A recent editorial in the Brazilian Journal of Psychiatry highlighted the hopes, problems, and disappointments of anti-amyloid antibody treatments in the management of Alzheimer’s disease.1 The amyloid cascade hypothesis of Alzheimer’s disease has predominated for 4 decades, coupled to hyperphosphorylated tau to form amyloid-β plaques and tangles, respectively. This has provided no significant benefits to the management of Alzheimer’s disease pathophysiology. The editorial highlights the plethora of other factors now linked to dementia pathophysiology, including alterations in the gut microbiome and gut permeability. The amyloid hypothesis is further challenged by the antimicrobial effects of amyloid-β, possibly indicative that amyloid-β is “too much of a good thing.” This article highlights the role of the melatonergic pathway, providing a more integrated dementia pathophysiology, including alterations in gut microbiome/permeability, and proposing that aging-associated factors which suppress the melatonergic pathway underpin dementia.
The melatonergic pathway is evident in all mitochondria-containing cells, where it is induced by two transcription factors that drive amyloid-β production, namely nuclear factor kappa B (NF-κB) and Yin Yang 1 (YY1) via β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) induction. The suppressed capacity of NF-κB and YY1 to synchronize melatonin production and release in association with BACE1 and amyloid-β prolongs amyloid-β production whilst maintaining astrocyte and microglia reactivity, thereby driving microbial/alarmin signaling. The loss of synchronized autocrine and paracrine melatonin contributes to ongoing inflammation. The elimination of amyloid-β by anti-amyloid antibodies would not be expected to impact such dysregulated inflammatory processes.
The above provides a framework to link a wide array of diverse bodies of data on dementia pathophysiology by highlighting the importance of the astrocyte melatonergic pathway and how it can become desynchronized from BACE1 induction, resulting in excessive amyloid-β production and the maintenance of inflammatory interactions of astrocytes, neurons, and microglia in the course of dementia pathophysiology.
The melatonergic pathway
Melatonin production occurs predominantly within mitochondria, allowing melatonin to have mitochondrial, intracrine, autocrine, and paracrine effects. The ancient bacteria that became endosymbionts of proto-eukaryotes 2 billion years ago produced melatonin, gradually evolving into mitochondria, with the mitochondrial melatonergic pathway evident in all animals, plants, and fungi.2 This maintained presence of the melatonergic pathway highlights its importance for multicellular life on earth.
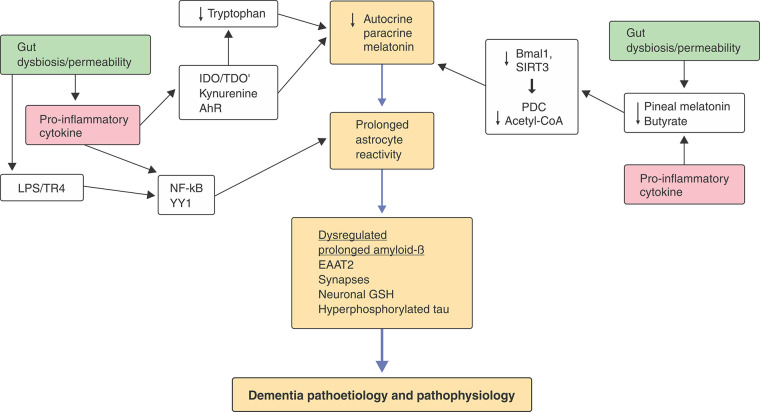
Astrocytes are the most important neuroregulatory cells. The melatonergic pathway was first shown to be present in astrocytes in 2007.3 The astrocytic melatonergic pathway involves tryptophan uptake and the stabilization of tryptophan hydroxylase 2 by 14-3-3ɛ to produce serotonin, which is converted by arylalkylamine N-acetyltransferase (AANAT) to N-acetylserotonin (NAS). AANAT requires stabilization by 14-3-3ζ, as well as the availability of acetyl coenzyme A (acetyl-CoA), for NAS production. NAS is then converted to melatonin by acetylserotonin methyltransferase. Factors regulating tryptophan, tryptophan uptake, 14-3-3ɛ, 14-3-3ζ, tryptophan hydroxylase 2, AANAT, acetylserotonin methyltransferase and acetyl-CoA can therefore modulate astrocytic capacity to produce melatonin (Figure 1).
Figure 1. How different processes can impact mitochondrial function by regulating the conversion of tryptophan to melatonin. Tryptophan uptake allows it to be converted to serotonin by tryptophan hydroxylase 2, which requires 14-3-3ɛ for stabilization. Serotonin’s conversion to N-acetylserotonin, 14-3-3ζ stabilization of arylalkylamine N-acetyltransferase, as well as acetyl-CoA as a co-substrate are all necessary to initiate the melatonergic pathway. Factors attenuating the availability of these 14-3-3 isoforms limit mitochondrial melatonergic pathway induction. A number of activated receptors, including the aryl hydrocarbon receptor (AhR), can suppress 14-3-3, thereby suppressing the melatonergic pathway. The induction of CYP1B1 by the AhR can back-convert melatonin to N-acetylserotonin via O-demethylation. Some of the complex, and sometimes contradictory, effects of the AhR in aging and associated medical conditions may arise from variations in the availability of the mitochondrial melatonergic pathway. Gut dysbiosis and permeability suppress butyrate and increase lipopolysaccharide (LPS) in interaction with heightened pro-inflammatory cytokines, which then increase indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO), further depleting tryptophan via its conversion to kynurenine, an endogenous AhR ligand, coupled to increasing neuroregulatory kynurenine pathway products. LPS and pro-inflammatory cytokines also inhibit pineal melatonin production, thereby suppressing circadian resetting of mitochondria by pineal melatonin’s upregulation of Bmal1 and sirtuin-3 (SIRT3), as does butyrate. Mitochondrial resetting is mediated by the disinhibition of pyruvate dehydrogenase complex (PDC) by SIRT3 and Bmal1, thereby enhancing the conversion of pyruvate to acetyl-CoA, which is required for melatonergic pathway induction. LPS, high-mobility group box 1, and heat shock protein 70, via toll-like receptor 4 (TLR4), induce the transcription factors nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and Yin Yang 1 (YY1), as do pro-inflammatory cytokines, leading to β-site amyloid precursor protein-cleaving enzyme 1 and amyloid-β induction, which is normally synchronized with the anti-inflammatory effects of sequentially induced melatonin. Such alterations in astrocytes change neuronal regulation and transmitter release, coupled to enhanced plaques and tangles. Core processes are highlighted in orange, upon which many genetic and epigenetic factors can act, including the gut microbiome (green) and pro-inflammatory cytokines (pink). EAAT = excitatory amino acid transporter; GSH = glutathione.
Melatonergic pathway induction is intimately linked to optimized mitochondrial function. Activation of the pyruvate dehydrogenase complex, including by circadian Bmal1 and longevity-linked sirtuin-3 (SIRT3), enhances the conversion of pyruvate to acetyl-CoA, thereby optimizing adenosine triphosphate production from the tricarboxylic acid cycle and oxidative phosphorylation. Acetyl-CoA is also a necessary co-substrate for converting serotonin to NAS, allowing optimized mitochondrial function to be intimately linked to melatonergic pathway upregulation.4 Notably, pineal melatonin production decreases 10-fold between 18 and 80 years of age in humans. It is unknown whether this aging-associated melatonin decrement is evident in other body and brain cells, including neurons and glia.
Linking the melatonergic pathway with dementia pathophysiology
BACE1 induction and amyloid-β production follow toll-like receptor 2/4 activation, including by lipopolysaccharide (LPS) and herpes simplex virus 1, as well as by endogenous “alarmins,” such as heat shock protein 70 and high-mobility group box 1. Consequently, local inflammation-linked processes, as well as gut permeability-derived LPS, upregulate BACE1 and amyloid-β via YY1 and NF-κB. However, both YY1 and NF-κB temporally limit glia reactivity via synchronized melatonergic pathway induction.5,6
Given that melatonin treatment in human dementia and animal models shows benefits,7 it is surprising that its local production in the central nervous system has not been clinically investigated. Notably, exogenous melatonin induces the mitochondrial melatonergic pathway, partly via Bmal1, SIRT1, and SIRT3 induction,8 thereby disinhibiting the pyruvate dehydrogenase complex and the conversion of pyruvate to acetyl-CoA, which is required as an AANAT co-substrate to initiate the melatonergic pathway (Figure 1).
Gut microbiome and gut permeability in modulation of the melatonergic pathway
The gut microbiome and gut permeability are at the cutting edge of research in a growing body of diverse medical conditions.9,10 Gut microbiome/permeability is intimately linked to melatonergic pathway regulation, including by the gut microbiome-derived short-chain fatty acid, butyrate. Butyrate is an epigenetic regulator and histone deacetylase inhibitor (HDACi), with HDACs detrimentally regulating dementias by modifying gene promotors, thereby upregulating YY1-driven transcriptions.11 The loss of butyrate’s pan-HDACi capacity potentiates YY1, including YY1’s suppression of excitatory amino acid transporter 2, thereby contributing to glutamatergic excitotoxicity in dementia.12 Butyrate upregulates SIRT3 and the melatonergic pathway in intestinal epithelial cells, suggesting PDC disinhibition, which clearly requires investigation in central nervous system cells, including the impact of factors regulating 14-3-3 isoforms and tryptophan hydroxylase 2, as well as the effects of butyrate on YY1 and NF-κB.13
Gut dysbiosis and permeability commonly co-occur, with gut permeability allowing the release of many immune and glia regulatory factors, including LPS, aryl hydrocarbon receptor (AhR) ligands, and tiny fragments of partially digested food. LPS, via toll-like receptor 2/4, induces YY1 and NF-κB with associated immune and glia inflammation. NF-κB induction in macrophages and microglia also induces melatonin release and autocrine effects, thereby limiting inflammation and shifting to an M2-like phenotype to clear debris after a time-limited activation.5 Clearly, this requires investigation over the course and dampening of astrocyte reactivity and highlights the crucial importance of astrocyte mitochondrial melatonergic pathway availability. Exogenous melatonin prevents tau hyperphosphorylation,14 indicating that the loss of local melatonin production is intimately linked to amyloid-β and hyperphosphorylated tau overproduction and neurotoxicity. The decades-old conceptualizations of the core processes underpinning dementia, namely plaques and tangles, may arise due to suppressed local melatonin production.
The melatonergic pathway, amyloid-β, and other medical conditions associated with aging
The above provides a framework for integrating many previously disparate bodies of data on dementia, including suppressed serotonergic signaling in the pathophysiology of early dementia. Amyloid-β is not definitive of dementia, as heightened amyloid-β is evident in diverse medical conditions, such as glioblastoma and breast cancers, which implicates a suppressed mitochondrial melatonergic pathway in the pathophysiology of many diseases associated with aging, including the tumor microenvironment.13 This may have relevance to how aging is associated with the complex effects of the AhR, given data showing its capacity to suppress 14-3-3 in some cells, as well as to back-convert melatonin to NAS, which can act as a brain-derived neurotrophic factor (BDNF) mimic via BDNF-TrkB activation.15 The AhR is also expressed in the outer membrane of mitochondria, and it is important that its complex effects, which arise from different ligands and in different cells, are placed within its capacity to regulate the mitochondrial melatonergic pathway.
The above would indicate clear evolutionary-driven pressure for the association of the melatonergic pathway with mitochondria in the three kingdoms of life on earth. The astrocyte pathways linked to dementia, namely LPS/YY1-NF-κB/HDAC/BACE1/amyloid-β are relevant to many other conditions associated with inflammation. Importantly, the LPS/YY1-NF-κB/HDAC pathway is also significantly associated with regulation of the melatonergic pathway, indicating that suppression of the synchronized induction of the melatonergic pathway with BACE1 and amyloid-β production explains, and links, a host of previously disparate data on dementia. This has relevance to a wide array of other poorly conceptualized and treated medical conditions, including amyotrophic lateral sclerosis and cancers, as well as to the many psychiatric conditions linked to suboptimal mitochondrial function. Within this framework, it could be predicted that anti-amyloid antibody therapy would afford little protection against dementia, which the data broadly support.1
Disclosure
The author reports no conflicts of interest.
Acknowledgements
The author would like to thank Jonathan Harris of Harris Associates for support in the production of this article.
Footnotes
How to cite this article: Anderson G. Why do anti-amyloid beta antibodies not work? Time to reconceptualize dementia pathophysiology by incorporating astrocyte melatonergic pathway desynchronization from amyloid-beta production. Braz J Psychiatry. 2023;45:89-92. http://doi.org/10.47626/1516-4446-2022-2949
References
- 1.Loureiro JC, Silva LFAL, Pais MV, Forlenza OV. Anti-amyloid agents for treating incipient Alzheimer’s disease: a new hope? Braz J Psychiatry. 2022;44:368–9. doi: 10.47626/1516-4446-2022-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan DX, Manchester LC, Liu X, Rosales-Corral SA, Acuna-Castroviejo D, Reiter RJ. Mitochondria and chloroplasts as the original sites of melatonin synthesis: a hypothesis related to melatonin’s primary function and evolution in eukaryotes. J Pineal Res. 2013;54:127–38. doi: 10.1111/jpi.12026. [DOI] [PubMed] [Google Scholar]
- 3.Liu YJ, Zhuang J, Zhu HY, Shen YX, Tan ZL, Zhou JN. Cultured rat cortical astrocytes synthesize melatonin: absence of a diurnal rhythm. J Pineal Res. 2007;43:232–8. doi: 10.1111/j.1600-079X.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson G, Rodriguez M, Reiter RJ. Multiple sclerosis: melatonin, orexin, and ceramide interact with platelet activation coagulation factors and gut-microbiome-derived butyrate in the circadian dysregulation of mitochondria in glia and immune cells. Int J Mol Sci. 2019;20:5500. doi: 10.3390/ijms20215500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markus RP, Fernandes PA, Kinker GS, da Silveira Cruz-Machado S, Marçola M. Immune-pineal axis - acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br J Pharmacol. 2018;175:3239–50. doi: 10.1111/bph.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nowak K, Lange-Dohna C, Zeitschel U, Günther A, Lüscher B, Robitzki A, et al. The transcription factor Yin Yang 1 is an activator of BACE1 expression. J Neurochem. 2006;96:1696–707. doi: 10.1111/j.1471-4159.2006.03692.x. [DOI] [PubMed] [Google Scholar]
- 7.Tseng PT, Zeng BY, Chen YW, Yang CP, Su KP, Chen TY, et al. The dose and duration-dependent association between melatonin treatment and overall cognition in Alzheimer’s dementia: a network meta- analysis of randomized placebo-controlled trials. Curr Neuropharmacol. 2022;20:1816–33. doi: 10.2174/1570159X20666220420122322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cucielo MS, Cesário RC, Silveira HS, Gaiotte LB, Dos Santos SAA, de Campos Zuccari DAP, et al. Melatonin reverses the Warburg-type metabolism and reduces mitochondrial membrane potential of ovarian cancer cells independent of MT1 receptor activation. Molecules. 2022;27:4350. doi: 10.3390/molecules27144350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarawneh R, Penhos E. The gut microbiome and Alzheimer’s disease: complex and bidirectional interactions. Neurosci Biobehav Rev. 2022;141:104814. doi: 10.1016/j.neubiorev.2022.104814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson G, Seo M, Berk M, Carvalho AF, Maes M. Gut permeability and microbiota in Parkinson’s disease: role of depression, tryptophan catabolites, oxidative and nitrosative stress and melatonergic pathways. Curr Pharm Des. 2016;22:6142–51. doi: 10.2174/1381612822666160906161513. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Zheng D, Weng F, Jin Y, He L. Sodium butyrate ameliorates the cognitive impairment of Alzheimer’s disease by regulating the metabolism of astrocytes. Psychopharmacology (Berl) 2022;239:215–27. doi: 10.1007/s00213-021-06025-0. [DOI] [PubMed] [Google Scholar]
- 12.Wang XM, Gu P, Saligan L, Iadarola M, Wong SSC, Ti LK, Cheung CW. Dysregulation of EAAT2 and VGLUT2 spinal glutamate transports via Histone Deacetylase 2 (HDAC2) contributes to paclitaxel-induced painful neuropathy. Mol Cancer Ther. 2020;19:2196–209. doi: 10.1158/1535-7163.MCT-20-0006. [DOI] [PubMed] [Google Scholar]
- 13.Anderson G, Maes M. Gut dysbiosis dysregulates central and systemic homeostasis via suboptimal mitochondrial function: assessment, treatment and classification implications. Curr Top Med Chem. 2020;20:524–39. doi: 10.2174/1568026620666200131094445. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Lan G, Li R, Mei Y, Shui X, Gu, et al. Melatonin ameliorates tau-related pathology via the miR-504-3p and CDK5 axis in Alzheimer’s disease. Transl Neurodegener. 2022;11:27. doi: 10.1186/s40035-022-00302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19:184–97. doi: 10.1038/s41577-019-0125-8. [DOI] [PubMed] [Google Scholar]