:0 (4.16) | |||
| 11C6-E3 | 4:1 | 4:0 (3.28) | 0 (4.03) | 0:33 | 0 (2.99) | (3.37) |
The number of R:S mutations in the expressed V-region genes of the antimyosin MAbs compared to the most homologous germline gene.
Numbers in parentheses are the expected random R:S ratios for the respective germline genes.
FIG. 2.
Correlation of somatic mutation rates with apparent affinities to myosin. The rate of somatic mutation for each MAb was for combined VH and Vk gene segments and was determined by comparison with the most homologous germline genes in the database.
Cross-reactive anti-GlcNAc, anti-myosin serum response.
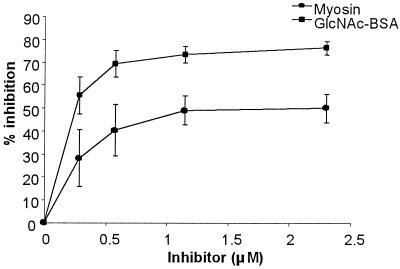
The fact that all the anti-myosin MAbs bound GlcNAc made us wonder whether myosin and GlcNAc were almost always antigenic mimics and whether a T-cell-dependent response to GlcNAc would result in antimyosin reactivity. Mice immunized with GlcNAc-BSA developed an antimyosin response (data not shown), and myosin significantly inhibited the anti-GlcNAc reactivity (Fig. 3). Thus, in the T-cell-dependent response to GlcNAc-BSA, GlcNAc and myosin are antigenic mimics.
FIG. 3.
Inhibitions of reactivity of serum of GlcNAc-BSA-immunized BALB/c mice to immobilized GlcNAc-BSA. Constant amounts of serum were incubated with equal volumes of increasing amounts of soluble myosin or GlcNAc-BSA. The amount of inhibition was determined by comparing serum reactivity in the presence and absence of the inhibitor. Each group represents serum samples from five mice.
DISCUSSION
Molecular mimicry is thought to give rise to cross-reactive antistreptococcal, antimyosin autoantibodies in mice as well as in humans (reviewed in reference 29). Our studies suggest that myosin and GlcNAc have significant antigenic homology and that this cross-reactivity may characterize a large percentage of the antimyosin antibody repertoire. Myosin inhibition of anti-GlcNAc reactivity of MAbs from mice with cardiac myosin-induced myocarditis suggests that GlcNAc and myosin are recognized by the same Ab binding site, and the cross-reactivity of the MAbs with GlcNAc and MF1 suggests that GlcNAc mimics structural epitopes in the head region of myosin. A significant amount of cross-reactivity between GlcNAc and myosin was also detectable in the serum of GlcNAc-BSA-immunized mice. The shared antigenic cross-reactivity of autoantibodies from mice with cardiac myosin-induced myocarditis and those from humans with rheumatic carditis may account for the overlapping histopathologic features in these diseases.
The unrestricted usage of VH and Vk genes by antimyosin, anti-GlcNAc B cells was surprising both because the MAbs share antigenic specificity and because the anti-GlcNAc Abs made in response to the streptococcal carbohydrate are highly restricted with respect to V-gene usage (18). Molecular analysis of human antistreptococcal, antimyosin MAbs from rheumatic carditis patients shows that they display heterogeneous usage of heavy- and light-chain V-region genes (1, 25, 31), similar to the antibodies reported on here. We would speculate that a genetically restricted anti-GlcNAc response occurs when the response is T cell independent and that a genetically diverse antimyosin, anti-GlcNAc response occurs when T-cell help is present (i.e., when a peptide mimic of GlcNAc or myosin itself is the eliciting antigen). The T-cell-independent response does not undergo affinity maturation but includes antibodies that at inception have a higher affinity for GlcNAc than do those that are activated in the T-cell-dependent antimyosin response. The T-cell-dependent antibodies undergo affinity maturation but, at inception, have a lower affinity for GlcNAc than the canonical, protective antibody. Presumably, T-cell help is required for the activation of these low-affinity B cells.
From the studies of MAbs from rheumatic carditis patients, it is evident that somatic mutation is not required to generate specificity to myosin. In independent studies, Adderson et al. (1) and Wu et al. (31) have shown that these MAbs are multireactive and have little somatic mutation in their V-region genes. Wu et al. further showed that they had reduced light-chain N additions, which is characteristic of Abs found in the fetal repertoire. The MAbs analyzed by Adderson et al. (1) were derived from V-region genes preferentially expressed in the early repertoire and showed no or little somatic mutation. It was suggested that these MAbs might derive from natural autoantibodies. Only one of the antimyosin, antistreptococcal MAbs derived from rheumatic patients was an IgG Ab (31). Five of the six antimyosin MAbs from cardiac myosin-immunized mice in this report are IgG; all express late V-region genes and overall display some degree of somatic mutation. Our studies suggest that somatic mutation may be involved in producing higher-affinity antimyosin, anti-GlcNAc Abs. It is interesting that affinity for both antigens increases with somatic mutation. The naïve cross-reactive B cell may survive in the naïve repertoire because myosin is a sequestered self antigen. Since IgM antimyosin Abs cannot penetrate normal cardiac tissue (15), they only become potentially pathogenic upon isotype switching to IgG.
It has been reported that only patients with rheumatic fever, and not normal individuals infected with group A streptococcus, develop elevated titers of IgG antimyosin antibodies (12). One provocative hypothesis to arise from the current study is that the generation of pathogenic antimyosin reactivity in rheumatic carditis may depend on a preceding anticarbohydrate response becoming a cross-reactive antimyosin, anti-GlcNAc response. We would speculate that this can occur if the anticarbohydrate response that is usually T cell independent becomes T cell dependent and undergoes class switching, affinity maturation, and diversification of V-region gene usage. All these T-cell-dependent features are properties of the cross-reactive MAbs reported here. The T-cell-dependent response that we suggest can occur in selected individuals is cross-reactive with self antigen and potentially pathogenic. That both MAbs and serum antibodies show extensive cross-reactivity between myosin and GlcNac lends credence to this hypothesis.
ACKNOWLEDGMENTS
We thank C. Kowal, S. Harris, and A. P. Kuan for critical review of the manuscript and Sylvia Jones for secretarial assistance.
This study was supported by grant 43018 from NIAMS.
REFERENCES
- 1.Adderson E E, Shikhman A R, Ward K E, Cunningham M W. Molecular analysis of polyreactive monoclonal antibodies from rheumatic carditis: human anti-N-acetylglucosamine/anti-myosin antibody V region genes. J Immunol. 1998;161:2020–2031. [PubMed] [Google Scholar]
- 2.Alanen A, Weiss S. Sequence and linkage of the V kappa 21A and G germline gene segments in the mouse. Eur J Immunol. 1989;19:1961–1963. doi: 10.1002/eji.1830191031. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez F L, Neu N, Rose N R, Craig S W, Beisel K W. Heart-specific autoantibodies induced by coxsackievirus B3: identification of heart autoantigens. Clin Immunol Immunopathol. 1987;43:129–139. doi: 10.1016/0090-1229(87)90164-4. [DOI] [PubMed] [Google Scholar]
- 4.Barry W H. Mechanisms of immune-mediated myocyte injury. Circulation. 1994;89:2421–2432. doi: 10.1161/01.cir.89.5.2421. [DOI] [PubMed] [Google Scholar]
- 5.Boyd R T, Goldrick M M, Gottlieb P D. Structural differences in a single gene encoding the V kappa Ser group of light chains explain the existence of two mouse light-chain genetic markers. Proc Natl Acad Sci USA. 1986;83:9134–9138. doi: 10.1073/pnas.83.23.9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caforio A L, Goldman J H, Haven A J, Baig K M, Libera L D, McKenna W J. Circulating cardiac-specific autoantibodies as markers of autoimmunity in clinical and biopsy-proven myocarditis. The Myocarditis Treatment Trial Investigators. Eur Heart J. 1997;18:270–275. doi: 10.1093/oxfordjournals.eurheartj.a015230. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J B, Givol D. Allelic immunoglobulin VH genes in two mouse strains: possible germline gene recombination. EMBO J. 1983;2:2013–2018. doi: 10.1002/j.1460-2075.1983.tb01693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudding B A, Ayoub E M. Persistence of streptococcal group A antibody in patients with rheumatic valvular disease. J Exp Med. 1968;128:1081–1098. doi: 10.1084/jem.128.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Froude J, Gibofsky A, Buskirk D R, Khanna A, Zabriskie J B. Cross-reactivity between streptococcus and human tissue: a model of molecular mimicry and autoimmunity. Curr Top Microbiol Immunol. 1989;145:5–26. doi: 10.1007/978-3-642-74594-2_2. [DOI] [PubMed] [Google Scholar]
- 10.Geliebter J, Zeff R A, Melvold R W, Nathenson S G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci USA. 1986;83:3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herzum M, Maisch B. Humoral and cellular immune reactions to the myocardium in myocarditis. Herz. 1992;17:91–96. [PubMed] [Google Scholar]
- 12.Jambotkar S M, Shastry P, Kamat J R, Kinare S G. Elevated levels of IgG specific anti-myosin antibodies in acute rheumatic fever (ARF): differential profiles of antibodies to myosin and soluble myocardial antigens in ARF, acute glomerulonephritis and group A streptococcal pharyngitis. J Clin Lab Immunol. 1993;40:149–161. [PubMed] [Google Scholar]
- 13.Kettleborough C A, Saldanha J, Ansell K H, Bendig M M. Optimization of primers for cloning libraries of mouse immunoglobulin genes using the polymerase chain reaction. Eur J Immunol. 1993;23:206–211. doi: 10.1002/eji.1830230132. [DOI] [PubMed] [Google Scholar]
- 14.Kim S O, Sanz I, Williams C, Capra J D, Gottlieb P D. Polymorphism in V kappa 10 genes encoding L chains of antibodies bearing the Ars-A and A48 cross-reactive idiotypes. Immunogenetics. 1991;34:231–241. doi: 10.1007/BF00215258. [DOI] [PubMed] [Google Scholar]
- 15.Kuan A P, Zuckier L, Liao L, Factor S M, Diamond B. Immunoglobulin isotype determines pathogenicity in antibody-mediated myocarditis in naïve mice. Circ Res. 2000;86:281–285. doi: 10.1161/01.res.86.3.281. [DOI] [PubMed] [Google Scholar]
- 16.Lauer B, Padberg K, Schultheiss H P, Strauer B E. Autoantibodies against human ventricular myosin in sera of patients with acute and chronic myocarditis. J Am Coll Cardiol. 1994;23:146–153. doi: 10.1016/0735-1097(94)90513-4. [DOI] [PubMed] [Google Scholar]
- 17.Liao L, Sindhwani R, Rojkind M, Factor S, Leinwand L, Diamond B. Antibody-mediated autoimmune myocarditis depends on genetically determined target organ sensitivity. J Exp Med. 1995;181:1123–1131. doi: 10.1084/jem.181.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz C T, Bartholow T L, Greenspan N S, Fulton R J, Monafo W J, Perlmutter R M, Huang H V, Davie J M. Molecular dissection of the murine antibody response to streptococcal group A carbohydrate. J Exp Med. 1987;165:531–545. doi: 10.1084/jem.165.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahm M H, Clevinger B L, Davie J M. Monoclonal antibodies to streptococcal group A carbohydrate. I. A dominant idiotypic determinant is located on VK. J. Immunol. 1982;129:1513–1518. [PubMed] [Google Scholar]
- 20.Neu N, Beisel K W, Traystman M D, Rose N R, Craig S W. Autoantibodies specific for the cardiac myosin isoform are found in mice susceptible to coxsackievirus B3-induced myocarditis. J Immunol. 1987;138:2488–2492. [PubMed] [Google Scholar]
- 21.Neu N, Rose N R, Beisel K W, Herskowitz A, Gurri-Glass G, Craig S W. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 22.Neumann D A, Burek C L, Baughman K L, Rose N R, Herskowitz A. Circulating heart-reactive antibodies in patients with myocarditis or cardiomyopathy. J Am Coll Cardiol. 1990;16:839–846. doi: 10.1016/s0735-1097(10)80331-6. [DOI] [PubMed] [Google Scholar]
- 23.Ng K H, Lavigueur A, Ricard L, Boivrette M, Maclean S, Cloutier D, Gibson D M. Characterization of allelic V kappa-1 region genes in inbred strains of mice. J Immunol. 1989;143:638–648. [PubMed] [Google Scholar]
- 24.Pollack P S, Malhotra A, Fein F S, Scheuer J. Effects of diabetes on cardiac contractile proteins in rabbits and reversal with insulin. Am J Physiol. 1986;251:448–454. doi: 10.1152/ajpheart.1986.251.2.H448. [DOI] [PubMed] [Google Scholar]
- 25.Quinn A, Adderson E E, Shackelford P G, Carroll W L, Cunningham M W. Autoantibody germ-line gene segment encodes VH and VL regions of a human anti-streptococcal monoclonal antibody recognizing streptococcal M protein and human cardiac myosin epitopes. J Immunol. 1995;154:4203–4212. [PubMed] [Google Scholar]
- 26.Rathbun G A, Otani F, Milner E C, Capra J D, Tucker P W. Molecular characterization of the A/J J558 family of heavy chain variable region gene segments. J Mol Biol. 1988;202:383–395. doi: 10.1016/0022-2836(88)90272-0. [DOI] [PubMed] [Google Scholar]
- 27.Retter M W, Cohen P L, Eisenberg R A, Clarke S H. Both Sm and DNA are selecting antigens in the anti-Sm B cell response in autoimmune MRL/1pr mice. J Immunol. 1996;156:1296–1306. [PubMed] [Google Scholar]
- 28.Schiff C, Milili M, Hue I, Rudikoff S, Fougereau M. Genetic basis for expression of the idiotypic network. One unique Ig VH germline gene accounts for the major family of Ab1 and Ab3 (Ab1′) antibodies of the GAT system. J Exp Med. 1986;163:573–587. doi: 10.1084/jem.163.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shikhman A R, Greenspan N S, Cunningham M W. A subset of mouse monoclonal antibodies cross-reactive with cytoskeletal proteins and group A streptococcal M proteins recognizes N-acetyl-beta-d-glucosamine. J Immunol. 1993;151:3902–3913. [PubMed] [Google Scholar]
- 30.Thomas J W. Anti-insulin and regulatory anti-idiotypic antibodies use the same germ-line VHIX gene. Eur J Immunol. 1992;22:2445–2448. doi: 10.1002/eji.1830220938. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Liu B, Van der Merwe P L, Kalis N N, Berney S M, Young D C. Myosin-reactive autoantibodies in rheumatic carditis and normal fetus. Clin Immunol Immunopathol. 1998;87:184–192. doi: 10.1006/clin.1998.4531. [DOI] [PubMed] [Google Scholar]
- 32.Yancopoulos G D, Alt F W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985;40:271–281. [PubMed] [Google Scholar]