Abstract
Purpose
Oxaliplatin, a component of the capecitabine plus oxaliplatin (XELOX) regimen, has a more favorable toxicity profile than cisplatin in patients with advanced gastric cancer (GC). However, oxaliplatin can induce sensory neuropathy and cumulative, dose-related toxicities. Thus, the capecitabine maintenance regimen may achieve the maximum treatment effect while reducing the cumulative neurotoxicity of oxaliplatin. This study aimed to compare the survival of patients with advanced GC between capecitabine maintenance and observation after 1st line XELOX chemotherapy.
Materials and Methods
Sixty-three patients treated with six cycles of XELOX for advanced GC in six hospitals of the Catholic University of Korea were randomized 1:1 to receive capecitabine maintenance or observation. The primary endpoint was progression-free survival (PFS), analyzed using a two-sided log-rank test stratified at a 5% significance level.
Results
Between 2015 and 2020, 32 and 31 patients were randomized into the maintenance and observation groups, respectively. After randomization, the median number of capecitabine maintenance cycles was 6. The PFS was significantly higher in the maintenance group than the observation group (6.3 vs. 4.1 months, P=0.010). Overall survival was not significantly different between the 2 groups (18.2 vs. 16.5 months, P=0.624). Toxicities, such as hand-foot syndrome, were reported in some maintenance group patients. Maintenance treatment was a significant factor associated with PFS in multivariate analysis (hazard ratio, 0.472; 95% confidence interval, 0.250–0.890; P=0.020).
Conclusions
After 6 cycles of XELOX chemotherapy, capecitabine maintenance significantly prolonged PFS compared with observation, and toxicity was manageable. Maintenance treatment was a significant prognostic factor associated with PFS.
Trial Registration
ClinicalTrials.gov Identifier: NCT02289547
Keywords: Stomach neoplasm, Capecitabine, Maintenance chemotherapy, Clinical trial
INTRODUCTION
The prognosis for metastatic gastric cancer (GC) remains poor, indicating a great unmet need [1,2]. To date, the 1st line standard treatment for advanced human epidermal growth factor receptor 2 (HER2)-negative GC is systemic chemotherapy with platinum/fluoropyrimidine [3]. In most patients, chemotherapy is difficult to administer for more than 6–8 cycles due to cumulative side effects and reduced performance status. It has been reported that only 20%–40% of patients in the West receive second-line therapy because failure of first-line therapy leads to further cancer progression and makes it difficult for patients to endure the side effects of chemotherapy [4]. Of the 964 patients enrolled in a randomized phase III trial performed by Cunningham et al. [5], 135 (14%) received second-line chemotherapy. However, in the East, it has been reported that 60%–80% of patients receive second-line therapy. In a phase III trial comparing S-1 plus cisplatin treatment with S-1 alone performed in Japan, 74%–75% of patients underwent second-line chemotherapy [6]. In Korea, a study that analyzed the real-world treatment pattern of patients with advanced GC reported that approximately 80% of patients underwent second-line chemotherapy [7].
It is clinically critical to improve the effectiveness of first-line treatment and delay tumor progression. Maintenance therapy is important in oncology. It is mainly used in colorectal cancers (CRCs) and non-small cell lung cancers (NSCLCs) [8]. Candidate agents for maintenance therapy in NSCLCs include agents used in first-line chemotherapy and new cytotoxic agents with fewer toxicities than the first-line chemotherapy agents or target agents [9].
There is no definite recommendation of maintenance chemotherapy for metastatic HER2-negative GC according to current clinical guidelines, such as the National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) guidelines [10,11]. However, many retrospective and observational studies have shown the efficacy and safety of maintenance fluoropyrimidine treatment compared to observation alone [10,11].
Park et al. [12] observed that oxaliplatin as part of the capecitabine plus oxaliplatin (XELOX) regimen has a more favorable toxicity profile than cisplatin in patients with advanced GC. However, oxaliplatin can induce sensory neuropathy and cumulative, dose-related toxicities. After 6 cycles of XELOX chemotherapy, chemotherapy-induced peripheral neuropathy occurs in a significant number of patients due to the accumulation of chemotherapy doses. Therefore, it might be possible to devise a capecitabine maintenance regimen to achieve the maximum treatment effect before cumulative neurotoxicity appears. The objective of this study was to report the results of a randomized phase III study of patients with advanced gastric adenocarcinoma that did not progress after six cycles of XELOX followed by capecitabine maintenance or clinical observation.
MATERIALS AND METHODS
Inclusion and exclusion criteria
Inclusion criteria were: patients with 1) histologically proven GC, stage IV (regardless of the presence or absence of measurable disease according to Response Evaluation Criteria in Solid Tumors [RECIST] criteria) or recurrent after curative surgery and at least stable disease (SD) following 6 cycles of 1st line XELOX chemotherapy; 2) a minimum age of 18 years; 3) HER2-negative GC [13]; 4) Eastern Cooperative Oncology Group Performance status 0–2; and 5) adequate bone marrow, renal, and hepatic functions. Exclusion criteria were: 1) patients who were previously exposed to any chemotherapy except XELOX for advanced disease; 2) those who underwent R0 or R1 resection for metastatic or recurrent GC without evaluable/measurable disease; 3) those who had disease relapse during or within 4 months after adjuvant therapy; 4) those who had central nervous system and meningeal metastases; 5) those who had significant neurologic or psychiatric disorders; and 6) those with active infection, severe heart disease, uncontrollable hypertension or diabetes mellitus, myocardial infarction during the preceding 6 months, pregnancy, or breast feeding. Any previous or concurrent malignancy was not included, except for adequately treated non-melanoma skin cancer, in situ cancer of the uterine cervix, non-muscle invasive bladder cancer, or malignancy without evidence of recurrence within 5 years.
Study design and treatment
This study (ClinicalTrials.gov identifier: NCT02289547) was a multicenter randomized controlled phase III open-label trial. Study subjects were randomized into two groups at a ratio of 1:1 and stratified by the presence of measurable lesions and tumor response to the initial six cycles of XELOX chemotherapy (partial response [PR]/complete response [CR] vs. SD). Group A, the maintenance group, was treated with 1,000 mg/m2 capecitabine bis in die (b.i.d.) on days 1–14 every 3 weeks until disease progression or an unacceptable toxicity occurred. Group B, the control group, underwent observation. The primary objective of this study was to compare the progression-free survival (PFS) between the 2 groups. We predicted that the median number of cycles for the maintenance group after randomization would be up to eight, and the median follow-up period of patients after progression would be up to 5 months.
All procedures performed involving human participants were conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Institutional Review Board of the Catholic Medical Center approved this study (number: XC14MIMS0024H). The study subjects provided informed consent to participate in the study.
Assessments
A response was evaluated radiologically every 2 cycles until 18 weeks and then every 3 cycles thereafter, or when progression was suspected by RECIST version 1.1 [14]. Overall response rate (ORR) was defined as the proportion of patients whose best response was either PR or CR. The disease control rate was defined as the proportion of patients whose best response was CR, PR, or SD. Safety was evaluated after every treatment using the National Cancer Institute-Common Terminology Criteria for Adverse Events version 4.0 guidelines [15]. The primary endpoint was PFS. The secondary endpoints were overall survival (OS), ORR, toxicity profile of chemotherapy, and quality of life.
Statistical analysis
We anticipated that the difference in PFS between the maintenance and observation groups would be three months. The type 1 error probability associated with testing this null hypothesis was 0.05, and the power was 80%. Assuming that approximately 2% of the subjects dropped out, the required number in each group was 40 and a total patient accrual of 80 was warranted [16].
This study was designed to provide evidence to support either the null hypothesis H0: λ=1 or to reject it in favor of the alternative hypothesis HA: λ≠1, where λ is the hazard ratio (HR) of PFS in the experimental arm/control arm. Additionally, this study was designed to provide evidence for a similar hypothesis for OS, although OS was not the primary endpoint. All analyses were conducted using a 2-sided log-rank test, stratified at an overall significance level of 5%.
Relative dose intensity (RDI) was defined as the actual dose divided by the standard dose. PFS was defined as the time from the date of randomization to the earliest date of disease progression or death due to any cause before the documentation of progression. For subjects who withdrew from study treatment, tumor assessments followed afterwards to appropriately capture the date of disease progression. These patients were not treated as censored because of treatment discontinuation. PFS was summarized using Kaplan-Meier curves and compared between the 2 arms using a stratified log-rank test. OS was defined as the time from the date of randomization to death due to any cause. The same statistical methods were used to analyze the OS and PFS. Univariate and multivariate analyses for PFS were performed using the Cox proportional hazards model to analyze the independent prognostic factors. Analysis was performed using the SPSS software (version 20.0; IBM Corp., Armonk, NY, USA).
RESULTS
Patient characteristics
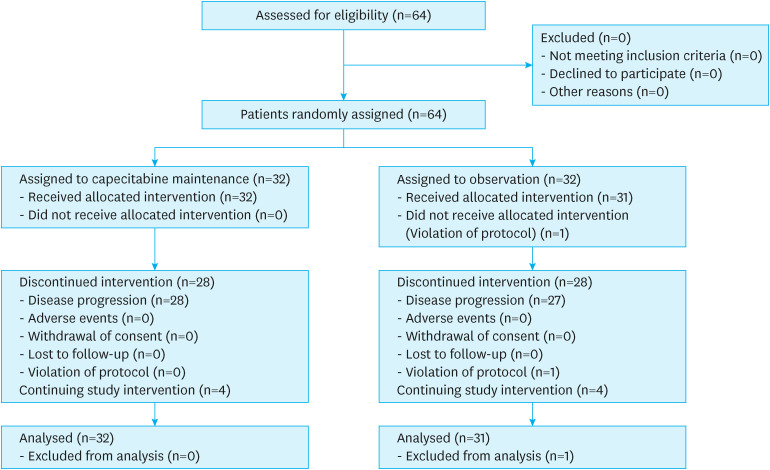
Patients were enrolled from six hospitals of the Catholic University of Korea between 2015 and 2020. Patient registration for this study was delayed. Because of the slow pace of patient recruitment, the Data Safety Monitoring Committee recommended stopping patient enrollment and analyzing the data. Overall, 64 patients were treated per protocol, and 32 and 31 patients were randomized into the maintenance and observation groups, respectively (Fig. 1). One patient in the observation group dropped out because of a recent myocardial infarction, which was considered a study violation.
Fig. 1. Study flow chart showing the selection of study subjects.
Poor tissue differentiation was noticeably more frequent in the maintenance group than in the observation group (P=0.013). Other clinical characteristics were similar between the 2 groups. There was no statistical difference between the two groups in terms of whether subsequent chemotherapy was performed (P=0.890) (Table 1).
Table 1. Clinical characteristics of subjects in the maintenance or observation groups.
| Characteristics | Maintenance group (n=32) | Observation group (n=31) | P-value | |
|---|---|---|---|---|
| Median age (range) | 64.5 (41–81) | 61 (40–79) | 0.674 | |
| Age <60 years | 13 (40.6) | 11 (35.5) | ||
| Age ≥60 years | 19 (59.4) | 20 (64.5) | ||
| Underlying disease | 0.059 | |||
| No | 12 (37.5) | 19 (61.3) | ||
| Yes | 20 (62.5) | 12 (38.7) | ||
| Smoking history | 0.982 | |||
| Never or ex-smoker | 31 (96.9) | 30 (96.8) | ||
| Current | 1 (3.1) | 1 (3.2) | ||
| ECOG performance status | 0.238 | |||
| 0–1 | 31 (96.9) | 31 (100) | ||
| ≥2 | 1 (3.1) | 0 (0) | ||
| Serum CEA (ng/mL) | 0.916 | |||
| <7.5 | 20 (62.5) | 20 (64.5) | ||
| ≥7.5 | 6 (18.8) | 4 (12.9) | ||
| Unknown | 6 (18.8) | 7 (22.6) | ||
| Histology subtype | 0.013 | |||
| Well to moderate | 3 (9.4) | 11 (35.5) | ||
| Poor | 29 (90.6) | 20 (64.5) | ||
| Disease status | 0.476 | |||
| Initial metastasis | 20 (62.5) | 22 (71.0) | ||
| Recurrence | 12 (37.5) | 9 (29.0) | ||
| Previous adjuvant chemotherapy | 0.414 | |||
| No | 23 (71.9) | 25 (80.6) | ||
| Yes | 9 (28.1) | 6 (19.4) | ||
| FP | 4 | - | ||
| S-1 | 3 | - | ||
| XELOX | 2 | - | ||
| Chemo response to XELOX | 0.674 | |||
| Stable disease | 22 (68.7) | 17 (54.8) | ||
| Partial response | 9 (29.1) | 12 (38.7) | ||
| Complete response | 1 (3.1) | 2 (6.5) | ||
| Neuropathy after XELOX | 0.501 | |||
| Grade 1 | 8 (25.0) | 6 (19.4) | ||
| Grade 2 | 1 (3.1) | 3 (9.7) | ||
| Grade 3 | 0 (0) | 1 (3.2) | ||
| Other adverse event after XELOX | 0.183 | |||
| Grade 1 | 9 (28.1) | 8 (25.8) | ||
| Grade 2 | 3 (9.4) | 8 (25.8) | ||
| Grade 3 | 3 (9.4) | 4 (12.9) | ||
| Grade 4 | 1 (3.1) | 1 (3.2) | ||
| Sites of metastasis | 0.145 | |||
| 1 | 17 (53.1) | 22 (71.0) | ||
| ≥2 | 15 (46.9) | 9 (29.0) | ||
| Liver metastasis | 7 (21.9) | 7 (22.6) | 0.946 | |
| Peritoneal metastasis | 21 (65.6) | 20 (64.5) | 0.926 | |
| Lung metastasis | 5 (15.6) | 2 (6.5) | 0.247 | |
| Non-regional lymph node metastasis | 12 (37.5) | 7 (22.6) | 0.197 | |
| Bone metastasis | 5 (15.6) | 2 (6.5) | 0.426 | |
| Measurable lesion | 0.904 | |||
| No | 17 (53.1) | 16 (51.6) | ||
| Yes | 15 (46.9) | 15 (48.4) | ||
| Subsequent chemotherapy | 0.890 | |||
| No | 6 (18.8) | 5 (16.1) | ||
| Yes | 26 (81.2) | 26 (83.9) | ||
Values are presented as number of patients (%) not otherwise specified.
ECOG = Eastern Cooperative Oncology Group; CEA = carcinoembryonic antigen; FP = 5-fluorouracil plus cisplatin; XELOX = capecitabine plus oxaliplatin.
Treatment exposure
The median number of cycles of capecitabine maintenance after randomization was 6 (range, 1–32). The median RDI of capecitabine was 0.9 (range, 0.7–1).
Efficacy
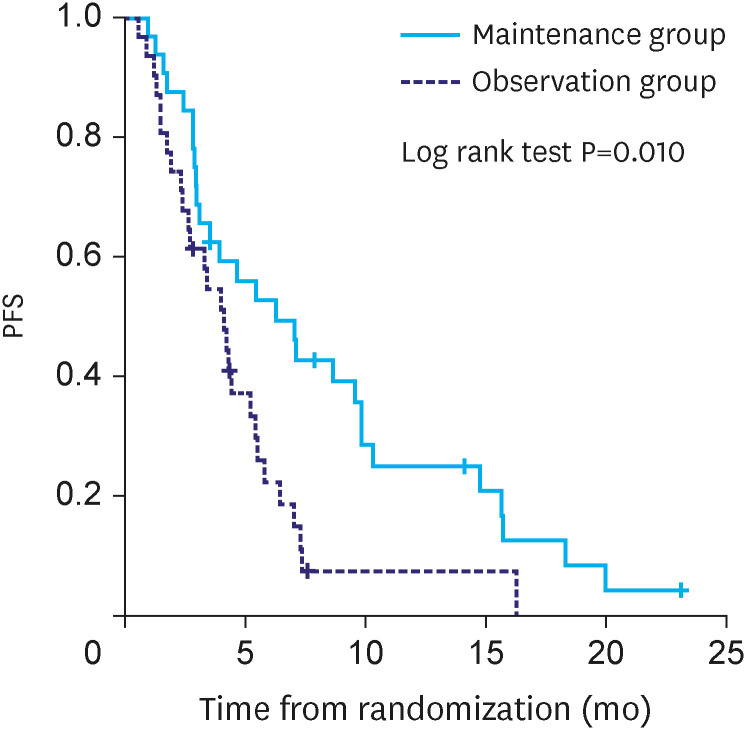
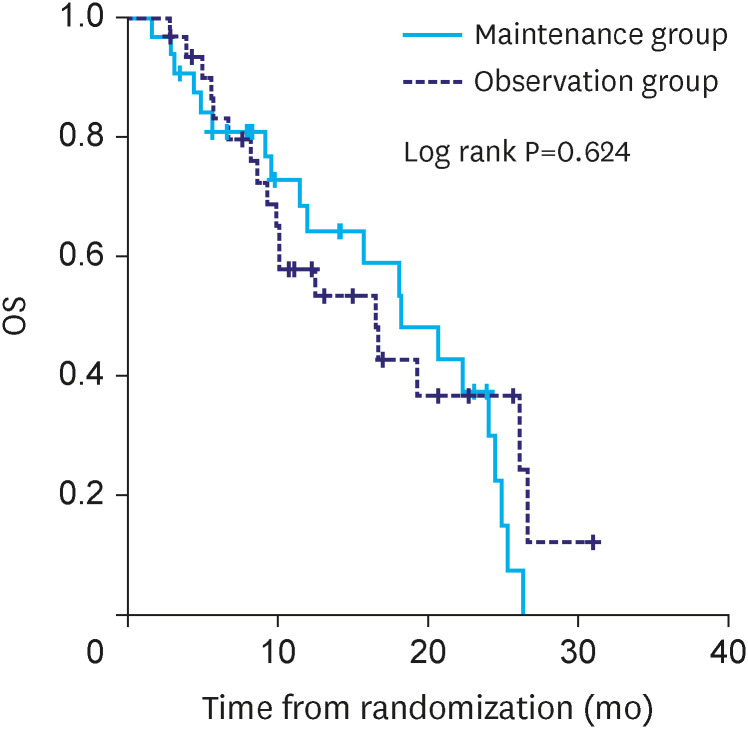
The median duration of follow-up was 14.1 months in the maintenance group and 16.5 months in the observation group. PFS was significantly different between maintenance and observation groups (median PFS: 6.3 vs. 4.1 months, P=0.010) (Fig. 2). However, OS was not significantly different between the two groups (median OS:18.2 vs. 16.5 months, P=0.624) (Fig. 3).
Fig. 2. Kaplan-Meir analysis of PFS from randomization.
PFS = progression-free survival.
Fig. 3. Kaplan-Meir analysis of OS from randomization.
OS = overall survival.
Safety
Treatment-related adverse events (AEs) are summarized in Table 2. In the maintenance group, all grades of hand-foot syndrome (HFS) were found in 8 (25%) patients. Among the higher than grade 3 AEs, 4 (12.5%) patients had grade 3 HFS, 1 (3.1%) had grade 3 thrombocytopenia, and 1 (3.1%) had grade 3 liver dysfunction in the maintenance group. One patient (3.2%) in the observation group had grade 3 asthenia.
Table 2. Treatment-related adverse events in study patients (n=63).
| Adverse event | Maintenance group (n=32) | Observation group (n=31) | |||||
|---|---|---|---|---|---|---|---|
| All grades | Grade 3 | Grade 4 | All grades | Grade 3 | Grade 4 | ||
| Hematologic AEs | |||||||
| Neutropenia | |||||||
| Anemia | |||||||
| Thrombocytopenia | 2 (6.3) | 1 (3.1) | |||||
| Non-hematologic AEs | |||||||
| Asthenia | 1 (3.2) | 1 (3.2) | |||||
| Anorexia | |||||||
| Nausea | 1 (3.1) | ||||||
| Vomiting | 1 (3.1) | ||||||
| Dizziness | |||||||
| Stomatitis | 2 (6.3) | ||||||
| Diarrhea | 1 (3.2) | ||||||
| Hand-foot syndrome | 8 (25.0) | 4 (12.5) | |||||
| Skin rash | |||||||
| Neuropathy | 1 (3.1) | ||||||
| Peripheral edema | 1 (3.1) | ||||||
| Liver dysfunction | 1 (3.1) | 1 (3.1) | |||||
| Renal dysfunction | |||||||
Values are presented as number of patients (%) not otherwise specified.
AE = adverse event.
Survival analysis
Maintenance treatment was the only significant factor associated with PFS in the univariate analysis (HR, 0.486; 95% confidence interval [CI], 0.277–0.852). Sex, disease status, and measurable lesions were not statistically significant factors in the multivariate analysis (HR, 1.264; 95% CI, 0.718–2.226; HR, 0.614; 95% CI, 0.317–1.190; and HR, 1.468; 95% CI, 0.788–2.735, respectively). Maintenance treatment was the only significant factor in multivariate analysis (HR, 0.472; 95% CI, 0.250–0.890; P=0.020) (Table 3).
Table 3. Univariate and multivariate analyses of progression-free survival.
| Variables | Univariate analysis | ||||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age (years) | 0.650 | ||||
| <60 | 1.000 | ||||
| ≥60 | 1.135 (0.657–1.962) | ||||
| Sex | 0.864 | 0.417 | |||
| Female | 1.000 | 1.000 | |||
| Male | 0.972 (0.705–1.341) | 1.264 (0.718–2.226) | |||
| Disease status | 0.174 | 0.148 | |||
| Initial metastasis | 1.000 | 1.000 | |||
| Recurrence | 0.667 (0.372–1.195) | 0.614 (0.317–1.190) | |||
| Previous adjuvant chemotherapy | 0.658 | ||||
| No | 1.000 | ||||
| Yes | 1.152 (0.617–2.151) | ||||
| Number of metastases | 0.990 | ||||
| 1 | 1.000 | ||||
| ≥2 | 1.003 (0.579–1.738) | ||||
| Liver metastasis | 0.342 | ||||
| No | 1.000 | ||||
| Yes | 1.357 (0.723–2.546) | ||||
| Peritoneal metastasis | 0.401 | ||||
| No | 1.000 | ||||
| Yes | 0.791 (0.457–1.368) | ||||
| Lung metastasis | 0.611 | ||||
| No | 1.000 | ||||
| Yes | 1.231 (0.553–2.737) | ||||
| Non-regional lymph node metastasis | 0.581 | ||||
| No | 1.000 | ||||
| Yes | 1.182 (0.653–2.138) | ||||
| Bone metastasis | 0.695 | ||||
| No | 1.000 | ||||
| Yes | 0.853 (0.384–1.893) | ||||
| Measurable lesion | 0.454 | ||||
| No | 1.000 | 1.000 | |||
| Yes | 1.226 (0.720–2.088) | 1.468 (0.788–2.735) | |||
| Smoking history | 0.668 | ||||
| Never or Ex-smoker | 1.000 | ||||
| Current smoker | 1.367 (0.328–5.698) | ||||
| Previous response to XELOX chemotherapy | 0.830 | 0.226 | |||
| Stable disease | 1.000 | ||||
| Partial response/Complete response | 1.061 (0.617–1.826) | ||||
| Histology subtype | 0.248 | ||||
| Well to moderate | 1.000 | ||||
| Poor | 1.204 (0.879–1.649) | ||||
| Serum CEA (ng/mL) | 0.754 | ||||
| ≤7.5 | 1.000 | ||||
| >7.5 | 0.930 (0.590–1.466) | ||||
| Maintenance treatment | 0.012 | 0.020 | |||
| No | 1.000 | 1.000 | |||
| Yes | 0.486 (0.277–0.852) | 0.472 (0.250–0.890) | |||
HR = hazard ratio; CI = confidence interval; CEA = carcinoembryonic antigen.
DISCUSSION
The hypothesis of this study was that PFS might be prolonged by continually exposing patients to chemotherapy agents compared to fixed cycles of XELOX. We selected PFS as the primary endpoint. A meta-analysis of randomized trials showed that improvements in PFS in advanced GC were closely associated with improvements in OS [17]. PFS has been used as a surrogate endpoint in many randomized clinical trials evaluating 1st line chemotherapy for metastatic GC.
The main findings of this population-based study were as follows. The primary endpoint of this study was that the capecitabine maintenance group showed significantly prolonged PFS compared with the observation group, although these two groups did not show statistically significant differences in OS. Regarding HFS toxicity, there were all-grade AEs (25.0%) and grade 3 AEs (12.5%) in the maintenance group. However, most of the toxicities were manageable. There was no interruption of capecitabine maintenance due to side effects. Maintenance treatment was the only significant factor associated with PFS in both the univariate and multivariate analyses.
Maintenance fluoropyrimidine therapy has been used to treat patients with advanced CRCs. The OPTIMOX2 study [18] showed that there was a significant difference in the duration of disease control between 5-fluorouracil (5-FU)/leucovorin (LV) maintenance and chemotherapy-free interval group (13.1 vs 9.2 months, P=0.046). This study provides an important clue that maintenance treatment could be an effective strategy to relieve the neuropathy induced by the cumulative effect of oxaliplatin.
In a phase II study published in 2011 [19], the authors evaluated the efficacy and safety of short-course XELOX followed by maintenance capecitabine in advanced CRCs. In the 34 patients allocated to the capecitabine maintenance group, the median PFS and OS were 8.1 and 23.1 months, respectively. Although it was not a randomized clinical trial, an OS of more than 20 months was much longer compared to the survival of previous studies.
The initial support for maintenance regimens in advanced GC comes from observational and retrospective studies, which show that maintenance fluoropyrimidine treatment might improve PFS compared to observation alone [20]. Petrioli et al. [21] studied maintenance chemotherapy with LV bolus and continuous infusion of 5-FU after administration of the 5-FU plus oxaliplatin (FOLFOX) regimen in patients with advanced esophagogastric cancer aged 75 years or older with impaired performance status. The results showed modest efficacy and tolerable toxicity of maintenance therapy. The authors selected LV bolus and continuous infusion of 5-FU as maintenance chemotherapy because elderly patients with impaired performance status might have severe difficulties with oral intake. Notably, the frequency of grade 3 neutropenia was considerable (15.7%), although it was not associated with treatment-related deaths.
Park et al. [22] reported a randomized phase II study of continuous versus stop-and-go S-1 plus oxaliplatin following disease stabilization in first-line chemotherapy in patients with metastatic GC. Continuing chemotherapy showed a PFS benefit in reducing the risk of progression or death by 45% compared to the stop-and-go strategy and increased median PFS by 3.3 months. (10.5 vs. 7.2 months, HR, 0.55; 95% CI, 0.37–0.81; P=0.002). However, maintenance chemotherapy improved PFS but not the duration of disease control or OS. In addition, maintenance treatment has a negative impact on quality of life. Therefore, the authors insisted that continuous maintenance with the same doublet regimen was not recommended in unselected patients with metastatic GC. Platinum induces cumulative toxicities, such as neuropathy; therefore, if it is maintained continuously, it will have a negative effect on the patient’s quality of life. Capecitabine is used with platinum in the treatment of 1st line chemotherapy and has the advantages of safety and ease of administration. Therefore, maintenance treatment with capecitabine may be a strategy to reduce toxicity while maintaining the effects of existing chemotherapy. To date, proven efficacy has not been found with several maintenance treatments for GC, although there have been positive results for capecitabine maintenance treatment in several retrospective or phase II trials [8].
A multicenter, open-label phase II trial [23] was conducted in China to compare the efficacy and safety of capecitabine as a maintenance treatment after 1st line chemotherapy for patients with advanced esophagogastric junction adenocarcinoma. When the initial disease was controlled after capecitabine-based first-line combination chemotherapy, 60 patients were randomly assigned to receive capecitabine treatment (oral capecitabine 1250 mg/m2 twice daily on days 1–14 every 3 weeks). Compared to controls, patients who received capecitabine maintenance therapy showed significantly prolonged PFS and OS (median PFS: 11 vs. 7 months, median OS: 17 vs. 11 months). There were no significant differences in the prevalence of AEs between the patients who received maintenance therapy and controls.
From 2008 to 2009, a prospective observational study [9] was performed to determine the role of capecitabine maintenance treatment after first-line chemotherapy in patients with advanced GC. Patients with grade 2 or higher neuropathies were enrolled after six cycles of oxaliplatin and capecitabine chemotherapy without disease progression. There was significant difference in median PFS between the maintenance and observation groups (11.4 vs. 7.1 months, P<0.001). In the multivariate analysis, the status of maintenance treatment was an independent prognostic factor, as well as the type of metastasis and the response to chemotherapy. Maintenance treatment showed mild hematologic toxicities with no febrile neutropenia or manageable non-hematologic toxicities.
Compared to previous studies on capecitabine maintenance treatment, the distinctive feature of our study was that it was the first randomized controlled phase III trial. This study compared the efficacy and toxicity of capecitabine maintenance and observation after 6 cycles of 1st line XELOX chemotherapy for HER2-negative GC. In this study, the dose of capecitabine was set to 1,000 mg/m2 b.i.d. Compared to the results of previous studies on capecitabine maintenance treatment, the frequency of more than grade 3 HFS (12.5%) was relatively low, and the toxicities were manageable.
The first limitation of this trial was that the sample size was small and did not meet the planned study recrutiment. Because there was a lack of evidence for maintenance chemotherapy in GC, patients tended to continue XELOX chemotherapy after six cycles. In addition, in some elderly patients, chemotherapy was stopped because of decreased patient performance and desire to continue treatment. Second, detailed quality of life data were not collected in this study.
Although the study sample size was small and the number of study subjects planned by the protocol was not met due to delayed patient registration, we found that the PFS of the maintenance group was statistically longer than that of the observation group. Considering that there were more patients with poor tissue differentiation in the maintenance group, the gain in PFS in the maintenance group was meaningful. However, there was not a statistically significant difference in the OS between the study and control groups. Maintenance treatment showed some side effects, such as HFS. The occurrence of HFS during capecitabine maintenance is thought to have a negative effect on quality of life. Capecitabine was administered with a slight dose reduction in the maintenance group given that the median RDI was 0.9.
Several studies evaluating the efficacy and side effects of maintenance treatment in HER2-negative advanced GC are currently underway. The Maintenance S-1 in Esophagogastric Cancer (MATEO) trial is a multinational, randomized phase II study that explores the role of S-1 maintenance therapy [24]. After 12 weeks of first-line platinum-fluoropyrimidine-based chemotherapy, patients without tumor progression were planned to be randomized in a 2:1 allocation to receive S-1 alone (Arm A) or continue with the same polychemotherapy regimen (Arm B) until tumor progression or limiting toxicity. Although the final results of the study have not been published, the results of non-Asian patients who participated in the MATEO trial have been reported [25]. Between November 2014 and April 2019, 165 patients were randomized after 12 weeks of induction therapy. S-1 as maintenance therapy showed comparable activity to prolonged platinum-based therapy with a favorable toxicity pattern. Therefore, it is necessary to wait for the results of the updated data.
Moreover, there is a need to incorporate new drugs into maintenance therapies. For immunotherapy, Moehler et al. [26] reported results from a phase III JAVELIN Gastric 100 trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with GC. Patients without progressive disease after 12 weeks of first-line chemotherapy with oxaliplatin plus fluoropyrimidine were randomly assigned to receive avelumab maintenance or continuation of 1st line chemotherapy. This trial did not meet the primary endpoint of OS. However, avelumab maintenance had a more favorable toxicity profile than continuation of chemotherapy. It showed a prolonged duration of response and potentially increased benefits in some subgroups. In the future, it will be necessary to confirm the efficacy and safety of novel agents for the maintenance therapy of advanced GC.
In conclusion, this phase III trial demonstrated that maintenance with capecitabine could significantly prolong PFS. Considering its effect and acceptable toxicity, maintenance capecitabine may be a reasonable option after stabilization following 6 cycles of XELOX. However, the increase in OS due to maintenance therapy was not proven in this study. Further clinical trials on novel maintenance treatments are warranted to improve the survival of patients with advanced GC.
ACKNOWLEDGMENTS
The authors thank all patients and investigators who participated in this study. We also thank Roche, Ltd. (Korea) for supplying the capecitabine.
Footnotes
Funding: This study was partly supported by Roche, Ltd. (Korea). This study was an investigator-initiated trial. The company was not involved in the interpretation of the data; nor the preparation, review, or approval of the manuscript; nor the decision to submit this study for publication. The corresponding author has full authority over the research data. The corresponding author was responsible for the final decision to submit this study for publication.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: L.G.J., A.H.J., K.Y.H., R.S.Y., S.B.Y.
- Data curation: L.G.J., K.H., C.S.S., P.H.S., A.H.J., B.J.H., H.J.H., S.D.S., J.J.Y., P.J.C., K.I.H., S.B.Y.
- Formal analysis: L.G.J., K.H., A.H.J., S.B.Y.
- Investigation: L.G.J., K.H., A.H.J., S.B.Y.
- Methodology: L.G.J., A.H.J., K.Y.H., R.S.Y., S.B.Y.
- Resources: L.G.J., K.H., A.H.J., W.I.S., K.Y.H., W.H.S., J.J.Y., P.J.C., R.S.Y., S.B.Y.
- Software: L.G.J., K.H., A.H.J., W.I.S., K.Y.H., W.H.S., J.J.Y., P.J.C., R.S.Y., S.B.Y.
- Writing - original draft: L.G.J., K.H., S.B.Y.
- Writing - review & editing: L.G.J., K.H., S.B.Y.
References
- 1.Faivre J, Forman D, Estève J, Gatta G EUROCARE Working Group. Survival of patients with oesophageal and gastric cancers in Europe. Eur J Cancer. 1998;34:2167–2175. doi: 10.1016/s0959-8049(98)00329-3. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Muro K, Van Cutsem E, Narita Y, Pentheroudakis G, Baba E, Li J, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:19–33. doi: 10.1093/annonc/mdy502. [DOI] [PubMed] [Google Scholar]
- 4.Catalano V, Graziano F, Santini D, D’Emidio S, Baldelli AM, Rossi D, et al. Second-line chemotherapy for patients with advanced gastric cancer: who may benefit? Br J Cancer. 2008;99:1402–1407. doi: 10.1038/sj.bjc.6604732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 6.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 7.Carter GC, Kaltenboeck A, Ivanova J, Liepa AM, San Roman A, Koh M, et al. Real-world treatment patterns among patients with advanced gastric cancer in South Korea. Cancer Res Treat. 2017;49:578–587. doi: 10.4143/crt.2016.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eren OO, Ozturk MA, Sonmez OU, Oyan B. Safety, feasibility, and efficacy of capecitabine maintenance in patients with advanced gastric cancer: a retrospective study. Am J Ther. 2016;23:e1493–e1497. doi: 10.1097/MJT.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 9.Qiu MZ, Wei XL, Zhang DS, Jin Y, Zhou YX, Wang DS, et al. Efficacy and safety of capecitabine as maintenance treatment after first-line chemotherapy using oxaliplatin and capecitabine in advanced gastric adenocarcinoma patients: a prospective observation. Tumour Biol. 2014;35:4369–4375. doi: 10.1007/s13277-013-1574-5. [DOI] [PubMed] [Google Scholar]
- 10.Yao Y, Deng R, Liao D, Xie H, Zuo J, Jia Y, et al. Maintenance treatment in advanced HER2-negative gastric cancer. Clin Transl Oncol. 2020;22:2206–2212. doi: 10.1007/s12094-020-02379-7. [DOI] [PubMed] [Google Scholar]
- 11.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition) Gastric Cancer. 2021;24:1–21. doi: 10.1007/s10120-020-01042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park YH, Lee JL, Ryoo BY, Ryu MH, Yang SH, Kim BS, et al. Capecitabine in combination with oxaliplatin (XELOX) as a first-line therapy for advanced gastric cancer. Cancer Chemother Pharmacol. 2008;61:623–629. doi: 10.1007/s00280-007-0515-7. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Liu YJ, Zhu GP, Guan XY. Comparison of the NCI-CTCAE version 4.0 and version 3.0 in assessing chemoradiation-induced oral mucositis for locally advanced nasopharyngeal carcinoma. Oral Oncol. 2012;48:554–559. doi: 10.1016/j.oraloncology.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Lakatos E. Sample sizes based on the log-rank statistic in complex clinical trials. Biometrics. 1988;44:229–241. [PubMed] [Google Scholar]
- 17.Shitara K, Ikeda J, Yokota T, Takahari D, Ura T, Muro K, et al. Progression-free survival and time to progression as surrogate markers of overall survival in patients with advanced gastric cancer: analysis of 36 randomized trials. Invest New Drugs. 2012;30:1224–1231. doi: 10.1007/s10637-011-9648-y. [DOI] [PubMed] [Google Scholar]
- 18.Chibaudel B, Maindrault-Goebel F, Lledo G, Mineur L, André T, Bennamoun M, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol. 2009;27:5727–5733. doi: 10.1200/JCO.2009.23.4344. [DOI] [PubMed] [Google Scholar]
- 19.Waddell T, Gollins S, Soe W, Valle J, Allen J, Bentley D, et al. Phase II study of short-course capecitabine plus oxaliplatin (XELOX) followed by maintenance capecitabine in advanced colorectal cancer: XelQuali study. Cancer Chemother Pharmacol. 2011;67:1111–1117. doi: 10.1007/s00280-010-1322-0. [DOI] [PubMed] [Google Scholar]
- 20.Roviello G, Rodriquenz MG, Aprile G, D’Angelo A, Roviello F, Nobili S, et al. Maintenance in gastric cancer: new life for an old issue? Crit Rev Oncol Hematol. 2021;160:103307. doi: 10.1016/j.critrevonc.2021.103307. [DOI] [PubMed] [Google Scholar]
- 21.Petrioli R, Francini E, Roviello F, Marrelli D, Miano ST, Fiaschi AI, et al. Treatment of advanced oesophagogastric cancer with FOLFOX-4 regimen followed by leucovorin/bolus and continuous infusion 5-FU as maintenance chemotherapy in patients aged ≥ 75 years with impaired performance status. J Geriatr Oncol. 2015;6:380–386. doi: 10.1016/j.jgo.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Park SR, Kim MJ, Nam BH, Kim CG, Lee JY, Cho SJ, et al. A randomised phase II study of continuous versus stop-and-go S-1 plus oxaliplatin following disease stabilisation in first-line chemotherapy in patients with metastatic gastric cancer. Eur J Cancer. 2017;83:32–42. doi: 10.1016/j.ejca.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Lu B, Bao LB, Sun Z, Hua ZL, Wang X, Qu CP. Efficacy and safety of capecitabine as maintenance therapy after capecitabine-based combination chemotherapy for patients with advanced esophagogastric junction adenocarcinoma. Eur Rev Med Pharmacol Sci. 2015;19:3605–3612. [PubMed] [Google Scholar]
- 24.Haag GM, Stocker G, Quidde J, Jaeger D, Lordick F. Randomized controlled trial of S-1 maintenance therapy in metastatic esophagogastric cancer - the multinational MATEO study. BMC Cancer. 2017;17:509. doi: 10.1186/s12885-017-3497-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haag GM, Stocker G, Lorenzen S, Ettrich TJ, Longo F, Kiani A, et al. 1447P S-1 maintenance therapy in non-Asian patients with advanced, HER-2 negative esophagogastric adenocarcinoma – first results of the international MATEO trial initiated by the AIO. Ann Oncol. 2020;31:S910. [Google Scholar]
- 26.Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu MH, Muntean AS, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN Gastric 100. J Clin Oncol. 2021;39:966–977. doi: 10.1200/JCO.20.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]