Abstract
Objectives:
Ketamine has a fast onset of action that may offer a paradigm change for depression management at the end of life. We aimed to synthesize evidence regarding the safety and efficacy of ketamine in depression treatment within a broad palliative care concept.
Methods:
We searched seven databases and included studies on the safety and efficacy of ketamine for depression treatment in patients diagnosed with any life-threatening disease. We also conducted a narrative review of the evidence.
Results:
Among 2,252 screened titles and abstracts, we included 32 studies in our final synthesis: 14 case reports, two case series, two quasi-experimental studies, and seven randomized clinical trials (RCTs), as well as data from three unpublished clinical trials and seven cases from four larger case series. Most case reports reported a robust effect; however, the larger studies reported conflicting findings. Five RCTs reported positive outcomes; however, four of them were focused on a perioperative setting. Two negative studies did not primarily focus on depression and did not apply severity cutoffs.
Conclusion:
Although ketamine is generally safe and potentially useful, its efficacy in palliative care settings remains unclear. It may be a reasonable alternative for perioperative depression in oncological patients.
Keywords: Ketamine, antidepressive agents, palliative care, depression, mood disorders
Introduction
Depressive disorders are highly prevalent in the adult population, with major depressive disorder (MDD) having an estimated worldwide annual prevalence of 4.7%.1 Moreover, they exert a significant burden, with MDD contributing to 2.5% of global disability-adjusted life years (DALYs).2 Chronic medical conditions and pain increase susceptibility to depression; accordingly, the estimated prevalence of MDD among patients with cancer undergoing palliative care is 7-49%.3
Monoaminergic antidepressants have been the primary pharmacological treatment for MDD for > 80 years. Their efficacy over placebo has been described in meta-analyses of adult patients with depression in the general population4 and in palliative care settings.5 However, their unfavorable remission rates and extended time-to-remission windows (≥ 4-8 weeks)6 limit their utility at the end of life, which strongly features depression and requires prompt symptom reduction given patients’ short life expectancy.
Accordingly, there have been extensive efforts to shorten the response time of depression treatment in this setting. Methylphenidate, which is routinely used to treat mood disorders in the general population, is frequently prescribed as an off-label intervention for rapid relief of depressive symptoms. Some studies on patients receiving palliative care have reported an early response, starting at 3 days.7 However, there have only been a few randomized clinical trials (RCTs), with inconsistent results.7-9
Glutamatergic drugs are a potential new standard treatment for depression. Ketamine, an N-methyl-D-aspartate (NMDA)-receptor noncompetitive antagonist, was developed as an anesthetic10 and has been increasingly used as an antidepressant in recent practice, even though this indication was first reported > 20 years ago.11,12 Intranasal esketamine has been approved for depression treatment by the United States Food and Drug Administration (FDA), as well as other regulatory agencies.
Ketamine has a higher response rate (65-70%)13 and a faster onset of action than monoaminergic antidepressants. Although the antidepressant effects of ketamine can appear as early as < 1 h after administration,14 they peak at approximately 24 h and last for 7 days.15 Additionally, these profiles vary according to the administration route, with oral ketamine having a more delayed onset.16
Ketamine has been extensively studied in palliative care settings, mainly as a continuous subcutaneous (SC) infusion for adjunctive treatment of opioid-unresponsive oncologic pain.17 Moreover, two reviews examined different aspects of administering ketamine and other psychedelic drugs in end-of-life care, including studies on pain and mental disorders.18,19 Although these studies focused on the potential of ketamine, its utility in depression remains unclear, since it has been underused and reserved for treatment-resistant scenarios, probably due to a lack of robust safety data.
Given the growing body of evidence regarding the use of ketamine in psychiatry, we aimed to describe the use of this agent as an antidepressant in palliative care settings, since a fast-acting drug may provide a paradigm change in such a time-sensitive scenario. Specifically, we aimed to review studies on the safety and/or efficacy of ketamine (or any of its enantiomers) for depression treatment within a broad concept of palliative care according to the World Health Organization (WHO) definition, i.e., interventions promoting the quality of life of people suffering from life-threatening illnesses.20
Methods
Search strategy
We searched seven databases (PubMed/MEDLINE, Cochrane, Web of Science, EMBASE, Scopus, PsycINFO, and ClinicalTrials.gov) using strategies developed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.21 Moreover, we screened the references of the selected articles, contacted several authors, and investigated the gray literature. Appendix 1 (112.4KB, pdf) shows the search strategies.
Inclusion criteria
Study type
Clinical trials, cohort studies, case-control studies, case series, and case reports were included.
Mental health diagnosis
Participants must present depressive symptoms or be at risk of depression. Symptoms must be measured using validated symptom scales and/or qualitatively described (in case reports).
Condition subject to palliative care
To capture the broad spectrum of palliative care, we searched terms regarding the most common chronic, progressive, life-threatening illnesses, rather than only specific terms such as “palliative care” and “end-of-life.” Accordingly, we selected 10 conditions that may render the patient a candidate for palliative care, based on the minimal estimate proposed by Rosenwax et al.22 This list was used to broaden our search. Nonetheless, we included studies involving any life-threatening illness at any stage.
Intervention
Pharmacological treatment with ketamine or its enantiomers (S-ketamine, also known as esketamine, or R-ketamine, also known as arketamine) via any route of administration and with any treatment/follow-up duration, as monotherapy or adjunctive medication. We included studies with placebo controls, drug comparisons, or no controls.
Outcomes
We included studies that reported depressive symptom outcomes, including changes in severity scale scores, response or remission rates, or subjective improvements in mood and behavior.
Language
We included studies published in English, Portuguese, or Spanish.
Exclusion criteria
We excluded studies only published as conference posters or oral sessions, letters to the editor without complete descriptions of the original data, review articles, clinical trial protocols, studies involving patients not clearly subject to palliative care (including patients undergoing elective non-cancer surgery), and studies not available in the selected languages.
Procedures
This review was registered in PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021240763). The initial and subsequent literature searches were performed in May 2021 and February 2022, respectively. Abstract screening, full-text screening, and quality assessment were performed independently by two authors (MGB and GTG), with conflicts being resolved by a third author (APJ). The authors performed data extraction and synthesis. All review stages were performed using the Covidence23 application. For quality assessment, we used the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Reports, Case Series, and Quasi-Experimental Studies,24 as well as version 2 of the Cochrane Risk-of-Bias (RoB 2) tool for randomized trials.25
Results
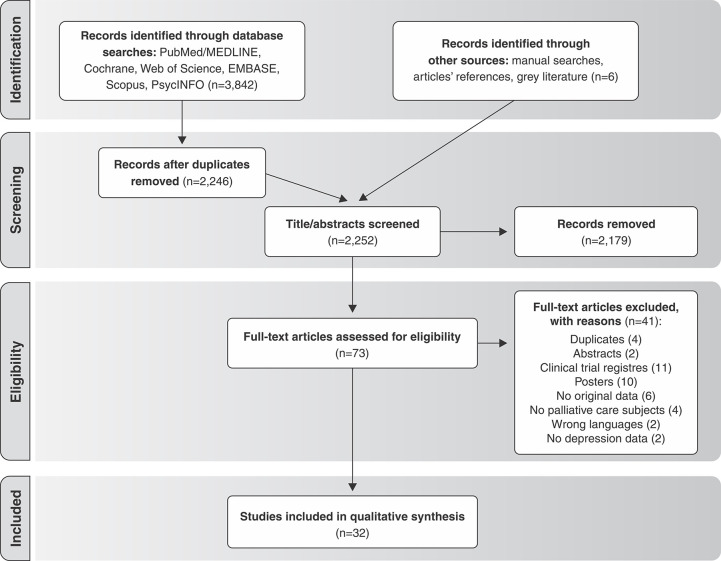
We initially imported 3,933 entries into Covidence. After removing duplicates, 2,246 titles and abstracts were screened. We also added three new records via manual searches, and retrieved unpublished data from three trials at ClinicalTrials.gov and by contacting the authors. Subsequently, 73 entries were included in the full-text review, with 32 studies being ultimately included in the analysis. Figure 1 shows the PRISMA flowchart.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Risk-of-bias (RoB) assessment
The included studies showed a generally low RoB considering the specific limitations of some study designs, including case reports and series. Table 1 summarizes the RoB assessment of the RCTs.
Table 1. Risk-of-bias (RoB) assessment for the included randomized clinical trials (RCTs).
| Author | Randomization processes | Deviations from intended interventions | Missing outcome data | Outcome measurement | Selection of result | Overall RoB |
|---|---|---|---|---|---|---|
| Salas26 | Low | Low | Low | Low | Low | Low |
| Wang27 | Low | Low | Low | Low | Low | Low |
| Fan28 | Low | Low | Low | Low | Low | Low |
| Xu29 | Low | Low | Low | Low | Low | Low |
| Fallon30 | Low | Low | Low | Low | Low | Low |
| Liu31 | Low | Low | Low | Low | Low | Low |
| Zhou32 | Low | Low | Low | Low | Low | Low |
Case reports
We included 14 case reports on 16 patients (one study reported two patients, one study reported three patients, and two reports described the same patient at different time points).33-45 These are summarized in Table 2.
Table 2. Summary of findings from case reports.
| Author | n | Age (years) | Sex | Palliative care diagnosis | Intervention (dose, route, duration) | Results (depression) | Side effects | Comments and follow-up |
|---|---|---|---|---|---|---|---|---|
| Zanicotti33,42 | 1 | 36 | Female | Metastatic ovarian cancer | Repeated IM ketamine (weekly for 10 months, 1 mg/kg) | MADRS reduction from 24 at baseline to 7 at 1 h after the first injection (71% improvement) | Moderate but tolerable dissociative effects Improved tolerability over time and stable vital signs | Sustained remission (MADRS <10) for 10 months on ketamine and 8 more months after discontinuation |
| Rocha40 | 1 | 76 | Female | Alzheimer’s disease | Repeated SC ketamine (eight doses, 0.5-0.75 mg/kg) | CGI-S reduction from 7 at baseline to 1 after two doses (3 days) | Light somnolence during ketamine sessions, no other side effects | Sustained remission for six more doses and 1 month after discontinuation |
| Stefanczyk-Sapieha45 | 1 | 50 | Male | Hormone-refractory prostate cancer | Repeated IV ketamine (two doses, 10 days interval, 0.5 mg/kg) | BDI and HAMD-17 reduction from 25 and 30 at baseline to 11 and 15 at 6 h after the second dose (56% and 50% improvement) | A brief episode of visual hallucinations during the second dose | Effects wore off quickly |
| Rodríguez-Mayoral & Domínguez-Ocadio35 | 1 | 39 | Female | Cervical epidermoid carcinoma | Single dose of IV ketamine (0.5 mg/kg) | BEDS reduction from 18 at baseline to 5 on day 17 (72%) | No side effects | |
| Swiatek36 | 1 | 62 | Male | Liver failure | Repeated oral ketamine (nine daily doses, 0.25-0.5 mg/kg) | HADS-D reduction from 16 at baseline to 10 after 48 h (38% improvement) | No safety measures reported | Mood worsened due to aggravated clinical condition Treatment was discontinued to focus on comfort |
| Barbosa37 | 1 | 65 | Male | Undifferentiated metastatic abdominal tumor | Repeated SC ketamine (four doses, twice a week, 0.5-0.75 mg/kg) | MADRS reduction from 30 at baseline to 9 on day 7 (70% improvement) | Stable vital signs, intense dissociative symptoms without discomfort | Patient died on day 11 |
| CortiñaS-Saenz43 | 1 | 42 | Female | Breast cancer with bone metastasis | Repeated IV ketamine (a single dose followed by five daily doses, 0.3-0.5 mg/kg) | HAMD-17 reduction from 22 at baseline to 13 (41% improvement) 4 days after discontinuation | Mild nausea and confusion | |
| Sexton38 | 1 | 64 | Male | Metastatic anaplastic thyroid cancer | A single continuous IV ketamine infusion (2 weeks, 0.2 mg/kg/h) | PHQ-9 24 at baseline, not reported at follow-up Remission of suicidal ideation and significant qualitative improvement on day 5 | Two episodes of worsening pain during the infusion | Sustained improvement for 2 weeks until his death |
| Rajagukguk & Lee39 | 1 | Late 40s | Male | Jejunal adenocarcinoma | A single infusion of IV ketamine (0.5 mg/kg) | Self-assessment of depression decreased from 8/10 to 0/10 in 24 h Significant qualitative improvement | Drowsiness | Worsened after 5 days, refused treatment |
| Litvan41 | 1 | 74 | Female | Mixed dementia (Alzheimer’s + vascular) | 12 sessions of ECT with esketamine anesthesia | Remission of catatonic symptoms | No safety measures reported | |
| Irwin34 | 2 | 64 | Female | COPD, respiratory failure | Repeated oral ketamine (two doses, 30 days interval, 0.5 mg/kg) | HAM-D-17 and HADS-D reduction of 66 and 50% at day 15 | No side effects reported | No response to the second dose |
| 70 | Male | Metastatic prostate cancer | A single dose of oral ketamine (0.5 mg/kg) | HAMD-17 and HADS-D reduction of 57 and 45% on day 8 | No changes in scores for adverse effects and cognitive status | Physical deterioration on day 13, could not participate in the assessments | ||
| McNulty & Hahn44 | 1 | 44 | Male | Heart failure and COPD | A single SC ketamine injection (0.5 kg/kg) followed by daily oral ketamine (0.5 mg/kg) | Improvement in self-assessed depression from 8/10 to 0/10 in 60 min | No side effects | Sustained remission under oral ketamine for 2 months |
BDI = Beck Depression Inventory; BEDS = Brief Edinburgh Depression Scale; CGI = Clinical Global Impression; COPD = chronic obstructive pulmonary disease; ECT = electroconvulsive therapy; HADS = Hospital Anxiety and Depression Rating Scale; HAMD-17 = Hamilton Depression Rating Scale; IM = intramuscular; IV = intravenous; MADRS = Montgomery-Asberg Depression Rating Scale; PHQ-9 = Patient Health Questionnaire; SC = subcutaneous.
We also included seven relevant patients from other case series. A series of five cases on the effect of ketamine in reducing L-DOPA-induced dyskinesia included one patient with depression.46 This 84-year-old man with Parkinson’s disease received a 65-h intravenous (IV) infusion of ketamine, which was titrated from 0.05 mg/kg/h to 0.15 mg/kg/h. The patient showed subjective “dramatic improvement” (the patient was suicidal before infusion and only mildly depressed on subsequent follow-up visits). No adverse effects were observed.
Another series of five cases reported the effectiveness of ketamine in patients resistant to electroconvulsive therapy or transcranial magnetic stimulation. Among them, one was diagnosed with breast cancer.47 This 51-year-old woman received a single 0.5 mg/kg IV ketamine infusion. Her Montgomery-Asberg Depression Rating Scale (MADRS)48 score was 38 at 2 h before infusion, which dropped to 27 at 120 min after infusion but returned to 39 after 24 h upon treatment discontinuation. She showed mild dissociative effects during infusion, with no other side effects.
A series of four cases reported the effect of low-dose ketamine on critical illness, with one patient having depression.49 This 55-year-old man with acute respiratory distress syndrome received a 70-h infusion of 3 µg/kg/min IV ketamine. His depression subscale of the Hospital Anxiety and Depression Scale (HADS-D)50 score was 12 at baseline, 9 after 24 h of treatment, and 8 after 24 h of treatment. There were no psychotomimetic effects during infusion.
Bryant et al.51 reported six patients treated with 0.5 kg/kg IV ketamine for geriatric depression. Among them, four patients had diseases eligible for palliative care. Infusions were administered twice weekly for two to four infusions and then every 2-6 weeks.
One patient responded to ketamine but relapsed with alcohol dependence. Two patients responded initially, but this effect was not sustained. The fourth patient received five infusions and did not show any improvement. Treatment was discontinued in all patients.51
Case series
We included three case series that reported quantitative and qualitative depressive symptom assessments, which are summarized in Table 3.
Table 3. Summary of findings from case series.
| Author(s) | n | Age (years) | Sex | Palliative care diagnosis | Intervention (dose, route, duration) | Depression measures | Results (depression) | Side effects | Comments and follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Iglewicz52 | 31 | 44-89 | Both | Multiple (cancer and non-cancer) | Single (n=22) or multiple (n=9) doses of 0.5 mg/kg oral (n=29), SC (n=1) or oral + SC (n=1) ketamine | CGI score at baseline and days 0-1, 2-3, 4-7, and 8-21 | Positive therapeutic outcomes for depression were more common (93%) than negative therapeutic outcomes at days 0-1 (p < 0.001), 2-3 (p < 0.001), and 4-7 (p < 0.05) | No serious side effects More patients had positive therapeutic outcomes than negative therapeutic outcomes (p < 0.001 at days 0-1 and 8-21, and p < 0.05 at days 2-3 and 4-7) | |
| Latuga53 | 3 | 76 | Male | Metastatic pancreatic adenocarcinoma | A single dose of SC ketamine (0.5 mg/kg) followed by repeated oral ketamine doses (daily, 0.5 mg/kg) | PHQ-4 | Reduction from PHQ-4 12 at baseline to PHQ-4 0 on day 7 | Somnolence, nausea, drowsiness, fatigue, sleepiness, feeling “strange” and “floating” Increased blood pressure at 20 min post-injection in one patient | Sustained remission with 0 on PHQ-4, died on day 50 |
| 83 | Male | End-stage alcoholic cirrhosis | Reduction on PHQ-4 from 6 at baseline to 1 on day 7 | PHQ-4 remained between 1 and 3, died on day 139 | |||||
| 49 | Female | Metastatic ovarian cancer | Reduction of PHQ-4 from 12 on day 3 to 1 on day 19 | Sustained until she died on day 37 | |||||
| Rosenblat & Li54 | 3 | 67 | Female | Cancer | Repeated IM ketamine (three doses, 3 days interval, 50-150 mg) | Improved guilt, indecisiveness and strengthened desire for MAiD | Transient drowsiness and vivid dreams | ||
| 60 | Female | Ovarian cancer | Subjective “a lot better,” improved guilt and self-esteem, withdrew request for MAiD | Transient drowsiness and dizziness | |||||
| 80 | Male | Prostate cancer | Subjective improvement of depression, improved sleep Received MAiD |
Transient drowsiness and confusion |
CGI = Clinical Global Impression; IM = intramuscular; MAiD = medical assistance in dying; PHQ-4 = Patient Health Questionnaire; SC = subcutaneous.
Iglewicz et al.52 reported on 31 hospice inpatients aged 44-89 years (mean = 68 years) who received ketamine for depression. Among them, 77% had a cancer diagnosis, while the remaining patients had coronary disease, liver failure, and other conditions. Positive therapeutic outcomes on the Clinical Global Impression (CGI)55 scale were more common than negative ones at days 0-1, 2-3, and 4-7 after dosing. Regarding side effects, more patients had positive outcomes than negative outcomes at all time points, and all side effects were psychiatric (disorientation, sedation, and hallucinations).
Latuga et al.53 reviewed the charts of three hospice patients aged 49-83 years. Two patients had a cancer diagnosis while one had a cirrhosis diagnosis. Patients received a single dose of 0.5 mg/kg SC ketamine injection followed by daily 0.5 mg/kg oral doses of ketamine. One patient had a PHQ-456 of 12 at baseline; after 2 days, he reported a notable increase in energy and sense of well-being. On day 7, his PHQ-4 score was 0 and remained 0 despite his worsening physical disability.
Another patient had a PHQ-4 score of 6 at baseline. On day 4, the oral ketamine dose was decreased from 30 mg to 5 mg due to somnolence. His PHQ-4 score was 1 on day 7 and remained between 1 and 3 until day 50, when he began experiencing mood fluctuations until he died. At baseline, the last patient had a depression score of 10/10, with no PHQ-4 scores being recorded. On day 3, her depression rating and PHQ-4 scores were 6/10 and 12, respectively. On day 12, her depression rating and PHQ-4 scores were 6/10 and 7, respectively. On day 19, her PHQ-4 score was 1. She developed delirium and lethargy on day 37 and died. These patients reported nausea, drowsiness, fatigue, sleepiness, and feeling “strange” and “floating.” One patient showed increased blood pressure at 20 minutes post-injection. Overall, ketamine was well tolerated, and the side effects were self-limited.
Rosenblat & Li54 reported on three hospice patients aged 60-80 years who requested medical assistance in dying (MAiD) and received intranasal ketamine doses (50-150 mg) at 3-day intervals. One patient with cancer, who had a poor prognosis, responded to ketamine and had improved motivation, drive, and energy. She went to a movie and restaurant as well as enjoyed playing games on a smartphone. She improved her guilt and indecisiveness; furthermore, she had a strengthened desire for MAiD. The patient reported transient drowsiness and vivid dreams. Another patient with ovarian cancer, who had a poor prognosis, reported, “Wow, I feel a lot better. I am so glad I finally decided to do the study,” after the first ketamine dose, which was confirmed by her daughter. She withdrew her request for MAiD after realizing that it was largely driven by guilt and low self-worth. The patient reported transient drowsiness and dizziness. A third patient reported significantly reduced depressive symptoms after ketamine administration. He entertained friends in his hospital room and agreed to engage in a psychotherapeutic life review. He remained firm in his decision to receive MAiD.54 The patient reported transient drowsiness and confusion.
Overall, all case series reported positive therapeutic effects. Iglewicz et al.52 reported an early response (1-3 days), which was sustained for 7 days; Latuga et al.53 reported a robust and sustained improvement that lasted 1-7 weeks; Rosenblat & Li54 reported subjective mood improvement for all patients within the treatment timeframe (9 days).
Quasi-experimental studies
We included two quasi-experimental studies, which are summarized in Table 4. A non-randomized study by Falk et al.51 included 16 patients in a specialized palliative care unit. Among them, eight patients received the intervention (a single IV infusion of 0.25 mg/kg esketamine), and eight patients were used as controls (those not requiring ketamine for pain control, who received standard care). The mean age was 52 and 55 years in the intervention and control groups, respectively. The main diagnoses were multiple cancer types and HIV infection. There was no significant effect on State Trait Anxiety Depression Inventory (STADI)57 score for depression (esketamine vs. control: F1,14 = 0.31, p = 0.59, 1-4 post-injection days vs. baseline: F1,14 = 1.80, p = 0.20). There was no evidence of a persistent psychotomimetic effect of esketamine.58
Table 4. Summary of findings from quasi-experimental studies.
| Authors | Design | n | Palliative care diagnosis | Intervention (dose, route, duration) | Control | Main outcomes | Secondary outcomes | Depression measures | Results (depression) | Side effects |
|---|---|---|---|---|---|---|---|---|---|---|
| Falk58 | Observational, retrospective chart review | 16 (eight intervention, eight control) | Inpatient/palliative care unit; cancer (various) | Single dose of 0.25 mg/kg IV S-ketamine | Standard care (does not require ketamine for pain) | Anxiety | Depression | STADI score before and 1-4 days after treatment | No significant effect of S-ketamine on depression, compared with control (F1,14 = 0.31, p = 0.59) and baseline (F1,14 = 1.80, p = 0.20) | No serious side effects, changes in restlessness/anxiety or persistent psychotomimetic effects |
| Irwin16 | Open label trial | 14 (intervention) | Hospice; multiple (cancer and non-cancer) | Daily oral doses of 0.5 mg/kg ketamine for 28 days | None | Depression | Anxiety | HADS score at baseline and days 3, 7, 14, 21, and 28 | HADS-D scores on days 14 (mean = 3.5, 95%CI 1.09-5.90, p = 0.01), 21 (mean = 4.1, 95%CI 2.0-6.2, p = 0.002), and 28 (mean = 4, 95%CI 2.3-5.9, p = 0.001) were significantly lower than the baseline scores | No vital sign changes or serious side effects Mild side effects (diarrhea, trouble sleeping, and trouble sitting) were observed in 12.5% of the patients |
HADS = Hospital Anxiety and Depression Rating Scale; IV = intravenous; STADI = State Trait Anxiety Depression Inventory.
Irwin et al.16 conducted an open-label trial of 14 hospice patients with various chronic conditions eligible for palliative care who received daily oral ketamine doses for 28 days (up to 0.5 mg/kg per dose). Four participants withdrew from the study after 14 days due to no improvement, and two withdrew due to complications unrelated to ketamine treatment. The remaining patients showed ≥ 30% improvement in the HADS-D score. Among the six patients who withdrew, none showed improved HADS-D scores. Post-hoc analyses revealed significantly lower HADS-D scores on day 14 (mean D = 3.5, d = 1.14, 95%CI 1.09-5.90, p = 0.01), day 21 (mean D = 4.1, d = 1.364, 95%CI 2.0-6.2, p = 0.002), and day 28 (mean D = 4, d = 1.34, 95%CI 2.3-5.9, p = 0.001) compared with the baseline scores. There were no changes in vital signs or serious adverse events. Mild symptoms, including diarrhea and trouble sleeping, occurred in 12.5% of the patients.
Among these quasi-experimental studies, Irwin et al.16 reported sustained improvement for 28 days, whereas Falk et al.58 reported negative results. Notably, Falk et al.58 used a lower IV ketamine dose than most of the other studies,59 which might have affected the efficacy of the drug. Furthermore, the control group comprised patients who were not prescribed ketamine because they did not have uncontrolled pain and showed a trend toward lower depression scores, which indicates a better mood due to more adequate pain control. Additionally, the study reported five deaths in the ketamine group and one death in the placebo group, which indicates better overall health in the control group.
Randomized controlled trials (RCTs)
We included seven RCTs on 1,125 participants. Most studies were conducted in the perioperative setting of tumor resection surgery.
Salas et al.26 conducted a randomized, double-blind, placebo-controlled trial on 20 patients in palliative care units. The patients had locally advanced or metastatic cancer and pain refractory to opioids. The main objective was assessing pain control; however, depressive symptoms were included as a secondary objective. Eleven patients (active group) received a single 48-h infusion of IV ketamine, which was titrated from 0.5 mg/kg per day for the first 24 h to 1 mg/kg per day for the next 24 h.
The Edmonton Symptom Assessment Scale (ESAS)60 was used to assess depressive symptoms at baseline (T0), 2 h (T1), 24 h (T2), and 48 h (T3). There were no differences in the ESAS subscale scores at T2 and T3 compared with the baseline scores (T2: ketamine = 0.27-2.41, placebo = -1.13-2.53, p = 0.13; T3: ketamine = 0.60-0.43, placebo = -2.38-3.50, p = 0.08). There were no adverse effects related to ketamine administration.
Wang et al.27 reported the use of ketamine to treat depression after laparoscopic total hysterectomy. They included 417 women with cervical carcinoma and mild/moderate depression (HAM-D score: 8-24) who underwent hysterectomy and were randomized to receive 0.5 mg/kg IV racemic ketamine (104 patients), 0.5 mg/kg (high-dose) S-ketamine (104 patients), 0.25 mg/kg (low-dose) S-ketamine (104 patients), or placebo (50 mL normal saline, 105 patients). A single ketamine infusion was administered after 1 h of analgesia.
Depression was assessed using the 17-item Hamilton Depression Rating Scale (HAMD-17)61 at 1, 2, 3, 5, and 7 postoperative days. All treatment groups showed lower HAMD-17 scores at 1, 2, and 3 days than the control group (p < 0.05). The high-dose S-ketamine (0.5 mg/kg) group had the lowest HAMD-17 score (p < 0.05). There was no among-group difference at 5 postoperative days in HAMD-17, operative time, bleeding, or hospitalization time, nor in the 1-month complication rate (nausea, dizziness, and vomiting).
Fan et al.28 conducted an RCT including 37 patients with newly diagnosed cancer. The patients were randomized to receive a single infusion of placebo (0.05 mg/kg midazolam) or IV 0.5 mg/kg racemic ketamine). After 7 days, they were assessed for depression, using the MADRS, and for suicidal ideation, using the Beck Scale for Suicidal Ideation (BSI)62 and the corresponding subscale of the MADRS (MADRS-SI).
Ketamine treatment showed antidepressant effects on day 1 (24.46±8.04 vs. 31.89±7.39, p = 0.0339) and day 3 (25.09±7.07 vs. 32.03±7.21, p = 0.0546); however, this effect was not significant on day 7. Additionally, the BSI and MADRS-SI scores were significantly decreased in the ketamine group on day 1 (BSI, p = 0.0474; MADRS-SI, p = 0.0119) and day 3 (BSI, p = 0.0265; p = 0.0107). There were no significant adverse psychiatric symptoms during the follow-up period.
Xu29 conducted an RCT on 50 women who underwent modified radical mastectomy for unilateral breast cancer and were randomized to receive intraoperative infusions of 0.5 mg/kg ketamine IV or isotonic saline for depression (HAMD-17 score ≥ 17). The HAMD-17 scores were significantly lower in the ketamine group at day 1 (12.55±4.50 vs. 18.64±3.83, p < 0.05) and day 3 (10.64±4.33 vs. 16.27±4.45, p < 0.05), but not at day 7 after operation (13.45±5.21 vs 17.36±6.25, p>0.05). Moreover, the HAMD-17 scores in the ketamine group were lower (p < 0.05) on days 1, 3, and 7 than the baseline scores.
There were reports of nausea, irritability, and mild respiratory depression, with no significant between-group difference in the incidence of adverse reactions. Compared with the control group, the ketamine group had a non-significantly longer duration of extubation (p > 0.05).
An RCT conducted by Fallon30 reported the effect of oral ketamine for neuropathic pain in patients with cancer, with depressive symptoms being a secondary measure. They randomized 214 patients to receive placebo or a daily dose of oral ketamine (titrated from 40 mg to 400 mg daily for 2 weeks and then maintained for 16 days). There was no between-group difference in HADS-D score improvement (ketamine = -3.481, placebo = -3.654, median difference = 0.173 [-0.500 to 0.958]). There were eight and 10 adverse events (cognitive disturbance, dizziness, fatigue, nausea, and somnolence) in the ketamine and placebo groups, respectively.
An RCT conducted by Liu31 reported on 303 women with breast cancer who underwent modified radical mastectomy and were randomized to receive an IV injection of 0.125 mg/kg racemic ketamine (n=102), 0.125 mg/kg of S-ketamine (n=101), or placebo (normal saline, n=100) for mild/moderate depression (HAMD-17 score = 8-24). They underwent follow-up assessment using the HAMD-17 for 3 months.
Compared with the placebo group, both ketamine groups showed significantly lower HAMD-17 scores at 3 days (S-ketamine = 11.4±2.2; racemic ketamine = 13.2±2.5; placebo = 16.4±2.0, p < 0.001), 1 week (S-ketamine = 9.4±3.0; racemic ketamine = 10.5±2.9; placebo = 11.2±3.6, p < 0.001) and 1 month (S-ketamine = 6.9±2.8; racemic ketamine = 9.5±2.9; placebo = 11.0±3.8, p < 0.001) after surgery. Further, the S-ketamine group showed lower scores than the racemic ketamine group (p < 0.05). At 3 months postoperatively, there were no significant among-group differences in HAMD-17 scores (S-ketamine = 6.5±3.3; racemic ketamine = 7.5±3.2; placebo = 7.5±3.0, p = 0.050). There were no significant among-group differences in mean operative time (p = 0.562); mean bleeding volume (p = 0.556); and nausea, dizziness, or vomiting (p = 0.986).
An RCT conducted by Zhou32 reported on 84 patients undergoing elective surgical resection of supratentorial brain tumors who had moderate/severe depression (Patient Health Questionnaire 963 [PHQ-9] score ≥ 10 and MADRS score ≥ 22) and received a single intraoperative infusion of 0.5 mg/kg IV ketamine (n=41) or placebo (normal saline, n=43). Patients were assessed on days 1 and 3 as well as at discharge. Compared with the placebo group, the ketamine group showed a higher treatment response rate on day 3 (41.5 vs. 16.3%, relative risk [RR]: 2.25, 95%CI 1.18-5.50) and remission rate on discharge (29.3 vs. 7.0%; RR: 4.20, 95%CI 1.28-13.80). Further, compared with the placebo group, the ketamine group showed nonsignificantly lower postoperative depressive scores. There were no significant between-group differences in the number of patients with manic, psychotic, or dissociative symptoms, nor in delirium, prolonged hospital stay, or blood loss.
Among the seven included RCTs, two reported negative results. These two RCTs26,30 used pain control and depressive symptoms as the primary and secondary objectives, respectively. Accordingly, there were no specified cutoff values for minimum depression severity, and the samples might have included individuals without depression as well as those with severe depression, which might have resulted in weaker findings regarding efficacy. This is consistent with the findings of a large negative RCT conducted by Mashour et al.,64 which we did not include due to incompatible outcomes. Its primary objective was to use intraoperative ketamine to prevent depressive symptoms after major surgeries. They included 670 patients aged ≥ 60 years who underwent cardiac and non-cardiac surgeries and were randomized to receive 1 mg/kg IV ketamine (high dose, 223 patients), 0.5 mg/kg (low dose, 226 patients), or saline placebo (221 patients). There was no significant among-group difference in the severity of depressive symptoms on days 3 (p = 0.773) and 30 (p = 0.271) after surgery.
The limited efficacy of ketamine as a preventive intervention could have contributed to the negative results of the two aforementioned studies. The patients with depression may have responded to the intervention; however, euthymic individuals may also develop mood disorders, which counterbalances the effect.
Four studies described the perioperative use of ketamine in patients with clinically significant depressive symptoms,27,29,31,32 which reported an overall positive effect that lasted for 5-30 days. Among them, two compared the use of S-ketamine and R/S-ketamine, with S-ketamine showing a more robust effect in both studies.27,31 Moreover, Wang et al.27 described that the 0.5 mg/kg dose had higher efficacy than the 0.25 mg/kg dose. This is consistent with the previously reported higher potency of S-ketamine, owing to its higher affinity for NMDA receptors.65
Fan et al.28 demonstrated the treatment efficacy of ketamine against depressive symptoms (more specifically, against suicidal ideation). Compared with placebo, ketamine showed a positive effect that started at day 1 and was sustained for at least 3 days. Additionally, ketamine showed a fast-acting effect on suicidal ideation,66 which is highly beneficial for specific populations. Given the inherent urgency in palliative care as well as the urgency required for controlling suicidal behavior, ketamine is a potential game-changer.
Gray literature
We report data available for three unpublished clinical trials. An RCT conducted by Bright67 (https://clinicaltrials.gov/ct2/show/study/NCT01680172) recruited five patients with cancer who received a single oral dose of 0.5 mg/kg ketamine solution or placebo for depression (HADS-D score > 11). The mean (SD) HADS-D score for the ketamine group (n=3) was 12.7 (1.5) at baseline and 11.7 (2.3) at 120 min, while the corresponding values in the placebo group (n=2) were 14.5 (2.1) and 10.5 (3.5), respectively. All participants had minor side effects (e.g., rapid heart rate, vomiting, headache, drowsiness, and shortness of breath).
An open-label trial conducted by Singh68 (https://clinicaltrials.gov/ct2/show/results/NCT03146806) enrolled 10 patients who received ketamine for uncontrolled cancer pain and depression, using the MADRS score as a secondary outcome. The patients were followed for five visits at 2-to-5-day intervals. On the first visit, the patients received 10 mg intranasal ketamine, with the MADRS scores (SD) being 18.7 (6.57) at baseline and 13.5 (4.38) at 180 min. On the second visit, they received 10 mg IV ketamine, with the MADRS scores (SD) being 13.4 (6.40) at baseline and 13.3 (5.31) at 180 min. On the third visit, they received 30 mg intranasal ketamine, with the MADRS scores (SD) being 12 (5.05) at baseline and 11.3 (6.15) at 180 min. On the fourth visit, they received 50 mg intranasal ketamine, with the MADRS scores (SD) being 11.89 (8.34) at baseline and 9.67 (8.34) at 180 min. The fifth visit served only as a follow-up, with the MADRS scores being 14.78 (7.31).
Rodríguez-Mayoral69 conducted an RCT (https://clinicaltrials.gov/ct2/show/study/NCT04471818) on eight patients with cancer who were randomized to receive 0.5 mg/kg IV ketamine (n=4) or placebo (n=4) for depression. Weekly injections were administered for 4 weeks, with assessment using the Brief Edinburgh Depression Scale (BEDS).70 The ketamine group had lower BEDS scores than the placebo group at 3 weeks post-intervention (3.0 [SD: 2.160] vs. 11.75 [SD: 3.594]; p = 0.006). The most common adverse events were drowsiness and nausea; there were no serious adverse events.
Among these unpublished studies, Rodriguez-Mayoral69 reported a robust response to weekly IV ketamine. Similarly, the remaining unpublished data demonstrated a progressive decrease in symptom severity after repeated ketamine dosing.
Discussion
Across all the included studies, the most consistent finding was safety. There were no reports of serious adverse effects leading to treatment discontinuation. The most common adverse effects were dissociative symptoms, nausea, vomiting, dizziness, somnolence, and confusion, with most RCTs reporting similar rates between the active and placebo groups. Further, studies that used ketamine in perioperative settings did not report differences in complication rates, including operative time, blood loss, and length of hospital stay.27,29-31 Hypertension and hemodynamic instability, which are common concerns regarding the use of ketamine,71 were not limiting factors in the reported studies. One case report described a long follow-up period with 10 months of repeated doses and a progressive improvement in tolerability.42
Our study selection approach allowed analysis of a large spectrum of palliative-care patients with low or high life expectancies, as well as patients with cancer and non-cancer diagnoses. Ketamine appeared to be safe even among patients with very poor prognoses; however, treatment was frequently interrupted due to complications of the primary disease. These tolerability findings could inform the widespread use of ketamine for depression in palliative care settings, since its use in this scenario is mainly impeded by the lack of safety data.
Regarding efficacy, most case reports described a large positive effect, with an early onset of action from as early as 1 h to approximately 48 h. Maintenance of effect was variable, with only a few participants showing sustained remission after a single ketamine dose.35 However, most patients relapsed after 1-7 days and required follow-up doses, with some patients not responding. The routes of administration, dosing regimen, and reported follow-up measures varied widely.
Nonetheless, numerous aspects regarding ketamine treatment within palliative care remain unclear. As mentioned above, only one small-scale RCT has focused on patients with depression outside the perioperative context,28 with only a few other small-scale unpublished clinical trials. Conversely, the two other available studies not conducted in a surgical context primarily focused on pain and reported mixed results.
A 2017 Cochrane review72 included three studies on the use of ketamine for cancer pain, with various regimens (intrathecal ketamine, IV bolus ketamine, and SC ketamine); the largest study used SC ketamine.73 The intervention was generally safe, but prominent psychomimetic effects were reported more frequently in the ketamine group. Other minor side effects, such as drowsiness, nausea, hallucinations, cognitive disturbance, and injection site reaction, as well as two major adverse reactions (bradyarrhythmia and cardiac arrest), were also reported. Therefore, safety evidence in this area remains scarce, and efficacy for the treatment of pain was extrapolated from trials of acute perioperative pain.
Furthermore, data regarding route of administration are highly heterogeneous. Findings with the SC route from pain studies cannot be extrapolated for the treatment of depressive symptoms, where most utilize IV or oral administration (Table 5). Even more scarce are studies that used an intranasal route of administration, which is the only label-approved route for the treatment of depression. Although robust data are emerging on the comparison of ketamine enantiomers for depression in the general population,74 there is very little information on the preferred route.
Table 5. Summary of findings from RCTs.
| Authors | n | Setting and palliative care diagnosis | Intervention (dose, route, duration) | Control | Main outcomes | Secondary outcomes | Depression measures | Results (depression) | Side effects |
|---|---|---|---|---|---|---|---|---|---|
| Salas26 | 20 (11 intervention, nine control) | Palliative care unit; locally advanced or metastatic cancer and pain refractory to opioids | A single continuous 48-h IV infusion of morphine + ketamine (0.5-1.0 mg/kg) | A single continuous 48-h IV infusion of morphine + placebo (not described) | Pain | Depression, tiredness, nausea, anxiety, drowsiness, lack of appetite, well-being, shortness of breath, sedation, satisfaction | ESAS score at baseline (T0), 2 h (T1), 24 h (T2), and 48 h (T3) | No differences were found (T2: ketamine = 0.27-2.41 placebo = -1.13-2.53 p = 0.13; T3: ketamine = 0.60-1.43 placebo = -2.38-3.50 p = 0.08) | One patient died in the placebo group No ketamine-related adverse effects |
| Wang27 | 417 (313 intervention, 104 control) | Hospital; women with cervical carcinoma undergoing laparoscopic total hysterectomy | A single IV injection 1 h after analgesia of 0.5 mg/kg R/S-ketamine (n=104), 0.5 mg/kg S-ketamine (n=104); or 0.25 mg/kg S-ketamine (n=104) | A single IV injection 1 h after analgesia of 50 mL normal saline (104 patients) | Depression, pain | None | HAMD-17 score at baseline and days 1, 2, 3, 5, and 7 | In all treatment groups, the HAMD-17 scores were lower at 1, 2, and 3 days than in the control group (p < 0.05)The high-dose S-ketamine (0.5 mg/kg) group had the lowest HAMD-17 scores (p < 0.05) No difference at 5 postoperative days |
No difference in operative time, bleeding, length of hospital stay, or 1-month complication rate (nausea, dizziness, vomiting) |
| Fan28 | 37 (20 intervention, 17 control) | Hospital; newly diagnosed cancer (various) | A single 40-min IV infusion of 0.5 mg/kg IV R/S-ketamine | A single 40-min IV infusion of 0.05 mg/kg midazolam | Suicidal ideation | Depression | MADRS score at baseline and days 1, 3, and 7 | The ketamine group was less depressed on days 1 (MADRS = 24.46±8.04 vs. 31.89±7.39, p = 0.0339) and 3 (25.09±7.07 vs. 32.03±7.21, p = 0.0546) No difference on day 7 |
No increase in emergent psychiatric symptoms |
| Xu29 | 50 (25 intervention, 25 control) | Hospital; female patients undergoing modified radical mastectomy of unilateral breast cancer | A single 10-min IV infusion of 0.5 mg/kg ketamine 1 h after the start of anesthesia | A single 10-min IV infusion of isotonic saline 1 h after the start of anesthesia | Depression | Pain | HAMD-17 score at baseline as well as days 1, 3 and 7 | The ketamine group had significantly lower HAMD-17 scores than the control group at 1 and 3 postoperative days (p < 0.05) At postoperative day 7, there was no significant between-group difference (p > 0.05) |
No between-group difference in the incidence of adverse reactions The ketamine group had one case of nausea, two cases of irritability, and one case of mild respiratory depression |
| Fallon30 | 214 (107 intervention, 107 control) | Unspecified setting; cancer-related neuropathic pain | Daily doses of 40-400 mg oral ketamine (2-week titration followed by fixed dose for 16 days) | Placebo (not described) | Pain | Depression, anxiety | HADS score (time points not specified) | No between-group difference Mean HADS (ketamine) = -3.481; mean HADS (placebo) = -3.654; median difference = 0.173 (-0.500 to 0.958) |
Eight and 10 adverse events in the ketamine and placebo groups, respectively (cognitive disturbance, dizziness, fatigue, nausea, somnolence) |
| Liu31 | 303 (203 intervention, 100 control) | Hospital; female breast cancer patients who received modified radical mastectomy | A single IV infusion of 0.125 mg/kg of R/S-ketamine (n=102); or 0.125 mg/kg of S-ketamine (n=101) | A single IV infusion of normal saline (n=100) | Depression | Pain | HAMD-17 at baseline and days 3, 7, 30 and 90 | HAMD-17 scores were lower at three days, 1 week, and 1 month after surgery in both ketamine groups when compared to the control group Scores in the S-ketamine group were lower than in the R/S-ketamine group (p < 0.05)No difference at 3 months |
No side effects are reported |
| Zhou32 | 84 (41 intervention, 43 control) | Hospital; patients undergoing elective surgical resection of supratentorial brain tumor | A single 40-min IV infusion of 0.5 mg/kg ketamine | A single 40-min IV infusion of normal saline | Depression | Anxiety, delirium, pain | PHQ-9 and MADRS scores at baseline; days 1, 2, and 3; and at discharge. | The ketamine group had a higher proportion of patients with treatment response on day 3 than the placebo group (41.5 vs 16.3%, respectively; RR: 2.25, 95%CI 1.18-5.50) The rate of remission on discharge was higher with ketamine than with placebo (29.3 vs. 7.0%; RR: 4.20, 95%CI 1.28-13.80) |
Three days after surgery, there was no significant between-group difference in the number of patients who experienced manic symptoms (4.9 vs. 2.3%), psychotic symptoms (7.3 vs. 9.3%), or dissociative symptoms (7.3 vs. 2.3%) |
ESAS = Edmonton Symptom Assessment Scale; HADS = Hospital Anxiety and Depression Rating Scale; HAMD-17 = Hamilton Depression Rating Scale; IV = intravenous; MADRS = Montgomery-Asberg Depression Rating Scale; PHQ-9 = Patient Health Questionnaire; RCT = randomized clinical trials; RR = relative risk.
Apart from psychomimetic, neurological, and gastrointestinal adverse effects, there are other concerns regarding the use of ketamine in complex patients. Through catecholamine release and reuptake blockade, ketamine can increase blood pressure and pose a risk to patients with coronary artery disease and hypertension. Other rare adverse effects are laryngospasm and apnea.75 Subsequently, there are specific concerns for the use of ketamine in oncologic patients. Some of the mechanisms of action hypothesized to contribute to the antidepressant effect of ketamine are the activation of neuroplasticity-related signaling pathways such as brain-derived neurotrophic factor and mechanistic target of rapamycin (mTOR) complex 1, which promote protein synthesis.76 This same mechanism is implicated in tumor growth, and activating mutations of mTOR have been linked to multiple cancer types, whereas mTOR inhibitors have been used for cancer treatment.77
However, these concerns are not strongly supported by evidence, as there are no studies describing progression of malignancy that may be attributed to ketamine use. Furthermore, in vitro studies also suggest a reduction of mTOR activity in ketamine-treated cells, resulting in increased apoptosis of tumoral cells. This finding was also replicated in an animal model, which showed a reduction in tumor volume.78 Finally, a point can be made that even if the use of ketamine for the treatment of depression results in accelerated tumor growth, the benefits may still outweigh the risks in patients with very short prognoses.
Currently, ketamine can be considered for the treatment of depression in patients with cancer receiving palliative care who are undergoing tumor resection under general anesthesia. In this setting, ketamine could be a safe and effective option for rapid symptom relief. Based on the available evidence, the preferred regimen would be a single IV infusion of 0.5 mg/kg S-ketamine after anesthesia induction.
Evidence to support the efficacy of ketamine in non-surgical patients receiving palliative care is now mainly derived from case reports and open-label studies. Therefore, we cannot draw specific conclusions for daily clinical use. Nevertheless, the safety data are reassuring; additionally, there is a need for large-scale RCTs aimed at depressive symptom relief within various palliative care populations.
Finally, our findings indicate that ketamine is not appropriate as a preventive measure for depressive symptoms in patients without depression receiving palliative care.
In conclusion, ketamine is a potential therapeutic option for depression treatment in palliative care settings. It appears to be safe, well-tolerated, and effective for perioperative depression. The remaining evidence is still conflicting; however, case reports and open-label studies have provided promising results. Future studies should focus on non-surgical and non-cancer palliative care populations and seek to determine the optimal therapeutic regimen.
Disclosure
The authors report no conflicts of interest.
Acknowledgements
This study was funded in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, Finance Code 001. MGB receives financial support from CAPES, Finance Code 001.
Footnotes
How to cite this article: Barbosa MG, Garcia GT, Sarin LM, Jackowski AP. Efficacy and safety of ketamine for the treatment of depressive symptoms in palliative care: a systematic review. Braz J Psychiatry. 2023;45:182-195. http://doi.org/10.47626/1516-4446-2022-2876
References
- 1.Ferrari AJ, Somerville AJ, Baxter AJ, Norman R, Patten SB, Vos T, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychol Med. 2013;43:471–81. doi: 10.1017/S0033291712001511. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, et al. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker J, Hansen CH, Martin P, Sawhney A, Thekkumpurath P, Beale C, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol. 2013;24:895–900. doi: 10.1093/annonc/mds575. [DOI] [PubMed] [Google Scholar]
- 4.Rayner L, Price A, Evans A, Valsraj K, Hotopf M, Higginson IJ. Antidepressants for the treatment of depression in palliative care: systematic review and meta-analysis. Palliat Med. 2011;25:36–51. doi: 10.1177/0269216310380764. [DOI] [PubMed] [Google Scholar]
- 5.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaynes BN, Rush AJ, Trivedi MH, Wisniewski SR, Spencer D, Fava M. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 2008;75:57–66. doi: 10.3949/ccjm.75.1.57. [DOI] [PubMed] [Google Scholar]
- 7.Ng CG, Boks MP, Roes KC, Zainal NZ, Sulaiman AH, Tan SB, et al. Rapid response to methylphenidate as an add-on therapy to mirtazapine in the treatment of major depressive disorder in terminally ill cancer patients: a four-week, randomized, double-blinded, placebo-controlled study. Eur Neuropsychopharmacol. 2014;24:491–8. doi: 10.1016/j.euroneuro.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan DR, Mongoue-Tchokote S, Mori M, Goy E, Ganzini L. Randomized, double-blind, placebo-controlled study of methylphenidate for the treatment of depression in SSRI-treated cancer patients receiving palliative care. Psychooncology. 2017;26:1763–9. doi: 10.1002/pon.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centeno C, Sanz A, Cuervo MA, Ramos D, Hernansanz S, Gonzalez J, et al. Multicentre, double-blind, randomised placebo-controlled clinical trial on the efficacy of methylphenidate on depressive symptoms in advanced cancer patients. BMJ Support Palliat Care. 2012;2:328–33. doi: 10.1136/bmjspcare-2011-000093. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Vlisides PE. Ketamine: 50 years of modulating the mind. Front Hum Neurosci. 2016;10:612. doi: 10.3389/fnhum.2016.00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rot MAH, Zarate CA, Jr, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biol Psychiatry. 2012;72:537–47. doi: 10.1016/j.biopsych.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 13.Lener MS, Kadriu B, Zarate CA., Jr Ketamine and beyond: investigations into the potential of glutamatergic agents to treat depression. Drugs. 2017;77:381–401. doi: 10.1007/s40265-017-0702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thakurta RG, Das R, Bhattacharya AK, Saha D, Sen S, Singh OP, et al. Rapid response with ketamine on suicidal cognition in resistant depression. Indian J Psychol Med. 2012;34:170–5. doi: 10.4103/0253-7176.101793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bobo WV, Voort JLV, Croarkin PE, Leung JG, Tye SJ, Frye MA. Ketamine for treatment-resistant unipolar and bipolar major depression: critical review and implications for clinical practice. Depress Anxiety. 2016 Aug;33(8):698–710. doi: 10.1002/da.22505. [DOI] [PubMed] [Google Scholar]
- 16.Irwin SA, Iglewicz A, Nelesen RA, Lo JY, Carr CH, Romero SD, et al. Daily oral ketamine for the treatment of depression and anxiety in patients receiving hospice care: a 28-day open-label proof-of-concept trial. J Palliat Med. 2013;16:958–65. doi: 10.1089/jpm.2012.0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bredlau AL, Thakur R, Korones DN, Dworkin RH. Ketamine for pain in adults and children with cancer: a systematic review and synthesis of the literature. Pain Med. 2013;14:1505–17. doi: 10.1111/pme.12182. [DOI] [PubMed] [Google Scholar]
- 18.Goldman N, Frankenthaler M, Klepacz L. The efficacy of ketamine in the palliative care setting: a comprehensive review of the literature. J Palliat Med. 2019;22:1154–61. doi: 10.1089/jpm.2018.0627. [DOI] [PubMed] [Google Scholar]
- 19.Schimmel N, Breeksema JJ, Smith-Apeldoorn SY, Veraart J, van den Brink W, Schoevers RA. Psychedelics for the treatment of depression, anxiety, and existential distress in patients with a terminal illness: a systematic review. Psychopharmacology (Berl) 2022;239:15–33. doi: 10.1007/s00213-021-06027-y. [DOI] [PubMed] [Google Scholar]
- 20.Borasio GD. Translating the World Health Organization definition of palliative care into scientific practice. Palliat Support Care. 2011;9:1–2. doi: 10.1017/S1478951510000489. [DOI] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenwax LK, McNamara B, Blackmore AM, Holman CD. Estimating the size of a potential palliative care population. Palliat Med. 2005;19:556–62. doi: 10.1191/0269216305pm1067oa. [DOI] [PubMed] [Google Scholar]
- 23.Veritas Health Innovation [Internet] Covidence systematic review software. www.covidence.org [Google Scholar]
- 24.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetc R, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. JBI; 2020. https://synthesismanual.jbi.global [Google Scholar]
- 25.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 26.Salas S, Frasca M, Planchet-Barraud B, Burucoa B, Pascal M, Lapiana JM, et al. Ketamine analgesic effect by continuous intravenous infusion in refractory cancer pain: considerations about the clinical research in palliative care. J Palliat Med. 2012;15:287–93. doi: 10.1089/jpm.2011.0353. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Wang Y, Xu X, Peng S, Xu F, Liu P. Use of various doses of s-ketamine in treatment of depression and pain in cervical carcinoma patients with mild/moderate depression after laparoscopic total hysterectomy. Med Sci Monit. 2020;26:e922028. doi: 10.12659/MSM.922028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan W, Yang H, Sun Y, Zhang J, Li G, Zheng Y, et al. Ketamine rapidly relieves acute suicidal ideation in cancer patients: a randomized controlled clinical trial. Oncotarget. 2017;8:2356–60. doi: 10.18632/oncotarget.13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu R, Zhan Y, Chen S. Effect of intraoperative single administration of sub-anesthesia ketamine on breast cancer patients with depression. Biomed Res. 2017:552–6. [Google Scholar]
- 30.Fallon MT, Wilcock A, Kelly CA, Paul J, Lewsley LA, Norrie J, et al. Oral ketamine vs placebo in patients with cancer-related neuropathic pain: a randomized clinical trial. JAMA Oncol. 2018;4:870–2. doi: 10.1001/jamaoncol.2018.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu P, Li P, Li Q, Yan H, Shi X, Liu C, et al. Effect of pretreatment of s-ketamine on postoperative depression for breast cancer patients. J Invest Surg. 2021;34:883–8. doi: 10.1080/08941939.2019.1710626. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Sun W, Zhang G, Wang A, Lin S, Chan MTV, et al. Ketamine alleviates depressive symptoms in patients undergoing intracranial tumor resection: a randomized controlled trial. Anesth Analg. 2021;133:1588–97. doi: 10.1213/ANE.0000000000005752. [DOI] [PubMed] [Google Scholar]
- 33.Zanicotti CG, Perez D, Glue P. Mood and pain responses to repeat dose intramuscular ketamine in a depressed patient with advanced cancer. J Palliat Med. 2012;15:400–3. doi: 10.1089/jpm.2011.0314. [DOI] [PubMed] [Google Scholar]
- 34.Irwin SA, Iglewicz A. Oral ketamine for the rapid treatment of depression and anxiety in patients receiving hospice care. J Palliat Med. 2010;13:903–8. doi: 10.1089/jpm.2010.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodríguez-Mayoral O, Pérez-Esparza R, Domínguez-Ocadio G, Allende-Pérez S. Ketamine as augmentation for the treatment of major depression and suicidal risk in advanced cancer: Case report. Palliat Support Care. 2020;18:110–2. doi: 10.1017/S1478951519000580. [DOI] [PubMed] [Google Scholar]
- 36.Swiatek KM, Jordan K, Coffman J. New use for an old drug: oral ketamine for treatment-resistant depression. BMJ Case Rep. 2016;2016:bcr2016216088. doi: 10.1136/bcr-2016-216088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barbosa MG, Delfino RS, Sarin LM, Jackowski AP. Repeated subcutaneous esketamine administration for depressive symptoms and pain relief in a terminally ill cancer patient: a case report. Palliat Med. 2020;34:822–5. doi: 10.1177/0269216320910351. [DOI] [PubMed] [Google Scholar]
- 38.Sexton J, Atayee RS, Bruner HC. Case report: ketamine for pain and depression in advanced cancer. J Palliat Med. 2018;21:1670–3. doi: 10.1089/jpm.2017.0551. [DOI] [PubMed] [Google Scholar]
- 39.Rajagukguk S, Lee T. Intravenous ketamine as an effective and safe treatment in a suicidal patient with cancer who was nil per os. Psychosomatics. 2020;61:371–4. doi: 10.1016/j.psym.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Rocha FL, Cunha UGV, Paschoalin RC, Hara C, Thomaz DP. Use of subcutaneous ketamine to rapidly improve severe treatment-resistant depression in a patient with Alzheimer's disease. Int Clin Psychopharmacol. 2021;36:104–5. doi: 10.1097/YIC.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 41.Litvan Z, Bauer M, Kasper S, Frey R. Electroconvulsive therapy with S-ketamine anesthesia for catatonia in coexisting depression and dementia. Int Psychogeriatr. 2017;29:1223–5. doi: 10.1017/S104161021700014X. [DOI] [PubMed] [Google Scholar]
- 42.Zanicotti CG, Perez D, Glue P. Case report: long-term mood response to repeat dose intramuscular ketamine in a depressed patient with advanced cancer. J Palliat Med. 2013;16:719–20. doi: 10.1089/jpm.2013.0057. [DOI] [PubMed] [Google Scholar]
- 43.Cortiñas-Saenz M, Alonso-Menoyo MB, Errando-Oyonarte CL, Alférez-Garcia I, Carricondo-Martínez MA. Efecto antidepresivo de dosis subanestésicas de ketamina en el postoperatorio de una paciente con depresión no controlada. Rev Esp Anestesiol Reanim. 2013;60:110–3. doi: 10.1016/j.redar.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 44.McNulty JP, Hahn K. Compounded oral ketamine. Int J Pharm Compd. 2012;16:364–8. Erratum in: Int J Pharm Compd. 2019;23:438. [PubMed] [Google Scholar]
- 45.Stefanczyk-Sapieha L, Oneschuk D, Demas M. Intravenous ketamine “burst” for refractory depression in a patient with advanced cancer. J Palliat Med. 2008;11:1268–71. doi: 10.1089/jpm.2008.9828. [DOI] [PubMed] [Google Scholar]
- 46.Sherman SJ, Estevez M, Magill AB, Falk T. Case reports showing a long-term effect of subanesthetic ketamine infusion in reducing l-DOPA-induced dyskinesias. Case Rep Neurol. 2016;8:53–8. doi: 10.1159/000444278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gosek P, Chojnacka M, Bieńkowski P, Swiecicki Ł. [Effectiveness of ketamine in depressed patients resistant to ECT or rTMS therapy] Psychiatr Pol. 2014;48:49–58. [PubMed] [Google Scholar]
- 48.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 49.Moitra VK, Patel MK, Darrah D, Moitra A, Wunsch H. Low-dose ketamine in chronic critical illness. J Intensive Care Med. 2016;31:216–20. doi: 10.1177/0885066615587868. [DOI] [PubMed] [Google Scholar]
- 50.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 51.Bryant KA, Altinay M, Finnegan N, Cromer K, Dale RM. Effects of repeated intravenous ketamine in treatment-resistant geriatric depression: a case series. J Clin Psychopharmacol. 2019;39:158–61. doi: 10.1097/JCP.0000000000001006. [DOI] [PubMed] [Google Scholar]
- 52.Iglewicz A, Morrison K, Nelesen RA, Zhan T, Iglewicz B, Fairman N, et al. Ketamine for the treatment of depression in patients receiving hospice care: a retrospective medical record review of thirty-one cases. Psychosomatics. 2015;56:329–37. doi: 10.1016/j.psym.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Latuga NM, Luczkiewicz DL, Grant PC, Levy K, Hansen E, Kerr CW. Single subcutaneous ketamine dose followed by oral ketamine for depression symptoms in hospice patients: a case series. J Pain Palliat Care Pharmacother. 2021;35:106–2. doi: 10.1080/15360288.2021.1883182. [DOI] [PubMed] [Google Scholar]
- 54.Rosenblat JD, Li M. Is ketamine a litmus test for capacity in assisted dying with depression? Psychooncology. 2021;30:417–20. doi: 10.1002/pon.5586. [DOI] [PubMed] [Google Scholar]
- 55.Guy W. ECDEU assessment manual for psychopharmacology. Maryland: US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976. [Google Scholar]
- 56.Löwe B, Wahl I, Rose M, Spitzer C, Glaesmer H, Wingenfeld K, et al. A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord. 2010;122:86–95. doi: 10.1016/j.jad.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 57.Renner KH, Hock M, Bergner-Köther R, Laux L. Differentiating anxiety and depression: the State-Trait Anxiety-Depression Inventory. Cogn Emot. 2018;32:1409–23. doi: 10.1080/02699931.2016.1266306. [DOI] [PubMed] [Google Scholar]
- 58.Falk E, Schlieper D, van Caster P, Lutterbeck MJ, Schwartz J, Cordes J, et al. A rapid positive influence of S-ketamine on the anxiety of patients in palliative care: a retrospective pilot study. BMC Palliat Care. 2020;19:1. doi: 10.1186/s12904-019-0499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marcantoni WS, Akoumba BS, Wassef M, Mayrand J, Lai H, Richard-Devantoy S, et al. A systematic review and meta-analysis of the efficacy of intravenous ketamine infusion for treatment resistant depression: January 2009 – January 2019. J Affect Disord. 2020;277:831–41. doi: 10.1016/j.jad.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 61.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beck AT, Steer RA, Ranieri WF. Scale for Suicide Ideation: psychometric properties of a self-report version. J Clin Psychol. 1988;44:499–505. doi: 10.1002/1097-4679(198807)44:4<499::aid-jclp2270440404>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 63.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mashour GA, Abdallah AB, Pryor KO, El-Gabalawy R, Vlisides PE, Jacobsohn E, et al. Intraoperative ketamine for prevention of depressive symptoms after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Br J Anaesth. 2018;121:1075–83. doi: 10.1016/j.bja.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muller J, Pentyala S, Dilger J, Pentyala S. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol. 2016;6:185–92. doi: 10.1177/2045125316631267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Witt K, Potts J, Hubers A, Grunebaum MF, Murrough JW, Loo C, et al. Ketamine for suicidal ideation in adults with psychiatric disorders: A systematic review and meta-analysis of treatment trials. Aust N Z J Psychiatry. 2020;54:29–45. doi: 10.1177/0004867419883341. [DOI] [PubMed] [Google Scholar]
- 67.Bright RP. Oral ketamine in the treatment of depression and anxiety in patients with cancer [Internet] ClinicalTrials.gov: NCT01680172. 2012 Sep 7 https://clinicaltrials.gov/ct2/show/study/NCT01680172 [Google Scholar]
- 68.Singh V. Intranasal (NAS) ketamine for cancer pain [Internet] ClinicalTrials.gov: NCT03146806. 2017 May 10 https://clinicaltrials.gov/ct2/show/results/NCT03146806 [Google Scholar]
- 69.Mayoral ORR. Ketamine or placebo in patients with major depression and advanced cancer (KODIAC) [Internet] ClinicalTrials.gov: NCT04471818. 2020 Jul 15 https://clinicaltrials.gov/ct2/show/study/NCT04471818 [Google Scholar]
- 70.Lloyd-Williams M, Shiels C, Dowrick C. The development of the Brief Edinburgh Depression Scale (BEDS) to screen for depression in patients with advanced cancer. J Affect Disord. 2007;99:259–64. doi: 10.1016/j.jad.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 71.Short B, Fong J, Galvez V, Shelker W, Loo CK. Side-effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65–78. doi: 10.1016/S2215-0366(17)30272-9. [DOI] [PubMed] [Google Scholar]
- 72.Bell RF, Eccleston C, Kalso EA. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst Rev. 2012;11:CD003351. doi: 10.1002/14651858.CD003351.pub2. [DOI] [PubMed] [Google Scholar]
- 73.Hardy J, Quinn S, Fazekas B, Plummer J, Eckermann S, Agar M, et al. Randomized, double-blind, placebo-controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. J Clin Oncol. 2012;30:3611–7. doi: 10.1200/JCO.2012.42.1081. [DOI] [PubMed] [Google Scholar]
- 74.Bahji A, Vazquez GH, Zarate CA., Jr Comparative efficacy of racemic ketamine and esketamine for depression: A systematic review and meta-analysis. J Affect Disord. 2021;278:542–55. doi: 10.1016/j.jad.2020.09.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Midega TD, Chaves RCF, Ashihara C, Alencar RM, Queiroz VNF, Zelezoglo GR, et al. Ketamine use in critically ill patients: a narrative review. Rev Bras Ter Intensiva. 2022;34:287–94. doi: 10.5935/0103-507X.20220027-en. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23:801–11. doi: 10.1038/mp.2017.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murugan AK. mTOR: Role in cancer, metastasis and drug resistance. Semin Cancer Biol. 2019;59:92–111. doi: 10.1016/j.semcancer.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Zhao S, Shao L, Wang Y, Meng Q, Yu J. Ketamine exhibits anti-gastric cancer activity via induction of apoptosis and attenuation of PI3K/Akt/mTOR. Arch Med Sci. 2019;16:1140–9. doi: 10.5114/aoms.2019.85146. [DOI] [PMC free article] [PubMed] [Google Scholar]