Abstract
The use of electronic nicotine delivery systems (ENDS), specifically electronic cigarettes (E-cig), has risen dramatically within the last few years; the demographic purchasing these devices is now predominantly adolescents that are not trying to quit the use of traditional combustible cigarettes, but rather are “new users”. The composition and appearance of these devices has changed since their first entry into the market in the late 2000s, but they remain composed of a battery and aerosol delivery system that is used to deliver breakdown products of propylene glycol/vegetable glycerin, flavorings and potentially nicotine or other additives. Manufacturers have also adjusted the type of nicotine that is used within the liquid to make the inhalation more palatable for younger users, further affecting the number of youth who use these devices. While the full spectrum of cardiovascular (CV) and cardiometabolic consequences of e-cig use is not fully appreciated, data is beginning to show that e-cigs can cause both short- and long-term issues on cardiac function, vascular integrity and cardiometabolic issues. This review will provide an overview of the cardiovascular, cardiometabolic, and vascular implications of the use of e-cigs, and the potential short- and long-term health effects. A robust understanding of these effects is important in order to inform policy makers on the dangers of e-cigs use.
Keywords: electronic nicotine delivery systems, nicotine, vaping, cardiovascular, cardiometabolic
Subject Terms: Risk Factors, Lifestyle, Cardiovascular Disease
Introduction:
Smoking is the leading cause of preventable disease and death in the United States1,2 and has been responsible for approximately 20 million deaths in the United States over the past 60 years3. Electronic nicotine delivery systems (ENDS), including electronic cigarettes (E-cig), were invented as a healthier alternative to traditional cigarettes and were marketed as a smoking cessation aid. E-cigs are considered a modern technological replacement for traditional (combustible) tobacco cigarettes and were introduced for commercial use in 2004. The term e-cig includes a range of battery-powered devices that use a variety of aerosol delivery methods that have changed over time, as summarized in Figure 1. E-cig liquids or cartridges usually contain a mixture of propylene glycol (PG) and vegetable glycerin (VG) in combination (PG/VG), flavorants, and various nicotine concentrations, typically ranging from 1.6 to 19 mg/cartridge4,5 or potentially other additives. In August 2016, a World Health Organization (WHO) report concluded that it was not currently possible to measure the relative risk of ENDS in comparison to combustible tobacco products due to the large variances in e-cig ingredients and device elements6. Therefore, it is not known to what extent the use of these products is safer, in terms of relative or absolute risk reduction, when compared to traditional cigarette smoking.
Figure 1. Electronic Nicotine Delivery Systems.
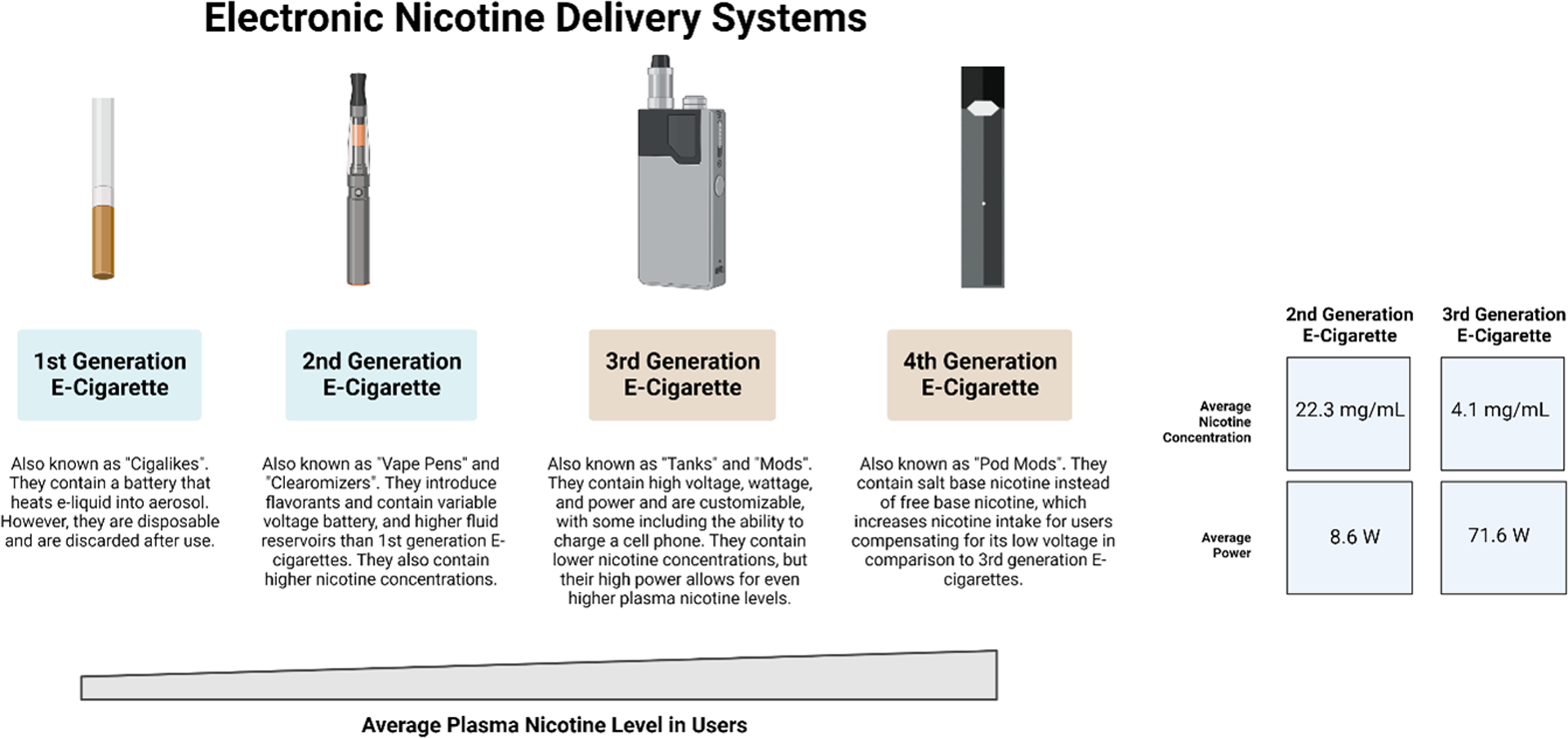
1st Generation e-cig or “Cigalike”, as well as representative 2nd-4th generations of e-cigs with design characteristics5,83,84. Created with BioRender.com.
ENDS use is increasing rapidly among youth (age 18–24 years old) and adolescents (grades 6–12)7–9, with current e-cig use doubling among middle (grade 6–8) and high school (grade 9–12) students from 3.3% to 6.8%10. Current e-cig use is higher amongst adult occasional smokers and heavy smokers (20 cigarettes/day) than among daily smokers and less frequent smokers11. About 6% of all US adults and 21% of US adult smokers have tried e-cigs12 and data from the US, UK, Canada, and Australia shows that nearly half of current smokers and former smokers are aware of e-cigs. As expected, awareness is higher in countries where these products are legal (73% in the US compared to 20% in Australia)11. Furthermore, greater than 75% of smokers and former smokers believe that e-cigs are less harmful than traditional cigarettes.
Compared to traditional combustible cigarettes that burn continuously during use, e-cigs release aerosols only upon inhalation. E-cig particles are small enough to penetrate the alveoli, allowing for nicotine absorption13 and deeper penetration into the lungs and systemic circulation14. E-cig vapor may induce oxidative stress in lung endothelial cells15 and lead to serious inflammatory pulmonary diseases16. In addition, e-cig aerosols may also increase the risk of acute coronary syndrome, particularly in patients with pre-existing risk factors17. Since e-cig ingredients, route of administration, and methods of use differ from those of conventional cigarettes, it is likely that their cardiopulmonary effects may also differ. While there is evidence that carbon-based cigarette particles from conventional cigarettes are harmful when inhaled, little is known about the effects of inhaling liquid-based particles from e-cigs, as well as the health effects of heating these components. The process of heating the e-cig liquid may elicit chemical reactions, potentially leading to chemical changes differing from the original e-cig liquid, unintentionally leading to health effects different from those caused by cigarette smoke. The use of e-cigs has been shown to cause dysregulation of the autonomic nervous system (ANS) homeostasis via the stimulant effects of nicotine, and it has also been suggested that there may be a risk of cardiomyopathy18. Short-term e-cig usage also demonstrated effects on blood vessel tone, heart rate, heart rate variability and rhythm19. A recent study showed that e-cig smoking affects arterial function in a similar manner to smoking traditional cigarettes in both non-smokers and smokers20. Therefore, this review intends to elucidate the current understanding of adverse cardiovascular and metabolic consequences of e-cig use.
Effects on Myocardial Health:
The number of clinical trials and prospective studies conducted to investigate the effects of e-cig use on myocardial function and structure is limited. However, data suggest that e-cig exposure and sequelae may combine as essential drivers of cardiovascular disease (CVD) in a similar manner by which traditional tobacco smoking and hypertension are essential drivers of CVD. E-cig-related sequelae include increased sympathetic activity and oxidative stress, altered metabolism, as well as increased vascular stiffness and endothelial dysfunction (discussed below). The combination of complications influences the development of CVD via aneurysm formation, atherosclerosis, and free radical formation.
A cross-sectional analysis has shown that daily e-cig use was independently associated with higher odds of myocardial infarction (OR=1.79 [95% CI: 1.20–2.66]), as was daily conventional cigarette smoking (OR=2.29 [95% CI: 2.29–3.24])21. This analysis further highlighted how underlying risk factors for CVD such as age, diabetes, hypertension, hypercholesterolemia, and chronic pulmonary and vascular changes can influence alterations of the myocardium in e-cig users similarly to conventional cigarette smoking, albeit with decreased risk in this study. Acute clinical studies, using echocardiography, reported no changes in myocardial function in adult smokers using e-cigs22; however, that is to be expected as short-term studies are not likely to detect cardiac remodeling and current cigarette smoking use may conceal changes induced by e-cigs. Animal studies further support chronic e-cig use as a potential driver for CVD with acute e-cig use yielding mixed results. Interestingly, animal models have provided insights into how the presence of comorbidities, e-cig flavorings, and other forms of e-cig exposure factor into structural and functional changes in cardiac tissues that serve as a pathway for CVD development.
Acute exposures (2 weeks) to e-cigs showed cardiac function changes via echocardiography23. This study showed that e-cig exposure for 3 hours a day for 14 days, similar to a casual-use model24, found no significant effects on ejection fraction, but did elicit significantly increased angiogenesis in mouse heart tissue and significantly increased collagen content, but not tissue fibrosis23. This study implies that acute e-cig exposure had no significant effect on contractile function or fibrosis but did induce cardiac angiogenesis. While it is known that traditional combustible cigarettes inhibit angiogenesis, nicotine alone stimulates angiogenesis, and increased angiogenesis may be beneficial in instances of myocardial infarction (MI)23,25; however, the role of increased angiogenesis following e-cig exposure necessitates further study to fully understand risks and benefits of increased angiogenesis.
In contrast, a chronic exposure (i.e., 12-weeks) study in mice found that e-cig use concomitant with a high fat diet (HFD) resulted in decreased left ventricle (LV) fractional shortening, ejection fraction, and velocity of circumferential fiber shortening in mice exposed to nicotine containing e-cig, compared to no nicotine and saline exposed mice26. HFD mice exposed to nicotine-containing e-cigs also exhibited LV structural abnormalities such as lipid accumulation (ventricular steatosis), myofibrillar derangement and destruction, and mitochondrial hypertrophy on microscopy26. These structural changes were likely due to the accompanied increases in oxidative stress, plasma free fatty acids, cardiomyocyte apoptosis, and inactivation of AMP-activated protein kinase and activation of its downstream target, acetyl-CoA-carboxylase.
Similarly, another 12-week study in apolipoprotein-E knockout (ApoE−/−) mice (used as a model of lipid metabolism dysregulation, atherosclerosis, and obesity) demonstrated that nicotine-containing e-cig exposure resulted in decreased LV fractional shortening and ejection fraction compared to nicotine-free and saline-exposed ApoE−/− mice27. ApoE−/− mice also showed changes in ventricular transcriptomic analysis of genes related to metabolism, circadian rhythm, and inflammation as well as increased oxidative stress and mitochondrial DNA mutations. Electron microscopy also showed ultrastructural myocyte abnormalities potentially indicative of cardiomyopathy, or the development of cardiomyopathy27. These myocyte abnormalities included nuclear abnormalities such as shrunken nuclei, chromatin condensation and fragmentation, and nuclear malformation with convoluted nuclear membranes as well as cytoplasmic abnormalities such as myofibrillar derangement, thinning, and destruction alongside intramyocardial lipid accumulation and mitophagy. Chronic combustible tobacco smoking has previously been associated with the development of cardiomyopathy28 with Gvozdjăk et al. coining the term “smoker’s cardiomyopathy” in 198729. More recent data has shown that cigarette smoke exposure resulted in increased LV end diastolic and systolic diameters30, which are associated with the development of cardiomyopathy31. The extent to which e-cigs may induce these structural changes requires more investigation. However, chronic studies utilizing ApoE−/− mice over the span of 6 months found that the cardiovascular effects of e-cig exposure were reduced relative to combustible tobacco smoking32. These findings indicate that e-cig use alongside other comorbidities (i.e., obesity) may induce or potentiate e-cig-associated CV adverse effects.
Secondary forms of e-cig exposures exist such as perinatal nicotine exposure, which has recently been shown to reduce viability of human embryonic stem cells (hESC) and induce minor changes in cell-type distribution upon nicotine containing e-cig exposure when analyzed via single-cell RNA sequencing33. hESC exposure to nicotine resulted in disrupted intracellular Ca2+ handling, as characterized by increased gene expression of HMGB1 and TLR4 in hESC-derived myocytes. Increased expression of HMGB1 proteins resulted in impaired cardiac excitation-contraction via sarcoplasmic reticulum leakage of Ca2+ through TLR4-ROS signaling, increasing the risk for Ca2+-associated arrhythmias33. Further, perinatal ENDS exposure, and other forms of nicotine exposure, may cause gender-dependent increases in CV adverse events. Male offspring from pregnant rats exposed to nicotine via subcutaneous osmotic minipumps (at concentrations representative of ENDS and traditional tobacco smoking use) from gestational day 4 through postnatal day 10 developed cardiac dysfunction in adulthood34; this was not seen in female offspring. Male offspring displayed enhanced I/R-induced cardiac dysfunction and infarction associated with the overexpression of miR-181a in LV tissues, which was not seen in female offspring. The downstream effects of miR-181a overexpression altered target genes, such as enhanced cardiac angiotensin receptor expression, upregulated transforming growth factor beta protein (TGF-β)/Smads proteins, upregulated autophagy-related protein, and decreased cardiac IncRNA H19 levels34. The disruption of these target genes has been shown to alter regulation of cardiac remodeling, hypertrophy, and interstitial fibrosis, as well as increased expression of ischemia-sensitive signaling proteins and facilitation of cell death34–38.
Sex-dependent differences have also been observed in adolescent mice following 3-month e-cig exposure, with male mice exhibiting greater reductions of LV fractional shortening24. Adolescent mice exposed to e-cigs had reduced end-systolic elastance independent of nicotine concentration and reduced preload-recruitable stroke work in e-cigs with nicotine, indicative of reduced contractile capacity24. Male adolescent mice following 3 months of e-cig use showed increased perivascular fibrosis in e-cig liquid without nicotine, further supported by increased, although non-significant, type I collagen24. This same study also showed that gene expression of Col1a1 and Col3a1 was increased following e-cig use with nicotine at 3 weeks, but was reversed by 3 months, suggesting that perhaps nicotine may play a protective role.
Interestingly, the female adolescent mice exposed in the same study did not show alterations in cardiac function and serum biomarker profiles only showed elevated IFN-γ levels, whereas males had increased IL-18, CCL2, macrophage inflammatory protein 1β, stem cell factor, and vascular endothelial growth factor A levels24. While e-cig exposure concentrations of nicotine were consistent between male and female mice, the 3-hydroxycotinine (3HC) to cotinine (cot) ratio was increased in female mice24. The 3HC/cot ratio is a measure of cytochrome P450 2A5 (CYP2A5) activity and is responsible for nicotine metabolism. These elevations suggest that female mice metabolize nicotine at a higher rate than male mice and were cardioprotective of the deleterious effects of nicotine observed in the male counterparts potentially due to upregulation of CYP2A5 activity or expression. Further research on sex-based differences in e-cig nicotine metabolism are required to better understand how health outcomes may be influenced by sex.
Direct changes in cardiac tissues following conventional cigarette smoking have been well documented, but research on e-cig effects concerning cardiac tissue changes remain sparse. Mouse models utilizing subcutaneous nicotine and angiotensin-II pump infusions for 4 weeks demonstrated that nicotine augmented cardiac remodeling via increased matrix metalloproteinase-2 (MMP-2) activity and resulted in cardiac growth and increased aortic wall thickness, indicating a possible cardiotoxic interaction between nicotine and systemic hypertension39. Research on zebrafish embryos in non-tissue culture plates containing e-cig extracts investigated the effects of e-cigs on cardiac structure changes40. It was discovered that exposure to both cigarettes and e-cigs are associated with pericardial edema, reduced heart function, and cardiac fibrosis. E-cig exposure in rats revealed increased expression of TGF-β in cardiac tissue41. TGF-β is responsible for several molecular changes that promote fibrotic changes in the heart, including activation of collagen synthesis, up-regulation of connective tissue growth factor (CTGF) expression, and increased matrix protein production42, leading to increased cardiac fibrosis. While specific changes in the pericardium have not been thoroughly investigated, changes in TGF-β expression are a reliable indicator of cardiovascular pathology such as arterial hypertension, atherosclerosis, and coronary artery disease.
Furthermore, while more research is needed to understand the mechanisms behind these adverse cardiac structural changes, this same study showed reductions in transcriptional levels of cardiac myosin light chain 2 (cmlc2; the zebrafish orthologue of MYL7), cardiac muscle troponin T (tnnt2), and gap junction protein connexin-43 (cx43), which may be of importance for understanding how e-cigs influence cardiac contractile function, as these proteins are of great importance in maintaining cardiac function40. However, more research is needed concerning structural changes.
Similarly, in vitro e-cig aerosol exposure in human embryonic stem cells showed reduced expression of sarcomere genes MLC2v and MYL6 that regulate myosin light chains in a similar fashion to the cmlc2 gene in zebrafish40. These studies collectively provide evidence that important cardiac regulatory genes are downregulated after e-cig exposure, suggesting that e-cigs have the potential to severely reduce both sarcomere assembly and cardiac contractility contributing to CVD, such as hypertrophic cardiomyopathy43. More studies are needed to bridge the connection between e-cigs and CVD, as well as the mechanisms that occur through the numerous transcriptional changes that have been proven.
Effects on Cardiometabolic Health, Adipose Tissue and Metabolic Activity:
Tobacco smoking and nicotine use are known to impact metabolic health and adipose tissue function44. Nicotine suppresses appetite, and cessation of tobacco use is associated with increased body weight. Nicotine affects metabolism in various tissues, including adipose tissue45. Nicotine consumption decreases lipogenesis and increases adipose tissue lipolysis, increasing the risk of developing type 2 diabetes44. However, the effects of e-cigs on metabolic function and adipose tissue have not been thoroughly investigated.
There are conflicting results on body weight and glucose tolerance following e-cig use in rodents. Multiple studies have shown that 6–12 weeks of varying amounts of e-cig exposure reduced body weight and improved glucose tolerance in male mice fed either a control chow46 or high-fat diet26,46. These effects are mediated in part by adaptations to the liver, including increased expression of glucose transporters (GLUT2 and GLUT4), fatty acid synthesis (FASN)46, and cytokines (IL-10)47. In contrast, an earlier study found that 12 weeks of e-cig exposure did not affect body weight or glucose tolerance in male chow-fed mice48. These studies all used different exposure chambers, sources of e-cig vapors, duration of exposure, and strains of mice, which could contribute to the different experimental outcomes; therefore, future studies should take each of these factors into consideration. The role of diet and adiposity in interacting or compounding e-cig exposure has not been fully investigated and is likely a contributing factor to effects on glucose metabolism. Finally, all previous studies were performed in male mice; investigating the metabolic effect of e-cigs in female mice will be important to determine sex-specific differences.
Recent studies have investigated the effects of e-cigs on white adipose tissue (WAT). Acute exposure to e-cigs in male and female mice decreased their respiratory exchange ratio, suggesting acute e-cigs increase whole-body lipid metabolism49. Only 6 weeks of e-cig exposure has a direct effect on WAT. Expression of numerous inflammatory chemokines were increased in the serum of mice and gene expression of these chemokines were increased in visceral WAT46. The investigators hypothesized that e-cigs could increase whole-body inflammation by activating inflammatory resident macrophages in adipose tissue, but this has not been thoroughly investigated. Interestingly, this increase in chemokines was observed in mice exposed to nicotine-free e-cigs, indicating that the inflammatory effects of e-cig vapor exposure may not be directly caused by nicotine consumption, but by the other substances produced by the e-cig vaporization. In addition to the more commonly studied flavorants and PG/VG base, e-cigs may contain additional chemicals that are not well characterized and are triggering an inflammatory response. Future investigations may investigate which chemicals of the e-cig vapor are responsible for the increase in inflammation. In vitro studies using 3T3-L1 cells, an adipocyte cell line, showed that while tobacco smoke decreased cell viability and impaired adipocyte differentiation, e-cig exposure does not directly impact cell proliferation and differentiation into mature adipocytes, which contrasts with what is seen following exposure to cigarette smoke50. Overall, these limited data would suggest that e-cig exposure does not directly affect WAT50, but instead alters the resident immune cells within WAT47. Further research is required to determine what immune cells are affected, if e-cig exposure affects WAT function in vivo, and the underlying mechanisms for these adaptations. It is essential to determine if changes are due to intrinsic changes in adipocytes or if e-cig exposure influence adipose tissue to immune cell crosstalk.
In humans, recent studies showed that chronic e-cig exposure has a direct impact on whole-body metabolism. The effects of e-cig use on glucose metabolism in humans have shown conflicting results48,51,52. e-cig use is associated with an increased odds ratio of self-reported prediabetes51. and higher fasting glucose52, while data analysis from the annual cross-sectional National Health and Nutrition Examination Survey (NHANES) showed no effect of sole e-cig consumption in glucose tolerance and HOMA-IR48. It is unclear why there are discrepancies between these observational studies; however, only 0.88% (30/3415) of the subjects from the NHANES cohort were e-cig users. E-cig usage impacts the lipid profile of humans52–54 and is also correlated with decreased serum levels of ω−6 fatty acids, including linoleic and arachidonic acid, as well as their bioactive metabolites, the oxylipins 9-HODE and 13-HODE53. However, e-cig use does not affect total levels of triglycerides, low-density (LDL) and high-density (HDL) lipoprotein52,54. These initial data suggest that e-cig use affects the metabolic profile of humans by altering the levels of different free fatty acids and their metabolites. Altered levels of oxylipins suggest that WAT function may be affected by e-cig exposure in humans, since WAT is the main source of fatty acid and lipid storage and production. Future studies are required to determine if e-cig usage alters whole-body metabolic function, as well as to directly study their effect on WAT function. The effects of e-cigs on adipose tissue and CV dysfunction are summarized in Figure 2.
Figure 2. E-cig-induced Adipose Tissue Changes and CV Dysfunction.
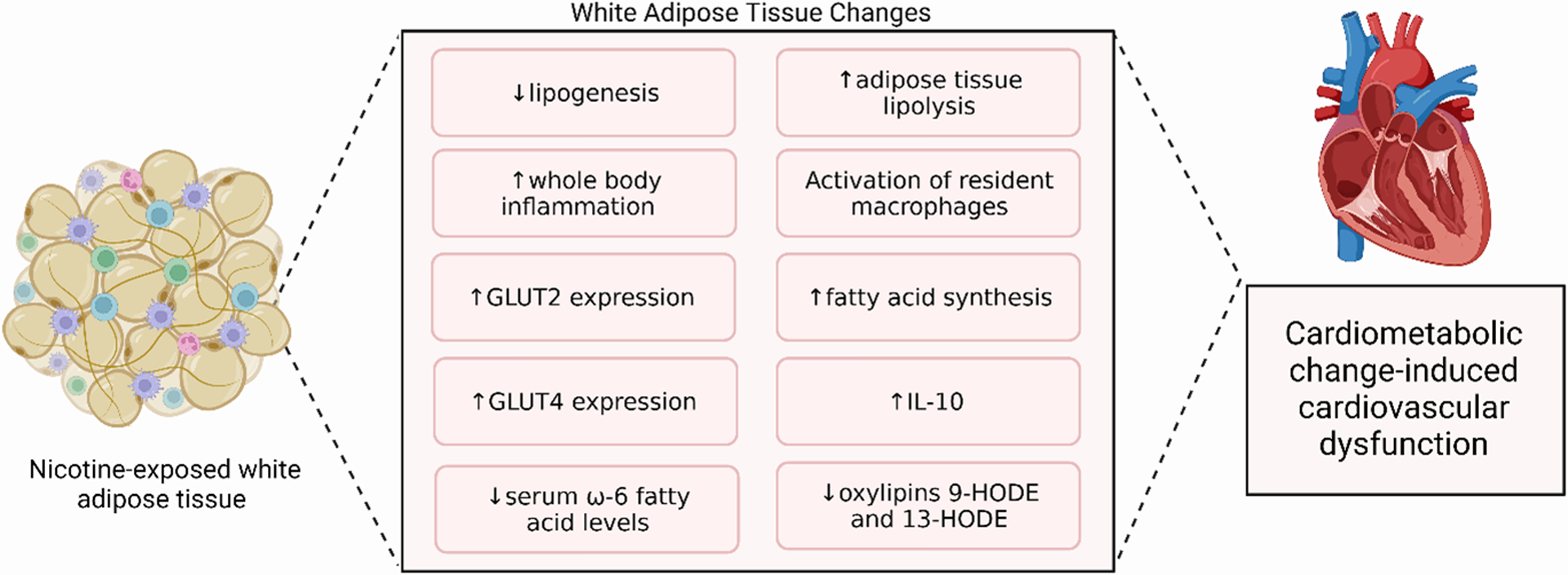
Nicotine exposure leads to changes in WAT. These changes include decreased lipogenesis, serum levels of ω−6 fatty acids, and oxylipins 9- and 13-HODE, as well as increased adipose tissue lipolysis, fatty acid synthesis, whole body inflammation, GLUT2 and GLUT4 expression, IL-10 levels, and activation of resident macrophages. Created with BioRender.com.
Effects on Vascular Health:
Due to the chemical makeup of inhaled toxins in the e-cig aerosols, endothelial dysfunction is a common contributor to the development of CVD within the heart and corresponding vessels. E-cig users display diminished flow-mediated vasodilation, often resulting in endothelial dysfunction even after a single use of e-cigs55. Studies also demonstrate that nitric oxide (NO) bioavailability plays a significant role in vascular health and reductions in NO bioavailability can induce the release of inflammatory factors, leading to endothelial dysfunction and dysregulation of blood pressure in in vivo randomized control trials56,57. E-cig liquid (e-liquid) composition and flavorings also elicit adverse effects on endothelial membranes58. Apple/mint and tobacco flavored e-cigs induce cytotoxic effects on endothelial cells that can eventually lead to cell death and heart disease58. Similarly, a recent study that utilized human induced pluripotent stem cell-derived endothelial cells (iPSC-ECs) found that cinnamon-flavored e-cigs exhibited potent cytotoxicity, leading to decreased cell viability, increased ROS and activation of ROS stress-related pathways, caspase 3/7 activity, and low-density lipoprotein uptake, and impaired tube formation and migration following acute e-cig exposure59. These findings suggest that acute exposure to flavored e-cigs may induce or exacerbate endothelial dysfunction. E-cigs have also been demonstrated to increase arterial stiffness following acute use of e-cigs60. Arterial stiffness and endothelial dysfunction often occur in concert with one another61. Using pulse wave technology, one study of 70 current adult smokers showed that both nicotine-free and nicotine-containing e-cig use resulted in increased arterial stiffness, but relatively less than conventional cigarettes61.
Exposure to e-cig vapor has been shown to increase the risk of thrombosis with short term usage, with platelet dysfunction being a common explanation for this increase. JUUL e-cigs have been used specifically to test the potential effects of e-cigs on thrombosis. The results, analyzing blood taken directly from the heart, showed decreased duration in occlusion time within mice exposed to JUUL, indicating thrombosis62. ADP- or thrombin-induced (1 μM and 0.1 U/mL, respectively) platelet aggregation was also significantly increased when compared to controls. Mice exposed to JUUL e-cigs had enhanced ADP- and thrombin-induced P-selection expression and increased agonist-mediated activation of GPIIb/IIIa. These factors both play a large role in platelet adhesion and coagulation63, negatively impacting the cardiovascular system. When these results were compared to the levels of GPIIb/IIIa in other e-cigs, the findings demonstrated that JUUL had greater detrimental effects on ADP-triggered aggregation62. Although it is not entirely clear, it is conceivable that endothelial dysfunction and oxidative stress likely contribute to this increase in platelet aggregation64.
Oxidative stress may play a role in endothelial dysfunction and thrombosis64. A study showed decreased prostaglandin production in the vascular endothelium as well as microvascular endothelial dysfunction following exposure to e-cigs containing nicotine64. This study concludes that endothelial dysfunction could be induced by a NO-independent mechanism or plasma myeloperoxidase (MPO), affecting heart rhythm, heart rate, and blood pressure64. Interestingly, e-cig-induced endothelial dysfunction could be rescued with antioxidants which increased flow-mediated vasodilation, indicating that issues with oxidative stress could be an underlying cause for the condition64. Similarly, acute e-cig usage is associated with other serum markers of oxidative stress, such as increased 8-isoprostanes and NOX2 levels, suggesting that nitric oxide may be an underlying mechanism for e-cig-induced endothelial dysfunction, affecting heart rate, blood pressure, and arterial pressure55. The effects of e-cig-induced oxidative stress and subsequent platelet aggregation and thrombosis are summarized in Figure 3.
Figure 3. Effects of E-cig-induced Oxidative Stress on Platelet Aggregation and Thrombosis.
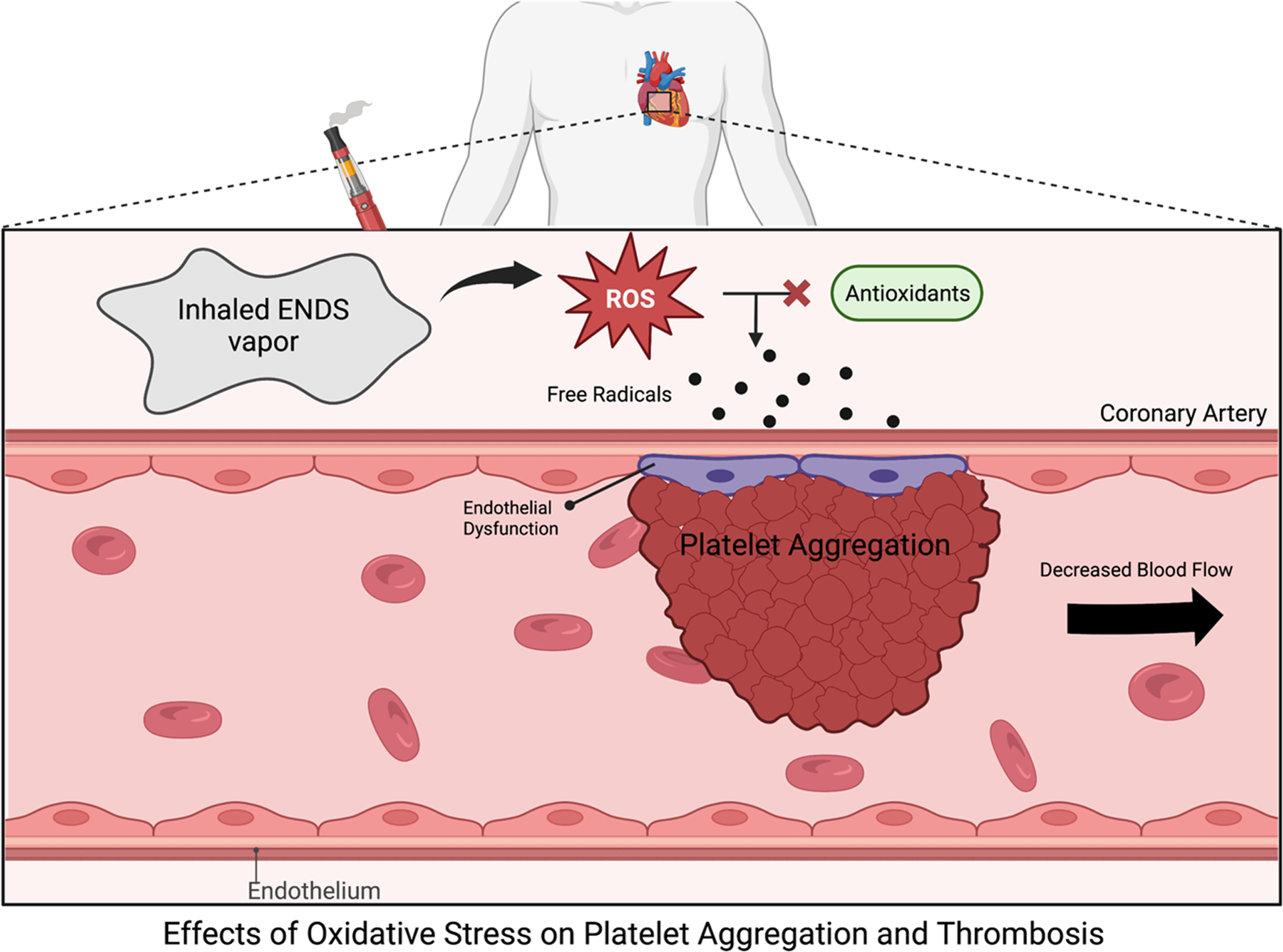
Inhaled e-cig vapor increases levels of ROS. This increase in ROS affects the buffering capacity of antioxidants, inducing oxidative stress85. More specifically, within endothelial cells, NO is the primary antioxidant85. When NO reacts with superoxide anion, free radicals form to disrupt proteins within the endothelium85, leading to endothelial dysfunction and platelet aggregation, further predisposing individuals to thrombosis, and decreased blood flow beyond the site of platelet aggregation. Created with BioRender.com.
Oxidative Stress and Potential Biomarkers:
While some research has shown that ENDS products induce fewer mediators of oxidative stress than traditional cigarettes, e-cig use may still produce some absolute risk for oxidative stress-induced adverse health effects. e-cigs contain a variety of components that contribute to their diversity across thousands of different e-cig designs, possessing many different cofactors that contribute to the degree of oxidative stress produced by e-cigs. These factors include the following: vapor volume, voltage setting, type of heating system, nicotine concentration, type of atomizers, battery size, e-liquid composition, and the type of refillable flavoring being used65. The effects of e-cig-induced oxidative stress, and sequential inflammatory markers, may impact cardiometabolic and cardiovascular function. The individual effects of each of these components remains understudied. However, many studies have shown that the type of e-liquid used in e-cigs has several detrimental effects on oxidative stress, such as the release of reactive oxygen species (ROS). One study investigated the impact that e-cig flavorings have on ROS levels from analyzing ROS handling proteins. They found that three flavors (tobacco, apple/mint, and vanilla) induced cytotoxic behavior alongside varied nicotine concentrations (2.5%, 2.5%, and 5%, respectively)58. Vanilla and tobacco flavored e-cig liquids showed a greater overall increase in ROS levels than apple/mint flavored e-liquid. Vanilla and apple/mint e-cig flavorings, both containing nicotine concentrations of 12 mg/ml, resulted in increased glutathione (GSH) levels by 110% and 107%, respectively58. GSH is an antioxidant that is released in response to oxidative stress66 and increased GSH levels following e-cig exposure suggest that e-cigs may elicit oxidative stress. A similar study also displayed increased ROS levels following e-cig exposure containing 20 different flavors67. However, the increases in ROS levels varied by e-cig flavorant. The effects of different e-cig flavorants on ROS levels from this study are summarized in Table 1.
Table 1.
E-cig Flavorant-Induced Increases in ROS levels. The effects of 49 different commercially available e-cig flavorants on lung cells were captured and analyzed for their effects on free radical generation via measurements of secondary lipid peroxidation of Thiobarbituric acid reactive substance (TBARS) assay67. Of the 49 e-cig flavorants investigated, 20 e-cig flavorants showed significant increases in ROS handling enzymes, expressed as % increase in TBARS levels.
| Commercial E-cig Flavorants | ROS Handling Enzyme Increases |
|---|---|
| Vanilla Custard | 122% |
| Cotton Candy | 114% |
| Butterscotch | 106% |
| Subtle Cinnamon | 105% |
| Rainbow Candy | 102% |
| Real Watermelon | 94% |
| Ripe Strawberry | 80% |
| Bubblegum | 76% |
| Dark Raz | 74% |
| Raspberry | 72% |
| Tootie Frootie Cereal | 80% |
| Pear | 70% |
| Real Honey | 67% |
| Kiwi | 66% |
| Sweet Tea | 65% |
| Root Beer | 61% |
| Coffee | 58% |
| Grape | 56% |
| Blue Raz | 49% |
| Lemon | 46% |
Thiobarbituric acid reactive substance (TBARS) functions as another biomarker of oxidative stress, as it is involved in lipid peroxidation58. Tobacco and vanilla flavored e-cigs with nicotine concentrations of 12 mg/ml increased TBARS levels by 38% and 71%, respectively, while tobacco flavored e-cigs without nicotine increased TBARS levels by 40%, when compared to controls58. These results indicate that flavored e-cigs elicit oxidative stress mediator release and increased ROS handling proteins with and without nicotine. While many studies have shown that e-cig flavorings are associated with an increase in ROS, some studies have shown that e-cig flavorings may also provide some protective benefits. Apple/mint flavored e-cigs (12 mg/ml nicotine concentration) decreased TBARS levels by 22%58. The addition of the chemical ethyl vanillin to e-cig flavorings has also been shown to decrease ROS levels compared to baseline data67. Still, the overall usage of e-cigs has been shown to induce oxidative stress.
Clinical trials have shown that following e-cig exposure, plasma levels of MPO were increased64. MPO is an enzyme normally released to induce oxidative stress and inflammation64, suggesting that inhaled e-cigs containing nicotine and flavorings activate leukocytes to promote the toxic release of MPO. Additionally, NADPH oxidase (NOX2), an enzyme involved in the quenching of oxidative stress64, was increased following e-cig exposure, as were hydrogen peroxide (H2O2) and 8-iso- PGF2α56. However, levels of vitamin E and activity of enzymes responsible for H2O2 breakdown were reduced, demonstrating that e-cig use increased oxidative stress while also depleting antioxidant levels56. It is hypothesized that reduction in most antioxidant proteins is due to oxidative DNA damage caused by e-cig use68. More research is still needed to identify which components of e-cigs are responsible for inducing oxidative stress and depleting antioxidant levels. However, it remains apparent that these devices pose a risk for inducing oxidative stress, predisposing for negative impacts on cardiometabolic health and cardiovascular function.
Effects on Neurological Activation of the Cardiovascular System:
The ANS is responsible for the involuntary physiological processes that maintain heart rate (HR) and blood pressure (BP) among other autonomic functions. The ANS is comprised of the parasympathetic (PNS or vagal) and the sympathetic (SNS) nervous systems, which are largely responsible for maintaining cardiovascular homeostasis. Extensive research has shown that e-cig use, with and without nicotine, alters the balance between PNS and SNS, resulting in skewed SNS overactivation, posing a risk for HR and BP elevations as well as arrhythmias. Extensive clinical research regarding the effects of vaping on HR have yielded mixed results, with several clinical trials showing that acute e-cig use results in significantly elevated HR56,60,64,69–71, while other clinical trials and exposure methods showed no significant changes following acute e-cig use60,61,70. Trials that demonstrated significantly increased HR following e-cig use showed that HR increases exist almost independently of nicotine concentration, with HR elevations present at 0,64 1.5,60 3,64 18,71 and 24 mg/mL69 of nicotine. However, the increases in HR following nicotine-free e-cig use were short lived64. Further, these same trials showed elevations in systolic BP56,60,64,69,70 and diastolic BP56,64,70. Other studies focusing on e-cig effects on heart rate variability (HRV) and sympathetic tone found that acute e-cig exposure resulted in decreased HRV72 and increased sympathetic tone73; both studies further suggest an acute shift towards sympathetic predominance and blunting of vagal activity. Animal models support these epidemiological findings and further suggest that nicotine and e-cig liquid alone may not be the only constituents that alter sympathetic activation of the cardiovascular system and that protonation of nicotine and/or e-cig flavorings may impact ANS homeostasis.
The effects of e-cig devices on ANS function have more recently been compared to oral nicotine packs (nicotine lozenges) and traditional tobacco smoke in a small clinical trial (n = 17), with observed alterations in HRV as well as increased biomarkers of physiological stress (i.e., salivary cortisol and serum catecholamines)74. It was shown that HRV following nicotine lozenge use changed the least when compared to both traditional tobacco and e-cig, suggesting that hydrophilicity of nicotine salts may play a role in the variable ANS reactivity. E-cigs, such as JUUL, often contain protonated nicotine salts, which are more hydrophilic and closer to physiological pH; this allows for slower binding to nicotinic receptors and slower diffusion than unprotonated nicotine74,75. HRV remained unchanged in participants during the first hour of e-cig exposure; however, after 1.5 hours, HRV decreased to nearly 20% of the value of smoking traditional cigarettes, and 50% at 2 hours74. Traditional tobacco heating systems showed HRV decreased by 80% compared to baseline almost instantaneously, whereas e-cig changes in HRV were subtle and gradual in onset74. These findings suggest that while e-cigs may have lowered relative risks for instantaneous changes in HRV when compared to traditional tobacco smoke, they also have increased relative risk compared to lozenges and absolute risks compared to abstaining from e-cig use, potentially because of nicotine salt protonation.
Preclinical studies using animal models demonstrated that various e-cig flavorings similar to those discussed in the Oxidative Stress and Potential Biomarkers section alter ANS homeostasis. Exposure to vanillin aldehyde flavored e-cig vapor in mice over a 10-week span resulted in increased sympathetic predominance in HRV measurements76. When compared to control air groups at 5 and 10 weeks of chronic exposure to vanillin aldehyde flavored e-cigs, e-cig-exposed mice showed significantly decreased pNN0676; which is a measurement of cardiac parasympathetic activity via the percentage of adjacent NN intervals (pNN) that differ from one another in a given time frame (e.g., 6 ms)77,78. Decreased pNN06 values following exposure suggest that aldehyde-containing flavorings decrease cardiac parasympathetic activity leading to the predominance of sympathetic activity, which has been linked to poor cardiovascular outcomes. Given the use of aldehydes (e.g., vanillin aldehyde) in e-cig flavorings and aldehyde byproducts of combustion (e.g., acetaldehyde), the pathological mechanisms by which these compounds induce cardiac dysfunction is of great concern. Aldehydes are metabolized by aldehyde dehydrogenase 2 (ALDH2), with approximately 8% of the population having an inactivating ALDH2 genetic variant (ALDH2*2) that reduces their ability to metabolize aldehydes. ALDH2*2 mice showed greater increases in HR following 10 days of e-cig exposure when compared to ALDH2 control mice exposed to e-cig and ALDH2 and ALDH2*2 exposed to air79. These alterations in aldehyde metabolism led to cardiovascular oxidative stress and may have potentially altered sympathetic activity that could be a symptom of oxidative stress or an additive insult to e-cigs containing flavorings.
Interestingly, studies have also demonstrated that biological sex may be yet another determinant in sympathetic activation following e-cig exposure. Mice exposed to various e-liquid constituents and aerosols (PG, VG, PG/VG, and tobacco- and menthol-flavored commercial e-liquids) showed significantly decreased HR during flavored e-cig exposures80. PG/VG-derived e-liquids caused the greatest changes in HR in male mice and showed no changes in female mice. Male mice also showed significantly increased HRV during flavored e-cig exposures, again with no changes observed in female mice. These changes were then followed by increased HR and decreased HRV between exposures and post-exposure in males with no observable changes in females80. A recent study from Nabavizadeh et al. suggested that no individual constituent of e-cigs or traditional cigarettes was responsible for endothelial dysfunction but that inhaled particles induce vagal nerve signaling initiated by airway irritation81. These studies indicate that different components of e-cigs, including but not limited to e-liquid and nicotine, as well as biological characteristics, such as sex and metabolic co-morbidities, all interact to predispose e-cig users to adverse cardiovascular outcomes. Thus, assessment of the physiological mechanisms that direct inhalants elicit as well as the interplay with social determinants of health and comorbidities could potentially provide greater benefit to public health, rather than research focusing solely on the toxic effects of individual e-cig components.
Conclusions, Limitations, and Future Directions:
While it is well known that the usage of and exposure to e-cig devices is fundamentally different from that of traditional cigarette combustion, the differences in cardiac, vascular, and cardiometabolic outcomes requires more investigation to better discern relative and absolute risks of e-cig use, as well as the mechanisms by which e-cigs elicit adverse cardiovascular outcomes. Additionally, e-cig effects on the cardiovascular and metabolic outcomes of individuals with comorbidities are of paramount concern when considering the relative and absolute risks of e-cig use in these individuals. Further, many studies have focused on how the essential components of e-cigs (i.e., delivery vehicle (PG, VG, PG/VG) with or without nicotine or other additives) elicit cardiovascular and metabolic effects; however, how additional e-cig constituents (e.g., nicotine salts (protonation/hydrophilicity status) and added flavorings) influence cardiovascular and metabolic health remains understudied (summarized in Table 2). While the United States Food and Drug Association (FDA) banned the sale of flavored e-cig cartridges and pod-based in February 2020, there is still extensive use of menthol-flavored e-cig liquids amongst e-cig users82. Furthermore, the ban was implemented as a means of reducing the appeal of e-cigs to adolescent users; however, the ban does not prevent e-cig users from mixing unflavored e-liquids with other purchased flavorants and necessitates further study.
Table 2.
GSH = glutathione, TBARS = Thiobarbituric acid reactive substances, MPO = Myeloperoxidase, NOX2 = NADPH oxidase, 8-iso-PGF2α = urinary 8-isoprostane, HR = Heart Rate, BP = Blood Pressure, HRV = Heart Rate Variability, pNN06 = the percentage of adjacent NN intervals (pNN) that differ from one another in more than 6 ms, FASN = fatty acid synthase, RER = Respiratory Exchange Ratio, HODE = Hydroxyoctadecadienoic acid, LDL = low-density lipoprotein, HDL = high-density lipoprotein, COL1A1 = Collagen (type 1, Alpha 1), COL3A1 = Collagen (Type 3, Alpha 1), EF = Ejection Fraction, LVFS = Left Ventricle Fractional Shortening, VCF = Velocity of circumferential fiber shortening, PWV = Pulse Wave Velocity, Alx75 = Augmentation Index corrected for heart rate, NO = Nitric Oxide, ADP = Adenosine diphosphate, GPIIb/IIIa = Glycoprotein IIb/IIIa, wks = week1.
| Condition | Biomarker | Change | Device Generation | Nicotine Concentration (mg/mL) | Flavor |
|---|---|---|---|---|---|
| Oxidative Stress | GSH | ↑58 | Unclear | 12 | Vanilla, Apple/Mint |
| No Change58 | Unclear | 0 | Tobacco | ||
| 18 | Apple/Mint | ||||
| TBARS | ↑58 | Unclear | 0, 12, 18 | Tobacco, Vanilla | |
| No Change58 | Unclear | 6 | Vanilla, Apple/Mint | ||
| ↓58 | Unclear | 12 | Apple/Mint | ||
| MPO | ↑64 | 3rd | 3 | None | |
| No Change64 | 3rd | 0 | None | ||
| NOX2 | ↑56 | 2nd | 16 | Tobacco | |
| H2O2 | ↑56 |
2nd | 16 | Tobacco | |
| 8-iso- PGF2α | ↑56 | 2nd | 16 | Tobacco | |
| Vitamin E | ↓56 | 2nd | 16 | Tobacco | |
| Sympathetic Effects | HR | ↑56,60,64,69–71 | 1st70, 2nd56,71, 3rd60,69 | 064, 1.560, 364, 1656, 1871, 2469, Several70 | None60,64, Tobacco56,69,71, Several70 |
| No Change60,61,70 | 1st70, 2nd61, 3rd60 | 060,61, 1261, Several70 | None60, Several70, Unclear61 | ||
| Systolic BP | ↑56,60,64,69,70 | 1st70, 2nd56, 3rd60,64,69 | 060,64, 1.560, 364, 1656, 2469, Several70 | None60,64, Tobacco56,69, Several70 | |
| Diastolic BP | ↑56,64,70 | 1st70, 2nd56, 3rd64 | 064, 364, 1656, Several70 | None64, Tobacco56, Several70 | |
| HRV | ↓72,74 | 1st72, 3rd74, 4th74 | 1872, Unclear74 | Tobacco72, Menthol72, Unclear74 | |
| Sympathetic Tone | ↑73 | 1st, 2nd | 12, Unclear | Tobacco, Strawberry | |
| pNN06 | ↓76 | 3rd | 6 | Vanilla-Custard | |
| Adipose Tissue Effects | Body Weight | ↓26,46 | 3rd | 046, 2426 | Tobacco |
| No Change26,46,48 | 3rd | 026, 1846, 3648 | Tobacco26,46, Unclear48 | ||
| Glut2 | ↑46 | 3rd | 0, 18 | Tobacco | |
| Glut4 | ↑46 | 3rd | 0 | Tobacco | |
| FASN | ↑46 | 3rd | 0, 18 | Tobacco | |
| Cytokines (Il10) | ↑47 | 3rd | 0 | Tobacco | |
| RER | ↓49 | 2nd | 18 | None | |
| serum ω−6 fatty acid levels | ↓53 | Several | Several | Several | |
| oxylipins 9-HODE & 13-HODE | ↓53 | Several | Several | Several | |
| LDL, HDL | No Change52,54 | 4th52, Unclear54 | Several | Several | |
| Myocardial Effects | End-Systolic Elastance | ↓24 | 3rd | 0, 20.2 | None |
| Preload-Recruitable Stroke work | ↓24 | 3rd | 20.2 | None | |
| Perivascular Fibrosis | ↑24 | 3rd | 0 | None | |
| COL1A1, COL3A1 | ↑(3 wks)24 | 3rd | 20.2 | None | |
| No Change (3 mo)24 | 3rd | 20.2 | None | ||
| EF | ↓(12 wks)26,27 | 3rd | 24 | Tobacco | |
| No Change (2 wks)23 | Unclear | 24 | None | ||
| LVFS | ↓26,27 | 3rd | 24 | Tobacco | |
| VCF | ↓26,27 | 3rd | 24 | Tobacco | |
| Vascular Effects | Arterial Stiffness PWV | ↑61,64 | 2nd61, 3rd64 | 061, 364, 1261 | None64, Unclear61 |
| Alx75 | ↑61,64 | 2nd61, 3rd64 | 061, 364, 1261 | None64, Unclear61 | |
| Flow-Mediated Vasodilation | ↑20 | Unclear | 16 | Tobacco | |
| NO Bioavailability | ↓20,56 | 2nd56, Unclear20 | 16 | Tobacco | |
| Thrombosis Effects | Duration in Occlusion Time | ↓62 | 4th | 18 | Menthol |
| ADP-induced platelet aggregation | ↑62 | 4th | 18 | Menthol | |
| ADP | ↑62 | 4th | 18 | Menthol | |
| P-Selectin | ↑62 | 4th | 18 | Menthol | |
| GPIIb/IIIa | ↑62 | 4th | 18 | Menthol |
Sources of Funding:
The authors thank the sponsors of research in the Wold laboratory, including the National Institutes of Health (R01 HL139348, R01 AG057046) and the American Heart Association (20YVNR35490079) to LEW.
Nonstandard Abbreviations and Acronyms:
- ALDH2
aldehyde dehydrogenase 2
- ANS
autonomic nervous system
- BP
blood pressure
- CTGF
connective tissue growth factor
- CVD
cardiovascular disease
- E-cig
electronic cigarettes
- ENDS
electronic nicotine delivery systems
- GLUT
glucose transporters
- H2O2
hydrogen peroxide
- HDL
high-density lipoprotein
- HR
heart rate
- HRV
HR variability
- IL
interleukin
- LDL
low-density lipoprotein
- LV
left ventricle
- MI
myocardial infarction
- MMP-2
matrix metalloproteinase 2
- MPO
myeloperoxidase
- NOX2
NADPH oxidase
- PG
propylene glycol
- ROS
reactive oxygen species
- TGF-β
transforming growth factor β
- VG
vegetable glycerin
- WAT
white adipose tissue
Footnotes
Disclosures:
Nothing to disclose.
References:
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442–e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samet JM. Tobacco Smoking. Thorac Surg Clin. 2013;23:103–112. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder MJ, Hoffman AC. Electronic cigarettes and nicotine clinical pharmacology. Tob Control. 2014;23 Suppl 2:ii30–ii35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine Levels in Electronic Cigarettes. Nicotine & Tobacco Research. 2012;15:158–166. [DOI] [PubMed] [Google Scholar]
- 5.Soule E, Bansal-Travers M, Grana R, McIntosh S, Price S, Unger JB, Walton K. Electronic cigarette use intensity measurement challenges and regulatory implications. Tob Control. 2023;32:124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FCTC W Electronic Nicotine Delivery Systems and Electronic Non-Nicotine Delivery Systems (ENDS / ENNDS). Cop7. 2016;7–12. [Google Scholar]
- 7.Corey CG, Ambrose BK, Apelberg BJ, King BA. Flavored Tobacco Product Use Among Middle and High School Students — United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:1066–1070. [DOI] [PubMed] [Google Scholar]
- 8.King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever-use of electronic cigarettes among U.S. adults, 2010–2011. Nicotine Tob Res. 2013;15:1623–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu S-H, Gamst A, Lee M, Cummins S, Yin L, Zoref L. The use and perception of electronic cigarettes and snus among the U.S. population. PLoS One. 2013;8:e79332–e79332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cobb NK, Byron MJ, Abrams DB, Shields PG. Novel nicotine delivery systems and public health: the rise of the “e-cigarette.” Am J Public Health. 2010;100:2340–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adkison SE, O’Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong H-H, Cummings KM, McNeill A, Thrasher JF, Hammond D, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. Am J Prev Med. 2013;44:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi K, Forster J. Awareness, perceptions and use of snus among young adults from the upper Midwest region of the USA. Tob Control. 2013;22:412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebbert JO, Agunwamba AA, Rutten LJ. Counseling Patients on the Use of Electronic Cigarettes. Mayo Clin Proc. 2015;90:128–134. [DOI] [PubMed] [Google Scholar]
- 14.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129:1972–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaisar MA, Prasad S, Liles T, Cucullo L. A decade of e-cigarettes: Limited research & unresolved safety concerns. Toxicology. 2016;365:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua M, Talbot P. Potential health effects of electronic cigarettes: A systematic review of case reports. Prev Med Rep. 2016;4:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lippi G, Favaloro E, Meschi T, Mattiuzzi C, Borghi L, Cervellin G, Lippi G. E-Cigarettes and Cardiovascular Risk: Beyond Science and Mysticism. Semin Thromb Hemost. 2013;40:60–65. [DOI] [PubMed] [Google Scholar]
- 18.Nelluri B, Murphy K, Mookadam F, Mookadam M. The current literature regarding the cardiovascular effects of electronic cigarettes. Future Cardiol. 2016;12:167–179. [DOI] [PubMed] [Google Scholar]
- 19.Orellana-Barrios MA, Payne D, Mulkey Z, Nugent K. Electronic Cigarettes—A Narrative Review for Clinicians. Am J Med. 2015;128:674–681. [DOI] [PubMed] [Google Scholar]
- 20.Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, Perri L, Peruzzi M, Marullo AGM, de Falco E, Chimenti I, et al. Acute Impact of Tobacco vs Electronic Cigarette Smoking on Oxidative Stress and Vascular Function. Chest. 2016;150:606–612. [DOI] [PubMed] [Google Scholar]
- 21.Alzahrani T, Pena I, Temesgen N, Glantz SA. Association Between Electronic Cigarette Use and Myocardial Infarction. Am J Prev Med. 2018;55:455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farsalinos KE, Tsiapras D, Kyrzopoulos S, Savvopoulou M, Voudris V. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: Comparison with the effects of regular cigarettes. BMC Cardiovasc Disord. 2014;14:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi H, Fan X, Horton A, Haller ST, Kennedy DJ, Schiefer IT, Dworkin L, Cooper CJ, Tian J. The Effect of Electronic-Cigarette Vaping on Cardiac Function and Angiogenesis in Mice. Sci Rep. 2019;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neczypor EW, Saldaña TA, Mears MJ, Aslaner DM, Escobar Y-NH, Gorr MW, Wold LE. e-Cigarette Aerosol Reduces Left Ventricular Function in Adolescent Mice. Circulation. 2022;145:868–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain RK. Clearing the smoke on nicotine and angiogenesis. Nat Med. 2001;7:775–7. [DOI] [PubMed] [Google Scholar]
- 26.Hasan KM, Friedman TC, Parveen M, Espinoza-Derout J, Bautista F, Razipour MM, Shao XM, Jordan MC, Roos KP, Mahata SK, et al. Electronic cigarettes cause alteration in cardiac structure and function in diet-induced obese mice. PLoS One. 2020;15:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Espinoza-Derout J, Hasan KM, Shao XM, Jordan MC, Sims C, Lee DL, Sinha S, Simmons Z, Mtume N, Liu Y, et al. Chronic intermittent electronic cigarette exposure induces cardiac dysfunction and atherosclerosis in apolipoprotein-E knockout mice. American Journal of Physiology-Heart and Circulatory Physiology. 2019;317:H445–H459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartz AJ, Anderson AJ, Brooks HL, Manley JC, Parent GT, Barboriak JJ. The association of smoking with cardiomyopathy. N Engl J Med. 1984;311:1201–6. [DOI] [PubMed] [Google Scholar]
- 29.Gvozdjăk J, Gvozdjáková A, Kucharská J, Bada V. The effect of smoking on myocardial metabolism. Czech Med. 1987;10:47–53. [PubMed] [Google Scholar]
- 30.Gu L, Pandey V, Geenen DL, Chowdhury SAK, Piano MR. Cigarette smoke-induced left ventricular remodelling is associated with activation of mitogen-activated protein kinases. Eur J Heart Fail. 2008;10:1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaplan A, Abidi E, Ghali R, Booz GW, Kobeissy F, Zouein FA. Functional, Cellular, and Molecular Remodeling of the Heart under Influence of Oxidative Cigarette Tobacco Smoke. Oxid Med Cell Longev. 2017;2017:3759186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szostak J, Wong ET, Titz B, Lee T, Wong SK, Low T, Lee KM, Zhang J, Kumar A, Schlage WK, et al. A 6-month systems toxicology inhalation study in ApoE−/− mice demonstrates reduced cardiovascular effects of E-vapor aerosols compared with cigarette smoke. Am J Physiol Heart Circ Physiol. 2020;318:H604–H631. [DOI] [PubMed] [Google Scholar]
- 33.Guo H, Tian L, Zhang JZ, Kitani T, Paik DT, Lee WH, Wu JC. Single-Cell RNA Sequencing of Human Embryonic Stem Cell Differentiation Delineates Adverse Effects of Nicotine on Embryonic Development. Stem Cell Reports. 2019;12:772–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jian J, Zhang P, Li Y, Liu B, Zhang Y, Zhang L, Shao XM, Zhuang J, Xiao D. Reprogramming of miR-181a/DNA methylation patterns contribute to the maternal nicotine exposure-induced fetal programming of cardiac ischemia-sensitive phenotype in postnatal life. Theranostics. 2020;10:11820–11836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. [DOI] [PubMed] [Google Scholar]
- 38.Tian J, Popal MS, Zhao Y, Liu Y, Chen K, Liu Y. Interplay between Exosomes and Autophagy in Cardiovascular Diseases: Novel Promising Target for Diagnostic and Therapeutic Application. Aging Dis. 2019;10:1302–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colombo ES, Davis J, Makvandi M, Aragon M, Lucas SN, Paffett ML, Campen MJ. Effects of nicotine on cardiovascular remodeling in a mouse model of systemic hypertension. Cardiovasc Toxicol. 2013;13:364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palpant NJ, Hofsteen P, Pabon L, Reinecke H, Murry CE. Cardiac development in zebrafish and human embryonic stem cells is inhibited by exposure to tobacco cigarettes and ecigarettes. PLoS One. 2015;10:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayyas F, Aldawod H, Alzoubi KH, Khabour O, Shihadeh A, Eissenberg T. Comparison of the cardiac effects of electronic cigarette aerosol exposure with waterpipe and combustible cigarette smoke exposure in rats. Life Sci. 2020;251:117644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dabek J, Kułach A, Monastyrska-Cup B, Gasior Z. Transforming growth factor beta and cardiovascular diseases: the other facet of the “protective cytokine”. Pharmacol Rep. 2006;58:799–805. [PubMed] [Google Scholar]
- 43.Chen Z, Huang W, Dahme T, Rottbauer W, Ackerman MJ, Xu X. Depletion of zebrafish essential and regulatory myosin light chains reduces cardiac function through distinct mechanisms. Cardiovasc Res. 2008;79:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kos K Cardiometabolic Morbidity and Mortality with Smoking Cessation, Review of Recommendations for People with Diabetes and Obesity. Curr Diab Rep. 2020;20:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Górna I, Napierala M, Florek E. Electronic Cigarette Use and Metabolic Syndrome Development: A Critical Review. Toxics. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen H, Li G, Chan YL, Zhang HE, Gorrell MD, Pollock CA, Saad S, Oliver BG. Differential Effects of “Vaping” on Lipid and Glucose Profiles and Liver Metabolic Markers in Obese Versus Non-obese Mice. Front Physiol. 2021;12:755124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen H, Chan YL, Thorpe AE, Pollock CA, Saad S, Oliver BG. Inhaled or Ingested, Which Is Worse, E-Vaping or High-Fat Diet? Front Immunol. 2022;13:913044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orimoloye OA, Uddin SMI, Chen L-C, Osei AD, Mirbolouk M, Malovichko M v, Sithu ID, Dzaye O, Conklin DJ, Srivastava S, et al. Electronic cigarettes and insulin resistance in animals and humans: Results of a controlled animal study and the National Health and Nutrition Examination Survey (NHANES 2013–2016). PLoS One. 2019;14:e0226744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crawford DL, Phillips AR, Williams TR. Evaluation of secondary electronic cigarette inhalation on lipid metabolism in C57BL/6J mice using indirect calorimetry. Metabol Open. 2021;12:100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zagoriti Z, el Mubarak MA, Farsalinos K, Topouzis S. Effects of Exposure to Tobacco Cigarette, Electronic Cigarette and Heated Tobacco Product on Adipocyte Survival and Differentiation In Vitro. Toxics. 2020;8(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Atuegwu NC, Perez MF, Oncken C, Mead EL, Maheshwari N, Mortensen EM. E-cigarette use is associated with a self-reported diagnosis of prediabetes in never cigarette smokers: Results from the behavioral risk factor surveillance system survey. Drug Alcohol Depend. 2019;205:107692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majid S, Keith RJ, Fetterman JL, Weisbrod RM, Nystoriak J, Wilson T, Stokes AC, Blaha MJ, Srivastava S, Robertson RM, et al. Lipid profiles in users of combustible and electronic cigarettes. Vascular Medicine. 2021;26:483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gupta R, Lin Y, Luna K, Logue A, Yoon AJ, Haptonstall KP, Moheimani R, Choroomi Y, Nguyen K, Tran E, et al. Electronic and Tobacco Cigarettes Alter Polyunsaturated Fatty Acids and Oxidative Biomarkers. Circ Res. 2021;129:514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okafor CN, Okafor N, Kaliszewski C, Wang L. Association between electronic cigarette and combustible cigarette use with cardiometabolic risk biomarkers among U.S. adults. Ann Epidemiol. 2022;71:44–50. [DOI] [PubMed] [Google Scholar]
- 55.Fetterman JL, Hamburg NM. A cautionary note on electronic cigarettes and vascular health. Vascular Medicine (United Kingdom). 2018;23:426–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Biondi-Zoccai G, Sciarretta S, Bullen C, Nocella C, Violi F, Loffredo L, Pignatelli P, Perri L, Peruzzi M, Marullo AGM, et al. Acute Effects of Heat-Not-Burn, Electronic Vaping, and Traditional Tobacco Combustion Cigarettes: The Sapienza University of Rome-Vascular Assessment of Proatherosclerotic Effects of Smoking (SUR-VAPES) 2 Randomized Trial. J Am Heart Assoc. 2019;8(6):e010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fetterman JL, Weisbrod RM, Feng B, Bastin R, Tuttle ST, Holbrook M, Baker G, Robertson RM, Conklin DJ, Bhatnagar A, et al. Flavorings in Tobacco Products Induce Endothelial Cell Dysfunction. Arterioscler Thromb Vasc Biol. 2018;38:1607–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kerasioti E, Veskoukis AS, Skaperda Z, Zacharias A, Poulas K, Lazopoulos G, Kouretas D. The flavoring and not the nicotine content is a decisive factor for the effects of refill liquids of electronic cigarette on the redox status of endothelial cells. Toxicol Rep. 2020;7:1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee WH, Ong S-G, Zhou Y, Tian L, Bae HR, Baker N, Whitlatch A, Mohammadi L, Guo H, Nadeau KC, et al. Modeling Cardiovascular Risks of E-Cigarettes With Human-Induced Pluripotent Stem Cell-Derived Endothelial Cells. J Am Coll Cardiol. 2019;73:2722–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaumont M, Tagliatti V, Channan EM, Colet J-M, Bernard A, Morra S, Deprez G, van Muylem A, Debbas N, Schaefer T, et al. Short halt in vaping modifies cardiorespiratory parameters and urine metabolome: a randomized trial. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2020;318:L331–L344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ikonomidis I, Vlastos D, Kourea K, Kostelli G, Varoudi M, Pavlidis G, Efentakis P, Triantafyllidi H, Parissis J, Andreadou I, et al. Electronic cigarette smoking increases arterial stiffness and oxidative stress to a lesser extent than a single conventional cigarette. Circulation. 2018;137:303–306. [DOI] [PubMed] [Google Scholar]
- 62.Ramirez JEM, Karim ZA, Alarabi AB, Hernandez KR, Taleb Z ben, Rivera JO, Khasawneh FT, Alshbool FZ. The JUUL E-Cigarette Elevates the Risk of Thrombosis and Potentiates Platelet Activation. J Cardiovasc Pharmacol Ther. 2020;25:578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.French DL, Seligsohn U. Platelet glycoprotein IIb/IIIa receptors and Glanzmann’s thrombasthenia. Arterioscler Thromb Vasc Biol. 2000;20:607–10. [DOI] [PubMed] [Google Scholar]
- 64.Chaumont M, de Becker B, Zaher W, Culié A, Deprez G, Mélot C, Reyé F, van Antwerpen P, Delporte C, Debbas N, et al. Differential Effects of E-Cigarette on Microvascular Endothelial Function, Arterial Stiffness and Oxidative Stress: A Randomized Crossover Trial. Sci Rep. 2018;8:10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andrikopoulos GI, Zagoriti Z, Topouzis S, Poulas K. Oxidative stress induced by electronic nicotine delivery systems (ENDS): Focus on respiratory system. Curr Opin Toxicol. 2019;13:81–89. [Google Scholar]
- 66.Kwon DH, Cha HJ, Lee H, Hong SH, Park C, Park SH, Kim GY, Kim S, Kim HS, Hwang HJ, et al. Protective effect of glutathione against oxidative stress-induced cytotoxicity in RAW 264.7 macrophages through activating the nuclear factor erythroid 2-related factor-2/heme oxygenase-1 pathway. Antioxidants. 2019;8(4):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bitzer ZT, Goel R, Reilly SM, Elias RJ, Silakov A, Foulds J, Muscat J, Richie, John PJ. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic Biol Med. 2018;120:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ganapathy V, Manyanga J, Brame L, McGuire D, Sadhasivam B, Floyd E, Rubenstein DA, Ramachandran I, Wagener T, Queimado L. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS One. 2017;12:e0177780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franzen KF, Willig J, Cayo Talavera S, Meusel M, Sayk F, Reppel M, Dalhoff K, Mortensen K, Droemann D. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: A randomized, double-blinded pilot study. Vasc Med. 2018;23:419–425. [DOI] [PubMed] [Google Scholar]
- 70.Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regulatory Toxicology and Pharmacology. 2015;71:24–34. [DOI] [PubMed] [Google Scholar]
- 71.Kerr DMI, Brooksbank KJM, Taylor RG, Pinel K, Rios FJ, Touyz RM, Delles C. Acute effects of electronic and tobacco cigarettes on vascular and respiratory function in healthy volunteers: A cross-over study. J Hypertens. 2019;37:154–166. [DOI] [PubMed] [Google Scholar]
- 72.Lee M-S, Rees VW, Koutrakis P, Wolfson JM, Son Y-S, Lawrence J, Christiani DC. Cardiac Autonomic Effects of Secondhand Exposure to Nicotine from Electronic Cigarettes. Environmental Epidemiology. 2019;3:e033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moheimani RS, Bhetraratana M, Peters KM, Yang BK, Yin F, Gornbein J, Araujo JA, Middlekauff HR. Sympathomimetic effects of acute e-cigarette use: Role of nicotine and non-nicotine constituents. J Am Heart Assoc. 2017;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menshov VA, Trofimov A v., Zagurskaya A v., Berdnikova NG, Yablonskaya OI, Platonova AG. Influence of Nicotine from Diverse Delivery Tools on the Autonomic Nervous and Hormonal Systems. Biomedicines. 2022;10:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shao XM, Friedman TC. Pod-mod vs. conventional e-cigarettes: nicotine chemistry, pH, and health effects. J Appl Physiol (1985). 2020;128:1056–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abouassali O, Chang M, Chidipi B, Martinez JL, Reiser M, Kanithi M, Soni R, McDonald T v., Herweg B, Saiz J, et al. In vitro and in vivo cardiac toxicity of flavored electronic nicotine delivery systems. Am J Physiol Heart Circ Physiol. 2021;320:H133–H143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thireau J, Zhang BL, Poisson D, Babuty D. Heart rate variability in mice: a theoretical and practical guide. Exp Physiol. 2008;93:83–94. [DOI] [PubMed] [Google Scholar]
- 78.Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health. 2017;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu X, Zeng X, Xiao F, Chen R, Sinharoy P, Gross ER. E-cigarette aerosol exacerbates cardiovascular oxidative stress in mice with an inactive aldehyde dehydrogenase 2 enzyme. Redox Biol. 2022;54:102369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carll AP, Arab C, Salatini R, Miles MD, Nystoriak MA, Fulghum KL, Riggs DW, Shirk GA, Theis WS, Talebi N, et al. E-cigarettes and their lone constituents induce cardiac arrhythmia and conduction defects in mice. Nat Commun. 2022;13:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nabavizadeh P, Liu J, Rao P, Ibrahim S, Han DD, Derakhshandeh R, Qiu H, Wang X, Glantz SA, Schick SF, et al. Impairment of Endothelial Function by Cigarette Smoke Is Not Caused by a Specific Smoke Constituent, but by Vagal Input From the Airway. Arterioscler Thromb Vasc Biol. 2022;42:1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yingst JM, Bordner CR, Hobkirk AL, Hoglen B, Allen SI, Krebs NM, Houser KR, Livelsberger C, Foulds J. Response to Flavored Cartridge/Pod-Based Product Ban among Adult JUUL Users: “You Get Nicotine However You Can Get It”. Int J Environ Res Public Health. 2020;18(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rüther T, Hagedorn D, Schiela K, Schettgen T, Osiander-Fuchs H, Schober W. Nicotine delivery efficiency of first- and second-generation e-cigarettes and its impact on relief of craving during the acute phase of use. Int J Hyg Environ Health. 2018;221:191–198. [DOI] [PubMed] [Google Scholar]
- 84.Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep. 2014;4:4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Incalza MA, D’Oria R, Natalicchio A, Perrini S, Laviola L, Giorgino F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19. [DOI] [PubMed] [Google Scholar]


