Abstract
Objective
We aim to: 1) investigate whether starting cardiac rehabilitation (CR) during the COVID-19 period was influential on new-onset depressive symptoms, 2) examine the relationship between sociodemographic and medical factors with the new-onset of depressive symptoms before and during the COVID-19 period in UK patients commencing CR.
Methods
The national audit of cardiac rehabilitation (NACR) data were used and the two years of data before COVID-19 and during COVID-19 were analysed (Feb,2018 – Nov,2021). Hospital Anxiety and Depression Scale measurement was used to assess depressive symptoms. Bivariate analysis and logistic regression were conducted to examine the influence of the COVID-19 period on new-onset depressive symptoms and the patient characteristics associated with it.
Results
71055 patients screened for new-onset depressive symptoms were included in the analysis. Based on multivariate analysis, patients commencing CR during COVID-19 were 8% more likely to have new onset depressive symptoms compared to patients commencing before COVID-19. Smoking (OR: 1.26, 95%CI: 1.11, 1.43), physical inactivity (OR: 1.86, 95%CI: 1.74, 1.98), high anxiety (OR: 1.45, 95%CI: 1.44, 1.46), being male (OR: 1.21, 95%CI: 1.12, 1.30), single (OR: 1.25, 95%CI: 1.16, 1.35), having comorbidities of arthritis, diabetes, chronic bronchitis, emphysema, claudication (OR range: 1.19 to 1.60), receiving CABG treatment (OR: 1.47, 95%CI: 1.25, 1.73), and having heart failure (OR: 1.33, 95%CI: 1.19, 1.48) were the factors associated with having new-onset depressive symptoms at the start of CR.
Conclusion
Our findings have shown that starting CR during the COVID-19 period was associated with increased odds of having new-onset depressive symptoms.
Keywords: Cardiac rehabilitation, Cardiovascular disease, COVID-19, New onset depressive symptoms, Observational study
1. Introduction
Depression has been found to be associated with an increased risk of mortality and poor prognosis in patients with cardiovascular disease (CVD) [1], and recommendations have been made for depression to be recognized as a risk factor for mortality and worse cardiac outcomes [2]. In the general population, the prevalence of depression was estimated to be around 5% [3], whereas in cardiac patients this prevalence was noticeably higher at around 20% [4].
Cardiac rehabilitation (CR) is a multimodal intervention aiming at providing comprehensive care and secondary prevention for patients in CR [5]. Depression is a widely recognized risk factor that has a negative impact on cardiac prognosis and outcomes [2]. Recent guidelines acknowledge this and suggest that evaluating and managing depression should be an integral component of core CR services [5,6].
Due to the rapid spread of the coronavirus illness 2019 (COVID-19), many people in the UK have been advised to stay at home for extended periods. The COVID-19 pandemic and its containment measures have already had far-reaching consequences for many parts of society [7]. COVID-19's incidence and fatality rates, the virus's pathogenesis, its negative impacts on physical health, and its growing influence on the global economy have all been widely published and integrated into the public consciousness [[8], [9], [10]]. However, people are becoming more conscious of the pandemic's impact on depression [11]. The pervasive impact of COVID-19 related stressors, limited access to psychosocial health interventions, and widespread worries about psychosocial and physical health in the general community underline the significance of determining who is most at risk and how their experiences are changing as the pandemic progresses [12].
Recent studies have shown the association between COVID-19 and depression in the general population [[12], [13], [14]] and in healthcare workers [15]. However, to our knowledge, there is a lack of studies investigating the association of starting CR during the COVID-19 period with new-onset depressive symptoms in cardiac patients. Hitherto the influence of societal constraints associated with limiting the influence of COVID-19 has not been studied on new-onset depressive symptoms in cardiac patients at the commencement of CR employing Hospital Anxiety and Depression Scale (HADS) measurements. Thus, the aim of this research is to: 1) investigate whether starting CR during the COVID-19 period was influential on new-onset depressive symptoms, 2) examine the relationship between sociodemographic and medical factors with the new-onset of depressive symptoms before and during the COVID-19 period in UK patients undergoing CR. We hypothesise that patients who are starting the CR during COVID-19, are smokers, physically inactive, single, have high anxiety, have comorbidities, and present with heart failure will be more likely to experience new-onset depressive symptoms compared to those who do not have these characteristics. The findings of this study could enhance our comprehension of specific patient characteristics that make patients commencing CR vulnerable to new-onset depressive symptoms. This, in turn, could result in greater personalizing of CR services.
2. Methods
This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline [16].
2.1. Data collection
The National Audit of Cardiac Rehabilitation (NACR)’s goal is to keep track of UK CR programmes in order to improve the quality of service delivery and outcomes in CR centres, as well as to design and carry out studies to evaluate the influence of routinely provided CR services on patient-agreed outcomes, cardiovascular disease risk profiles, and the use of health care. NACR provided the study population and data. Data at the patient level were extracted and analysed. An observational research method was employed using routinely acquired NACR data. Individual patient data are gathered under section 251 of the NHS Act 2006 and submitted into the NHS Digital data storage system. It was not essential to obtain specific consent from each patient because NHS Digital has the approval to gather patient-identifiable data that are anonymized and then made available for NACR. Additionally, research done to improve services is acceptable as long as it falls under the scope of the 251 exemption and the audit's aim. Due to these factors, ethical approval was not required before carrying out and submitting this study. There are 233 CR programmes accessible for NACR in the UK, encompassing centres from England, Northern Ireland, and Wales, with 194 of them having electronic NACR registration for data submission, allowing for better audit coverage [17]. For people who attend CR in the United Kingdom, the data includes demographics, medication, risk factors, and treatment. In a recent NACR report, a detailed description of NACR is available [17].
2.2. Participants
The data period was from the NACR between 1st February 2018 and 31st January 2020 for the pre-COVID-19 period and between 01 February 2020 and 31 November 2021 for the COVID-19 period. Starting CR before or during COVID-19 was determined based on the date that COVID-19 spread was confirmed in the UK, which was at the end of January. As a result, the two years preceding the spread of COVID-19 were designated as pre-COVID, and the years following the disease's spread were designated as the COVID-19 period. Patients with myocardial infarction (MI), heart failure (HF), and percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) treatment were included in the study since it was suggested in National Institute for Health and Care Excellence (NICE) clinical guidelines [18,19]. The NACR dataset was used to identify patients who had no prior history of depression and had valid pre-CR HADS measures, resulting in an eligible patient population for the study sample during the study period in preCOVID-19 and during the COVID-19 period (N = 71,055).
2.3. Measures
Patients who did not present with a prior history of depression and had baseline HADS assessments recorded were chosen from the NACR data set as eligible patients for the study sample using this method. Case note review and examination of the patient's medical record are used by CR practitioners to confirm the pre-diagnosis of depression.
2.4. Psychological covariates
2.4.1. Hospital anxiety and depression scale
The HADS is a self-administered questionnaire that is used to test for depression symptoms in healthcare practice. The HADS is a recommended instrument for assessing psychosocial health in patients undergoing CR in order to provide them with a personalised CR program based on their needs [5]. HADS contains 14 items: seven for anxiety symptoms and seven for depressive symptoms. Each item is given a score from 0 to 3, with a minimum of 0 and a maximum of 21. Anxiety and depression scores are given separately. Higher scores indicate more severe symptoms. The HADS is suggested for use with cardiac patients since it has been demonstrated to be a reliable and valid tool for assessing anxiety and depressive symptoms [20,21]. The current study's analysis compared patients with HADS <8 to patients with HADS ≥8 in a subset of patients without a history of depression and comparisons were made between individuals who commenced CR during COVID-19 to those who commenced prior to COVID-19. At the start of CR, patients were classified using a clinical cut-off point of 8, with <8 indicating patients with the absence of new-onset depressive symptoms and ≥ 8 indicating patients with the presence of new-onset depressive symptoms. According to a systematic review, the optimal balance between specificity and sensitivity for the HADS assessment tool was consistently reached at a cut-off score of 8 (specificity and sensitivity for both scales around 0.80) both for depression and anxiety [20]. In addition, baseline HADS anxiety scores were examined to understand whether it was associated with new-onset depressive symptoms at the commencement of CR.
2.4.2. Demographics
The age, sex (male/female), and partner status (single/partnered) of the patients were used in the study. Another patient demographic was the English Index of Multiple Deprivation (IMD), a deprivation metric used in England [22]. In the current study, IMD was used as a dichotomous variable (most deprived areas/less deprived areas) as described in previous studies [23]. The IMD measure is created using seven different areas of consideration: work opportunities, health issues, financial status, criminal activity, access to housing and resources, overall living conditions, and education skills and training [24]. In total, 32,844 specific regions are ranked from the most to least disadvantaged. IMD was used to classify patients into two quintiles. The first quintile, referred to as the “lowest quintile,” represents the most deprived areas, while the remaining quintiles were considered to be less deprived.
2.5. Medical covariates
Patients were classified as current smokers or non-smokers based on their baseline smoking measurements. Comorbidities, medical history of conditions (confirmed by CR providers reviewing patient medical records), including arthritis, diabetes, chronic bronchitis, and others were included in the analysis. In addition to assessing whether participants met the UK physical activity guideline of engaging in moderate physical activity for 150 min per week [25], the study examined their body mass index (BMI), cardiac treatment (PCI/CABG/other/none), and whether they had a presence of heart failure (yes/no).
To be able to investigate if starting CR during COVID-19 was associated with new-onset depressive symptoms in CR attendees, a covariate based on whether the patient underwent CR prior to or during the COVID-19 period was used in the regression model. The factors included in this study were chosen based on previous research and baseline clinical variables performed by CR practitioners, which were described in detail in earlier publications. [23,[26], [27], [28]].
2.6. Statistical analysis
The IBM SPSS program statistics V-28 was used to analyse the data (New York, USA). The level of statistical significance was set at P < 0.05. To present summary data, percentages, means, and standard deviations were used. The pre-COVID-19 and during COVID-19 baseline characteristics were compared using the chi-square test for categorical variables and t-tests for continuous variables. For continuous variables, Cohen's d effect size was calculated, whereas for categorical variables, the phi effect size was provided. Further comparisons were made for patients who started CR before COVID-19 and those who started during the COVID-19 period. A logistic regression was used to scrutinize the factors associated with new-onset depressive symptoms at the commencement of CR. Thus, a binary logistic regression model was constructed to ascertain the association of the starting CR during the COVID-19 period and other patient characteristics including age, sex, partner status, IMD, anxiety, smoking, physical activity, BMI, presence of heart failure, cardiac treatment, comorbidities of arthritis, diabetes, chronic bronchitis, emphysema, and claudication with the likelihood of having new-onset depressive symptoms.
3. Results
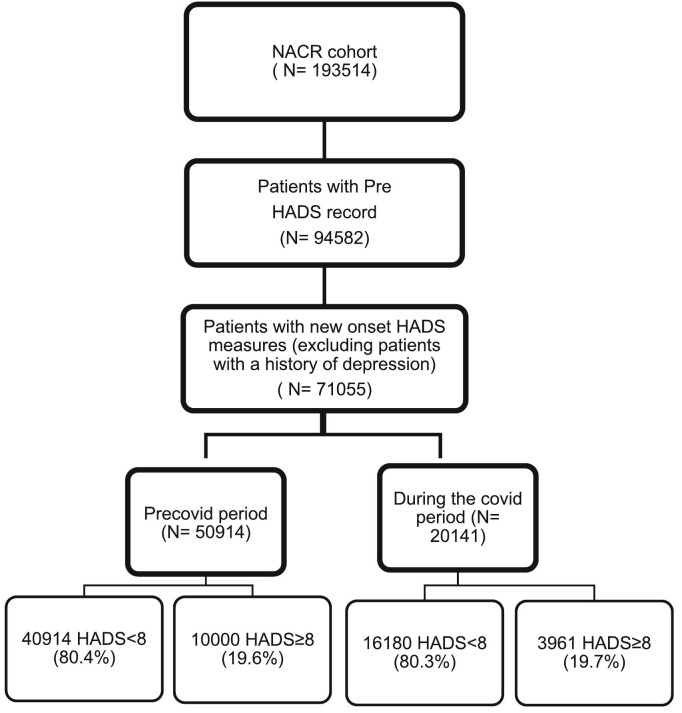
71,055 patients with new onset HADS measures were included in the study with valid baseline assessments. Of these patients, 50,914 (71.7%) were assessed prior to CR during pre COVID-19 period, and 20,141 (28.3%) participants during the COVID-19 period. The percentage of participants who presented with new-onset depressive symptoms was 19.6% in pre COVID-19 period and this was 19.7% during the COVID-19 period. The flow diagram displays the total population during the study period, including the pre- and during-COVID-19 time periods, as well as the rates of depressive symptoms observed during these periods ( Fig. 1 ). Baseline characteristics of patients were compared based on commencing CR in the pre COVID-19 period and during the COVID-19 period in Table 1 . In Table 2 , baseline patient characteristics were presented based on their depressive symptom levels according to HADS, and the pre-COVID-19 and COVID-19 period were also compared.
Fig. 1.
Study flow diagram.
Table 1.
Baseline characteristics for pre COVID-19 era and during COVID-19 era groups.
| Variables | Pre COVID-19 Era (n = 50,914) |
During COVID-19 Era (n = 20,141) |
P | Effect Size | ||
|---|---|---|---|---|---|---|
| n | Mean + SD | n | Mean + SD | |||
| Age | 50,914 | 66.02 ± 11.34 | 20,141 | 65.73 ± 11.52 | 0.002 | 0.02 |
| BMI | 47,445 | 28.42 ± 5.25 | 18,240 | 28.24 ± 5.21 | <0.001 | 0.03 |
| HADS Anxiety Score Measurement | 50,878 | 5.43 ± 4.14 | 20,094 | 5.22 ± 4.15 | <0.001 | 0.05 |
| HADS Depression Score Measurement | 50,914 | 4.50 ± 3.67 | 20,141 | 4.40 ± 3.74 | 0.003 | 0.03 |
| Variables | % | % | P | Effect size | ||
| Sex (female) | 13,062 | 25.7 | 5253 | 26.2 | 0.23 | 0.00 |
| 150 min. Physical Activity a Week (Yes) | 22,102 | 50 | 8151 | 47.8 | <0.001 | 0.02 |
| Smoking (Yes) | 2980 | 6.1 | 1371 | 7.1 | <0.001 | 0.02 |
| Single | 8575 | 21.6 | 3633 | 23.5 | <0.001 | 0.02 |
| IMD (Most deprived) | 6432 | 15 | 2567 | 14.6 | 0.28 | 0.00 |
| Heart Failure (Yes) | 4982 | 9.8 | 1704 | 8.5 | <0.001 | 0.02 |
| Cardiac treatment No treatment PCI CABG Other treatment |
||||||
| 3946 | 7.8 | 1005 | 5.0 | <0.001 | 0.07 | |
| 25,123 | 49.3 | 11,427 | 56.7 | |||
| 6483 | 12.7 | 2060 | 10.2 | |||
| 15,362 | 30.2 | 5649 | 28.0 | |||
| Comorbidities | ||||||
| Arthritis (Yes) | 8007 | 15.7 | 2903 | 14.4 | <0.001 | 0.02 |
| Diabetes (Yes) | 11,181 | 22.0 | 4502 | 22.4 | 0.26 | 0.00 |
| Chronic bronchitis (Yes) | 1600 | 3.1 | 664 | 3.3 | 0.29 | 0.00 |
| Emphysema (Yes) | 1233 | 2.4 | 453 | 2.2 | 0.17 | 0.00 |
| Claudication (Yes) | 1013 | 2.0 | 356 | 1.8 | 0.052 | 0.01 |
HADS, Hospital Anxiety and Depression Scale; SD, standard deviation; BMI, body mass index.
Table 2.
Baseline characteristics comparing patients with presence and absence of new-onset depressive symptoms using t-tests.
| Pre COVID-19 Era |
COVID-19 Era |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | HADS < 8 group (n = 40,914) |
HADS ≥ 8 group (n = 10,000) |
P | d | HADS < 8 group (n = 16,180) |
HADS ≥ 8 group (n = 3961) |
P |
d |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | b | ||||
| Age | 66.46 ± 11.19 | 64.22 ± 11.77 | < 0.001 | 0.19 | 66.08 ± 11.40 | 64.30 ± 11.86 | < 0.001 | 0.15 |
| BMI | 28.28 ± 5.09 | 29.06 ± 5.82 | < 0.001 | 0.14 | 28.08 ± 5.07 | 28.90 ± 5.70 | < 0.001 | 0.15 |
| HADS Anxiety Score Measurement | 4.35 ± 3.36 | 9.84 ± 4.15 | <0.001 | 1.45 | 4.13 ± 3.36 | 9.67 ± 4.13 | <0.001 | 1.47 |
| Pre COVID-19 Era |
COVID-19 Era |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variables | HADS < 8 Group % (n = 40,914) |
HADS ≥ 8 Group % (n = 10,000) |
P | Effect size | HADS < 8 Group % (n = 16,180) |
HADS ≥ 8 Group % (n = 3961) |
P | Effect size |
| Female | 24.4 | 31.2 | < 0.001 | 0.06 | 24.8 | 31.9 | < 0.001 | 0.07 |
| 150 min. Physical Activity a Week (Yes) | 53.7 | 34.0 | < 0.001 | 0.16 | 51.1 | 33.9 | < 0.001 | 0.14 |
| Smoking (Yes) | 5.2 | 9.6 | < 0.001 | 0.07 | 6.2 | 10.5 | < 0.001 | 0.07 |
| Single | 20.6 | 25.6 | < 0.001 | 0.05 | 22.1 | 29.5 | < 0.001 | 0.07 |
| IMD (Most deprived) | 13.5 | 21.2 | < 0.001 | 0.09 | 13.7 | 18.8 | < 0.001 | 0.06 |
| Heart Failure (Yes) | 9.0 | 13.2 | < 0.001 | 0.06 | 8.3 | 9.2 | 0.049 | 0.01 |
| Cardiac treatment No treatment PCI CABG Other treatment |
||||||||
| 7.5 | 8.8 | < 0.001 | 0.04 | 4.8 | 5.9 | < 0.001 | 0.04 | |
| 50.3 | 45.6 | 57.7 | 52.9 | |||||
| 12.9 | 12.2 | 10.1 | 10.7 | |||||
| 29.4 | 33.5 | 27.4 | 30.5 | |||||
| Comorbidities | ||||||||
| Arthritis (Yes) | 15.4 | 17.0 | < 0.001 | 0.02 | 13.9 | 16.7 | < 0.001 | 0.03 |
| Diabetes (Yes) | 20.5 | 27.8 | < 0.001 | 0.07 | 21.2 | 27.2 | < 0.001 | 0.06 |
| Chronic bronchitis (Yes) | 2.8 | 4.7 | < 0.001 | 0.04 | 2.9 | 4.9 | < 0.001 | 0.04 |
| Emphysema (Yes) | 2.1 | 3.6 | < 0.001 | 0.04 | 2.0 | 3.4 | < 0.001 | 0.04 |
| Claudication (Yes) | 1.8 | 2.7 | < 0.001 | 0.03 | 1.5 | 2.8 | < 0.001 | 0.04 |
SD = standard deviation, d = Cohen's d.
Chi-square test results for presence and absence of new-onset depressive symptoms groups.
In Table 1, the baseline characteristics of the patients were compared between the pre COVID-19 period and during the COVID-19 period. Patients starting CR during the COVID-19 period were more likely to be younger, single, smoking, and more likely to receive PCI treatment compared to patients starting during the pre-COVID-19 period (P < 0.001). However, they had lower BMI, HADS anxiety, less physical activity, were less likely to present with heart failure and receive CABG treatment and have a comorbidity of arthritis (P < 0.001).
In Table 2, patients with new-onset depressive symptoms were more likely to be younger, female, single, from areas of higher social deprivation, have a higher BMI, HADS anxiety, more likely to smoke, present with heart failure, and have comorbidities of arthritis, diabetes, chronic bronchitis, emphysema, and claudication (P < 0.001). However, they were less likely to receive cardiac treatments and be physically active (P < 0.001). And these findings were similar in both the pre COVID-19 period and during the COVID-19 period.
A binary logistic regression was conducted to ascertain the association of the COVID-19 period and other patient characteristics with the likelihood of having new-onset depressive symptoms. The logistic regression model was statistically significant, X2(18) = 10,383.833, p < 0.001. The model correctly classified 85.6% of the cases. Of the sixteen variables, fourteen were statistically significant: Age, sex, partner status, HADS anxiety, smoking, physical activity, presence of heart failure, cardiac treatment, comorbidities of arthritis, diabetes, chronic bronchitis, emphysema, claudication, and COVID-19 (as shown in Table 3 ).
Table 3.
Multivariable adjusted odds ratios for having new-onset depressive symptoms.
| Variable | B | SE | Odds Ratio | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|
| Age | 0.005 | 0.002 | 1.005 | 1.002 | 1.009 |
| Sex (Male) | 0.192 | 0.038 | 1.212 | 1.125 | 1.305 |
| Partner status (Single) | 0.224 | 0.039 | 1.251 | 1.159 | 1.349 |
| HADS anxiety score measurement | 0.372 | 0.005 | 1.450 | 1.436 | 1.464 |
| 150 min. a week physical activity (No) | 0.619 | 0.034 | 1.858 | 1.739 | 1.984 |
| Smoking (Yes) | 0.231 | 0.066 | 1.260 | 1.107 | 1.433 |
| BMI | 0.004 | 0.003 | 1.004 | 0.998 | 1.010 |
| IMD (most deprived) | 0.051 | 0.046 | 1.052 | 0.961 | 1.152 |
| Arthritis (Yes) | 0.173 | 0.045 | 1.189 | 1.089 | 1.297 |
| Diabetes (Yes) | 0.322 | 0.038 | 1.380 | 1.280 | 1.488 |
| Chronic bronchitis (Yes) | 0.257 | 0.087 | 1.293 | 1.089 | 1.534 |
| Emphysema (Yes) | 0.292 | 0.102 | 1.339 | 1.096 | 1.636 |
| Claudication (Yes) | 0.467 | 0.107 | 1.596 | 1.295 | 1.967 |
| Heart Failure (Yes) | 0.286 | 0.055 | 1.331 | 1.194 | 1.483 |
| Cardiac treatment (reference: No treatment) | |||||
| PCI | −0.102 | 0.073 | 0.903 | 0.784 | 1.041 |
| CABG | 0.388 | 0.083 | 1.474 | 1.253 | 1.734 |
| Other treatment | 0.215 | 0.073 | 1.240 | 1.076 | 1.430 |
| Year (COVID-19 period) | 0.076 | 0.037 | 1.079 | 1.004 | 1.159 |
B, regression coefficient; SE, standard error; CI, confidence interval for odds ratio; IMD (Index of Multiple Deprivation), PCI (Percutaneous Coronary Intervention), CABG (Coronary Artery Bypass Graft), BMI (Body Mass Index).
Commencing the CR journey during the COVID-19 period was associated with an 8% increase in the odds of having new-onset depressive symptoms (OR: 1.08, 95%CI: 1.01, 1.16). Patients who were physically inactive were 86% more likely to have new onset depressive symptoms at the start of CR (OR: 1.86, 95%CI: 1.74, 1.98). One unit increase in the HADS anxiety score was associated with a 45% increase in the odds of having new-onset depressive symptoms (OR: 1.45, 95%CI: 1.44, 1.46). Patients who are smoking were 26% more likely to have new onset depressive symptoms (OR: 1.26, 95%CI: 1.11, 1.43). Receiving CABG or other treatments were associated with increase in the odds of having new-onset depressive symptoms at the start of CR with 47% and 24% respectively (OR: 1.47, 95%CI: 1.25, 1.73; OR: 1.24, 95%CI: 1.08, 1.43). Patients who had heart failure were 33% more likely to have new onset depressive symptoms (OR: 1.33, 95%CI: 1.19, 1.48). Patients who have a comorbidity of claudication had 1.59 times higher odds of having new-onset depressive symptoms and this was followed by the comorbid conditions of diabetes, emphysema, chronic bronchitis, and arthritis with 1.38,1.34,1.29 and 1.19 times respectively. Being single and male were also associated with an increased likelihood of having new-onset depressive symptoms with 25% and 21% (OR: 1.25, 95%CI: 1.16, 1.35; OR: 1.21, 95%CI: 1.13, 1.31).
4. Discussion
The present nationally representative study investigated the characteristics of UK patients undergoing CR who experienced new-onset depressive symptoms. Notably, the analysis also compared the association of starting prior to and during the COVID-19 period for the first time. Previous studies conducted in the general population have examined the association of COVID-19 with depressive symptoms and were limited to gathering data through online questionaries [13,14]. However, no prior studies have focused on a specific population of patients with new-onset depressive symptoms and examined the influence of starting CR during the COVID-19 period in cardiac patients. The factors associated with new-onset depressive symptoms at the commencement of CR, and whether starting CR during the COVID-19 was influential on new-onset depressive symptoms were investigated in the current study. According to the results of the multivariate analysis, we found that the patient characteristics that were associated with new-onset depressive symptoms were smoking, physical inactivity, high anxiety, being male, single, having comorbidities of arthritis, diabetes, chronic bronchitis, emphysema, claudication, receiving CABG treatment, and having heart failure. Furthermore, starting CR during the COVID-19 period was also associated with presenting with new-onset depressive symptoms.
Considering patient demographics, being single and male were found to be associated with an increased likelihood of having new-onset depressive symptoms with 25% and 21% respectively. Although IMD was found to be associated with depression in some studies conducted in the general population [29], this association was not confirmed in others [30,31]. In the current study, IMD was not found to be associated with new-onset depressive symptoms in patients with CVD attending CR. However, comorbidities and cardiac risk factors were adjusted for in multivariate analysis which may have had an influence on this finding.
Patients with comorbidity of claudication and arthritis were 59% and 19% more likely to have new onset depressive symptoms. A previous study has shown that at the start of CR, patients with musculoskeletal comorbidities had a worse health profile than those without such comorbidities, with lower levels of cardiovascular fitness and physical activity [32]. Although the patients with musculoskeletal comorbidities require comprehensive risk factor management, a few were referred to CR programmes. Hence, including patients with musculoskeletal comorbidities in CR programs may be necessary and could also be beneficial for improving new-onset depressive symptoms. However, additional studies are required to investigate whether this is indeed the case.
Having the comorbidity of diabetes was associated with a 38% increased likelihood of experiencing new-onset depressive symptoms at the commencement of CR. When compared to non-diabetic patients, diabetic patients in cardiac rehabilitation exhibited worse physical fitness and higher cardiac risk factors [33]. Patients with diabetes who participated in or completed CR had a lower risk of mortality than non-participants or non-completers [34]. When the state of diabetic patients was considered in terms of having a higher cardiac risk profile and a lower CR participation rate, CR programs were advised to target diabetic patients and involve them in CR. Furthermore, given the continuous rise in diabetes prevalence over time [35], managing diabetes may be necessary and could potentially alleviate depressive symptoms.
Our findings indicate that patients with comorbidities of emphysema and chronic bronchitis had 1.34 and 1.29 times higher odds of experiencing new-onset depressive symptoms at the start of CR, respectively. In the context of multimorbidity, patients with chronic obstructive pulmonary disease (COPD) and cardiovascular disease (CVD) have problems with breathlessness and disability; therefore, recommendations have been made to CR programmes to adopt tailored interventions for COPD patients [36]. COPD patients may benefit from multifaceted and integrated disease management by CR providers aware of multimorbidity care [36].
Recent research has shown that patients with COVID-19 and comorbidities are a vulnerable group that is negatively influenced by the disease, and requires special protection [37]. Our findings also indicate that having certain comorbidities is associated with new-onset depressive symptoms in patients commencing CR. Given that depression is strongly linked to higher mortality and a worse cardiac prognosis in cardiac patients [1,2], effective management of comorbidities could play a crucial role in protecting not only against COVID-19-related deaths, but also against new-onset depressive symptoms in cardiac patients. Further investigation is needed to explore this connection in greater depth.
Not surprisingly, physical inactivity was found to be associated with increased odds of having new-onset depressive symptoms. The association of physical activity with depressive symptoms is well-established, and has been emphasized in a recent physical activity guideline [25]. Patients are encouraged to engage in at least 150 min of moderate physical activity per week to improve their psychosocial health. The findings of a recent Chinese cross-sectional study conducted in cardiac patients were in line with our study [38]. The relationship between physical activity and depressive symptoms can also be bidirectional [39]. A recent systematic review of the general population, which included around 42,000 people from 21 studies, found that those who engaged in regular physical activity and maintained a regular physical activity schedule during the COVID-19 pandemic had a reduced likelihood of displaying depressive symptoms, with a 12–32% [40]. This finding showing the importance of maintaining moderate physical activity was supported by our investigation. Conditions unique to COVID-19 are quite likely. Social distance, for example, made it difficult to exercise in a group environment. The COVID-19 epidemic also reduced opportunities for physical activity by forcing the closure of gyms, sports clubs, and other popular indoor and outdoor spaces for physical activity. While some individuals were still permitted to jog and walk on the streets, others were not. In general, there may have been less physical activity during the pandemic due to a lack of opportunity. A systematic review conducted in the general population has also shown that physical activity can help to prevent the development of depressive symptoms [41]. However, patients with new-onset depressive symptoms who started CR during COVID-19 were not included in these studies. Additionally, smoking at baseline was associated with 1.26 times increase in the odds of having new-onset depressive symptoms at the start of CR which was in line with the findings of previous studies [42].
Another finding was having a higher HADS anxiety score was associated with a 45% increased likelihood of presenting with new-onset depressive symptoms in patients undergoing CR. This finding supports the interrelationship between anxiety and depression which is associated with worse outcomes. This finding was supported by a recent Australian retrospective cohort research examining patients starting CR programs [43]. The Depression Anxiety Stress Scale (DASS-21) measurement tool was used by the authors to evaluate the patients' levels of anxiety and depressive symptoms. They found that anxiety was strongly linked to depressive symptoms. However, because this study was performed before COVID-19, it was not possible to include patient data from that time [43].
Patients receiving cardiac treatments of CABG and other treatments were more likely to have new onset depressive symptoms with 47% and 24% at the commencement of CR respectively. In addition, patients presented with heart failure had a 33% increased likelihood of having new-onset depressive symptoms.
A finding of note was that patients starting CR during the COVID-19 service delivery period were 8% more likely to have new onset depressive symptoms at the start of CR. Previous studies have shown that in the general population, COVID-19 was associated with increased depressive symptoms in terms of increased prevalence rates [14,44]. Yet, they were unable to be inclusive of cardiac patients and investigate new-onset depressive symptoms in the CR setting. Although a recent study has investigated the new onset depressive symptoms in female patients with an implantable cardioverter-defibrillator [45], it was unable to investigate the association of COVID-19 service delivery with new-onset depressive symptoms or focus on a wider cardiac population starting CR.
4.1. Limitations
The current study included a representative sample of UK patients undergoing CR in UK CR services. This study's strength was its use of an observational approach to comprehend the real world by applying routinely obtained large data. However, a limitation was that the study analysis could not account for the diagnosis of depression during the CR period, antidepressant medication treatment, or family member loss due to COVID-19 because these variables were not recorded in the NACR data set. Therefore, future studies are recommended to account for these variables. In addition, because this is a registry-based study, it's important to remember that observational studies only allow for the association, not causation findings. COVID-19 was confirmed to be spreading in the UK by the end of January 2020. Therefore, we decided to define the COVID-19 period starting from 1 February 2020. Yet, it could be argued to be an early starting point for the COVID-19 period as the first UK lockdown did not happen until March 2020. However, there are previous studies applying a similar approach as in the current study.
5. Conclusions
The purpose of this study was to examine baseline patient characteristics associated with new-onset depressive symptoms at the start of CR during COVID-19 service delivery periods. Starting CR during the COVID-19 period was associated with an increased likelihood of presenting with new onset depression symptoms in patients undergoing CR. Our findings imply that once CR programs reach full capacity, the initial CR baseline assessment should include new-onset depression symptoms and patients starting CR during COVID-19 need to be assessed carefully due to their increased likelihood of developing new-onset depressive symptoms. In addition, their baseline characteristics linked to increased depressive symptoms should also be considered. These patient characteristics emphasize the necessity of devising techniques to identify at-risk persons, reallocate psychosocial support systems in CR programmes to those who need them, and deliver psychosocial management options to alleviate new-onset depressive symptoms.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author's contributions
SS, PD, AH contributed to the conception or design of the work. SS, PD, and AH contributed to the acquisition, analysis, or interpretation of data for the work. SS, PD, and AH drafted and critically revised the manuscript. All authors gave final approval to be accountable for all aspects of the work, ensuring accuracy and integrity. In addition, all authors have read and approved the final manuscript.
Declaration of Competing Interest
The authors have no competing interests to report.
Acknowledgements
The authors would like to acknowledge the support of NACR Team.
Footnotes
“All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation"
Data availability statement
The data that support the findings of this study are available from the National Audit of Cardiac Rehabilitation, but restrictions apply for the availability of this data, which were used under license for the current study, as the data being link anonymised with NHS Digital under Section 251 approval, cannot be shared publicly.
References
- 1.Meijer A., Conradi H.J., Bos E.H., Anselmino M., Carney R.M., Denollet J., Doyle F., Freedland K.E., Grace S.L., Hosseini S.H., Lane D.A., Pilote L., Parakh K., Rafanelli C., Sato H., Steeds R.P., Welin C., de Jonge P. Adjusted prognostic association of depression following myocardial infarction with mortality and cardiovascular events: individual patient data meta-analysis. Br. J. Psychiatry. 2013;203:90–102. doi: 10.1192/bjp.bp.112.111195. [DOI] [PubMed] [Google Scholar]
- 2.Lichtman J.H., Froelicher E.S., Blumenthal J.A., Carney R.M., Doering L.V., Frasure-Smith N., Freedland K.E., Jaffe A.S., Leifheit-Limson E.C., Sheps D.S., Vaccarino V., Wulsin L. Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the american heart association. Circulation. 2014;129:1350–1369. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- 3.WHO . World Health Organisation; 2021. Depression Factsheet.https://www.who.int/news-room/fact-sheets/detail/depression (accessed May 6, 2022) [Google Scholar]
- 4.Thombs B.D., Bass E.B., Ford D.E., Stewart K.J., Tsilidis K.K., Patel U., Fauerbach J.A., Bush D.E., Ziegelstein R.C., Sources D. 2006. Prevalence of Depression in Survivors of Acute Myocardial Infarction Review of the Evidence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.BACPR . 2017. The BACPR Standards and Core Components for Cardiovascular Disease Prevention and Rehabilitation. [Google Scholar]
- 6.M. Ambrosetti, A. Abreu, U. Corrà, C.H. Davos, D. Hansen, I. Frederix, M.C. Iliou, R.F.E. Pedretti, J.P. Schmid, C. Vigorito, H. Voller, M. Wilhelm, M.F. Piepoli, B. Bjarnason-Wehrens, T. Berger, A. Cohen-Solal, V. Cornelissen, P. Dendale, W. Doehner, D. Gaita, A.B. Gevaert, H. Kemps, N. Kraenkel, J. Laukkanen, M. Mendes, J. Niebauer, M. Simonenko, A.D.O. Zwisler, Secondary prevention through comprehensive cardiovascular rehabilitation: From knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology, Eur. J. Prev. Cardiol. 28 (2021) 460–495. doi: 10.1177/2047487320913379. [DOI] [PubMed]
- 7.Holmes E.A., O’Connor R.C., Perry V.H., Tracey I., Wessely S., Arseneault L., Ballard C., Christensen H., Cohen Silver R., Everall I., Ford T., John A., Kabir T., King K., Madan I., Michie S., Przybylski A.K., Shafran R., Sweeney A., Worthman C.M., Yardley L., Cowan K., Cope C., Hotopf M., Bullmore E. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science, lancet. Psychiatry. 2020;7:547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang Y., Li H., Zhang R. Effects of pandemic outbreak on economies: evidence from business history context. Front. Public Health. 2021;9 doi: 10.3389/fpubh.2021.632043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stockwell S., Trott M., Tully M., Shin J., Barnett Y., Butler L., McDermott D., Schuch F., Smith L. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc. Med. 2021;7 doi: 10.1136/bmjsem-2020-000960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strain T., Sharp S.J., Spiers A., Price H., Williams C., Fraser C., Brage S., Wijndaele K., Kelly P. 2022. Population Level Physical Activity before and during the First National COVID-19 Lockdown: A Nationally Representative Repeat Cross-Sectional Study of 5 Years of Active Lives Data in England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bueno-Notivol J., Gracia-García P., Olaya B., Lasheras I., López-Antón R., Santabárbara J. Prevalence of depression during the COVID-19 outbreak: a meta-analysis of community-based studies. Int. J. Clin. Health Psychol. 2021;21 doi: 10.1016/j.ijchp.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iob E., Frank P., Steptoe A., Fancourt D. Levels of severity of depressive symptoms among at-risk groups in the UK during the COVID-19 pandemic. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlis R.H., Ognyanova K., Santillana M., Baum M.A., Lazer D., Druckman J., Volpe J. della. Association of Acute Symptoms of COVID-19 and symptoms of depression in adults. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ettman C.K., Abdalla S.M., Cohen G.H., Sampson L., Vivier P.M., Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olaya B., Pérez-Moreno M., Bueno-Notivol J., Gracia-García P., Lasheras I., Santabárbara J. Prevalence of depression among healthcare workers during the covid-19 outbreak: a systematic review and meta-analysis. J. Clin. Med. 2021;10 doi: 10.3390/jcm10153406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Elm E., Egger M., Altman D.G., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement : guidelines for reporting observational studies. Br. Med. J. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NACR . 2020. The National Audit of Cardiac Rehabilitation Quality and Outcomes Report 2020. [Google Scholar]
- 18.NICE . Vol. 106. National Clinical Guideline Centre. NG; 2018. Chronic Heart Failure in Adults: Diagnosis and Management. [DOI] [PubMed] [Google Scholar]
- 19.NICE . Vol. 172. National Clinical Guideline Centre. CG; 2013. Secondary Prevention in Primary and Secondary Care for Patients Following a Myocardial Infarction. [Google Scholar]
- 20.Bjelland I., Dahl A.A., Haug T.T., Neckelmann D. The validity of the hospital anxiety and depression scale. J. Psychosom. Res. 2002;52:69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 21.Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 22.Department for Communities and Local Government . 2015. The English Indices of Deprivation 2015. [Google Scholar]
- 23.Sever S., Doherty P., Golder S., Harrison A.S. Is improvement in depression in patients attending cardiac rehabilitation with new-onset depressive symptoms determined by patient characteristics? Open Heart. 2020;7 doi: 10.1136/openhrt-2020-001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The English Indices of Deprivation 2015: Technical report. 2015. [Google Scholar]
- 25.DHSC . 2019. UK Chief Medical Officers’ Physical Activity Guidelines. [Google Scholar]
- 26.Sever S., Golder S., Doherty P. Factors associated with acute depressive symptoms in patients with comorbid depression attending cardiac rehabilitation. BMC Cardiovasc. Disord. 2018;18:1–8. doi: 10.1186/s12872-018-0974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sever S., Harrison A.S., Golder S., Doherty P. Determinants of depression in patients with comorbid depression following cardiac rehabilitation. Open Heart. 2019;6 doi: 10.1136/openhrt-2018-000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sever S., Harrison A.S., Doherty P. Levels of depressive symptoms in cardiac patients attending cardiac rehabilitation with a history of depression: pre Covid-19 and Covid-19 period comparison. BMC Cardiovasc. Disord. 2022;22:427. doi: 10.1186/s12872-022-02867-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi X., Jia Y., Pan C., Li C., Wen Y., Hao J., Liu L., Cheng B., Cheng S., Yao Y., Zhang F. Index of multiple deprivation contributed to common psychiatric disorders: a systematic review and comprehensive analysis. Neurosci. Biobehav. Rev. 2022;140 doi: 10.1016/j.neubiorev.2022.104806. [DOI] [PubMed] [Google Scholar]
- 30.Remes O., Lafortune L., Wainwright N., Surtees P., Khaw K.T., Brayne C. Association between area deprivation and major depressive disorder in British men and women: a cohort study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-027530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garman E.C., Avendano M., Araya R., Evans-Lacko S., McDaid D., Zimmerman A., Lund C. Understanding the complex relationship between multidimensional poverty and depressive symptoms among young south Africans: a cross-sectional study. J. Affect. Disord. 2022;319:352–360. doi: 10.1016/j.jad.2022.09.101. [DOI] [PubMed] [Google Scholar]
- 32.Marzolini S., Candelaria H., Oh P. Prevalence and impact of musculoskeletal comorbidities in cardiac rehabilitation. J. Cardiopulm. Rehabil. Prev. 2010;30:391–400. doi: 10.1097/HCR.0b013e3181e174ac. [DOI] [PubMed] [Google Scholar]
- 33.Mourot L., Boussuges A., Maunier S., Chopra S., Rivière F., Debussche X., Blanc P. Cardiovascular Rehabilitation in Patients with Diabetes. 2010. www.jcrpjournal.com [DOI] [PubMed]
- 34.Armstrong M.J., Sigal R.J., Arena R., Hauer T.L., Austford L.D., Aggarwal S., Stone J.A., Martin B.J. Cardiac rehabilitation completion is associated with reduced mortality in patients with diabetes and coronary artery disease. Diabetologia. 2015;58:691–698. doi: 10.1007/s00125-015-3491-1. [DOI] [PubMed] [Google Scholar]
- 35.Einarson T.R., Acs A., Ludwig C., Panton U.H. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc. Diabetol. 2018;17 doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borghi-Silva A., Garcia-Araújo A.S., Winkermann E., Caruso F.R., Bassi-Dibai D., Goulart C. de L., Dixit S., Back G.D., Mendes R.G. Exercise-based Rehabilitation delivery models in comorbid chronic pulmonary disease and chronic heart failure. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.729073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., Hosein Z., Padda I., Mangat J., Altaf M. Comorbidity and its impact on patients with COVID-19. SN Compr. Clin. Med. 2020;2:1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu Y., Yu X., Wu Y., Shi C., Zhang A., Jiang R., Li S., Guo G., Gao R., Blumenthal J.A. Association of Depression and Unhealthy Lifestyle Behaviors in Chinese patients with acute coronary syndromes. J. Cardiopulm. Rehabil. Prev. 2019;39:E1–E5. doi: 10.1097/HCR.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sin N.L., Kumar A.D., Gehi A.K., Whooley M.A. Direction of association between depressive symptoms and lifestyle behaviors in patients with coronary heart disease: the heart and soul study. Ann. Behav. Med. 2016;50:523–532. doi: 10.1007/s12160-016-9777-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolf S., Seiffer B., Zeibig J.M., Welkerling J., Brokmeier L., Atrott B., Ehring T., Schuch F.B. Is physical activity associated with less depression and anxiety during the COVID-19 pandemic? A Rapid Systematic Review. Sports Med. 2021;51:1771–1783. doi: 10.1007/s40279-021-01468-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuch F.B., Vancampfort D., Firth J., Rosenbaum S., Ward P.B., Silva E.S., Hallgren M., de Leon A.P., Dunn A.L., Deslandes A.C., Fleck M.P., Carvalho A.F., Stubbs B. Physical activity and incident depression: a meta-analysis of prospective cohort studies. Am. J. Psychiatr. 2018;175:631–648. doi: 10.1176/appi.ajp.2018.17111194. [DOI] [PubMed] [Google Scholar]
- 42.Fluharty M., Taylor A.E., Grabski M., Munafò M.R. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob. Res. 2017;19:3–13. doi: 10.1093/ntr/ntw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao A., Zecchin R., Newton P.J., Phillips J.L., DiGiacomo M., Denniss A.R., Hickman L.D. The prevalence and impact of depression and anxiety in cardiac rehabilitation: a longitudinal cohort study. Eur. J. Prev. Cardiol. 2020;27:478–489. doi: 10.1177/2047487319871716. [DOI] [PubMed] [Google Scholar]
- 44.Han X., Chen S., Bi K., Yang Z., Sun P. Depression following COVID-19 lockdown in severely, moderately, and mildly impacted areas in China. Front. Psychiatry. 2021;12:1–10. doi: 10.3389/fpsyt.2021.596872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedersen S.S., Nielsen J.C., Wehberg S., Jørgensen O.D., Riahi S., Haarbo J., Philbert B.T., Larsen M.L., Johansen J.B. New onset anxiety and depression in patients with an implantable cardioverter defibrillator during 24 months of follow-up (data from the national DEFIB-WOMEN study) Gen. Hosp. Psychiatry. 2021;72:59–65. doi: 10.1016/j.genhosppsych.2021.07.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the National Audit of Cardiac Rehabilitation, but restrictions apply for the availability of this data, which were used under license for the current study, as the data being link anonymised with NHS Digital under Section 251 approval, cannot be shared publicly.