Abstract
In this study vaccines prepared from culture filtrate proteins (CFP) of Mycobacterium bovis and interleukin-2 (IL-2) were tested in cattle for their capacity to stimulate immune responses and to protect against an intratracheal challenge with virulent M. bovis. Nine groups of cattle were vaccinated with combinations of different doses of CFP and bovine IL-2 mixed with a monophosphoryl lipid A (MPL) adjuvant. An additional group was vaccinated with M. bovis BCG. Immune responses in CFP–IL-2-vaccinated animals differed from those seen in BCG-vaccinated animals by inducing high antigen-specific antibody responses and low levels of gamma interferon and IL-2 released from purified protein derivative-stimulated whole-blood cultures. In a concurrent experiment, additional animals were added to the high-dose CFP–IL-2, MPL control, and BCG groups and these expanded groups of animals were challenged intratracheally with virulent M. bovis. Although the lung lesion scores were significantly lower for both the CFP–IL-2-and BCG-vaccinated groups compared to the MPL control group, the overall level of protection was greatest for the BCG-vaccinated animals. There were more animals with extrathoracic spread of disease in the CFP–IL-2 group than in the other groups. While vaccination of cattle with M. bovis CFP gave an encouraging reduction in tuberculous lesions and did not induce a delayed-type hypersensitivity response to PPD, future CFP vaccines must prevent any extrathoracic spread of disease.
The widespread occurrence of bovine tuberculosis in developing countries and its potential contribution to the prevalence of human tuberculosis has raised concerns. Tuberculosis, predominantly caused by Mycobacterium tuberculosis, is a major opportunistic infection in human immunodeficiency virus-infected people, particularly in sub-Saharan Africa (26). The epidemic of human immunodeficiency virus infection in developing countries, together with the widespread incidence of M. bovis infection in domestic and wild animals and conditions that favor zoonotic transmission, provides opportunity for zoonotic tuberculosis to become a serious public health problem (12, 23). Programs to eradicate bovine tuberculosis based on a “test and slaughter” approach eliminate animals identified as being infected with M. bovis. However, these programmes have been only partially effective in countries such as the United Kingdom, Ireland, and New Zealand, which have a wildlife reservoir of infected animals. Furthermore, this approach to the control of bovine tuberculosis is economically and socially unacceptable in many African countries. Therefore, in both industrialized countries where there is persistence of infected wildlife and in developing countries, the use of a vaccine against bovine tuberculosis warrants serious consideration.
The attenuated BCG strain of M. bovis has been used widely as a vaccine against human tuberculosis and for experimental studies in cattle (8, 9). However, the inconsistencies in the effectiveness of BCG in both humans and cattle and its interference with the detection of infected animals by the intradermal tuberculin test necessitate the development of better vaccines. An improved tuberculosis vaccine for animals will probably also have an application for use in humans.
A major research effort to develop new vaccines against human and bovine tuberculosis has recently been initiated. One approach has centered on the development of live attenuated strains of M. tuberculosis and M. bovis (13, 19, 21). An alternative approach, which focuses on the use of protective protein antigens released from live mycobacteria, has the potential not to compromise diagnostic tests and also perhaps to be unaffected by prior sensitization to environmental mycobacteria. Culture filtrates prepared from M. tuberculosis contain proteins that are highly stimulatory to T cells of human tuberculosis patients (14), mice (1, 27), and cattle (25) experimentally infected with tuberculosis. Several studies using small-animal models have demonstrated the protective potential of antigens contained in culture filtrates. Immunization of mice and guinea pigs with culture filtrate proteins (CFP) from M. tuberculosis gave high levels of protection against aerogenic challenge with M. tuberculosis (3, 27). Similarly, a CFP vaccine derived from M. bovis induced significant protection in mice against aerogenic challenge with virulent M. bovis (5).
Interleukin-2 (IL-2), a cytokine secreted by activated helper T cells, modulates the proliferation and differentiation of helper T cells, cytotoxic T cells, activated B cells, and natural killer (NK) cells. The expression of IL-2 by antigen-activated CD4+ T cells during a primary immune response has been linked to enhanced memory/effector function with increased antigenic sensitivity and expression of effector cytokines in secondary responses (29). Studies in animals have shown that IL-2 can act as an adjuvant for enhancing cellular immune responses to inactivated or subunit vaccines (24, 34). Coadministration of IL-2 with a CFP vaccine enhances the vaccine-induced protection against tuberculosis in a guinea pig model (3).
To date the potential of CFP as an effective vaccine against bovine tuberculosis in a natural host for M. bovis has not been determined. In the present study, immune responses induced by M. bovis CFP during experimentally induced tuberculosis in cattle were investigated and the ability of a CFP vaccine to protect cattle against intratracheal challenge with virulent M. bovis was determined. Cattle were vaccinated with combinations of different doses of M. bovis CFP and bovine IL-2 mixed with a monophosphoryl lipid A (MPL) adjuvant. MPL was chosen as a suitable adjuvant for the M. bovis CFP based on encouraging results in mice and guinea pigs which showed that M. tuberculosis CFP vaccines administered with this adjuvant were protective (3). Immune responses in the CFP–IL-2-vaccinated cattle were distinguished from those in BCG-vaccinated animals by the high antigen-specific antibody responses and low levels of gamma interferon (IFN-γ) and IL-2 released from purified protein derivative (PPD)-stimulated whole-blood cultures. A delayed-type hypersensitivity (DTH) response to PPD was not induced by vaccination with CFP–IL-2. Furthermore, vaccination of cattle with CFP–IL-2 reduced the severity of tuberculosis lung lesions. However, in comparison with control or BCG-vaccinated animals, these animals had more extrathoracic tuberculous lesions.
MATERIALS AND METHODS
Animals.
Seventy-two Friesian cross female calves, approximately 6 months old, were obtained from tuberculosis-free accredited herds from an area of New Zealand where both farmed and feral animals were free of tuberculosis. Prior to the experiment, the cattle tested negative for reactivity to bovine PPD in the whole-blood IFN-γ assay (28). The cattle were grazed on pasture in an isolation unit.
Bacterial strains and growth conditions.
M. bovis BCG Pasteur 1172P2 was used as the vaccine strain and has been used in previous vaccination-challenge trials in cattle (8, 9). The challenge strain, M. bovis WAg201 (previously designated 83/6235), was originally isolated from a tuberculous possum in New Zealand and has been used in previous studies in cattle (6, 8, 9). The vaccine and challenge strains were grown to mid-log phase in Tween albumin broth (Dubos broth base [Difco Laboratories, Detroit, Mich.]) supplemented with 0.006% (vol/vol) alkalinized oleic acid, 0.5% (wt/vol) albumin fraction V, and 0.25% (wt/vol) glucose. Dilutions were made in Tween albumin broth to obtain the appropriate doses for inoculating the cattle. The number of CFU inoculated was determined retrospectively by plating 10-fold dilutions on Middlebrook 7H11 (Difco) supplemented with 0.5% (wt/vol) albumin, 0.2% (wt/vol) glucose, and 1% (wt/vol) sodium pyruvate.
CFP.
The CFP used to vaccinate cattle in these experiments was prepared as described previously (5) from three strains of M. bovis (strains 862422, 896146, and 903053; a gift from R. Ellis, Colorado State University, Fort Collins, Colo.), since this gave a broader range of proteins than that produced from only one strain. Briefly, each strain was grown separately in sodium pyruvate alanine salts medium (0.03% [wt/vol] Bacto Casitone [Difco], 0.005% [wt/vol] ferric ammonium citrate, 0.4% [wt/vol] K2HPO4, 0.2% [wt/vol] citric acid, 0.1% [wt/vol] l-alanine, 0.12% [wt/vol] MgCl2·6H2O, 0.06% [wt/vol] K2SO4, 0.2% [wt/vol] NH4Cl, 0.18% [vol/vol] 10 N NaOH, 0.5% [wt/vol] sodium pyruvate) for 2 weeks (until mid-log phase) with gentle rolling and culture supernatants were separated from the cells by filtration through a 0.22-μm-pore-size filter. Each CFP was concentrated by ultrafiltration using an ultrafiltration stirred cell (Amicon, Danvers, Mass.) with a PM10 membrane (Millipore, Bedford, Mass.) and dialyzed against phosphate-buffered saline (PBS). The protein concentration was determined using bicinchoninic acid (Pierce, Rockford, Ill.). The final vaccine was prepared by pooling equal amounts of CFP from each strain, and the preparation was lyophilized. Analysis of the CFP by two-dimensional gel electrophoresis showed that the vaccine consisted of a complex mixture of proteins.
Recombinant bovine IL-2.
Bovine IL-2 was prepared in the yeast Pichia pastoris as described previously (33) and had a specific activity of 2,500 U/μg of protein. Briefly, the cDNA encoding the mature IL-2 (amino acids 21 to 155) was isolated by reverse transcription-PCR of RNA prepared from concanavalin A (ConA)-stimulated bovine lymphocytes. A construct which placed the mature IL-2 protein, with a six-histidine tag at the C terminus, in frame with the yeast MF-α signal sequence was made in the expression plasmid pPIC9 (Invitrogen Corp., Carlsbad, Calif.). Recombinant bovine IL-2 was produced from batch cultures of P. pastoris transformed with this construct by methods described previously (32). The protein was purified by immobilized metal-chelating affinity chromatography with Talon (Clontech Laboratories Inc., Palo Alto, Calif.), and the biological activity was determined using an IL-2 bioassay as described previously (8). The amount of endotoxin in the IL-2 was <0.1 endotoxin unit/μg of protein as determined by a Limulus amebocyte lysate assay (E. Toxate; Sigma Chemical Co., St. Louis, Mo.).
Adjuvant and preparation of vaccines.
The adjuvant formulation used in the vaccines was based on MPL (Ribi ImmunoChem Research Inc., Hamilton, Mont.), which is a nontoxic derivative of lipid A from Salmonella enterica serovar Minnesota. Lyophilized MPL was reconstituted to a concentration of 1 mg/ml in water containing 2 μl of triethylamine per ml. Vaccines were prepared by diluting the MPL in sterile saline and combining it with different amounts of CFP and IL-2, as shown in Table 1.
TABLE 1.
Vaccination groups used to assess the effects of dose of CFP and IL-2 on immune responses in cattle (experiment 2)
| Vaccine groupa | Dose of CFPb | Dose of IL-2 (μg) | Dose of BCG (CFU) |
|---|---|---|---|
| 1 | |||
| 2 | 50 | ||
| 3 | 250 | ||
| 4 | 200 μg | ||
| 5 | 200 μg | 50 | |
| 6 | 200 μg | 250 | |
| 7 | 1 mg | ||
| 8 | 1 mg | 50 | |
| 9 | 1 mg | 250 | |
| 10 | 106 |
Animals in groups 1 to 9 were vaccinated three times at 2-week intervals, and animals in group 10 were vaccinated twice with BCG with a 6-week interval.
M. bovis CFP. Vaccines were prepared by combining CFP and IL-2 with MPL adjuvant. Each dose contained 200 μg of MPL.
Vaccination, M. bovis challenge, and necropsy procedure. (i) Effect of vaccination with CFP on DTH responses (experiment 1).
Three groups of four calves were either vaccinated subcutaneously three times at 2-week intervals with high-dose CFP–IL-2 (as for group 9 in Table 1), once subcutaneously with 106 CFU of BCG, or not vaccinated to assess the effect of vaccination on the intradermal tuberculin test. The intradermal tuberculin test was carried out 13 weeks after the initial vaccination. For this test, the animals were inoculated intradermally with 0.1 ml containing 0.1 mg of bovine PPD (Ministry of Agriculture and Forestry, Central Animal Health Laboratory, Upper Hutt, New Zealand) (this is the PPD used for intradermal testing of cattle in New Zealand) in the right side of the neck. DTH responses were expressed as the difference in skin thickness between the time of inoculation and 72 h later.
(ii) Effect of different amounts of CFP and IL-2 on immune responses (experiment 2).
Forty calves were randomly divided into 10 groups each containing four calves. Animals in groups 1 to 9 were inoculated with combinations of nil, low (200-μg), or high (1-mg) doses of CFP and nil, low (50 μg), or high (250 μg) doses of recombinant bovine IL-2 in a 3 by 3 Latin square arrangement as shown in Table 1. The preparations were injected three times, subcutaneously in the left side of the neck, 2 weeks apart. Animals in group 10 were injected with BCG at 106 CFU subcutaneously, and this was repeated 6 weeks after the initial vaccination. Clotted and heparinized (sodium heparin) blood samples were collected from the cattle at 0, 2, 4, 7, and 11 weeks after the initial vaccination.
(iii) Protective efficacy of CFP vaccines (experiment 3).
An additional 20 calves were allocated to three of the groups in experiment 2 to increase the group size to a sufficient level to assess the protective efficacy of the vaccines. Experiment 3 was run concurrently with experiment 2. Seven of these calves were assigned to group 1 (adjuvant alone), seven calves were assigned to group 9 (high-dose CFP–IL-2), and six calves were assigned to group 10 (BCG). These calves were vaccinated in the same manner and at the same times as the corresponding calves from groups 1, 9, and 10. The expanded groups of calves from group 1 (11 calves), group 9 (11 calves) and group 10 (10 calves), together with the original four calves from group 5 (low-dose CFP–IL-2), were challenged intratracheally with 5 × 103 CFU of virulent M. bovis at 13 weeks after the initial vaccination as previously described (8). Briefly, an 80-cm endotracheal tube containing a fine cannula was inserted per os into the trachea of an anesthetized animal. A 1.5-ml inoculum containing the M. bovis strain was injected through the cannula and flushed out with 2 ml of saline. Clotted and heparinized blood samples were collected from the animals at 0, 2, 5, 10, and 17 weeks after challenge. All cattle were killed by use of a captive bolt and severance of the carotid artery and were necropsied 17 weeks after inoculation. Procedures for identifying macroscopic tuberculous lesions and processing for histopathologic testing have been described previously (8). Samples from four thoracic lymph nodes (left and right bronchial and anterior and posterior mediastinal) were collected from all of the animals for bacterial culture. Additional samples were collected from any tuberculous lesions observed in other lymph nodes or organs. For bacterial culture, the tissue samples were homogenized in a Tenbroeck grinder (Wheaton, Millville, N.J.), decontaminated in 0.75% cetylpyridium chloride for 1 h, centrifuged at 3,500 × g for 20 min, and processed for isolation of mycobacteria as described previously (8).
Antibody ELISA.
The M. bovis culture filtrate was prepared from M. bovis strain AN5, the strain used for the production of bovine PPD for skin testing in New Zealand. The culture filtrate was diluted to 3 μg/ml in carbonate buffer (pH 9.6); 100 μl per well was added to 96-well enzyme-linked immunosorbent assay (ELISA) plates (Maxisorb; Nunc, Roskilde, Denmark), and the plates were incubated overnight at 4°C. The antibody ELISA was carried out as described previously (35). Sera were stored at −20°C until tested. The results are expressed as an absorbance index, calculated by expressing the value found for the test serum as a fraction of the binding of a strong positive reference serum (9) multiplied by 100.
IFN-γ and IL-2 assays.
Heparinized blood was dispersed in two 1.5-ml aliquots within 4 h of collection, and 100 μl of either PBS or bovine PPD (300 μg/ml; supplied by CSL Ltd., Parkville, Victoria, Australia, in the IFN-γ assay kit) was added to the blood in separate wells. The blood cultures were incubated at 37°C for 24 h, and the IFN-γ levels in the plasma supernatants were measured using a sandwich ELISA kit (supplied by CSL Ltd.) as described previously (28). Results were expressed as optical density (OD) indices (OD for the bovine PPD sample/OD for the PBS sample). Bovine PPD was used in these assays rather than the vaccine CFP since PPD is the standard antigen for testing cattle in the field. Furthermore, the IL-2 and IFN-γ responses of cattle vaccinated with a M. tuberculosis CFP vaccine were similar with both CF and bovine PPD antigens (B. M. Buddle and D. N. Wedlock, unpublished observations).
IL-2 in the plasma supernatants from the whole-blood cultures was assayed by a bioassay as described previously (8). Briefly, triplicate wells containing 104 ConA-stimulated 4-day lymphoblasts were incubated in 200 μl of supplemented RPMI 1640 medium with 1:10 dilutions of supernatant. After the wells were incubated for 24 h, 0.25 μCi of [3H]thymidine was added to each well and the cultures were harvested 16 h later. The amount of tritiated thymidine incorporated was determined using a liquid β-scintillation counter (LS600 IC; Beckman Instruments, Inc., Fullerton, Calif.). The results were expressed as a stimulation index, defined as mean counts per minute (cpm) for bovine PPD plasma supernatant/mean cpm for PBS plasma supernatant. Addition of a monoclonal antibody against bovine IL-2 to the ConA-stimulated lymphoblasts immediately before addition of the plasma supernatants has been shown to block the IL-2 bioactivity.
Statistical analyses.
Analyses of antibody, IFN-γ, and IL-2 responses and bacterial counts were done by analysis of variance on log10-transformed data, while the analysis of intradermal tuberculin test responses and lung lesion scores was done by analysis of variance on raw data. The proportions of cattle with lung lesions and lesioned lymph nodes in different treatment groups were compared using Fisher's exact test, while the comparison of the percentage of culture-positive thoracic lymph nodes was calculated using χ2 test.
RESULTS
Vaccination with CFP does not induce DTH responses in cattle (experiment 1).
DTH responses to bovine PPD were measured by the intradermal tuberculin test at 13 weeks after vaccination. The four animals vaccinated with BCG produced DTH responses to PPD of 3.5 to 6 mm, and the mean response and standard error (SE) (4.6 ± 0.6) for these animals was greater than the means for the high-dose CFP–IL-2 (0.0 ± 0.0) and nonvaccinated (0.1 ± 0.1) groups (P < 0.05). None of the animals in the CFP–IL-2 or control groups produced DTH responses of 1 mm or greater.
Vaccination with CFP–IL-2 induces a strong antibody response (experiment 2).
Cattle vaccinated with either a low or high dose of CFP, combined with IL-2, produced a strong serum antibody response to M. bovis culture filtrate by 7 weeks after the first vaccination (Table 2). The mean responses of these groups were higher than for the control MPL-alone group at this time point (P < 0.05). Antibody responses were low in animals vaccinated with either CFP alone or BCG.
TABLE 2.
Changes in the IL-2 and IFN-γ responses of peripheral blood cultures to bovine PPD and mean antibody responses to M. bovis culture filtrate following vaccination (experiment 2)
| Vaccine formulation
|
Group | Ratio of mean response at 4 wk to response prevaccinationa
|
Mean antibody response 7 wk postvaccinationad | ||
|---|---|---|---|---|---|
| M. bovis CFP | IL-2 | IL-2b | IFN-γc | ||
| Nil | Nil | 1 | 1.1 ± 0.2 | 1.3 ± 0.3 | 6.9 ± 1.6 |
| Nil | Low | 2 | 0.9 ± 0.3 | 0.9 ± 0.3 | 6.9 ± 2.3 |
| Nil | High | 3 | 0.8 ± 0.2 | 1.0 ± 0.4 | 5.5 ± 1.4 |
| Low | Nil | 4 | 1.6 ± 0.4 | 1.7 ± 0.7 | 6.9 ± 1.4 |
| Low | Low | 5 | 5.3 ± 0.3* | 7.4 ± 3.2* | 29.0 ± 6.2* |
| Low | High | 6 | 2.0 ± 0.6 | 2.0 ± 0.7 | 22.5 ± 5.5* |
| High | Nil | 7 | 3.1 ± 1.0 | 5.2 ± 2.9 | 9.4 ± 1.3 |
| High | Low | 8 | 2.2 ± 1.2 | 2.5 ± 0.9 | 19.3 ± 1.9* |
| High | High | 9 | 2.1 ± 0.4 | 1.9 ± 0.4 | 17.8 ± 4.5* |
| Nil (BCG) | Nil | 10 | 21.9 ± 13.6† | 29.8 ± 21.2† | 9.0 ± 0.9 |
*, significantly greater than control group 1 (P < 0.05); †, significantly greater than all other groups (P < 0.05).
IL-2 responses are expressed as stimulation indices; data are presented as the ratio of the mean response at 4 weeks to the response prevaccination ± SE.
IFN-γ responses are expressed as OD indices; data are presented as the ratio of the mean response at 4 weeks to the response prevaccination ± SE.
Maximum antibody responses were measured 7 weeks after the first vaccination; data are expressed as absorbance indices ± SE.
Development of T-cell responses following vaccination (experiment 2).
T-cell responses in vaccinated animals were measured by the release of IFN-γ and IL-2 from bovine PPD-stimulated whole-blood cultures. The induction of T-cell responses was analyzed by comparing the mean responses 4 weeks after vaccination with the prevaccination responses, since these responses were at their highest values at 4 weeks after vaccination. Changes in the IL-2 and IFN-γ responses in cattle vaccinated with different combinations of CFP and IL-2 or BCG (experiment 1, groups 1 to 10) are given in Table 2. The low-dose CFP–IL-2 group was the only CFP group to show significant increases in the induction of IL-2 and IFN-γ responses after vaccination, but these increases were small compared to those induced by BCG vaccination (P < 0.05).
Pathological and bacteriological findings following challenge with M. bovis (experiment 3).
The protective efficacy of the BCG, high-dose CFP–IL-2, and low-dose CFP–IL-2 vaccines was determined in a vaccination-challenge experiment. The low-dose CFP–IL-2-vaccinated group was added to the vaccination-challenge trial when this group produced the highest cellular immune responses for the CFP groups. The cattle were killed and examined postmortem 17 weeks after the M. bovis challenge. The number of animals with tuberculous lung lesions, the classification of lesions, and the mean lung lesion scores are shown in Table 3. The lung lesions consisted of multiple small lesions (3 mm in diameter) or a large consolidated lesion (up to 100 mm in diameter). There were significantly fewer BCG-vaccinated animals with lung lesions than those in the control group, and the mean lung lesion scores were lower for both the BCG- and high-dose CFP–IL-2-vaccinated groups than for the control group (P < 0.05). There were no significant differences between the mean lung bacterial counts of the vaccinated groups (Table 3).
TABLE 3.
Presence of macroscopic tuberculous lung lesions and lung lesion scores and bacterial counts in cattle following challenge with virulent M. bovis (experiment 3)
| Group | No. of cattle with lung lesions/total no. | Lung lesion scorea | Lung bacterial countb |
|---|---|---|---|
| Control | 11/11 | 3.82 ± 0.12 | 2.53 ± 0.14 |
| BCG-vaccinated | 5/10* | 1.00 ± 0.39* | 1.96 ± 0.32 |
| High-dose CFP–IL-2 | 8/11 | 2.09 ± 0.48* | 2.63 ± 0.20 |
| Low-dose CFP–IL-2 | 4/4 | 2.75 ± 0.63 | 2.62 ± 0.32 |
Mean lung lesion scores in vaccinated and control animals ± SE. Lung lesion score: 4, ≥100 small lesions or a consolidated lesion >60 mm in diameter; 3, 25 to 99 small lesions or a consolidated lesion 20 to 60 mm in diameter; 2, 10 to 24 small lesions; 1, 1 to 9 small lesions; 0, no lesions. *, significantly different from the control (MPL adjuvant only) group (P < 0.05).
Geometric mean for bacterial counts (log10 CFU per gram of tissue) ± SE.
Although there were no major differences between the vaccine groups for the number of animals with macroscopic tuberculous lymph nodes, the BCG-vaccinated group had a lower mean number of lesioned lymph nodes per animal than all of the other groups (Table 4) (P < 0.05). There was a major difference in the location of the lesioned lymph nodes for the different groups. The high-dose CFP–IL-2 group contained significantly more animals with lesioned lymph nodes outside the thoracic cavity than did the BCG-vaccinated and control groups (P < 0.05). In the high-dose CFP–IL-2 group, 11 of the 37 lesioned lymph nodes were found outside the thoracic cavity (10 nodes in the head and neck region and 1 mesenteric lymph node). In contrast, only two lesioned lymph nodes were found outside the thoracic cavity in animals from the other groups (two head lymph nodes from one control animal).
TABLE 4.
Presence of macroscopic tuberculous lymph node lesions in cattle following challenge with virulent M. bovis (experiment 3)
| Group | No. of cattle with lesioned lymph nodes/total no.a
|
No. of lesioned lymph nodes/animalb | Lymph node bacterial countsc | % of thoracic lymph nodes M. bovis culture positive | |
|---|---|---|---|---|---|
| All sites | Outside thoracic cavity | ||||
| Control (MPL) | 11/11 | 1/11 | 3.18 ± 0.38 | 2.42 ± 0.14 | 84 |
| BCG vaccinated | 7/10 | 0/10 | 1.20 ± 0.33* | 1.86 ± 0.18‡ | 53* |
| High-dose CFP–IL-2 | 11/11 | 5/11§ | 3.36 ± 0.28 | 2.53 ± 0.12 | 73 |
| Low-dose CFP–IL-2 | 4/4 | 0/4 | 3.5 ± 0.29 | 2.41 ± 0.14 | 81 |
Macroscopic tuberculous lesions were observed in the thoracic, head, and mesenteric lymph nodes. §, significantly greater than the control and BCG-vaccinated groups (P < 0.05).
Mean number of lesioned lymph nodes per animal ± SE. *, significantly less than the control (MPL adjuvant only) group (P < 0.05).
Geometric mean for bacterial counts (log10 CFU per gram of tissue) ± SE. ‡, significantly less than the high-dose CFP–IL-2 group (P < 0.05).
Histologically, the tuberculous granulomata in the lung and lymph node lesions had a central necrotic area, which was usually mineralized. Mantles of epithelioid macrophages and lymphocytes surrounded the necrotic areas, and the lesions were walled off by fibrosis. The lung lesions in the BCG-vaccinated animals generally had less necrosis and appeared to have been established more recently as evidenced by limited mineralization. No major difference was observed between lesions from control and CFP–IL-2-vaccinated groups.
Samples from the four main thoracic lymph nodes of each animal were cultured for the presence of M. bovis. M. bovis was cultured from at least one thoracic lymph node from all animals in the trial including the three BCG-vaccinated animals, which had no visible macroscopic lesions. The only significant difference between these counts was that the mean bacterial count of the infected lymph nodes for the BCG-vaccinated group was lower than the count of the corresponding nodes for the high-dose CFP–IL-2 group (Table 4) (P < 0.05). The percentage of thoracic lymph nodes in the BCG-vaccinated group which were culture positive was lower than that in the control group (Table 4) (P < 0.05).
Antibody responses following vaccination and challenge.
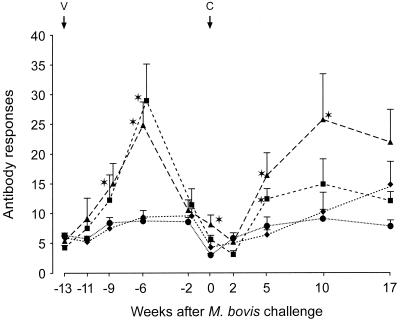
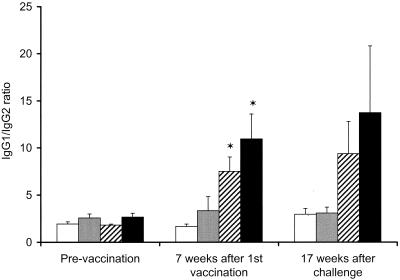
In the challenge experiment, antibody responses were measured after both vaccination and challenge. Higher antibody responses in serum (P < 0.05) were measured in the CFP–IL-2 groups at 4, 7, and 13 weeks after vaccination than in both the BCG-vaccinated and the adjuvant control groups (Fig. 1). Following challenge with virulent M. bovis, there was a marked anamnestic increase in antibody levels in serum in the animals that were vaccinated with CFP–IL-2, particularly in the high-dose group. The mean antibody responses in cattle in this group were higher (P < 0.05) at 5 and 10 weeks after challenge than were the responses in both the control and the BCG-vaccinated groups. Antibody responses remained low in the BCG-vaccinated animals throughout the duration of the experiment, while antibody responses in the control animals were raised only at 17 weeks after challenge. Following vaccination, the ratio of immunoglobulin G1 (IgG1) to IgG2 increased in animals vaccinated with either dose of CFP–IL-2 (Fig. 2). Mean ratios at 7 weeks after the first vaccination in these groups were greater than the corresponding ratios prevaccination (P < 0.05) and remained elevated following the challenge.
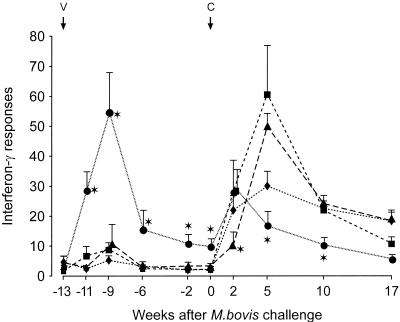
FIG. 1.
Antibody responses to M. bovis culture filtrate (experiment 3). Mean antibody responses of animals vaccinated with high-dose CFP–IL-2 (▴), low-dose CFP–IL-2 (■), BCG (●), or adjuvant alone (⧫) and challenged with virulent M. bovis at 13 weeks after the initial vaccination (n = 11 for the high-dose CFP–IL-2 and adjuvant groups, n = 10 for the BCG group, and n = 4 for the low-dose CFP–IL-2 group) are shown. Data are expressed as absorbance indices and SE. ∗, means were significantly greater than the means of the BCG and adjuvant control groups (P < 0.05). V, vaccination; C, challenge.
FIG. 2.
Changes in the mean IgG1-to-IgG2 antibody ratio to M. bovis culture filtrate in animals vaccinated with high-dose CFP–IL-2 (■), low-dose CFP–IL-2 (▨), BCG ( ), or adjuvant alone (□) and challenged with virulent M. bovis at 13 weeks after the initial vaccination (experiment 3). Error bars indicate SE. ∗, means were significantly greater than the means of the adjuvant control group (P < 0.05).
), or adjuvant alone (□) and challenged with virulent M. bovis at 13 weeks after the initial vaccination (experiment 3). Error bars indicate SE. ∗, means were significantly greater than the means of the adjuvant control group (P < 0.05).
T-cell responses following vaccination and challenge.
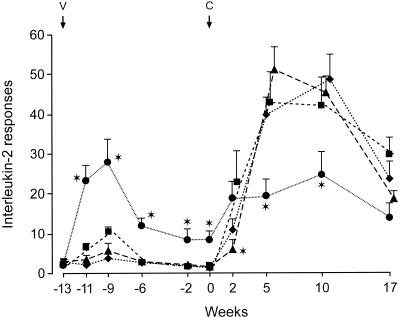
High IL-2 (Fig. 3) and IFN-γ (Fig. 4) responses were observed in cattle vaccinated with BCG, and low responses were seen in the CFP–IL-2 groups. The mean responses in the BCG group were greater at 2 to 13 weeks after the initial vaccination than were the responses in all other groups (P < 0.05). The IL-2 and IFN-γ responses in the BCG-vaccinated animals increased rapidly by 2 weeks after challenge, but responses at 5 and 10 weeks after challenge were lower than those in the other groups at these times (P < 0.05). For the high-dose CFP–IL-2-vaccinated animals, the mean IL-2 and IFN-γ responses at 2 weeks after the M. bovis challenge were lower than the responses in the other groups (P < 0.05) but rose to high levels by 5 to 10 weeks. Responses in the control MPL-alone group rose rapidly at 2 weeks and peaked at 5 to 10 weeks after challenge.
FIG. 3.
IL-2 released from bovine PPD-stimulated peripheral blood cultures from animals vaccinated with high-dose CFP–IL-2 (▴), low-dose CFP–IL-2 (■), BCG (●), or adjuvant alone (⧫) and challenged with virulent M. bovis at 13 weeks after initial vaccination (experiment 3). Data are expressed as mean stimulation indices and SE. ∗, means were significantly different from the means of the adjuvant control group (P < 0.05). V, vaccination; C, challenge.
FIG. 4.
IFN-γ released from bovine PPD-stimulated peripheral blood lymphocytes from animals vaccinated with high-dose CFP–IL-2 (▴), low-dose CFP–IL-2 (■), BCG (●), or adjuvant alone (⧫) and challenged with virulent M. bovis at 13 weeks after the initial vaccination (experiment 3). Data are expressed as mean OD indices and SE. ∗, means were significantly different from the means of the other groups (P < 0.05). V, vaccination; C, challenge.
DISCUSSION
The results from this study provide evidence that immunization with a nonliving CFP–IL-2 vaccine can reduce the incidence of tuberculous lung lesions in cattle resulting from a subsequent M. bovis challenge, although the level of protection was lower than that conferred by BCG. The type of immune response induced following vaccination with CFP included a substantial humoral component, in comparison to a strong IFN-γ response induced by vaccination with BCG. This could imply that the immune mechanisms mediating the reduction in the pulmonary pathology may be different for the CFP–IL-2- and BCG-vaccinated groups. However, immunization with this nonliving vaccine was also associated with an undesirable feature, the extrathoracic spread of the disease. Together, these findings may provide valuable insights into the immunological processes associated with protection and disease.
Although CFP–IL-2-vaccinated cattle had lower lung lesion scores, there was no apparent change in the type of histopathology of the lung lesions. This contrasted with the lung lesions of the BCG-vaccinated animals, which had less necrosis and limited mineralization and appeared to have been established more recently. Mineralization of lesions has been detected as early as 35 days after experimental challenge of cattle with M. bovis (11). Recent studies on antigen recognition have also suggested that BCG vaccination of cattle can have an effect on tuberculous lesion development (10). Studies using a similar CFP–IL-2 vaccine in guinea pigs have produced results which are different from those seen in cattle. The lung lesions in the CFP–IL-2-vaccinated guinea pigs resembled those seen in the BCG-vaccinated animals and had more lymphocytic infiltration and less caseation than did those in the controls (3). These vaccinated guinea pigs also had better survival, but the bacterial counts in the lungs were not reduced below those of placebo controls 30 days after aerosol challenge. In the present study, there was similarly no reduction in the bacterial counts from infected lymph node and lungs of the CFP–IL-2-vaccinated cattle compared to the controls.
The rapid anamnestic antibody response in the CFP–IL-2-vaccinated animals after challenge is an indicator that this type of vaccine is predominantly priming a Th2 type response. This was further confirmed with the increase in the ratio of the antigen-specific IgG1 to IgG2 responses after vaccination and challenge. In cattle, IL-4 enhances IgG1 and IgE production while IFN-γ enhances IgG2 production (15, 16). In humans, IgG1 antibodies have been associated with progressive disease and may enhance the chronic release of tumor necrosis factor alpha from PPD-stimulated monocytes (20). In the present study, an increase in IgG2 production was not observed in the BCG-vaccinated cattle, since vaccination of cattle with low doses of BCG does not induce an antibody response (8).
Cellular immune responses are considered necessary for protection against tuberculosis, and there is little evidence that antibody plays an important role in protective immunity. However, this is currently being reevaluated (18, 30). The induction of partial protection in the lungs of the CFP–IL-2-vaccinated animals in the absence of a high IL-2 and IFN-γ response in peripheral blood T cells but the presence of antibody is an interesting and unexpected finding. However, the induction of an antibody response in these vaccinated animals may have contributed to the dissemination of the extrathoracic disease. Antibodies may have served to opsonize M. bovis, promoting the phagocytosis of the bacteria by monocytes. The infected cells could readily traffic through the lymphatics, leading to the development of tuberculous granulomata in lymph nodes outside the thoracic cavity.
There was an initial delay in the induction of the peripheral blood cellular immune response after challenge in the high-dose CFP–IL-2-vaccinated animals. This may have resulted from the sequestration of M. bovis-sensitized T cells to the site of infection in the lungs. The localization of protective T cells in the lungs of these vaccinated animals could account for the reduction in the severity of the pulmonary pathology. Sequestration of protective T cells may also have occurred in the BCG-vaccinated animals but did not produce an initial delay in induction of peripheral blood cellular immune responses, since there was an excess of circulating M. bovis-sensitized T cells. By 5 weeks after challenge, high levels of IFN-γ and IL-2 were released from antigen-stimulated blood cultures of the CFP–IL-2 and control group animals while the levels in the BCG-vaccinated cattle had decreased. Previous studies have shown that high persistent peripheral blood cellular immune responses after challenge are associated with an active M. bovis infection while the responses wane in BCG-vaccinated animals, which have controlled the infection (8, 9, 31).
Our preliminary dose-response study in cattle indicated that IL-2 had to be included in the CFP vaccine for induction of immune responses to M. bovis antigens. The dose of CFP or IL-2 was not critical for induction of an antibody response. Low doses of CFP and IL-2 were more effective in inducing cellular immune responses, although these responses were weak compared to those induced by BCG. The low-dose CFP–IL-2-vaccinated group was added to the vaccination-challenge trial on the basis of inducing higher cellular immune responses. Although there were only four animals in this group, there was no evidence that this vaccine was protective, but additional animals would have to be tested to confirm this finding.
A useful characteristic of an improved tuberculosis vaccine for human and veterinary purposes would be the inability to induce DTH reactions to PPD, thus retaining the use of the intradermal tuberculin test for diagnosis. The present study has shown that immunization with a nonliving vaccine did not induce a DTH reaction to PPD. This result concurs with similar vaccines prepared from M. tuberculosis and M. bovis CFP tested in mice (5), guinea pigs (3), and monkeys (2).
There are a number of similarities between tuberculosis in humans and cattle, including the slow development of the disease, the predominant respiratory involvement, and the strong granulomatous response and walling off of the infection (4, 22). Testing of tuberculosis vaccines in cattle may provide useful information for the development of a human vaccine, particularly since cattle are a natural host for tuberculosis and an outbred population. As in humans, the efficacy of BCG vaccination for protection against tuberculosis has been variable and BCG vaccination has not been protective in cattle which have been previously sensitized to environmental mycobacteria (7). Exposure to environmental mycobacteria has been considered an explanation for the failure of BCG to protect humans against tuberculosis (17). Adjuvants which are effective in one species may not enhance immune responses in another species. For example, dimethyl-dioctyldecyl ammonium bromide (DDA), which induces immune responses and protection against tuberculosis in mice when used with CFP vaccines (1, 5), has not been shown to induce immune responses in cattle (Buddle and Wedlock, unpublished). The addition of cytokines to CFP vaccines could produce different effects in cattle and rodent models. Although it is important to use mouse and guinea pig models as primary screens for tuberculosis vaccines, cattle may give a better indication of the efficacy of tuberculosis vaccines for humans.
The CFP vaccines used in the present study have not provided an alternative to the BCG vaccine, but they have given some unexpected results, which have the potential to lead to an improved understanding of protective immunity against bovine tuberculosis. CFP vaccines for the control of bovine tuberculosis have an advantage in greater likely acceptance by regulatory authorities as well as in not compromising the use of the intradermal tuberculin test, but the effectiveness of these vaccines must be improved. Improvements could be made by formulation with stronger adjuvants and incorporation of other cytokines such as IL-12 to promote antigen-specific IFN-γ responses.
ACKNOWLEDGMENTS
This work was supported by a grant from the New Zealand Ministry of Agriculture and Forestry (Policy Management). B.V. is supported by an USDA training grant to Colorado State University. I.M.O. is supported by NIH grant AI-75320.
We thank Denise Keen, Natalie Parlane, Allison McCarthy, Leong Goh, Robyn Midwinter, and Gary Yates for excellent technical assistance; Oliver Turner and Joseph Cassidy for histopathological examinations; and Lilian Morrison for statistical analyses.
REFERENCES
- 1.Anderson P. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect Immun. 1994;62:2536–2544. doi: 10.1128/iai.62.6.2536-2544.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attanasio R, Pehler K, McClure H M. Immunogenicity and safety of Mycobacterium tuberculosis culture filtrate proteins in non-human primates. Clin Exp Immunol. 2000;119:84–91. doi: 10.1046/j.1365-2249.2000.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin S L, D'Souza C, Roberts A D, Kelly B P, Frank A A, Lui M A, Ulmer J B, Huygen K, McMurray D N, Orme I M. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66:2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates J H. Transmission, pathogenesis, pathology and clinical manifestations of tuberculosis Mycobacterium tuberculosis complex. In: Kubica G P, Wayne L G, editors. The mycobacteria: a sourcebook, part B. New York, N.Y: Marcel Dekker, Inc.; 1984. pp. 999–1005. [Google Scholar]
- 5.Bosio C M, Orme I M. Effective, nonsensitizing vaccination with culture filtrate proteins against Mycobacterium bovis infections in mice. Infect Immun. 1998;66:5048–5051. doi: 10.1128/iai.66.10.5048-5051.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buddle B M, Aldwell F E, Pfeffer A, de Lisle G W, Corner L A. Experimental Mycobacterium bovis infection of cattle: effect of dose of M. bovis and pregnancy on immune responses and distribution of lesions. N Z Vet J. 1994;42:167–172. doi: 10.1080/00480169.1994.35814. [DOI] [PubMed] [Google Scholar]
- 7.Buddle B M, Aldwell F E, Wedlock D N. Vaccination of cattle and possums against bovine tuberculosis. In: Griffin F, De Lisle G, editors. Tuberculosis in wildlife and domestic animals. Otago Conference Series no. 3. Dunedin, New Zealand: University of Otago Press; 1995. pp. 113–115. [Google Scholar]
- 8.Buddle B M, de Lisle G W, Pfeffer A, Aldwell F E. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine. 1995;13:1123–1130. doi: 10.1016/0264-410x(94)00055-r. [DOI] [PubMed] [Google Scholar]
- 9.Buddle B M, Keen D, Thomson A, Jowett G, McCarthy A R, Heslop J, deLisle G W, Stanford J L, Aldwell F E. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res Vet Sci. 1995;59:10–16. doi: 10.1016/0034-5288(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 10.Buddle B M, Parlane N A, Keen D L, Aldwell F E, Pollock J M, Lightbody K, Andersen P. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin Diagn Lab Immunol. 1999;6:1–5. doi: 10.1128/cdli.6.1.1-5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassidy J P, Bryson D G, Pollock J M, Evans R T, Forster F, Neill S D. Early lesion formation in cattle experimentally infected with Mycobacterium bovis. J Comp Pathol. 1998;19:27–44. doi: 10.1016/s0021-9975(98)80069-8. [DOI] [PubMed] [Google Scholar]
- 12.Cosivi O, Grange J M, Daborn C J, Raviglione M C, Fujikura T, Cousins D, Robinson R A, Huchzermeyer H F, de Kantor I, Meslin F X. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg Infect Dis. 1998;4:59–70. doi: 10.3201/eid0401.980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lisle G W, Wilson T, Collins D M, Buddle B M. Vaccination of guinea pigs with nutritionally impaired avirulent mutants of Mycobacterium bovis protects against tuberculosis. Infect Immun. 1999;67:2624–2626. doi: 10.1128/iai.67.5.2624-2626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demissie A, Ravn P, Olobo J, Doherty T M, Eguale T, Geletu M, Hailu W, Andersen P, Britton S. T-cell recognition of Mycobacterium tuberculosis culture filtrate fractions in tuberculosis patients and their household contracts. Infect Immun. 1999;67:5867–5971. doi: 10.1128/iai.67.11.5967-5971.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estes D M, Closser N M, Allen G K. IFN-γ stimulates IgG2 production from bovine B cells costimulated with anti-μ and mitogen. Cell Immunol. 1994;154:287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- 16.Estes D M, Hirano A, Heussler V T, Dobberlaere D A E, Brown W C. Expression and biological activities of bovine interleukin 4: effects of recombinant interleukin 4 on T cell proliferation and B cell differentiation and proliferation in vitro. Cell Immunol. 1995;163:268–279. doi: 10.1006/cimm.1995.1126. [DOI] [PubMed] [Google Scholar]
- 17.Fine P E, Vynnycky E. The effect of heterologous immunity upon the apparent efficacy of (eg. BCG) vaccines. Vaccine. 1998;16:1923–1928. doi: 10.1016/s0264-410x(98)00124-8. [DOI] [PubMed] [Google Scholar]
- 18.Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin Microbiol Rev. 1998;11:514–532. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guleria I, Teitelbaum R, McAdam R A, Kalpana G, Jacobs W R, Jr, Bloom B R. Auxotrophic vaccines for tuberculosis. Nat Med. 1996;2:334–337. doi: 10.1038/nm0396-334. [DOI] [PubMed] [Google Scholar]
- 20.Hussain R, Shiratsuchi H, Ellner J J, Wallis R S. PPD-specific IgG1 antibody subclass upregulate tumour necrosis factor expression in PPD-stimulated monocytes: possible link with disease pathogenesis in tuberculosis. Clin Exp Immunol. 2000;119:449–455. doi: 10.1046/j.1365-2249.2000.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson M, Phalen S W, Lagranderie M, Ensergueix D, Chavarot P, Marchal G, McMurray D N, Gicquel B, Guilhot C. Persistence and protective efficacy of a Mycobacterium tuberculosis auxotroph vaccine. Infect Immun. 1999;67:2867–2873. doi: 10.1128/iai.67.6.2867-2873.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jubb K V F, Kennedy P C, Palmer N. Pathology of domestic animals. 3rd ed. Vol. 2. Orlando, Fla: Academic Press, Inc.; 1985. p. 582. [Google Scholar]
- 23.Moda G, Daborn C J, Grange J M, Cosivi O. The zoonotic importance of Mycobacterium bovis. Tubercle Lung Dis. 1996;77:103–108. doi: 10.1016/s0962-8479(96)90022-2. [DOI] [PubMed] [Google Scholar]
- 24.Nunberg J H, Doyle M V, York S M. Interleukin-2 acts as an adjuvant to increase the potency of inactivated rabies virus vaccine. Proc Natl Acad Sci USA. 1989;86:4240–4243. doi: 10.1073/pnas.86.11.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollock J M, Andersen P. Predominant recognition of the ESAT-6 protein in the first phase of infection with Mycobacterium bovis in cattle. Infect Immun. 1997;65:2587–2592. doi: 10.1128/iai.65.7.2587-2592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raviglione M C, Snider D E, Kochi A. Global epidemiology of tuberculosis. JAMA. 1995;273:220–226. [PubMed] [Google Scholar]
- 27.Roberts A D, Sonnenberg M G, Ordway D J, Furney S K, Brennan P J, Belisle J T, Orme I M. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995;85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 28.Rothel J S, Jones S L, Corner L A, Cox J C, Wood P R. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust Vet J. 1990;67:134–137. doi: 10.1111/j.1751-0813.1990.tb07730.x. [DOI] [PubMed] [Google Scholar]
- 29.Saparov A, Wagner F H, Zheng R, Oliver J R, Maeda H, Hockett R D, Weaver C T. Interleukin-2 expression by a subpopulation of primary T cells is linked to enhanced memory/effector function. Immunity. 1999;11:271–280. doi: 10.1016/s1074-7613(00)80102-8. [DOI] [PubMed] [Google Scholar]
- 30.Teitelbaum R, Glatman-Freedman A, Chen B, Robbins J B, Unanue E, Casadevall A, Bloom B R. A mAb recognising a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc Natl Acad Sci USA. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wedlock D N, Aldwell F E, Collins D M, de Lisle G W, Wilson T, Buddle B M. Immune responses induced in cattle by virulent and attenuated Mycobacterium bovis strains: correlation of delayed-type hypersensitivity with ability of strains to grow in bovine macrophages. Infect Immun. 1999;67:2172–2177. doi: 10.1128/iai.67.5.2172-2177.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wedlock D N, Goh L P, McCarthy A R, Midwinter R G, Parlane N A, Buddle B M. Physiological effects and adjuvanticity of recombinant brushtail possum TNF-α. Immunol Cell Biol. 1999;77:28–33. doi: 10.1046/j.1440-1711.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- 33.Wedlock D N, Doolin E E, Parlane N A, Lacy-Hulbert S J, Woodford M W, Buddle B M. The effect of yeast expressed recombinant interleukin-2 and interferon-γ on physiological changes in bovine mammary glands and on bactericidal activity of neutrophils. J Dairy Res. 2000;67:189–197. doi: 10.1017/s0022029900004131. [DOI] [PubMed] [Google Scholar]
- 34.Weinberg A, Merigan T C. Recombinant interleukin-2 as an adjuvant for vaccine-induced protection: immunization of guinea pigs with herpes simplex virus subunit vaccines. J Immunol. 1988;140:294–299. [PubMed] [Google Scholar]
- 35.Wood P R, Corner L A, Rothel J S, Ripper J L, Fifis T, McCormick B S, Francis B, Melville L, Small K, De Witte K, Tolson J, Ryan T J, de Lisle G W, Cox J C, Jones S L. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet Microbiol. 1992;31:71–79. doi: 10.1016/0378-1135(92)90142-g. [DOI] [PubMed] [Google Scholar]